Abstract
Background
Ionizing radiation is an established carcinogen, but risks from low-dose exposures are controversial. Since the Biological Effects of Ionizing Radiation VII review of the epidemiological data in 2006, many subsequent publications have reported excess cancer risks from low-dose exposures. Our aim was to systematically review these studies to assess the magnitude of the risk and whether the positive findings could be explained by biases.
Methods
Eligible studies had mean cumulative doses of less than 100 mGy, individualized dose estimates, risk estimates, and confidence intervals (CI) for the dose-response and were published in 2006–2017. We summarized the evidence for bias (dose error, confounding, outcome ascertainment) and its likely direction for each study. We tested whether the median excess relative risk (ERR) per unit dose equals zero and assessed the impact of excluding positive studies with potential bias away from the null. We performed a meta-analysis to quantify the ERR and assess consistency across studies for all solid cancers and leukemia.
Results
Of the 26 eligible studies, 8 concerned environmental, 4 medical, and 14 occupational exposure. For solid cancers, 16 of 22 studies reported positive ERRs per unit dose, and we rejected the hypothesis that the median ERR equals zero (P = .03). After exclusion of 4 positive studies with potential positive bias, 12 of 18 studies reported positive ERRs per unit dose (P = .12). For leukemia, 17 of 20 studies were positive, and we rejected the hypothesis that the median ERR per unit dose equals zero (P = .001), also after exclusion of 5 positive studies with potential positive bias (P = .02). For adulthood exposure, the meta-ERR at 100 mGy was 0.029 (95% CI = 0.011 to 0.047) for solid cancers and 0.16 (95% CI = 0.07 to 0.25) for leukemia. For childhood exposure, the meta-ERR at 100 mGy for leukemia was 2.84 (95% CI = 0.37 to 5.32); there were only two eligible studies of all solid cancers.
Conclusions
Our systematic assessments in this monograph showed that these new epidemiological studies are characterized by several limitations, but only a few positive studies were potentially biased away from the null. After exclusion of these studies, the majority of studies still reported positive risk estimates. We therefore conclude that these new epidemiological studies directly support excess cancer risks from low-dose ionizing radiation. Furthermore, the magnitude of the cancer risks from these low-dose radiation exposures was statistically compatible with the radiation dose-related cancer risks of the atomic bomb survivors.
The evidence for cancer risks provided by epidemiological studies of low-dose ionizing radiation exposure is of key relevance to radiation protection because a large fraction of the population is exposed to low doses of ionizing radiation from diagnostic medical procedures or occupationally, in addition to natural background radiation. Careful and sophisticated interpretation of the results from these studies is required, however, because the risks are likely to be small compared with those associated with nonradiation risk factors, studies may have power below 80% (the conventional threshold of adequacy in this respect), dose estimation may be limited and/or retrospective, and studies may suffer from biases typical of observational studies such as confounding.
The last major US review of the epidemiological and experimental evidence for cancer risks from low-dose exposures (which we denote as <100 mGy) in 2006 concluded that “the available scientific evidence is consistent with a linear dose-response relationship between ionizing radiation and the development of cancer in humans” (1). This conclusion was largely based on studies of populations exposed to higher doses combined with experimental data. Subsequent to 2006, several new epidemiological studies of populations exposed primarily to low doses have been published, and several existing studies have reported new results from extended follow-up. Most of these new publications report excess cancer risks from low-dose radiation exposures. The aim of this monograph is to systematically evaluate whether there is direct human evidence of excess cancer risks from low-dose (<100 mGy) radiation exposure, and if so, what the magnitude of the risk is and whether the positive findings could be explained by biases.
Here, we provide a synthesis from our in-depth systematic assessments of the methodology for the eligible studies published during 2006–2017 that we evaluated and the associated potential for the risk estimates to be biased because of dose error, confounding, selection bias, or outcome misclassification (2–5). We assess for each study the direction of the biases from any of these sources. As a general evaluation of whether the studies support cancer risks from low-dose ionizing radiation, we conducted a sign test for whether the median of the excess relative risks (ERRs) equals zero and then calculated the impact of excluding the positive studies identified as being biased away from the null. Finally, to quantify the magnitude of the estimated risks, we conduct a meta-analysis for all solid cancers and for leukemia from childhood or adulthood exposure to low-dose ionizing radiation.
Methods
We included epidemiological studies published since the Biological Effects of Ionizing Radiation VII report in 2006 (1) and before 2018. Studies were eligible for inclusion if they were based on human populations exposed to low-dose, predominantly low-linear energy transfer, radiation (mean cumulative dose < 100 mGy). We required individualized dose estimates for the study participants and that the publications provided risk estimates and confidence intervals (CI) for the dose-response for cumulative radiation dose. For a full description of the eligible studies, see the overview paper in this monograph (6). In brief, we used the results of the methodological assessments conducted in this monograph [dosimetry (2), confounding (5), outcome (4)] to derive, for each aspect, an assessment of the potential for bias in the risk estimate and the direction of the bias.
Summarizing the evaluations of the different study aspects was as follows. We assessed the strengths and weaknesses of dosimetry systems with respect to the directness, complexity, and completeness of the dosimetry, the dosimetric uncertainty, and the validity of dose estimates (2). This process identified studies with a known or suspected bias in dose estimates and the likely direction of the bias in the risk estimate.
In assessing the evidence for confounding and selection bias, we summarized methods to control confounding and assessed the likelihood of uncontrolled confounding as well as its direction (5). This assessment was based on available data from the eligible studies and related publications including some examples of quantitative bias assessment to examine the potential magnitude of the bias.
The outcome evaluation paper in this monograph reviewed the possible impact of differential outcome ascertainment across radiation dose levels (4). The evaluation also considered loss to follow-up, under- or overascertainment of cancer outcomes, misclassification of outcomes, and changing classifications over time. The main objective was to identify studies whose outcome ascertainment was differential regarding exposure level and, hence, to bias the relative risk estimate.
We then performed a summary of the assessments of different biases for each study and carefully considered both the direction of the observed effect and the direction of the bias. For our general question of whether the studies overall support excess cancer risks (as opposed to the question of the magnitude of the risk), our priority was to identify the positive studies with bias in the positive direction or bias of uncertain direction. If there were several biases acting in different directions, or it was not possible to determine the potential direction, we classified these studies as potentially biased away from the null. Because the magnitude of bias is difficult to determine from the published reports, we conservatively counted any study with a negative ERR estimate as negative regardless of any potential for bias. We also indicated whether the estimated power (if available) was low (<50%) or reasonable (≥50%) (3).
We performed a one-sided sign test for the reported ERRs, separately for solid cancers and for leukemia, to evaluate the hypothesis that the median of the ERRs per unit dose equals zero vs the alternative that the median ERR per unit dose exceeds zero (7). The sign test excluded the INWORKS study (8, 9) because it is a pooled analysis of UK, French, and US nuclear workers, which were included separately. To assess the impact of the studies identified as potentially biased, we then repeated the sign test after excluding the studies where bias adjustment could move a positive ERR toward the null. A one-sided P value less than .05 was considered statistically significant.
