Abstract
Background
Acute myocardial infarction (AMI) is the leading cause of death in developed countries, and current treatment modalities have failed to regenerate the dead myocardium resulting from the ischemic damage. Stem cells have the potential to regenerate the damaged myocardium. These cells can be mobilized from the bone marrow by factors such as granulocyte colony stimulating factor (G‐CSF).
Objectives
To assess the effects of stem cell mobilization following granulocyte colony stimulating factor therapy in patients with acute myocardial infarction.
Search methods
We searched CENTRAL (The Cochrane Library Issue 4, 2010), MEDLINE (1950 to November week 3, 2010), EMBASE (1980 to 2010 week 48), BIOSIS Previews (1969 to 30 November 2010), ISI Science Citation Index Expanded (1970 to 4 December 2010) and ISI Conference Proceedings Citation Index ‐ Science (1990 to 4 December 2010). We also checked reference lists of articles.
Selection criteria
We included randomized controlled trials including participants with a clinical diagnosis of AMI who were randomly allocated to the subcutaneous administration of G‐CSF through a daily dose of 2.5, 5 or 10 microgram/kg for four to six days or placebo. No age or other restrictions were applied for the selection of patients.
Data collection and analysis
Two authors independently selected trials, assessed trials for eligibility and methodological quality, and extracted data regarding the clinical efficacy and adverse outcomes. Disagreements were resolved by the third author.
Main results
We included seven trials reported in 30 references in the review (354 participants). In all trials, G‐CSF was compared with placebo preparations. Dosage of G‐CSF varied among studies, ranging from 2.5 to 10 microgram/kg/day. Regarding overall risk of bias, data regarding the generation of randomization sequence and incomplete outcome data were at a low risk of bias; however, data regarding binding of personnel were not conclusive. The rate of mortality was not different between the two groups (RR 0.64, 95% CI 0.15 to 2.80, P = 0.55). Regarding safety, the limited amount of evidence is inadequate to reach any conclusions regarding the safety of G‐CSF therapy. Moreover, the results did not show any beneficial effects of G‐CSF in patients with AMI regarding left ventricular function parameters, including left ventricular ejection fraction (RR 3.41, 95% CI ‐0.61 to 7.44, P = 0.1), end systolic volume (RR ‐1.35, 95% CI ‐4.68 to 1.99, P = 0.43) and end diastolic volume (RR ‐4.08, 95% CI ‐8.28 to 0.12, P = 0.06). It should also be noted that the study was limited since the trials included lacked long enough follow up durations.
Authors' conclusions
Limited evidence from small trials suggested a lack of benefit of G‐CSF therapy in patients with AMI. Since data of the risk of bias regarding blinding of personnel were not conclusive, larger RCTs with appropriate power calculations and longer follow up durations are required in order to address current uncertainties regarding the clinical efficacy and therapy‐related adverse events of G‐CSF treatment.
Plain language summary
Granulocyte colony stimulating factor treatment following a heart attack
People who suffer a heart attack (due to a blockage in the artery supplying blood to the heart) are usually affected by the damage to a portion of their heart muscle. Current treatment options are unable to restore the damaged section of the heart. Recently, stem cells have been shown to be able to restore and replace the damaged tissue in patients with heart attack. These cells could be mobilized to the heart with agents such as granulocyte colony stimulating factor (G‐CSF).
In this review, analysis of seven included studies with low risk of bias using G‐CSF to improve the function of damaged heart of patient with heart attack failed to show any beneficial effects of this treatment. The rate of mortality was not different between the two groups (RR 0.64, 95% CI 0.15 to 2.80, P = 0.55). Also, left ventricular parameters including left ventricular ejection fraction (RR 3.41, 95% CI ‐0.61 to 7.44, P = 0.1), end systolic volume (RR ‐1.35, 95% CI ‐4.68 to 1.99, P = 0.43) and end diastolic volume (RR ‐4.08, 95% CI ‐8.28 to 0.12, P = 0.06) did not show significant changes between the treatment and the control groups. There was no evidence that the study was associated with serious adverse effects, however it should be noted that the study was limited since the trials included lacked long enough follow up durations. Additionally four studies had either high or unclear risk of bias for blinding. Therefore, based on the results of the current study, G‐CSF treatment should not be administered for patients with heart attack.
Background
Description of the condition
Acute myocardial infarction (AMI) is the most common cause of morbidity from ischemic heart disease and is the leading cause of death in developed countries (BHF 2004). Worldwide more than seven million people suffer from AMI each year (White 2008). Following occlusion of a coronary artery, the inadequate supply of blood to the myocardium causes necrosis of the affected area. This in turn can lead to complications such as cardiogenic shock, cardiac perforations, embolism, heart failure, papillary muscle rupture, rhythm disturbances or autoimmune pericarditis, all of which can result in the death of affected individuals (Burton 1996).
Current pharmacologic and interventional strategies have been shown to be effective in terms of improved survival in patients with AMI (Lindquist 2003; Stone 2003). However, such treatment options can only limit the ongoing process and have failed to regenerate the dead myocardium resulting from the ischemic damage (Hartwell 2005). While these revascularization therapies such as catheterization and balloon angioplasty or stenting can reestablish the epicardial blood flow, the damage to the myocardium is usually unavoidable and may result in heart failure caused by adverse left ventricular remodeling (Hartwell 2005). Therefore, given the current advances, new therapeutic approaches are needed in order to target the lost cells during the ischemic damage and to restore the normal myocardial function.
While measurement of ejection fraction has been considered as a measure for cardiac function, echocardiographic assessment of function may have inherent limitations because of two‐dimensional imaging as compared to both radionuclide blood pool imaging (with a three‐dimensional component) and volumetric magnetic resonance imaging (Martin‐Rendon 2008). However, since this method has been applied by most studies, it was considered in the present review as the main assessment tool for cardiac function evaluation.
A similar systematic review was published in 2008 assessing the role of stem cells in the treatment of AMI (Martin‐Rendon 2008). The authors of the review found beneficial effects of stem cell therapy by direct implantation of cells into the ischaemic regions for patients suffering from AMI. Our review was developed to address the question of whether mobilization of stem cells from the bone marrow by granulocyte colony stimulating factor (G‐CSF), which is a growth factor, could show similar beneficial effects without direct implantation.
Description of the intervention
In recent years, both animal and human studies have suggested that stem cells derived from the bone marrow have the potential to differentiate into specialized cells such as cardiomyocytes, endothelial cells, and smooth muscle cells (Asahara 1999; Kawamoto 2001). Based on this finding, a novel approach to treat AMI has developed from the observation in animal models that bone marrow‐derived stem cells may regenerate myocardium by inducing neovascularization and myogenesis in the ischemic myocardium, and improve cardiac function after AMI (Kocher 2001; Martin‐Rendon 2008; Orlic 2001). Later, preliminary human studies demonstrated that cardiac function was improved following infusion of bone marrow stem cells into the infracted myocardium (Assmus 2002; Fernandez‐Aviles 2004; Meyer 2006). However, since this approach requires a sizable bone marrow aspiration in a potentially hemodynamically unstable patient, less invasive methods to repopulate the damaged myocardium with stem cells would be required.
Alternatively, stem cell mobilization with cytokines such as G‐CSF may be a viable option, since it obviates the need for bone marrow aspiration and repeated cardiac catheterization (Abdel‐Latif 2008). Several studies being conducted in different regions of the world have demonstrated the recruitment of mobilized stem cells to the ischemic myocardium and their differentiation into myoblasts and endothelial cells following G‐CSF administration (Ellis 2006; Engelmann 2006; Ince 2005b; Leone 2007; Ripa 2006). The dosage of G‐CSF administration in these studies have been between 2.5 and 10 microgram/kg for four to six days. However, most of these studies have been performed on a low number of patients, and have therefore yielded disparate results. While some of these studies have demonstrated beneficial effects of G‐CSF treatment (Ince 2005b; Leone 2007), others have not yielded such results (Ellis 2006; Engelmann 2006; Ripa 2006). Therefore this novel approach may constitute a potential, new clinical treatment strategy for patients suffering from AMI.
Early clinical studies have raised some safety issues associated with G‐CSF administration (Hill 2005; Kang 2004). These include initiation or exaggeration of plaque instability, myocardial infarction and rupture and increased risk of neointima formation and restenosis (Hill 2005; Kang 2004). However, further clinical trials have failed to address these side effects in patients treated with G‐CSF (Ince 2005b; Kuethe 2005; Ripa 2006).
