Abstract
Endometriosis is a widespread gynecologic condition affecting up to 15% of women of reproductive age. The Janus kinase/signal transducer and activator of transcription (JAK/STAT3) pathway is upregulated in endometriosis and is a therapeutic target. Here we sought to determine the effect of Tofacitinib, a JAK inhibitor in widespread clinical use, on JAK/STAT signaling in endometriosis and lesion growth. Endometriosis was surgically induced in C57BL/6 mice using homologous uterine horn transplantation. Lesions were allowed to form over 4 weeks followed by Tofacitinib (10 mg/kg) or vehicle administered by oral gavage over 4 weeks. Tofacitinib treatment in vivo led to endometriosis lesion regression and reduced adhesion burden compared to vehicle treatment. In vitro studies on Ishikawa cells showed that Tofacitinib reduced hypoxia-inducible factor 1α and vascular endothelial growth factor mRNA levels at 12 and 24 h. Western blot analysis showed that Tofacitinib effectively reduced STAT3 phosphorylation in Ishikawa cells and human primary stromal and epithelial cells from eutopic endometrium of patients with and without endometriosis. This study suggests that the inhibition of JAK/STAT signaling using Tofacitinib may be a viable method for the treatment of endometriosis.
Keywords: endometriosis, Tofacitinib, JAK/STAT, STAT3 phosphorylation, endometrium, hypoxia-inducible factor 1α, vascular endothelial growth factor
Introduction
Endometriosis is a widespread gynecologic condition affecting up to 15% of women of reproductive age (Gurates and Bulun, 2003). It is a common cause of dysmenorrhea and dyspareunia and up to 50–60% of women with pelvic pain or unexplained infertility are afflicted with this disease (Giudice and Kao, 2004; Bulun, 2009; Zondervan et al., 2018). Inflammation is a fundamental part of the pathogenesis of endometriosis. The disease is characterized by the increased production of pro-inflammatory cytokines such as IL-6 and tumor necrosis factor alpha (TNFα), which can encourage endometrial cell proliferation and adhesion (Lebovic et al., 2001; Weiss et al., 2009).
In addition to abnormal immune signaling, there are clear aberrations in the distributions of immune cells in patients with endometriosis. Peritoneal macrophage levels are known to be increased in endometriosis and they, paradoxically, stimulate endometriotic lesions (Haney et al., 1981, Olive et al., 1985; Braun et al., 1994). Although total lymphocyte numbers are unchanged in the peripheral blood, peritoneal fluid lymphocyte numbers, especially regulatory T-cell concentrations, are increased in endometriosis (Steele et al., 1984; Slabe et al., 2013).
Given the substantial contribution of inflammation to the pathogenesis of endometriosis, numerous attempts have been made to use immunomodulation as a potential treatment for endometriosis (Kotlyar et al., 2019). Hydroxychloroquine (HCQ), a drug commonly used to treat systemic lupus erythematosus, was examined in human stromal cells and a mouse model of endometriosis. HCQ treatment led to decreased cell survival and decreased lesion number in treated mice (Ruiz et al., 2016). A binding protein for TNFα, one of the cytokines typically overexpressed in endometriosis, has also been tested in baboons with improvement in lesion size, number, and overall severity of disease (D'Hooghe et al., 2006). These studies demonstrate the value of exploring immunomodulatory therapy as a new avenue for endometriosis treatment. All current approved endometriosis therapies use sex steroid hormones or agents designed to reduce estradiol levels; these therapies have significant off-target side effects and prevent conception in a population where fertility is a paramount concern (Bulun et al., 2019).
To identify immune modulators that are effective in endometriosis, we considered the molecular mechanisms that are aberrantly upregulated in endometriosis. Numerous inflammatory cytokines convey their message through the Janus kinase/Signal transducer and activator of transcription (JAK/STAT) pathway. This pathway is essential for immune cell signaling. Upon cytokine binding, JAKs phosphorylate STAT proteins which then translocate to the nucleus and alter transcription (Villarino et al., 2017). However, recent work has also shown that the JAK/STAT pathway, specially STAT3 phosphorylation, is upregulated in the eutopic endometrium of patients with endometriosis (Kim et al., 2015). This suggests that ectopic lesions may also exhibit STAT3 overactivity, which may influence lesion formation and survival as STAT3 is well-known to influence these cellular processes. Numerous genes are known to be activated by STAT3, including hypoxia-inducible factor 1 alpha (HIF1α) and vascular endothelial growth factor (VEGF) (Pawar et al., 2013).
HIF1α is a transcription factor that mediates a cell’s response to hypoxia. It is typically composed of two proteins in a heterodimer, HIF1α and HIF1β. When a cell is exposed to normoxic conditions, HIF1α is rapidly degraded; however, under hypoxic conditions, HIF1α is stabilized and then translocates to the nucleus where it binds to its target transcription sites (Majmundar et al., 2010). HIF1α-modulated genes are known to be involved in angiogenesis (i.e. production of VEGF) and cell invasion (Chen and Han, 2008). Angiogenesis plays a substantial role in endometriosis with suppression of this pathway leading to lesion regression (Rocha et al., 2013).
A new class of immunomodulatory drugs called JAK inhibitors presents a tool to investigate the effect of JAK inhibition and reduction of STAT3 phosphorylation and STAT3 transcriptional activity. JAK inhibitors represent a method potentially of treating endometriosis by not only an immunomodulatory and antiangiogenic effect but also by an effect on the eutopic and ectopic endometrial cells themselves. The first drug of this class to be approved by the US Food and Drug Administration is Tofacitinib, which is currently used to treat rheumatoid arthritis. Therefore, we sought to study the activity of Tofacitinib in endometriosis. We aimed to determine the effect of Tofacitinib on STAT3 activity in cell culture and on lesion growth in a mouse model of endometriosis.
Materials and methods
Animals
Six- to eight-week-old C57BL/6J female mice (Jackson Laboratories, Bar Harbor, ME, USA) were obtained. Mice were maintained in the animal facility at the Yale School of Medicine. Three to four animals per cage were housed in a 12-h light and 12-h dark cycle with access to food and water ad libitum. All mice studies were performed under a protocol approved by the Yale University Institutional Animal Care and Use Committee.
Induction of endometriosis in mice
Endometriosis was induced in 20 mice using a modified version of the syngeneic endometriosis protocol that has been previously used by our laboratory (Lee et al., 2009). This model involved suturing identical sizes of uterine tissue fragments onto the peritoneal surface. Ten mice in oestrus stage were sacrificed using a CO2 chamber and both uterine horns were excised from each mouse and cut longitudinally. Each uterine horn was then divided into equal fragments measuring approximately 4 mm2. These fragments were then kept on ice in HBSS (Gibco-BRL, Gaithersburg, MD, USA) until transplantation. Upon implantation, 20 mice were anaesthetized using inhalation of isoflurane (Isothesia; Henry Schein, OH, USA) and then a midline laparotomy was performed. Two uterine fragments were sutured on each side of the peritoneal surface in each mouse using 5-0 polyglactin suture (Vicryl; Ethicon, Somerville, NJ, USA). Subsequently, the peritoneum was closed with 5-0 polyglactin suture and the skin was closed with 4-0 polyglactin suture.