Finally, we conducted a meta-analysis of the published ERR estimates at 100 mGy to quantify the magnitude of the risk and to assess the consistency across studies for both all solid cancers and leukemia. Here we excluded the INWORKS study (8, 9) because of overlap as described above and the US Radiologic Technologists (USRT) studies (10–12) because only site-specific ERRs were reported. All other studies were included regardless of potential bias because, as described above, we generally could not quantify the magnitude of the bias and hence the magnitude of bias-corrected ERRs and because only excluding the subset of positive studies with positive biases could bias the summary risk estimate toward the null. We generated standard errors of the ERR at 100 mGy based on the upper (UL) and lower limit (LL) of the confidence interval as (UL–LL)/2*1.96 or (UL–LL)/2*1.645, depending on whether 95% or 90% confidence intervals were reported, respectively. We acknowledge that this is an approximation that may not be fully adequate given the skewed dose distributions and small numbers of cases in some of the studies. The meta-ERR estimate was derived from a random effects model using the iterative method of Paule and Mandel (13) as outlined in DerSimonian and Kacker (14). We also computed Cochran's Q, which is the weighted sum of squared differences between individual study effects and the pooled effect across studies, to test homogeneity, as well as the I2 statistic [variance due to heterogeneity (15)]. If homogeneity was rejected, we excluded the studies with the largest signed contribution to the Q statistic. Meta-ERRs were calculated separately for studies on adult solid cancers, adult leukemia, and childhood leukemia. We did not calculate meta-ERR estimates for childhood solid cancers two studies reported ERRs.
Results
Of the 26 eligible studies, we found that 3 studies had a known or suspected bias in dose estimates that could bias the risk estimate away from the null because of possible recall or selection bias [Chornobyl residents (16), Chornobyl liquidators (17), Ukrainian Chornobyl liquidators (18); Tables 1 and 2] and 1 study that was likely biased toward the null [Three Mile Island, leukemia (19)]. The direction of potential dose bias was uncertain in the USRT study on breast cancer (12). Among the 3 case-control studies of leukemia in Chornobyl liquidators (17, 18) and residents (16), individual dose estimates relied extensively on information obtained by interview after case ascertainment. In 2 of these studies there was evidence from the manuscripts that the risk estimate was reduced after exclusions of a study center [Chornobyl residential childhood leukemia study (16)] and proxy respondents [Ukrainian liquidators leukemia study (18)].
Table 1.
Assessment of bias from several sources for studies of solid cancers
| Study name | Reference | ERR at 100 mGy (95% CI) | ERR per unit of dose bias |
Could bias adjustment move ERR toward null?* | Estimated power† | ||
|---|---|---|---|---|---|---|---|
| Dose error | Confounding/selection bias | Outcome misclassification | |||||
| Environmental | |||||||
| Three Mile Island | Han et al., 2011 (19) | −1 (−6 to 3) | ↓ | — | — | Yes. Adjustment possibly moves ERR toward null.‖ | NC |
| Chinese background | Tao et al., 2012 (20) | −0.101 (−0.253 to 0.095) | — | — | ↕ | Uncertain. Adjustment could move ERR toward or away from null. | Low |
| GB background | Kendall et al., 2013 (21) | 2 (−2.0 to 6.0) | — | — | — | — | NC |
| Swiss background | Spycher et al., 2015 (22) | 2.8 (0.8 to 4.8) | — | — | — | — | NC |
| Techa River | Davis et al., 2015 (23) | 0.077 (0.013 to 0.150) | — | — | — | — | Reasonable |
| Taiwanese residents | Hsieh et al., 2017 (24) | 0.04 (0.01 to 0.08)‡ | — | — | — | — | Low |
|
Medical | |||||||
| Canadian cardiac imaging | Eisenberg et al., 2011 (25) | 0.3 (0.2 to 0.4) | — | ↕ | ↑ | Uncertain. Adjustment could move ERR toward or away from null. | NC |
| French Pediatric CT (brain tumors) | Journy et al., 2016 (26) | 0.7 (−0.1 to 1.0) | — | — | — | — | Low |
| UK Pediatric CT (brain tumors) | Berrington et al., 2016 (27) | 1.2 (0.4 to 3.1) | — | — | — | — | Reasonable |
| PIRATES (thyroid cancer) | Lubin et al., 2017 (28) | 0.96 (0.37 to 1.70) | — | — | — | — | Reasonable |
|
Occupational | |||||||
| Korean workers | Ahn et al., 2008 (29) | 0.72 (−0.5 to 2.1)‡ | — | ↓ | ↑ | Uncertain. Adjustment could move ERR toward or away from null. | NC |
| UKNRRW | Muirhead et al., 2009 (30) | 0.03 (0 to 0.056) | — | ↓ | — | No. Adjustment would move ERR away from null. | Reasonable |
| Korean nuclear workers | Jeong et al., 2010 (31) | 0.21 (−0.19 to 0.9) | — | ↓ | — | No. Adjustment would move ERR away from null. | Low |
| Rocketdyne workers | Boice et al., 2011 (32) | −0.02 (−0.18 to 0.17) | — | ↓ | — | No. Adjustment would move ERR away from null. | Low |
| Japanese workers | Akiba et al., 2012 (33) | 0.13 (−0.03 to 0.30) | — | ↕ | ↓ | Uncertain. Adjustment could move ERR toward or away from null. | Low |
| Canadian nuclear workers§ | Zablotska et al., 2014 (34) | −0.12 (<-0.15 to 0.24) | — | ↓ | — | Yes. Adjustment possibly moves ERR toward null.‖ | NC |
| German nuclear workers | Merzenich et al., 2014 (35) | −0.1 (−0.4 to 0.1) | — | ↓ | — | Yes. Adjustment possibly moves ERR toward null.‖ | NC |
| US nuclear workers | Schubauer-Berigan et al., 2015 (36) | 0.01 (−0.02 to 0.05) | — | ↓ | — | No. Adjustment would move ERR away from null. | Low |
| INWORKS | Richardson et al., 2015 (9) | 0.047 (0.018 to 0.079)‡ | — | ↓ | — | No. Adjustment would move ERR away from null. | Reasonable |
| USRT (breast cancer) | Preston et al., 2016 (12) | 0.07 (−0.005 to 0.19) | ↕ | — | — | Uncertain. Adjustment could move ERR toward or away from null. | Low |
| USRT (brain cancer) | Kitahara et al., 2017 (10) | 0.1 (<-0.3 to 1.5) | — | — | — | — | Low |
| USRT (skin cancer) | Lee et al., 2015 (11) | −0.001 (−0.04 to 0.05) | — | — | — | — | Reasonable |
| French nuclear workers | Leuraud et al 2017 (37) | 0.04 (−0.04 to 0.13)‡ | — | ↓ | — | No. Adjustment would move ERR away from null. | Low |
Reflects an assessment of the presence of bias of the ERR and its likely direction, but not statistical significance. ↓ = bias in the negative direction; ↑ = bias in the positive direction; ↕ = bias of unclear direction; CI = confidence interval; ERR = excess relative risk; NC = not calculated because dose distributions needed for power calculations were not available.
Low: <50%; reasonable: ≥50% based on Life Span Study ERR and published dose distributions.
90% CI.
The Canadian Study is restricted to the cohort excluding early Atomic Energy of Canada Limited (AECL) workers.
If bias is sufficiently large and additive, adjustment could increase the ERR above null. Because the magnitude of bias is difficult to determine from the published reports, we conservatively assume that adjustment of bias will not increase the ERR to positive.
Table 2.
