Abstract
Background
The cervicovaginal microbiota, including sexually transmitted infections (STIs), have not been well described in female genital schistosomiasis (FGS).
Methods
Women (aged 18–31, sexually active, nonpregnant) were invited to participate at the final follow-up of the HPTN 071 (PopART) Population Cohort in January–August 2018. We measured key species of the cervicovaginal microbiota (Lactobacillus crispatus, L. iners, Gardnerella vaginalis, Atopobium vaginae, and Candida) and STIs (Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium) using quantitative PCR (qPCR). We evaluated associations of the microbiota and STI presence and concentration with FGS (qPCR-detected Schistosoma DNA in any of 3 genital specimens).
Results
The presence and concentration of key cervicovaginal species did not differ between participants with (n = 30) or without FGS (n = 158). A higher proportion of participants with FGS had T. vaginalis compared with FGS-negative women (P = .08), with further analysis showing that T. vaginalis was more prevalent among women with ≥2 Schistosoma qPCR-positive genital specimens (50.0%, 8/16) than among FGS-negative women (21.5%, 34/158; P = .01).
Conclusions
We found weak evidence of an association between the presence of T. vaginalis and FGS, with a stronger association in women with a higher-burden FGS infection. Additional research is needed on potential between-parasite interactions, especially regarding HIV-1 vulnerability.
Keywords: cervicovaginal microbiota, female genital schistosomiasis, Schistosoma haematobium, sexually transmitted infection
Female genital schistosomiasis (FGS) is caused when eggs from the waterborne parasite S. haematobium are entrapped in genital tissue [1]. FGS is prevalent in Sub-Saharan Africa and is associated with adverse reproductive outcomes, including ectopic pregnancy, infertility, and prevalent HIV-1 [1, 2]. The cervicovaginal environment has been described as “optimal” when it is dominated by lactic acid–producing lactobacilli, commensal microorganisms that adhere to an intact vaginal squamous epithelium [3, 4], protecting against pathogens by acidifying the vagina and producing antimicrobial substances such as bacteriocins [3]. Bacterial vaginosis (BV) and vulvovaginal candidiasis are examples of “nonoptimal” microbiota. BV is prevalent in women in Sub-Saharan Africa [5, 6] and is characterized by a shift from lactobacilli dominance to an increase in anaerobic species or yeast [4]. BV has important sexual and reproductive health consequences, including increased risk of pelvic inflammatory disease [5]. Additionally, BV has been associated with adverse pregnancy outcomes such as preterm delivery [5, 7], a leading cause of under-5 mortality in Sub-Saharan Africa [8]. BV is also strongly associated with HIV-1 acquisition and other sexually transmitted pathogens infecting the genital tract [5, 9, 10]. Studies employing 16S rRNA sequencing to evaluate the cervicovaginal microbiota suggest that high-intensity urinary S. haematobium infection, in the absence of investigation for genital involvement, may alter cervicovaginal microbiota diversity [11]. However, the relationship between cervicovaginal microbiota and FGS is not well characterized.
Prevalent sexually transmitted infections (STIs) have been reported in women with [12] or in populations endemic for [13, 14] urinary schistosomiasis. However, evaluation of genital involvement (FGS) in studies of urinary S. haematobium and STI co-infection is not universally performed or reported [12–14]. The biopsy prevalence of FGS in participants with urinary S. haematobium infection ranges from 23.0% to 75.0% ([15, 16]), making the assumption of genital involvement (FGS) in urinary S. haematobium infection tenuous. A reference standard diagnostic does not exist for FGS. Thus, an FGS evaluation is often based on a combination of diagnostic tests including circulating anodic antigen (CAA), urine microscopy, colposcopy, tissue-based diagnostics (biopsy, Papanicolaou smear, wet prep), and polymerase chain reaction (PCR) [17–19]. Where an FGS evaluation has been performed, studies reporting PCR-defined FGS have either not investigated or reported STI prevalence [20–22] or STI prevalence has been correlated with visual FGS findings [23]. In this cross-sectional study, we utilized PCR to detect Schistosoma DNA in the female genital tract and evaluated the association of PCR-defined FGS with the concentration and presence of key markers of the cervicovaginal microbiota, including STI.