Tofacitinib treatment
The 20 animals with experimentally induced endometriosis were randomly divided into two groups of 10 mice in each. The mice were allowed to heal for 4 weeks to allow for distinct endometriosis lesion formation. One group of mice (treatment) was given Tofacitinib (MedChemExpress, Monmouth Junction, NJ, USA) dissolved in 0.5% methylcellulose/0.025% Tween 20 (Sigma-Aldrich, St. Louis, MO, USA) and administered at a dose of 10 mg/kg/day based on previous studies (Milici et al., 2008; Furumoto et al., 2017). Dosages below 5 mg/kg/day did not show any response in mice when assessing rheumatoid arthritis disease burden and dosages beyond 12.5 mg/kg/day were noted to lead to more substantial immune suppression (Milici et al., 2008, Okiyama et al., 2014). The second group of mice were given 0.5% methylcellulose/0.025% Tween 20 without any Tofacitinib (vehicle group). Both groups were treated via oral gavage for 4 weeks.
Macroscopic and microscopic evaluation of lesions, adhesive burden, and excision of uteri
Following 4 weeks of treatment with Tofacitinib, animals were sacrificed with a CO2 chamber, laparotomy was performed, and endometriotic lesions were removed from the peritoneum of the mice. All lesions were individually measured, and lesion volumes were calculated using the smallest diameter2 × largest diameter/2 formula, as described (Sahin et al., 2018). Half of each lesion was kept in RNA stabilization solution (RNA later; Qiagen, Hilden, Germany) for mRNA isolation to determine the gene expression by quantitative RT-PCR (qRT-PCR) analysis, and the other half was kept in 4% paraformaldehyde solution for immunohistochemistry studies. For each mouse, one uterine horn was placed into RNA stabilization solution and the other horn was placed into 4% paraformaldehyde. After hematoxylin and eosin staining, all lesions were evaluated under light microscopy to confirm endometriosis. The area of each lesion was calculated using the NIS Elements Imaging software program (3.10, Nikon, Brighton, MI, USA) by subtracting the inner circumference of the lesion from the outer lesion circumference.
Adhesion scoring was performed according to (Yesildaglar et al. 1999). However, their scoring system was modified to take into account the type and severity of adhesions for each endometriotic lesion in the mouse, as shown in Equation (1). Equation (1) represents an average of adhesion scores for all of the four implanted lesions in each mouse. If a mouse had less than four lesions, then this was factored into the total adhesion score using an average score. Adhesion type referred to whether a lesion was filmy, firm, or vascular and firm, with a corresponding score of 1, 2, or 3, respectively. If the lesion did not have any adhesions, it received a score of 0. Adhesion tenacity referred to the ease with which the adhesion was removed. A score of 1 meant the adhesion was lysed upon opening of the abdomen, a score of 2 signified that only tension was needed, and a score of 3 indicated that sharp excision was needed to lyse the adhesion.
| Equation (1) |
Extraction of protein from formalin-fixed tissues
Lesions and uteri excised from mice treated with vehicle or Tofacitinib that were fixed in 4% paraformaldehyde were kept in 70% ethanol for 24 h. Protein was extracted using the QProteome FFPE Tissue Kit (QIAGEN, Hilden, Germany). Protein was extracted in the supplied QIAGEN EXB buffer according to the manufacturer’s protocol and protein concentrations were assessed using a Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA).
Tissue procurement and homogenization
Eutopic endometrial tissue was obtained from women with and without any surgical evidence of endometriosis (n = 11, aged 24–45 years). None of the patients had received any hormonal treatments for at least 3 months prior to tissue collection. All of the specimens were confirmed as being in the mid- to late-proliferative phases according to pathological observation and menstrual cycle history. This study was approved by the institutional review board of Yale University and written informed consent was obtained from all patients.
Endometrial stromal cells (eSCs) and endometrial epithelial cells (eECs) were isolated from eutopic endometrial tissues, respectively, by enzymatic digestion with collagenase B (Sigma-Aldrich) and DNase I (Roche Diagnostics, Switzerland), as previously described (Cho et al., 2016). Isolated eSC and eECs were cultured in Dulbecco’s modified eagle medium supplemented with 100 IU/ml of penicillin, 50 mcg/ml of streptomycin, and 1% Amphotericin B and 10% heat-inactivated fetal bovine serum (all obtained from Gibco-BRL) at 37°C in 5% CO2 in air. eSCs and eECs were initially cultured in 25 cm2 and 10 cm2 plates, respectively, prior to reaching 80–90% confluence after which they were trypsinized and replated into 12-well plates.
Cell culture and tofacitinib treatment
The eEC line, Ishikawa (endometrial adenocarcinoma), was grown in 12-well plates in Dulbecco’s modified Eagle’s Medium with F12 (Company), supplemented with 10% v/v of fetal bovine serum (Gibco-BRL), 50 IU/ml of Penicillin, and 50 mcg/mL of streptomycin (Gibco-BRL) in an atmosphere of 5% CO2 and 95% air at 37°C. Given that Ishikawa cells have been previously studied in assessing the JAK/STAT pathway in endometrial tissue, we proceeded with this cell line (Kim et al., 2015). Cells from patient-derived eutopic endometrium and ectopic endometrium were grown in grown in 12-well plates in Dulbecco’s modified Eagle’s Medium with low glucose (1 mg/mL) supplemented with 10% v/v of fetal bovine serum (Gibco-BRL), 50 IU/ml of Penicillin, and 50 mcg/mL of streptomycin (company) at the same atmospheric conditions as for Ishikawa cells. The cells were incubated with IL-6 at concentration of 25 ng/mL for 24 h and beta-estradiol at 100 nM to induce STAT3 activation. Tofacitinib treatment was performed for 24 h since this time period was needed to observe substantial STAT3 phosphorylation, as described previously by Kim et al. (2015). Two concentrations of Tofacitinib were tested in vitro, 0.02 and 0.2 µM, corresponding to 10-fold subtherapeutic and therapeutic concentrations of Tofacitinib.
