Abstract
Fecal microbiota transplantation (FMT) is effective for induction of remission in ulcerative colitis (UC). Diet has potential to augment the efficacy and durability of FMT by encouraging engraftment of transplanted microorganisms. A trial of FMT combined with a defined diet was undertaken as salvage therapy for a 71‐year‐old woman with active steroid‐refractory extensive UC. A multidimensional sulfide‐reducing diet (4‐SURE diet) was commenced followed by single‐donor FMT administered by colonoscopy and then enemas over 7 days. Dietary adherence, clinical evaluation, and stool samples for metagenomic profiling were undertaken at weeks 0, 4, 8, and 24. Colonoscopy was performed 8 weeks post‐FMT. Shotgun metagenomic profiling of the donor fecal suspension was also performed. A rapid clinical response to FMT and 4‐SURE diet was observed with normalization of stool frequency (≤2 motions/day) and resolution of rectal bleeding within 2 weeks. Dietary adherence was excellent. Colonoscopy at week 8 revealed no evidence of active colitis (Mayo endoscopic sub‐score 0) with histology showing no evidence of acute or chronic lamina propria inflammatory cell infiltrate. Sustained clinical and endoscopic remission out to 24 weeks was observed. Metagenomic sequencing confirmed sustained engraftment of beneficial donor microbiota with increased alpha‐diversity and capacity for short‐chain fatty acid production, including Faecalibacterium prauznitzii and Eubacterium hallii. This case report supports the rationale of prescribed diet therapy to support engraftment of donor microbiota following FMT for UC. Further large trials with a diet‐arm control group are needed to evaluate FMT augmented by a defined diet in UC.
Keywords: diet, fecal microbiota transplantation, metagenomics, refractory ulcerative colitis
Durable efficacy and sustained remission to 24 weeks associated with microbial engraftment of induction fecal microbiota transplantation augmented by diet are observed in a patient with refractory ulcerative colitis (UC). Metagenomic sequencing confirmed sustained engraftment of beneficial donor microbiota with increased alpha‐diversity and capacity for short‐chain fatty acid production, including Faecalibacterium prauznitzii and Eubacterium hallii. Mechanistic insights provided by functional microbiome analysis illustrate a synergistic combined approach to microbial manipulation in UC.

Introduction
Ulcerative colitis (UC) is a lifelong relapsing and remitting inflammatory disease of rising global prevalence.1 The gut microbiota contribute to the pathogenesis of UC, however, current therapies for UC are “immunocentric,” solely targeting the immune response to the gut microbiota without altering the luminal environment. Fecal microbiota transplantation (FMT) involves transferring gut microbiota from a healthy individual to another with disease to restore diversity and function. Early data show FMT can be used for induction of remission in UC with rates of clinical and endoscopic remission comparable to available biologic therapies.2
Fermentable dietary substrate has a key role in shaping the composition and function of the gut microbiota, including modulation of potentially harmful microbial metabolites such as hydrogen sulfide (H2S). Colonic fermentation of excess animal protein increases production of H2S, whereas fermentation of dietary fibers suppresses and attenuates H2S production via the gut microbiota preferentially fermenting complex carbohydrate over protein sources that would otherwise produce H2S.3, 4 Emerging observational data suggest modulation of fermentable fibers, total protein, and sulfur proteins, and sulfated food additives, strategies central to the 4‐SURE diet (FOUR strategies to Sulphide‐REduction), are efficacious in inducing clinical and endoscopic response in UC.5 Moreover, a single case report used combined FMT and 4‐SURE diet as therapy and reported induction and maintenance of clinical and endoscopic remission out to 12 months.6
It is biologically plausible that a specific diet may augment the efficacy and durability of FMT by providing a favorable luminal milieu, encouraging engraftment of transplanted microorganisms and restoring luminal homeostasis.
Case Report
A 71‐year‐old woman with steroid‐refractory extensive UC underwent microbial manipulation with FMT and 4‐SURE diet, resulting in sustained clinical and endoscopic remission out to 24 weeks. Metagenomic sequencing confirmed sustained engraftment of beneficial donor microbiota.
