Abstract
Sixteen amikacin-resistant clinical Acinetobacter baumannii isolates from nine different hospitals in Spain were investigated to determine whether the high incidence of amikacin-resistant A. baumannii was due to the dissemination of an amikacin-resistant strain or to the spread of an amikacin resistance gene. The epidemiological relationship studied by repetitive extragenic palindromic PCR and low-frequency restriction analysis of chromosomal DNA showed that the same clone was isolated in eight of nine hospitals, although other clones were also found. The strains were studied for the presence of the aph(3′)-VIa and aac(6′)-I genes, which encode enzymes which inactivate amikacin, by PCR. All 16 clinical isolates had positive PCRs with primers specific for the amplification of the aph(3′)-VIa gene, whereas none had a positive reaction for the amplification of the aac(6′)-I gene. Therefore, the high incidence of amikacin resistance among clinical A. baumannii isolates in Spain was mainly due to an epidemic strain, although the spread of the aph(3′)-VI gene cannot be ruled out.
Several outbreaks of nosocomial infections caused by amikacin-resistant Acinetobacter baumannii have been documented (3, 4, 11, 20). The most frequent cause of resistance to aminoglycosides in A. baumannii is the modification of hydroxyl or amino groups of the antibiotic by aminoglycoside-modifying enzymes (1, 5–7, 14, 15, 21), although other mechanisms such as diminished permeability or alteration of the binding sites have been suggested (21). Until recently, amikacin remained the most active aminoglycoside in the treatment of infections caused by Acinetobacter spp. The 6′-aminoglycoside-acetylating enzyme found in Acinetobacter spp. inactivates amikacin (13); however, the most frequently found amikacin-modifying enzyme in A. baumannii is aminoglycoside-3′-phosphotransferase VI [APH(3′)-VI], a type of 3′-O-phosphotransferase which also inactivates amikacin (8). Buisson et al. (3) found a significant correlation between amikacin consumption and the emergence of amikacin resistance mediated by APH(3′)-VI in Acinetobacter species. The main purpose of this study was to develop a PCR method for the detection of the aph(3′)-VIa gene and to determine if the spread of amikacin resistance in A. baumannii strains isolated in nine Spanish hospitals was due to an epidemic strain carrying the aph(3′)-VIa or aac(6′)-I gene.
MATERIALS AND METHODS
Bacterial strains.
Sixteen amikacin-resistant clinical isolates of A. baumannii were collected from nine Spanish hospitals (Hospital Clinic, Barcelona; Hospital Valle Hebrón, Barcelona; Hospital de Sant Joan de Reus, Reus, Tarragona; Hospital Virgen del Rocio, Seville; Hospital Clinico, Seville; Hospital de La Princesa, Madrid; Hospital 12 de Octubre, Madrid; Hospital Ramón y Cajal, Madrid; and Hospital Nuestra Señora [Ntra. Sra.] del Pino, Las Palmas, Grand Canary Islands). The identification of A. baumannii was performed by standard biochemical reactions by following the criteria of Bouvet and Grimont (2). Moreover, all the strains analyzed in this study were causing pneumonia in patients in the intensive care units of each hospital.
Susceptibility testing.
Susceptibility testing was performed by using an agar dilution method in accordance with the guidelines established by the National Committee for Clinical Laboratory Standards (16). Approximately 104 CFU of each isolate was inoculated onto freshly prepared media containing serial dilutions of amikacin (Bristol-Myers Laboratories, Hounslow, United Kingdom) with a multipoint replicator.
REP-PCR and low-frequency restriction analysis of chromosomal DNA (PFGE).
The repetitive extragenic palindromic PCR (REP-PCR) was performed with the primers and under the conditions described previously (22). Samples (5 μl) of each PCR end product were analyzed by polyacrylamide gel electrophoresis with Genephor precast 12.5% polyacrylamide gels run at 600 V and 25 mA. After that, the gel was stained with silver. The analysis of chromosomal DNA by digestion with low-frequency-of-cleavage restriction enzymes and separation of the fragments by pulsed-field gel electrophoresis (PFGE) was performed with the ApaI enzyme under the conditions mentioned elsewhere (12).