METHODS
Study Setting and Participants
The cross-sectional bilharzia and HIV (BILHIV) study [18] was nested in HPTN 071 (PopART), a cluster randomized trial to measure the impact of an HIV-1 combination prevention package [24]. In HPTN 071 (PopART), HIV-1 incidence was measured in a population cohort at baseline and 12, 24, and 36 months [24]. Between January and August 2018, after the 36-month HPTN 071 (PopART) visit, community workers made home visits to women expressing interest in the BILHIV study [18]. Eligible women were aged 18–31 years, not pregnant, sexually active, and resident in 1 of 2 urban communities that participated in HPTN 071 (PopART) in Livingstone, Zambia.
Home and Clinic-Based Sample Collection
The home visit included written informed consent, a questionnaire, genital self-sampling (cervical and vaginal), and urine specimen collection, as previously described [18]. Enrolled women not currently menstruating were invited to attend Livingstone Central Hospital cervical cancer clinic, where midwives performed cervicovaginal lavage (CVL). After speculum insertion, a bulb syringe was used to flush normal saline (10 mL) across the cervix and vaginal walls for 1 minute. CVL fluid was collected from the posterior fornices (Supplementary Data). CVL and vaginal and cervical swab specimens were used for quantitative PCR (qPCR) detection of Schistosoma; cervical swabs were used for characterization of the microbiota and STI by qPCR; urine was used for detection of CAA and S. haematobium eggs by microscopy.
Cervicovaginal images were captured with a portable colposcope (MobileODT, Tel Aviv, Israel) and were evaluated (by E.F.K.) for any of the 4 recognized FGS cervicovaginal manifestations: grainy sandy patches, homogenous yellow sandy patches, rubbery papules, and abnormal blood vessels [25]. Women with at least 1 of these manifestations [25] or with any positive urine or genital Schistosoma diagnostic were treated free of charge with 40 mg/kg of praziquantel. Testing for STI was not performed at the point of care, and participants with suspected STI were offered syndromic management, as per local guidelines [26].
HIV-1
Laboratory-based fourth-generation HIV-1 testing (Abbott Architect HIV Ag/Ab Combo Assay) was performed for HPTN 071 (PopART) Population Cohort participants at each study visit [24].
Urine Microscopy and Circulating Anodic Antigen
Urine was centrifuged and examined by microscopy for S. haematobium eggs. The participant was considered positive if a pellet contained at least 1 S. haematobium egg [18]. A lateral flow assay utilizing up-converting reporter particles for the quantification of CAA was performed on urine samples, as previously described [18, 27]. Analyzing the equivalent of 417 µL of urine (wet reagent, UCAAhT417), a test result indicating a CAA value >0.6 pg/mL was considered positive [28].
qPCR for Detection of Schistosoma DNA
Detection of the Schistosoma-specific internal-transcribed-spacer-2 (ITS2) target by qPCR was performed at LUMC, as previously described (Supplementary Data) [18, 29]. DNA extraction of 200 µL of CVL or cervical or vaginal swab fluid was done with QIAamp spin columns (QIAGEN Benelux, Venlo, the Netherlands) according to manufacturer’s guidelines. The qPCR output was reported in cycle threshold values (Ct values), and parasite DNA loads were categorized by the following prespecified values: high (Ct < 30), moderate (30 ≤ Ct < 35), low (35 ≤ Ct < 50), and negative (no amplification) [30].
