Abstract
Schizophrenia has been hypothesized to be a human-specific condition, but experimental approaches to testing this idea have been limited. Because Neanderthals, our closest evolutionary relatives, interbred with modern humans prior to their disappearance from the fossil record, leaving a residual echo that survives in our DNA today, we leveraged new discoveries about ancient hominid DNA to explore this hypothesis in living people in three converging ways. First, in four independent case-control datasets totaling 9362 individuals, individuals with schizophrenia had less Neanderthal-derived genetic variation than controls (p=0.044). Second, in 49 unmedicated inpatients with schizophrenia, having more Neanderthal admixture predicted less severe positive symptoms (p=0.046). Finally, using 18F-fluorodopa PET scanning in 172 healthy individuals, having greater Neanderthal introgression was significantly associated with lower dopamine synthesis capacity in the striatum and pons (p’s<2×10−5), which is fundamentally important in the pathophysiology and treatment of psychosis. These results may help to elucidate the evolutionary history of a devastating neuropsychiatric disease by supporting the notion of schizophrenia as a human-specific condition. Additionally, the relationship between Neanderthal admixture and dopamine function suggests a potential mechanism whereby Neanderthal admixture may have affected our gene pool to alter schizophrenia risk and/or course.
Keywords: Neanderthal Admixture, Introgression, Psychosis, Dopamine Function, Neuroleptic Response
Introduction:
Schizophrenia is a severe, debilitating mental illness characterized by disordered thought, psychotic symptoms such as hallucinations and paranoid delusions, emotional blunting, and cognitive impairments. Because analogues of this constellation of symptoms do not naturally occur in other species (Crow, 2000), it has been hypothesized that this disease is a human-specific condition and that its evolutionary origins may be related to the emergence of abilities that characterize modern humans, such as language (Crow, 2000), social functioning (Burns, 2006), and/or creativity (Power et al., 2015). The fact that the prevalence of schizophrenia has remained relatively stable at about 1% in human populations throughout the world (McGrath, Saha, Chant, & Welham, 2008) despite substantially decreased fecundity in patients (Power et al., 2013; van Dongen & Boomsma, 2013) suggests that some of these abilities that have been associated with the emergence of this illness may be under positive selection pressure, and understanding how natural selection is linked to the genetic risk for schizophrenia may provide important insights (Pardinas et al., 2018). The heritability of schizophrenia, now estimated to be up to 80% (Sullivan, Kendler, & Neale, 2003), has been of primary interest – with regard to its potential to inform diagnosis, prediction of disease, and treatment – since the 1971 seminal studies by Seymour Kety and colleagues of adopted-away patients and children of patients (Ingraham & Kety, 2000; Kety, Rosenthal, Wender, & Schulsinger, 1971; Rosenthal, Wender, Kety, Welner, & Schulsinger, 1971). The search for the genetic underpinnings of schizophrenia has, therefore, received considerable attention in the research portfolio throughout the field of neuropsychiatry.
Recent work within this portfolio has highlighted the polygenic nature of schizophrenia, demonstrating that a large number of genetic variants, each of which alone conveys a small risk, converge to collectively produce the phenotypic characteristics of this patient population (Schizophrenia Working Group of the Psychiatric Genomics, 2014). Prior work has also suggested that the pattern of polygenic risk may even uncover various subgroups of disease (Dickinson et al., 2020). Such endeavors may have implications for how we understand schizophrenia and disease risk and for how we think about and treat patients.
In addition to contributing to fundamental disease-related knowledge, the genetic revolution has also allowed unprecedented insights into evolutionary biology through evaluation of ancient hominid DNA. As a case in point, sequencing of the Neanderthal genome has revealed that, before they disappeared from the fossil record, Neanderthals mixed with the ancestors of modern humans ancestors some 47,000 to 65,000 years ago (Sankararaman, Patterson, Li, Paabo, & Reich, 2012). The result of this admixture lives on today, accounting for approximately 2% of the non-African genome (Prufer et al., 2017; Prufer et al., 2014). It has been shown that this inheritance is not just an idle part of the human genome, but that it, indeed, carries functional ramifications. For example, Neanderthal-derived variation is associated with risk for autoimmune disorders (Sankararaman et al., 2014) and affects keratin filaments, which may have helped to modify skin and hair to adapt to non-African climates (Sankararaman et al., 2014; Vernot & Akey, 2014). Of particular relevance for understanding uniquely human characteristics, Neanderthal admixture biases the skull shape of living humans to more resemble Neanderthal fossil remains (Gregory et al., 2017), affects morphological characteristics of the modern human brain (Gregory et al., 2017; Gregory et al., 2020; Kochiyama et al., 2018), is associated with neuronal gene expression (Simonti et al., 2016), and is overrepresented in genes known to be responsible for neurogenesis (Akkuratov, Gelfand, & Khrameeva, 2018). In contrast, other evidence has shown that the genetics underlying schizophrenia risk emerged recently in hominid evolution, after modern humans and Neanderthals diverged (Srinivasan et al., 2017), and is enriched in human accelerated genetic regions (Xu, Schadt, Pollard, Roussos, & Dudley, 2015). This new information about ancient DNA, together with an enhanced understanding of the genetic mechanisms governing brain development and mental illnesses, paves the way for exploring the evolutionary nature of neuropsychiatric conditions such as schizophrenia.
Because of speculations that schizophrenia is a human-specific condition and because Neanderthals, whose genetic information we still carry, are our closest evolutionary relatives, here, we first tested whether the amount of Neanderthal DNA an individual harbors relates to risk for schizophrenia in four independent case-control samples comprising a total of 9362 people. Expecting that if schizophrenia is indeed a human-specific condition and if Neanderthal-introgressed loci in humans intersect with schizophrenia genetic risk, then afflicted individuals might have less Neanderthal introgression, we next tested whether the degree of such Neanderthal-derived genetic variation relates both to the severity of the psychotic symptoms patients experience and to their response to antipsychotic medications during a blinded, placebo-controlled, crossover study. Finally, as these antipsychotic medications typically act through dopaminergic mechanisms, we also examined whether Neanderthal admixture in the general population was related to dopamine synthesis capacity, as measured by 18F-fluorodopa (FDOPA) PET scanning.