How the intervention might work
The exact mechanism of action of G‐CSF administration has not been fully elucidated. Providing stem cells through mobilization of these cells from the bone marrow and homing of them into the damaged myocardium is the most clearly known mechanism of G‐CSF administration (Kawada 2004). It has also been proposed that G‐CSF accelerates the healing process by inducing growth factors and attenuates early ventricular expansion after AMI through collagen deposition in the infracted area (Minatoguchi 2004; Sugano 2005). Also through activation of specific receptors in the heart, G‐CSF may enhance the survival of cardiomyocytes and reduce the rate of apoptosis (Hasegawa 2006). Thus, it seems that by supplying endothelial progenitor cells and providing multiple angiogenic factors or cytokines, G‐CSF may improve the cardiac function and reverse the ischemic status of the affected myocardium (Ince 2005a).
Why it is important to do this review
Since administration of G‐CSF in combination with already established therapeutic weapons for patients suffering from AMI has emerged as a novel intervention in clinical practice, it is important that a systematic review is undertaken in order to assess the safety and efficacy of this intervention. Therefore, we performed the current systematic review in order to investigate the potential therapeutic benefits of G‐CSF therapy for AMI.
Objectives
To determine the safety, feasibility, tolerability and efficacy of stem cell mobilization following granulocyte colony stimulating factor as a treatment for acute myocardial infarction.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials of patients suffering from AMI who received G‐CSF treatment in comparison to placebo or no intervention in addition to routine treatment. Studies published in all languages were eligible.
Types of participants
Any participants (of any age) with a clinical diagnosis of AMI.
Types of interventions
Studies involving the subcutaneous administration of G‐CSF through a daily dose of 2.5, 5 or 10 microgram/kg for four to six days as treatment for AMI.
Participants in the control treatment arm would have had either no intervention or placebo such as isotonic saline infusion. Trials in which surgery (e.g. coronary artery bypass graft (CABG)) or percutaneous coronary intervention (PCI) were administered were eligible for inclusion.
Types of outcome measures
Primary outcomes
All‐cause mortality.
Left ventricular ejection fraction (LVEF).
Secondary outcomes
Cardiovascular morbidity (a composite outcome) including reinfarction, incidence of arrhythmias, incidence of restenosis, hospital readmission, congestive heart failure (CHF) requiring rehospitalization, tamponade, cardiac perforation, cardiogenic shock and target vessel revascularisation.
Left ventricular end‐systolic volume (LVESV).
Left ventricular end‐diastolic volume (LVEDV).
Economic costs.
Patient‐reported outcomes including pain‐free walking distance (PFWD) and the total amount of pain measured by visual analogue scale (VAS).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Issue 4, 2010), MEDLINE (1950 to November week 3, 2010), EMBASE (1980 to 2010, week 48), BIOSIS Previews (1969 to 30 November 2010), ISI Science Citation Index Expanded (1970 to 4 December 2010) and ISI Conference Proceedings Citation Index ‐ Science (1990 to 4 December 2010). See Appendix 1 for the search strategies for all databases.
We used the Cochrane sensitive‐maximizing search strategy for identifying randomized trials in searching MEDLINE and EMBASE (Lefebvre 2009).
Searching other resources
We checked the bibliographic references of relevant studies and reviews. We contacted the authors of the studies and experts in the field for information about other possible trials.
We also looked for unpublished and ongoing studies by searching the metaRegister of controlled trials (including International Standard Randomised Controlled Trial Number Register (ISRCTN) and National Institutes of Health (NIH) ‐ randomized trial records) at www.controlled-trials.com/. Finally we also attempted to obtain individual patient level data.
No language restrictions were applied.
Data collection and analysis
Selection of studies
The titles and abstracts of references identified by the search were screened by KM and their eligibility for inclusion in the review was assessed independently by two review authors (KM and AR). Any disagreements were resolved by a third author (BM). We obtained full versions of articles that potentially met the inclusion criteria based on the title or abstract, and assessed them independently against the inclusion criteria. We recorded the reasons for exclusion of any study previously considered for inclusion in the Characteristics of excluded studies table.
Data extraction and management
KM and AR independently extracted both dichotomous and continuous data concerning outcome measures. Disagreements were resolved by a third author (BM). Once disagreements were resolved, we recorded the consensus data extracted on a third data extraction form. We sought any additional information necessary from trial authors.
Assessment of risk of bias in included studies
We assessed the risk of bias in included studies according to the Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011):
Sequence generation
Allocation concealment
Blinding of participants, personnel and outcome assessors
Incomplete outcome data
Selective outcome reporting
Other sources of bias
Accordingly, each specified risk of bias item was assigned as low, unclear or high risk.
Measures of treatment effect
We used relative risk as the measure of effect for each dichotomous outcome. Where continuous scales of measurement were used to assess the effects of treatment, we analyzed these data using mean difference (MD). If different scales were used in the different studies, the results were standardized, where possible, and then combined (i.e. standardized mean difference).
Assessment of heterogeneity
We explored and assessed clinical heterogeneity using the I² and Q statistics, and by subjective judgment of comparability of patients, interventions, and outcomes. An I² greater than 30% or Q statistic with a P value less than 0.1 was considered indicative of heterogeneity. Where there was significant heterogeneity among the studies, we explored the reasons for such heterogeneity and discussed in the review possible explanations for the observed heterogeneity.
Assessment of reporting biases
We used funnel plots to assess publication bias.
Data synthesis
We used both random‐effects and fixed‐effect models for robustness of results. If heterogeneity did not exist, we reported a fixed‐effect model. If statistical, but not clinical, heterogeneity existed, we reported a random‐effects model.
Subgroup analysis and investigation of heterogeneity
If sufficient trials were available, we would have performed subgroup analyses using age, sex, the mean LVEF at baseline, the dose of G‐CSF, and the peak white blood cell (WBC) and CD34+ cell counts as indicators of bone marrow cell mobilization efficacy with G‐CSF therapy.
Sensitivity analysis
Had sufficient studies been identified, we would have conducted sensitivity analyses to examine the robustness of the observed findings in relation to a number of factors including study quality and patient type.
Results
Description of studies
See Figure 1.
1.
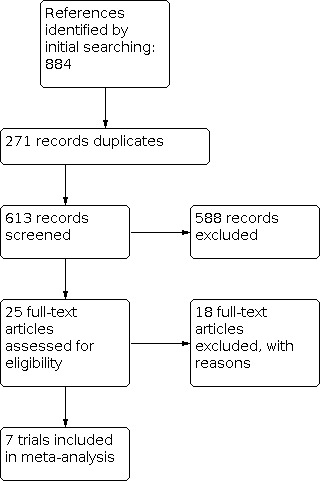
Study flow diagram.
We identified 884 references from the searches (442 from ISI Science Citation Index Expanded, 154 from ISI BIOSIS Previews, 143 from EMBASE, 102 from MEDLINE and 43 from CENTRAL). Following de‐duplication, 613 records were left. Initial screening of the citations excluded 548 references. All remaining references were assessed on the basis of their full text for inclusion or exclusion against the Criteria for considering studies for this review.
Included studies
See table of Characteristics of included studies.
Seven trials were included in the review (354 participants) (Ellis 2006; Ince 2005a; Leone 2007; Ripa 2006; Takano 2007; Valgimigli 2005; Zohlnhöfer 2006). The number of participants included ranged from six to 58. We provide data regarding the proportion of each sex and the range of age for each trial in the table Characteristics of included studies.
All trials use percutaneous coronary intervention as primary treatment for AMI. Follow up was variable, from one month to one year.
Dosage of G‐CSF varied among studies, ranging from 2.5 to 10 microgram/kg/day. In two trials, dosages of 2.5 (Takano 2007) and 5 (Zohlnhöfer 2006) microgram/kg/day were administered, respectively. All other trials administered the dosage of 10 microgram/kg/day for study subjects. Duration of G‐CSF treatment ranged from four to six days.
The trials included in the review were conducted in five countries: USA, Germany, Denmark, Japan and Italy. The trials were published between 2005 and 2007. All trials were presented as full journal articles and all of them were published in English. All trials were parallel RCTs. None of studies were funded by industry.
All trials administrated a standard set of drugs including aspirin, clopidogrel, heparin, B‐blockers, statins, angiotensin converting enzyme (ACE) inhibitors, nitrates and/or diuretics. However, different trials used different sets of drugs for their patients. ACE‐I were administered in six trials (Ellis 2006; Ince 2005a; Ripa 2006; Takano 2007; Valgimigli 2005; Zohlnhöfer 2006), aspirin in five trials (Ince 2005a; Leone 2007; Takano 2007; Valgimigli 2005; Zohlnhöfer 2006), B‐ blockers and statins in five trials (Ellis 2006; Ripa 2006; Takano 2007; Valgimigli 2005; Zohlnhöfer 2006), clopidogrel in three trials (Ince 2005a; Leone 2007; Ripa 2006), and nitrates in one trial (Zohlnhöfer 2006).
