Abstract
We commonly see patients presenting with either portal hypertensive gastropathy (PHG) or radiation gastritis. Radiation-induced hemorrhagic gastritis is an unusual lethal complication postradiation. Patients with preexisting PHG have very friable mucosa that can easily bleed after radiation for cancer treatment. There is an increased risk of bleeding with both entities present together. Our aim is to focus on treatment and possible prevention of gastrointestinal bleeding in patients with preexisting PHG undergoing radiation therapy for newly diagnosed cancer. Several therapies like prednisolone, argon plasma coagulation, laser coagulation have been proposed. There are no set guidelines for treatment. In these patients, if radiation therapy is indicated either for hepatic or gastrointestinal malignancy, it is suggested to premedicate with proton pump inhibitors or sucralfate. We describe a case of 73-year-old female who presented with upper gastrointestinal bleeding. She had liver cirrhosis secondary to nonalcoholic fatty liver disease and diagnosed with pancreatic cancer, for which she received chemoradiation. She was found to have both radiation gastritis and PHG with diffuse erythematous, edematous, congested mucosa with diffuse oozing blood in the antrum making it very challenging to treat.
Keywords: Portal hypertensive gastropathy, Radiation-induced hemorrhagic gastritis, Liver cirrhosis
Introduction
Radiation-induced hemorrhagic gastritis is an unusual complication postradiation for gastrointestinal malignancies. Patients with cirrhosis can present with upper GI bleeding secondary to portal hypertensive gastropathy (PHG). We commonly see patients presenting with either PHG or with radiation-induced hemorrhagic gastritis. In our case, we noticed an increased risk of bleeding due to both entities present together. Our aim is to focus on treatment and possible prevention of gastrointestinal bleeding in patients with preexisting PHG undergoing radiation therapy for newly diagnosed cancer.
Case Description
We have a 73-year-old female with past medical history of insulin-dependent diabetes mellitus, hypertension, liver cirrhosis secondary to nonalcoholic fatty liver disease, pancreatic cancer with liver metastasis diagnosed 8 months prior to presentation, completed chemoradiation therapy 3 months ago presented to the hospital with bright red blood in stool and 3 episodes of hematemesis quantified as a cupful each time starting on the day of presentation. Patient also complained of palpitations, lightheadedness, and chills for the past day. Vitals on admission showed blood pressure 90/50 mm Hg, heart rate 110 beats/min, afebrile, saturating 94% on room air. On abdominal examination mild tenderness in the mid epigastric region was present, positive bowel sounds, no distention, no guarding or rigidity. Rectal examination revealed bright red blood. Laboratory studies are mentioned in Table 1. Calculated MELD score was 9. Patient was adequately resuscitated with intravenous fluids, blood transfusion and started on pantoprazole and octreotide infusion. Emergent esophagogastroduodenoscopy (EGD) was done, results as below. Chronic liver disease workup is negative for viral hepatitis panel, autoimmune diseases. CT abdomen and pelvis with IV contrast showed pancreatic neck adenocarcinoma, cirrhosis of the liver with portal hypertension but no evidence of Hepatocellular carcinoma. US Doppler showed patent portal veins with normal directionality of flow, patent hepatic veins, and hepatic artery.
Table 1.
Laboratory values on admission
| Patient data | Normal range | |
|---|---|---|
| Hematology | ||
| WBC, K/µL | 1.33 | 3.8–10.5 |
| Hemoglobin, g/dL | 6.9 | 11.5–15.5 |
| Hematocrit, % | 21.6 | 34.5–45.0 |
| PLT, K/µL | 71 | 150–400 |
| BMP | ||
| Sodium, mmol/L | 143 | 135–145 |
| Potassium, mmol/L | 4.0 | 3.5–5.3 |
| BUN, mg/dL | 6.0 | 7–23 |
| Creatinine, mg/dL | 0.6 | 0.5–1.3 |
| Coagulation profile | ||
| PT, s | 13.6 | 9.8–13.1 |
| PTT, s | 28.8 | 27.5–36.3 |
| INR | 1.22 | 0.88–1.17 |
| Liver function tests | ||
| Total protein, g/dL | 4.6 | 6–8.3 |
| Albumin, g/dL | 2.7 | 3.3–5 |
| Total bilirubin, mg/dL | 0.4 | 0.2–1.2 |
| ALP, U/L | 61 | 40–120 |
| AST, U/L | 31 | 4–32 |
| ALT, U/L | 32 | 4–33 |
WBC, white cell count; PLT, platelet count; BMP, basic metabolic profile; BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; PT, prothrombin time; PTT, partial thromboplastin time.