RNA extraction and qRT-PCR
Cells in each incubation condition for in vivo studies were lysed and homogenized in 400 μL of TRIzol reagent (Invitrogen, Carlsbad, CA, USA). All samples were then chloroform-extracted and then precipitated in isopropyl alcohol. Following two washes in 70% ethanol, pellets were dried and dissolved in 30–50 μL of RNase-free water. Total RNA was purified using the RNeasy cleanup kit (Qiagen, Valencia, CA, USA), as per the manufacturer’s protocol, treated using recombinant shrimp DNase (USB, Cleveland, OH, USA) to eliminate DNA contamination and quantified using a NanoDrop spectrophotometer. Purified RNA was immediately used for cDNA synthesis or stored at −80°C until use later. For cDNA synthesis, purified RNA (1000 ng) was reverse transcribed using an iScript cDNA synthesis kit (Bio-Rad Laboratories). qRT-PCR was performed using SYBR Green (Bio-Rad Laboratories) and optimized using the MyiQ single‐color real‐time PCR detection system (Bio‐Rad Laboratories). Primer sequences (5′ to 3′) used for qRT-PCR are Hif1-α Forward-′TTCACCTGAGCCTAATAGCCC′, Reverse-′CAAGTCTAAATCTGTGTCCTG′; Vegf-A: F-′CTGCCGTCCGATTGAGACC′, R-′CCCCTCCTTGTACCACTGTC′. Amplified transcript specificity and absence of primer dimers were determined via a melting curve analysis. Gene expression was normalized to that of GAPDH as an internal control. Relative mRNA expression was calculated using the comparative cycle threshold (Ct) method (2−ΔΔCT) (Livak and Schmittgen, 2001). All experiments were performed in duplicate for three instances.
Western blot analysis
Cells were lysed in cell lysis buffer (Cell Signaling, Danvers, MA, USA) with 1 mM PMSF (Sigma-Aldrich) with 1 mM NaF (Sigma-Aldrich). 4x sodium dodecyl sulphate—polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (Bio-Rad Laboratories) was added to the sample, which was then boiled at 95°C for 3–5 min. Samples (15 μL each) were then loaded onto 4–20% SDS-PAGE gels (Bio-Rad Laboratories) and run at 60 V until adequate protein separation was achieved based on ladder appearance. Protein was then transferred onto nitrocellulose membranes and incubated in 5% bovine serum albumin (Sigma-Aldrich) for blocking. Membranes were then incubated in primary antibody at a dilution of 1:1000 for anti-STAT3, anti-pSTAT3, and anti-HIF1α. Anti-GAPDH (1:2000) was used as the house-keeping protein. Membranes were washed in TBS-T and then incubated in secondary antibody conjugated to horse-radish peroxidase goat/anti-rabbit/anti-mouse (1:1000) (Cell Signaling). Membranes were then washed in TBST-T and treated with enhanced chemiluminescence plus western-blot substrate (Thermofisher, Waltham, MA, USA) and then visualized using an Amersham 680 imager.
Statistical analysis
GraphPad Prism 7.0 software (GraphPad Software, La Jolla, CA, USA) was utilized for statistical analyses. For in vitro studies, all experiments were performed in triplicate, and the mean for each individual animal was used for statistical analysis. The quantitative data were tested for normality using the Shapiro–Wilk test. Normally distributed data were evaluated using Student’s t-test, and Mann–Whitney U test was used for non-normally distributed data (adhesion score). A P value of <0.05 was considered statistically significant.
Results
In vivo testing of Tofacitinib in a mouse model of endometriosis
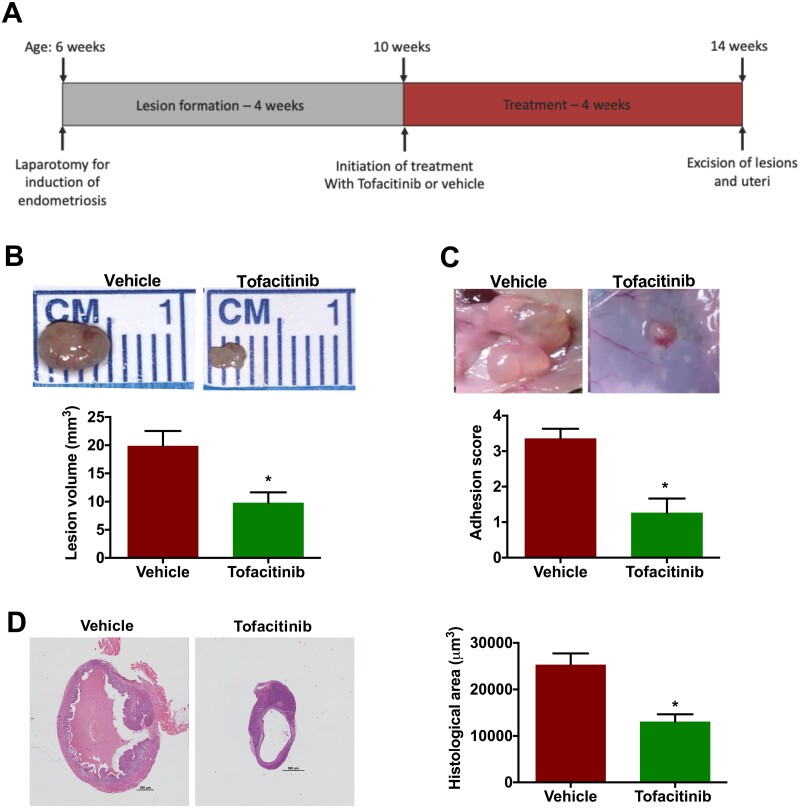
Endometriosis was induced in C57BL/6 mice (n = 20) as described in the “Materials and methods” section. Lesions were allowed to form over the span of 4 weeks. After this period, the mice were randomized to treatment with Tofacitinib versus vehicle via once daily oral gavage. Once the mice completed 4 weeks of gavage, they were sacrificed and underwent laparotomy (Fig. 1A). We observed that the lesions in the Tofacitinib-treated mice were significantly smaller in size than mice that were given the vehicle (Fig. 1B). The lesion volume was reduced by more than 2-fold (P < 0.01). Furthermore, the adhesion score in mice was 3-fold lower in the Tofacitinib-treated group compared to vehicle (P < 0.001) (Fig. 1C). As a large portion of the lesion may consist of cystic fluid, histological assessment of lesions measured the size of active endometriosis tissue within the lesion. The area comprising endometriosis was also significantly decreased by 2-fold (P < 0.01) (Fig. 1D).
Figure 1.
Tofacitinib treatment reduced lesion growth and adhesions in mice. (A) Depiction of the timeline of induction of endometriosis, lesion formation followed by Tofacitinib treatment and lesion collection. (B) Lesion size reduced significantly in mice with endometriosis treated with Tofacitinib compared to vehicle. Bar graph shows mean lesion volume in mm3. (C) The strength of the lesion adherence to the peritoneal cavity is significantly reduced in mice with endometriosis treated with Tofacitinib (n = 8) compared to vehicle (n = 12). Bar graph shows the strength of adherence in score from 0 to 5. (D) Histologic area of actual endometriosis excludes the fluid-filled cysts. The area between the outer and inner lines layers was used to calculate the histologic area of lesions. A 500 µm bar is placed to the right of each lesion as a measure of relative scale. Each bar represents the mean ± SEM of lesions. *P < 0.05, between Tofacitinib versus vehicle treatments.