Assessment of bias from several sources for studies of leukemia
| Study name | Reference | ERR at 100 mGy (95% CI) | ERR per unit of dose bias |
Could bias adjustment move ERR toward null?† | Estimated power* | |||
|---|---|---|---|---|---|---|---|---|
| Dose error | Confounding/ selection bias | Outcome misclassification | ||||||
| Environmental | ||||||||
| Chornobyl residents | Davis et al., 2006 (16) | 3.2 (0.9 to 8.4) | ↑ | ↑ | — | Yes. Exclusion of subgroup with potential recall bias reduced risk to null. | NC | |
| Three Mile Island | Han et al., 2011 (19) | 19 (−3 to 45) | ↓ | — | — | No. Adjustment would move ERR away from null. | NC | |
| Chinese background | Tao et al., 2012 (20) | 1.068 (<0 to inf) | — | — | ↕ | Uncertain. Adjustment could move ERR toward or away from null. | Low | |
| GB background | Kendall et al., 2013 (21) | 12 (3.0 to 22.0) | — | — | — | — | Reasonable | |
| Swiss background | Spycher et al., 2015 (22) | 3.6 (−0.3 to 7.7) | — | — | — | — | Low | |
| Finnish background | Nikkila et al., 2016 (38) | −3 (−11 to 6) | — | — | — | — | NC | |
| Taiwanese residents | Hsieh et al., 2017 (24) | 0.15 (0.03 to 0.24)‡ | — | — | — | — | Low | |
|
Medical |
||||||||
| French Pediatric CT | Journy et al., 2016 (26) | 1.6 (−2.3 to 2.7) | — | — | — | — | Low | |
| UK Pediatric CT | Berrington et al., 2016 (27) | 3 (0.3 to 10.9) | — | — | — | — | Low | |
|
Occupational |
||||||||
| Korean workers | Ahn et al., 2008 (29) | 1.68 (−3.4 to 14.9)‡ | — | ↓ | ↑ | Uncertain. Adjustment could move ERR toward or away from null. | NC | |
| Chornobyl liquidators | Kesminiene et al., 2008 (17) | 0.5 (−0.38 to 5.70)‡ | ↑ | — | — | Yes. Adjustment possibly moves ERR to null. | Low | |
| UKNRRW | Muirhead et al., 2009 (30) | 0.18 (−0.006 to 0.50) | — | — | — | — | Low | |
| Rocketdyne workers | Boice et al., 2011 (32) | 0.06 (−0.50 to 1.23) | — | — | — | — | Low | |
| Japanese workers | Akiba et al., 2012 (33) | −0.19 (−0.61 to 0.86) | — | ↓ | ↓ | Yes. Adjustment possibly moves ERR toward null.‖ | Low | |
| Ukrainian Chornobyl liquidators | Zablotska et al., 2013 (18) | 0.221 (0.005 to 0.761) | ↑ | — | — | Yes. Adjustment possibly moves ERR toward null. | NC | |
| Canadian nuclear workers§ | Zablotska et al., 2014 (34) | 1.44 (<−0.15 to 14.6) | — | — | — | — | NC | |
| German nuclear workers | Merzenich et al., 2014 (35) | 0.4 (−0.3 to 1.1) | — | ↓ | — | No. Adjustment would move ERR away from null. | NC | |
| US nuclear workers | Schubauer-Berigan et al., 2015 (36) | 0.17 (−0.02 to 0.47) | — | — | — | — | Reasonable | |
| INWORKS | Richardson et al., 2015 (9) | 0.3 (0.12 to 0.52)‡ | — | — | — | — | Reasonable | |
| US atomic veterans | Caldwell et al., 2016 (39) | −0.5 (−14 to 4) | — | — | — | — | Low | |
| French nuclear workers | Leuraud et al., 2017 (37) | 0.35 (<0 to 1.6)‡ | ↓ | — | No. Adjustment would move ERR away from null. | Low | ||
Low: <50%; reasonable: ≥50%. ↓ = bias in the negative direction; ↑ = bias in the positive direction; CI = confidence interval; ERR = excess relative risks; NC = not calculated because dose distributions needed for power calculations were not available.
Reflects an assessment of the presence of bias of the ERR and its likely direction, but not statistical significance.
90% CI.
The Canadian Study is restricted to the cohort excluding early AECL workers.
If bias is sufficiently large and additive, adjustment could increase the ERR above null. Because the magnitude of bias is difficult to determine from the published reports, we conservatively assume that adjustment of bias will not increase the ERR to positive.
Our evaluation of confounding and selection bias identified several sources that could bias the risk estimates: clinical indication for studies of medical diagnostic exposures during childhood and lifestyle factors for environmental, medical, and occupational studies of adult cancers, and for occupational studies, other workplace exposures and healthy worker survivor bias. In addition to the impact of a potential dose error considered above (2), the Chornobyl residential case-control study (16) may also have suffered from control selection bias that may have upwardly biased the risk estimate. We assessed that a potential for uncontrolled confounding biasing the risk estimate toward the null from healthy worker survivor bias was especially likely for the Korean workers (29) and Japanese nuclear workers (33) studies, which did not adjust for socioeconomic status or duration of employment, and the German nuclear workers study (35), which did not adjust for birth cohort and socioeconomic status (Tables 1 and 2). For the solid cancer results in the Canadian (34) and German nuclear workers study (35) and the leukemia findings in the Japanese nuclear workers study (33), bias adjustment would move the ERR toward the null. For the Japanese nuclear workers, the direction of the bias was uncertain, given that smoking may be a positive confounder in this cohort (40). Therefore, we could not draw a definitive conclusion on the impact of bias adjustment with the available data.
Four studies may have had cancer ascertainment possibly differential by radiation exposure, which could have biased the risk estimate. These include the Japanese nuclear workers (33) with bias toward the null through early loss to follow-up during periods with higher radiation exposure; the Chinese background study (20) with possible bias away from the null because of a higher loss to follow-up in regions with lower background radiation exposure compared with regions with high background radiation exposure; the cardiovascular imaging study (25), possibly biased away from the null because those undergoing diagnostic or therapeutic procedures with imaging were more likely to have cancer outcomes detected; and the Korean radiation workers population (29), where medical surveillance was required for radiation workers but not for the general population or for the worker comparison group (manufacture of motor vehicles) (Tables 1 and 2).
When considering the 3 potential biases for the 22 studies of solid cancers, there were 3 studies with negative ERR estimates where adjustment would likely move the ERR toward the null [Three Mile Island (19), Canadian (34), and German nuclear workers (35)] and 4 studies with positive ERR estimates where it was uncertain whether adjustment would move the ERR toward or away from the null [Canadian cardiac imaging (25), Korean (29) and Japanese workers (33), and USRT breast cancer (12); Table 1]. For leukemia, there was 1 study with a negative ERR where adjustment would likely have moved the ERR toward the null [Japanese workers (33)], 3 studies with a positive ERR where adjustment would likely have moved the ERR toward the null [Chornobyl residents (16), Chornobyl liquidators (17), and Ukrainian Chornobyl liquidators (18)], and 2 positive studies where the direction of the bias was uncertain [Chinese background (20) and Korean workers (29); Table 2].