Cervicovaginal Microbiota Characterization and STI Detection
We quantified Lactobacillus crispatus as a key marker of vaginal health. Additionally, we characterized markers of a “nonoptimal” cervicovaginal microbiota (Gardnerella vaginalis and Atopobium vaginae), as well as L. iners (an enigmatic and highly prevalent lactobacillus), Candida spp. and STI (Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, and Trichomonas vaginalis). STI were quantified by qPCR using the S-DiaCTNG (for C. trachomatis and N. gonorrhea) and S-DiaMGTV kits (for M. genitalium and T. vaginalis; Diagenode Diagnostics, Seraing, Belgium) on DNA from cervical swabs at Ghent University (Ghent, Belgium) according to the manufacturer’s instructions. Quantification of A. vaginae, G. vaginalis, L. crispatus, L. iners, and Candida species was performed in the Laboratory Bacteriology Research, Ghent, using LightCycler480, version 1.5 (Roche, Basel, Switzerland) (Supplementary Data). The concentration of each species was expressed as genome-equivalents per mL (ge/mL) [31].
FGS Definitions
In this study, we employed various diagnostic tests to evaluate urinary schistosome infection (CAA and urine microscopy) and FGS (portable colposcopy and Schistosoma DNA on CVL and genital swabs). As previously described [32], participants were grouped by diagnostic test results into 3 mutually exclusive categories: FGS, at least 1 positive Schistosoma qPCR on a genital specimen (cervical swab, vaginal swab, and/or CVL); FGS negative, negative results on all diagnostic methods; probable FGS, genital Schistosoma qPCRs negative but urinary schistosomiasis positive (as defined above), in combination with 1 of 4 clinical findings suggestive of FGS on any colposcope-obtained photograph [25].
Patient Consent
The study was approved by the University of Zambia Biomedical Research Ethics Committee (011-08-17), the Zambia National Health Research Authority, and the London School of Hygiene and Tropical Medicine Ethics Committee (14506). Permission to conduct the study was given by Livingstone District Health Office and the Livingstone Central Hospital superintendent. Participants provided written informed consent.
Statistical Methods
All participants with FGS (n = 30) and all participants with probable FGS (n = 25) were selected for characterization of the cervicovaginal microbiota and STI by qPCR on cervical swabs. Three FGS-negative participants were selected for every FGS and probable FGS participant using a random number generator. The FGS-negative participants were frequency-matched by age to participants with FGS (age groups 18–19, 20–21, 22–23, 24–25, 26–27, 28–29, 30–31).
Participant characteristics were summarized by median and interquartile range (IQR) for continuous variables and by frequency and percentage for categorical variables. Differences in characteristics between FGS categories were evaluated using the Fisher exact or chi-square test. For cervicovaginal microbiota and STI species with at least 20% of sample results detectable by qPCR (ie, ≥20% prevalence), P values for comparison of presence, median (IQR), and log10 concentration mean between FGS and FGS-negative groups were calculated using the chi-square test, rank-sum test, and t test, respectively. For species with <20% prevalence, species presence was compared between FGS groups using the Fisher exact test. To enable investigation of potential confounding, concentrations of cervicovaginal microbiota with ≥20% prevalence were log-transformed to normalize their distribution, and linear regression was used to evaluate the association between FGS and mean log-concentration of each organism (ge/mL) in univariable and multivariable analysis. We developed a causal framework (Supplementary Figure 1) to inform our minimal adjustment set and adjusted for age, education, and community of residence. For all species, logistic regression was used to calculate the crude and adjusted odds ratio (OR) for presence vs absence by FGS group; due to the relatively low number of participants with detectable concentrations, logistic regression analyses only adjusted for age. Given the exploratory nature of this work, we did not correct for multiple comparisons.
Our primary analysis focused on the detection of Schistosoma DNA in the genital tract (FGS vs FGS negative). A secondary analysis compared the FGS and probable FGS groups with the FGS-negative participants. To evaluate the possible association between FGS burden and changes in presence and concentration of cervicovaginal key species, 2 ad hoc analyses were performed: (1) participants with ≥2 genital samples with detectable Schistosoma DNA were compared with the FGS-negative group, (2) participants with a moderate/high genital Schistosoma DNA concentration (Ct < 35 in at least 1 of 3 samples) were compared with the FGS-negative group. Data were analyzed using STATA 15.1 (Stata Corporation, College Station, TX, USA).