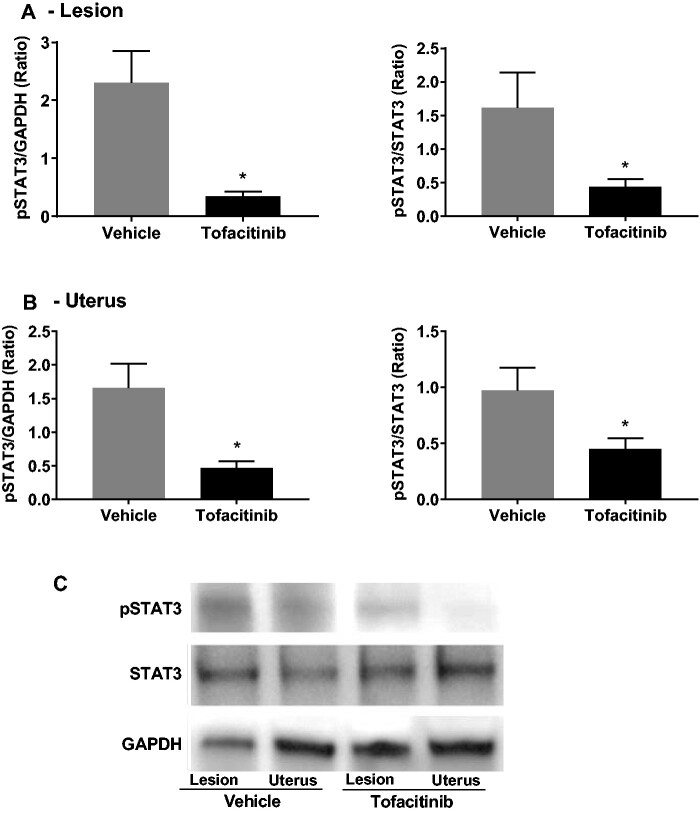
Tofacitinib abrogates STAT3 phosphorylation in murine tissues
To determine the effect of oral Tofacitinib administration on STAT3 phosphorylation in murine tissue, protein was extracted from formalin-fixed lesions and uteri from mice treated with vehicle or Tofacitinib. This protein level was then analyzed using western blotting (Fig. 2C). Densitometry analysis of protein bands showed that in endometriotic lesions, pSTAT3 levels were decreased in Tofacitinib-treated mice compared to vehicle when normalized to GAPDH (P < 0.05) and total STAT3 levels (P < 0.05) (Fig. 2A). When assessing uteri, pSTAT3 levels were also significantly decreased in Tofacitinib-treated mice when compared to vehicle when normalized to GAPDH (P < 0.05) and total STAT3 levels (P < 0.05) (Fig. 2B).
Figure 2.
Tofacitinib reduced phoshorylation of STAT3 in murine endometriotic lesions and uteri. (A) The ratio of phosphorylated signal transducer and activator of transcription pathway (pSTAT3) to GAPDH and pSTAT3 to STAT3 in endometriotic lesions. (B) The ratio of pSTAT3 to GAPDH and pSTAT3 to STAT3 in excised uterine horns. (C) Representative western blot for phosphorylated STAT3 (pSTAT3) levels, GAPDH, and total-STAT3 following treatment with vehicle or Tofacitinib via oral gavage for 4 weeks (see Supplementary Information for full western blot image). Results shown as mean ± SEM. *P < 0.05 versus stimulation only.
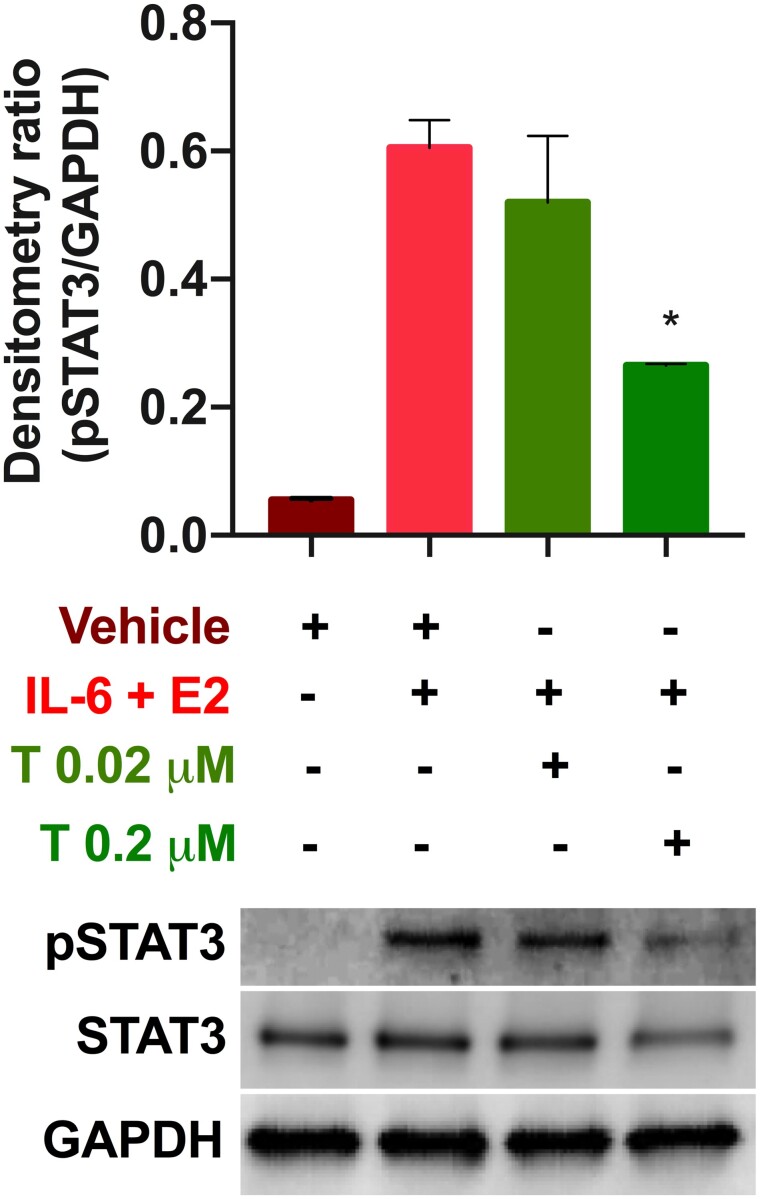
Tofacitinib abrogates STAT3 signaling in Ishikawa cells
To assess the effect of Tofacitinib on endometrial cells, we first assessed the action of the drug in cell culture. Ishikawa cells underwent stimulation of the JAK/STAT pathway with IL-6 and estradiol (E2) as described by Kim et al. (2015). Tofacitinib was added at two different concentrations as described in the methods section. STAT3 phosphorylation levels were not significantly decreased by the lower dosage of Tofacitinib; however, they were significantly decreased by approximately 2-fold (P < 0.05) at the therapeutic dosage of Tofacitinib compared to treatment only with IL-6+E2 (Fig. 3).
