Abstract
Purpose:
Resistance to treatment with inhibitors of mammalian target of rapamycin (mTOR) is partially mediated by activation of epidermal growth factor receptor (EGFR). We conducted a phase I study to determine the recommended phase II dose (RP2D) and dose-limiting toxicities (DLT) of temsirolimus (mTOR inhibitor) combined with erlotinib (EGFR inhibitor) in patients with refractory solid tumors.
Methods:
Standard “3+3” design was used for dose escalation. An expansion cohort at RP2D included only patients with squamous histology or mutations relevant to PI3K or EGFR pathway activation. Patients started daily erlotinib 7 days prior to starting temsirolimus on cycle 1. Intravenous temsirolimus was then administered weekly. Starting dose levels were 15 mg for temsirolimus and 100 mg for erlotinib.
Results:
Forty-four patients received treatment on this study (28 in dose escalation and 16 in the expansion cohort). The RP2D was temsirolimus 25 mg IV weekly and erlotinib 100 mg orally daily. Two patients experienced DLTs (G3 dehydration and G4 renal failure). The most common drug-related adverse events (all grades) were rash, mucositis/stomatitis, diarrhea, nausea and fatigue. No complete or partial responses was observed. The median duration on this study was 69 days (range 3–770) for escalation and 88 days (range 25–243) for expansion cohorts. Among 11 response evaluable patients in the expansion cohort, 9 (82%) had stable disease and 2 (18%) had progressive disease.
Conclusion:
The combination of temsirolimus and erlotinib at the RP2D was well-tolerated, and the regimen resulted in prolonged disease stabilization in selected patients. NCT00770263
Keywords: mTOR inhibitor, EGFR inhibitor, phase 1 study
Introduction
Growth factor receptors such as epidermal growth factor receptor (EGFR) and phosphoinositide 3-kinase (PI3K)-AKT-mTOR play important roles in regulation of proliferation, differentiation, and cell death in many malignancies. Receptor tyrosine kinases, such as EGFR can activate PI3K heterodimer by phosphorylating adaptor proteins. Activated PI3K phosphorylates a membrane phospholipid, phosphatidylinositol bisphosphate (PIP2) to phosphatidylinositol trisphosphate (PIP3), leading to phosphorylation of AKT and mTORC1, and regulation of a host of cellular proteins relevant to cell growth, cell cycle entry, and protein synthesis[1]. Several agents that inhibit either EGFR or PI3K-AKT-mTOR pathways are now in clinical use, and more are in various stages of clinical development. mTORC1 inhibitors, such as temsirolimus and everolimus have been approved by FDA for selected cancers based on phase III clinical trials in kidney cancer, neuroendocrine tumors and metastatic breast cancer[2–4]. EGFR inhibitors, including erlotinib, afatinib, gefitinib, dacomitinib, and osimertinib are currently approved for treatment of non-small cell lung cancer[5–9].
EGFR tyrosine kinase inhibitors (TKIs), especially earlier generation agents, are prone to therapeutic resistance. Mechanisms of resistance are well studied, and include presence of constitutively active form of EGFR or oncogenic shifts involving HER2, HER3 or other members of EGFR family of receptor tyrosine kinases[10]. Deleterious mutations or loss of PTEN, activates AKT/mTOR signaling and has been implicated in resistance to EGFR TKIs[11]. MET amplification leading to activation of HER3 mediated PI3K/AKT/mTOR has also been seen in EGFR TKI resistant lung cancer models [12]. Upregulation of IGF-1R upon EGFR inhibition can also lead to activation of PI3K/AKT signaling and acquired resistance to EGFR TKIs[13]. These put together support the importance of PI3K/AKT/mTOR signaling pathway in resistance to EGFR TKIs.
Tyrosine kinase receptors including EGFR play a role in acquired resistance to mTORC1 inhibition. Suppression of negative feedback loops and subsequent activation of other signaling pathways is one of the well-known resistance mechanisms to mTORC1 inhibition. mTORC1 inhibition leads to alleviation of mTORC1 mediated negative feedback inhibition of other mitogenic signaling, such as Erk, via insulin like growth factor-1, and subsequently activates PI3K/Akt signaling[14–17]. Activation of Akt then leads to the stimulation of anti-apoptotic pathways and promotes tumor cell survival. In addition, mTORC1 inhibition can lead to tyrosine phosphorylation of EGFR via activation of c-Src or release of FOXO1/3a, then activates Erk1/2 signaling[18,19].
Synergistic effects of mTORC1 inhibitors in combination with erlotinib were observed in several cancer cell lines, including non-small cell lung, pancreatic, colon and breast cancers[14,20–25]. In gefitinib-resistant non-small cell lung cancer cell lines, addition of everolimus led to growth inhibition[20,21,25]. A similar observation has been extended in vivo using colon cancer xenograft models[20]. This mechanistic evidence provides rationale for combining EGFR inhibition and mTORC1 inhibition to overcome resistance to either therapy and to enhance anti-tumor activity in solid tumors.
Several trials combining mTOR inhibitors and EGFR inhibitors have been ongoing or completed since this study was designed [26–33]. Temsirolimus is given intravenously at 25mg weekly for treatment of renal cell carcinoma [34]. Erlotinib is dosed orally daily at 100mg for pancreatic cancer indication and 150mg for lung cancer indication[35]. At the time of this study planning, limited toxicity data was available for the combination of erlotinib and temsirolimus in patients with recurrent malignant gliomas, which was later published[36]. The maximum tolerated dose (MTD) for this combination is proposed to be 150mg/day of erlotinib and 15mg/week for temsirolimus with dose limiting toxicities of rash and mucositis. No objective responses were in glioblastoma cohort, but 2 out of 16 patients had responses in anaplastic glioma cohort. In addition to this clinical trial, another preclinical study evaluated the impact of the dosing sequence of mTOR inhibitor in combination with EGFR inhibitor in pancreatic cancer cell lines. Interestingly, synergic effects in terms of cell growth inhibition and apoptosis induction were only observed when EGFR inhibitor was administered before mTOR inhibitor[37]. Considering that the half-life of erlotinib was 36 hours, a more rational design was to initiate erlotinib treatment one week prior to the administration of temsirolimus. Therefore, we proposed to evaluate the combination of temsirolimus with erlotinib in a dose escalation phase I trial reflecting the new dosing schedule in patients with refractory, recurrent solid tumor malignancies.