Patient underwent EGD and colonoscopy at an outside hospital for similar complaints 2 weeks ago. EGD showed small varices, multiple bleeding distal gastric and duodenal vascular ectasias. Colonoscopy showed diffuse vascular ectasia in colon with flecks of blood but no active bleeding, so no intervention was performed. Liver biopsy showed steatohepatitis in the background of moderate steatosis, trichrome stain highlighting portal, and periportal fibrosis with bridging and vague nodule formation suggestive of cirrhosis.
During hospitalization, the patient underwent series of endoscopies due to persistent bleeding. First EGD on day 1 of hospitalization showed nonbleeding grade 1, esophageal varices, PHG, portal hypertensive duodenopathy, diffuse active oozing noted in the distal stomach and duodenum not amenable to endoscopic techniques. Patient was continued on pantoprazole and octreotide infusion to complete a total of 5-day course of therapy. Patient was also started on nadolol for portal hypertension which made him hypotensive with a systolic blood pressure in 80 s in two instances due to which it had to be discontinued. Due to findings of PHG and duodenopathy on EGD with episodes of GI bleeding requiring transfusions and patient not tolerating nadolol, trans-jugular intrahepatic portosystemic shunt (TIPS) procedure was considered. As patient continued to have hematochezia, colonoscopy was performed on day 9, which showed 4 nonbleeding colonic angiectasias treated with argon plasma coagulation (APC), erythematous mucosa in transverse colon, nonbleeding rectal varices, and diverticulosis in ascending colon.
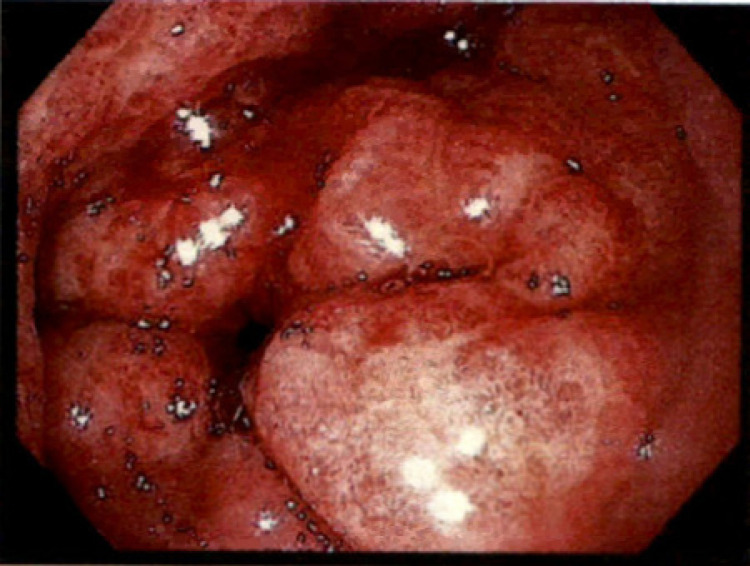
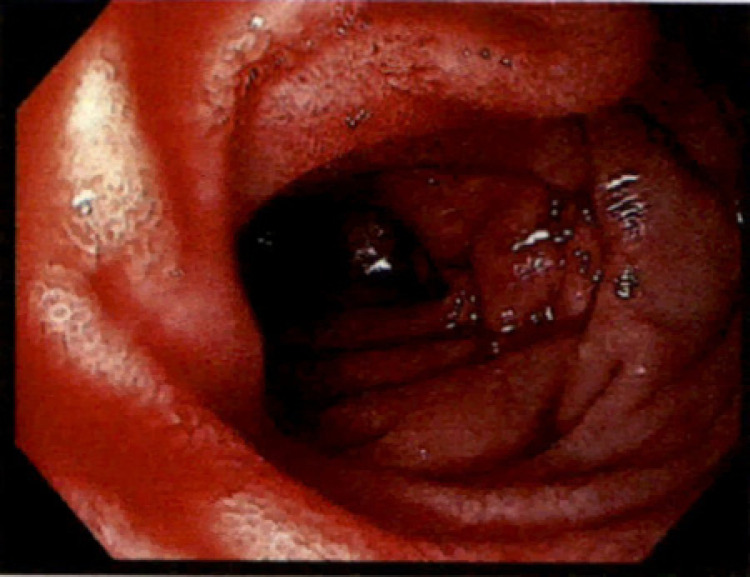
Portal pressures measured by hepatic vein catheterization showed wedge hepatic venous pressure of 17 mm Hg, free hepatic venous pressure of 5 mm Hg, and hepatic venous pressure gradient of 12 mm Hg. Considering the pressures were not high enough, TIPS was not performed. It was also evident that patient had persistent bleeding from a secondary cause other than PHG. On day 15 of hospitalization, repeat EGD showed diffuse erythematous, edematous, congested mucosa with diffusely oozing blood in the antrum (Fig. 1) consistent with hemorrhagic gastritis. A small area in the antrum which was bleeding more than others was treated with APC. Hemostasis at that spot was achieved, but continuous oozing was present from other areas. Active oozing of blood was also noted in duodenal bulb, duodenal sweep, and second portion of duodenum consistent with hemorrhagic duodenopathy (Fig. 2). Due to the extent of involvement, decision was made to try medical management as endoscopic intervention is not amenable. Video capsule endoscopy showed diffuse oozing from stomach and duodenum with clots seen throughout the small bowel until approximately 2 h 30 min. There were no other lesions in rest of the small bowel and colon. Considering the timing of presentation after radiation therapy and EGD findings, this was believed to be likely secondary to radiation gastritis with overlying PHG. Patient was medically managed on sucralfate 4 times daily, subcutaneous octreotide injections twice daily, twice daily pantoprazole. Bleeding subsequently improved and patient had no significant bleeding in 3-month outpatient follow-up.
