In a geographically diverse U.S. population, the coronavirus disease 2019 (COVID-19) pandemic was not associated with a significant difference in adverse pregnancy outcomes, even when considering severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection status.
OBJECTIVE:
To examine whether the coronavirus disease 2019 (COVID-19) pandemic altered risk of adverse pregnancy-related outcomes and whether there were differences by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection status among pregnant women.
METHODS:
In this retrospective cohort study using Epic's Cosmos research platform, women who delivered during the pandemic (March–December 2020) were compared with those who delivered prepandemic (matched months 2017–2019). Within the pandemic epoch, those who tested positive for SARS-CoV-2 infection were compared with those with negative test results or no SARS-CoV-2 diagnosis. Comparisons were performed using standardized differences, with a value greater than 0.1 indicating meaningful differences between groups.
RESULTS:
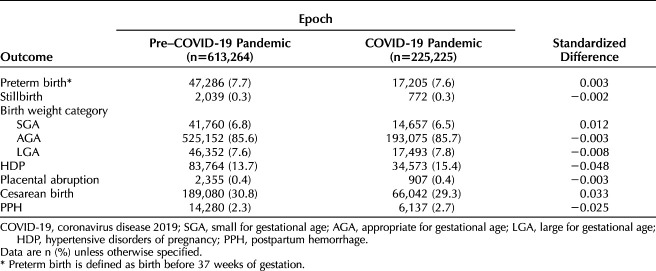
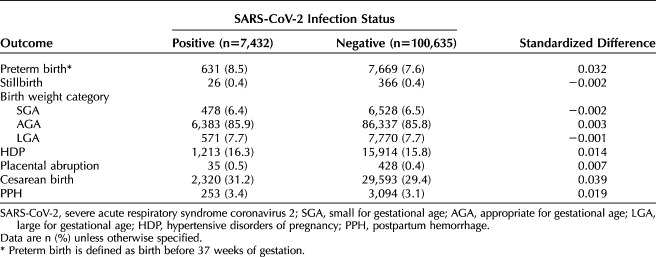
Among 838,489 women (225,225 who delivered during the pandemic), baseline characteristics were similar between epochs. There were no significant differences in adverse pregnancy outcomes between epochs (standardized difference<0.10). In the pandemic epoch, 108,067 (48.0%) women had SARS-CoV-2 testing available; of those, 7,432 (6.9%) had positive test results. Compared with women classified as negative for SARS-CoV-2 infection, those who tested positive for SARS-CoV-2 infection were less likely to be non-Hispanic White or Asian or to reside in the Midwest and more likely to be Hispanic, have public insurance, be obese, and reside in the South or in high social vulnerability ZIP codes. There were no significant differences in the frequency of preterm birth (8.5% vs 7.6%, standardized difference=0.032), stillbirth (0.4% vs 0.4%, standardized difference=−0.002), small for gestational age (6.4% vs 6.5%, standardized difference=−0.002), large for gestational age (7.7% vs 7.7%, standardized difference=−0.001), hypertensive disorders of pregnancy (16.3% vs 15.8%, standardized difference=0.014), placental abruption (0.5% vs 0.4%, standardized difference=0.007), cesarean birth (31.2% vs 29.4%, standardized difference=0.039), or postpartum hemorrhage (3.4% vs 3.1%, standardized difference=0.019) between those who tested positive for SARS-CoV-2 infection and those classified as testing negative.
CONCLUSION:
In a geographically diverse U.S. cohort, the frequency of adverse pregnancy-related outcomes did not differ between those delivering before compared with during the pandemic, nor between those classified as positive compared with negative for SARS-CoV-2 infection during pregnancy.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected millions of people in the United States,1 and the coronavirus disease 2019 (COVID-19) pandemic has created an enormous health burden, significantly affecting health care delivery and utilization.2,3 It is unknown whether the stressors and disruptions associated with the COVID-19 pandemic altered the risk of adverse pregnancy outcomes for women as a whole or if risk for such outcomes was conferred only to women acquiring SARS-CoV-2 infection during pregnancy.
To date, most studies examining pregnancy outcomes related to the COVID-19 pandemic compare birth outcomes among all pregnant women in the prepandemic and pandemic periods without assessing the SARS-CoV-2 infection status of included individuals.4–11 Studies specifically assessing pregnant women with and without SARS-CoV-2 infection12–14 are limited by their inability to distinguish outcomes between those with true-negative results and those untested for infection. This is a noteworthy limitation because there may be selectivity in the pregnant women referred for testing. In addition, the current literature has not yet distinguished between the downstream effects of societal disruptions caused by the pandemic and SARS-CoV-2 infection on pregnancy-related outcomes.