Methods
This was an open-label, non-randomized, phase I study that employed a “3+3” dose-escalation design (NCT00770263) performed at Siteman Cancer Center at Washington University School of Medicine in Saint Louis. The protocol was approved by the Institutional Review Board. The trial was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All participants provided informed consent prior to entering the clinical trial.
Eligibility
Patients with histologically confirmed solid tumor that was resistant to standard treatments were enrolled. Patients without measurable disease were allowed to participate. Patients were required to ECOG performance status of 0 – 1, ages 18 or older, and have preserved organ functions including absolute neutrophil count of 1,500/mm3 or greater, platelets 100,000/mm3 or greater, hemoglobin 9.0g/dL or greater, total bilirubin of 1.5 × upper limit of normal (ULN) or less, transaminases of 3 × ULN or less, creatinine of 2 × ULN or less or creatinine clearance 60mL/min or greater, and fasting serum cholesterol 350mg/dL or less. Patients who were concomitantly on CYP3A4 inducers were not allowed as temsirolimus is primarily metabolized by CYP3A4. Other exclusions included known hypersensitivity to macrolide antibiotics; active anti-retroviral therapy for HIV infection; active central nervous system disease; or uncontrolled intercurrent illness. Additional eligibility requirements for the expansion cohort included 1) available archival tissue, 2) known tumor mutational status including PTEN loss, PIK3CA mutation, and/or EGFR mutation, without KRAS or BRAF mutations as determined by next generation sequencing performed at the Genomic and Pathology Services at Washington University or other CLIA-certified laboratories, 3) squamous carcinoma histology, papillary thyroid carcinoma, and adenoid cystic carcinoma regardless of genetic alterations based on durable stable disease seen during dose escalation phase in these histology types, and 4) consent to mandatory post-cycle 1 biopsy. Dose expansion phase was planned to enroll up to 10 patients evaluable for post-treatment biopsies as well as to better define the toxicity profile at the RP2D; if evaluability criteria were not met, patients were to be replaced.
Treatment
Patients were enrolled at 4 dose levels. The starting doses were erlotinib 100mg orally daily with temsirolimus 15mg IV weekly, and doses were increased according to a standard “3+3” dose escalation design (Table 1). Prophylactic minocycline 50mg orally twice daily was started on cycle 1 day 1 for erlotinib-induced rash. The first cycle included 7 days of erlotinib alone, followed by weekly temsirolimus infusions on days 8, 15, 22, and 29; therefore, the first cycle was considered to be 35 days. All subsequent cycles were 28 days. Additional patients were added to the dose expansion phase once the recommended dose was defined. Patients continued treatment until they experienced disease progression or intolerable toxic effects.
Table 1.
Dose levels and DLTs in the phase I study of temsirolimus and erlotinib in patients with advanced cancer
| Dose Level | Erlotinib (oral, daily) | Temsirolimus (IV, weekly) | Number of patients treated | Number of patients eligible for DLT evaluation | Number of patients experiencing DLT |
|---|---|---|---|---|---|
| 1A | 100 mg | 10 mg | 1 | 1 | 0 |
| 1 | 100 mg | 15 mg | 15 | 9 | 2 (G3 dehydration, n=1; G3 acute renal failure, n=1) |
| 2 | 100 mg | 20 mg | 5 | 3 | 0 |
| 3 | 100 mg | 25 mg | 4 | 3 | 0 |
| Dose Expansion Phase | 100 mg | 25 mg | 16 | 16 | 0 |
DLT, dose limiting toxicity; G, grade
Toxicity assessment and dose modifications
Dose modifications were allowed for treatment-related toxicities. Toxicity grades followed Common Terminology Criteria for Adverse Events (CTCAE) version 3. Temsirolimus dose reductions were allowed to as low as 10mg weekly; erlotinib dose reduction was not allowed below 100mg daily, but dose holds per protocol-defined criteria were allowed.
Definition of dose limiting toxicity (DLT) and maximum tolerated dose (MTD)
Patients who received at least one dose of temsirolimus following the 7-day erlotinib lead-in period were included for toxicity evaluation. Those who had DLT or completed the first cycle without DLT were evaluable for DLT analysis. Hematologic DLT was defined as grade 4 neutropenia with greater than 7 days duration during the first cycle, or febrile neutropenia of any duration, or grade 4 anemia or thrombocytopenia which required transfusion therapy on more than two occasions in 7 days during the first cycle. Non-hematologic DLT was defined as any possibly, probably, or definitely related grade 3 or grade 4 non-hematologic toxicity that occurred during the first cycle with the specific exceptions of grade 3 or 4 nausea, vomiting, or anorexia which returned to grade 1 prior to the next treatment cycle. Grade 3 triglyceride elevation despite appropriate lipid-lowering drug therapy and grade 3 rash with no improvement after two weeks of supportive therapy were also defined as DLTs. Any treatment-related toxicity requiring a delay of 14 days prior to beginning cycle 2 was also considered a DLT.
Correlative studies
Serum and plasma samples were collected for proteomic evaluation. Archived formalin-fixed paraffin embedded tissue samples were collected when available. Fresh tumor biopsy was optional for patients during dose escalation, and was required for the expansion cohort. Services from the Genomic and Pathology Service at Washington University were used for molecular profiling of the tumor tissue.
Statistical considerations
This study used a traditional “3+3” design dose escalation schema with primary objectives 1) to define the maximum tolerated dose (MTD) and dose-limiting toxicities (DLT) of temsirolimus in combination with erlotinib in patients with resistant solid malignancies; and 2) to determine the incidence and severity of other toxicities of temsirolimus in combination with erlotinib in patients with advanced solid malignancies. Secondary objectives included assessment of tumor response in patients with response-evaluable disease. Tumor response to therapy was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 [38].