Excluded studies
See the table Characteristics of excluded studies.
We excluded 18 studies for the following reasons:
In 11 trials, the diagnoses of patients were other than AMI (Engelmann 2006; Gloekler 2009; Huttmann 2006; Hyun‐Jae 2003; Li 2004; Meier 2009; Subramaniyam 2009; Suzuki 2006; Wnag 2005; Wolfram 2007; Zbinden 2005);
In three trials, the study had more than one active arm of investigation which was out of the scope of the current review (De Lezo 2007; Kang 2006; Suarez 2004);
In three trials, the study was not randomized (Joseph 2008; Kuethe 2004; Kuo 2009);
One study included patients with AMI and leukopenia (Guo 2008).
Risk of bias in included studies
In summary, the overall risk of bias was considered low among the studied trials. Since only seven trials were included in the review, no funnel plots were generated (see Table 1, Figure 2 and Figure 3).
1. Summary table of risk of bias.
| Bias element | Overall risk of bias |
| Random sequence generation (selection bias) | Low risk |
| Allocation concealment (selection bias) | Low risk |
| Blinding of participants and personnel (performance bias) | High risk |
| Blinding of outcome assessment (detection bias) | Low risk |
| Incomplete outcome data (attrition bias) | Low risk |
| Selective reporting (reporting bias) | Unclear risk |
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Six trials provided details as to the generation of the randomization sequence (Ellis 2006; Ince 2005a; Ripa 2006; Takano 2007; Valgimigli 2005; Zohlnhöfer 2006). The methods included permutated block design in densely opaque envelopes in one trial (Ellis 2006), closed envelope In one trial (Ince 2005a), sealed envelope in two trials (Ripa 2006; Zohlnhöfer 2006); minimization method in one trial (Takano 2007); and computer‐based randomization in one trial (Valgimigli 2005).
The generation of the randomization sequence was defined as unclear in one trial (Leone 2007) . No description was given in this publication as to which methods were used to generate the random sequence.
Blinding
In three trials, the blinding of all trial personnel (participants, clinicians and outcome assessors) was adequate (Ellis 2006; Ripa 2006; Zohlnhöfer 2006). In one trial, the blinding of participants and outcome assessors was adequate, but the blinding of clinicians was unclear (Ince 2005a). In three trials, the blinding of outcome assessors was adequate, but the blinding of participants and clinicians was unclear (Leone 2007; Takano 2007; Valgimigli 2005).
Incomplete outcome data
In one trial, all participants randomized to the trial were included in the final analysis of outcome data and the studies did not lose any participants during follow‐up (Ellis 2006). In four trials, not all participants were included in the outcome data analysis (Leone 2007; Ripa 2006; Takano 2007; Zohlnhöfer 2006). In these studies between 2% and 15% of participants initially screened for the study were lost during follow up. In Leone 2007, one patient in the treatment group was lost during the follow up. In Ripa 2006, 6 and 2 patients in the control and comparator group were lost during the follow up, respectively. In another study, three and two patients in the control and treatment group were lost during the follow up, respectively (Takano 2007). Finally, one study lost 11 and 7 participants in the control and treatment group, respectively (Zohlnhöfer 2006).
In the remaining two studies, description of follow‐up and withdrawals was incomplete (Ince 2005a; Valgimigli 2005).
Selective reporting
No reporting bias was identified in the current study. However, selective reporting is difficult to rule out in most of the cases.
Other potential sources of bias
Equal use of co‐interventions in each trial arm
None of the trials reported the use of any other co‐intervention in their trial.
Power calculation
Four trials reported power calculations (Ellis 2006; Leone 2007; Ripa 2006; Zohlnhöfer 2006). In Leone 2007, while 60 participants were included in order to detect a possible significant difference of 5% in LV ejection fraction, the study enrolled 41 patients and acknowledged the limited power of the study to discriminate a potential significant difference between patients in each treatment arm. In Ellis 2006, a statistical power of 59% was calculated in order to see a trend in LVEF improvement. In another trial (Ripa 2006), a sample size of 50 was estimated in order to yield an expected power of 90% to detect a difference of 15% between the treated and the placebo groups, with a 2‐sided significance level of 0.05. Finally, Zohlnhöfer 2006 calculated a total sample size of 90 patients to detect a difference of 6% or higher with a power of 80% and a 2‐sided error of 0.05.
Effects of interventions
Mortality
Six trials (341 participants) reported the incidence of mortality (Ellis 2006; Ince 2005a; Leone 2007; Ripa 2006; Takano 2007; Zohlnhöfer 2006). In three studies, no deaths were reported (Ince 2005a; Leone 2007; Zohlnhöfer 2006). In the four remaining studies, one death was reported per study; in two of these trials, the patient belonged to the control group and in the other two trials (Ellis 2006; Ripa 2006), the patient from the experimental group died during the follow up period (Takano 2007; Zohlnhöfer 2006).
Overall, the rate of mortality was not statistically significant (RR 0.64, 95% CI 0.15 to 2.80, P = 0.55, Analysis 1.1). No heterogeneity was identified.
1.1. Analysis.

Comparison 1: GCSF versus placebo, Outcome 1: Mortality
Left ventricular ejection fraction (LVEF)
All seven trials (354 participants) measured left ventricular ejection fraction (LVEF) (Ellis 2006; Ince 2005a; Leone 2007; Ripa 2006; Takano 2007; Valgimigli 2005; Zohlnhöfer 2006). Different methods were applied in order to measure the LVEF. In four studies, echocardiography was applied in isolation (Ellis 2006; Leone 2007; Ripa 2006) or in combination with angiography (Ince 2005a). Two trials used SPECT in order to study the left ventricular parameters (Takano 2007; Valgimigli 2005). Finally, in one trial, both MRI and angiography was used as the method of choice for measuring LVEF (Zohlnhöfer 2006).
All studies reported the timing of LVEF measurement outcomes of LV differences at short (less than 6 months) follow up durations.
Overall, no significant difference was observed between the two groups regarding LVEF (RR 3.41, 95% CI ‐0.61 to 7.44, P = 0.1, Analysis 1.2). Substantial statistical heterogeneity was observed (I2 = 84%) among the two groups. The reasons for the observed heterogeneity could be attributed to the different modalities of measurement used and also different timing of outcome measurements (from one to six months).
1.2. Analysis.

Comparison 1: GCSF versus placebo, Outcome 2: Left Ventricular Ejection Fraction
Cardiovascular morbidity outcomes
Six trials measured cardiovascular outcomes as a treatment outcome (343 participants). Five trials measured only the observed side effects in short term follow up (Ince 2005a; Leone 2007; Ripa 2006; Takano 2007; Valgimigli 2005; Zohlnhöfer 2006). In only one study, the outcomes were reported at one year following AMI (Ellis 2006).
Incidence of reinfarction
Incidence of reinfarction was reported in four trials (244 participants) (Ellis 2006; Ripa 2006; Takano 2007; Zohlnhöfer 2006). In two trials, none of the patients developed reinfarction during the follow up period (Ripa 2006; Takano 2007). In the other two trials, one patient developed reinfarction in each study (Ellis 2006; Zohlnhöfer 2006). In the Ellis 2006 study, the patient who developed reinfarction was reported to be in the study group at three weeks after G‐CSF therapy, while in the other study (Zohlnhöfer 2006), one patient in the comparator arm developed reinfarction following MI.
Overall, there was no significant difference regarding the incidence of reinfarction in these trials (RR 1.02, 95% CI 0.15 to 6.86, P = 0.99, Analysis 1.3). No heterogeneity was observed among studies.
1.3. Analysis.

Comparison 1: GCSF versus placebo, Outcome 3: Incidence of reinfarction
Incidence of arrhythmia
Three trials reported the incidence of arrhythmia among participants (232 participants) (Ripa 2006; Takano 2007; Zohlnhöfer 2006). In only one trial arrhythmia was observed in one patient in the study group (Zohlnhöfer 2006). This patient died of ventricular fibrillation 12 days after enrolment.
Incidence of restenosis
Six trials reported the incidence of restenosis (343 participants) (Ince 2005a; Leone 2007; Ripa 2006; Takano 2007; Valgimigli 2005; Zohlnhöfer 2006). In all of these trials, restenosis was observed among participants. In one trial, only one patient in the comparator arm developed restenosis following MI treatment (Valgimigli 2005). In the other studies, restenosis was observed among both the study and comparator arms.