Figure 3.
Tofacitinib reduced IL-6+E2-stimulated phosphorylation of STAT3 in Ishikawa cells. Representative western blot for pSTAT3 levels, GAPDH, and total STAT3 following 24 -h treatment (see Supplementary Information For full western blot image). The ratio of pSTAT3 to GAPDH is compared in the bar graph for exposure with vehicle (dimethylsulphoxide: DMSO), stimulation with IL-6 and estradiol (E2), and treatment with 0.02 or 0.2 µM Tofacitinib. Results shown as mean ± SEM. *P < 0.05 versus stimulation only.
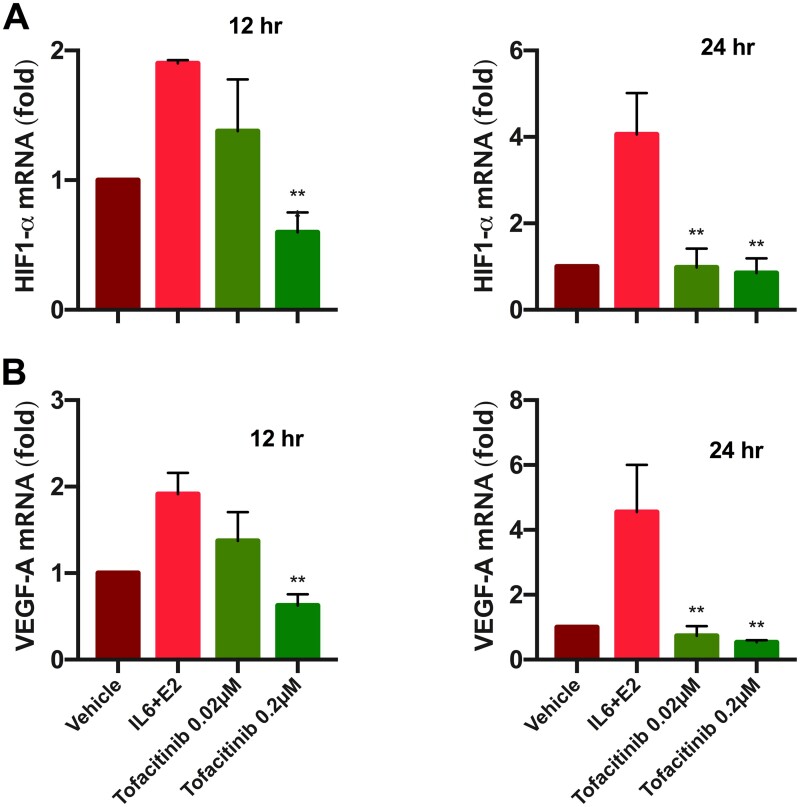
Once we established that Tofacitinib abrogates STAT3 phosphorylation, we sought to assess mRNA levels of HIF1α and VEGF, which are the downstream genes for STAT3. Gene expression was measured at two time points (12 and 24 h) of Tofacitinib treatment. qRT-PCR results at both time points showed highest levels of HIF1α and VEGF mRNA in the group treated with IL6+E2 compared to all other conditions. The lower concentration of Tofacitinib (0.02 µM) did not lead to a significant decrease in HIF1α mRNA levels at 12 h. However, at 24 h, Tofacitinib treatment did lead to a significant decrease in both HIF1α and VEGF mRNA levels at this subtherapeutic level, which was 4-fold lower than treatment only with IL6+E2 (Fig. 4A and B). The therapeutic concentration of Tofacitinib led to a 2- to 3-fold decrease in HIF1α and VEGF mRNA at 12 h and an even greater 4- to 5-fold decrease in these mRNA levels at 24 h compared to treatment only with IL6+E2 (Fig. 4A and B).
Figure 4.
Tofacitinib reversed the stimulatory effect of IL-6 + E2 on HIF1-α and VEGF-A mRNA level in Ishikawa cells, assessed by quantitative RT-PCR. (A) and (B) show hypoxia-inducible factor 1 alpha (HIF1α) and vascular endothelial growth factor (VEGF) mRNA expression levels, respectively, measured in Ishikawa cells at 12 and 24 h of exposure to vehicle (DMSO), IL-6 and E2, and Tofacitinib (0.02 and 0.2 µM). IL-6 and E2 stimulated the mRNA levels, while Tofacitinib inhibited the stimulation effect significantly at both concentrations for 24 h and at 12 h only for higher concentration (0.2 µM). Each bar represents the mean ± SEM, for data from two individual experiments, and each experiment was performed in triplicate. **P < 0.01 versus stimulation only.