Results
Patient characteristics
From June 2009 to June 2014, a total of 67 patients were consented and were assessed for eligibility. Of those, 58 patients met eligibility, and 41 were started on erlotinib and temsirolimus combination treatment. Three patients who were started on erlotinib, but did not proceed with temsirolimus and erlotinib combination were excluded from DLT analysis. All patients were treated at the Siteman Cancer Center at Washington University in St. Louis. The reasons for not starting treatment after enrollment include insurance coverage issues, patient preference and clinical deterioration as shown in Figure 1. For the 41 patients who started treatment on the trial, demographic and clinical characteristics are as shown in Table 2. Median age of patients was 62 years, and approximately 60% were male. Most patients were non-Hispanic and Caucasian. Eighteen different tumor types were included with non-small cell lung cancer, pancreatic cancer, and adenoid cystic carcinoma being most common tumor types.
Figure 1.
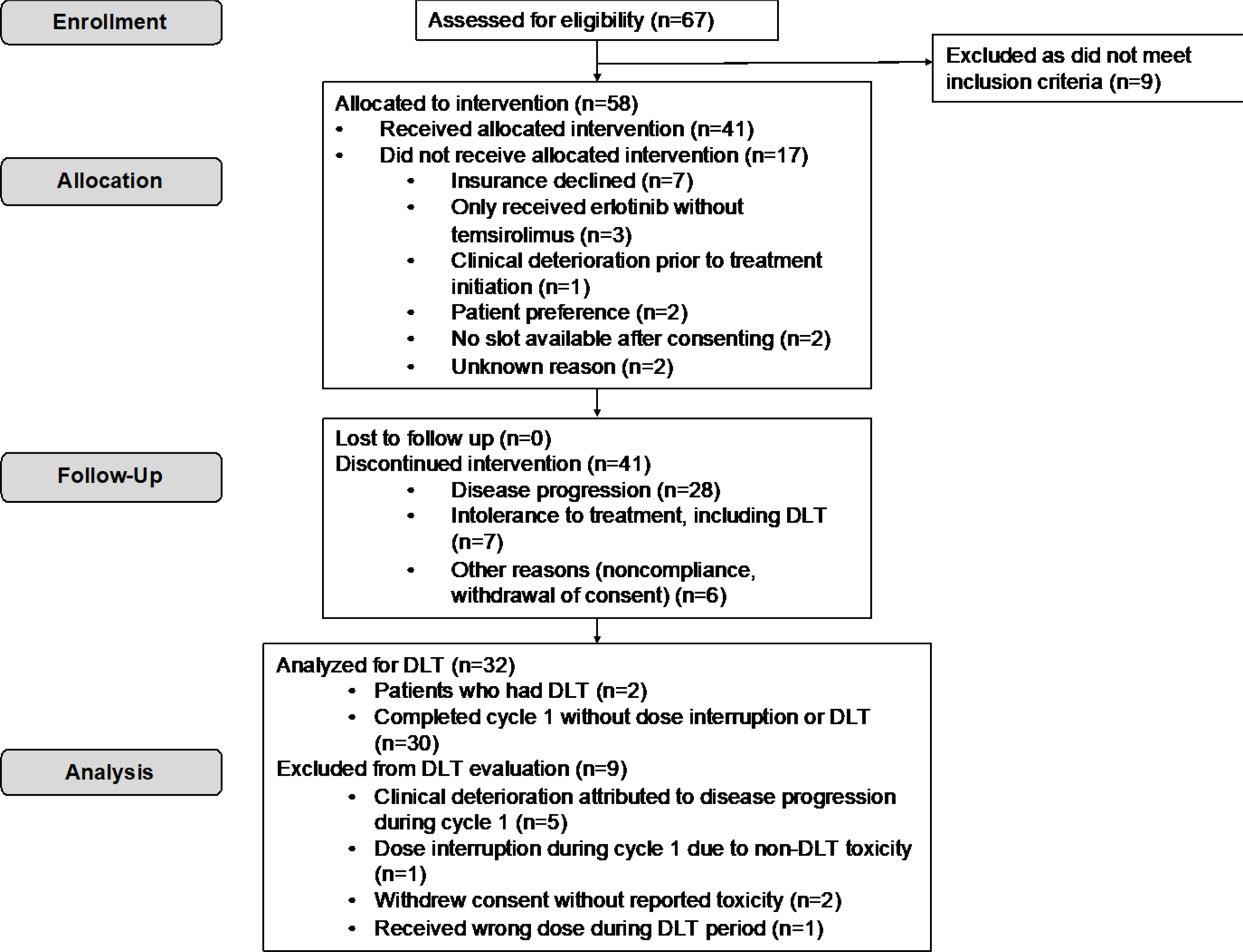
Flow of patients on the trial with temsirolimus and erlotinib in patients with advanced solid tumors. Intervention is defined as having received at least one dose of both erlotinib and temsirolimus.
Table 2.
Demographic and clinical characteristics of patients with advanced cancer in the phase I study of temsirolimus and erlotinib (N=41). Non-small cell lung cancer included adenocarcinoma (n=4), squamous cell carcinoma (n=5) and sarcomatoid carcinoma (n=1).
