Abstract
Background
In 2017, Massachusetts General Hospital implemented the Severe Immunotherapy Complications (SIC) Service, a multidisciplinary care team for patients hospitalized with immune-related adverse events (irAEs), a unique spectrum of toxicities associated with immune checkpoint inhibitors (ICIs). This study’s objectives were to evaluate the intervention’s (1) effect on patient outcomes and healthcare utilization, and (2) ability to collect biological samples via a central infrastructure, in order to study the mechanisms responsible for irAEs.
Methods
A hospital database was used to identify patients who received ICIs for a malignancy and were hospitalized with severe irAEs, before (April 2, 2016–October 3, 2017) and after (October 3, 2017–October 24, 2018) SIC Service initiation. The primary outcome was readmission rate after index hospitalization. Secondary outcomes included length of stay (LOS) for admissions, corticosteroid and non-steroidal second-line immunosuppression use, ICI discontinuation, and inpatient mortality.
Results
In the pre-SIC period, 127 of 1169 patients treated with ICIs were hospitalized for irAEs; in the post-SIC period, 122 of 1159. After SIC service initiation, reductions were observed in irAE readmission rate (14.8% post-SIC vs 25.9% pre-SIC; OR 0.46; 95% CI 0.22 to 0.95; p=0.036) and readmission LOS (median 6 days post-SIC vs 7 days pre-SIC; 95% CI −16.03 to –0.14; p=0.046). No significant pre-initiation and post-initiation differences were detected in corticosteroid use, second-line immunosuppression, ICI discontinuation, or inpatient mortality rates. The SIC Service collected 789 blood and tissue samples from 234 patients with suspected irAEs.
Conclusions
This is the first study to report that establishing a highly subspecialized care team focused on irAEs is associated with improved patient outcomes and reduced healthcare utilization. Furthermore, the SIC Service successfully integrated blood and tissue collection safety into routine care.
Keywords: immunotherapy, biomarkers, tumor, cytotoxicity, immunologic, drug therapy, combination, inflammation
Background
As of November 2020, there are 55 Food and Drug Administration-approved indications for immune checkpoint inhibitors (ICIs) in the treatment of cancer.1 Concordant with the increase in approvals, the number of patients eligible for ICI therapy has exponentially grown; whereas only 1.5% of patients with cancer were eligible for ICI therapy in 2011, by 2019 the percentage had risen to 36% or 233,790 patients annually.2 The actual number of patients treated with ICIs may be underestimated as a result of new approved indications and enrollment in clinical trials, and this number will continue to increase as new ICIs and combination immune and non-immune treatment regimens are developed.3 4 Despite these successes, ICI therapy may be associated with the development of treatment-related toxicities—called immune-related adverse events (irAEs)—which are thought to reflect autoinflammatory sequelae of immune activation and downstream effects that occur with ICI administration.5 6 In clinical trials, approximately 60%–85% of participants receiving single-agent ICI therapy develop irAEs (of any grade), with even higher numbers for those administered combination therapy.7–14 Approximately 10%–30% of participants develop grade ≥3 irAEs,7 with a recent meta-analysis reporting 14% with programmed death-1 (PD-1) single agent, 34% with cytotoxic T-lymphocyte antigen-4 (CTLA-4) monotherapy and as high as 55% with combination ICI therapy.8 These serious toxicities may require hospitalization and high-dose immunosuppression. To date, the molecular mechanisms responsible for irAE development remain poorly understood, and we currently lack predictive markers to identify individuals at risk of developing these toxicities, which are fatal in 0.4%–1.2% of patients.15 As ICI therapy becomes a core pillar of cancer care, the incidence of irAEs will significantly increase alongside ICI use. Therefore, it is essential that the healthcare community is aware of and educated on the identification and management of irAEs.
Managing irAEs represents a major clinical challenge in oncologic care, even in large academic centers.16 17 Although the dermatological, gastrointestinal (GI), endocrine, and hepatic systems are most commonly involved, any organ system can be affected,9 and different subspecialist consultations may be required for each specific organ toxicity.10 The National Comprehensive Cancer Network (NCCN) currently recommends subspecialty consultation for (1) grade ≥2 colitis, hepatitis, or pancreatitis; (2) grade ≥2 pneumonitis; (3) grade ≥2 renal failure; (4) moderate myositis; (5) myasthenia gravis; (6) hyperglycemia >200, primary adrenal insufficiency, or hypophysitis, (7) mild eye changes; (8) grade 3 or 4 rash; and (9) for any potential cardiac toxicity. Moreover, it is critical that irAEs be detected early18 through proper assessment and diagnostic testing, and that specialist care be coordinated rapidly to prevent irAEs from progressing to more severe grade ≥3 events. In recent years, several institutions have described the multidisciplinary team approaches they have developed for responding to irAEs.19–21 However, the effect of such approaches on healthcare utilization and patient outcomes has not yet been reported.