For solid cancers, 16 of 22 studies reported a positive ERR, leading to rejection of the hypothesis that the median ERR per unit dose equals zero (P = .03) from the sign test (Supplementary Table 1, available online). After exclusion of the 4 studies for which bias adjustment could move a positive ERR toward null [Canadian cardiac imaging (25), Korean workers (29), Japanese nuclear workers (33), and USRT breast cancer (12)], 12 of the remaining 18 studies were positive (P = .12). For leukemia, 17 of 20 studies were positive, and we rejected the hypothesis that the median ERR per unit dose equals zero (P = .001) (Supplementary Table 2, available online). This conclusion was not changed by the exclusion of 5 studies [Chornobyl residents (16), Chinese background (20), Korean workers (29), Chornobyl liquidators (17), and Ukrainian Chornobyl liquidators (18)] for which bias adjustment could move a positive ERR toward null (P = .02).
Power to reject the null for these studies was evaluated under atomic bomb survivor–based alternative hypotheses. For all cancers except leukemia, studies with a statistically significantly elevated ERR (at the 5% level) had reasonable power (≥50%), whereas studies with statistically nonsignificant ERR estimates had low power (<50%). The only exception was the basal cell carcinoma analysis within the USRT study (11), with a statistically nonsignificant negative ERR estimate in the presence of reasonable power (Table 1). For leukemia, the pattern was less clear. Although the power of studies with statistically nonsignificant ERR estimates was generally low, 3 studies with statistically significant ERR estimates were estimated to have low power [UKNRRW (30), UK pediatric CT (27), and Taiwanese residents (24)] and 2 studies to have reasonable power [Great Britain (GB) background (21) and INWORKS (8)] (Table 2).
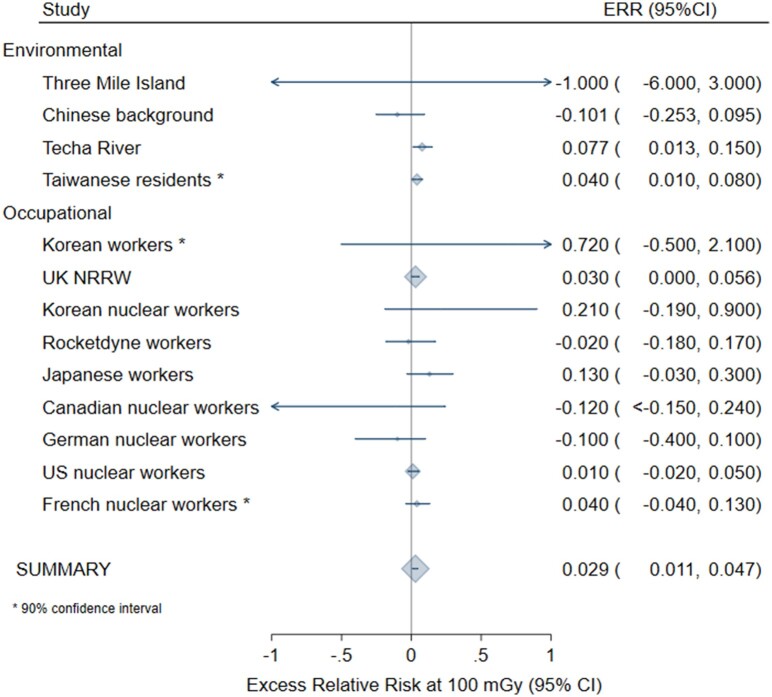
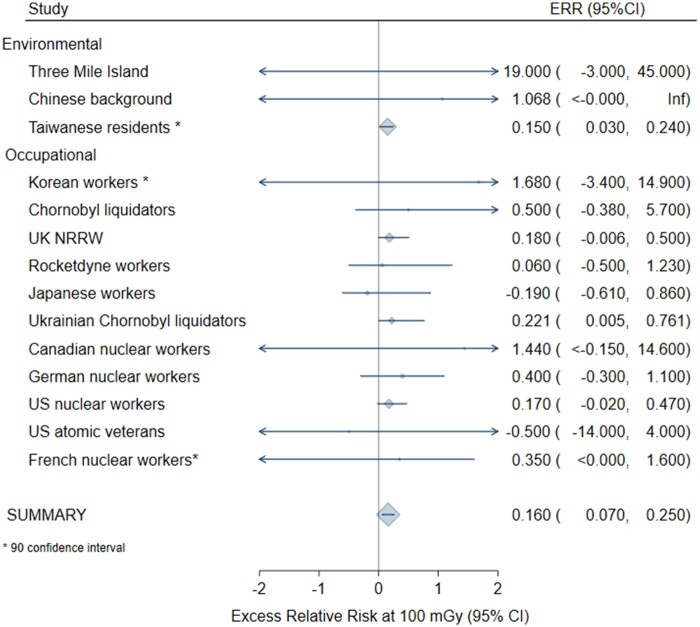
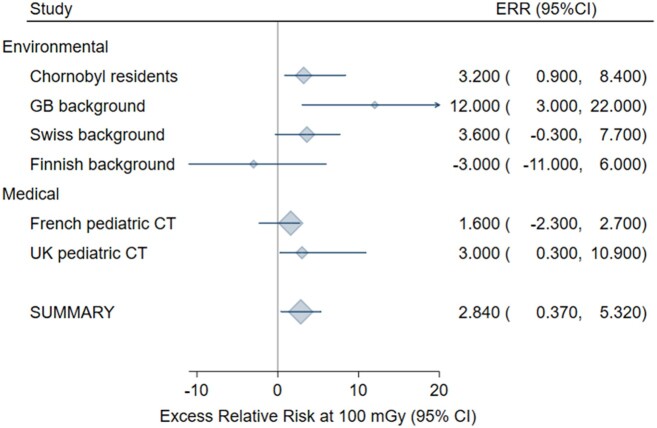
In the meta-anaylsis for all solid cancers following adulthood exposure, homogeneity was not rejected only after exclusion of the Canadian cardiac imaging study (25) (P = .63), which introduced statistically significant heterogeneity because of the very small standard deviation in relation to the size of the ERR. Based on the remaining 13 studies, the meta-ERR at 100 mGy was 0.029 (95% CI = 0.011 to 0.047) with 10% of the variability explained by heterogeneity (Figure 1; Table 3). For leukemia following adulthood exposure (n = 14 studies), the meta-ERR at 100 mGy was 0.16 (95% CI = 0.07 to 0.25) with no indication of heterogeneity (P = .99) (Table 3; Figure 2). Homogeneity was not rejected for 6 studies of leukemia after childhood exposure (P = .27) with a meta-ERR at 100 mGy of 2.84 (95% CI = 0.37 to 5.32) (Table 3; Figure 3).
Figure 1.
Meta-analysis of the excess relative risk (ERR) at 100 mGy for all solid cancers after adulthood radiation exposure. The size of the ERR symbol is proportional to the inverse variance of the study-specific ERR. CI = confidence interval.
Table 3.
Meta-analysis of excess relative risks (ERR) at 100 mGy for all solid cancers and leukemia
| Outcome | No. of studies | ERR at 100 mGy (95% CI) | P | Cochran Q (P) | I 2 |
|---|---|---|---|---|---|
| Adult solid cancer | 14* | 0.055 (−0.0027, 0.112) | .03 | 37.25 (<.001) | 0.65 |
| Adult solid cancer excluding the Canadian cardiovascular imaging study due to heterogeneity | 13* | 0.029 (0.011, 0.047) | <.001 | 9.89 (.63) | NA |
| Adult leukemia | 14† | 0.160 (0.070 to 0.250) | <.001 | 4.12 (.99) | NA |
| Childhood leukemia | 6 | 2.840 (0.370 to 5.320) | .01 | 6.40 (.27) | 0.22 |
Excluding INWORKS and site-specific results from US Radiologic Technologists.