PCR amplification of the aph(3′)-VIa and aac(6′)-I genes.
Two oligonucleotide primers were designed on the basis of the nucleotide sequence of the aph(3′)-VIa gene in comparison with the sequences of different phosphotransferases in an attempt to find specific sequences of this gene which do not anneal with the other genes. These primers were 5′-ATACAGAGACCACCATACAGT-3′ (from nucleotides 140 to 159) and 5′-GGACAATCAATAATAGCAAT-3′ (from nucleotides 355 to 374) (Genosys Biotechnologies, Cambridge, United Kingdom). The PCR was performed as follows. One colony grown on MacConkey agar was resuspended in 25 μl of sterile distilled water and boiled for 10 min. After a centrifugation step at 15,000 × g for 1 min, 25 μl of the reaction mixture containing 20 mM Tris-HCl (pH 8.8), 100 mM potassium chloride, 3.0 mM magnesium chloride, 0.1% (wt/vol) gelatin, 400 μM deoxynucleoside triphosphates, and 1 μM (each) primer was added, together with 2.5 U of Taq polymerase (BRL, Life Technologies Inc., Gaithersburg, Md.). Each reaction mixture was overlaid with mineral oil and amplified with the following temperature profiles: 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. Amplification was performed in a DNA thermal cycler (model 480; Perkin-Elmer Cetus). The amplified DNA products were resolved by electrophoresis in 1.5% NuSieve and 1% agarose gels. In order to confirm that the amplified product belongs to the aph(3′)-VIa gene, the fragment was recovered from the reaction mixture with the QIAquick Spin PCR purification kit (Qiagen, Chatsworth, Calif.) and processed with a DNA sequencing kit (Taq DyeDeoxy Terminator Cycle Sequencing Kit; Applied Biosystems, Foster City, Calif.) and analyzed in an automatic DNA sequencer (model 373A; Applied Biosystems).
Eight clinical isolates carrying the genes for other aminoglycoside phosphotransferases were used as controls; they were Escherichia coli carrying the gene for APH(3′)-I, E. coli carrying the gene for APH(3′)-II, Enterobacter cloacae carrying the gene for APH(3′)-II, Staphylococcus epidermidis carrying the gene for APH(3′)-II, E. cloacae carrying the gene for APH(3′)-III, Staphylococcus aureus carrying the gene for APH(3′)-IV, and Enterococcus faecalis carrying the gene for APH(3′)-IV.
Detection of the aac(6′)-Ib and aac(6′)-Ih genes was performed by PCR by following the procedure described by Ploy et al. (17).
RESULTS
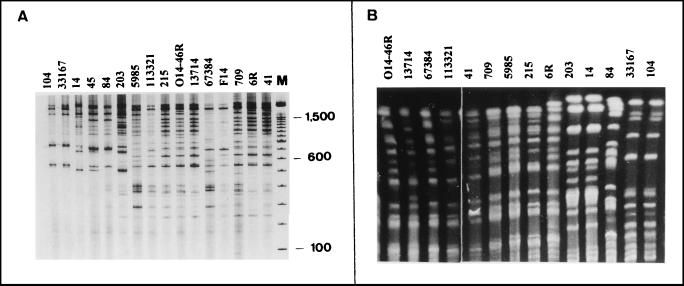
The 16 amikacin-resistant clinical A. baumannii isolates were epidemiologically analyzed by REP-PCR and by low-frequency-of-cleavage restriction enzyme analysis of chromosomal DNA and PFGE. The analysis by REP-PCR allowed the distribution of the strains into five different groups with DNA bands ranging from 200 to 2,000 bp (Fig. 1A). Nine of the 16 strains belonged to the same type (type 1) and came from seven different hospitals, whereas two strains each were of types 2, 3, and 4 and one strain was of type 5. The strains belonging to type 2 were isolated in a Madrid hospital and in another hospital in Seville, whereas strains belonging to type 4 were isolated in two different hospitals in Seville (Table 1). The analysis of chromosomal DNA by PFGE confirmed the results obtained by REP-PCR (Table 1 and Fig. 1B) with one exception. The PFGE pattern of the strain determined to be of type 5 by REP-PCR had the same PFGE pattern as the strains determined to be of type 1 by REP-PCR; therefore, all these strains must be considered the same type (type A by PFGE). The results were analyzed by following the criteria described by Tenover et al. (19).