Overall, meta‐analysis of data revealed no significant difference in restenosis between participants in the treatment and control arms (RR 0.97, 95% CI 0.65 to 1.46, P = 0.89, Analysis 1.4). There was no statistical heterogeneity.
1.4. Analysis.

Comparison 1: GCSF versus placebo, Outcome 4: Incidence of restenosis
Hospital readmission
Two studies reported the incidence of hospital readmission (118 participants) (Ripa 2006; Takano 2007). In one study, (Takano 2007), there were no cases of hospital readmission during the follow up period. In the other study (Ripa 2006), two patients in the control group were readmitted to the hospital following MI treatment.
Congestive heart failure (CHF) requiring rehospitalization
Two studies reported data regarding the number of CHF cases requiring rehospitalization after MI (58 participants) (Ellis 2006; Takano 2007). In one study, none of the patients developed CHF following treatment (Takano 2007). In another study two patients, one in each arm developed CHF after MI treatment (Ellis 2006). There were no data regarding the timing of CHF development. However, the screening was performed 12 months after MI.
Tamponade
Only one study reported the incidence of tamponade in participants following treatment (18 participants) (Ellis 2006). None of the patients in this trial developed tamponade following treatment.
Cardiac perforation
None of the studies reported data regarding the occurrence of cardiac perforation.
Cardiogenic shock
The incidence of cardiogenic shock was assessed in one trial (78 participants) (Ripa 2006). In this trial, only one patient in the control group developed cardiogenic shock after the primary PCI and died 2.5 days later, despite aggressive treatment (intra‐aortic balloon pump, dialysis, and ventilator therapy).
Target vessel revascularization
The rate of target vessel revascularization was assessed in two trials (192 participants) (Ripa 2006; Zohlnhöfer 2006). The trials reported the incidence of target vessel revascularization by six months in Ripa 2006, and four to six months in Zohlnhöfer 2006. The incidence was not shown to be significantly different between the treatment and comparator group (RR 0.94, 95% CI 0.56 to 1.57, P = 0.8, Analysis 1.5). No heterogeneity was reported between the trials.
1.5. Analysis.

Comparison 1: GCSF versus placebo, Outcome 5: Incidence of revascularization
Left ventricular end‐systolic volume (LVESV)
Left ventricular end‐systolic volume (LVESV) was measured in five trials (285 participants) (Ellis 2006; Leone 2007; Ripa 2006; Takano 2007; Zohlnhöfer 2006). All studies reported the timing of LVESV measurement at short (less than six months) follow up durations.
Overall, no significant difference was observed between the two groups regarding LVESV (RR ‐1.35, 95% CI ‐4.68 to 1.99, P = 0.43, Analysis 1.6). Statistical heterogeneity was observed (I2 = 34%) among the two groups. This heterogeneity could be attributed to different modalities for measurement of systolic volume.
1.6. Analysis.

Comparison 1: GCSF versus placebo, Outcome 6: Left Ventricular End‐Systolic Volume
Left ventricular end‐diastolic volume (LVEDV)
Six trials reported changes in LVEDV between the study and control groups (343 participants) (Ince 2005a; Leone 2007; Ripa 2006; Takano 2007; Valgimigli 2005; Zohlnhöfer 2006). The reported measurements were performed at short follow up durations.
Meta‐analysis revealed no significant difference between the two groups (RR ‐4.08, 95% CI ‐8.28 to 0.12, P = 0.06, Analysis 1.7). Significant heterogeneity was present between the groups (I2 = 55%). As for LVEF and LVESV, different measurement procedures could contribute to the observed heterogeneity between the studies.
1.7. Analysis.

Comparison 1: GCSF versus placebo, Outcome 7: Left Ventricular End‐Diastolic Volume
Discussion
Summary of main results
Despite the fact that several clinical trials have investigated the effects of G‐CSF therapy on cardiac repair, the role of this cytokine remains controversial. In the present systematic review, seven trials were eligible to be considered in the final analysis (354 participants). In summary, our results indicate that regarding efficacy, G‐CSF therapy did not show any evidence of beneficial effects in patients with MI following reperfusion. The parameters of LV function including LVEF, LVESV, and LVEDV did not show any further improvement in patients receiving G‐CSF compared to the control group.
Regarding safety, the limited amount of evidence is inadequate to reach any conclusions regarding the safety of G‐CSF therapy.
Overall completeness and applicability of evidence
While the total number of participants may be adequate to reach final conclusions, the sample sizes of each individual study included in the present review were small. Four trials used power calculations to estimate the minimum number of participants to be randomized in the trial (Ellis 2006; Leone 2007; Ripa 2006; Zohlnhöfer 2006). The follow up duration varied in different studies, and none of the studies had long follow up duration (more than 18 months). Therefore, data regarding safety should be interpreted with caution, as longer follow up data should be assessed to fully determine any adverse effects of therapy.
Quality of the evidence
In the current review, six out of seven trials reported details of their method of randomization and were considered to be at low risk for this parameter. In only three trials were all trial personnel adequately blinded. Finally, while two trials did not report details regarding the number of patients lost to follow up, in the other five trials, less than 20% of participants were lost to follow up.
In conclusion, while the overall data regarding the generation of randomization sequence and incomplete outcome data were at low risk of bias, data regarding binding of personnel are not conclusive. Therefore, the results of the current review should be interpreted with caution.
Potential biases in the review process
While we conducted a comprehensive search, the possibility of publication bias cannot be ruled out completely. Selection of studies and extraction of data were performed independently by two authors in order to minimize the risk of introducing bias. Finally, individual patient level data could not be retrieved and the results were based solely on summary reports.
Agreements and disagreements with other studies or reviews
A number of meta‐analyses have investigated the role of G‐CSF treatment in patients with AMI (Abdel‐Latif 2008; Fan 2008; Ince 2008; Kang 2007; Zohlnhöfer 2008). Regarding safety outcomes, all these reviews have documented G‐CSF to be a safe modality and associated with minor side effects which are similar to the results of our findings. Moreover, in a meta‐analysis performed to investigate the incidence of coronary restenosis or progression of coronary lesions in patients with AMI following G‐CSF therapy, the results were in line with our findings, indicating that G‐CSF does not elevate the risk for coronary restenosis (Ince 2008).
All of these meta‐analyses except one have found similar results to those of our review regarding clinical efficacy (Abdel‐Latif 2008; Fan 2008; Ince 2008; Zohlnhöfer 2008). In these studies, G‐CSF did not enhance the improvement of LV function parameters at follow‐up in comparison with the control group. In one systematic review, however, the mean LVEF was significantly increased in the G‐CSF group in comparison to the control group (3.46%; 95% CI 0.60 to 6.32; P = 0.018) (Kang 2007). The observed discrepancy may be attributed to the fact that Kang 2007 included two studies which were not included in our study. We excluded one study (Kang 2006) because it investigated more than one active arm. The other study used granulocyte‐macrophage colony stimulating factor, which was out of the scope of our study (Deng 2006).
Authors' conclusions
Implications for practice.
There is no evidence from the included studies indicating that G‐CSF treatment in dosage from 2.5 to 10 microgram/kg for four to six days is not safe or associated with major side effects. However, the limited amount of evidence is inadequate to reach any conclusions regarding the safety of G‐CSF therapy. Regarding clinical efficacy, the results do not show this modality to be beneficial in patients with AMI in terms of both mortality and left ventricular functional parameters. However, the results of the current study should be interpreted with caution given the low number of studies and participants, and potential risk of bias.
Implications for research.
