Abstract
Islet transplantation has been shown to restore normoglycemia clinically. One of the current limitations to the widespread clinical use of islet transplantation is culturing and preserving more than 1 million islet equivalents in preparation for transplant. One possible solution is to bank frozen islets and use them when needed. Although promising, the standard islet freezing protocol introduces stress and cell death, resulting in high variability of islet quality post thawing. This study aimed to develop an improved cryopreservation protocol using alginate-encapsulated islets to improve islet survival and function for future transplants. Our data showed that encapsulation improved islet survival and function after thawing the frozen islets. Frozen encapsulated islets have an islet yield recovery of 84% when compared to non-encapsulated islets at 72% after thawing. Post-thaw viability was 78% for non-encapsulated islets compared to 88% for encapsulated islets. The stimulation index values after a static glucose test following thawing were 1.9 ± 0.5, 2.9 ± 0.1, and 3.3 ± 0.3 for the non-encapsulated, 1.75% alginate, and 2.5% alginate groups, respectively. In a transplant study, the mice that received 1.75% alginate-encapsulated cryopreserved islets achieved normoglycemia on average 5 days after transplant. In comparison, control mice that received fresh islets took 4 days, while those receiving unencapsulated cryopreserved islets took 18 days. In conclusion, encapsulating islets in 1.75% alginate prior to freezing was shown to improve islet survival, function post thawing, and graft response significantly when compared to islets frozen without encapsulation.
Keywords: islet, alginate, diabetes, encapsulation, cryopreservation
Introduction
While effective islet transplantation has been available since 2000 with the development of the Edmonton protocol for islet isolation and transplantation1, it is still hampered by a lack of deceased donor pancreas suitable for islet isolation and transplant. One method to increase the availability of pancreatic islets is to incorporate cryogenic banking of islets from multiple donors2. Cryobanking has also been reported to reduce the immunogenicity of islets3,4, thus increasing the potential for graft survival.
While the Edmonton protocol currently requires immunosuppression to delay the onset of islet rejection, the immunosuppression regimen itself is toxic to the islets. As such, alginate encapsulation has been studied as a physical alternative to immunosuppression to prevent transplant rejection5. In this study, we evaluated the potential to freeze islets within the alginate capsule to maintain their structural integrity, as well as providing a convenient method for banking islets for transplantation.
Materials and Methods
Ethical Compliance
All animal procedures in this study were conducted in accordance to the Institutional Animal Care and Use Committee at University of California Irvine approved protocols AUP-17-129 and AUP-17-241.
Islet Isolation
Sprague-Dawley rats (Envigo Harlan, Houston, TX, USA) were used as the islet donor. Islet isolation was done using standard collagenase digestion and gradient purification5. After isolation, the islets were cultured in CMRL-1066 (Mediatech, Manassas, VA, USA) supplemented with 10% newborn calf serum (Equitech Bio, Kerrville, TX, USA) and 1% penicillin/streptomycin (Fisher Scientific, Hampton, NH, USA) in a 37°C 5% CO2 incubator.
Alginate Encapsulation
Islets were encapsulated in either 1.75% or 2.5% ultrapure low viscosity mannuronate (UPLVM; Novamatrix, Sandvika, Norway) alginate using a gas-driven electrostatic encapsulator (Nisco Engineering AG, Zurich, Switzerland), as previously described5 (Fig. 1). Alginate-encapsulated islets were on average about 400 µm in diameter (Fig. 2).
Fig. 1.

Alginate encapsulation system. The setup for the gas-driven electrostatic encapsulator used to create the alginate-encapsulated islets.
Fig. 2.

Alginate-encapsulated islets. A representative picture was taken of the Sprague-Dawley rat islets after encapsulation in 2.5% ultrapure low viscosity mannuronate alginate, prior to the cryopreservation process. Scale = 1,000 µm.