In addition, the molecular mechanisms driving treatment-induced toxicity are poorly understood, in part because it has been challenging to study irAEs using mouse models.22 23 An improved understanding of the mechanisms underlying irAEs development and the identification of predictive biomarkers may allow for improved management protocols and even preventive strategies for these toxicities.5 24 25 Our lack of mechanistic understanding of irAEs has led experts in the field to call for international registries and translational efforts to collect real-world data on irAEs.5
In October 2017, the Massachusetts General Hospital (MGH) altered the inpatient oncology clinical practice model to implement the Severe Immunotherapy Complications (SIC) Service—a service dedicated to caring for patients with suspected serious irAEs. This multidisciplinary effort was launched by medical oncologists who partnered with dedicated subspecialty expert consultants from 11 subspecialties to refine the clinical identification and management of irAEs. In addition to providing clinical care, the SIC Service also supports a clinical–translational research effort to study these novel toxicities. To achieve the translational goals, a standardized infrastructure was implemented that enrolls patients with suspected irAEs into studies focused on the collection of relevant clinical data and paired blood and tissue specimens.
Here, we present data on the SIC Service’s multidisciplinary clinical and translational research effort with the goals of (1) evaluating this intervention’s effect on healthcare utilization and outcomes for patients experiencing irAEs; and (2) determining the feasibility of using a central infrastructure to collect blood and tissue samples to study the mechanisms responsible for irAEs across toxicity types.
Methods
Study population
Using pharmacy and hospital admission databases, a list of patients was identified who received ICI (ipilimumab, pembrolizumab, nivolumab, atezolizumab, durvalumab, avelumab, or any ICI combination) for a malignancy and were hospitalized at MGH between April 2, 2016 and October 24, 2018. The start date, April 2, 2016, was selected to maintain consistency of data storage as that was the implementation date of the new EPIC electronic medical record at MGH. The SIC Service was established on October 3, 2017 and patients whose admission and discharge dates spanned this date were excluded from analysis. To ensure at least a 1-year follow-up, October 24, 2018 was chosen as the end date. The ‘pre-SIC period’ was therefore defined as an admission between April 2, 2016 through October 3, 2017 (before SIC Service implementation), and the ‘post-SIC period’ was defined as an admission from October 3, 2017 through October 24, 2018 (after SIC service implementation).
Data collection
Admissions underwent a two-stage review process where each hospitalization was first screened for the presence of a potential irAE based on documentation in the electronic health record. Subsequently, specialists (allergy: JRF; cardiology: TGN, DZ; dermatology: STC; endocrinology: ATF, MR; gastroenterology/hepatology: MD, MFT; hematology: RSKL; nephrology: MES; neurology: ACG; pulmonology: DO, BDM; rheumatology: MN, MK, SS) followed published organ-specific diagnostic criteria to identify cases of suspected or confirmed irAEs admitted to the hospital.26 In patients with multiple confirmed toxicities, the primary irAE was defined as that which prompted hospitalization and/or determined treatment.
SIC Service intervention
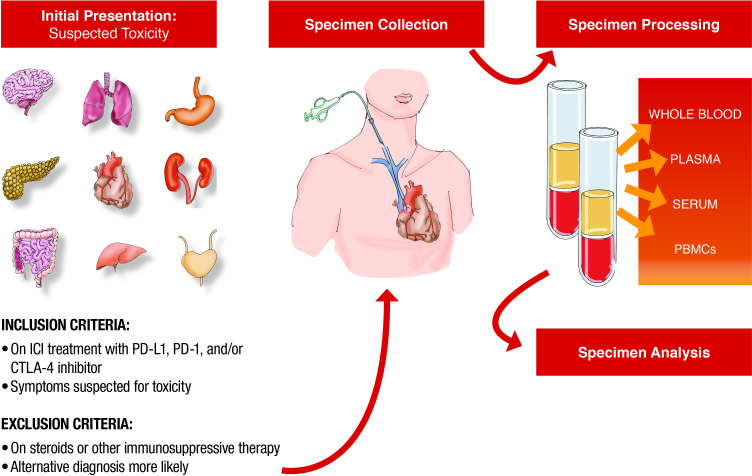
On October 3, 2017, the SIC Service was established to care for patients hospitalized with severe irAEs. All patients who present to MGH with suspected irAEs are evaluated by 1 of 12 SIC Service oncologists with expertise in irAEs. SIC oncologists coordinate patient care with ward services and expert subspecialists that belong to the broader SIC Service care team and represent providers in allergy/immunology, cardiology, dermatology, endocrinology, gastroenterology, hematology, nephrology, neurology, ophthalmology, pulmonology, and rheumatology. Since the time the SIC Service began, efforts have been made to inform all emergency department providers, hospitalists, trainees, internal medicinephysicians, and MGH Cancer Center providers of this team of subspecialists with irAE expertise so that patients with suspected toxicity can be referred accordingly. All admitted patients are then captured by an electronic report generated every morning that identifies all patients treated with ICIs admitted to any floor of the hospital. Every morning, these patients’ charts are reviewed for suspected toxicity by a nurse practitioner or an oncologist and added to the SIC Service list if there is clinical suspicion of toxicity (figure 1). On discharge, patients are scheduled for outpatient follow-up with the disease-specific subspecialist, when appropriate, as well as the oncologist. Finally, referrals to outpatient subspecialty clinics are streamlined to permit urgent evaluation with irAEs experts in order to avoid hospitalization when possible.