Larger RCTs with appropriate power calculations are needed in order to address current uncertainties regarding the clinical efficacy of G‐CSF treatment. In order to clearly define therapy‐related adverse events, studies with longer follow up durations are needed. Moreover, future studies should also evaluate economic costs and patient‐reported outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 21 September 2021 | Review declared as stable | This Cochrane Review has had low usage is therefore not a priority for updating. |
History
Protocol first published: Issue 11, 2010 Review first published: Issue 5, 2013
| Date | Event | Description |
|---|---|---|
| 11 June 2013 | Amended | Affiliatin detail for Aria Roohi and Bobak Moazzami amended |
Appendices
Appendix 1. Search strategies
CENTRAL (The Cochrane Library)
#1 MeSH descriptor Myocardial Infarction explode all trees #2 myocard* next infarct* #3 ami #4 coronary near/3 occlusion* #5 cardiac next infarct* #6 heart next attack* #7 heart near/2 infarct* #8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) #9 MeSH descriptor Granulocyte Colony‐Stimulating Factor explode all trees #10 MeSH descriptor Granulocyte‐Macrophage Colony‐Stimulating Factor explode all trees #11 G‐CSF #12 Colony next Stimulating next Factor* #13 neupogen #14 filgrastim #15 pegfilgrastim #16 lenograstim #17 molgramostim #18 (#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17) #19 (#8 AND #18)
MEDLINE (OVID)
1. exp Myocardial Infarction/ 2. myocard* infarct*.tw. 3. ami.tw. 4. (coronary adj3 occlusion*).tw. 5. cardiac infarct*.tw. 6. heart attack*.tw. 7. (heart adj2 infarct*).tw. 8. or/1‐7 9. exp Granulocyte Colony‐Stimulating Factor/ 10. exp Granulocyte‐Macrophage Colony‐Stimulating Factor/ 11. G‐CSF.tw. 12. Colony‐Stimulating Factor*.tw. 13. neupogen.tw. 14. filgrastim.tw. 15. pegfilgrastim.tw. 16. lenograstim.tw. 17. molgramostim.tw. 18. or/9‐17 19. 8 and 18 20. randomized controlled trial.pt. 21. controlled clinical trial.pt. 22. randomized.ab. 23. placebo.ab. 24. drug therapy.fs. 25. randomly.ab. 26. trial.ab. 27. groups.ab. 28. or/20‐27 29. exp animals/ not humans.sh. 30. 28 not 29 31. 19 and 30
EMBASE (OVID)
1. exp heart infarction/ 2. ami.tw. 3. cardiac infarct*.tw. 4. (coronary adj3 occlusion*).tw. 5. heart attack*.tw. 6. (heart adj2 infarct*).tw. 7. myocard* infarct*.tw. 8. or/1‐7 9. granulocyte colony stimulating factor/ 10. recombinant granulocyte colony stimulating factor/ 11. granulocyte macrophage colony stimulating factor/ 12. recombinant granulocyte macrophage colony stimulating factor/ 13. G‐CSF.tw. 14. Colony Stimulating Factor*.tw. 15. neupogen.tw. 16. filgrastim.tw. 17. pegfilgrastim.tw. 18. lenograstim.tw. 19. molgramostim.tw. 20. 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21. 8 and 20 22. random$.tw. 23. factorial$.tw. 24. crossover$.tw. 25. cross over$.tw. 26. cross‐over$.tw. 27. placebo$.tw. 28. (doubl$ adj blind$).tw. 29. (singl$ adj blind$).tw. 30. assign$.tw. 31. allocat$.tw. 32. volunteer$.tw. 33. crossover procedure/ 34. double blind procedure/ 35. randomized controlled trial/ 36. single blind procedure/ 37. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 38. (animal/ or nonhuman/) not human/ 39. 37 not 38 40. 21 and 39 41. limit 40 to embase
BIOSIS Previews (ISI Web of Science)
#19 #18 AND #17 #18 TS=(random* or blind* or placebo* or trial or trials or mask* or singl* or doubl* or trebl* or tripl*) #17 #16 AND #8 #16 #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 #15 TS=molgramostim #14 TS=lenograstim #13 TS=pegfilgrastim #12 TS=filgrastim #11 TS=neupogen #10 TS=G‐CSF #9 TS="Colony Stimulating Factor*" #8 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #7 TS="cardiac infarct*" #6 TS="myocard* infarct*" #5 TS="heart attack*" #4 TS=ami #3 TS=(heart SAME infarct*) #2 TS=(coronary SAME occlusion*) #1 TS=cardiac infarct*
Science Citation Index Expanded and Conference Proceedings Citation Index – Science (ISI Web of Science)
#21 #20 OR #19 #20 #17 AND Document Type=(Meeting Abstract OR Meeting Summary OR Meeting‐Abstract) #19 #18 AND #17 #18 TS=(random* or blind* or placebo* or trial or trials or mask* or singl* or doubl* or trebl* or tripl*) #17 #16 AND #8 #16 #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 #15 TS=molgramostim #14 TS=lenograstim #13 TS=pegfilgrastim #12 TS=filgrastim #11 TS=neupogen #10 TS=G‐CSF #9 TS="Colony Stimulating Factor*" #8 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #7 TS="cardiac infarct*" #6 TS="myocard* infarct*" #5 TS="heart attack*" #4 TS=ami #3 TS=(heart SAME infarct*) #2 TS=(coronary SAME occlusion*) #1 TS=cardiac infarct*
Data and analyses
Comparison 1. GCSF versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mortality | 6 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.15, 2.80] |
| 1.2 Left Ventricular Ejection Fraction | 7 | 354 | Mean Difference (IV, Random, 95% CI) | 3.41 [‐0.61, 7.44] |
| 1.3 Incidence of reinfarction | 4 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.15, 6.86] |
| 1.4 Incidence of restenosis | 6 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.65, 1.46] |
| 1.5 Incidence of revascularization | 2 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.56, 1.57] |
| 1.6 Left Ventricular End‐Systolic Volume | 5 | 285 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐4.68, 1.99] |
| 1.7 Left Ventricular End‐Diastolic Volume | 6 | 343 | Mean Difference (IV, Random, 95% CI) | ‐4.08 [‐8.28, 0.12] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ellis 2006.
| Study characteristics | ||
| Methods | Study design: stated as randomized Method of randomization: permutated block design in densely opaque envelopes Losses to follow up: No | |
| Participants | Country: USA
Participants: 18 randomized
Mean age: 62 and 60 years for control and treatment groups respectively
Sex (M/F): 6/0 and 11/1 for control and treatment groups respectively
Inclusion criteria: Patients 21 to 79 years of age with acute ST‐segment elevation MI re perfused (TIMI 3 flow) more than 4 hours after symptom onset with baseline left ventricular ejection fraction 20% to 39% eligible to receive study drug in less than 48 hours from symptom onset. Exclusion criteria: Patients with a mechanical complication of MI such as ventricular septal defect, severe mitral insufficiency or contained rupture, anatomy likely to require bypass surgery within 30 days, clinical features suggestive of extremely limited likelihood of survival to 30 days (eg, severe oliguria), known malignancy, sepsis, vasculitis, gout, sickle cell trait or disease, lithium use, or possible pregnancy. |
|
| Interventions | Treatment group: G‐CSF injected subcutaneously (one injection for the 5 microgram/kg dose, 2 injections for the 10 microgram/kg dose) once daily for 5 days. Control group: identical‐appearing normal saline placebo. |
|
| Outcomes | Primary end points:
Secondary end points:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a permutated block design |
| Allocation concealment (selection bias) | Low risk | By densely opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double blinded. The nurses and physicians caring for the patient were blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | In the hematology and core echocardiographic laboratories, technicians and physicians were fully blinded to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Stated that no patient was lost to follow up through 30 days. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in methods are reported in results. Selective reporting would be difficult to rule out. |
Ince 2005a.
| Study characteristics | ||
| Methods | Study design: stated as randomized Method of randomization: closed‐envelope method Losses to follow up: Not reported | |
| Participants | Country: Germany
Participants: 50 randomized
Mean age: 49 and 50 years for control and treatment groups respectively
Sex (M/F): 23/2 and 23/2 for control and treatment groups respectively
Inclusion criteria: Patients between 18 and 65 years of age and with first STEMI comprising 3 of 12 ECG leads were eligible Exclusion criteria: Cardiogenic shock (defined as systolic blood pressure 80mm Hg requiring intravenous pressors or intra‐aortic balloon counterpulsation), major bleeding requiring blood transfusion, a history of leukopenia, thrombocytopenia, hepatic or renal dysfunction, evidence of malignant disease, or unwillingness to participate were criteria for exclusion. |
|
| Interventions | Treatment group: subcutaneous G‐CSF at a dose of 10 microgram/kg body weight over a period of 6 days. Control group: not stated. |
|
| Outcomes | Baseline ejection fraction (LVEF) and volumes were calculated by use of the area‐length method; coronary angiograms were evaluated for binary restenosis, (in‐stent) late lumen loss, and minimal lumen diameter of the target lesion. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated. |
| Allocation concealment (selection bias) | Low risk | Patients were randomized by use of the closed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label application of G‐CSF. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded evaluation by expert readers unaware of patient group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in methods are reported in results. Selective reporting would be difficult to rule out. |
Leone 2007.