Cryopreservation and Recovery
Aliquots of 500 free or encapsulated islets were cryopreserved for this study, with each group done in 5 replicates. The cryopreservation process could be broken down into 3 steps: (1) the pre-freeze; (2) freezing, storage, and thawing; and (3) returning the islets to their physiological medium, as previously described by Rajotte et al.2. The free islets and 2 groups of encapsulated islets were first suspended at 22°C in 0.1 mL 2 M dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO, USA). After 5 min, 0.1 mL 2 M DMSO was added, and after 25 min at 0°C, 0.4 mL 3 M DMSO was added in a stepwise fashion so the islets could equilibrate with the cryoprotectant. After the stepwise addition of DMSO, the tubes of islets were transferred to a –7.5°C ethanol bath for 5 min to nucleate the samples and ensure uniform cooling. Touching the outside of the tube with a pre-frozen metal rod allows the nucleation step to occur, releasing the latent heat of fusion uniformly. Next, the samples were transferred to an evacuated freezing Dewar (Fisher Scientific, Hampton, NH, USA), which was set to cool the sample at 0.25°C/min, from –7.5°C to –40°C. Once at –40°C, the samples are submerged in liquid nitrogen until they reach –196°C. The islets were stored in the vapor phase of a liquid nitrogen Dewar for 4 weeks, after which they were thawed at a rate of 200°C/min using a 37°C water bath. The DMSO was stepwise removed at 22°C using increasing volumes of 0.75 M sucrose (Sigma, St. Louis, MO, USA)2. After thawing was completed, islets or encapsulated islets were cultured in CMRL-1066 (Mediatech, Manassas, VA, USA) supplemented with 10% newborn calf serum (Equitech Bio) and 1% penicillin/streptomycin (Fisher Scientific, Hampton, NH, USA) in a 37°C 5% CO2 incubator for 48 h.
Islet Counting
Islet counting was done using dithizone (Fisher Scientific, Hampton, NH, USA) staining, as previously described6. Counting was performed prior to freezing, after thawing, and 48 h after recovery culture post thawing.
Islet Viability
Islet viability was measured using fluorescein diacetate (Fisher Scientific, Hampton, NH, USA) and propidium iodide, Calcein AM, and 4′,6-diamidino-2-phenylindole (Fisher Scientific, Hampton, NH, USA) staining, as previously described7. Viability was measured prior to freezing, after thawing, and 48 h after recovery culture post thawing.
Islet Functional Testing
Glucose-stimulated insulin release (GSIR) was performed to test islet insulin secretory function in response to glucose stimuli, as previously described7. Insulin concentration was measured using insulin enzyme-linked immunosorbent assay kits (Mercodia AB, Uppsala, Sweden). GSIR was done prior to freezing, after thawing, and 48 h after recovery culture post thawing. Islet function was calculated as the stimulation index (SI)—the ratio of insulin concentration after high-glucose stimulation divided by that after low-glucose stimulation.
Animal Studies
A total of 20 athymic nude mice (Charles River, San Diego, CA, USA), 9 wk old, were used for this study. The mice were made diabetic using streptozotocin (STZ; Teva Parenteral Medicines, Irvine, CA, USA). Blood glucose was measured in all mice using a tail capillary prick (Bayer, Parsippany, NJ, USA). The blood glucose was measured in all 3 mice groups 10 days before (day –10), 5 days before (day –5), and on the day of (day 0) the STZ injection. Blood glucose levels were then monitored every day for the first week after STZ injection. After confirmation of diabetes (3 consecutive daily non-fasting blood glucose >300 mg/dL), the mice received a transplant of 1,000 IE of freshly isolated islets (in kidney capsule), cryopreserved islets (in kidney capsule), and alginate-encapsulated cryopreserved islets (intraperitoneally due to the large size). Two units of long-acting insulin (Lantus; Sanofi-Aventis, Bridgewater, NJ, USA) was administered on the day of the transplant to prevent hyperglycemic shock on the freshly transplanted islets. The blood glucose levels were monitored and recorded every day from day 10 to day 21, and then every other day from day 23 to day 50. Lastly, the islets or islet capsules were explanted on day 50. We defined normoglycemia is defined as non-fasting blood glucose levels <200 mg/dL.
Statistical Analysis
Analysis of variance with post hoc Tukey HSD was used to determine statistical significance, with a p-value of <0.05 regarded as statistically significant. Statistical calculation was performed using Excel (Microsoft, Redmond, WA, USA).