Fig. 1.
Endoscopic image of antrum showing diffuse edematous, erythematous mucosa with angiectasias that are diffusely actively oozing blood.
Fig. 2.
Endoscopic image of duodenum showing diffuse edematous, erythematous mucosa with angiectasias that are actively oozing blood.
Discussion
The most common complications of portal hypertension leading to upper GI bleeding are Varices and PHG. Though varices are more commonly seen, it is not uncommon to see PHG. Like varices, PHG also develop as a result of increased resistance in the portal circulation [1]. Up to 65% of patients with cirrhosis and portal hypertension develop PHG [2]. While varices are seen more commonly in the lower esophagus and fundus of stomach, PHG is seen in the mucosa of antrum and body of the stomach. The stomach mucosa is edematous, very friable, and bleeds easily as a result of chronic congestion in PHG. Histologically, PHG can be seen as marked dilatation of capillaries and collecting venules in the gastric mucosa. Clinically patients present with overt or chronic upper GI bleeding due to oozing from the dilated capillaries. Bleeding is uncommon in mild stage PHG than in severe PHG. Approximately 65–90% have mild PHG whereas 10–25% may present as severe PHG [2]. Certain cytokines are known to play a role in the pathogenesis of PHG like TNF and VEGF. TNF upregulates nitric oxide (NO) synthase which increases NO production leading to capillary dilation. High levels of VEGF have also been found in PHG which can lead to increased angiogenesis [1]. Endoscopically, PHG can include several lesions like fine pink speckling, petechia, multiple bleeding spots, snake-like pattern, and cherry red spots [3]. Gastric mucosa is extremely susceptible to damage in PHG. The best treatment recommended so far are β-blockers and octreotide which reduce gastric blood flow. Endoscopic treatment has not been proven effective as the lesions are widespread. In patients not responding to medical therapy, TIPS must be considered [1].
Radiation-induced hemorrhagic gastritis is an unusual lethal complication presenting 1–12-month postradiation with peak ulceration occurring within 1–2 months [4]. The severity of damage is directly related to the high total dose and high daily fraction of radiation used. Incidence of gastric ulcer occurs between 25 and 30% as the dose exceeds 45 Gy with risk of perforation when dose exceeds 60 Gy [5]. Damage to stomach mucosa is most commonly due to direct injury from radiation followed by vasculopathy which can progress to ischemia and ulceration. Histologically, mucosal edema, microscopic hemorrhages, and exudates with damage to parietal and chief cells can be seen [4]. Upper endoscopy should be performed 4–8 weeks after radiation therapy if symptoms of abdominal pain, nausea, vomiting, or GI bleeding are present [6]. Treatment with proton pump inhibitors, sucralfate must be initiated. There are no set guidelines for treatment and successful treatment can be very challenging. Hyperbaric oxygen has been tried in the past to increase neovascularization to help heal the ulcers [7]. Epsilon aminocaproic acid, a fibrinolytic inhibitor which acts by inhibiting plasminogen and antiplasmin activity has also been used [8]. Initial therapy with oral prednisolone [9] and rescue therapies like bevacizumab [10] have also been tried. Shukuwa et al. [11] described a case of hemorrhagic radiation gastritis after being treated for pancreatic cancer managed with APC successfully. In our patient, the mucosa was extensively involved that APC was ineffective in controlling the bleeding. After all the medical and endoscopic measures are exhausted, surgery with total gastrectomy will be the last resort for radiation injuries.