| Study characteristics | ||
| Methods | Study design: stated as randomized Method of randomization: Not stated Losses to follow up: one patient in the treatment group | |
| Participants | Country: Germany
Participants: 41 randomized
Mean age: 56 and 53 years for control and treatment groups respectively
Sex (M/F): 13/1 and 27/0 for control and treatment groups respectively Inclusion criteria: Patients with a first large anterior AMI and a LV ejection fraction 50% despite successful percutaneous revascularization of the infarct‐related artery Exclusion criteria: Cardiogenic shock, uncontrolled myocardial ischemias or arrhythmias, malignancies, severe infections, hematologic diseases, splenomegaly on abdominal echocardiography, and age 80 years. |
|
| Interventions | Treatment group: Subcutaneous G‐CSF at a dose of 10 microgram/kg body weight over a period of 5 days. Control group: Conventional therapy. |
|
| Outcomes | Left ventricular function studies. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly allocated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blind to the treatment outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient in the treatment group was lost to follow up |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in methods are reported in results. Selective reporting would be difficult to rule out. |
Ripa 2006.
| Study characteristics | ||
| Methods | Study design: stated as randomized Method of randomization: sequentially numbered, sealed envelopes Losses to follow up: 8 patients | |
| Participants | Country: Denmark Participants: 78 randomized Mean age: 54 and 57 years for control and treatment groups respectively Sex (M/F): 34/5 and 28/11 for control and treatment groups respectively Inclusion criteria: Patients treated successfully with primary PCI within 12 hours after the onset of symptoms were included in the study. STEMI was diagnosed from typical chest pain at rest lasting 30 minutes, the presence of cumulative ST‐elevations 0.4 mV in 2 contiguous leads on a standard 12‐lead ECG, and a significant rise in serum markers of myocardial infarction. Only patients who were between 20 and 70 years of age with a culprit lesion located in the proximal section of a large coronary artery branch, plasma creatine kinase‐MB more than 100 g/L, or development of significant Q waves in the ECG were included. Exclusion criteria: Patients with prior myocardial infarction, significant stenosis in a nonculprit coronary vessel, ventricular arrhythmia after PCI requiring treatment, pregnancy, unprotected left main stem lesion, diagnosed or suspected cancer, New York Heart Association class 3 to 4 heart failure symptoms, or known severe claustrophobia. |
|
| Interventions | Treatment group: subcutaneous G‐CSF at a dose of 10 microgram/kg body wt over a period of 6 days Control group: similar volume of placebo (isotonic sodium‐chloride) |
|
| Outcomes | Primary end point was change in regional systolic wall thickening from day 1 to 6 months evaluated with cardiac MRI. Secondary end points were: (1) change in ejection fraction, end‐systolic and end‐diastolic volumes, and infarct size by MRI and (2) change in ejection fraction and end‐systolic and end‐diastolic volumes by echocardiography. Safety end points were (1) death of any cause, reinfarction, and new revascularization; (2) other adverse events; (3) in‐stent restenosis; and (4) changes in inflammatory parameters (C‐reactive protein and erythrocyte sedimentation rate). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind, randomized, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double‐blind, randomized, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 participants, 2 and 6 in the treatment and control groups respectively |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in methods are reported in results. Selective reporting would be difficult to rule out. |
Takano 2007.
| Study characteristics | ||
| Methods | Study design: stated as randomized Method of randomization: sequentially numbered, sealed envelopes Losses to follow up: 5 patients | |
| Participants | Country: Japan Participants: 40 randomized Mean age: 63 and 61 years for control and treatment groups respectively Sex (M/F): 18/4 and 14/4 for control and treatment groups respectively Inclusion criteria: Patients were eligible if they were admitted within 12 hours after onset of AMI with total occlusion of LAD alone and underwent successful PCI with bare metal stent implantation Exclusion criteria: previous MI; angiographically significant lesions in right coronary artery and/or left circumflex coronary artery; persistent severe heart failure (greater than Killip class II); uncontrolled myocardial ischemia or ventricular tachycardia; culprit lesion of infarct related artery not feasible for PCI; age older than 80 years; malignant disease; serious current infection or hematological disorder. |
|
| Interventions | Treatment group: subcutaneous G‐CSF at a dose of 2.5 microgram/kg body weight over a period of 5 days. Control group: patients were subcutaneously injected with saline. |
|
| Outcomes | Primary end point was the changes between global LVEF, LVESV and LVEDV at baseline and those after 6 months follow‐up. Secondary end points were a change in defect scores and the difference in the incidence of major adverse cardiac events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated |
| Allocation concealment (selection bias) | Low risk | Minimization method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | After randomization, study processes were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomization was done by a blinded independent coordinator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants, 2 and 3 in the treatment and control groups respectively |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in methods are reported in results. Selective reporting would be difficult to rule out. |
Valgimigli 2005.
| Study characteristics | ||
| Methods | Study design: stated as randomized Method of randomization: Computer based Losses to follow up: Not stated | |
| Participants | Country: Italy Participants: 20 randomized Mean age: 61 and 62 years for control and treatment groups respectively Sex (M/F): 8/2 and 8/2 for control and treatment groups respectively Exclusion criteria: previous MI, any haematological disorder, age less than 21 or more than 80, and Killip class more than 1. |
|
| Interventions | Treatment group: subcutaneous G‐CSF at a dose of 5 microgram/kg body weight over a period of 4 days Control group: placebo |
|
| Outcomes | Major side effects, angiographic analysis to assess the rate of restenosis, LV function parameters | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated |
| Allocation concealment (selection bias) | Low risk | Computer‐based randomization |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Single blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in methods are reported in results. Selective reporting would be difficult to rule out. |
Zohlnhöfer 2006.
| Study characteristics | ||
| Methods | Study design: stated as randomized Method of randomization: Sealed envelope Losses to follow up: 18 participants | |
| Participants | Country: Germany Participants: 114 randomized Mean age: 59 and 59 years for control and treatment groups respectively Sex (M/F): 46/12 and 44/12 for control and treatment groups respectively Inclusion criteria: Patients were required to have had successful reperfusion by percutaneous coronary intervention (performed 12 hours from symptom onset) and an infarct size of at least 5% of the left ventricle in single‐photon emission computed tomography with technetium Tc 99m sestamibi (performed before randomization) Exclusion criteria: age younger than 18 years or older than 80 years, congestive heart failure defined as Killip class higher than II, electrical or hemodynamic instability, a history of prior myocardial infarction, autoimmune diseases, fructose intolerance, malignancies, incompatibility of G‐CSF, and known or suspected pregnancy. |
|
| Interventions | Treatment group: subcutaneous G‐CSF at a dose of 10 microgram/kg body wt over a period of 5 days Control group: placebo |
|
| Outcomes | The primary end point was the reduction of infarct size measured as the difference in left ventricular infarct size at baseline (study entry) and follow‐up by single‐photon emission computed tomography. Secondary end points were improvement in LVEF from baseline to follow‐up by MRI as well as angiographic restenosis defined as a diameter stenosis of 50% or greater by follow‐up angiography. Other measures assessed were left ventricular volumes by MRI, LVEF, and number of hypokinetic chords by angiography. We also monitored for the occurrence of the following major adverse cardiac events: death, recurrent myocardial infarction, and reintervention in the infarct‐related artery. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated as double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 participants lost to follow up, 7 and 11 in the treatment and control groups respectively |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in methods are reported in results. Selective reporting would be difficult to rule out. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| De Lezo 2007 | The study had more than one active arm of investigation which was out of the scope of the current review. |
| Engelmann 2006 | In this study, patients with subacute MI were included, not AMI. |
| Gloekler 2009 | The study investigates patients with coronary artery disease, not AMI. |
| Guo 2008 | The study included patients with MI and leukopenia. |
| Huttmann 2006 | The study included patients with chronic heart failure, not AMI. |
| Hyun‐Jae 2003 | The study Included patients with chronic heart failure, not AMI. |
| Joseph 2008 | The study was not randomized. |
| Kang 2006 | The study had more than one active arm of investigation which was out of the scope of the current review. |
| Kuethe 2004 | The study was not randomized. |
| Kuo 2009 | The study was not randomized. |
| Li 2004 | The study included patients with old MI, not AMI. |
| Meier 2009 | The study included patients with coronary artery disease, not AMI. |
| Suarez 2004 | The study had more than one active arm of investigation which was out of the scope of the current review. |
| Subramaniyam 2009 | The study included patients with peripheral artery disease not AMI |
| Suzuki 2006 | The study included patients with coronary heart disease, not AMI. |
| Wnag 2005 | The study included patients with severe chronic Ischaemic heart disease, not AMI. |
| Wolfram 2007 | The study included patients with coronary artery disease, not AMI. |
| Zbinden 2005 | The study included patients with coronary artery disease, not AMI. |
Contributions of authors
KM is the contact author and the guarantor of the review. He was responsible for drafting the protocol, obtaining copies of studies, selecting which studies to include, extracting data from studies, entering data into RevMan, and drafting the final review and will update the review. AR was responsible for drafting the protocol, obtaining copies of the studies, selecting which studies to include, extracting data from studies and drafting the final review. BM was responsible for drafting the protocol, extracting data from studies, entering data into RevMan, and drafting the final review and will update the review.