Figure 1.
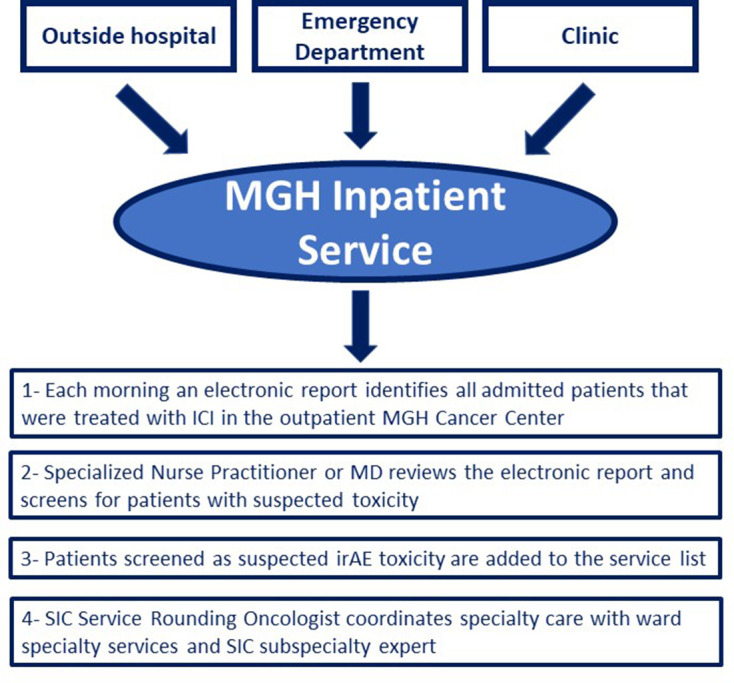
Patient identification for Severe Immunotherapy Complications (SIC) Service. ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; MD, Doctor of Medicine; MGH, Massachusetts General Hospital.
Translational research program
Under the SIC Service’s translational research infrastructure, patients with suspected irAEs are identified, communicated to research staff, and consented for blood and tissue collection to occur alongside preplanned diagnostic or therapeutic procedures (figure 2). For a subset of patients, sequential specimens are collected at various points during their toxicity course including at the time of presentation before initiation of immunosuppressive therapy, diagnosis, after immunosuppression initiation and/or escalation, and at time of irAE resolution or recurrence. In specific cases of irAE, the SIC Service partners with the MGH Rapid Autopsy program (principal investigator: DJ) to collect blood as well as tissue specimens immediately after death from tumor and all involved irAE sites. All specimens are immediately couriered to the laboratory for processing and storage on the day of collection.
Figure 2.
Standard operation procedure for sample collection and processing. CTLA-4, cytotoxic T-lymphocyte antigen-4; PBMCs, peripheral blood mononuclear cells; PD-1, programmed death-1; PD-L1, programmed death-ligand 1.
Primary and secondary outcomes
The primary outcome in the study was the rate of hospital readmissions for irAEs in the pre-SIC and post-SIC periods. Secondary outcomes included length of stay (LOS) for both initial irAE admissions and readmissions, use of corticosteroids and non-steroidal second-line immunosuppression, ICI discontinuation, and inpatient mortality in the pre-SIC and post-SIC periods.
Statistical analysis
Statistical analyses were performed using Stata, V.15.0 (StataCorp). Descriptive statistics with unpaired t-test for continuous data and Pearson χ2 test for categorical data were used for comparison of baseline characteristics. Multivariable linear and logistic regressions were used to analyze primary and secondary outcomes. Modeling covariates included age, sex, irAE confirmation status, malignancy, ICI class, primary toxicity type, and presence of multiple toxicities. P<0.05 was considered statistically significant.
Results
From April 2, 2016 through October 3, 2017 (18-month ‘pre-SIC’ study period), 1169 patients were treated with ICIs at our institution and 127 patients were hospitalized for irAEs. From October 3, 2017 through October 24, 2018 (12-month ‘post-SIC’ study period), our institution treated 1159 patients with ICIs and 122 patients were hospitalized for irAEs. There were no statistically significant differences in baseline characteristics of patients admitted for irAEs before and after SIC Service implementation (table 1).
Table 1.