Tofacitinib treatment of primary cells derived from eutopic endometrium
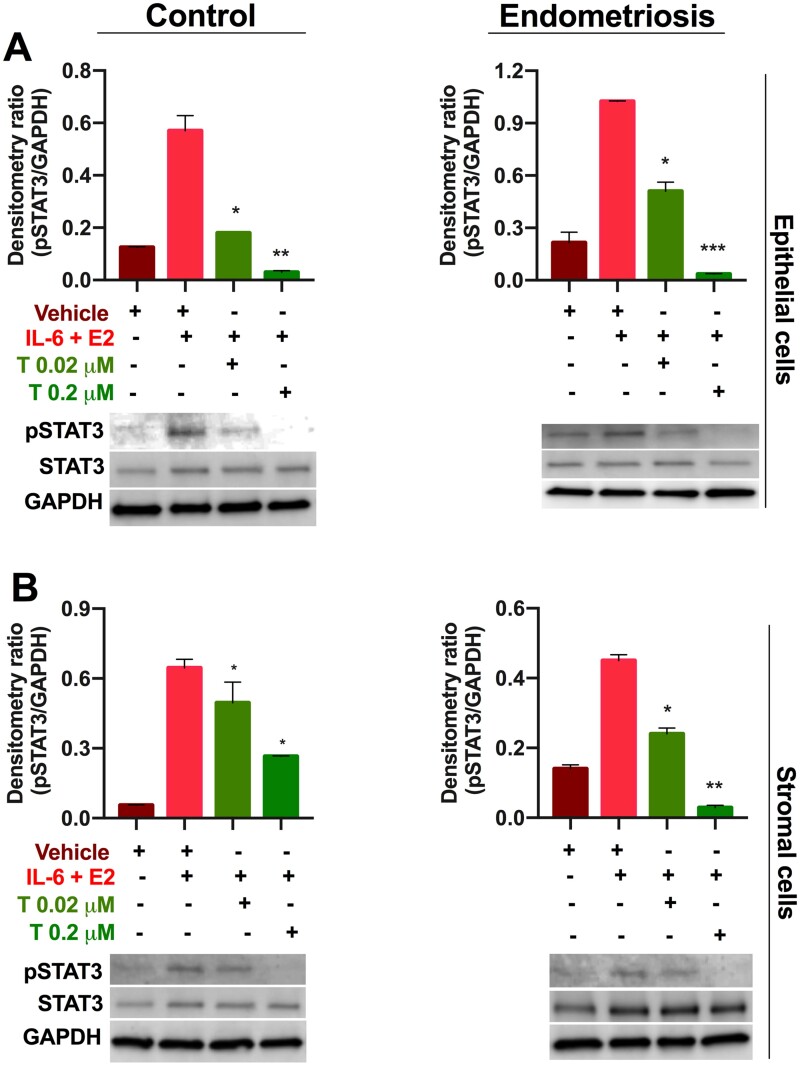
Given the effect of Tofacitinib on STAT3 phosphorylation in Ishikawa cells, we sought to determine whether this same effect occurred in primary cells obtained from patients with and without endometriosis. Western blot analysis of Tofacitinib-treated eutopic epithelial cells from control patients showed STAT3 phosphorylation was significantly inhibited at both doses of Tofacitinib (3-fold lower, P < 0.05 for 0.02 µM and 10-fold lower, P < 0.01 for 0.2 µM) (Fig. 5A). While in patients with endometriosis, we observed a similar pronounced effect of Tofacitinib with even the lower dose leading to significantly lower pSTAT3 levels (3-fold, P < 0.05) and a greater effect at therapeutic dosage (20-fold lower, P < 0.01) compared to no treatment (Fig. 5A). When looking at eutopic stromal cells from control patients, we observed a significant decrease in pSTAT3 levels at the lower as well as at the therapeutic dosage of Tofacitinib (1.3-fold lower, P < 0.05 and 2-fold lower, P < 0.01), respectively, as shown in Fig. 5B. For eutopic stromal cells from patients with endometriosis, we noted an even greater decrease in pSTAT3 levels at the lower dosage in addition to the therapeutic dosage of Tofacitinib (2-fold lower, P < 0.05 and 10-fold lower, P < 0.01), respectively, as shown in Fig. 5B.
Figure 5.
Tofacitinib treatment decreased pSTAT3 levels in primary human cells. (A) Epithelial cells and (B) stromal cells from patients with (endometriosis) and without (control) endometriosis show that pSTAT levels are increased by IL-6 and E2 treatment while Tofacitinib (0.02 and 0.2 µM) treatment significantly decreased the pSTAT levels stimulated by IL-6 + E2 compared to vehicle (DMSO). Representative western blot images are shown for pSTAT3, STAT3, and GAPDH detection (see Supplementary Information for full western blot images). Bar graph shows the ratio of pSTAT3 to GAPDH by densitometry analysis. Results shown as mean ± SEM. *P < 0.05; **<0.01, and ***<0.001 versus IL-6 + E2 stimulation.
Discussion
In this study, we investigated the role of a JAK inhibitor, Tofacitinib, on endometrial and endometriosis tissue. Our in vivo studies showed that daily treatment with Tofacitinib not only decreased lesion size in a murine model of endometriosis but also decreased the adhesion burden in a murine model. Tofacitinib consistently reduced STAT3-phoshorylation in Ishikawa cells. Furthermore, Tofacitinib reduced the mRNA expression levels of the STAT3-dependent genes, HIF1α and VEGF. We also determined that Tofacitinib can inhibit STAT3 phosphorylation in vitro in epithelial and stromal cells derived from eutopic endometrial tissue from control patients and patients with endometriosis. These results indicate that Tofacitinib can affect cells from both eutopic and ectopic endometrium and supports the notion that JAK-inhibition can limit lesion growth.
Concerning the effect on adhesion burden, Tofacitinib likely has an effect both on endometriotic cells, any immune cells interacting with the lesions, and fibroblast function. It is well established that Tofacitinib affects T-cell and macrophage function (Furumoto et al., 2017; De Vries et al., 2019; McInnes et al., 2019). The release of cytokines from these cells including IL-6, which can perpetuate STAT3 activation, and other pro-inflammatory cytokines is decreased with Tofacitinib treatment. In fibroblasts or fibroblast-like cells, Tofacitinib also inhibits the production of TNFα, MCP-1, and TGF1-β (Rosengren et al., 2012; Mehtap et al., 2018). Furthermore, Tofacitinib has been shown to reduce the proliferation of skin and liver fibroblasts in vitro at a therapeutic concentration (Mehtap et al., 2018).
These results strengthen the possibility of using JAK inhibitors as a treatment for endometriosis. The current mainstays of endometriosis treatment include hormonal suppression and surgery (Bulun et al., 2019). However, hormonal suppression treats endometriosis by lowering estrogen levels and/or by leading to decidualization of lesions via progestin effects (Taylor et al., 2017). Furthermore, these treatments typically preclude a patient from achieving pregnancy (Bulun et al., 2019). Surgery, while it can lead to pain relief and improve fecundity, does have a substantial recurrence rate with 40–45% of patients experiencing a return of their chronic pelvic pain (Vercellini et al., 2009). These limitations stress the need for new therapeutics that address additional pathogenic mechanisms underlying endometriosis.
Chief among these mechanisms is the widespread inflammation seen in women with endometriosis. Given the aforementioned increased levels of various pro-inflammatory cytokines and changes in immune cell populations, immunomodulation remains an attractive approach to treating endometriosis (Kotlyar et al., 2019). The inflammation seen in endometriosis can occur through various mechanisms including the downregulation of regulatory proteins, some of which are involved in the STAT3 pathway such as protein inhibitor of activated STAT3 (PIAS3) (Yoo et al., 2016). Tofacitinib has been shown to increase levels of PIAS3 thereby abrogating STAT3 signaling (Gao et al., 2016). Increased macrophage activity has also been implicated in endometriosis and coculture experiments of macrophages from patients with endometriosis have been shown to increase STAT3 signaling in eSCs (Itoh et al., 2013). An additional inflammatory target is the CXCR4-CXCL12 signaling pathway, which is upregulated in endometriosis and increasing evidence indicates it may also be dependent on the JAK/STAT pathway (Pfeiffer et al., 2009; Pluchino et al., 2018). The widespread inflammation seen in endometriosis has been implicated in numerous systemic effects, including cardiovascular and metabolic disease, and shows an association with various autoimmune conditions (Alderman et al., 2017).