To address these limitations, this study had two main objectives: 1) to compare pregnant women who delivered before the COVID-19 pandemic with those who delivered during the COVID-19 pandemic to investigate whether the pandemic and its disruptions were associated with changes in adverse pregnancy-related outcomes independent of individual SARS-CoV-2 infection status; and 2) among those with evidence of SARS-CoV-2 testing during pregnancy, to examine whether SARS-CoV-2 infection was associated with adverse pregnancy-related outcomes compared with those classified as negative for SARS-CoV-2 infection.
METHODS
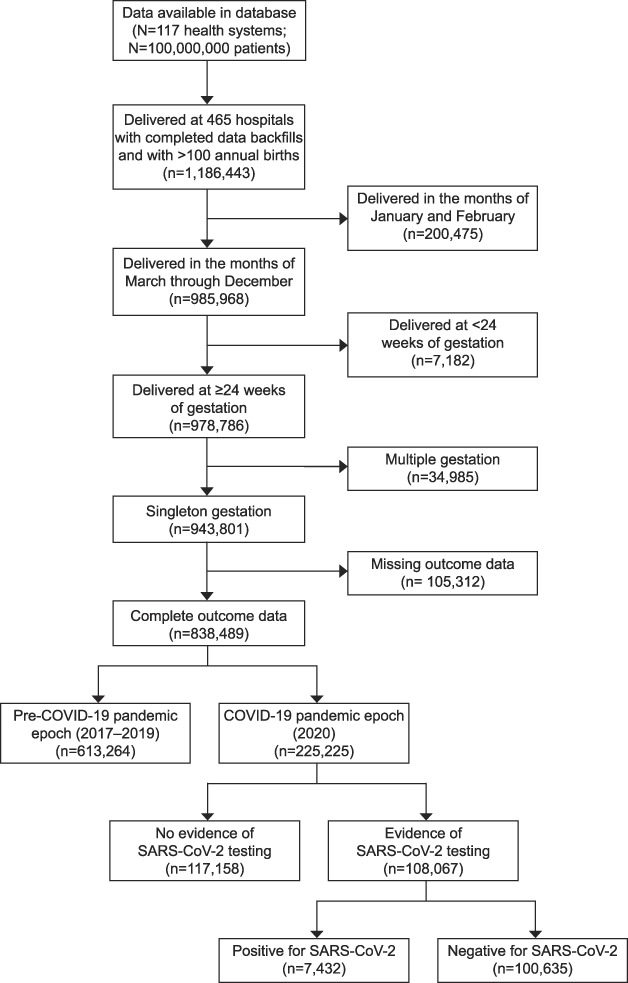
This retrospective cohort study was performed using data in Epic's Cosmos research platform.15 Epic Systems Corporation provides electronic health record (EHR) software and related services to roughly one third of the hospitals in the United States. Currently, 117 Epic health systems and their combined 100 million patients have contributed data to Cosmos. Cosmos collects a Health Insurance Portability and Accountability Act–defined limited data set from participating health systems that use Epic's software and aggregates these data to support research, public health, and health care operations activities. Patients with records at more than one health care organization are deduplicated across participating organizations in Cosmos. For this study, all hospital departments with more than 100 births annually that are part of health systems that had at least 3 years of prepandemic data in Cosmos were included, representing 79% of the health systems participating in Cosmos. All women who delivered after 24 weeks of gestation were included. Women with multiple gestation pregnancies and those with missing outcome data were excluded.
Two epochs were created: the pandemic epoch, spanning March 1, 2020 (when COVID-19 cases first became widely reported in the United States), to December 31, 2020; and the prepandemic epoch, inclusive of matched months (to account for seasonality) in the 3 years before the pandemic (2017–2019). Two comparisons were made: 1) all eligible pregnant women who delivered in the 3 years before the pandemic compared with all those who delivered during the COVID-19 pandemic; and 2) within the COVID-19 epoch, women classified as positive for SARS-CoV-2 infection compared with those classified as negative for SARS-CoV-2 infection during their pregnancies or their delivery hospitalizations.