| Characteristic | ||
|---|---|---|
| Sex, N (%) | ||
| Female | 17 | 41.5% |
| Male | 24 | 58.5% |
| Median age at study enrollment, years (range) | 62 (31 – 81) | |
| Race, N (%) | ||
| Caucasian | 34 | 82.9% |
| African-American | 4 | 9.8% |
| Asian | 2 | 4.9% |
| Other | 1 | 2.4% |
| Disease type, N (%) | ||
| Non-small cell lung cancer* | 10 | 24.4% |
| Pancreatic cancer | 9 | 22.0% |
| Adenoid cystic | 4 | 9.8% |
| Breast cancer | 2 | 4.9% |
| Gastric cancer | 2 | 4.9% |
| Cancer of unknown primary | 2 | 4.9% |
| Small cell lung cancer | 1 | 2.4% |
| Papillary thyroid cancer | 1 | 2.4% |
| Colon cancer | 1 | 2.4% |
| Cholangiocarcinoma | 1 | 2.4% |
| Ampullary adenocarcinoma | 1 | 2.4% |
| Sarcoma | 1 | 2.4% |
| Thymic cancer | 1 | 2.4% |
| Anal cancer | 1 | 2.4% |
| Mesothelioma | 1 | 2.4% |
| Vulvar cancer | 1 | 2.4% |
| Hepatocellular carcinoma | 1 | 2.4% |
| Head and neck squamous cell cancer | 1 | 2.4% |
| Number of prior therapies, median (range) | 2 (1 – 14) |
DLTs and MTD
Out of 6 DLT-evaluable patients that were enrolled in dose level 1, one patient had a DLT of grade 3 dehydration. Given the 3+3 rules defined in the protocol, dose escalation to the next dose level should have occurred; however, due to misinterpretation of protocol, the next patient was enrolled to the lower dose level (1A) and another patient was enrolled to dose level 1. Unfortunately, this seventh patient at dose level 1 experienced a DLT of grade 3 acute renal failure. These were not consistent with the 3+3 dose escalation design; thus, further review by the principal investigator and Quality Assurance and Safety Monitoring Committee at Siteman Cancer Center was performed. They determined that a total of 9 DLT evaluable patients would be enrolled in dose level 1; and no additional patients were to be added to the lower dose level unless additional DLTs were observed at dose level 1. As no other DLTs were observed at dose level 1, the study proceeded with further dose escalation. Subsequent dose levels followed 3+3 design without any reported DLTs. Therefore, MTD was not reached. Dose level 3 (erlotinib 100mg orally daily and temsirolimus 25 mg IV weekly) was determined to be recommended phase 2 dose (RP2D). A total of 16 patients were enrolled for the expansion cohort at the RP2D, as 7 patients who did not have post-treatment tumor biopsies were replaced per protocol; however, all 16 patients were included in DLT assessment.
Other toxicities
For most patients, treatment with temsirolimus and erlotinib was tolerated as expected. Most common toxicities were acneiform rash, reported in 26 patients (63.4%), but all were grades 1 or 2. Stomatitis, hyperglycemia, diarrhea, nausea, and hypercholesterolemia were also common. Most common grade 3 or worse toxicities included hyperglycemia (N=7, 17.1%), anemia (N=4, 9.8%), and lymphopenia (N=3, 7.3%). Seven patients (17.1%) discontinued treatment due to toxicities.
Efficacy
Twenty-six patients had at least one scans to evaluate disease response. No objective response was observed. Of 26 response evaluable patients, 9 had progressive disease and 17 had stable disease as best responses. Best responses are shown in Figure 2. Of the 17 patients who had stable disease as best response, 8 patients had more than 6 months of disease stability. In the expansion cohort of 16 patients, where patients were more homogeneous in nature, 2 patients experienced prolonged stable disease. Of these 2 patients, one patient had adenoid cystic cancer, and the other had high grade soft tissue sarcoma with PTEN loss. Duration of treatment for patients treated on the expansion dose is shown in Figure 3.
Figure 2.
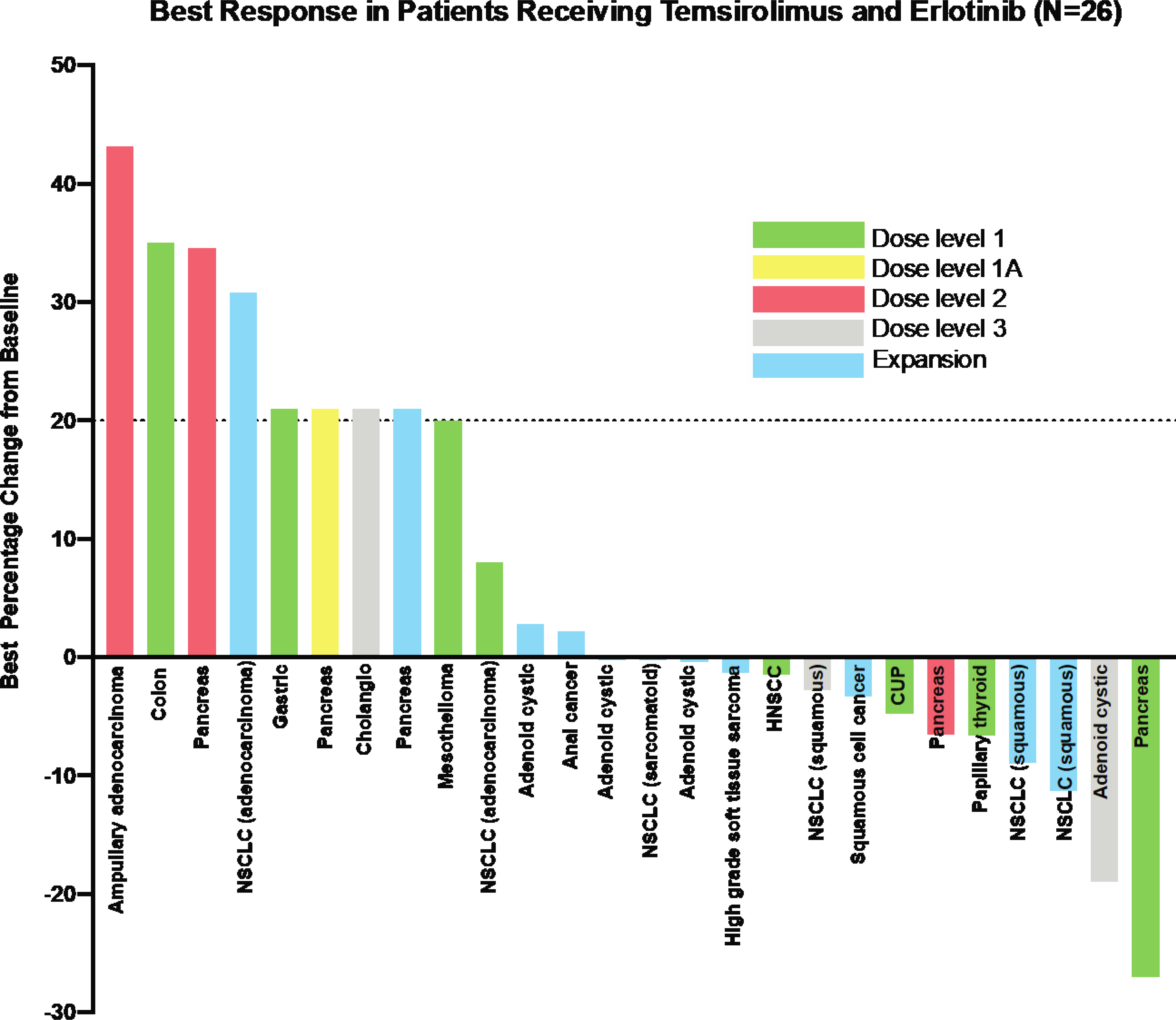
Best radiographic response in patients receiving temsirolimus and erlotinib by dose levels.