Results
In Vitro Testing
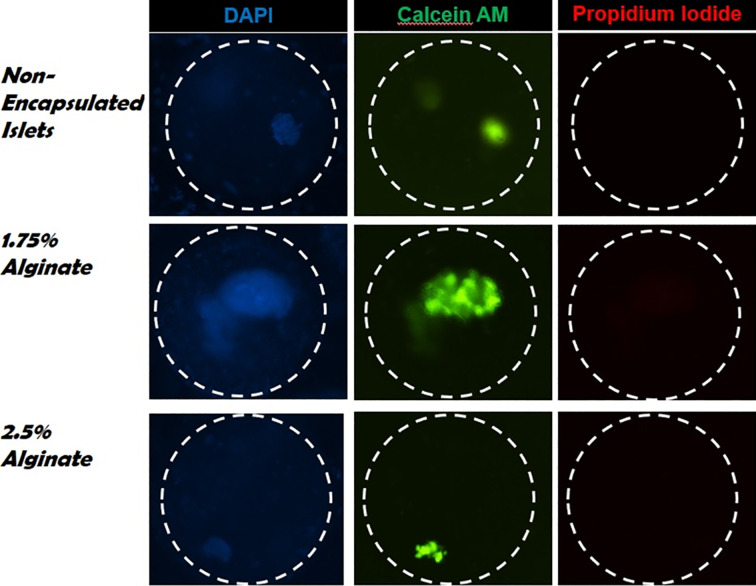
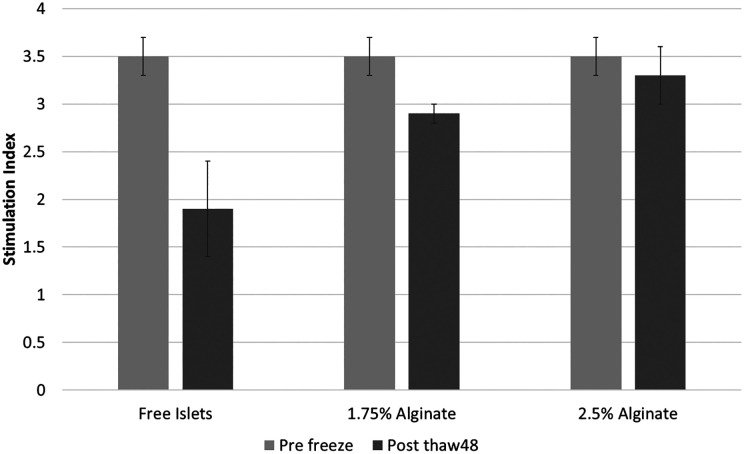
Table 1 summarizes the results of the in vitro results. The mean islet yields post cryopreservation (ratio of islet counts post thawing compared to pre freezing) were 72.6 ± 5.7%, 84.6 ± 4.1%, and 83.6 ± 3.3% for the standard CP, 1.75% alginate, and 2.5% alginate groups, respectively. After the 48 h culture, the mean yields were 48 ± 5.7%, 79 ± 1.67%, and 80.42.6% for the standard CP, 1.75% alginate, and 2.5% alginate groups, respectively (Fig. 3). Fig. 4 shows the viability percentages among the 3 groups, and Fig. 5 shows actual images of the islets. The pre-freeze, post-thaw, and post-48-h culture viability percentages for the standard CP group were 92%, 78%, and 52%, respectively. The pre-freeze, post-thaw, and post-48-h culture viability percentages for the 1.75% alginate group were 92%, 88%, and 83%, respectively. The pre-freeze, post-thaw, and post-48-h culture viability percentages for the 2.5% alginate group were 92%, 85%, and 80%, respectively. The SI values pre freeze were 3.5 ± 0.2 for all 3 groups, and 1.9 ± 0.5, 2.9 ± 0.1, and 3.3 ± 0.3 for the standard CP, 1.75% alginate, and 2.5% alginate groups, respectively (Fig. 6).
Table 1.