RESULTS
A total of 603 eligible women were enrolled in the BILHIV study, and 213 (35.3%) were included in the present study (Supplementary Figure 1). Of those included, 14.1% (30/213) had FGS, defined by a positive genital Schistosoma qPCR from any of the following sites: 9.4% (20/213) cervical swab, 7.0% (15/213) vaginal swab, and 6.6% (14/211) CVL. In participants with FGS, portable colposcopy revealed sandy patches in 20% (6/30), abnormal blood vessels in 20% (6/30), and no clinical signs of FGS in 50% (15/30). Two women with FGS did not present to the clinic for portable colposcopy, and 1 had uninterpretable images. Of participants with FGS, 53.3% (16/30) had Schistosoma qPCR detected from multiple sites, and 53.3% (16/30) had moderate/high genital Schistosoma DNA loads; these groups overlapped by 12 participants. Twenty-five women had probable FGS, and 74.2% (158/262) of the women who were negative on all diagnostic tests were randomly selected for inclusion in this study. Consistent with the FGS definitions, portable colposcopy in 100.0% (25/25) of the participants with probable FGS showed FGS-associated cervicovaginal manifestations. In the majority of participants with probable FGS, portable colposcopy showed sandy patches (76.0%, 19/25), with 24% (6/25) of participants having abnormal blood vessels.
Baseline Characteristics
The majority of the participants were married/cohabitating, had received some secondary education, and were using hormonal contraception (Table 1). At the conclusion of HPTN 071 (PopART), HIV-1 prevalence was 17.4% (37/213) among the women included in this study, and one-third had at least 1 STI (Table 1). Urinary schistosome infection, defined as either a positive urine microscopy (11.7%, 25/213) or positive CAA (20.7%, 44/213), was reported in 21.6% (46/213) of participants. There were differences between the 3 categories of FGS status for age (P = .002), marital status (P = .04), education (P = .06), and employment (P = .04), with participants in the probable FGS group being more likely to be older, employed, and married than FGS and FGS-negative participants. There was strong evidence of a difference in community of residence by FGS status (P < .001), with participants with FGS and probable FGS more likely to live in Community A than participants in the FGS-negative group (Table 1). Other characteristics were similar by FGS status.
Table 1.
Baseline Characteristics of the Study Population (n = 213) by FGS Status
| Participant Characteristics | FGS Negative, No. (%) (n = 158) |
Probable FGS, No. (%) (n = 25) |
FGS, No. (%) (n = 30) |
P Valuea | |
|---|---|---|---|---|---|
| Age, median (IQR), y | 23 (22–24) | 27 (23–31) | 22 (21–24) | .002b | |
| Marital status | Single | 72 (45.6) | 4 (16.0) | 13 (43.3) | .04 |
| Married or cohabitating | 81 (51.3) | 20 (80.0) | 17 (56.7) | ||
| Divorced or separated | 5 (3.2) | 1 (4.0) | 0 (0.0) | ||
| Education (highest level) | None or any primary school | 35 (22.2) | 12 (48.0) | 10 (33.3) | .06 |