Materials and Methods:
Participants:
Three substudies carried out to address the main questions defined above are described below. Table 1 lists demographic information for the cohorts investigated in each substudy. All procedures were carried out in accordance with United States National Institutes of Health (NIH) guidelines and were approved by the NIH Institutional Review Board. Participants in samples obtained from dbGaP (referenced below) provided informed consent to have data shared. Participants for the NIMH samples referenced below provided written informed consent for all procedures; diagnosis was based on DSM-IV criteria and was confirmed with clinician-administered structured clinical interview (SCID). These participants were found to be free of confounding psychiatric, neurological, substance-related, or major medical illness (except schizophrenia spectrum illness in the patient groups) by physician-conducted medical history and physical examination, and by structural magnetic resonance imaging (MRI) and routine laboratory tests including urine toxicology.
Table 1:
| SUBSTUDY | Sample Size (N) (SCZ/Control) | AgeSCZ | Agecontrol | SexSCZ (N) | Sexcontrol (N) |
|---|---|---|---|---|---|
|
| |||||
| Neanderthal DNA & Schizophrenia Diagnosis | |||||
| NIMH | 1367 (537/830) | 34.1±10.1 yrs | 31.1±9.7 yrs | 397 M, 140 F | 381 M, 449 F |
| Ashkenazi Jewish | 3052 (1027/2025) | 45.3±13.9 yrs | 42.4±16.7 yrs | 649 M, 378 F | 1482 M, 543 F |
| GAIN Schizophrenia | 2550 (1173/1377) | 43.8±11.3 yrs | 51.1±17.0 yrs | 820 M, 353 F | 633 M, 744 F |
| Non-GAIN Schizophrenia* | 2393 (1100/1293) | 43.0±12.0 yrs | 49.9±15.8 yrs | 766 M, 334 F | 644 M, 649 F |
| Total | 9362 (3837/5525) | 42.6±12.6 yrs | 44.6±17.1 yrs | 2632 M/1205 F | 3140 M, 2385 F |
|
| |||||
| Neanderthal DNA & Schizophrenia symptoms (NIMH subsample) | 49 (49/0) | 29.9±9.0 yrs | N/A | 37 M, 12 F | N/A |
|
| |||||
| Neanderthal DNA & FDOPA PET (NIMH subsample) | 173 (0/173) | N/A | 36.5±12.4 yrs | N/A | 86 M, 87 F |
Age was not provided for 1.2% of the samples from the non-GAIN Schizophrenia dataset.
Abbreviations:
SCZ = Schizophrenia
FDOPA = 18F-fluorodopa
PET = positron emission tomography
Yrs = years
M = Male F = Female
Genetic Analyses – Estimating Neanderthal Admixture:
For the NIMH sample, details of genotyping and imputation have been previously reported (Gregory et al., 2019). Briefly, SNP-based genetic information was derived from blood lymphocytes using Illumina genome-wide SNP chips (550K-2.5M SNPs). After genotype QC (Anderson et al., 2010), data were phased using Shapeit and imputed using Impute2 software. For the three other samples, genotype data were obtained from dbGaP, and the same QC, phasing, and imputation procedures were performed. For all samples, only unrelated individuals of European descent were included in further analyses.
Previous studies have taken varying approaches to estimate Neanderthal admixture in living individuals for associating with differing phenotypes. Some have used the proportion of the genome derived from Neanderthals (Sankararaman et al., 2014), while others have used selective sweeps to detect regions of Neanderthal admixture (Peyregne, Boyle, Dannemann, & Prufer, 2017). In this work, we employed an enhanced D statistic, the NeanderScore metric (Gregory et al., 2017), to estimate the degree of Neanderthal admixture in an individual. This score was calculated for each individual, as:
where ∑(nABBA) is the count of SNP locations at which the genotyped individual shares an allele with the Neanderthal sequence but differs from all Yoruba and primate sequences, and ∑(nAABA) is the count of locations at which the genotyped individual shares an allele with Yoruba and primate sequences but differs from the Neanderthal sequence (Figure 1). Enhanced D statistics for Neanderthal introgression have been previously shown to be linearly related to the amount of Neanderthal ancestry in an individual (Fu et al., 2016) and are termed “enhanced” because they improve the signal-to-noise ratio by restricting computation to sites where an isolated population carries only the ancestral allele (Meyer et al., 2012). This metric has also been validated by being associated with variations skull shape in living modern humans that recapitulate Neanderthal-to-modern human differences in cranial remains (Gregory et al., 2017) and has been related to brain structure and function (Gregory et al., 2017; Gregory et al., 2020). Ancestry-related components were calculated from the genetic data using the ‘pca’ function in Plink (version 1.9, https://www.cog-genomics.org/plink2) for each sample separately.
Figure. 1: Derivation of the NeanderScore metric.

Phylogenetic tree demonstrating the evolutionary relationship between Yorubans, genotyped individuals of European ancestry, Neanderthals, and primates. Note that the tree is not drawn to scale. For each SNP used in the NeanderScore metric, sites were identified where Yoruban and primate sequences contained only the ancestral allele (A), whereas only the derived allele (B) is found in the Neanderthal genome, implying the B allele emerged during Neanderthal speciation (red line). For each individual genotyped in this study, either the ancestral allele shared with Yorubans and primates (A) or the derived allele introgressed through admixture with Neanderthals (B) might be found. This SNP could be either AABA or ABBA (reading the genotype column/gray box from top to bottom) for a given individual. NeanderScore, an enhanced D statistic representing the percentage of Neanderthal-derived genetic variation in a given individual, was calculated as per the equation in the text.
Neanderthal DNA and Schizophrenia Diagnosis:
Participants:
Four independent samples, together comprising 9362 individuals (3837 patients with schizophrenia and 5525 controls), were included in a case-control analysis of schizophrenia diagnosis. The NIMH cohort was collected at the NIH Clinical Center, under NIH protocol 95-M-0150, NCT00001486, ZIAMH002942. Three additional cohorts were obtained from the publicly available dbGaP repository (https://www.ncbi.nlm.nih.gov/gap/). These included an Ashkenazi Jewish case-control schizophrenia sample (dbGaP accession #: phs000448), the Genetic Association Information Network (GAIN) schizophrenia sample (dbGaP accession #: phs000021), and the Molecular Genetics of Schizophrenia non-GAIN sample (dbGaP accession #: phs000167).