In Vitro Characterization of Pre-freezing, Post-thawing, and Post-recovery Culture of Cryopreserved Islets.
| Yield | Viability (IE) | Stimulation index | ||
|---|---|---|---|---|
| Free islets | Pre freeze | 100% | 92 ± 4.1% | 3.5 ± 0.2 |
| Post thaw | 72.6 ± 5.7% | 78 ± 2.4% | ||
| Post 48 h culture | 48 ± 5.7% | 52 ± 7.4% | 1.9 ± 0.5 | |
| 1.75% alginate | Post thaw | 84.6 ± 4.1% | 88 ± 2.0% | |
| Post 48 h culture | 79 ± 1.7% | 83 ± 3.7% | 2.9 ± 0.1 | |
| 2.5% alginate | Post thaw | 83.6 ± 3.3% | 85 ± 2.9% | |
| Post 48 h culture | 80.4 ± 2.6% | 80 ± 1.7% | 3.3 ± 0.3 |
Islet yield was calculated by counting dithizone staining and expressed as percentage recovery from the initial number prior to freezing. Viability was shown as a count of viable islets using fluorescein diacetate and propidium iodide staining. Stimulation index was calculated as the ratio of insulin produced after high glucose stimulation compared to low glucose stimulation of the islets. Each result represents the average value for 5 samples.
Fig. 3.
Islet percentage yield post thaw and post culture. The in vitro percentage yield of islets after the thaw compared to after the 48 h culture is depicted. Islets were stained using dithizone and counted under a microscope. The islet yield was calculated as the ratio of the islet count post thaw or post-recovery culture compared to the pre-freeze count. *p < 0.05.
Fig. 4.
Islet viability pre freeze, post thaw, and post culture. In vitro islet viability among the 3 groups including free islets, the islets encapsulated in 1.75% alginate, and the islets encapsulated in 2.5% alginate. A viability test was done using fluorescein diacetate and propidium iodide staining of the islets and measured using a fluorescence plate reader. *p < 0.05.
Fig. 5.
Images of islet viability after thawing. Non-encapsulated, 1.75%, and 2.5% encapsulated islets were cryopreserved and thawed after 4 weeks. Viability staining was done with Calcein AM (green for viable cells), propidium iodide (red for dead cells), and 4′,6-diamidino-2-phenylindole (blue as nuclear counterstain). A representative image was taken using a confocal microscope after reading the fluorescence intensity on a fluorescent plate reader.
Fig. 6.
Islet glucose-stimulated insulin release pre freeze and post culture. The islets were stimulated using low glucose (3 mM) and high glucose (28 mM). Then, the insulin concentration was measured. The stimulation index was calculated from the ratio of insulin concentration after high glucose compared to that from low glucose. *p < 0.05.
In Vivo Mice Study
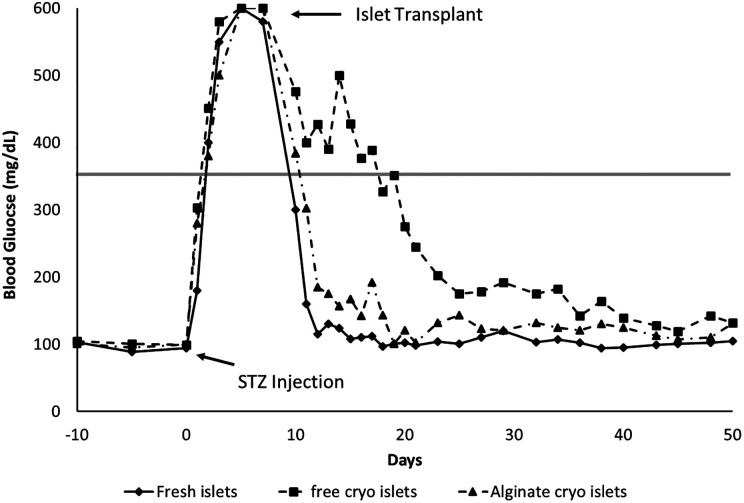
The 3 groups of mice are defined as follows: group 1, the fresh tissue transplanted mice; group 2, the cryopreserved islet transplanted mice; and group 3, the alginate-encapsulated cryopreserved mice. The blood glucose results of the 3 groups are shown in Fig. 7. There was no significant difference between the mean blood glucose values between the 3 groups of mice prior to the STZ injection. The mean blood glucose values were 95.33 ± 2.47, 101.67 ± 3.07, and 98.67 ± 2.73 mg/dL for groups 1, 2, and 3, respectively. All 3 groups received the STZ injection at day 0, and all 3 groups showed hyperglycemia beginning on day 2 until day 7 with no significant difference. Group 1 showed normoglycemia on day 11, group 2 on day 25, and group 3 on day 12. The time to normoglycemia between groups 1 and 3 was statistically significant (p < 0.01), but the differences between groups 1 and 2 and between groups 2 and 3 were not.