| Any secondary school | 1111 (70.3) | 13 (52.0) | 19 (63.3) | ||
| Trade, degree or higher | 12 (7.6) | 0 (0.0) | 1 (3.3) | ||
| District | Community A | 66 (41.8) | 20 (80.0) | 22 (73.3) | <.01 |
| Community B | 92 (58.2) | 5 (20.0) | 8 (26.7) | ||
| Household members | 1–3 | 51 (32.3) | 4 (16.0) | 12 (40.0) | .3 |
| 4–5 | 61 (38.6) | 13 (52.0) | 8 (26.7) | ||
| 6+ | 46 (29.1) | 8 (32.0) | 10 (33.3) | ||
| Employment status | Not working | 117 (74.1) | 14 (56.0) | 26 (86.7) | .04 |
| Working | 41 (25.9) | 11 (44.0) | 4 (13.3) | ||
| Sexual behavior characteristics | |||||
| Age at sexual debut, y | 8–16 | 64 (40.5) | 11 (44.0) | 17 (56.7) | .4 |
| 17–19 | 74 (46.8) | 13 (52.0) | 10 (33.3) | ||
| 20–24 | 20 (12.7) | 1 (4.0) | 3 (10.0) | ||
| Lifetime sexual partners | 1 | 57 (36.1) | 8 (32.0) | 5 (16.7) | .3 |
| 2 | 36 (22.8) | 6 (24.0) | 9 (30.0) | ||
| 3 | 23 (14.6) | 6 (24.0) | 8 (26.7) | ||
| 4+ | 42 (26.6) | 5 (20.0) | 8 (26.7) | ||
| Currently sexually activec,d | No | 26 (16.6) | 2 (8.0) | 3 (10.0) | .5 |
| Yes | 131 (83.4) | 23 (92.0) | 27 (90.0) | ||
| Condom use with last sexe | No | 120 (76.9) | 16 (66.7) | 22 (73.3) | .6f |
| Yes | 36 (23.1) | 8 (33.3) | 8 (26.7) | ||
| HIV-1 status | Not detected | 132 (83.5) | 20 (80.0) | 24 (80.0) | .8 |
| Detected | 26 (16.5) | 5 (20.0) | 6 (20.0) | ||
| Any STIg | Not detected | 106 (67.1) | 14 (56.0) | 18 (60.0) | .5 |
| Detected | 52 (32.9) | 11 (44.0) | 12 (40.0) | ||
| Contraceptive use | |||||
| Condoms | No | 132 (83.5) | 20 (80.0) | 26 (86.7) | .8 |
| Yes | 26 (16.5) | 5 (20.0) | 4 (13.3) | ||
| OCP | No | 148 (93.7) | 22 (88.0) | 29 (96.7) | .5 |
| Yes | 10 (6.3) | 3 (12.0) | 1 (3.3) | ||
| Injectable | No | 82 (51.9) | 13 (52.0) | 15 (50.0) | 1.0f |
| Yes | 76 (48.1) | 12 (48.0) | 15 (50.0) | ||
| Implant | No | 144 (91.1) | 23 (92.0) | 28 (93.3) | 1.0 |
| Yes | 14 (8.9) | 2 (8.0) | 2 (6.7) | ||
| Any hormonal contraceptionh | No | 58 (36.7) | 8 (32.0) | 10 (35.7) | .9f |
| Yes | 100 (63.3) | 17 (68.0) | 18 (64.3) | ||
| Schistosomiasis-related factors | |||||
| Urine microscopy | Not detected | 158 (100.0) | 19 (76.0) | 11 (36.7) | <.001i |
| Detected | 0 (0.0) | 6 (24.0) | 19 (63.3) | ||
| Urine CAA | Negative | 158 (100.0) | 0 (0.0) | 11 (36.7) | <.001i |
| Positive | 0 (0.0) | 25 (100.0) | 19 (63.3) |
Abbreviations: CAA, circulating anodic antigen; FGS, female genital schistosomiasis; IQR, interquartile range; OCP, oral contraceptive pill; STI, sexually transmitted infection.
aFisher’s exact P value unless otherwise indicated.
bKruskal-Wallis P value.
cAny sexual activity in the last 6 months.
dParticipants who responded with “no answer” (n = 1) are not shown in the table.
eParticipants who responded with “no answer” (n = 3) are not shown in the table.
fChi-square P value.
gAny STI defined as the presence of at least 1 of N. gonorrhoeae, C. trachomatis, M. genitalium, or T. vaginalis.
hAny hormonal contraception is defined as use of injectable agents, implants, or oral contraceptive pills.
iPart of the definition for FGS categories.