Characteristics of patients admitted for irAE before and after SIC Service implementation
| Characteristic | Pre-SIC* | Post-SIC† | P value‡ |
| (n=127 patients) | (n=122 patients) | ||
| Age, mean (SD), years | 62.6 (13.9) | 64.6 (11.1) | 0.216 |
| Female sex | 44 (34.7%) | 55 (45.1%) | 0.093 |
| Cancer type | |||
| Melanoma | 48 (37.8%) | 31 (25.4%) | 0.156 |
| Thoracic | 35 (27.6%) | 38 (31.2%) | |
| Gastrointestinal | 14 (11.0%) | 26. (21.3%) | |
| Genitourinary | 8 (6.3%) | 9 (7.4%) | |
| Hematologic | 3 (2.4%) | 7 (5.7%) | |
| Gynecologic | 5 (3.9%) | 3 (2.5%) | |
| Head and neck | 5 (3.9%) | 2 (1.6%) | |
| Neurologic | 3 (2.4%) | 4 (3.3%) | |
| Breast | 5 (3.9%) | 2 (1.6%) | |
| Sarcoma | 1 (0.8%) | 0 | |
| ICI type | |||
| CTLA-4 | 9 (7.1%) | 3 (2.5%) | 0.147 |
| PD-1 | 84 (66.1%) | 92 (75.4%) | |
| PD-L1 | 8 (6.3%) | 10 (8.2%) | |
| CTLA-4+PD-1 | 26 (20.5%) | 17 (13.9%) | |
| irAE type | |||
| Allergy | 3 (2.4%) | 1 (0.8%) | 0.311 |
| Cardiac | 9 (7.1%) | 11 (9.0%) | |
| Dermatologic | 9 (7.1%) | 3 (2.5%) | |
| Endocrine | 15 (11.8%) | 13 (10.7%) | |
| Gastrointestinal | 28 (22.1%) | 20 (16.4%) | |
| Hepatic | 20 (15.8%) | 23 (18.9%) | |
| Hematologic | 4 (3.2%) | 2 (1.6%) | |
| Neurologic | 10 (7.9%) | 14 (11.5%) | |
| Pulmonary | 26 (20.5%) | 26 (21.3%) | |
| Renal | 1 (0.8%) | 7 (5.7%) | |
| Rheumatologic | 2 (1.6%) | 2 (1.6%) |
*Data are presented as number (percentage) of patients, unless otherwise indicated; pre-SIC date range is April 2, 2016–October 2, 2017.
†Data are presented as number (percentage) of patients, unless otherwise indicated; post-SIC date range is October 3, 2017–October 24, 2018.
‡Unpaired t-test for continuous data; Pearson χ2 test for categorical data.
CTLA-4, cytotoxic T-lymphocyte antigen-4; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; SIC, Severe Immunotherapy Complications.
The majority of admitted patients both pre-SIC and post-SIC had melanoma, a thoracic malignancy, or a GI malignancy and were treated with either anti-PD-1 monotherapy or combination anti-CTLA-4 and anti-PD-1 therapy. There was a diverse mix of primary toxicity types leading to admission before and after SIC service initiation, with pulmonary, GI, and hepatic irAEs representing the most common toxicities (table 1).
Impact of SIC Service on the primary and secondary outcomes
Critical outcomes data after SIC Service implementation are shown in table 2.
Table 2.
Impact of SIC Service implementation on key outcomes—logistic regressions
| Outcome | Pre-SIC* | Post-SIC† | Coefficient/OR (95% CI)‡ | P value |
| (n=166 admits) | (n=149 admits) | |||
| Length of stay, median (IQR), days | 5.5 (3–11) | 5 (3–9) | −1.7 (−3.56 to 0.19)§ | 0.078 |
| Discharged on corticosteroids¶ | 121 (75.6%) | 96 (69.1%) | 0.60 (0.33 to 1.10)** | 0.101 |
| Use of non-steroidal immunosuppression | 24 (14.5%) | 18 (12.1%) | 0.87 (0.43 to 1.77)** | 0.702 |
| ICI discontinuation for irAE†† | 74 (66.1%) | 61 (67.0%) | 1.04 (0.55 to 1.98)** | 0.897 |
| Died during irAE admission | 11 (6.6%) | 13 (8.7%) | 1.46 (0.60 to 3.55)** | 0.398 |
| IrAE readmission | 43 (25.9) | 22 (14.8) | 0.46 (0.22 to 0.95)‡‡ | 0.036 |
| Length of stay of irAE readmission, median (IQR), days | 7 (3–16) | 6 (3–10) | −8.08 (−16.03 to 0.14)§ | 0.046 |
Bold values are statistically significant,
*Data are presented as number (percentage) of admissions, unless otherwise indicated; pre-SIC date range is April 2, 2016–October 2, 2017.
†Data are presented as number (percentage) of admissions, unless otherwise indicated; post-SIC date range is October 3, 2017–October 24, 2018.
‡Data are presented as coefficient (95% CI) for linear regressions (continuous variables) and OR (95% CI) for logistic regressions (categorical variables).
§Multivariable linear regression with covariates: age, sex, irAE confirmation status, malignancy, ICI class and primary toxicity type.
¶Excludes patients with thyroid toxicities or diabetes mellitus (given steroids are not indicated) as the irAE. Pre-SIC n=160; post-SIC n=139.
**Multivariable logistic regression with covariates: age, sex, irAE confirmation status, malignancy, ICI class, primary toxicity type and presence of multiple toxicities.
††Excludes patients with endocrine toxicities (given ICI discontinuation is not indicated) as well as patients who previously discontinued ICI prior to admission or discontinued ICI for any non-irAE reason (disease progression). Pre-SIC n=112; post-SIC n=91.