Fig. 7.
Blood glucose profiles of streptozotocin (STZ) mice transplanted with fresh islets, cryopreserved islets, and cryopreserved encapsulated islets. Daily blood glucose in STZ-induced diabetic nude mice. Day –10 indicated the first day of blood glucose testing prior to any intervention. On day 0, the arrow indicates the STZ injection the mice received with the subsequent hyperglycemia. On day 7 after STZ injection, the arrow indicates the 1,000 islets transplanted intraperitoneally (for encapsulated islets due to size) or in the kidney capsule (for free islets). The remainder of the figure shows the blood glucose among the 3 experimental groups until day 50. *p < 0.05.
Discussion
Currently, the incidence of type 1 diabetes is rising, with 1 in 300 children in the USA affected8,9. Islet transplantation technology is improving with each development, and the prospect of finding a cure for type 1 diabetes is no longer implausible. The process of isolating islets and preparing them for transplant is one that dramatically stresses the tissue, leading to a 15–50% reduction in functional islets10. In order to observe physiological changes in human allografts, the average dose that has demonstrated the most efficient benefits in clinical trials has been approximately 10,000 IE/kg of body weight, and in most cases, one patient usually requires islets from multiple donors to provide a sufficient dose11. The human pancreas contains about 1 million islet cells. It would be highly advantageous to be able to bank islets from multiple isolations so that sufficient cells could be accumulated for a single transplant.
We hypothesized about the cryoprotective role alginate would have on the islets based on its protective role seen during transportation12 and in vivo5,13,14. To determine the percentage of alginate that would yield the best results, we first did islet yield, viability, and GSIR testing in vitro. We found that 1.75% alginate had a small but significant improvement in viability, yield, and islet function when compared to 2.5% alginate, while both encapsulated islet groups were significantly better than non-encapsulated islets post thawing.
When transplanted into diabetic nude mice, we found that there was no statistical difference between transplanting fresh islets and those encapsulated with 1.75% alginate. These mice achieved normoglycemia within 4 and 5 days, respectively, and could sustain normoglycemia for 50 days until the conclusion of the study.
More recently, human islets have been shown to be functional after 20 years of cryopreservation15. This suggests the high viability of cryogenic banking of pancreatic islets from multiple isolations, including those that were found to be suboptimal in yield and thus not allocated for transplantation. This can establish more immediate availability of islets on demand for transplantation. We believe that our method may improve on islet cryobanking practices in support of the above goal.
Conclusions
Based on our results, encapsulating islets with 1.75% alginate prior to cryopreservation leads to a higher survival and viability than without encapsulation. There was no significant difference between the mice receiving fresh tissue or alginate encapsulated islets, which shows that alginate encapsulation prior to cryopreservation protects the islet’s ability to restore normoglycemia in mice. These results suggest additional research using transplantation of marginal dose of islets to compare the groups is necessary for better measurement of the efficacy of encapsulation prior to cryopreservation for rate of cure after transplantation, with the goal being the ability to bank islets indefinitely.
Acknowledgments
The authors gratefully acknowledge the support from the Departments of Surgery and Biomedical Engineering at University of California Irvine and the Sue and Bill Gross Stem Cell Research Center for all the material and facility support in the conduct of this study.