Statistical Analyses:
NeanderScore, as defined above, was calculated for each individual in each cohort. It is important to note that although the process of calculating NeanderScore is the same in each cohort, the mean and standard deviations of the measure across cohorts will be different as it is dependent on the SNPs-arrays used for genotyping. For this reason, analyses were undertaken separately in each cohort and combined using a meta-analytic approach after standardizing measures within each group. After controlling for ancestry-related components using linear regression, standardized mean and standard deviation NeanderScore values for both schizophrenia and control groups were calculated for each sample separately. The ‘metafor’ R package was used to calculate the meta-analytic standard mean difference and level of significance for the combined group.
Neanderthal DNA and Psychotic Symptoms:
Participants:
Forty-nine patients with schizophrenia who were a part of the NIMH cohort described above completed a dual-armed, blinded, balanced, crossover medication withdrawal study while inpatients on the NIMH schizophrenia research ward at the NIH Clinical Center. Here, diagnosis was confirmed, symptoms were rated by trained observers, and medication compliance was carefully observed under NIH protocol 89-M-0160, NCT00001247, ZIAMH002652. Demographic details are shown in Table 1.
Medication Withdrawal Protocol:
Full details of the clinical protocol have been previously reported (Eisenberg et al., 2017). Briefly, following stabilization on monotherapy with neuroleptic medication, each patient entered a blinded, dual-arm, balanced cross-over, medication withdrawal protocol. Symptoms were assessed with the Positive and Negative Syndrome Scale (PANSS),(Kay, Fiszbein, & Opler, 1987) as administered by blinded raters after at least 3 weeks of both active medication and medication-free treatment arms, and total, positive, and negative symptoms scores were calculated (Wallwork, Fortgang, Hashimoto, Weinberger, & Dickinson, 2012).
Statistical Analyses:
Change in PANSS symptom clusters was calculated as the medication-free rating minus the active medication rating. Linear regression in SPSS (IBM Corp, Released 2019, IBM SPSS Statistics Subscription, Armonk, NY) was performed to determine the relationship between NeanderScore and both the medication-free ratings and the change in symptoms, controlling for ancestry-related components.
Neanderthal DNA and Dopamine Synthesis Capacity:
Participants:
A subset of 173 healthy adults of from the NIMH sample described above participated in an FDOPA PET scanning study under NIH protocol 01-M-0232, NCT00024622, ZIAMH002717. Demographic details of this subgroup are given in Table 1.
FDOPA PET Scanning Procedures and Processing:
FDOPA procedures and image processing methods have been described previously (Eisenberg et al., 2016). Briefly, PET scanning included an 8-minute transmission scan for attenuation correction and a 90-minute series of 27 dynamically acquired emission scans immediately following intravenous injection of ~16 mCi FDOPA for each participant. Filtered back-projection was performed, and adjustments for radioactive decay, dead time, and scatter were applied. A registration attenuation correction algorithm additionally accounted for head motion. Participants also underwent structural brain imaging to obtain a T1-weighted structural brain scan used for coregistration purposes. ANTS software (http://stnava.github.io/ANTs) was used to warp each participant’s anatomic and PET data to standardized MNI space. Resulting data were spatially smoothed with a 10mm FWHM gaussian kernel using SPM software (https://www.fil.ion.ucl.ac.uk/spm/). The kinetic rate constant (Ki), representing specific tracer uptake and a measure of dopamine synthesis capacity, was calculated on a voxel-wise basis using the Patlak-Gjedde method (Patlak & Blasberg, 1985), as implemented in PMOD software (http://www.pmod.com) with the time-activity data from a cerebellar reference region serving as the image-derived input function.
Statistical Analyses:
Linear regression tested for the association between FDOPA Ki and NeanderScore, controlling for age, sex, and ancestry-related components in whole-brain analysis at each voxel using AFNI tools (https://afni.nimh.nih.gov). Results were thresholded at p<0.05, FWE-corrected for multiple comparisons, using AFNI’s 3dClustSim function to compute a cluster threshold based on 100,000 Monte Carlo simulations of synthesized white Gaussian noise, taking into account the smoothing and resampling parameters of the PET data using the ACF method (Cox, Chen, Glen, Reynolds, & Taylor, 2017).
Results:
Neanderthal DNA and Schizophrenia Diagnosis
To test whether the load of Neanderthal-derived genetic variants was related to schizophrenia diagnosis, we first performed a case-control analysis on four independent schizophrenia samples, together totaling 9362 individuals (3837 patients with schizophrenia and 5525 controls), controlling for ancestry-related components. In all samples, patients with schizophrenia had less Neanderthal admixture (lower “NeanderScores”) than did controls (standard mean difference=0.043 [confidence interval 0.0012–0.084], p=0.044). The relationship was significant in the combined meta-analytic group, but not in any single sample alone, though the direction of the finding was the same in all samples (Figure 2). This result did not appear to be significantly related to heterogeneity as Q(df=3)=0.48 (p=0.92) and I2=0%, suggesting that the variance between cohorts was predominantly due to sampling.