Primary Comparison: FGS vs FGS Negative
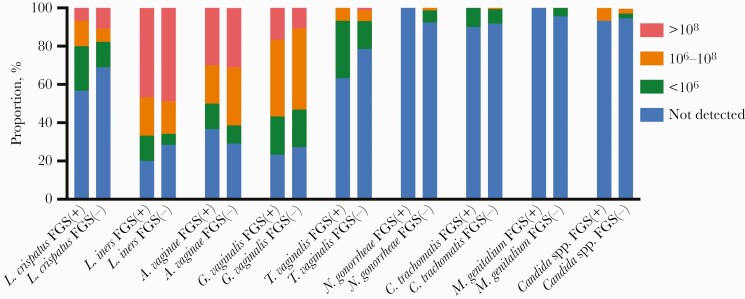
Concentrations of evaluated species are shown in Figure 1. Compared with FGS-negative women, there was no evidence of a difference in the presence or concentration of cervicovaginal L. crispatus, L. iners, A. vaginae, G. vaginalis, or C. albicans in participants with FGS (Table 2). A higher proportion of participants with FGS had T. vaginalis present, although there was not strong evidence of an association (OR, 2.11; 95% CI, 0.92–4.86; P = .08) (Supplementary Table 1). This result was similar after adjusting for age (Supplementary Table 1). Otherwise, compared with FGS-negative women, the presence and concentration of other STIs were similar in women with FGS (Table 2).
Figure 1.
Stacked bar chart of concentrations of cervicovaginal microbiota and taxa causing sexually transmitted infection by female genital schistosomiasis status. Abbreviation: FGS, female genital schistosomiasis.
Table 2.
Presence and Concentration of Vaginal Lactobacilli, Other Key Microbiota, and Sexually Transmitted Infection, Overall and by FGS Status
| Organism | No. (%) and Concentrationa | All Participants (n = 213) | FGS Negative (n = 158) | FGS (n = 30) | P Valuea |
|---|---|---|---|---|---|
| L. crispatus | Presence | 69 (32.4) | 49 (31.0) | 13 (43.3) | .19 |
| Median (IQR) | 8.7×106 (2.6×105–4.2×108) | 1.1×107 (2.6×105–7.0×108) | 7.5×105 (1.9×105–2.7×107) | .20 | |
| Log-concentration mean | 16.01 | 16.38 | 14.82 | .19 | |
| L. iners | Presence | 156 (73.2) | 113 (71.5) | 24 (80.0) | .34 |
| Median (IQR) | 2.7×108 (3.8×107–1.5×109) | 2.8×108 (3.9×107–1.6×109) | 1.7×108 (1.6×107–1.0×109) | .39 | |
| Log-concentration mean | 18.93 | 18.98 | 18.36 | .33 | |
| G. vaginalis | Presence | 156 (73.2) | 115 (72.8) | 23 (76.7) | .66 |
| Median (IQR) | 7.7×106 (8.3×105–5.2×107) | 8.1×106 (7.7×105–4.8×107) | 3.8×106 (6.8×105–6.4×107) | .93 | |
| Log-concentration mean | 15.85 | 15.74 | 15.91 | .79 | |
| A. vaginae | Presence | 152 (71.4) | 112 (70.9) | 19 (63.3) | .41 |
| Median (IQR) | 5.8×107 (8.2×106–2.1×108) | 5.8×107 (8.7×106–2.0×108) | 3.0×107 (1.06×106–2.8×108) | .83 | |
| Log-concentration mean | 17.31 | 17.33 | 17.06 | .66 | |
| T. vaginalis | Presence | 53 (24.9) | 34 (21.5) | 11 (36.7) | .08 |
| Median (IQR) | 4.2×104 (173.0–2.3×106) | 1.7×105 (56.9–6.3×106) | 1.0×104 (5030.0–4.5×105) | .53 | |
| Log-concentration mean | 10.39 | 10.88 | 10.19 | .69 | |
| Vaginal microbiota with prevalence <20% | |||||
| N. gonorrheae | Presence | 13 (6.1) | 12 (7.6) | 0.0 (0) | .22 |
| C. trachomatis | Presence | 17 (8.0) | 13 (8.2) | 3 (10.0) | .72 |
| M. genitalium | Presence | 8 (3.8) | 7 (4.4) | 0 (0.0) | .60 |
| C. albicans | Presence | 12 (5.63) | 8 (5.1) | 2 (6.7) | .66 |
Abbreviations: FGS, female genital schistosomiasis; IQR, interquartile range.