Figure 3.
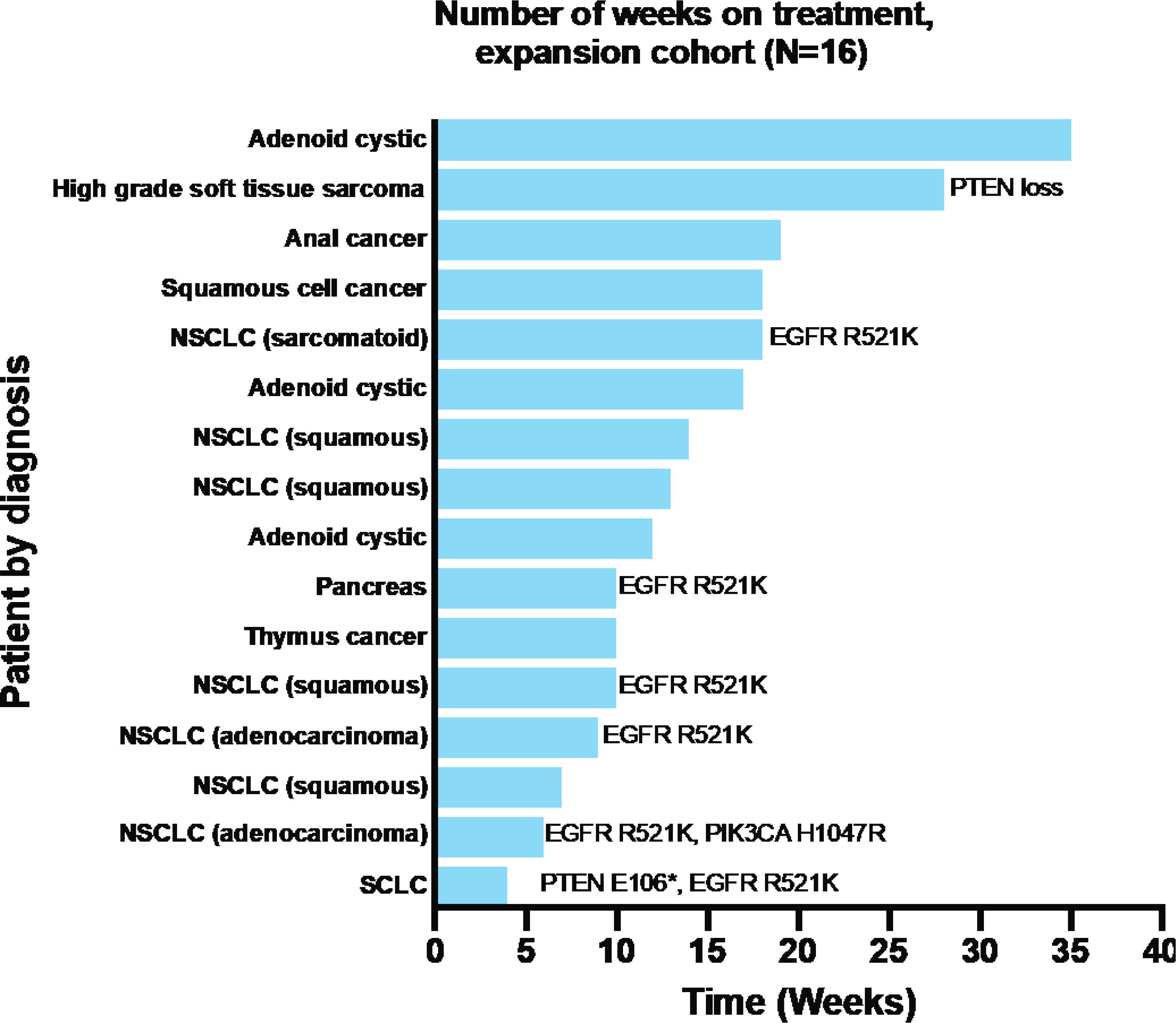
Duration of study treatment in the expansion cohort
Molecular analysis
Seven patients out of 16 in expansion cohort had tumor sequencing information, as shown in Figure 3. Reported molecular aberrations included PTEN loss/mutation, EGFR mutations, or PIK3CA mutations. No significant correlation between disease response, treatment duration and these mutations were noted. Only 3 patients in escalation cohorts, all in dose level 3, had sequencing information available. Among 3 patients treated at dose level 3, one patient with adenoid cystic cancer had KIT M541L and DNMT3A T44M identified, and had prolonged stable disease beyond 6 months; another patient had EGFR L747P and TP53 P72R, and also had prolonged stable disease; the last patient with TP53 P72R, RET G6915S had progressive disease as best response. Additional molecular analyses or proteomic studies in collected patient samples were not done due to incomplete collection and lack of funding.
Discussion
Combination of 25 mg of weekly temsirolimus and 100mg of daily erlotinib was well-tolerated without unexpected toxicities and MTD was not reached. While no objective responses were observed among multiple advanced patients with heavily pretreated solid tumors, there were two patients experienced prolonged stable disease. One of those had a tumor type without established standard treatment (adenoid cystic carcinoma) while the other also had an aggressive rare tumor (sarcoma).