Figure 2:
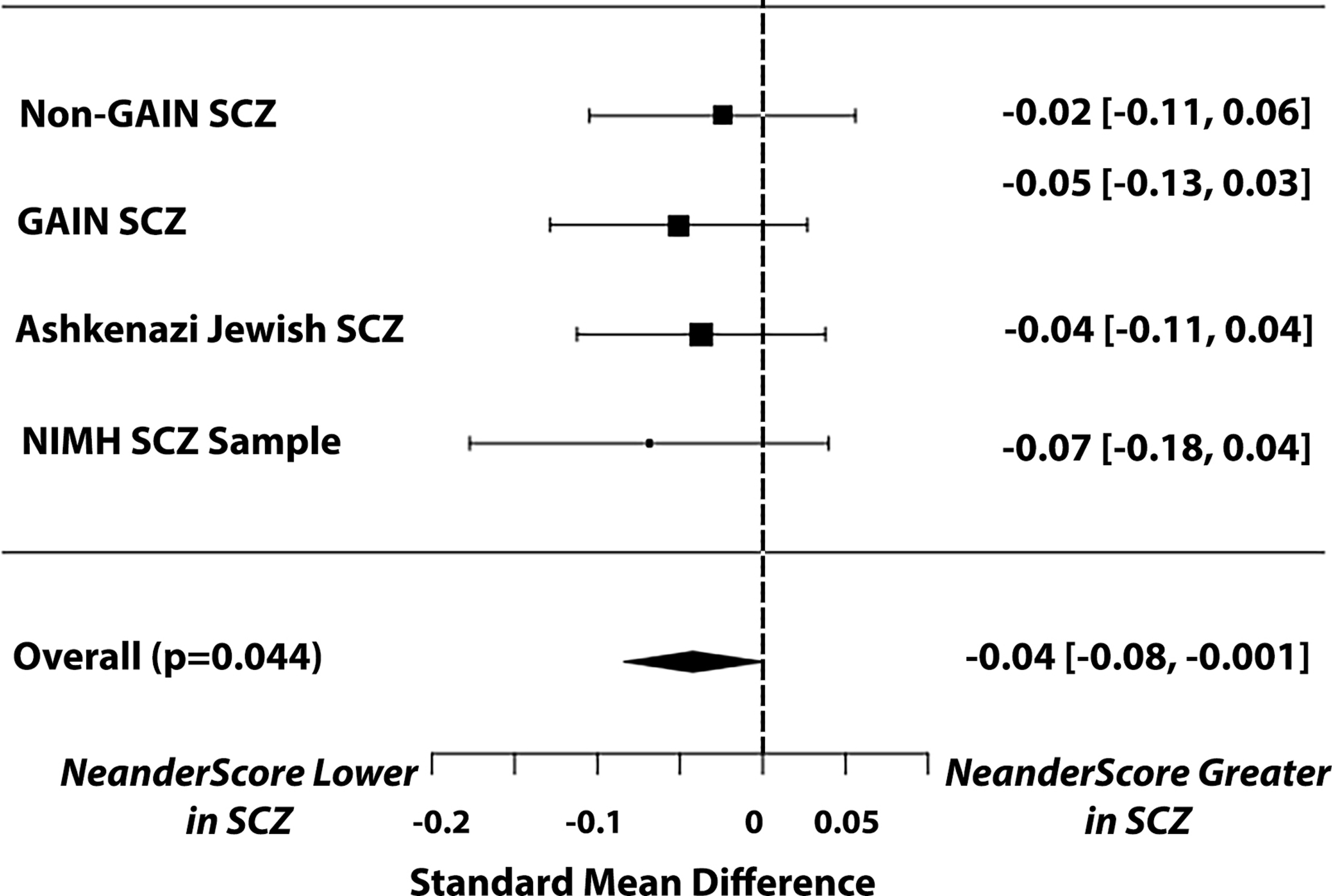
Relationship between NeanderScore and schizophrenia diagnosis in four independent case-control datasets. Plot shows the standard mean difference (SMD) of the individual datasets and confidence intervals. Flat diamond shows the overall meta-analytic effect of significantly lower NeanderScore in individuals with schizophrenia (SMD=0.043, p=0.044). Note that the direction of the effect is the same in all samples, such that individuals with schizophrenia in each sample had lower NeanderScores than controls.
Neanderthal DNA and Psychotic Symptoms
Given that the degree of Neanderthal admixture was lower in patients with schizophrenia, offering some support for the notion that schizophrenia may be a human-specific condition, we next tested whether NeanderScore variability was related to symptom severity. After controlling for ancestry components, PANSS scores during the medication-free arm of the protocol correlated with NeanderScores such that patients with higher NeanderScores had lower positive symptom scores, which include hallucinations and delusions (Figure 3A, R2=0.079, p=0.046). A trend toward a relationship between higher NeanderScore and fewer total symptoms (Figure 3B, R2=0.063, p=0.073) was also observed, but no relationship was found with negative symptoms, which include affective blunting and cognitive symptoms (R2=0.026, p=0.266). Additionally, higher NeanderScores also showed a trend-level association with less improvement in patients’ positive symptoms when they were treated with antipsychotic medications (Figure 3C, R2=0.067, p=0.055). It is notable that, among the manifestations of schizophrenia, positive symptoms are thought to be particularly ameliorated by medications whereas negative symptoms are not, and that we found no relationship with change in total symptoms (change in R2=0.034, p=0.172) or with change in negative symptoms (R2=0.015, p=0.367).
Figure 3:
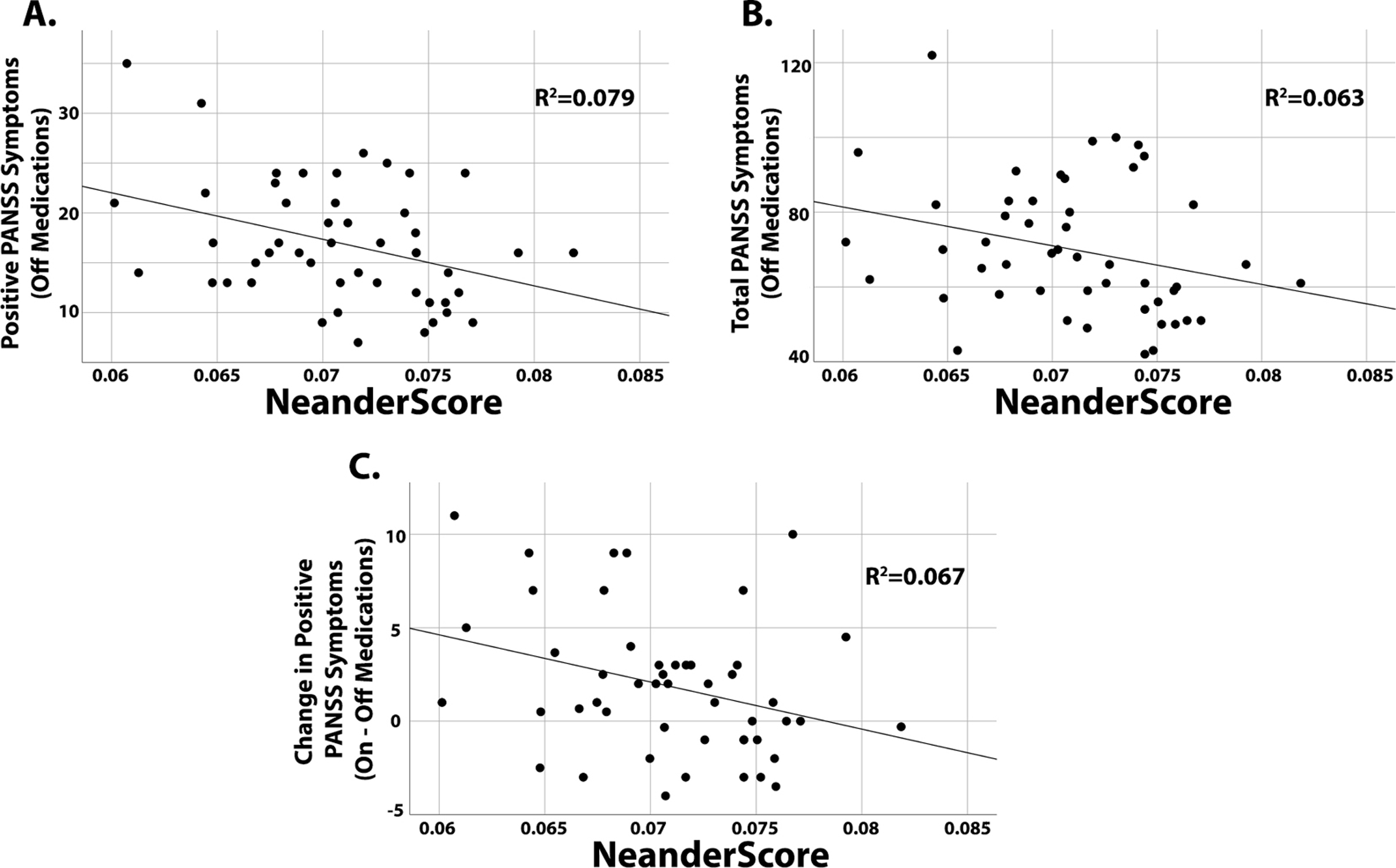
Relationships of NeanderScore with schizophrenia symptoms, as measured by the PANSS. A) The relationship of NeanderScore with positive symptoms in patients during the off-medications arm of the protocol. B) The relationship of NeanderScore with total PANSS scores in patients while off medications. C) The relationship of NeanderScore with the change in positive symptoms individuals experienced between antipsychotic treatment and off-medication arms.
Neanderthal DNA and Dopamine Synthesis Capacity
Finally, because dopaminergic mechanisms are thought to play a major role in the pathophysiology of positive symptoms in schizophrenia and antipsychotic medications predominantly act through blockade of dopamine receptors, we tested whether NeanderScore also related to dopamine synthesis capacity, as measured by FDOPA PET scanning (Figure 4). In a subgroup of 173 healthy adults from the NIMH sample who underwent FDOPA PET scanning and were genotyped, we found that NeanderScore was negatively correlated with FDOPA uptake in the left caudate, extending to the ventral striatum (peak MNIx,y,z= −8,2,3; Zmax=−4.29, p=1.8×10−5), and in the pons (peak MNIx,y,z= −11,−33,−32; Zmax=−4.35, p=1.4×10−5). Interestingly, dopamine synthesis in the striatum has also been found to be altered in schizophrenia. Additionally, this striatal region is known to be important for reward processing, and the pontine region is known to subserve pain pathways (Neurosynth Online Meta-Analytic Functional Imaging Atlas, https://neurosynth.org).
Figure 4:

Relationship of NeanderScore with dopamine synthesis capacity in 179 healthy participants. Sagittal slice (MNI x = −9) shows brain regions where NeanderScore was correlated with dopamine synthesis capacity (p<0.05, FWE-corrected), as measured by 18F-fluorodopa PET uptake (Ki); color bar shows z-values (blues indicate a negative relationship between NeanderScore and FDOPA Ki). Peak of the striatal region was MNIx,y,z= −8,2,3 and peak of the pontine region was MNIx,y,z= −11,−33,−32. Graphs show the relationship between FDOPA uptake and NeanderScore for striatal and pontine regions. Note that for both regions, individuals with higher NeanderScores had lower dopamine synthesis capacity.
Discussion:
In this work, we sought evidence that schizophrenia is a human-specific condition by testing whether the degree of genetic information inherited from our closest evolutionary relatives was related to the presence of schizophrenia, to the severity of schizophrenia symptoms, and to the amelioration of these symptoms by dopamine blockade with antipsychotic medications. In these complementary investigations, we showed that overall schizophrenia risk and the severity of psychotic symptomatology were reduced in individuals with the greatest proportion of Neanderthal-derived genetic variation. Further, exploring one potential mechanism for these effects, we demonstrated that healthy individuals with higher NeanderScores had lower dopamine synthesis capacity in the striatum and pons and that symptoms in patients with higher NeanderScores responded less robustly to dopamine blockade with neuroleptics.
The finding that individuals with schizophrenia tend to have less Neanderthal admixture than unaffected individuals was observed by examining four relatively large case-control samples. Though this result was not significant in any one sample alone, the directionality was the same in all samples and, when the samples were grouped together, the meta-analysis was statistically significant. The fact that the effect size was modest, accounting for about 4% of the variability in diagnosis, is not unexpected given the multifactorial genetic and environmental processes likely underlying schizophrenia. Nonetheless, these results point to an important evolutionary role in the emergence of schizophrenia and lend support to the long-held theory that schizophrenia is a condition specific to modern (as opposed to archaic) humans. This notion is further reinforced by our findings regarding psychotic symptoms, where individuals with a greater degree of Neanderthal admixture had less severe symptoms of disease when not receiving pharmacologic treatment.
Consistent with our case-control results, prior research investigating the evolutionary origins of schizophrenia has shown that the genetic risk for this disorder arose recently in hominid evolution after the time that H. Neanderthalensis and H. Sapiens diverged (Srinivasan et al., 2017), that this genetic risk is enriched in human accelerated regions of our genome (Xu et al., 2015), and that there is a negative association between schizophrenia risk and metrics of Neanderthal selective sweep scores (Srinivasan et al., 2016). While another previous study examined whether specific SNPs with high posterior probability of being derived from Neanderthals are associated with the heritability of schizophrenia risk and did not find a significant relationship (Pardinas et al., 2018), that result is difficult to compare to the present findings, because the measure of Neanderthal inheritance applied in that work, based on the individual contributions of Neanderthal-derived alleles to schizophrenia risk, differs substantially our measure, which estimates the total degree of admixture an individual harbors across the entire genome. Together, this body of work provides insights about the repercussions of the residual echo of ancient DNA that is present in modern humans. Our results extend previous investigations by assessing the effects of Neanderthal admixture on schizophrenia diagnosis and on the severity of schizophrenia symptoms in carefully observed inpatients cared for on a research ward at the National Institutes of Health.