Inflammation in the setting of endometriosis can not only contribute to a patient’s pelvic pain but also limit fecundity. Patients with endometriosis are more likely to experience infertility (Bulletti et al., 2010). In addition, the inflammation seen in endometriosis is associated with decreased endometrial receptivity (Lessey and Kim, 2017). Inflammation can elevate levels of aromatase in the endometrium and these endometrial aromatase levels have been associated with poor IVF outcomes (Brosens et al., 2004). STAT3 activity has been implicated in aromatase transcription based upon promoter analysis (Zhao et al., 2016). Therefore, modulation of STAT3 activity may have the additional benefits of improving fecundity in patients with endometriosis in addition to anti-inflammatory effects and antiproliferative effects on the lesions themselves.
Future studies will assess the effect of Tofacitinib on detailed functional outcomes, such as fecundity, in a mouse model of endometriosis. In previous studies, the presence of endometriosis and the number of lesions was shown to affect pregnancy rates and litter size in mice (Bilotas et al., 2015; Rosa-E-Silva et al., 2019). Tofacitinib may ameliorate these negative effects on fecundity by decreasing any inflammatory effects, reducing anatomical distortion, or ameliorating any deficit in endometrial receptivity. Furthermore, additional studies will be necessary to understand the exact downstream signaling following inhibition of STAT3 phosphorylation by Tofacitinib. This will involve not only understanding global gene expression but also protein interactions at the level of STAT3. An avenue for differential gene expression downstream of pSTAT3 is subsequent dimerization. STAT proteins are known to make homodimers following phosphorylation; however, they can make heterodimers depending upon cell type, timing, and the cytokine initiating the signaling (Delgoffe and Vignali, 2013). In fact, STAT3 was first identified making a heterodimer with STAT1 (Zhong et al., 1994). Differential expression and dimerization patterns of STAT proteins in endometriosis may alter levels of gene expression and this presents another avenue of investigation into the biology behind endometriosis.
In summary, this study assessed the in vitro and in vivo effect of a JAK inhibitor, Tofacitinib, on endometriosis. Treatment of mice with surgically induced endometriosis led to both lesion regression and decreased adhesive burden. Tofacitinib treatment of Ishikawa cells demonstrated both inhibition of STAT3 phosphorylation and a decrease in the expression of STAT3-mediated genes, such as HIF1α and VEGF. Finally, treatment of primary cells showed a consistent inhibition of STAT3 phosphorylation in patients both with and without endometriosis and for both epithelial and stromal cells. Tofacitinib overall poses a tantalizing new agent to be further explored for the treatment of endometriosis.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the help of Shutaro Habata in performing the mouse studies.
Authors’ roles
A.M.K., R.M., and H.S.T. were involved in the design, analysis, and interpretation of data. V.A.F. aided in acquiring patient tissue used for culture.
Funding
The authors wish to acknowledge the financial support of the Society of Reproductive Investigation via the SRI-Bayer Discovery Grant and NIH/NICHD R01 HD100336 and the Nezhat Family Foundation.
Conflict of interest
Authors declare that they have no conflicts to disclose.
Supplementary data
Supplementary data are available at Molecular Human Reproduction online.
Data availability
The data used to generate the results for this this article will be shared upon request to the corresponding author.
References
- Alderman MH III, Yoder N, Taylor HS. The systemic effects of endometriosis. Semin Reprod Med 2017;35:263–270. [DOI] [PubMed] [Google Scholar]
- Bilotas MA, Olivares CN, Ricci AG, Baston JI, Bengochea TS, Meresman GF, Baranao RI. Interplay between endometriosis and pregnancy in a mouse model. PloS One 2015;10:e0124900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DP, Muriana A, Gebel H, Rotman C, Rana N, Dmowski WP. Monocyte-mediated enhancement of endometrial cell proliferation in women with endometriosis. Fertil Steril 1994;61:78–84. [DOI] [PubMed] [Google Scholar]
- Brosens J, Verhoeven H, Campo R, Gianaroli L, Gordts S, Hazekamp J, Hagglund L, Mardesic M, Varila E, Zech J et al. High endometrial aromatase P450 mRNA expression is associated with poor IVF outcome. Hum Reprod 2004;19:352–356. [DOI] [PubMed] [Google Scholar]
- Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet 2010;27:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun S. Endometriosis. N Eng J Med 2009;360: [DOI] [PubMed] [Google Scholar]
- Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, Kohlmeier A, Yin P, Milad M, Wei J. Endometriosis. Endocr Rev 2019;40:1048–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Han ZC. STAT3: a critical transcription activator in angiogenesis. Med Res Rev 2008;28:185–200. [DOI] [PubMed] [Google Scholar]
- Cho S, Mutlu L, Zhou Y, Taylor HS. Aromatase inhibitor regulates let-7 expression and let-7f induced cell migration in endometrial cells from women with endometriosis. Fertil Steril 2016;106:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hooghe TM, Nugent NP, Cuneo S, Chai DC, Deer F, Debrock S, Kyama CM, Mihalyi A, Mwenda JM. Recombinant human TNFRSF1A (r-hTBP1) inhibits the development of endometriosis in baboons: a prospective, randomized, placebo- and drug-controlled study. Biol Reprod 2006;74:131–136. [DOI] [PubMed] [Google Scholar]
- De Vries LCS, Duarte JM, De Krijger M, Welting O, Van Hamersveld PHP, Van Leeuwen-Hilbers FWM, Moerland PD, Jongejan A, D'Haens GR, De Jonge WJ et al. A JAK1 selective kinase inhibitor and tofacitinib affect macrophage activation and function. Inflamm Bowel Dis 2019;25:647–660. [DOI] [PubMed] [Google Scholar]
- Delgoffe GM, Vignali DA. STAT heterodimers in immunity: a mixed message or a unique signal? JAKSTAT 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumoto Y, Smith CK, Blanco L, Zhao W, Brooks SR, Thacker SG, Abdalrahman Z, Sciumè G, Tsai WL, Trier AM et al. Tofacitinib ameliorates murine lupus and its associated vascular dysfunction. Arthritis Rheumatol 2017;69:148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, McGarry T, Orr C, McCormick J, Veale DJ, Fearon U. Tofacitinib regulates synovial inflammation in psoriatic arthritis, inhibiting STAT activation and induction of negative feedback inhibitors. Ann Rheum Dis 2016;75:311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC, Kao LC. Endometriosis. The Lancet 2004;364:1789–1799. [DOI] [PubMed] [Google Scholar]
- Gurates B, Bulun SE. Endometriosis: the ultimate hormonal disease. Semin Reprod Med 2003;21:125–134. [DOI] [PubMed] [Google Scholar]