Our cohort of patients did not show measurable tumor decrease on temsirolimus and erlotinib. Lack of radiographic disease response, despite sound mechanistic rationale, is in accordance with other clinical studies that combined mTORC1 and EGFR inhibitors. A study evaluating everolimus and erlotinib in patients with head and neck squamous cell cancer showed one partial response and 11 patients with disease stabilization; thus, concluding no significant clinical benefit in this population[26]. Similar lack of efficacy was noted in studies combining gefitinib and everolimus [28,31]. This may be in part due to incomplete inhibition of PI3K/AKT/mTOR pathway as well as Erk pathway with temsirolimus and erlotinib; as well as activation of alternative signaling pathways that may drive disease progression. Inconclusive roles of potentially predictive biomarkers such as aberrations in PTEN or other molecules in PI3K pathway make patient selection challenging. It should be noted that patients with non-small cell lung cancer harboring activating EGFR mutations, such as exon 19 deletion or exon 21 L858R mutations, were not included in our study, as they would have previously received erlotinib per standard of care. At the time of this study, erlotinib was the standard EGFR inhibitor for that population, not osimertinib.
We did observe favorable toxicity profile with 100mg of daily erlotinib and 25 mg of weekly temsirolimus dose combination. This is somewhat different from previous studies. One study that evaluated temsirolimus and erlotinib combination in patients with head and neck squamous cell carcinoma resulted in early closure of the study due to intolerable toxicities including fatigue, diarrhea, and infection [33]. Another study using 150mg of erlotinib, DLTs of rash and mucositis precluded further dose escalation of temsirolimus beyond 15mg [36,33]. While rash and stomatitis were common toxicities reported in our study, none met the DLT criteria. Furthermore, with advances in supportive management of mTOR inhibitor-induced stomatitis with dexamethasone mouthwash [39], stomatitis likely is no longer the most common toxicity. In our study, we kept erlotinib at 100mg, which is the dose used in pancreatic cancer; but instead, escalated the dose of temsirolimus to full single agent dose of 25mg.
We were not able to complete planned correlative studies including pharmacokinetic studies, proteomic analysis of patient samples, or detailed molecular characteristics of fresh tumor samples that were collected in some patients. Despite all our efforts, tissue collection was was incomplete; and further funding resources were insufficient. This remains a key limitation of our study, and makes it difficult to assess potential pharmacodynamic changes, or lack thereof, that may explain the limited efficacy of the combination observed in our study. This has also regrettably led to a significant delay in publishing the results.
Despite limited efficacy results from our study as well as some of previously reported studies, both EGFR family of TKIs and PI3K/AKT/mTOR pathways remain key mechanisms leading to tumor growth and survival. Ongoing trials (NCT04108858, NCT02705859, NCT04042051) are evaluating combination of PI3K inhibition with HER2 inhibitors in breast cancer. Future studies in other solid tumors and better identification of predictive biomarkers are necessary.
Table 3.
Commonly reported treatment-related toxicities (>10%) in patients receiving temsirolimus and erlotinib (N=41)
| Level 1 (N=15) | 1A (N=1) | 2 (n=5) | 3 (n=4) | Expansion (n=16) | All patients (N=41) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any grade, N | G3 or worse, N | Any grade, N | G3 or worse, N | Any grade, N | G3 or worse, N | Any grade, N | G3 or worse, N | Any grade, N | G3 or worse, N | Any grade, N (%) | G3 or worse, N (%) | |||
| Non-Hematologic | ||||||||||||||
| Acneiform rash | 12 | 0 | 1 | 0 | 4 | 0 | 2 | 0 | 7 | 0 | 26 | 63.4% | 0 | 0.0% |
| Stomatitis | 7 | 1 | 1 | 0 | 3 | 0 | 3 | 0 | 11 | 1 | 25 | 61.0% | 2 | 4.9% |
| Hyperglycemia | 8 | 1 | 1 | 0 | 4 | 2 | 1 | 1 | 7 | 3 | 21 | 51.2% | 7 | 17.1% |
| Diarrhea | 8 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 10 | 0 | 21 | 51.2% | 0 | 0.0% |
| Nausea | 6 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 9 | 0 | 18 | 43.9% | 1 | 2.4% |
| Hypercholesterolemia | 6 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 7 | 0 | 17 | 41.5% | 0 | 0.0% |
| Fatigue | 3 | 1 | 1 | 0 | 2 | 0 | 3 | 0 | 8 | 0 | 17 | 41.5% | 1 | 2.4% |
| Anorexia | 4 | 1 | 1 | 0 | 2 | 0 | 2 | 0 | 6 | 0 | 15 | 36.6% | 1 | 2.4% |
| Dysgeusia | 2 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 6 | 0 | 14 | 34.1% | 0 | 0.0% |
| Hypertriglyceridemia | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 10 | 1 | 11 | 26.8% | 1 | 2.4% |
| Hypoalbuminemia | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 5 | 0 | 10 | 24.4% | 0 | 0.0% |
| Rash, not otherwise specified | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 7 | 1 | 8 | 19.5% | 1 | 2.4% |
| Weight loss | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 4 | 0 | 7 | 17.1% | 0 | 0.0% |
| Hand-foot syndrome | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 6 | 0 | 7 | 17.1% | 0 | 0.0% |
| Dry skin | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 5 | 0 | 7 | 17.1% | 0 | 0.0% |
| Vomiting | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 6 | 14.6% | 1 | 2.4% |
| Hypokalemia | 2 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 6 | 14.6% | 2 | 4.9% |
| Fever | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 5 | 12.2% | 0 | 0.0% |
| Alopecia | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 5 | 12.2% | 1 | 2.4% |
| Nail changes | 1 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 5 | 12.2% | 0 | 0.0% |
| Elevated ALT | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 5 | 12.2% | 0 | 0.0% |
| Hematologic | 0 | 0.0% | ||||||||||||