Excluding INWORKS.
Figure 2.
Meta-analysis of the excess relative risk (ERR) at 100 mGy for leukemia after adulthood radiation exposure. The size of the ERR symbol is proportional to the inverse variance of the study-specific ERR. CI = confidence interval.
Figure 3.
Meta-analysis of the excess relative risk (ERR) at 100 mGy for leukemia after childhood radiation exposure. The size of the ERR symbol is proportional to the inverse variance of the study-specific ERR. CI = confidence interval.
Discussion
This summary report combines the results of our detailed assessments of potential biases in risk estimates for the 26 eligible human studies on low-dose radiation exposure and cancer risk. Most of the studies reported positive ERRs: 16 of 22 studies of solid cancers and 17 of 20 studies of leukemia. After a systematic assessment of methodological issues that may be associated with the potential for bias in the risk estimate, we concluded that only a small subset of the positive studies had biases where adjustment would move the risk estimate toward the null. The sign test rejected the hypothesis of no radiation effect for solid cancers and leukemia; for leukemia, this is true even after exclusion of the positive studies that were possibly biased away from the null (n = 5). For solid cancer, the evidence was borderline after exclusion of positive studies that were possibly biased away from the null (n = 4). Finally, a meta-analysis of the published risk estimates yielded statistically significantly elevated risks for leukemia separately among children and among adults and a statistically significantly elevated risk for solid cancers among adults.
For solid cancers following adulthood exposure, the summary risk estimate from our meta-analysis of 0.029 was very similar to the recent estimate from the Life Span Study (LSS) for males of 0.027 at 100 mGy but lower than the estimate for females of 0.064 (41). Because most of the studies of solid cancers following adulthood exposure were from nuclear workers, the comparison with the males from the LSS is probably most appropriate. For leukemia, our summary risk estimate for adulthood exposure of 0.16 at 100 mGy is double the most recent risk estimate from the LSS of 0.08 at 100 mGy for males and females combined, although statistically compatible with the 95% CI (0.003 to 0.19) (42). Our estimate is very similar to the meta-estimate of 0.19 (95% CI = 0.07 to 0.32) based on 10 studies of protracted exposure to low-dose radiation (43), which included earlier follow-up of cohorts in several studies also included in this report (9, 17, 36).
Several studies were ineligible for our review (6). Some details are presented for studies that had conducted an internal dose-response analysis but were ineligible because they then failed on an additional criterion (Table 4). One reason for exclusion was that the mean cumulative dose exceeded 100 mGy. This led to the exclusion of (borderline) statistically significantly positive studies such as Techa River (46), Mayak worker (47), US scoliosis (44), Chornobyl liquidators (48), and Chinese medical workers (49) and the statistically nonsignificantly negative study of background exposure in Kerala (45). Although the proportion of subjects with cumulative doses exceeding 100 mGy in these excluded studies ranged between 20% and 80%, it was below 10% among all included studies except for 5 studies (16–18, 20, 23) for which it ranged between 11% and 22% (6). Excluding these latter 5 studies from our test of whether the median ERR per unit dose equals zero still resulted in rejection of this hypothesis (solid cancer: P = .02 for 15 of 20 positive studies; leukemia: P = .01 for 13 of 16 positive studies). As an alternative, we recommend future analyses of individual subjects exposed to cumulative doses less than 100 mGy, as recently done for leukemia after childhood exposure (57). The 5 studies which were excluded because they only published risk estimates for categories of dose were mostly null (50–54). It is possible that the nonincreasing categorical risks were the reason for not presenting risks per continuous dose, which could be a form of reporting bias. Finally, 2 background radiation studies used dose rate instead of cumulative dose (55, 56). Both were largely null.
Table 4.
Studies with an internal dose-response analysis that were ineligible for only one reason, reason for exclusion, and summary of findings
| Population | 1st author | Publication year | Reason for exclusion | Findings for solid cancers and leukemia (or primary cancer site) |
|---|---|---|---|---|
| Breast cancer in US scoliosis | Ronckers (44) | 2008 | Mean cumulative dose = 120 mGy | Borderline statistically significant positive dose-response relationship for breast cancer incidence (Ptrend = .06). |
| Kerala background | Nair (45) | 2009 | Mean cumulative dose = 161 mGy | Statistically nonsignificant negative dose-response for incidence of all solid cancers excluding leukemia (Ptrend > .5). |
| Techa river | Krestinina (46) | 2013 | Mean cumulative dose = 410 mGy | Statistically significant positive dose-response for incidence of all leukemias (Ptrend < .001). |
| Mayak workers | Sokolnikov (47) | 2015 | Mean cumulative dose = 354 mGy | Statistically significant positive dose-response for mortality of all solid cancers excluding lung, liver, and bone cancers (Ptrend = .01). |
| Chornobyl clean-up workers | Kashcheev (48) | 2015 | Mean cumulative dose = 132 mGy | Statistically significant positive dose-response for total cancer incidence (Ptrend = .03) and mortality (Ptrend = .05). |
| Chinese medical workers | Sun (49) | 2016 | Mean cumulative dose = 250 mGy | Statistically significant positive dose-response for incidence of all solid cancers (Ptrend = .002). |
| US Shipyard workers | Matanoski (50) | 2008 | Categorical risk estimates | Statistically non-significant increased risk of leukemia mortality in highest dose category. No trend tests presented. |
| Australian nuclear test | Gun (51) | 2008 | Categorical risk estimates | No increased risk of solid cancer incidence or leukemia incidence across dose categories (Ptrend > .05). |
| French biology researchers | Guseva (52) | 2008 | Categorical risk estimates | Statistically significant increasing trend for all cancer deaths across dose categories (P = .03 5-year lag). |
| Finnish reindeer herders | Kurttio (53) | 2010 | Categorical risk estimates | No overall increased risk of cancer incidence across dose-categories (Ptrend = .28), but statistically significant increased risk with dose for exposure aged younger than 15 years (Ptrend = .003). |
| Childhood X-rays | Hammer (54) | 2009 | Categorical risk estimates | No increased risk of solid cancer incidence (Ptrend = .32) or leukemia incidence (Ptrend = .26) across dose categories. |
| French background (Geocap case-control) | Demoury (55) | 2017 | Risk for dose rate not cumulative dose | Acute leukemia incidence not related to background radiation exposure (Ptrend > .05). |
| German background | Spix (56) | 2017 | Risk for dose rate not cumulative dose | Statistically nonsignificant positive increased risk of lymphoid leukemia incidence (Ptrend = .54). |
A recent review conducted by the National Council on Radiation Protection and Measurements (NCRP) (58) assessed the different, albeit related, question of whether the recent epidemiological data from 29 low-dose and low-dose rate studies support the linear-no-threshold (LNT) model for radiation protection purposes. There were considerable differences between the studies included in the NCRP review and ours; only 12 of the studies were in both reviews. One reason for the difference in eligible studies is that we restricted our review to low-dose studies, defined as mean cumulative dose less than 100 mGy. The approach to reviewing the evidence was also different. NCRP adopted the traditional approach to assessing study quality and excluded several studies because they were classified as low quality. As we have shown in this monograph, there is not always a direct relationship between study quality and bias. Some of the studies that were classified as high quality could still have been subject to bias [eg, the Japanese nuclear workers study (33)], and those judged as poor quality are not necessarily biased [eg, the GB background study (21) and the Taiwanese residents study (24)]. We showed formally that mostly these methodological issues and errors are unlikely to have resulted in biases that would have impacted the interpretation of the study results. Nevertheless, after their exclusions, the NCRP committee still concluded that “twenty studies [of the 29 reviewed, with the committee designating 4 studies as inconclusive] (80%) provided some support for the LNT model, including five studies (20%) providing strong support and four (16%) providing moderate support” (58). As mentioned previously, the Biological Effects of Ionizing Radiation VII (1) and other earlier reviews of evidence for cancer risks from low doses mostly depended on epidemiological studies of higher dose exposures and then invoked the LNT assumption from experimental data to support the conclusion that low doses are likely to cause cancer. Although we cannot rule out the possibility that the risks are influenced by higher doses, our results, derived from studies with a mean cumulative dose of less than 100 mGy, apply to populations that are mostly exposed to low doses and therefore directly address the question of whether there is epidemiological evidence for cancer risks from low-dose exposures. Despite the different approaches and different studies included, the 2 previous reviews (1, 58) and the pooled analysis of leukemia after childhood exposure to doses less than 100 mGy (57) are all in general agreement with our result that there is evidence of cancer risks from low-dose ionizing radiation. Our summary risk estimates from the meta-analysis are broadly consistent with the Life Span Study of atomic bomb survivors (41, 42).