FIG. 1.
Patterns obtained by REP-PCR (A) and PFGE (B). The numbers above the lanes indicate the strains. Lane M, DNA molecular size marker (100-bp DNA Ladder; Life Technologies Inc.).
TABLE 1.
Epidemiological characteristics of amikacin-resistant A. baumannii strains
| Strain no. | Hospitala | PFGE pattern | REP-PCR pattern | AKb MIC (μg/ml) | PCR result for aph(3′)-VIa |
|---|---|---|---|---|---|
| F14 | VH, Barcelona | A | 1 | 256 | + |
| O14-46R | VH, Barcelona | A | 1 | 256 | + |
| 5985 | HC, Barcelona | A | 1 | 64 | + |
| 41 | HC, Barcelona | A | 1 | 64 | + |
| 113321 | HNSP, Canarias | A | 1 | 128 | + |
| 6R | 12O, Madrid | A | 1 | 128 | + |
| 67384 | RC, Madrid | A | 5 | 256 | + |
| 13714 | VR, Seville | A | 1 | 64 | + |
| 215 | HLP, Madrid | A | 1 | 64 | + |
| 709 | Re, Tarragona | A | 1 | 64 | + |
| 203 | HLP, Madrid | B | 2 | 32 | + |
| 84 | HLP, Madrid | C | 3 | 32 | + |
| 45 | HLP, Madrid | C | 3 | 64 | + |
| 14 | HCP, Seville | B | 2 | 256 | + |
| 104 | HCP, Seville | D | 4 | 128 | + |
| 33167 | VR, Seville | D | 4 | 64 | + |
VR, Hospital Virgen del Rocio; RC, Hospital Ramón y Cajal; HNSP, Hospital Ntra. Sra. del Pino; VH, Hospital del Valle Hebrón; Re, Hospital San Joan de Reus; HC, Hospital Clinic; HLP, Hospital La Princesa; HCP, Hospital Clinico Provincial; 12O, Hospital 12 de Octubre.
AK, amikacin.
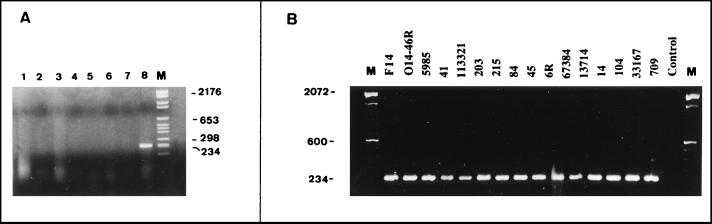
For all amikacin-resistant clinical A. baumannii isolates tested in this study, the amikacin MIC was >32 μg/ml (Table 1). To investigate whether the resistance was due to the synthesis of the aminoglycoside-modifying enzyme APH(3′)-VIa, a PCR for the amplification of the aph(3′)-VIa gene was used. A DNA fragment of the expected size of 234 bp, from nucleotides 140 to 374 of the aph(3′)-VIa gene, was obtained (Fig. 2A). Its nucleotide sequence showed 100% homology with that described by Martin et al. (13). This DNA fragment was not obtained from the other strains which carried the genes corresponding to four different phosphotransferases (Fig. 2A), showing that the primers were specific for aph(3′)-VIa. All the strains analyzed in our study were positive for the aph(3′)-VIa gene by PCR (Fig. 2B), whereas when primers specific for the amplification of the aac(6′)-Ib and aac(6′)-Ih genes were used, the genes were not amplified from any of the strains.