We also sought to uncover neuromechanistic insights by testing for a relationship of Neanderthal admixture to dopamine systems biology and to the response of schizophrenia symptoms to neuroleptic medications. In view of the findings that individuals with greater Neanderthal introgression have less dopamine synthesis capacity in known dopaminergic regions of the brain and that they also had the lowest response (i.e., change in positive symptoms) to pharmacologic dopamine blockade in the studied cohort, it is plausible that dopaminergic mechanisms might be important in the relationship between schizophrenia and Neanderthal admixture. Though the association between dopamine and Neanderthal DNA may be new, the idea that dopamine function might underlie schizophrenia pathophysiology is not a new one and was likely first postulated in the 1950s when chlorpromazine was found to provide symptomatic benefit (Delay, Deniker, & Harl, 1952). The dopamine hypothesis of schizophrenia has come to be an enduring foundation in psychiatry and has been modified over the years to account for novel information about the disease (Howes & Kapur, 2009). The anatomic localization of the findings, specifically to the striatum, is consistent with literature examining FDOPA PET scanning in schizophrenia, where striatal FDOPA uptake has been shown to be higher in some individuals with schizophrenia, but, as shown here, is lowest in individuals with the highest NeanderScores (Meyer-Lindenberg et al., 2002; Reith et al., 1994). Together, our results are consistent with the notion that dopaminergic mechanisms may play a role in the relationship of Neanderthal introgression with schizophrenia diagnosis and symptom severity.
A number of ethical considerations must be weighed when undertaking research that relies upon a medication withdrawal protocol (Carpenter, Schooler, & Kane, 1997). The independent IRB, the investigators, and the patients must weigh the risks (including the possible reemergence or worsening of psychotic symptoms) against the benefits to science and to the participants. The latter may include a better understanding of the need for medications, more complete characterization of symptoms, and optimization of pharmacologic regimens from the ground up. Our patients are closely monitored and evaluated on the NIH Clinical Center’s inpatient research ward by staff who are specifically trained, skilled, and experienced in treating medication-free patients, and our protocol is regularly reviewed by the independent NIH IRB and an independent safety monitor. Patients sign consent documents ensuring that they understand potential risks in accordance with IRB regulations. Nonetheless, choosing to participate in studies such as this, invariably, is a personal decision for patients requiring complex discussions and independent oversight to ensure that patients are truly informed to provide their consent.
Related to the clinical difficulty of studying medication-free patients with schizophrenia are the relatively modest sample size of this component of the work and the possibility that a longer placebo period may have allowed more complete symptomatic expression in some patients, both of which could have limited our ability to relate symptomatology to NeanderScore. In this context, it is worth noting that the statistical association between NeanderScore, psychotic symptoms, and response to medications, although consistent with hypothesized directions, was less robust than other reported relationships, with response to medications at trend level. Future work, especially studying patients suffering a first episode of psychosis, who may present in a medication-free state, may enable larger sample sizes within which to further test these associations.
It is important to note that although our current study highlights the relationships of Neanderthal admixture with schizophrenia diagnosis, symptom severity, and dopamine production, the NeanderScore metric utilized here is a genome-wide measure of total ‘burden’ of Neanderthal introgression, analogous to polygenic scores used in other modes of research. This method does not identify specific genes or genetic mechanisms that might drive the relationships; it only documents that the genetic relationship exists. The present work may help to focus future research on the functional implications of this ancient inheritance, particularly regarding genes important for schizophrenia that may have variation derived from admixture or that are implicated in dopaminergic neurotransmission.
While our work here was not designed to test for the importance of our findings to specific behavioral and cognitive domains, the neuroanatomical localization of the association between Neanderthal introgression and FDOPA uptake to the striatum and pons may allow for some inference. Not only is the highly dopaminergic striatal region identified here important in the pathophysiology of schizophrenia, it has also been implicated in reward pathways (Haber, 2016) that are known to be aberrant in schizophrenia. Though the pontine region is not historically thought of as dopaminergic and it is possible that other DOPA decarboxylase-containing neurons may contribute to the FDOPA signal, recent evidence indicates that dopamine plays a key role in pain processing in this region (Taylor et al., 2019). Based on these findings, it is interesting to speculate that the role of dopaminergic neurotransmission in the Neanderthal brain may have been different than in modern humans, particularly in relation to reward and pain processing. Future work could test whether individuals with a greater degree of Neanderthal-derived variation show altered neurophysiological and behavioral responses to reward and pain.
From a broad view, this work provides new insights into who and what we are as a species, about our remarkable interindividual variability, and about what may go awry to produce serious neuropsychiatric disorders. Our data align with the notion that schizophrenia is a uniquely modern human disease and suggest that having greater Neanderthal admixture is associated with reduced presence of the disease as well as reduced severity of symptoms. The data are also interesting when viewed from the perspective of the dopamine hypothesis of schizophrenia. Finally, this work also provides a lens through which to consider the neurobiology of our lost evolutionary cousins and the genetic mechanisms that make us the individuals we are today.