‡‡Multivariable logistic regression with covariates: age, sex, irAE confirmation status, malignancy, ICI class, primary toxicity type, presence of multiple toxicities and ICI discontinuation for toxicity.
ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; SIC, Severe Immunotherapy Complications.
In multivariable modeling, SIC Service implementation was associated with a significant reduction in irAE readmission rates (post-SIC 14.8% vs pre-SIC 25.9%; OR 0.46; 95% CI 0.22 to 0.95; p=0.036) and shorter LOS than pre-SIC readmissions (post-SIC median 6 days vs pre-SIC median 7 days; 95% CI −16.03 to –0.14; p=0.046). We observed a trend toward lower LOS (post-SIC 5 days vs pre-SIC 5.5 days (p=0.078)) but this was not statistically significant.
Overall rates of corticosteroid use, second-line immunosuppression, and ICI discontinuation for irAE, as well as inpatient mortality rates, were not significantly different before and after SIC Service implementation (table 2). A second analysis analyzing the data by first admission only also revealed no significant difference before and after SIC Service implementation in LOS (p=0.758), discharged on steroids (p=0.141), use of non-steroidal immunosuppression (p=0.878), ICI discontinuation for irAE (p=0.526), or inpatient mortality (p=0.361) indicating first admission acuity was similar among the two groups (online supplemental table 1).
jitc-2021-002886supp001.pdf (87KB, pdf)
Specimen collection
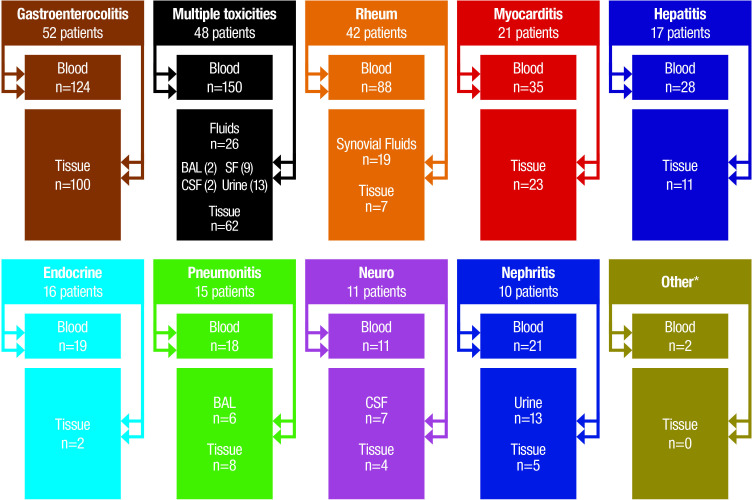
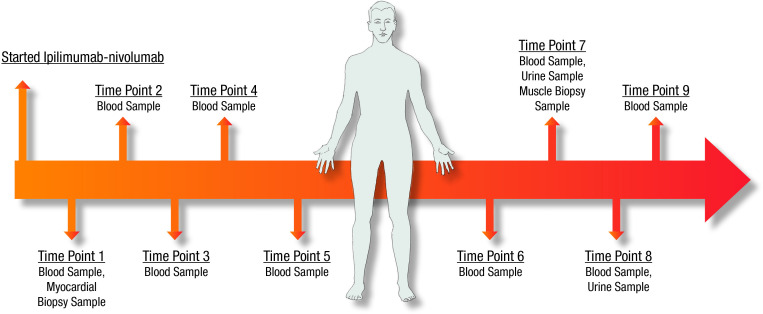
The sample collection effort began on January 1, 2018. All samples were collected after informed consent from each participant and only when performing clinically indicated diagnostic procedures or at the time of autopsy. The first sample was collected on January 12, 2018. From that date until December 28, 2019, a total of 789 samples were collected from 234 patients with suspected irAEs post-SIC. These samples include 496 blood specimens, 71 bodily fluids (bronchoalveolar lavage n=8, cerebrospinal fluid n=9, synovial fluid n=28, urine n=26) and 222 tissue samples collected during routine care (myocardial, liver, muscle, kidney and GI biopsies, as well as brain, lung and endocrine organ samples from autopsy) (figure 3). Specimens were either processed immediately on arrival to the lab (analysis ongoing), or frozen for future processing and analysis. An illustrative case example is a 53-year-old man with renal cell carcinoma treated with ipilimumab/nivolumab for two cycles who developed presumed ICI-related hepatitis requiring high-dose steroids. The hepatitis resolved and he was re-challenged with nivolumab for eight doses with a course complicated by hypothyroidism, myocarditis with congestive heart failure, and acute kidney injury with significant proteinuria. Nine blood, two urine, and two tissue samples (myocardium, muscle) were collected over the course of his illness (figure 4).
Figure 3.
Number of patient samples (blood, bodily fluids, tissues) collected. *Other=one case of hematological toxicity and one case of pancreatitis. BAL, bronchoalveolar lavage; CSF, cerebrospinal fluid.
Figure 4.