Diagnosis
She was diagnosed with left‐sided UC at the age of 54 years. Her disease was initially responsive to sulfasalazine therapy; however, over subsequent years she developed steroid‐dependent disease complicated by osteopenia. Six months before FMT she was hospitalized for severe Campylobacter jejuni gastroenteritis. Thereafter, her disease became steroid‐refractory, with persistent bloody diarrhea (>6 bloody motions/day). Colonoscopy 4 months following hospitalization revealed severe extensive colitis characterized as Mayo endoscopic sub‐score 3. Biopsies confirmed moderately active chronic colitis without features of cytomegalovirus infection, and both biopsies and serial stool cultures excluded persistent bacterial infection.
Escalation to immunomodulator or biologic therapy was precluded on account of concurrent medical comorbidities and patient reticence. Given salvage colectomy was the next most appropriate step in management, a trial of FMT combined with 4‐SURE diet was undertaken. Usual therapy with sulfasalazine was continued. Ethics approval was granted (Central Adelaide Local Health Network HREC/18/CALHN/646) and written informed consent gained from the patient.
Single‐donor FMT was prepared and administered as per a previously described protocol.6 Following a polyethylene glycol bowel preparation, 200 mL of thawed fecal suspension was instilled into the patient's caecum via colonoscopy. Two further 100 mL aliquots of the same fecal suspension were administered by enema over the following 7 days.
Prior to FMT, habitual diet was assessed using a 7‐day weighed food diary. A research dietitian provided individualized 4‐SURE dietary advice with a 1‐week set meal plan and recipes to be followed for 8 weeks.5 The 4‐SURE diet was commenced first, followed by FMT. Adherence was assessed at weeks 4, 8, and 24 using a 3‐day weighed food diary and self‐reported dietary adherence checklists (≥76–100% always adherent).5
Stool samples were collected prior to FMT (week 0), and at weeks 4, 8, and 24 for metagenomic profiling and calprotectin. Clinical evaluation and blood testing including C‐reactive protein were also undertaken at these time‐points. Colonoscopy was performed 8 weeks post‐FMT. Shotgun metagenomic profiling of the donor fecal suspension was performed as per established methods at Microba Life Sciences.
Outcome
Clinical
The patient reported a rapid clinical response to FMT and 4‐SURE diet, with normalization of stool frequency (≤2 motions/day) and resolution of rectal bleeding within 2 weeks. Corticosteroid taper and cessation were achieved within 6 weeks. She remained in steroid‐free clinical remission for 24 weeks following FMT and 4‐SURE diet. Concordant with clinical improvement, biomarkers of inflammation normalized by 8 weeks following FMT (Fig. 1). Colonoscopy at week 8 revealed no evidence of active colitis (Mayo endoscopic sub‐score 0) with histology showing no evidence of acute or chronic lamina propria inflammatory cell infiltrate (Fig. 1). Durable remission and persistent normalization of inflammatory biomarkers were evident 24 weeks post‐FMT and diet therapy (Fig. 1).
Figure 1.
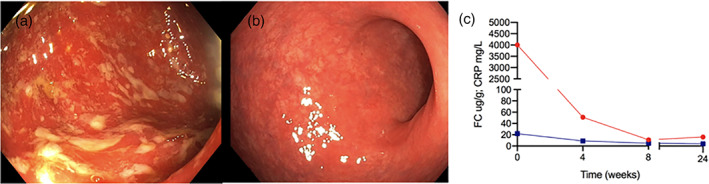
(a) Week 0 (day of fecal microbiota transplantation [FMT] and diet) showing ulceration, loss of vascular pattern, and erythema (endoscopic Mayo 3). (b). Week 8 post‐FMT and diet showing normalization of colonic mucosal appearances with no visible evidence of inflammation (Mayo 0). (c) Graph of fecal calprotectin  (FC) and C‐reactive protein
(FC) and C‐reactive protein  (CRP) over time course from commencement of FMT and dietary therapy (week 0) until week 24.
(CRP) over time course from commencement of FMT and dietary therapy (week 0) until week 24.
Diet
The 4‐SURE diet was well tolerated with excellent self‐reported adherence sustained to 24 weeks. This included maintaining a protein intake of 1.0–1.2 g/kg (75–90 g/day), increasing fermentable fibers to 25–30 g/day, and avoidance of specified food additives. Reduction of sulfur amino acids was not achieved. Food‐related quality‐of‐life score improved from 61 to 116 (poorer 29, greater 145).