Acknowledgements:
This work was supported by the Intramural Research Program (ZIAMH002942, ZIAMH002717, and ZIAMH002652), National Institute of Mental Health, National Institutes of Health, Bethesda, MD. All authors report no competing financial interests with regard to this manuscript. The data for the NIMH Sample were obtained under protocols 00M0085, 95M0150 and 01M0232 (NCT#s: NCT00001486, NCT00001247, NCT000024622). Funding support for the GAIN Genome-Wide Association of Schizophrenia Study was provided by the National Institute of Mental Health (R01 MH67257, R01 MH59588, R01 MH59571, R01 MH59565, R01 MH59587, R01 MH60870, R01 MH59566, R01 MH59586, R01 MH61675, R01 MH60879, R01 MH81800, U01 MH46276, U01 MH46289 U01 MH46318, U01 MH79469, and U01 MH79470) and the genotyping of samples was provided through the Genetic Association Information Network (GAIN). The datasets used for the analyses described in this manuscript were obtained from the database of Genotypes and Phenotypes (dbGaP) found at http://www.ncbi.nlm.nih.gov/gap through dbGaP accession number phs000021.v3.p2. Samples and associated phenotype data for the Genome-Wide Association of Schizophrenia Study were provided by the Molecular Genetics of Schizophrenia Collaboration (PI: Pablo V. Gejman, Evanston Northwestern Healthcare (ENH) and Northwestern University, Evanston, IL, USA). Funding support for the Molecular Genetics of Schizophrenia - nonGAIN Sample (MGS_nonGAIN), was provided by Genomics Research Branch at NIMH and the genotyping and analysis of samples was provided through the Genetic Association Information Network (GAIN) and under the MGS U01s: MH79469 and MH79470. Assistance with data cleaning was provided by the National Center for Biotechnology Information. The MGS dataset(s) used for the analyses described in this manuscript were obtained from dbGaP accession numbers phs000167.v1.p1. Samples and associated phenotype data for the MGS GWAS study were collected under the following grants: NIMH Schizophrenia Genetics Initiative U01s: MH46276 (CR Cloninger), MH46289 (C Kaufmann), and MH46318 (MT Tsuang); and MGS Part 1 (MGS1) and Part 2 (MGS2) R01s: MH67257 (NG Buccola), MH59588 (BJ Mowry), MH59571 (PV Gejman), MH59565 (Robert Freedman), MH59587 (F Amin), MH60870 (WF Byerley), MH59566 (DW Black), MH59586 (JM Silverman), MH61675 (DF Levinson), and MH60879 (CR Cloninger). The Ashkenazi Jewish dataset used for the analysis described in this manuscript were obtained from dbGaP accession number phs000448.v1.p1. Submission of the data to dbGaP was provided by Dr. Todd Lencz and on behalf of himself and his collaborator, Ariel Darvasi, Ph.D.. Support for the collection and analysis of the datasets was provided by RC2MH089964, R01 MH084098, the North Shore - LIJ Health System Foundation, and the Hebrew University Genetic Resource. Some of this work utilized the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov).
Footnotes
Competing Interests:
All authors report no competing financial interests with regard to this manuscript.
References:
- Akkuratov EE, Gelfand MS, & Khrameeva EE (2018). Neanderthal and Denisovan ancestry in Papuans: A functional study. J Bioinform Comput Biol, 16(2), 1840011. doi: 10.1142/S0219720018400115 [DOI] [PubMed] [Google Scholar]
- Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, & Zondervan KT (2010). Data quality control in genetic case-control association studies. Nat Protoc, 5(9), 1564–1573. doi: 10.1038/nprot.2010.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JK (2006). Psychosis: a costly by-product of social brain evolution in Homo sapiens. Prog Neuropsychopharmacol Biol Psychiatry, 30(5), 797–814. doi: 10.1016/j.pnpbp.2006.01.006 [DOI] [PubMed] [Google Scholar]
- Carpenter WT Jr., Schooler NR, & Kane JM (1997). The rationale and ethics of medication-free research in schizophrenia. Arch Gen Psychiatry, 54(5), 401–407. doi: 10.1001/archpsyc.1997.01830170015002 [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, & Taylor PA (2017). FMRI clustering in AFNI: False-positive rates redux. Brain Connect, 7(3), 152–171. doi: 10.1089/brain.2016.0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ (2000). Schizophrenia as the price that homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain Res Brain Res Rev, 31(2–3), 118–129. doi: 10.1016/s0165-0173(99)00029-6 [DOI] [PubMed] [Google Scholar]
- Delay J, Deniker P, & Harl JM (1952). [Therapeutic use in psychiatry of phenothiazine of central elective action (4560 RP)]. Ann Med Psychol (Paris), 110(2 1), 112–117. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12986408 [PubMed] [Google Scholar]
- Dickinson D, Zaidman SR, Giangrande EJ, Eisenberg DP, Gregory MD, & Berman KF (2020). Distinct polygenic score profiles in schizophrenia subgroups with different trajectories of cognitive development. Am J Psychiatry, 177(4), 298–307. doi: 10.1176/appi.ajp.2019.19050527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DP, Kohn PD, Hegarty CE, Ianni AM, Kolachana B, Gregory MD, … Berman KF (2016). Common variation in the DOPA Decarboxylase (DDC) gene and human striatal DDC activity in vivo. Neuropsychopharmacology, 41(9), 2303–2308. doi: 10.1038/npp.2016.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DP, Yankowitz L, Ianni AM, Rubinstein DY, Kohn PD, Hegarty CE, … Berman KF (2017). Presynaptic dopamine synthesis capacity in schizophrenia and striatal blood flow change during antipsychotic treatment and medication-free conditions. Neuropsychopharmacology, 42(11), 2232–2241. doi: 10.1038/npp.2017.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Posth C, Hajdinjak M, Petr M, Mallick S, Fernandes D, … Reich D (2016). The genetic history of Ice Age Europe. Nature, 534(7606), 200–205. doi: 10.1038/nature17993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MD, Kippenhan JS, Callicott JH, Rubinstein DY, Mattay VS, Coppola R, & Berman KF (2019). Sequence variation associated with SLC12A5 gene expression is linked to brain structure and function in healthy adults. Cereb Cortex, 29(11), 4654–4661. doi: 10.1093/cercor/bhy344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MD, Kippenhan JS, Eisenberg DP, Kohn PD, Dickinson D, Mattay VS, … Berman KF (2017). Neanderthal-derived genetic variation shapes modern human cranium and brain. Sci Rep, 7(1), 6308. doi: 10.1038/s41598-017-06587-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MD, Kippenhan JS, Kohn P, Eisenberg DP, Callicott JH, Kolachana B, & Berman KF (2020). Neanderthal-derived genetic variation is associated with functional connectivity in the brains of living humans. Brain Connect. doi: 10.1089/brain.2020.0809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN (2016). Corticostriatal circuitry. Dialogues Clin Neurosci, 18(1), 7–21. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27069376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, & Kapur S (2009). The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull, 35(3), 549–562. doi: 10.1093/schbul/sbp006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham LJ, & Kety SS (2000). Adoption studies of schizophrenia. Am J Med Genet, 97(1), 18–22. doi: [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, & Opler LA (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull, 13(2), 261–276. doi: 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- Kety SS, Rosenthal D, Wender PH, & Schulsinger F (1971). Mental illness in the biological and adoptive families of adpoted schizophrenics. Am J Psychiatry, 128(3), 302–306. doi: 10.1176/ajp.128.3.302 [DOI] [PubMed] [Google Scholar]