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Ellis 2006 {published data only}
- Ellis SG, Penn MS, Bolwell B, Garcia M, Chacko M, Wang T, et al. Granulocyte colony stimulating factor in patients with large acute myocardial infarction: results of a pilot dose-escalation randomized trial. American Heart Journal 2006;152(1051):e9-14. [DOI] [PubMed] [Google Scholar]
Ince 2005a {published data only}
- Ince H, Petzsch M, Kleine HD, Schmidt H, Rehders T, Körbern T, et al. Preservation from left ventricular remodeling by front-integrated revascularization and stem cell liberation in evolving acute myocardial infarction by use of granulocyte-colony-stimulating factor (FIRSTLINE-AMI). Circulation 2005;112:3097-106. [DOI] [PubMed] [Google Scholar]
Leone 2007 {published data only}
- Leone AM, Galiuto L, Garramone B, Rutella S, Giannico MB, Brugaletta S, et al. Usefulness of granulocyte colony-stimulating factor in patients with a large anterior wall acute myocardial infarction to prevent left ventricular remodeling (the Rigenera study). American Journal of Cardiology 2007;100:397-403. [DOI] [PubMed] [Google Scholar]
Ripa 2006 {published data only}
- Ripa RS, Jørgensen E, Wang Y, Thune JJ, Nilsson JC, Søndergaard L, et al. Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction: result of the double-blind, randomized, placebo-controlled Stem cells in Myocardial Infarction (STEMMI) trial. Circulation 2006;113:1983-92. [DOI] [PubMed] [Google Scholar]
Takano 2007 {published data only}
- Takano H, Hasegawa H, Kuwabara Y, Nakayama T, Matsuno K, Miyazaki Y, et al. Feasibility and safety of granulocyte colony-stimulating factor treatment in patients with acute myocardial infarction. International Journal of Cardiology 2007;122:41-7. [DOI] [PubMed] [Google Scholar]
Valgimigli 2005 {published data only}
- Valgimigli M, Rigolin GM, Cittanti C, Malagutti P, Curello S, Percoco G, et al. Use of granulocyte-colony stimulating factor during acute myocardial infarction to enhance bone marrow stem cell mobilization in humans: clinical and angiographic safety profile. European Heart Journal 2005;26:1838-45. [DOI] [PubMed] [Google Scholar]
Zohlnhöfer 2006 {published data only}
- Zohlnhöfer D, Ott I, Mehilli J, Schömig K, Michalk F, Ibrahim T, et al. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. JAMA 2006;295:1003-10. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
De Lezo 2007 {published data only}
- De Lezo JS, Herrera C, Pan M, Romero M, Pavlovic D, Segura J, et al. Regenerative therapy in patients with a revascularized acute anterior myocardial infarction and depressed ventricular function. Revista Espanola de Cardiologia 2007;60:357-65. [PubMed] [Google Scholar]
Engelmann 2006 {published data only}
- Engelmann MG, Theiss HD, Hennig-Theiss C, Huber A, Wintersperger BJ, Werle-Ruedinger AE, et al. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: final results from the G-CSF-STEMI (Granulocyte Colony-Stimulating Factor ST-Segment Elevation Myocardial Infarction) trial. Journal of the American College of Cardiology 2006;48:1712-21. [DOI] [PubMed] [Google Scholar]
Gloekler 2009 {published data only}
- Gloekler S, Meier P, Zbinden R, Marchi SF, Rutz T, Indermuehle A, et al. Granulocyte-colony stimulating factor (G-CSF) promotes coronary collateral growth and myocardial microvascular function in patients with coronary artery disease: a randomized, double-blind, placebo-controlled study. Journal of the American College of Cardiology 2009;53(10):A347. [Google Scholar]
Guo 2008 {published data only}
- Guo YH, He JG, Wu JL, Yang L, Zhang DS, Tan XY, et al. Hepatocyte growth factor and granulocyte colony-stimulating factor form a combined neovasculogenic therapy for ischemic cardiomyopathy. Cytotherapy 2008;10:857-67. [DOI] [PubMed] [Google Scholar]
Huttmann 2006 {published data only}
- Huttmann A, Duhrsen U, Stypmann J, Noppeney R, Nuckel H, Neumann T, et al. Granulocyte colony-stimulating factor-induced blood stem cell mobilisation in patients with chronic heart failure - feasibility, safety and effects on exercise tolerance and cardiac function. Basic Research in Cardiology 2006;101:78-86. [DOI] [PubMed] [Google Scholar]
Hyun‐Jae 2003 {published data only}
- Hyun-Jae K, Hyo-Soo K, Hyun-Jae C, Bon-Kwon K, Yong-Jin K, Byung-Hee O, et al. Granulocyte-colony stimulating factor-induced mobilization and intracoronary infusion of mobilized whole leukocytes is a feasible and safe method of stem cell transplantation in patients with myocardial infarction. American Journal of Cardiology 2003;92:107L. [Google Scholar]
Joseph 2008 {published data only}
- Joseph J, Mehta P, Rimawi A, Cottler-Fox M, Sinha A, Mansingh B, et al. Stem cell mobilization utilizing granulocyte colony stimulating factor in advanced chronic heart failure: lessons from a pilot study. European Heart Journal Supplements 2008;10:K24-6. [Google Scholar]
Kang 2006 {published data only}
- Kang H-J, Kim H-S, Na S-H, Zhang S-Y, Kang WJ, Youn T-J, et al. Six months follow up results of "granulocytes-colony stimulating factor" based stem cell therapy in patients with myocardial infarction: MAGIC cell randomized controlled trial. Korean Circulation Journal 2006;36:99-107. [Google Scholar]
Kuethe 2004 {published data only}
- Kuethe F, Figulla HR, Voth M, Richartz BM, Opfermann T, Sayer HG, et al. Mobilization of stem cells by granulocyte colony-stimulating factor for the regeneration of myocardial tissue after myocardial infarction. Deutsche Medizinische Wochenschrift 2004;129:424-8. [DOI] [PubMed] [Google Scholar]
Kuo 2009 {published data only}
- Kuo LT, Chen SJ, Cherng WJ, Yang NI, Lee CC, Cheng CW, et al. Late reperfusion of a totally occluded infarct-related artery increases granulocyte-colony stimulation factor and reduces stroma-derived factor-1alpha blood levels in patients with ongoing ischemia after acute myocardial infarction. International Heart Journal 2009;50:433-44. [DOI] [PubMed] [Google Scholar]
Li 2004 {published data only}
- Li YW, Genzou T, Hideshi O, Shusaku M, Masayau E, Rumi M, et al. G-CSF upregulates myocardial Akt and improves function of the hearts with established heart failure due to large old myocardial infarction. Journal of Cardiac Failure 2004;10:S190. [Google Scholar]
Meier 2009 {published data only}
- Meier P, Gloekler S, Marchi SF, Indermuehle A, Rutz T, Traupe T, et al. Myocardial salvage through coronary collateral growth by granulocyte colony-stimulating factor in chronic coronary artery disease: a controlled randomized trial. Circulation 2009;120:1355-63. [DOI] [PubMed] [Google Scholar]
Suarez 2004 {published data only}
- Suarez de Lezo J, Pan M, Torres A, Romero M, Herrera I, Segura J, et al. Functional recovery after regenerative treatment in patients with revascularized acute anterior myocardial infarction: a randomized study. Circulation 2004;110:Suppl. S. [Google Scholar]
Subramaniyam 2009 {published data only}
- Subramaniyam V, Waller EK, Murrow JR, Manatunga A, Lonial S, Kasirajan K, et al. Bone marrow mobilization with granulocyte macrophage colony-stimulating factor improves endothelial dysfunction and exercise capacity in patients with peripheral arterial disease. American Heart Journal 2009;158:53-60. [DOI] [PubMed] [Google Scholar]
Suzuki 2006 {published data only}
- Suzuki K, Nagashima K, Arai M, Uno Y, Misao Y, Takemura G, et al. Effect of granulocyte colony-stimulating factor treatment at a low dose but for a long duration in patients with coronary heart disease. Circulation Journal 2006;70:430-7. [DOI] [PubMed] [Google Scholar]
Wnag 2005 {published data only}
- Wang YZ, Tagil K, Ripa RS, Nilsson JC, Carstensen S, Jorgensen E, et al. Effect of mobilization of bone marrow stem cells by granulocyte colony stimulating factor on clinical symptoms, left ventricular perfusion and function in patients with severe chronic ischemic heart disease. International Journal of Cardiology 2005;100:477-83. [DOI] [PubMed] [Google Scholar]
Wolfram 2007 {published data only}
- Wolfram O, Jentsch-Ullrich K, Wagner A, Hammwohner M, Steinke R, Franke A, et al. G-CSF-induced mobilization of CD34(+) progenitor cells and proarrhythmic effects in patients with severe coronary artery disease. Pace-Pacing and Clinical Electrophysiology 2007;30:S166-9. [DOI] [PubMed] [Google Scholar]
Zbinden 2005 {published data only}
- Zbinden S, Zbinden R, Meier P, Windecker S, Seiler C. Safety and efficacy of subcutaneous-only granulocyte-macrophage colony-stimulating factor for collateral growth promotion in patients with coronary artery disease. Journal of the American College of Cardiology 2005;46:1636-42. [DOI] [PubMed] [Google Scholar]
Additional references
Abdel‐Latif 2008
- Abdel-Latif A, Bolli R, Zuba-Surma EK, Tleyjeh IM, Hornung CA, Dawn B. Granulocyte colony-stimulating factor therapy for cardiac repair after acute myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. American Heart Journal 2008;156:216-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Asahara 1999
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circulation Research 1999;85(3):221-8. [DOI] [PubMed] [Google Scholar]
Assmus 2002
- Assmus B, Schächinger V, Teupe C, Britten M, Lehmann R, Döbert N, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation 2002;106:3009-17. [DOI] [PubMed] [Google Scholar]
BHF 2004
- British Heart Foundation. Coronary heart disease statistics. http://www.heartstats.org/uploads/documents%5C2004pdf.pdf (accessed 15 August 2010).