Baseline patient and area-level characteristics were obtained, including maternal age, race and ethnicity, insurance type, prepregnancy body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), pre-existing medical comorbidities, overall Social Vulnerability Index, urbanicity, and Census region. Age was defined as the mother's age on the date she gave birth. Given the data that certain racial and ethnic groups are at increased risk for COVID-19 and its sequelae,16–18 race and ethnicity were examined as documented in the EHR. Race and ethnicity were classified as Non-Hispanic White, Non-Hispanic Black, Hispanic, Asian, or Other. The “Other” category was inclusive of “American Indian or Alaskan Native,” “Native Hawaiian or Other Pacific Islander,” or “Other,” which is a selectable category for some included hospital systems. Patients with public insurance were defined as those who had at least one insurance carrier with a financial class of Medicare or Medicaid documented at the birth admission. If the prepregnancy BMI was not available, it was estimated by subtracting the recommended weight gain during pregnancy per week in each trimester19 from the earliest weight obtained during pregnancy. Pre-existing medical comorbidities were determined using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnostic codes (Appendix 1, available online at http://links.lww.com/AOG/C409). Each patient's home address ZIP code was used to determine the Social Vulnerability Index, urbanicity, and Census region. The Centers for Disease Control and Prevention’s Social Vulnerability Index20 comprises indicators of socioeconomic status, household composition, disability, minority status, and language. For each ZIP code, the weighted average of composite Social Vulnerability Index across all Census tracts overlapping the ZIP code was calculated based on relative population. For urbanicity, mapping of ZIP codes to rural-urban commuting area codes was obtained from the U.S. Department of Agriculture.21 Rural-urban commuting area codes 1 through 3 were considered urban.
In the COVID-19 pandemic epoch, women were considered tested for SARS-CoV-2 infection during pregnancy if at least one SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) test result was available or if the presence of the ICD-10-CM code for confirmed COVID-19 (U07.1) was observed from the date of estimated conception through the delivery hospitalization encounter. Women without either of these indicators were considered untested for SARS-CoV-2 infection during pregnancy. Among those who were tested for SARS-CoV-2 infection during pregnancy, a woman was classified as positive if there was any positive RT-PCR result, independent of the number of tests performed during pregnancy, or if the ICD-10-CM code U07.1 was observed. If at least one negative RT-PCR test result and no positive RT-PCR test results were identified and no ICD-10-CM code U07.1 was recorded, the woman was considered tested negative.
The pregnancy-related outcomes were hypertensive disorders of pregnancy inclusive of gestational hypertension, preeclampsia, eclampsia, and hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome; placental abruption; cesarean birth; and postpartum hemorrhage. The neonatal outcomes were preterm birth at less than 37 weeks of gestation, stillbirth, and birth weights small or large for gestational age. Small for gestational age was defined as birth weight less than the 10th percentile for gestational age at birth; large for gestational age was defined as birth weight greater than the 90th percentile for gestational age at birth.22 Gestational age at birth, mode of delivery, and neonatal birth weight were available discretely in the EHR. The other outcome measures were determined using ICD-10-CM diagnostic codes recorded in the EHR (Appendix 1, http://links.lww.com/AOG/C409).
All comparisons between groups were made using standardized differences. Standardized differences compare the proportions—formulated as a series of one-vs-rest comparisons for categorical variables—in units of the pooled standard deviation.23,24 A standardized difference with an absolute value greater than 0.1 indicates meaningful difference between groups.23 With a large sample, hypothesis testing (eg, χ2 test) is likely to demonstrate a significant P-value even when the difference in outcome between groups is negligible or meaningless (due to chance).25,26 Standardized differences are not influenced by sample size and have been used to evaluate meaningful differences between groups in large cohort studies.27–29
Both comparisons (prepandemic vs pandemic epochs, positive vs negative for SARS-CoV-2 infection) were also stratified by race and ethnicity, high-risk Social Vulnerability Index, and public insurance type to test whether there were changes in the observed outcomes due to potential effect modification by these covariates.
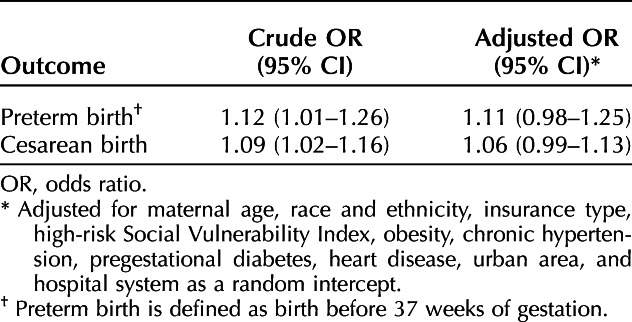
Although standardized difference analyses were favored for interpretation in this study, given the smaller cohort of women who had evidence of testing, χ2 tests (two-sided with P=.01) were also performed to compare women positive for SARS-CoV-2 infection with those negative for SARS-CoV-2 infection. Two post hoc mixed-effects logistic regression models were used to further examine the association between women with SARS-CoV-2 positivity during pregnancy and the risk for preterm birth at less than 37 weeks of gestation and the risk for cesarean birth, because these associations were found to have P-values less than .01 with χ2 analyses. The models were adjusted for relevant baseline characteristics (P<.01) and included hospital system as a random intercept.