The observation that most of the study findings were positive (ERR > 0) raises the question of whether there could be publication bias. Because these epidemiological studies required extensive effort and all had the primary (and usually the only) aim of evaluating whether low-dose ionizing radiation causes cancer, the likelihood that a null or statistically nonsignificant result would not be published is likely quite low in our view. Furthermore, the field of radiation epidemiology is relatively small and we are not aware of recent studies or updates that were not published, although a few were published or submitted for publication after the study period.
Confidence intervals and study power need to be considered in interpreting study findings. The confidence intervals for ERRs from all eligible studies included positive values; for the positive estimated ERRs, the upper confidence limits were often several times the estimate. Thus, even when the null hypothesis could not be rejected, findings were compatible with positive effects. It should also be noted that a statistically significant estimate in a low-power study may reflect a false-positive finding and suggest that the estimated ERR is biased. However, one should be cautious about the assessments of statistical power reported here [Tables 1 and 2; and see also Gilbert et al. (3)] because they are based on summary data reported by the study and several assumptions, most importantly that the ERR from the Life Span Study can be transported to other populations.
To our knowledge, this monograph provides the first systematic assessment of the impact of methodological issues and errors on the risk estimate in the studies of low-dose radiation exposure eligible for our analysis. More generally, we used a novel approach in this systematic review to formally assess biases based on published data combined with epidemiological and statistical theory. Traditionally, systematic reviews classify the quality of a study but without formally considering whether the quality of information translates into a bias. For example, low-quality dosimetry does not automatically result in bias [eg, the GB background study (21)]. In addition, the direction of the bias, and, if possible, the magnitude of the potential bias need to be assessed. Without these further considerations, exclusions based on quality or potential bias could result in substantial loss of information. Such an approach has been recently recommended over other approaches, such as the use of a “risk of bias” checklist (59).
There were, however, several limitations to our review. Primarily because we were working only with published data, there were several instances where we had insufficient information to assess the direction of the bias. As examples, this occurred in assessing the direction of confounding if the relationship between the exposure and the confounder was not published, the lack of quantitative estimates about the completeness of follow-up, and absence of information about the completeness and accuracy of vital statistics and cancer registry data for studies using linkage of the cohort with such databases. We also could not assess the magnitude of the bias in most situations because the required data were not available in the publications. Our summary analysis was therefore limited to the simple sign test, which does not take into account the size or precision of the estimated ERRs, because alternative tests are based on the actual values (t test) or their ranks (Wilcoxon signed rank test). We have made several recommendations in each of our papers that would facilitate assessments of bias in the risk estimates in the future. For example, we recommend the routine publication of assessments of dose uncertainty and levels of loss to follow-up by exposure and outcome. Some of the data to inform our assessments came from substudies, and we assumed that these applied to the full study population. For example, there was no evidence that smoking was related to occupational radiation exposure in a case-control study of leukemia in US nuclear workers (60). Because smoking data were not available for the full cohort, we assumed the findings from this sample were generalizable. In addition, the sign test requires that the distribution of the estimated ERRs per unit dose (under the assumption that the true ERRs are zero) has a median of zero, although it does not depend on the ERRs following approximately normal distributions. Our assessment of whether a study was positive or null as input for the sign test did not account for the width of the confidence intervals. Many of these studies had wide confidence intervals, which overlapped zero, and therefore one could argue that bias adjustment, which moves a statistically nonsignificant positive ERR toward the null, does not change the conclusion. Results on all solid cancers combine a diverse group of cancer sites. This heterogeneity has to be taken into account when comparing results with those for leukemia. Finally, the meta-analyses included all studies, even those identified as potentially biased and were based on the assumption that the estimated ERRs from the individual studies were approximately normally distributed. With the highly skewed dose distributions and small numbers of cases in many of these studies, these approximations may not be accurate (3). Thus, tests and confidence intervals could be distorted, especially because the meta-analyses are based on the Wald method. Furthermore, our assessment necessarily had a subjective component.
Age at exposure and time since exposure are important effect modifiers of cancer risk from ionizing radiation exposure, and the eligible studies were heterogeneous in these respects. A pooled analysis with individual subject data is necessary, therefore, to quantify the risk accounting for these effect modifiers. The childhood thyroid pooling project (28) included here and the more recently published pooled analysis of leukemia after childhood radiation exposure (57) provide the most reliable estimates of these cancer risks that account for these modifiers. Similarly, the INWORKS study (9) reviewed here provides a reliable, and we judge, minimally biased, estimate of the solid cancer and leukemia risks from adulthood occupational radiation exposures incorporating age and time since exposure (8, 9).
It is well established that moderate to high doses of ionizing radiation cause cancer. As discussed in the paper by Gilbert et al (3), the available low-dose human data also now satisfy most of Hill’s viewpoints on causality: consistency, temporality, biological gradient, plausibility, and coherence (61). The first of Hill’s viewpoints, strength of effect, or in other words, magnitude of association, is usually not met in studies of cancer risk after low-dose radiation exposure. Large effects are an important indicator of causality because they are less likely to be entirely due to bias. However, large effects are not considered a necessary aspect and indeed Hill explicitly cautions against dismissal of weak observed associations. Our systematic assessment in this monograph showed that these epidemiological studies of low-dose radiation and cancer risk are characterized by several limitations, but we found that only a small minority of the studies had biases whose correction could have moved a positive ERR toward the null. After exclusion of these studies, the majority of studies still reported positive risk estimates. We therefore conclude that there is now a large body of epidemiological data that supports excess cancer risks from low-dose ionizing radiation, and the magnitude of the excess relative cancer risk from these low-dose studies is statistically compatible with the atomic bomb survivors.