FIG. 2.
(A) Analysis of PCR-amplified DNA with primers specific for amplification of aph(3′)-VIa. Lanes: 1, E. coli carrying the gene for APH(3′)-I; 2, E. coli carrying the gene for APH(3′)-II; 3, E. cloacae carrying the gene for APH(3′)-II; 4, S. epidermidis carrying the gene for APH(3′)-II; 5, E. cloacae carrying the gene for APH(3′)-III; 6, S. aureus carrying the gene for APH(3′)-IV; 7, E. faecalis carrying the gene for APH(3′)-IV; 8, A. baumannii carrying the gene for APH(3′)-VIa; and M, DNA molecular mass marker VI (Boehringer Mannheim, Mannheim, Germany). (B) PCR analysis of the aph(3′)-VIa gene of the amikacin-resistant clinical A. baumannii isolates studied. Control indicates an amikacin-susceptible A. baumannii strain used as a negative control. Lane M, DNA molecular size marker (100-bp DNA Ladder; Life Technologies Inc.).
The number of beds in the hospitals from which the strains included in this study were obtained ranged from 274 to 1,150. All hospitals but one (Hospital San Joan de Reus, Reus, Tarragona, Spain) have both oncology and transplant patients. The distribution of the A. baumannii isolates by clinical source was quite similar in all hospitals, with respiratory specimens being the most common clinical specimens containing A. baumannii, followed by wound and urine specimens.
DISCUSSION
We studied whether the high incidence of amikacin resistance in A. baumannii strains isolated in Spain was due to the dissemination of the amikacin resistance gene aph(3′)-VIa or aac(6′)-I or to the spread of an amikacin-resistant strain of A. baumannii. The epidemiological relationship among the 16 selected clinical isolates of A. baumannii studied by REP-PCR and PFGE showed that clone A (as determined by PFGE) was isolated from patients in eight of nine hospitals. Therefore, the same clone has spread throughout Spain, even to the Canary Islands, which are far from the Spanish peninsula. Moreover, one clone (clone D4) spread between two hospitals in Seville. Another clone (clone B2) spread from a hospital in Seville to a hospital in Madrid or vice versa. Overall, four different clones of A. baumannii were identified.
Of the aminoglycoside-modifying enzymes detected in A. baumannii, so far, only APH(3′)-VI and AAC(6′)-I confer resistance to amikacin. APH(3′)-VI is primarily associated with Acinetobacter spp. and is less frequently observed in other gram-negative bacteria (18). The aph(3′)-VIa gene, which encodes APH(3′)-VI, has been cloned from A. baumannii (13). APH(3′)-VI production is characterized by resistance to kanamycin, neomycin, paromomycin, ribostamycin, butirosin, and gentamicin B, as well as to amikacin and isepamicin (9). AAC(6′)-I production confers resistance to amikacin, netilmicin, sisomicin, and tobramycin. Ten genes, named aac(6′)-Ia to aac(6′)-Ij, encoding AAC(6′)-I enzymes have been described (18). Of these genes, aac(6′)-Ib and Aac(6′)-Ih have frequently been found in A. baumannii (17). The resistance determinant of AAC(6′) was apparently chromosomally located (15), although recently it has been described to be plasmid mediated (10), and it has been suggested that the aph(3′)-VIa gene could reside on a transposon (9). These genes can therefore be easily transferred among strains carried on these genetic elements, contributing to the spread of amikacin resistance. In this study, we have developed a PCR for the specific detection of the aph(3′)-VIa gene, and we have detected this gene in 100% of the amikacin-resistant clinical A. baumannii isolates from different hospitals in different geographic areas in Spain, whereas these strains did not have a positive PCR result when primers specific for the aac(6′)-Ib and aac(6′)-Ih genes were used. Our data are consistent with the results of previous studies in which dot blot hybridization was used (9, 18). In those studies 82.7 and 95% of amikacin-resistant Acinetobacter strains hybridized with an aph(3′)-VIa probe (9, 18). In part, our results agree with those of Lambert et al. (9), who showed that in France the dissemination of amikacin resistance in Acinetobacter spp. was due to a gene, although the dissemination of an amikacin-resistant clone is also true in Spain.