Among women who were classified as positive for SARS-CoV-2 infection during pregnancy, we also compared women who were classified as positive during the first two trimesters of pregnancy (from estimated date of conception to 28 weeks of gestation) with those classified as positive during the third trimester of pregnancy (28 weeks of gestation and greater). For the outcome of preterm birth at less than 37 weeks of gestation, comparison between groups was made after excluding women who could not have reached term (37 0/7 weeks) gestation by the end of the study period and women who tested positive for SARS-CoV-2 infection at 37 weeks of gestation or later, because they could not have experienced the outcome of preterm birth after the exposure. Comparisons were made using standardized differences.
All calculations were based on the number of valid observations. Analysis was completed using Python v.3.8.5 (numpy v.1.19.1, pandas v.1.0.5, scipy v.1.5.0). This study was considered exempt from the Yale University Institutional Review Board because all data were deidentified.
RESULTS
A total of 838,489 women delivering at 465 U.S. hospitals were included in this analysis (Fig. 1). Overall 613,264 women delivered during the prepandemic epoch and 225,225 delivered during the pandemic epoch. Baseline sociodemographic characteristics and chronic medical comorbidities were similar between epochs (Table 1). There were no significant standardized differences in adverse pregnancy-related outcomes between the two epochs (Table 2).
Fig. 1. Study cohort identification flow diagram. COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Son. COVID-19 Pandemic and Pregnancy Outcomes. Obstet Gynecol 2021.
Table 1.
Maternal Characteristics Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic
Table 2.
Neonatal and Adverse Pregnancy Outcomes Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic
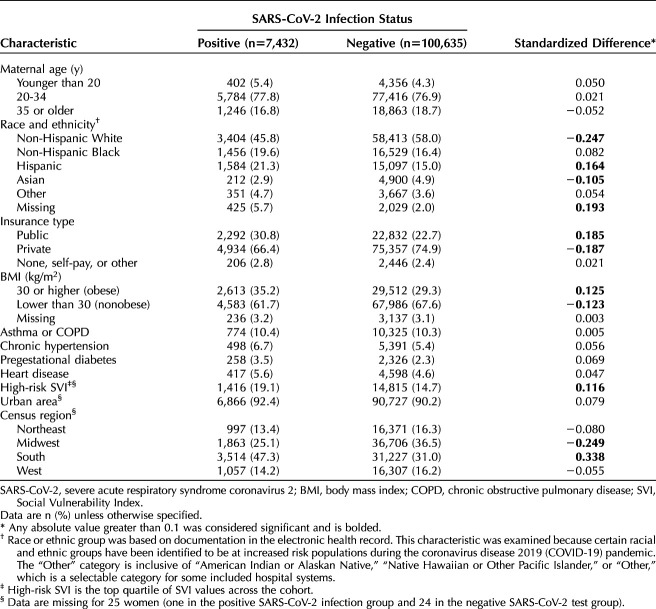
Among the 225,225 women who delivered during the COVID-19 pandemic, 108,067 (48.0%) had evidence of at least one RT-PCR test performed for SARS-CoV-2 infection or an ICD-10-CM code for confirmed COVID-19 present in their EHR. There was no evidence of SARS-CoV-2 testing in the antenatal or delivery encounter records for 52.0% of pregnant women delivering during the COVID-19 pandemic. Women with evidence of testing were more likely to live in ZIP codes in the top 25th percentile Social Vulnerability Index (the most vulnerable population) compared with those without evidence of testing (15.0% vs 11.2% respectively, standardized difference=0.112). Women who had evidence of testing during pregnancy were less likely to live in the South compared with those without evidence of testing (32.1% vs 44.8%, standardized difference=−0.262).