Functional microbiome analysis
A rapid change in the patient's microbiota was observed following FMT, with a shift toward the donor's microbiota profile (Appendix S1). Overall, the patient's microbiota remained stable in terms of most abundant organisms out to 24 weeks post‐FMT, illustrating stable engraftment of the donor's microbiota profile. Moreover, an increase in alpha‐diversity was observed over the follow‐up period associated with the 4‐SURE diet, with a steady increase in the patient's Shannon Diversity Index from 2.86 prior to FMT treatment, to 2.83 at 4 weeks, 3.19 at 8 weeks, and then 3.38 at 24 weeks, approximating the donor's Shannon Diversity Index of 3.32 at the time of stool donation.
At a species level, it was possible to categorize changes in the microbiota post‐FMT to better conceptualize shifts in the ecosystem (Appendices S1 and S2). Engrafted species (Group C) were those not detectable in the patient at baseline and engrafted following FMT. These organisms may be drivers of the therapeutic effect of FMT or benefactors of the therapy. This included avid producers of short‐chain fatty acids (SCFAs) such as Faecalibacterium prausnitzii and Eubacterium hallii. Suppressed species (Group D) were those present in the patient at baseline but contracted or became undetectable following FMT. Some of these organisms may be drivers of luminal inflammation or benefactors of an oxygen‐rich inflamed microenvironment, including species with toxigenic potential such as enterotoxigenic‐positive strains of Bacterioides fragilis.
The metabolic potential of organisms based on functional genes was extrapolated from metagenomic data. An increase in the relative abundance of organisms with capacity to biosynthesize SCFAs was observed in the patient following FMT, sustained over 24 weeks (Appendix S2). Concurrently, loss of capacity for both Bacteroides fragilis toxin production and histamine production was evident. Sulfate‐reducing capacity was diminished over the 24‐week study period, consistent with preferential carbohydrate fermentation and suppression of protein fermentation.
Discussion
This is the first report demonstrating durable efficacy and sustained remission associated with microbial engraftment of induction FMT augmented by a diet designed to modulate colonic fermentation and sulfide metabolism in a patient with refractory UC. Mechanistic insights provided by functional microbiome analysis illustrate an exciting synergistic combined approach to microbial manipulation in UC.
Higher rates of FMT efficacy are thought to be related to a greater biodiversity and abundance of microbes as presumed with pooled donor FMT compared with autologous FMT.7 Oxygen‐sensitive viable anaerobes such as F. prausnitzi are also more likely to be preserved through anaerobic preparation.7 As dietary substrate also directly influences microbial biodiversity and abundance, it is possible the diet of FMT recipients could affect engraftment and efficacy of FMT. The 4‐SURE diet is hypothesized to facilitate donor organism engraftment and microbial diversity and modulate excess H2S production at the colonic‐luminal interface by reducing availability of dietary protein for reduction by sulfate‐reducing bacteria and concurrently increasing the variety of slow and readily fermentable carbohydrates for degradation by SCFA‐producing organisms.5 This “switch” to preferential carbohydrate fermentation increases the bioavailability of SCFAs as an energy source for colonocytes.8 The sustained increase in SCFA‐producing organisms and sulfate‐reducing capacity observed is plausibly related to bioavailability of 4‐SURE dietary substrate and suppression of protein fermentation and yields insights of the possible mechanisms of efficacy of diet and FMT in UC.
Beyond a single pilot study, longevity of efficacy of FMT following an induction regimen is limited. Sood et al. reported significantly higher rates of endoscopic and histological remission using 8‐weekly FMT via colonoscopy following a rigorous multi‐session FMT induction, with no difference in steroid‐free remission between FMT and placebo groups at 48 weeks.6, 9 Only one other FMT study in UC has provided functional metagenomic data, similarly showing an increase in alpha‐diversity following FMT in those who achieved remission, as well as enrichment in SCFA‐producing organisms including E. hallii.10 Interestingly, in contrast to the findings of this case report, B. fragilis was associated with a favorable donor microbial profile. However, B. fragilis has also been described as a part of a UC microbial signature, and enterotoxigenic B. fragilis strains are associated with UC flares.
Collectively, the possibility of microbial manipulation using FMT and a defined diet to achieve durable remission in UC holds promise and warrants further study in high quality randomized‐controlled trials using a control diet‐arm.
Patient consent
Written informed consent was gained from the patient.
Supporting information
Appendix S1. Supporting information.