- Kochiyama T, Ogihara N, Tanabe HC, Kondo O, Amano H, Hasegawa K, … Akazawa T (2018). Reconstructing the Neanderthal brain using computational anatomy. Sci Rep, 8(1), 6296. doi: 10.1038/s41598-018-24331-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Saha S, Chant D, & Welham J (2008). Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev, 30, 67–76. doi: 10.1093/epirev/mxn001 [DOI] [PubMed] [Google Scholar]
- Meyer M, Kircher M, Gansauge MT, Li H, Racimo F, Mallick S, … Paabo S (2012). A high-coverage genome sequence from an archaic Denisovan individual. Science, 338(6104), 222–226. doi: 10.1126/science.1224344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, … Berman KF (2002). Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci, 5(3), 267–271. doi: 10.1038/nn804 [DOI] [PubMed] [Google Scholar]
- Pardinas AF, Holmans P, Pocklington AJ, Escott-Price V, Ripke S, Carrera N, … Walters JTR (2018). Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet, 50(3), 381–389. doi: 10.1038/s41588-018-0059-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak CS, & Blasberg RG (1985). Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab, 5(4), 584–590. doi: 10.1038/jcbfm.1985.87 [DOI] [PubMed] [Google Scholar]
- Peyregne S, Boyle MJ, Dannemann M, & Prufer K (2017). Detecting ancient positive selection in humans using extended lineage sorting. Genome Res, 27(9), 1563–1572. doi: 10.1101/gr.219493.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power RA, Kyaga S, Uher R, MacCabe JH, Langstrom N, Landen M, … Svensson AC (2013). Fecundity of patients with schizophrenia, autism, bipolar disorder, depression, anorexia nervosa, or substance abuse vs their unaffected siblings. JAMA Psychiatry, 70(1), 22–30. doi: 10.1001/jamapsychiatry.2013.268 [DOI] [PubMed] [Google Scholar]
- Power RA, Steinberg S, Bjornsdottir G, Rietveld CA, Abdellaoui A, Nivard MM, … Stefansson K (2015). Polygenic risk scores for schizophrenia and bipolar disorder predict creativity. Nat Neurosci, 18(7), 953–955. doi: 10.1038/nn.4040 [DOI] [PubMed] [Google Scholar]
- Prufer K, de Filippo C, Grote S, Mafessoni F, Korlevic P, Hajdinjak M, … Paabo S (2017). A high-coverage Neandertal genome from Vindija Cave in Croatia. Science, 358(6363), 655–658. doi: 10.1126/science.aao1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prufer K, Racimo F, Patterson N, Jay F, Sankararaman S, Sawyer S, … Paabo S (2014). The complete genome sequence of a Neanderthal from the Altai Mountains. Nature, 505(7481), 43–49. doi: 10.1038/nature12886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith J, Benkelfat C, Sherwin A, Yasuhara Y, Kuwabara H, Andermann F, … Gjedde A (1994). Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc Natl Acad Sci U S A, 91(24), 11651–11654. doi: 10.1073/pnas.91.24.11651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal D, Wender PH, Kety SS, Welner J, & Schulsinger F (1971). The adopted-away offspring of schizophrenics. Am J Psychiatry, 128(3), 307–311. doi: 10.1176/ajp.128.3.307 [DOI] [PubMed] [Google Scholar]
- Sankararaman S, Mallick S, Dannemann M, Prufer K, Kelso J, Paabo S, … Reich D (2014). The genomic landscape of Neanderthal ancestry in present-day humans. Nature, 507(7492), 354–357. doi: 10.1038/nature12961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankararaman S, Patterson N, Li H, Paabo S, & Reich D (2012). The date of interbreeding between Neandertals and modern humans. PLoS Genet, 8(10), e1002947. doi: 10.1371/journal.pgen.1002947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics, C. (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature, 511(7510), 421–427. doi: 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonti CN, Vernot B, Bastarache L, Bottinger E, Carrell DS, Chisholm RL, … Capra JA (2016). The phenotypic legacy of admixture between modern humans and Neandertals. Science, 351(6274), 737–741. doi: 10.1126/science.aad2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Bettella F, Hassani S, Wang Y, Witoelar A, Schork AJ, … Andreassen OA (2017). Probing the Association between Early Evolutionary Markers and Schizophrenia. PLoS One, 12(1), e0169227. doi: 10.1371/journal.pone.0169227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Bettella F, Mattingsdal M, Wang Y, Witoelar A, Schork AJ, … Andreassen OA (2016). Genetic Markers of Human Evolution Are Enriched in Schizophrenia. Biol Psychiatry, 80(4), 284–292. doi: 10.1016/j.biopsych.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, & Neale MC (2003). Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry, 60(12), 1187–1192. doi: 10.1001/archpsyc.60.12.1187 [DOI] [PubMed] [Google Scholar]
- Taylor NE, Pei J, Zhang J, Vlasov KY, Davis T, Taylor E, … Brown EN (2019). The role of glutamatergic and dopaminergic neurons in the periaqueductal gray/dorsal raphe: Separating analgesia and anxiety. eNeuro, 6(1). doi: 10.1523/ENEURO.0018-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen J, & Boomsma DI (2013). The evolutionary paradox and the missing heritability of schizophrenia. Am J Med Genet B Neuropsychiatr Genet, 162B(2), 122–136. doi: 10.1002/ajmg.b.32135 [DOI] [PubMed] [Google Scholar]
- Vernot B, & Akey JM (2014). Resurrecting surviving Neandertal lineages from modern human genomes. Science, 343(6174), 1017–1021. doi: 10.1126/science.1245938 [DOI] [PubMed] [Google Scholar]
- Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, & Dickinson D (2012). Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res, 137(1–3), 246–250. doi: 10.1016/j.schres.2012.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Schadt EE, Pollard KS, Roussos P, & Dudley JT (2015). Genomic and network patterns of schizophrenia genetic variation in human evolutionary accelerated regions. Mol Biol Evol, 32(5), 1148–1160. doi: 10.1093/molbev/msv031 [DOI] [PMC free article] [PubMed] [Google Scholar]