Of those with evidence of SARS-CoV-2 testing (n=108,067), 7,432 (6.9%) had a positive test result or a COVID-19 diagnosis code in their EHR and 100,635 (93.1%) had only negative test results and no recorded COVID-19 diagnosis code. Women positive for SARS-CoV-2 infection were more likely to be Hispanic, less likely to be non-Hispanic White or Asian, more likely to have public insurance, more likely to be socially vulnerable, more likely to live in the South, less likely to live in the Midwest, and more likely to be obese compared with those considered negative for SARS-CoV-2 infection (Table 3). There were no significant standardized differences in adverse pregnancy-related outcomes between women considered positive compared with negative for SARS-CoV-2 infection in pregnancy (Table 4). With logistic regression modeling, after adjustment for potential confounders, there was similarly no significant association between SARS-CoV-2 positivity and preterm birth or cesarean birth (Table 5).
Table 3.
Maternal Characteristics of the Coronavirus Disease 2019 (COVID-19) Pandemic Epoch Group by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection Status
Table 4.
Neonatal and Adverse Pregnancy Outcomes Among Women With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Testing
Table 5.
Mixed Effects Logistic Regression Models to Evaluate the Association Between Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Positivity and Pregnancy Outcomes
Of the 7,432 women positive for SARS-CoV-2 infection, the majority (n=6,842, 92%) were tested during the third trimester of pregnancy compared with the first or second trimesters (n=590, 8%). Compared with women positive for SARS-CoV-2 infection during the third trimester, those who were positive in the first or second trimester of pregnancy were more likely to have private insurance, more likely to be obese, more likely to have asthma or chronic obstructive pulmonary disease or pregestational diabetes, more likely to live in the Midwest, and less likely to live in an urban area (Appendix 2, available online at http://links.lww.com/AOG/C409). Women positive for SARS-CoV-2 infection during the first or second trimester were less likely to experience preterm birth at less than 37 weeks of gestation compared with those who were positive in the third trimester of pregnancy (12.9% vs 23.7%, standardized difference=−0.283) (Appendix 3, available online at http://links.lww.com/AOG/C409). There were no other significant differences in adverse pregnancy outcomes in the comparison between trimester of infection.
Analyses stratified by race and ethnicity, high-risk Social Vulnerability Index, and public insurance type did not show evidence of effect modification in adverse pregnancy outcomes across both comparisons (data not shown).
DISCUSSION
In this large, diverse U.S. cohort, the frequency of adverse pregnancy outcomes did not meaningfully differ between those who delivered before compared with during the COVID-19 pandemic. Notably, in this study, nearly half of women who delivered during the pandemic had SARS-CoV-2 testing, and there were no differences between those positive compared with negative for SARS-CoV-2 infection during pregnancy.
Our finding that there was no meaningful difference in the frequency of preterm birth between women who delivered before compared with during the COVID-19 pandemic is consistent with much of the existing literature.4–8 However, prior studies were limited in that they lacked data regarding SARS-CoV-2 testing status or included only those who tested positive for SARS-CoV-2 infection when making comparisons before and during the COVID-19 pandemic. Thus, this study is a significant contribution because we have SARS-CoV-2 testing results and diagnostic codes for almost half of the women who delivered in the pandemic epoch.
We found no significant changes in the frequency of other adverse pregnancy outcomes such as stillbirth during the COVID-19 pandemic. Although earlier case series and single-center data4,30 suggested a higher frequency of stillbirth during the pandemic, a larger cohort using National Health Service data in England31 did not find any increase in stillbirth frequency regionally or nationally. Notably, this larger cohort study did not have data available on maternal SARS-CoV-2 infection status, sociodemographic attributes, or access to medical care. With our large sample size inclusive of more than 7,000 women with SARS-CoV-2 infection and availability of more comprehensive data, we were able to more rigorously evaluate infrequent outcomes such as stillbirth.
Our data indicate that many U.S. hospitals were not employing universal SARS-CoV-2 screening for pregnant women during the pandemic. Still, almost half of the women who delivered during the COVID-19 epoch in our cohort had evidence of SARS-CoV-2 testing during pregnancy. We found that pregnant women living in ZIP codes with a high-risk (top 25th percentile) overall Social Vulnerability Index were significantly more likely to have evidence of testing for SARS-CoV-2 during their pregnancies or delivery hospitalizations. Selective testing patterns may have been influenced by the awareness that those who are socioeconomically vulnerable are at disproportionately higher risk of SARS-CoV-2 infection.32,33 Alternatively, hospitals that serve socially vulnerable populations may have prioritized or been better equipped to perform testing. Pregnant women delivering in the South were less likely to be tested for SARS-CoV-2 infection; those in the Northeast and Midwest were more likely to be tested. Regional testing patterns may have been influenced by the shifting geographical surges of SARS-CoV-2 infection during the study period, and there may have been regional differences in the prioritization or availability of resources for testing.