PCR for the detection of aminoglycoside-modifying enzymes may play a major role in delineating the types of genes involved in epidemics, may help define their modes of transmission, and may make large studies of the movement of specific resistance determinants during outbreaks of nosocomial and community-acquired infections possible. In the outbreaks caused by A. baumannii, the use of antibiotics can contribute to the persistence and spread of the outbreak. Sometimes the patients are only colonized with this microorganism and do not require antimicrobial therapy; therefore, it is important to differentiate between colonization and infection before therapy is begun. In conclusion, our study has shown the contribution of the aph(3′)-VIa gene to the incidence of amikacin resistance in A. baumannii studied by PCR. This, together with the molecular epidemiological analysis of strains isolated from different hospitals in Spain, has shown that the dissemination of amikacin resistance was due to an epidemic strain carrying the aph(3′)-VIa gene and demonstrates how easily this microorganism is spread from hospital to hospital.
ACKNOWLEDGMENTS
This work was supported in part by grant SAF97-0091 from Plan Nacional I+D, Madrid, Spain, and grant FIS 98/0526.
We thank R. Gómez-Lus for supplying us with the strains used as controls.
REFERENCES
- 1.Bergogne-Berezin E, Joly-Guillou M L, Moreau N, LeGoffic F. Aminoglycosides modifying enzymes in clinical isolates of Acinetobacter calcoaceticus. Curr Microbiol. 1980;4:361–364. [Google Scholar]
- 2.Bouvet P J M, Grimont P A D. Taxonomy of the genus Acinetobacter with the recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemlyticus sp. nov., Acinetobacter johnsoni sp. nov., and Acinetobacter junii sp. nov., emended description of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int J Syst Bacteriol. 1986;36:228–240. [Google Scholar]
- 3.Buisson Y, Tran Van Nhieu G, Ginot L, Bouvet P, Schill H, Driot L, Meyran M. Nosocomial outbreaks due to amikacin-resistant tobramycin-sensitive Acinetobacter species: correlation with amikacin usage. J Hosp Infect. 1990;15:83–93. doi: 10.1016/0195-6701(90)90024-i. [DOI] [PubMed] [Google Scholar]
- 4.Cefai C, Richards J, Gould F K, McPeake P. An outbreak of Acinetobacter respiratory tract infection resulting from incomplete disinfection of ventilatory equipment. J Hosp Infect. 1990;15:177–182. doi: 10.1016/0195-6701(90)90128-b. [DOI] [PubMed] [Google Scholar]
- 5.Devaud M, Kayser F H, Bachi B. Transposon-mediated multiple antibiotic resistance in Acinetobacter strains. Antimicrob Agents Chemother. 1982;22:323–329. doi: 10.1128/aac.22.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein G W, Labigne-Rousell A, Gerbaud G, Carlier C, Collatz E, Courvalin P. Transferable plasmid mediated antibiotic resistance in Acinetobacter. Plasmid. 1983;10:138–147. doi: 10.1016/0147-619x(83)90066-5. [DOI] [PubMed] [Google Scholar]
- 7.Gómez-Lus R, Larrad L, Rubio-Calvo M C, Navarro M, Lasierra M P. AAC(3) and AAC(6′) enzymes produced by R plasmids isolated in general hospital. In: Mitsushashi S, Rosival L, Kremery V, editors. Antibiotic resistance. Berlin, Germany: Springer-Verlag; 1980. pp. 295–303. [Google Scholar]