Serial blood and tissue samples in an irAE patient with multiple toxicities. irAE, immune-related adverse event.
Discussion
In this study, we evaluate the impact of altering a clinical practice model to care for patients with irAEs and creating a clinical–translational research model to study these events. Other services with multidisciplinary toxicity teams include Johns Hopkins University School of Medicine where an electronic referral system for immune-related toxicities was shown to be feasible and helpful to identify patterns of irAEs requiring subspecialist care.19 Dana-Farber Cancer Institute/Brigham Women’s Hospital has initiated a service through which they admitted 194 irAE patients in the first year and found the most common reasons for admission were colitis, hepatitis, and pneumonitis.27 To date, feasibility of these toxicity services has been demonstrated but evidence about the impact pre/post-implementation has not been reported.
Of note, our irAE admission rate of 10%–11% pre/post-SIC intervention is lower than the 41% reported for suspected irAEs and 23% rate for confirmed irAEs in another recent study, conducted at a major academic medical center over a much shorter 7-month period.28 This suggests that although a percentage of irAEs hospitalizations can be avoided, around 10% of patients receiving ICIs develop irAEs that inevitably end in hospitalization.
Importantly, the 6-month irAE readmission rate at our hospital decreased from 25.9% of patients to 14.8% after controlling for age, sex, irAE confirmation status, type of cancer, type of ICI regimen used, and primary toxicity type. Readmissions reduction is critically important as it is an opportunity to improve patient care and satisfaction, improve quality, and decrease healthcare costs. One potential explanation for the decrease in readmissions may have been the comprehensive inpatient care by SIC experts and consultants, the streamlined transition on discharge to outpatient care, and the network of outpatient subspecialists who provide significant continuity.
In addition, the post-SIC Service LOS for irAE readmissions also decreased from 7 to 6 days in comparison with the pre-SIC study period, a key finding which has implications for patients and healthcare systems. These findings are particularly important as readmission rates are commonly used measures for quality of care; it is estimated that Medicare spends $17 billion a year on avoidable readmissions, and in recent years, the US Hospital Readmissions Reduction Program has begun penalizing hospitals with high 30-day readmission rates.29 Thus, if a dedicated SIC Service can significantly improve these quality of care measures, it could result in meaningful gains for both patients and hospitals.
After instituting the SIC Service, the mortality rate for patients hospitalized for irAEs did not significantly change. Our pre-SIC mortality rate of 6.6% (over 12 months) and post-SIC mortality rate of 8.7% (over 18 months) are similar to the 7.3% mortality rate reported in a 7-month irAE study conducted by another major academic medical center where clinical outcomes on patients hospitalized for irAEs were also analyzed.28 Further steps to decrease inpatient mortality relating to irAEs likely await new developments in the treatment for toxicity.
Importantly, the data presented indicate SIC Service implementation was not associated with any negative outcomes despite integrating research blood and tissue collection into routine care. There were numerous samples collected but no sign of detrimental impact on clinical care—no difference in LOS, inpatient mortality, or readmission rate. Therefore, this study demonstrates safety and feasibility of collecting a wide range of biological samples from patients with irAE on an inpatient service. These specimens are being used for an ongoing effort to investigate predictive blood biomarkers for irAEs and identify mechanisms of irAE development. In this study, 789 blood and tissue samples were collected from 234 patients who developed irAEs in the first 2 years of the service. We found that the effort required to obtain timely patient consent for study participation and to collect the samples prior to steroids was considerable. Staff had to overcome a number of challenges including (1) seriously ill patients spread over 15 different floors of the hospital with staff often unfamiliar with the protocol; (2) patients who were often absent from their rooms to undergo imaging studies or procedures; (3) language barriers; (4) difficulty in obtaining blood; (5) delays and frequent rescheduling of inpatient procedures; (6) insufficient tissue quantity of specimen remaining for research after the necessary clinical samples were obtained; and (7) access to rapid and adequate processing in a timely fashion. Thus, if the oncology research community is to respond to the call for biomarker-based studies of irAE mechanisms and identification of potential biomarkers to detect patients at high risk of irAE, funding along with dedicated and coordinated services, or a central infrastructure, will likely be needed in order to scale efforts sufficiently.5 30 In order to propel this type of translational discovery, it will take a multi-institutional effort to pool samples in order to (1) uncover set of predictive factors for irAEs; (2) understand early mechanisms driving irAEs; (3) identify novel drug targets; and (4) develop better therapeutic strategies. This study is the first to report this type of effort is feasible and safe, and can be embedded in clinical care.
This study has several important limitations. First, it describes observations at a single institution and therefore results may not be representative of other institutions. Second, because our institution is an academic medical center housed within a general hospital, we were able to recruit numerous subspecialists focused on autoimmunity with an interest in irAEs across the spectrum; this may limit the study’s generalizability. Third, to ensure accuracy, in this study we only included data from patients who received both ICI therapy and irAE care at our institution; thus, our results do not reflect the experience of patients transferred to our hospital for irAE care, who tend to be in more critical condition, or patients who were treated with ICIs at our institution but hospitalized for irAEs elsewhere. Finally, a general improvement in knowledge and experience over time in managing irAEs may have led to the observed differences in the pre-SIC and post-SIC groups. During the time period of this study, new sets of guidelines from the American Society of Clinical Oncology and NCCN emerged. Therefore, we cannot exclude that increased irAE awareness and management skills among care providers, independent of the SIC service, affected the readmission rate and LOS.