Among women who had evidence of SARS-CoV-2 testing, the positivity rate was 7%, which is consistent with previous work from multiple hospitals with varying sampling rates.12 Many of the differences observed here in baseline characteristics between those considered positive compared with negative for SARS-CoV-2 infection have been similarly described in the existing literature.32,34,35
We did not find any meaningful differences in standardized differences in adverse pregnancy outcomes between women classified as positive compared with negative for SARS-CoV-2 infection, which differs from prior studies. However, it is important to note that our study was able to identify women with SARS-CoV-2 infection based mainly on laboratory testing and compare them with women confirmed negative for SARS-CoV-2 infection. This is a unique study strength because comparisons based solely on the presence or absence of a COVID-19 diagnosis code bias data with the inclusion of women with SARS-CoV-2 infection who are more likely to be symptomatic or exhibit more severe COVID-19 illness. A U.S. study14 using the Premier Healthcare database included 406,446 women hospitalized for childbirth during the COVID-19 pandemic, of whom 6,380 (1.6%) had a COVID-19 ICD-10-CM billing code. In their study, women with a COVID-19 diagnosis code had significantly higher risks of preeclampsia and preterm birth compared with those without a COVID-19 diagnosis code, but the study was limited by reliance on diagnosis codes to classify COVID-19 status and inability to distinguish between untested women and those testing negative. Similarly, the INTERCOVID multinational cohort study36 found that women with a COVID-19 diagnosis were at higher risk for preeclampsia and eclampsia, severe infections, intensive care unit admission, maternal mortality, preterm birth, and severe perinatal morbidity compared with unmatched, consecutive women without a COVID-19 diagnosis. In this study, however, women were considered positive for COVID-19 based on multiple criteria (varying laboratory tests, radiologic findings, or predefined symptoms), whereas those considered not to have COVID-19 were simply women who did not meet the aforementioned criteria, not those who were known to be tested with a negative result.
In our cohort, among women positive for SARS-CoV-2 infection, the overwhelming majority were tested during their third trimester of pregnancy. In part, this finding could result from the fact that the cohort included only women who delivered during the 10-month study period, reducing the number of women who could have been both tested early in their pregnancy and delivered during the study period. Though we found that women who tested positive for SARS-CoV-2 infection during the third trimester of pregnancy were more likely to experience preterm birth, given the aforementioned limitation, this finding should be interpreted with caution and data are presented to generate hypotheses and drive further research.
Strengths of this study include the large number of pregnant women and diversity of U.S. hospitals included in the analytic cohort. The size of our cohort enabled us to examine pregnant women who delivered before and during the COVID-19 pandemic as well as the SARS-CoV-2 testing status of all individuals included in the pandemic epoch. This is unique to our study; prior studies have been limited by use of only the presence or absence of diagnosis codes for COVID-19 infection14,36 or small numbers of confirmed tested women. Additionally, our data were obtained from 465 hospitals across all four U.S. Census Bureau regions. This is an important strength because SARS-CoV-2 cases have fluctuated significantly over time, with migratory geographical hotspots. In addition, we were able to include any SARS-CoV-2 testing results during the span of a pregnancy. This is valuable because certain outcomes (eg, preterm birth, hypertensive disorders of pregnancy, birth weight) would have been difficult to evaluate if SARS-CoV-2 infection status was determined only at the time of delivery hospitalization, given inadequate latency between exposure and outcome. We recognize that outpatient SARS-CoV-2 testing may have been more variable, and testing earlier in a pregnancy may have been more likely in women who were symptomatic or had suspected or known exposures.
Our study has limitations. First, we could not distinguish between asymptomatic and symptomatic SARS-CoV-2 infection nor severity of disease, which has been shown to have differential effects on pregnancy outcomes.37 Given the significant variability in how these data are reported (symptoms, clinical examination findings, and radiographic imaging results) and captured across health systems, these data could not be consistently abstracted from Cosmos. Second, positive SARS-CoV-2 infections may have been missed due to testing availability and testing practices, which likely varied across hospitals and over time. Patients who were tested for SARS-CoV-2 infection before their delivery hospitalizations also may have been missed if they were tested at a non-Epic facility or site that does not participate in Cosmos. Third, the gestational age at birth to determine preterm birth was based on the delivery date documented by the obstetrician, though there may have been deviations in accuracy based on the method used to determine gestational age for each included patient. Further, we were unable to determine whether a preterm birth was spontaneous or medically indicated; therefore, we cannot exclude the possibility that, although the overall preterm birth rate was not significantly different, indications for preterm births may have differed related to SARS-CoV-2 infection status or health care delivery and utilization. Encouragingly, however, a previous study did not show differences in preterm birth even when stratified by preterm birth phenotype.6 Fourth, we did not assess early pregnancy outcomes such as miscarriage or pregnancy loss due to the inability to accurately ascertain these outcomes using EHR data and our decision to exclude women delivering before 24 weeks of gestation. Last, some of the outcomes examined are still rare occurrences (eg, stillbirth and placental abruption), and we were likely underpowered to adequately assess these outcomes despite our very large sample size.