- 8.Lambert T, Gerbaud G, Courvalin P. Transferable amikacin resistance in Acinetobacter spp. due to a new type of 3′-aminoglycoside phosphotransferase. Antimicrob Agents Chemother. 1988;32:15–19. doi: 10.1128/aac.32.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert T, Gerbaud G, Bouvet P, Vieu J F, Courvalin P. Dissemination of amikacin resistance gene aphA6 in Acinetobacter spp. Antimicrob Agents Chemother. 1990;34:1244–1248. doi: 10.1128/aac.34.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert T, Gerbaud G, Courvalin P. Characterization of the chromosomal aac(6′)-Ij gene of Acinetobacter sp. 13 and the aac(6′)-Ih plasmid gene of Acinetobacter baumannii. Antimicrob Agents Chemother. 1994;38:1883–1889. doi: 10.1128/aac.38.9.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcos M A, Abdalla S, Pedraza F, Andreu A, Fernandez F, Gomez-Lus R, Jimenez de Anta M T, Vila J. Epidemiological markers of Acinetobacter baumannii clinical isolates from a spinal cord injury unit. J Hosp Infect. 1994;28:39–48. doi: 10.1016/0195-6701(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 12.Marcos M A, Jimenez de Anta M T, Vila J. Correlation of six methods for typing nosocomial isolates of Acinetobacter baumannii. J Med Microbiol. 1995;42:328–335. doi: 10.1099/00222615-42-5-328. [DOI] [PubMed] [Google Scholar]
- 13.Martin P, Jullien E, Courvalin P. Nucleotide sequence of Acinetobacter baumannii aphA-6 gene: evolutionary and functional implications of sequence homologies with nucleotide-binding proteins, kinases and other aminoglycoside-modifying enzymes. Mol Microbiol. 1988;2:615–625. doi: 10.1111/j.1365-2958.1988.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 14.Murray B E, Moellering R C., Jr Aminoglycoside-modifying enzymes among clinical isolates of Acinetobacter calcoaceticus subsp. anitratus (Herella vaginicola): explanation for high-level aminoglycoside resistance. Antimicrob Agents Chemother. 1979;15:190–199. doi: 10.1128/aac.15.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray B E, Moellering R C., Jr Evidence of plasmid-mediated production of aminoglycoside-modifying enzymes not previously described in Acinetobacter. Antimicrob Agents Chemother. 1980;17:30–36. doi: 10.1128/aac.17.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 17.Ploy M C, Giamarellon H, Bourlioux P, Courvalin P, Lambert T. Detection of aac(6′)-I genes in amikacin-resistant Acinetobacter spp. by PCR. Antimicrob Agents Chemother. 1994;38:2925–2928. doi: 10.1128/aac.38.12.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw K J, Rather P N, Hare R S, Miller G H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vila J, Almela M, Jimenez de Anta M T. Laboratory investigation of hospital outbreak caused by two different multiresistant Acinetobacter calcoaceticus subsp. anitratus strains. J Clin Microbiol. 1989;27:1086–1089. doi: 10.1128/jcm.27.5.1086-1089.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vila J, Marcos M A, Marco F, Abdalla S, Vergara Y, Reig R, Gomez-Lus R, Jimenez de Anta M T. In vitro antimicrobial production of β-lactamases, aminoglycoside-modifying enzymes, and chloramphenicol acetyltransferase by and susceptibility of clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 1993;37:138–141. doi: 10.1128/aac.37.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vila J, Marcos M A, Jimenez de Anta M T. A comparative study of different PCR-based DNA fingerprinting techniques for typing of the Acinetobacter calcoaceticus-A. baumannii complex. J Med Microbiol. 1996;44:482–489. doi: 10.1099/00222615-44-6-482. [DOI] [PubMed] [Google Scholar]