- Haney AF, Muscato JJ, Weinberg JB. Peritoneal fluid cell populations in infertility patients. Fertil Steril 1981;35:696–698. [DOI] [PubMed] [Google Scholar]
- Itoh F, Komohara Y, Takaishi H, Honda R, Tashiro H, Kyo S, Katabuchi H, Takeya M. Possible involvement of signal transducer and activator of transcription-3 in cell-cell interactions of peritoneal macrophages and endometrial stromal cells in human endometriosis. Fertil Steril 2013;99:1705–1713. [DOI] [PubMed] [Google Scholar]
- Kim BG, Yoo JY, Kim TH, Shin JH, Langenheim JF, Ferguson SD, Fazleabas AT, Young SL, Lessey BA, Jeong JW. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum Reprod 2015;30:1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlyar A, Taylor HS, D'Hooghe TM. Use of immunomodulators to treat endometriosis. Best Pract Res Clin Obstet Gynaecol 2019;60:56–65. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril 2001;75:1–10. [DOI] [PubMed] [Google Scholar]
- Lee B, Du H, Taylor HS. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod 2009;80:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA, Kim JJ. Endometrial receptivity in eutopic endometrium of women with endometriosis it is affected, let me show you why. Fertil Steril 2017;108:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 2010;40:294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes IB, Byers NL, Higgs RE, Lee J, Macias WL, Na S, Ortmann RA, Rocha G, Rooney TP, Wehrman T et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther 2019;21:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehtap S, Huseiyn A, Ahmet A, Mehmet Erim D, Ali S. The effects of the JAK-STAT signal pathway inhibition on collagen biosynthesis in fibroblast cell culture. Arthritis Rheumatol 2018;70:https://acrabstracts.org/abstract/the-effects-of-the-jak-stat-signal-pathway-inhibition-on-collagen-biosynthesis-in-fibroblast-cell-culture/. Accessed April 29, 2020. [Google Scholar]
- Milici AJ, Kudlacz EM, Audoly L, Zwillich S, Changelian P. Cartilage preservation by inhibition of Janus kinase 3 in two rodent models of rheumatoid arthritis. Arthritis Res Ther 2008;10:R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okiyama N, Furumoto Y, Villarroel VA, Linton JT, Tsai WL, Gutermuth J, Ghoreschi K, Gadina M, O'Shea JJ, Katz SI. Reversal of CD8 T-cell–mediated mucocutaneous graft-versus-host-like disease by the JAK inhibitor Tofacitinib. J Invest Dermatol 2014;134:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive DL, Weinberg JB, Haney AF. Peritoneal macrophages and infertility: the association between cell number and pelvic pathology. Fertil Steril 1985;44:772–777. [DOI] [PubMed] [Google Scholar]
- Pawar S, Starosvetsky E, Orvis GD, Behringer RR, Bagchi IC, Bagchi MK. STAT3 regulates uterine epithelial remodeling and epithelial-stromal crosstalk during implantation. Mol Endocrinol 2013;27:1996–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer M, Hartmann TN, Leick M, Catusse J, Schmitt-Graeff A, Burger M. Alternative implication of CXCR4 in JAK2/STAT3 activation in small cell lung cancer. Br J Cancer 2009;100:1949–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino N, Mamillapalli R, Moridi I, Tal R, Taylor HS. G-Protein-coupled receptor cxcr7 is overexpressed in human and murine endometriosis. Reprod Sci 2018;25:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha AL, Reis FM, Taylor RN. Angiogenesis and endometriosis. Obstet Gynecol Int 2013;2013:859619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-E-Silva A, Rosa-E-Silva J, Mamillapalli R, Taylor H. Dose-dependent decreased fertility in response to the burden of endometriosis in a murine model. Reprod Sci 2019;26:1395–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren S, Corr M, Firestein GS, Boyle DL. The JAK inhibitor CP-690,550 (tofacitinib) inhibits TNF-induced chemokine expression in fibroblast-like synoviocytes: autocrine role of type I interferon. Ann Rheum Dis 2012;71:440–447. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Rockfield S, Taran N, Haller E, Engelman RW, Flores I, Panina-Bordignon P, Nanjundan M. Effect of hydroxychloroquine and characterization of autophagy in a mouse model of endometriosis. Cell Death Dis 2016;7:e2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin C, Mamillapalli R, Yi KW, Taylor HS. microRNA Let‐7b: a novel treatment for endometriosis. J Cell Mol Med 2018;22:5346–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabe N, Meden-Vrtovec H, Verdenik I, Kosir-Pogacnik R, Ihan A. Cytotoxic T-cells in peripheral blood in women with endometriosis. Geburtshilfe Frauenheilkd 2013;73:1042–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele RW, Dmowski WP, Marmer DJ. Immunologic aspects of human endometriosis. American journal of reproductive immunology: AJRI: official journal of the American Society for the Immunology of Reproduction and the International Coordination Committee for. Immunology of Reproduction 1984;6:33–36. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Giudice LC, Lessey BA, Abrao MS, Kotarski J, Archer DF, Diamond MP, Surrey E, Johnson NP, Watts NB et al. Treatment of endometriosis-associated pain with Elagolix, an oral GnRH antagonist. N Engl J Med 2017;377:28–40. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Barbara G, Abbiati A, Somigliana E, Viganò P, Fedele L. Repetitive surgery for recurrent symptomatic endometriosis: what to do? Eur J Obstet Gynecol Reprod Biol 2009;146:15–21. [DOI] [PubMed] [Google Scholar]
- Villarino AV, Kanno Y, O'Shea JJ. Mechanisms and consequences of JAK-STAT signaling in the immune system. Nat Immunol 2017;18:374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Goldsmith LT, Taylor RN, Bellet D, Taylor HS. Inflammation in reproductive disorders. Reprod Sci 2009;16:216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesildaglar N, Ordonez JL, Laermans I, Koninckx PR. The mouse as a model to study adhesion formation following endoscopic surgery: a preliminary report. Hum Reprod 1999;14:55–59. [DOI] [PubMed] [Google Scholar]
- Yoo JY, Jeong JW, Fazleabas AT, Tayade C, Young SL, Lessey BA. Protein inhibitor of activated STAT3 (PIAS3) Is Down-regulated in eutopic endometrium of women with endometriosis. Biol Reprod 2016; 95:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Zhou L, Shangguan AJ, Bulun SE. Aromatase expression and regulation in breast and endometrial cancer. J Mol Endocrinol 2016;57:R19–R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Wen Z, Darnell JE. Jr., Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 1994;264:95–98. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis. Nat Rev Dis Primers 2018;4:9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to generate the results for this this article will be shared upon request to the corresponding author.