In conclusion, this study is, to the best of our knowledge, the first to report that establishing a highly subspecialized care team focused on irAEs can be associated with improved clinical outcomes for patients receiving ICI therapy, while also building the infrastructure needed to drive future clinical research on ICI toxicities. Such care teams are likely to serve as a model and may play an essential role in improving irAE care: defining phenotypes, identifying diagnostics for early detection, developing biomarkers to assess irAE severity, and generating preliminary data to guide next-generation clinical trials for the treatment of irAEs. In addition, maintaining a central registry of patients, fostering a collegial group dynamic, having regular meetings, and a focus on collaboration across subspecialties and oncology have the potential to foster that future discovery. These teams will also play a key role in bringing these advances back to the bedside to benefit patients.
Acknowledgments
LZ would like to acknowledge the Spanish Society of Medical Oncology (SEOM; Sociedad Española de Oncología Médica), her grant for a 2-year translational project at the Massachusetts General Hospital (MGH) Cancer Center.
Footnotes
LZ and GEM contributed equally.
A-CV and KLR contributed equally.
Correction notice: This article has been corrected since it was first published. Author name has been corrected to '
Rebecca K Leaf'.
Contributors: LZ and GEM devised the project idea, acquired and analyzed data, performed statistical analysis, and wrote the manuscript. MJM, JC, SD, LP, GMB, DJ, MD, MFT, ATF, MR, ACG, STC, DO, BDM, MN, MK, SS, RKL, MES, TGN, DZ, JRF, AB, RS, SB, YRS and A-CV provided critical intellectual input to study design and analysis and edited the manuscript. KLR created the registry of immunotherapy toxicity cases and samples, devised the project idea, designed the study, and supervised the statistical analysis and manuscript development.
Funding: This work was supported by the Spanish Society of Medical Oncology (SEOM; grant for a 2-year translational project at the MGH Cancer Center to LZ).
Competing interests: LZ—Merck consultant. JC—consultant for BMS and Sanofi-Genzyme. DJ—scientific advisory board fee from Eisai, EMD Serono, Genentech, Ipsen, Novartis, Guardant, Petra Pharma, Mapkure, Vibliome Therapeutics, and Relay Therapeutics; institutional research funds from Novartis, Genentech, EMD Serono, Eisai, Takeda, Placon Therapeutics, Syros, Ribon Therapeutics, Infinity Pharmaceuticals, InventisBio and Amgen. GMB—sponsored research agreements with Palleon Therapeutics and Olink Proteomics; scientific advisory board for Novartis and Nektar Therapeutics; consulting for Merck. MD—consultant for Moderna, Tillotts Pharma, ORIC Pharmaceuticals, and Partner Therapeutics; research funding from Novartis and Eli Lilly; scientific advisory board for Neoleukin Therapeutics. ACG—consultant for Momenta, Alexion, UCB/Ra pharma; royalties from Oakstone Publishing; research funding from Myasthenia Gravis Foundation of America, MGNET and Project Datasphere. BDM—consultant for Sanofi, AbbVie, and Celestial Pharmaceuticals. He has also received sponsored research agreements from Boehringer-Ingelheim, Bayer, and Bristol-Myers Squib. MK—consultant for Novartis and Mymee; speaker fees for Lilly. TGN—advisory fees from Parexel, BMS, H3 Biomedicine, AbbVie, and Intrinsic Imaging; grant support from AstraZeneca. JRF—research grants from Bristol Myers Squibb and X4 Pharmaceuticals. MJM—received honarium/acted as a consultant for AstraZeneca, Nektar Therapeutics, Catalyst Pharmaceuticals, Immunai Aditya Bardia; consulting or advisory role for bioTheranostics, Genentech, Genentech/Roche (Inst), Immunomedics, Immunomedics (Inst), Innocrin Pharma (Inst), Merck, Merck (Inst), Novartis, Novartis (Inst), Pfizer, Pfizer (Inst), Radius Health (Inst), Radius Pharma, Sanofi, Spectrum Pharmaceuticals; research funding from bioTheranostics. RS—consultant/scientific advisory board member for AstraZeneca, Bristol-Myers Squibb, Eisai, Iovance, Merck, Novartis, OncoSec, Pfizer, Replimune; research funding from Merck and Amgen. SB—consultant for Third Rock Consulting and Two River Consulting; equity holdings in Kronos Bio and Allogene Therapeutics. YRS—consultant for Castle Biosciences.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This cohort study conducted at MGH was approved by the Massachusetts General Brigham Institutional Review Board (#2017P000501).