Burton 1996
- Sobel BE. Acute myocardial infarction. In: Bennet C J, Plum F, editors(s). Cecil’s Textbook of Medicine. 20th edition. Philadelphia: WB Saunders, 1996. [Google Scholar]
Deng 2006
- Deng Z, Yang C, Deng H, Yang A, Geng T, Chen X, et al. Effects of GM-CSF on the stem cells mobilization and plasma C-reactive protein levels in patients with acute myocardial infarction. International Journal of Cardiology 2006;113:92-6. [DOI] [PubMed] [Google Scholar]
Fan 2008
- Fan L, Chen L, Chen X, Fu F. A meta-analysis of stem cell mobilization by granulocyte colony-stimulating factor in the treatment of acute myocardial infarction. Cardiovascular Drugs and Therapy 2008;22:45-54. [DOI] [PubMed] [Google Scholar]
Fernandez‐Aviles 2004
- Fernández-Avilés F, San Román JA, García-Frade J, Fernández ME, Peñarrubia MJ, la Fuente L, et al. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circulation Research 2004;95:742-8. [DOI] [PubMed] [Google Scholar]
Hartwell 2005
- Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al. Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation. Health Technology Assessment 2005;9(17):1-114. [DOI] [PubMed] [Google Scholar]
Hasegawa 2006
- Hasegawa H, Takano H, Iwanaga K, Ohtsuka M, Qin Y, Niitsuma Y, et al. Cardioprotective effects of granulocyte colony-stimulating factor in swine with chronic myocardial ischemia. Journal of the American College of Cardiology 2006;47:842-9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman, DG and Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane-handbook.org: The Cochrane Collaboration, 20011.
Hill 2005
- Hill JM, Syed MA, Arai AE, Powell TM, Paul JD, Zalos G, et al. Outcomes and risks of granulocyte colony-stimulating factor in patients with coronary artery disease. Journal of the American College of Cardiology 2005;46(9):1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ince 2005b
- Ince H, Petzsch M, Kleine HD, Eckard H, Rehders T, Burska D, et al. Prevention of left ventricular remodeling with granulocyte colony-stimulating factor after acute myocardial infarction: final 1-year results of the Front-Integrated Revascularization and Stem Cell Liberation in Evolving Acute Myocardial Infarction by Granulocyte Colony-Stimulating Factor (FIRSTLINE-AMI) trial. Circulation 2005;112(9 Suppl):173-80. [DOI] [PubMed] [Google Scholar]
Ince 2008
- Ince H, Valgimigli M, Petzsch M, Lezo S, Kuethe F, Dunkelmann S, et al. Cardiovascular events and re-stenosis following administration of G-CSF in acute myocardial infarction: systematic review and meta-analysis. Heart 2008;94:610-6. [DOI] [PubMed] [Google Scholar]
Kang 2004
- Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet 2004;363(9411):751-6. [DOI] [PubMed] [Google Scholar]
Kang 2007
- Kang S, Yang Y, Li C, Gao R. Effectiveness and tolerability of administration of granulocyte colony-stimulating factor on left ventricular function in patients with myocardial infarction: a meta-analysis of randomized controlled trials. Clinical Therapeutics 2007;29:2406-18. [DOI] [PubMed] [Google Scholar]
Kawada 2004
- Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood 2004;104(12):3581-7. [DOI] [PubMed] [Google Scholar]
Kawamoto 2001
- Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 2001;103(5):634-7. [DOI] [PubMed] [Google Scholar]
Kocher 2001
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nature Medicine 2001;7(4):430-6. [DOI] [PubMed] [Google Scholar]
Kuethe 2005
- Kuethe F, Figulla HR, Herzau M, Voth M, Fritzenwanger M, Opfermann T, et al. Treatment with granulocyte colony-stimulating factor for mobilization of bone marrow cells in patients with acute myocardial infarction. American Heart Journal 2005;150(1):115. [DOI] [PubMed] [Google Scholar]
Lefebvre 2009
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). The Cochrane Collaboration, 2009. [Google Scholar]
Lindquist 2003
- Lindquist R, Dupuis G, Terrin ML, Hoogwerf B, Czajkowski S, Herd JA, et al. Comparison of health-related quality-of-life outcomes of men and women after coronary artery bypass surgery through 1 year: findings from the POST CABG Biobehavioral Study. American Heart Journal 2003;146(6):1038-44. [DOI] [PubMed] [Google Scholar]
Martin‐Rendon 2008
- Martin-Rendon E, Brunskill S, Doree C, Hyde C, Mathur A, Stanworth S, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD006536. [DOI: 10.1002/14651858.CD006536.pub2] [DOI] [PubMed] [Google Scholar]
Meyer 2006
- Meyer GP, Wollert KC, Drexler H. Stem cell therapy: a new perspective in the treatment of patients with acute myocardial infarction. European Journal of Medical Research 2006;11:439-46. [PubMed] [Google Scholar]
Minatoguchi 2004
- Minatoguchi S, Takemura G, Chen XH, Wang N, Uno Y, Koda M, et al. Acceleration of the healing process and myocardial regeneration may be important as a mechanism of improvement of cardiac function and remodeling by postinfarction granulocyte colony-stimulating factor treatment. Circulation 2004;109:2572-80. [DOI] [PubMed] [Google Scholar]
Orlic 2001
- Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proceedings of the National Academy of Sciences of the United States of America 2001;98(18):10344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 2003
- Stone GW, Cox DA, Babb J, Nukta D, Bilodeau L, Cannon L, et al. Prospective, randomized evaluation of thrombectomy prior to percutaneous intervention in diseased saphenous vein grafts and thrombus-containing coronary arteries. Journal of the American College of Cardiology 2003;42(11):2007-13. [DOI] [PubMed] [Google Scholar]
Sugano 2005
- Sugano Y, Anzai T, Yoshikawa T, Maekawa Y, Kohno T, Mahara K, et al. Granulocyte colony-stimulating factor attenuates early ventricular expansion after experimental myocardial infarction. Cardiovascular Research 2005;65(2):446-56. [DOI] [PubMed] [Google Scholar]
White 2008
- White HD, Chew DP. Acute myocardial infarction. Lancet 2008;372:570-84. [DOI] [PubMed] [Google Scholar]
Zohlnhöfer 2008
- Zohlnhöfer D, Dibra A, Koppara T, Waha A, Ripa S, Kastrup J, et al. Stem cell mobilization by granulocyte colony-stimulating factor for myocardial recovery after acute myocardial infarction: a meta-analysis. Journal of the American College of Cardiology 2008;51:1429-37. [DOI] [PubMed] [Google Scholar]