In conclusion, in a large, geographically diverse U.S. cohort of pregnant women in which nearly half of women who delivered during the COVID-19 pandemic had evidence of SARS-CoV-2 testing, we found no meaningful differences in adverse pregnancy outcomes between those who delivered in the prepandemic and COVID-19 pandemic epochs. Pregnant women with evidence of SARS-CoV-2 infection had similar outcomes to those without evidence of viral infection. It is possible that COVID-19, disruption of health care delivery and utilization, or other unintended consequences may be offsetting one another with regard to pregnancy outcomes, or that symptomatic disease, severity of disease, or timing of disease during pregnancy may be more important prognostic factors.
Footnotes
Financial Disclosure Eric Lindgren, Kieran Gallagher, and Justin Y. Lo disclosed that they are employees of Epic Systems Corporation, a health IT software company that provides Cosmos, a research platform containing data from Epic's health care provider customer organizations. Epic licenses electronic health record software and provides related services to health care organizations that pay Epic for such software and services. The authors and Epic and have no conflicts of interest with respect to the subject matter of this study but would like to clarify that insights gleaned from this research may inform Epic's software design and development activities. Heather H. Burris disclosed that money was paid to her institution from Highmark Blue Cross Blue Shield Delaware's donor-advised fund (foundation grant), BluePrints for the Community, and Independence Blue Cross. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal's requirements for authorship.
Published online ahead-of-print August 9, 2021.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C410.
REFERENCES
- 1.Centers for Disease Control and Prevention COVID Data Tracker. Accessed July 5, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- 2.Czeisler MÉ, Marynak K, Clarke KEN, Salah Z, Shakya I, Thierry JM, et al. Delay or avoidance of medical care because of COVID-19-related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep 2020;69:1250–7. doi: 10.15585/mmwr.mm6936a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenthal D, Fowler EJ, Abrams M, Collins SR. Covid-19 - implications for the health care system. N Engl J Med 2020;383:1483–8. doi: 10.1056/NEJMsb2021088 [DOI] [PubMed] [Google Scholar]
- 4.Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O'Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA 2020;324:705–6. doi: 10.1001/jama.2020.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedermann G, Hedley PL, Bækvad-Hansen M, Hjalgrim H, Rostgaard K, Poorisrisak P, et al. Danish premature birth rates during the COVID-19 lockdown. Arch Dis Child Fetal Neonatal Ed 2021;106:93–5. doi: 10.1136/archdischild-2020-319990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Handley SC, Mullin AM, Elovitz MA, Gerson KD, Montoya-Williams D, Lorch SA, et al. Changes in preterm birth phenotypes and stillbirth at 2 Philadelphia hospitals during the SARS-CoV-2 pandemic, March-June 2020. JAMA 2021;325:87–9. doi: 10.1001/jama.2020.20991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matheson A, McGannon CJ, Malhotra A, Palmer KR, Stewart AE, Wallace EM, et al. Prematurity rates during the coronavirus disease 2019 (COVID-19) pandemic lockdown in Melbourne, Australia. Obstet Gynecol 2021;137:405–7. doi: 10.1097/AOG.0000000000004236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood R, Sinnott C, Goldfarb I, Clapp M, McElrath T, Little S. Preterm birth during the coronavirus disease 2019 (COVID-19) pandemic in a large hospital system in the United States. Obstet Gynecol 2021;137:403–4. doi: 10.1097/AOG.0000000000004237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Been JV, Burgos Ochoa L, Bertens LCM, Schoenmakers S, Steegers EAP, Reiss IKM. Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Health 2020;5:e604–11. doi: 10.1016/S2468-2667(20)30223-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kc A, Gurung R, Kinney MV, Sunny AK, Moinuddin M, Basnet O, et al. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet Glob Health 2020;8:e1273–e81. doi: 10.1016/S2214-109X(20)30345-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey EM, McNeer E, McDonald MF, Shapiro-Mendoza CK, Dupont WD, Barfield W, et al. Association of preterm birth rate with COVID-19 statewide stay-at-home orders in Tennessee. JAMA Pediatr 2021;175:635–7. doi: 10.1001/jamapediatrics.2020.6512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. doi: 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crovetto F, Crispi F, Llurba E, Pascal R, Larroya M, Trilla C, et al. Impact of SARS-CoV-2 infection on pregnancy outcomes: a population-based study. Clin Infect Dis 2021 Feb 8. [Epub ahead of print]. doi: 10.1093/cid/ciab104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jering KS, Claggett BL, Cunningham JW, Rosenthal N, Vardeny O, Greene MF, et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern Med 2021;181:714–7. doi: 10.1001/jamainternmed.2020.9241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarabichi Y, Frees A, Honeywell S, Huang C, Naidech AM, Moore JH, et al. The Cosmos collaborative: a vendor-facilitated electronic health record data aggregation platform. ACI Open 2021;05:e36–46. doi: 10.1055/s-0041-1731004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with covid-19. N Engl J Med 2020;382:2534–43. doi: 10.1056/NEJMsa2011686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, et al. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:759–65. doi: 10.15585/mmwr.mm6924e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu T, Mack JA, Salvatore M, Prabhu Sankar S, Valley TS, Singh K, et al. Characteristics associated with racial/ethnic disparities in COVID-19 outcomes in an academic health care system. JAMA Netw Open 2020;3:e2025197. doi: 10.1001/jamanetworkopen.2020.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: reexamining the guidelines. National Academies Press (US); 2009. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention, Agency for Toxic Substances and Disease Registry. CDC/ATSDR Social Vulnerability Index. Accessed March 22, 2021. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- 21.U.S. Department of Agriculture, Economic Research Service. Rural-urban commuting area codes. Accessed March 22, 2021. www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx
- 22.Fenton TR. A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr 2003;3:13. doi: 10.1186/1471-2431-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009;38:1228–34. doi: 10.1080/03610910902859574 [DOI] [Google Scholar]
- 24.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan GM, Feinn R. Using effect size-or why the P value is not enough. J Grad Med Educ 2012;4:279–82. doi: 10.4300/JGME-D-12-00156.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkson J. Some difficulties of interpretation encountered in the application of the chi-square test. J Am Stat Assoc 1938;33:526–36. doi: 10.1080/01621459.1938.10502329 [DOI] [Google Scholar]
- 27.Stephenson AL, Sykes J, Stanojevic S, Quon BS, Marshall BC, Petren K, et al. Survival comparison of patients with cystic fibrosis in Canada and the United States: a population-based cohort study. Ann Intern Med 2017;166:537–46. doi: 10.7326/M16-0858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol 2001;54:387–98. doi: 10.1016/s0895-4356(00)00321-8 [DOI] [PubMed] [Google Scholar]
- 29.Flythe JE, Assimon MM, Tugman MJ, Chang EH, Gupta S, Shah J, et al. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis 2021;77:190–203.e1. doi: 10.1053/j.ajkd.2020.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ 2020;369:m2107. doi: 10.1136/bmj.m2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stowe J, Smith H, Thurland K, Ramsay ME, Andrews N, Ladhani SN. Stillbirths during the COVID-19 pandemic in England, April-June 2020. JAMA 2021;325:86–7. doi: 10.1001/jama.2020.21369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1641–7. doi: 10.15585/mmwr.mm6944e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joseph NT, Stanhope KK, Badell ML, Horton JP, Boulet SL, Jamieson DJ. Sociodemographic predictors of SARS-CoV-2 infection in obstetric patients, Georgia, USA. Emerg Infect Dis 2020;26:2787–9. doi: 10.3201/eid2611.203091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lokken EM, Taylor GG, Huebner EM, Vanderhoeven J, Hendrickson S, Coler B, et al. Higher severe acute respiratory syndrome coronavirus 2 infection rate in pregnant patients. Am J Obstet Gynecol 2021;225:75.e1–16. doi: 10.1016/j.ajog.2021.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep 2020;69:769–75. doi: 10.15585/mmwr.mm6925a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr 2021 Apr 22. [Epub ahead of print]. doi: 10.1001/jamapediatrics.2021.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metz TD, Clifton RG, Hughes BL, Sandoval G, Saade GR, Grobman WA, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstet Gynecol 2021;137:571–80. doi: 10.1097/AOG.0000000000004339 [DOI] [PMC free article] [PubMed] [Google Scholar]