Footnotes
Ethical Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Statement on Human and Animal Rights: All animal procedures in this study were conducted in accordance to the Institutional Animal Care and Use Committee at University of California Irvine approved protocols AUP-17-129 and AUP-17-241.
Statement on Informed Consent: This are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jonathan RT Lakey  https://orcid.org/0000-0001-8553-4287
https://orcid.org/0000-0001-8553-4287
References
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. [DOI] [PubMed] [Google Scholar]
- 2.Rajotte RV, Lakey J, Warnock GL. Adult islet cryopreservation. In: Ricordi C, editor. Methods in cell transplantation. Austin (TX): R. G. Landes Company; 1995. p. 517–524. [Google Scholar]
- 3.Taylor MJ, Foreman J, Biwata Y, Tsukikawa S. Prolongation of islet allograft survival is facilitated by storage conditions using cryopreservation involving fast cooling and/or tissue culture. Transplant Proc. 1992;24(6):2860–2862. [PubMed] [Google Scholar]
- 4.Coulombe MG, Warnock GL, Rajotte RV. Prolongation of islet xenograft survival by cryopreservation. Diabetes. 1987;36(9):1086–1088. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan R, Arora RP, Alexander M, White SM, Lamb MW, Foster CE III, Choi B, Lakey JR. Noninvasive evaluation of the vascular response to transplantation of alginate encapsulated islets using the dorsal skin-fold model. Biomaterials. 2014;35(3):891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pisania A, Weir GC, O’Neil JJ, Omer A, Tchipashvili V, Lei J, Colton CK, Bonner-Weir S. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab Invest. 2010;90(11):1661–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamb M, Storrs R, Li S, Liang O, Laugenour K, Dorian R, Chapman D, Ichii H, Imagawa D, Foster C III, King S, et al. Function and viability of human islets encapsulated in alginate sheets: in vitro and in vivo culture. Transplant Proc. 2011;43(9):3265–3266. [DOI] [PubMed] [Google Scholar]
- 8.Rogers MAM, Kim C, Banerjee T, Lee JM. Fluctuations in the incidence of type 1 diabetes in the United States from 2001 to 2015: a longitudinal study. BMC Med. 2017;15(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan MJ, Albanese-O’Neill A, Haller MJ. Type 1 diabetes: current concepts in epidemiology, pathophysiology, clinical care, and research. Curr Probl Pediatr Adolesc Health Care. 2012;42(10):269–291. [DOI] [PubMed] [Google Scholar]
- 10.Al-Adra DP, Gill RS, Imes S, O’Gorman D, Kin T, Axford SJ, Shi X, Senior PA, Shapiro AM. Single-donor islet transplantation and long-term insulin independence in select patients with type 1 diabetes mellitus. Transplantation. 2014;98(9):1007–1012. [DOI] [PubMed] [Google Scholar]
- 11.The CITR.Coordinating Center and Investigators. 10th Collaborative Islet Transplant Registry 2017 annual report. Rockville (MD): The Emmes Corporation; 2017. [Google Scholar]
- 12.Li N, Zhang Y, Xiu Z, Wang Y, Chen L, Wang S, Li S, Guo X, Ma X. The preservation of islet with alginate encapsulation in the process of transportation. Biotechnol Appl Biochem. 2015;62(4):530–536. [DOI] [PubMed] [Google Scholar]
- 13.Kollmer M, Appel AA, Somo SI, Brey EM. Long-term function of alginate-encapsulated islets. Tissue Eng Part B Rev. 2016;22(1):34–46. [DOI] [PubMed] [Google Scholar]
- 14.Kuehn C, Fulop T, Lakey JR, Vermette P. Young porcine endocrine pancreatic islets cultured in fibrin and alginate gels show improved resistance towards human monocytes. Pathol Biol. 2014;62(6):354–364. [DOI] [PubMed] [Google Scholar]
- 15.Manning Fox JE, Lyon J, Dai XQ, Wright RC, Hayward J, van de Bunt M, Kin T, Shapiro AM, McCarthy MI, Gloyn AL, et al. Human islet function following 20 years of cryogenic biobanking. Diabetologia. 2015;58(7):1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]