| Anemia | 3 | 0 | 0 | 0 | 1 | 0 | 2 | 2 | 8 | 2 | 14 | 34.1% | 4 | 9.8% |
| Lymphopenia | 3 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 8 | 2 | 14 | 34.1% | 3 | 7.3% |
| Thrombocytopenia | 5 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 5 | 0 | 12 | 29.3% | 0 | 0.0% |
| Leukopenia | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 6 | 14.6% | 0 | 0.0% |
| Neutropenia | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 5 | 12.2% | 0 | 0.0% |
Acknowledgments
Financial support: This study was supported by Pfizer and partially supported by Washington University UL1 TR000448 and KL2 TR000450
References
- 1.Franke TF, Kaplan DR, Cantley LC (1997) PI3K: downstream AKTion blocks apoptosis. Cell 88 (4):435–437 [DOI] [PubMed] [Google Scholar]
- 2.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O’Toole T, Lustgarten S, Moore L, Motzer RJ, Global AT (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356 (22):2271–2281. doi: 10.1056/NEJMoa066838 [DOI] [PubMed] [Google Scholar]
- 3.Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366 (6):520–529. doi: 10.1056/NEJMoa1109653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Voi M, Brandt U, He W, Chen D, Capdevila J, de Vries EGE, Tomassetti P, Hobday T, Pommier R, Oberg K (2016) Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers From the Randomized, Phase III RADIANT-3 Study. J Clin Oncol 34 (32):3906–3913. doi: 10.1200/JCO.2016.68.0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, Zhou C, Hu CP, O’Byrne K, Feng J, Lu S, Huang Y, Geater SL, Lee KY, Tsai CM, Gorbunova V, Hirsh V, Bennouna J, Orlov S, Mok T, Boyer M, Su WC, Lee KH, Kato T, Massey D, Shahidi M, Zazulina V, Sequist LV (2015) Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 16 (2):141–151. doi: 10.1016/S1470-2045(14)71173-8 [DOI] [PubMed] [Google Scholar]
- 6.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Munoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L, Spanish Lung Cancer Group in collaboration with Groupe Francais de P-C, Associazione Italiana Oncologia T (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13 (3):239–246. doi: 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 7.Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, Migliorino MR, Pluzanski A, Sbar EI, Wang T, White JL, Nadanaciva S, Sandin R, Mok TS (2017) Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 18 (11):1454–1466. doi: 10.1016/S1470-2045(17)30608-3 [DOI] [PubMed] [Google Scholar]
- 8.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361 (10):947–957. doi: 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 9.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS, Investigators F (2018) Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 378 (2):113–125. doi: 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 10.Wheeler DL, Dunn EF, Harari PM (2010) Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol 7 (9):493–507. doi: 10.1038/nrclinonc.2010.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.She QB, Solit D, Basso A, Moasser MM (2003) Resistance to gefitinib in PTEN-null HER-overexpressing tumor cells can be overcome through restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3’-kinase/Akt pathway signaling. Clin Cancer Res 9 (12):4340–4346 [PubMed] [Google Scholar]
- 12.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Janne PA (2007) MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316 (5827):1039–1043. doi: 10.1126/science.1141478 [DOI] [PubMed] [Google Scholar]
- 13.Chakravarti A, Loeffler JS, Dyson NJ (2002) Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res 62 (1):200–207 [PubMed] [Google Scholar]
- 14.Buck E, Eyzaguirre A, Brown E, Petti F, McCormack S, Haley JD, Iwata KK, Gibson NW, Griffin G (2006) Rapamycin synergizes with the epidermal growth factor receptor inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and breast tumors. Mol Cancer Ther 5 (11):2676–2684. doi: 10.1158/1535-7163.MCT-06-0166 [DOI] [PubMed] [Google Scholar]
- 15.Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, Baselga J, Guichard S, Rosen N (2011) mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov 1 (3):248–259. doi: 10.1158/2159-8290.CD-11-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G (2004) Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431 (7005):200–205. doi: 10.1038/nature02866 [DOI] [PubMed] [Google Scholar]
- 17.Wei F, Liu Y, Bellail AC, Olson JJ, Sun SY, Lu G, Ding L, Yuan C, Wang G, Hao C (2012) K-Ras mutation-mediated IGF-1-induced feedback ERK activation contributes to the rapalog resistance in pancreatic ductal adenocarcinomas. Cancer Lett 322 (1):58–69. doi: 10.1016/j.canlet.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaturvedi D, Gao X, Cohen MS, Taunton J, Patel TB (2009) Rapamycin induces transactivation of the EGFR and increases cell survival. Oncogene 28 (9):1187–1196. doi: 10.1038/onc.2008.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei F, Zhang Y, Geng L, Zhang P, Wang G, Liu Y (2015) mTOR inhibition induces EGFR feedback activation in association with its resistance to human pancreatic cancer. Int J Mol Sci 16 (2):3267–3282. doi: 10.3390/ijms16023267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bianco R, Garofalo S, Rosa R, Damiano V, Gelardi T, Daniele G, Marciano R, Ciardiello F, Tortora G (2008) Inhibition of mTOR pathway by everolimus cooperates with EGFR inhibitors in human tumours sensitive and resistant to anti-EGFR drugs. Br J Cancer 98 (5):923–930. doi: 10.1038/sj.bjc.6604269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong S, Zhang XC, Cheng H, Zhu JQ, Chen ZH, Zhang YF, Xie Z, Wu YL (2012) Everolimus synergizes with gefitinib in non-small-cell lung cancer cell lines resistant to epidermal growth factor receptor tyrosine kinase inhibitors. Cancer Chemother Pharmacol 70 (5):707–716. doi: 10.1007/s00280-012-1946-3 [DOI] [PubMed] [Google Scholar]