Funding
This work was supported in part by the Intramural Research Program, National Cancer Institute, National Institutes of Health, and the US Department of Energy (grant no. DEHS0000091) (doe.gov)
Notes
Affiliations of authors: Institute of Biostatistics and Registry Research, Brandenburg Medical School Theodor Fontane, Neuruppin, Germany (MH); Department of Epidemiology and Biostatistics, Netherlands Cancer Institute, Amsterdam, The Netherlands (MH); Centers for Disease Control (CDC), National Institute for Occupational Safety and Health (NIOSH), OH (RDD); Radiation Programme, Institute for Global Health (ISGlobal), Barcelona, Spain (EC, IT-C); Department of Statistics, Radiation Effects Research Foundation, Hiroshima, Japan (HMC); Cancer Epidemiology Unit, Nuffield Department of Population Health, Oxford University, UK (GK); Institute of Radiation Protection and Nuclear Safety (IRSN), Fontenay-aux-Roses, France (DL); Radiation Epidemiology Branch, Division of Cancer Epidemiology & Genetics, National Cancer Institute, Bethesda, MD (MSL, MPL, JHL, ESG, ABdG); Hirosoft International, Eureka, CA (DLP); Department of Epidemiology, University of North Carolina, Chapel Hill, NC (DBR); Department of Preventive Medicine, School of Medicine, University of Southern California, Los Angeles, CA (DOS); Evidence Synthesis and Classification Section, International Agency for Research on Cancer, Lyon, France (MKS-B).
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of their respective institutions. The lead and corresponding authors had full access to the data in the study and final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.National Research Council. Health risks from exposure to low levels of ionizing radiation (BEIR VII) Phase 2. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 2.Daniels RD, Kendall GM, Thierry-Chef I, et al. Strengths and Weaknesses of Dosimetry Used in Studies of Low-Dose Radiation Exposure and Cancer. JNCI Monographs. 2020; 2020(56):114--132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert ES, Little MP, Preston DL, et al. Issues in Interpreting Epidemiologic Studies of Populations Exposed to Low-Dose, High-Energy Photon Radiation. JNCI Monographs. 2020; 2020(56):176--187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linet MS, Schubauer-Berigan MK, Berrington de Gonzalez A. Outcome Assessment in Epidemiological Studies of Low-Dose Radiation Exposure and Cancer Risks: Sources, Level of Ascertainment, and Misclassification. JNCI Monographs. 2020; 2020(56):154--175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schubauer-Berigan MK, Berrington de González A, Cardis E, et al. Evaluation of Confounding and Selection Bias in Epidemiological Studies of Populations Exposed to Low-Dose, High-Energy Photon Radiation. JNCI Monographs. 2020; 2020(56):(56):133--153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berrington de Gonzalez A, Daniels RD, Cardis E, et al. Epidemiological Studies of Low-Dose Ionizing Radiation and Cancer: Rationale and Framework for the Monograph and Overview of Eligible Studies. JNCI Monographs. 2020; 2020(56):97--113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sprent P, Smeeton NC. Applied Nonparametric Statistical Methods. 4th ed. Boca Raton: Chapman & Hall/CRC; 2007.
- 8.Leuraud K, Richardson DB, Cardis E, et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol. 2015;2(7):e276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson DB, Cardis E, Daniels RD, et al. Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ. 2015;351:h5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitahara CM, Linet MS, Balter S, et al. Occupational radiation exposure and deaths from malignant intracranial neoplasms of the brain and CNS in U.S. Radiologic Technologists, 1983-2012. AJR Am J Roentgenol. 2017;208(6):1278–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee T, Sigurdson AJ, Preston DL, et al. Occupational ionising radiation and risk of basal cell carcinoma in US radiologic technologists (1983-2005). Occup Environ Med. 2015;72(12):862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preston DL, Kitahara CM, Freedman DM, et al. Breast cancer risk and protracted low-to-moderate dose occupational radiation exposure in the US Radiologic Technologists Cohort, 1983-2008. Br J Cancer. 2016;115(9):1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paule RC, Mandel J. Consensus values and weighting factors. J Res Natl Bur Stand Technol. 1982;87(5):377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–114. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 16.Davis S, Day RW, Kopecky KJ, et al. Childhood leukaemia in Belarus, Russia, and Ukraine following the Chernobyl power station accident: results from an international collaborative population-based case-control study. Int J Epidemiol. 2006;35(2):386–396. [DOI] [PubMed] [Google Scholar]
- 17.Kesminiene A, Evrard AS, Ivanov VK, et al. Risk of hematological malignancies among Chernobyl liquidators. Radiat Res. 2008;170(6):721–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zablotska LB, Bazyka D, Lubin JH, et al. Radiation and the risk of chronic lymphocytic and other leukemias among Chornobyl cleanup workers. Environ Health Perspect. 2013;121(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han YY, Youk AO, Sasser H, et al. Cancer incidence among residents of the Three Mile Island accident area: 1982-1995. Environ Res. 2011;111(8):1230–1235. [DOI] [PubMed] [Google Scholar]
- 20.Tao Z, Akiba S, Zha Y, et al. Cancer and non-cancer mortality among inhabitants in the high background radiation area of Yangjiang, China (1979-1998). Health Phys. 2012;102(2):173–181. [DOI] [PubMed] [Google Scholar]
- 21.Kendall GM, Little MP, Wakeford R, et al. A record-based case-control study of natural background radiation and the incidence of childhood leukaemia and other cancers in Great Britain during 1980-2006. Leukemia. 2013;27(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spycher BD, Lupatsch JE, Zwahlen M, et al.; for the Swiss Pediatric Oncology Group. Background ionizing radiation and the risk of childhood cancer: a census-based nationwide cohort study. Environ Health Perspect. 2015;123(6):622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis FG, Yu KL, Preston D, et al. Solid cancer incidence in the Techa River incidence cohort: 1956-2007. Radiat Res. 2015;184(1):56–65. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh WH, Lin IF, Ho JC, et al. 30 years follow-up and increased risks of breast cancer and leukaemia after long-term low-dose-rate radiation exposure. Br J Cancer. 2017;117(12):1883–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenberg MJ, Afilalo J, Lawler PR, et al. Cancer risk related to low-dose ionizing radiation from cardiac imaging in patients after acute myocardial infarction. CMAJ. 2011;183(4):430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Journy N, Roue T, Cardis E, et al. Childhood CT scans and cancer risk: impact of predisposing factors for cancer on the risk estimates. J Radiol Prot. 2016;36(1):N1–7. [DOI] [PubMed] [Google Scholar]