Chon et al. [4] has demonstrated that gastroduodenal complications were higher in patients receiving radiation therapy with preexisting PHG secondary to liver cirrhosis like our patient. Mucosa is very friable due to increased blood supply to the stomach from increased portal venous pressure gradient. Portal hypertensive gastropathy can be a complication of portal hypertension leading to upper GI bleeding in cirrhotic patients. In patients receiving radiation therapy for newly diagnosed liver or pancreatic cancer, preexisting PHG can make them more susceptible to hemorrhagic gastritis. There are no existing guidelines currently for appropriate treatment and successful control of bleeding can be very challenging due to extensive involvement. In these patients, if radiation therapy is indicated either for hepatic or gastrointestinal malignancy, it is suggested to premedicate with proton pump inhibitors, somatostatin analogues, or sucralfate to reduce the risk of radiation-induced hemorrhagic gastritis.
Statement of Ethics
As per the institutional guidelines, review of medical records for publication of case reports of up to 3 patients is not considered human subjects research and does not require institutional review board review and approval. Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Conflict of Interest Statement
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Funding Sources
This manuscript did not receive any funding.
Author Contributions
Madala S. drafted the manuscript. Polavarapu A. followed the patient while admitted to the hospital. Gurala D. edited the manuscript; Gumaste V. approved the final manuscript.
References
- 1.Wong F. Portal hypertensive gastropathy. Gastroenterol Hepatol. 2007;3((6)):428–73. [PMC free article] [PubMed] [Google Scholar]
- 2.Piqué JM. Portal hypertensive gastropathy. Baillieres Clin Gastroenterol. 1997;11((2)):257–70. doi: 10.1016/s0950-3528(97)90039-7. [DOI] [PubMed] [Google Scholar]
- 3.Eleftheriadis E. Portal hypertensive gastropathy a clinically significant puzzle. Ann Gastroenterol. 2001;14((3)):196–204. [Google Scholar]
- 4.Chon YE, Seong J, Kim BK, Cha J, Kim SU, Park JY, et al. Gastroduodenal complications after concurrent chemoradiation therapy in patients with hepatocellular carcinoma: endoscopic findings and risk factors. Int J Radiat Oncol Biol Phys. 2011;81((5)):1343–51. doi: 10.1016/j.ijrobp.2010.07.1986. [DOI] [PubMed] [Google Scholar]
- 5.Sourati A, Ameri A, Malekzadeh M. Acute side effects of radiation therapy. Cham: Springer; 2017. Radiation gastritis. [Google Scholar]
- 6.Sangro B, Martínez-Urbistondo D, Bester L, Bilbao JI, Coldwell DM, Flamen P, et al. Prevention and treatment of complications of selective internal radiation therapy: expert guidance and systematic review. Hepatology. 2017;66((3)):969–82. doi: 10.1002/hep.29207. [DOI] [PubMed] [Google Scholar]
- 7.Kernstine KH, Greensmith JE, Johlin FC, Funk GF, De Armond DT, Van Natta TL, et al. Hyperbaric oxygen treatment of hemorrhagic radiation-induced gastritis after esophagectomy. Ann Thorac Surg. 2005;80((3)):1115–7. doi: 10.1016/j.athoracsur.2004.02.102. [DOI] [PubMed] [Google Scholar]
- 8.Grover N, Johnson A. Aminocaproic acid used to control upper gastrointestinal bleeding in radiation gastritis. Dig Dis Sci. 1997;42((5)):982–4. doi: 10.1023/a:1018828801379. [DOI] [PubMed] [Google Scholar]
- 9.Yun HG, Kim HY, Kim DY, Lim YJ. Successful treatment of intractable bleeding caused by radiation-induced hemorrhagic gastritis using oral prednisolone: a case report. Cancer Res Treat. 2015;47((2)):334–8. doi: 10.4143/crt.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maire F, Muller N, Lévy P. First case of radiation-induced diffuse hemorrhagic gastritis successfully treated with intravenous bevazicumab. Am J Gastroenterol. 2017;112((8)):1349–50. doi: 10.1038/ajg.2017.160. [DOI] [PubMed] [Google Scholar]
- 11.Shukuwa K, Kume K, Yamasaki M, Yoshikawa I, Otsuki M. Argon plasma coagulation therapy for a hemorrhagic radiation-induced gastritis in patient with pancreatic cancer. Intern Med. 2007;46((13)):975–7. doi: 10.2169/internalmedicine.46.0076. [DOI] [PubMed] [Google Scholar]