- 22.Herberger B, Berger W, Puhalla H, Schmid K, Novak S, Brandstetter A, Pirker C, Gruenberger T, Filipits M (2009) Simultaneous blockade of the epidermal growth factor receptor/mammalian target of rapamycin pathway by epidermal growth factor receptor inhibitors and rapamycin results in reduced cell growth and survival in biliary tract cancer cells. Mol Cancer Ther 8 (6):1547–1556. doi: 10.1158/1535-7163.MCT-09-0003 [DOI] [PubMed] [Google Scholar]
- 23.Dragowska WH, Weppler SA, Qadir MA, Wong LY, Franssen Y, Baker JH, Kapanen AI, Kierkels GJ, Masin D, Minchinton AI, Gelmon KA, Bally MB (2011) The combination of gefitinib and RAD001 inhibits growth of HER2 overexpressing breast cancer cells and tumors irrespective of trastuzumab sensitivity. BMC Cancer 11:420. doi: 10.1186/1471-2407-11-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Guerrab A, Bamdad M, Bignon YJ, Penault-Llorca F, Aubel C (2020) Co-targeting EGFR and mTOR with gefitinib and everolimus in triple-negative breast cancer cells. Sci Rep 10 (1):6367. doi: 10.1038/s41598-020-63310-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Monica S, Galetti M, Alfieri RR, Cavazzoni A, Ardizzoni A, Tiseo M, Capelletti M, Goldoni M, Tagliaferri S, Mutti A, Fumarola C, Bonelli M, Generali D, Petronini PG (2009) Everolimus restores gefitinib sensitivity in resistant non-small cell lung cancer cell lines. Biochem Pharmacol 78 (5):460–468. doi: 10.1016/j.bcp.2009.04.033 [DOI] [PubMed] [Google Scholar]
- 26.Massarelli E, Lin H, Ginsberg LE, Tran HT, Lee JJ, Canales JR, Williams MD, Blumenschein GR Jr., Lu C, Heymach JV, Kies MS, Papadimitrakopoulou V (2015) Phase II trial of everolimus and erlotinib in patients with platinum-resistant recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 26 (7):1476–1480. doi: 10.1093/annonc/mdv194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryce AH, Rao R, Sarkaria J, Reid JM, Qi Y, Qin R, James CD, Jenkins RB, Boni J, Erlichman C, Haluska P (2012) Phase I study of temsirolimus in combination with EKB-569 in patients with advanced solid tumors. Invest New Drugs 30 (5):1934–1941. doi: 10.1007/s10637-011-9742-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price KA, Azzoli CG, Krug LM, Pietanza MC, Rizvi NA, Pao W, Kris MG, Riely GJ, Heelan RT, Arcila ME, Miller VA (2010) Phase II trial of gefitinib and everolimus in advanced non-small cell lung cancer. J Thorac Oncol 5 (10):1623–1629. doi: 10.1097/JTO.0b013e3181ec1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moran T, Palmero R, Provencio M, Insa A, Majem M, Reguart N, Bosch-Barrera J, Isla D, Costa EC, Lee C, Puig M, Kraemer S, Schnell D, Rosell R (2017) A phase Ib trial of continuous once-daily oral afatinib plus sirolimus in patients with epidermal growth factor receptor mutation-positive non-small cell lung cancer and/or disease progression following prior erlotinib or gefitinib. Lung Cancer 108:154–160. doi: 10.1016/j.lungcan.2017.03.009 [DOI] [PubMed] [Google Scholar]
- 30.Hollebecque A, Bahleda R, Faivre L, Adam J, Poinsignon V, Paci A, Gomez-Roca C, Thery JC, Le Deley MC, Varga A, Gazzah A, Ileana E, Gharib M, Angevin E, Malekzadeh K, Massard C, Soria JC, Spano JP (2017) Phase I study of temsirolimus in combination with cetuximab in patients with advanced solid tumours. Eur J Cancer 81:81–89. doi: 10.1016/j.ejca.2017.05.021 [DOI] [PubMed] [Google Scholar]
- 31.Rathkopf DE, Larson SM, Anand A, Morris MJ, Slovin SF, Shaffer DR, Heller G, Carver B, Rosen N, Scher HI (2015) Everolimus combined with gefitinib in patients with metastatic castration-resistant prostate cancer: Phase 1/2 results and signaling pathway implications. Cancer 121 (21):3853–3861. doi: 10.1002/cncr.29578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gadgeel SM, Lew DL, Synold TW, LoRusso P, Chung V, Christensen SD, Smith DC, Kingsbury L, Hoering A, Kurzrock R (2013) Phase I study evaluating the combination of lapatinib (a Her2/Neu and EGFR inhibitor) and everolimus (an mTOR inhibitor) in patients with advanced cancers: South West Oncology Group (SWOG) Study S0528. Cancer Chemother Pharmacol 72 (5):1089–1096. doi: 10.1007/s00280-013-2297-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauman JE, Arias-Pulido H, Lee SJ, Fekrazad MH, Ozawa H, Fertig E, Howard J, Bishop J, Wang H, Olson GT, Spafford MJ, Jones DV, Chung CH (2013) A phase II study of temsirolimus and erlotinib in patients with recurrent and/or metastatic, platinum-refractory head and neck squamous cell carcinoma. Oral Oncol 49 (5):461–467. doi: 10.1016/j.oraloncology.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.TORISEL® Dosage and Administration. Available at https://www.pfizermedicalinformation.com/en-us/torisel/dosage-admin.2020
- 35.Tarceva prescribing information. Available at https://www.gene.com/download/pdf/tarceva_prescribing.pdf.2020
- 36.Wen PY, Chang SM, Lamborn KR, Kuhn JG, Norden AD, Cloughesy TF, Robins HI, Lieberman FS, Gilbert MR, Mehta MP, Drappatz J, Groves MD, Santagata S, Ligon AH, Yung WK, Wright JJ, Dancey J, Aldape KD, Prados MD, Ligon KL (2014) Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04–02. Neuro Oncol 16 (4):567–578. doi: 10.1093/neuonc/not247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azzariti A, Porcelli L, Gatti G, Nicolin A, Paradiso A (2008) Synergic antiproliferative and antiangiogenic effects of EGFR and mTor inhibitors on pancreatic cancer cells. Biochem Pharmacol 75 (5):1035–1044. doi: 10.1016/j.bcp.2007.11.018 [DOI] [PubMed] [Google Scholar]
- 38.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer 45 (2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 39.Rugo HS, Seneviratne L, Beck JT, Glaspy JA, Peguero JA, Pluard TJ, Dhillon N, Hwang LC, Nangia C, Mayer IA, Meiller TF, Chambers MS, Sweetman RW, Sabo JR, Litton JK (2017) Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol 18 (5):654–662. doi: 10.1016/S1470-2045(17)30109-2 [DOI] [PubMed] [Google Scholar]