- 27.Berrington de Gonzalez A, Salotti JA, McHugh K, et al. Relationship between paediatric CT scans and subsequent risk of leukaemia and brain tumours: assessment of the impact of underlying conditions. Br J Cancer. 2016;114(4):388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lubin JH, Adams MJ, Shore R, et al. Thyroid cancer following childhood low-dose radiation exposure: a pooled analysis of nine cohorts. J Clin Endocrinol Metab. 2017;102(7):2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn YS, Park RM, Koh DH. Cancer admission and mortality in workers exposed to ionizing radiation in Korea. J Occup Environ Med. 2008;50(7):791–803. [DOI] [PubMed] [Google Scholar]
- 30.Muirhead CR, O’Hagan JA, Haylock RG, et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer. 2009;100(1):206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong M, Jin YW, Yang KH, et al. Radiation exposure and cancer incidence in a cohort of nuclear power industry workers in the Republic of Korea, 1992-2005. Radiat Environ Biophys. 2010;49(1):47–55. [DOI] [PubMed] [Google Scholar]
- 32.Boice JD Jr, Cohen SS, Mumma MT, et al. Updated mortality analysis of radiation workers at Rocketdyne (Atomics International), 1948-2008. Radiat Res. 2011;176(2):244–258. [DOI] [PubMed] [Google Scholar]
- 33.Akiba S, Mizuno S. The third analysis of cancer mortality among Japanese nuclear workers, 1991-2002: estimation of excess relative risk per radiation dose. J Radiol Prot. 2012;32(1):73–83. [DOI] [PubMed] [Google Scholar]
- 34.Zablotska LB, Lane RS, Thompson PA. A reanalysis of cancer mortality in Canadian nuclear workers (1956-1994) based on revised exposure and cohort data. Br J Cancer. 2014;110(1):214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merzenich H, Hammer GP, Troltzsch K, et al. Mortality risk in a historical cohort of nuclear power plant workers in Germany: results from a second follow-up. Radiat Environ Biophys. 2014;53(2):405–416. [DOI] [PubMed] [Google Scholar]
- 36.Schubauer-Berigan MK, Daniels RD, Bertke SJ, et al. Cancer mortality through 2005 among a pooled cohort of U.S. nuclear workers exposed to external ionizing radiation. Radiat Res. 2015;183(6):620–631. [DOI] [PubMed] [Google Scholar]
- 37.Leuraud K, Fournier L, Samson E, et al. Mortality in the French cohort of nuclear workers. Radioprotection. 2017;52(3):199–210. [Google Scholar]
- 38.Nikkila A, Erme S, Arvela H, et al. Background radiation and childhood leukemia: a nationwide register-based case-control study. Int J Cancer. 2016;139(9):1975–1982. [DOI] [PubMed] [Google Scholar]
- 39.Caldwell GG, Zack MM, Mumma MT, et al. Mortality among military participants at the 1957 PLUMBBOB nuclear weapons test series and from leukemia among participants at the SMOKY test. J Radiol Prot. 2016;36(3):474–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kudo S, Ishida J, Yoshimoto K, et al. Direct adjustment for confounding by smoking reduces radiation-related cancer risk estimates of mortality among male nuclear workers in Japan, 1999-2010. J Radiol Prot. 2018;38(1):357–371. [DOI] [PubMed] [Google Scholar]
- 41.Grant EJ, Brenner A, Sugiyama H, et al. Solid cancer incidence among the life span study of atomic bomb survivors: 1958-2009. Radiat Res. 2017;187(5):513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu W-L, Preston DL, Soda M, et al. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950-2001. Radiat Res. 2013;179(3):361–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daniels RD, Schubauer-Berigan MK. A meta-analysis of leukaemia risk from protracted exposure to low-dose gamma radiation. Occup Environ Med. 2011;68(6):457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ronckers CM, Doody MM, Lonstein JE, et al. Multiple diagnostic X-rays for spine deformities and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(3):605–613. [DOI] [PubMed] [Google Scholar]
- 45.Nair RR, Rajan B, Akiba S, et al. Background radiation and cancer incidence in Kerala, India-Karanagappally cohort study. Health Phys. 2009;96(1):55–66. [DOI] [PubMed] [Google Scholar]
- 46.Krestinina LY, Davis FG, Schonfeld S, et al. Leukaemia incidence in the Techa River Cohort: 1953-2007. Br J Cancer. 2013;109(11):2886–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sokolnikov M, Preston D, Gilbert E, et al. Radiation effects on mortality from solid cancers other than lung, liver, and bone cancer in the Mayak worker cohort: 1948-2008. PLoS One. 2015;10(2):e0117784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kashcheev VV, Chekin SY, Maksioutov MA, et al. Incidence and mortality of solid cancer among emergency workers of the Chernobyl accident: assessment of radiation risks for the follow-up period of 1992-2009. Radiat Environ Biophys. 2015;54(1):13–23. [DOI] [PubMed] [Google Scholar]
- 49.Sun Z, Inskip PD, Wang J, et al. Solid cancer incidence among Chinese medical diagnostic X-ray workers, 1950-1995: estimation of radiation-related risks. Int J Cancer. 2016;138(12):2875–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matanoski GM, Tonascia JA, Correa-Villasenor A, et al. Cancer risks and low-level radiation in U.S. shipyard workers. J Radiat Res. 2008;49(1):83–91. [DOI] [PubMed] [Google Scholar]
- 51.Gun RT, Parsons J, Crouch P, et al. Mortality and cancer incidence of Australian participants in the British nuclear tests in Australia. Occup Environ Med. 2008;65(12):843–848. [DOI] [PubMed] [Google Scholar]
- 52.Guseva Canu I, Rogel A, Samson E, et al. Cancer mortality risk among biology research workers in France: first results of two retrospective cohorts studies. Int Arch Occup Environ Health. 2008;81(6):777–785. [DOI] [PubMed] [Google Scholar]
- 53.Kurttio P, Pukkala E, Ilus T, et al. Radiation doses from global fallout and cancer incidence among reindeer herders and Sami in Northern Finland. Occup Environ Med. 2010;67(11):737–743. [DOI] [PubMed] [Google Scholar]
- 54.Hammer GP, Seidenbusch MC, Schneider K, et al. A cohort study of childhood cancer incidence after postnatal diagnostic X-ray exposure. Radiat Res. 2009;171(4):504–512. [DOI] [PubMed] [Google Scholar]
- 55.Demoury C, Marquant F, Ielsch G, et al. Residential exposure to natural background radiation and risk of childhood acute leukemia in France, 1990-2009. Environ Health Perspect. 2017;125(4):714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spix C, Grosche B, Bleher M, et al. Background gamma radiation and childhood cancer in Germany: an ecological study. Radiat Environ Biophys. 2017;56(2):127–138. [DOI] [PubMed] [Google Scholar]
- 57.Little MP, Wakeford R, Borrego D, et al. Leukaemia and myeloid malignancy among people exposed to low doses (<100 mSv) of ionizing radiation during childhood: a pooled analysis of nine historical cohort studies. Lancet Haematol. 2018;5(8):e346–e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.NCRP. Implications of Recent Epidemiologic Studies for the Linear-Nonthreshold Model and Radiation Protection. Bethesda, MD: National Council on Radiation Protection and Measurements; 2018.
- 59.Savitz DA, Wellenius GA, Trikalinos TA. The problem with mechanistic risk of bias assessments in evidence synthesis of observational studies and a practical alternative: assessing the impact of specific sources of potential bias. Am J Epidemiol. 2019;188(9):1581–1585. [DOI] [PubMed] [Google Scholar]
- 60.Schubauer-Berigan MK, Daniels RD, Fleming DA, et al. Risk of chronic myeloid and acute leukemia mortality after exposure to ionizing radiation among workers at four U.S. nuclear weapons facilities and a nuclear naval shipyard. Radiat Res. 2007;167(2):222–232. [DOI] [PubMed] [Google Scholar]
- 61.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58(5):295–300. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.