Appendix S2. Supporting information.
Acknowledgments
The authors would like to acknowledge the generous assistance of The Queen Elizabeth Hospital Inflammatory Bowel Disease Nurses, Sangwoo Han and Serena Martin. The authors would also like to acknowledge both Microba and BiomeBank for funding of metagenomic analysis and provision of stool for FMT respectively.
Robert V Bryant and Alice S Day are the joint first authors.
Declaration of conflict of interest: Robert V Bryant has served as a speaker, a consultant, and an advisory board member for (all fees paid to employer for research support), and has received research funding from AbbVie, Ferring, Janssen, Shire, Takeda, Emerge Health. Robert V Bryant owns shares in BiomeBank. Alice S Day has served as a speaker for Emerge Health and AbbVie (fees paid to employer for research support). Ken C McGrath is an employee of Microba Life Sciences, who contributed the metagenomics analysis used in this study in‐kind. Karmen Telfer has no conflicts to declare. Chu K Yao has received research funding from Atmo Biosciences, Ferring Pharmaceuticals, Danone, and Yakult Australia. Her Department financially benefits from the sales of a digital application, booklets, and online courses on the FODMAP diet. Samuel P Costello has received advisory, speaking fees, or research support from Ferring, Falk, Microbiotica, Janssen. Samuel P Costello owns shares in BiomeBank.
Author contribution: Alice S Day, Robert V Bryant, Samuel P Costello contributed to the study concept and design. Robert V Bryant and Samuel P Costello provided study supervision. Alice S Day, Robert V Bryant, Samuel P Costello, Karmen Telfer, and Ken C McGrath contributed to the acquisition of data. All authors contributed to data analysis. All authors contributed to the interpretation of the data. Alice S Day and Robert V Bryant drafted the manuscript. All authors provided critical revision of the manuscript for important intellectual content. All authors approved the final version of the article, including the authorship list.
References
- 1.Molodecky NA, Soon IS, Rabi DMet al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012; 142: 46–54 e42 quiz e30. [DOI] [PubMed] [Google Scholar]
- 2.Zhou HY, Guo B, Lufumpa Eet al. Comparative of the effectiveness and safety of biological agents, tofacitinib, and fecal microbiota transplantation in ulcerative colitis: systematic review and network meta‐analysis. Immunol. Invest. 2021; 50: 323–37. [DOI] [PubMed] [Google Scholar]
- 3.Magee EA, Richardson CJ, Hughes R, Cummings JH. Contribution of dietary protein to sulfide production in the large intestine: an in vitro and a controlled feeding study in humans. Am. J. Clin. Nutr. 2000; 72: 1488–94. [DOI] [PubMed] [Google Scholar]
- 4.Yao CK, Rotbart A, Ou JZ, Kalantar‐Zadeh K, Muir JG, Gibson PR. Modulation of colonic hydrogen sulfide production by diet and mesalazine utilizing a novel gas‐profiling technology. Gut microbes. 2018; 9: 510–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day A, Yao CK, Costello SPet al. Evaluation of the tolerance and effect on disease activity of a new dietary strategy (4‐SURE diet) for mild‐moderately active ulcerative colitis. J. Gastroenterol. Hepatol. 2020; 35: 191–2. [Google Scholar]
- 6.Costello SP, Day A, Yao CK, Bryant RV. Faecal microbiota transplantation (FMT) with dietary therapy for acute severe ulcerative colitis. BMJ Case Rep. 2020; 13: e233135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costello SP, Hughes PA, Waters Oet al. Effect of fecal microbiota transplantation on 8‐week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019; 321: 156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Preter V, Arijs I, Windey Ket al. Impaired butyrate oxidation in ulcerative colitis is due to decreased butyrate uptake and a defect in the oxidation pathway. Inflamm. Bowel Dis. 2012; 18: 1127–36. [DOI] [PubMed] [Google Scholar]
- 9.Sood A, Mahajan R, Singh Aet al. Role of faecal microbiota transplantation for maintenance of remission in patients with ulcerative colitis: a pilot study. J. Crohns Colitis. 2019; 13: 1311–7. [DOI] [PubMed] [Google Scholar]
- 10.Paramsothy S, Nielsen S, Kamm MAet al. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology. 2019; 156: 1440–54.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.
Appendix S2. Supporting information.