References
- 1.Vaddepally RK, Kharel P, Pandey R, et al. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers 2020;12:738. 10.3390/cancers12030738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haslam A, Gill J, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open 2020;3:e200423. 10.1001/jamanetworkopen.2020.0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin S, Xu L, Yi M, et al. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer 2019;18:155. 10.1186/s12943-019-1091-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xin Yu J, Hodge JP, Oliva C, et al. Trends in clinical development for PD-1/PD-L1 inhibitors. Nat Rev Drug Discov 2020;19:163–4. 10.1038/d41573-019-00182-w [DOI] [PubMed] [Google Scholar]
- 5.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 6.Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers 2020;6:38. 10.1038/s41572-020-0160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haanen JBAG, Carbonnel F, Robert C, et al. Corrections to “Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann of Oncology 2018;29:iv264–6. 10.1093/annonc/mdy162 [DOI] [PubMed] [Google Scholar]
- 8.Arnaud-Coffin P, Maillet D, Gan HK, et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer 2019;145:639–48. 10.1002/ijc.32132 [DOI] [PubMed] [Google Scholar]
- 9.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network . Management of Immunotherapy-Related toxicities (version 2.2021). Available: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf [DOI] [PubMed]
- 11.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for immunotherapy of cancer (SITC) toxicity management Working group. J Immunother Cancer 2017;5:95. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883–95. 10.1016/S1470-2045(16)30098-5 [DOI] [PubMed] [Google Scholar]
- 13.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–17. 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med Overseas Ed 2019;381:1535–46. 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 15.Wang DY, Salem J-E, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721–8. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson DB, Reynolds KL, Sullivan RJ, et al. Immune checkpoint inhibitor toxicities: systems-based approaches to improve patient care and research. Lancet Oncol 2020;21:e398–404. 10.1016/S1470-2045(20)30107-8 [DOI] [PubMed] [Google Scholar]
- 17.Cole S, Zibelman M, Bertino E, et al. Managing immuno-oncology toxicity: top 10 innovative institutional solutions. Am Soc Clin Oncol Educ Book 2019;39:96–104. 10.1200/EDBK_100018 [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Zlotoff DA, Awadalla M, et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation 2020;141:2031–4. 10.1161/CIRCULATIONAHA.119.044703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naidoo J, Zhang J, Lipson EJ, et al. A multidisciplinary toxicity team for cancer Immunotherapy–Related adverse events. J Natl Compr Canc Netw 2019;17:712–20. 10.6004/jnccn.2018.7268 [DOI] [PubMed] [Google Scholar]
- 20.Läubli H, Dirnhofer S, Zippelius A. Immune tumor board: integral part in the multidisciplinary management of cancer patients treated with cancer immunotherapy. Virchows Archiv 2019;474:485–95. 10.1007/s00428-018-2435-9 [DOI] [PubMed] [Google Scholar]
- 21.Michot J-M, Lappara A, Le Pavec J, et al. The 2016–2019 ImmunoTOX assessment board report of collaborative management of immune-related adverse events, an observational clinical study. Eur J Cancer 2020;130:39–50. 10.1016/j.ejca.2020.02.010 [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Blake SJ, Smyth MJ, et al. Improved mouse models to assess tumour immunity and irAEs after combination cancer immunotherapies. Clin Trans Immunol 2014;3:e22. 10.1038/cti.2014.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson B, Li Y, Lin Y, et al. Mouse models for cancer immunotherapy research. Cancer Discov 2018;8:1358–65. 10.1158/2159-8290.CD-18-0044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esfahani K, Elkrief A, Calabrese C, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol 2020;17:504–15. 10.1038/s41571-020-0352-8 [DOI] [PubMed] [Google Scholar]
- 25.Weinmann SC, Pisetsky DS. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology 2019;58:vii59–67. 10.1093/rheumatology/kez308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molina GE, Zubiri L, Cohen JV, et al. Temporal trends and outcomes among patients admitted for immune-related adverse events: a single-center retrospective cohort study from 2011 to 2018. Oncologist 2021;26:514–22. 10.1002/onco.13740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abu-Shawer O, Singh P, Yenulevich E, et al. Novel platform Leveraging electronic medical record (EMR) to triage patients admitted with high-grade immune-related adverse events (irAEs) to the immune-toxicity (ITOX) service. J Immunother Cancer 2020;8:e000992. 10.1136/jitc-2020-000992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balaji A, Zhang J, Wills B, et al. Immune-related adverse events requiring hospitalization: spectrum of toxicity, treatment, and outcomes. J Oncol Pract 2019;15:e825–34. 10.1200/JOP.18.00703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boozary AS, Manchin J, Wicker RF. The Medicare Hospital readmissions reduction program: time for reform. JAMA 2015;314:347–8. 10.1001/jama.2015.6507 [DOI] [PubMed] [Google Scholar]
- 30.Zubiri L, Allen IM, Taylor MS, et al. Immune-Related adverse events in the setting of PD-1/L1 inhibitor combination therapy. Oncologist 2020;25:e398–404. 10.1634/theoncologist.2018-0883 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-002886supp001.pdf (87KB, pdf)
Data Availability Statement
Data are available upon reasonable request.