aConcentrations are expressed in genome equivalents/mL.
bFor species with >20% prevalence, P values for presence, median (IQR), and log-concentration mean are from the chi-square test, rank-sum test, and t test, respectively. For species with <20% prevalence, P values for presence are from the Fisher exact test.
Combining the FGS and probable FGS groups, participants with FGS/probable FGS similarly had a higher prevalence of T. vaginalis compared with FGS-negative participants (P = .05) (Supplementary Table 2). Otherwise, compared with FGS-negative women, the presence and concentrations of cervicovaginal microbiota and the presence of STI were similar in women with FGS/probable FGS compared with FGS-negative participants (Supplementary Table 2).
Ad Hoc Analysis—Schistosoma DNA Concentration
In participants (n = 16) with FGS and a moderate/high genital Schistosoma DNA concentration (Schistosoma qPCR Ct < 35), the presence of T. vaginalis was higher than among the FGS-negative participants (P = .01) (Table 3). Women with FGS and a moderate/high Schistosoma DNA concentration (Ct < 35) had a higher mean concentration of G. vaginalis compared with FGS-negative women (P = .03) (Table 3).
Table 3.
Ad Hoc Analysis of the Presence and Concentration of Vaginal Lactobacilli, Other Cervicovaginal Microbiota, and Sexually Transmitted Infection in Participants With a Moderate to High Concentration of Schistosoma DNA (Ct < 35) Compared With FGS-Negative Participants
| Organism | No. (%) and Concentrationa | FGS Negative (n = 158) | FGS With Ct < 35 (n = 16) | P Valueb |
|---|---|---|---|---|
| L. crispatus | Presence | 49 (31.0) | 6 (37.5) | .60 |
| Median (IQR) | 1.1×107 (2.6×105–7.0×108) | 1.2×106 (3.0×105–2.0×107) | .28 | |
| Log-concentration mean | 16.38 | 14.27 | .19 | |
| L. iners | Presence | 113 (71.5) | 11 (68.75) | .82 |
| Median (IQR) | 2.8×108 (3.9×107–1.6×109) | 7.5×107 (3.6×106–7.5×108) | .30 | |
| Log-concentration mean | 18.98 | 17.86 | .21 | |
| G. vaginalis | Presence | 115 (72.8) | 13 (81.3) | .46 |
| Median (IQR) | 8.1×106 (7.7×105–4.8×107) | 1.5×107 (3.8×106–3.1×108) | .11 | |
| Log-concentration mean | 15.74 | 17.45 | .03 | |
| A. vaginae | Presence | 112 (70.9) | 13 (81.3) | .38 |
| Median (IQR) | 5.8×107 (8.7×106–2.0×108) | 1.6×108 (1.6×107–2.8×108) | .70 | |
| Log-concentration mean | 17.33 | 17.38 | .95 | |
| T. vaginalis | Presence | 34 (21.5) | 8 (50.0) | .01 |
| Median (IQR) | 1.7×105 (56.9–6.3×106) | 8085.0 (5210.0–1.0×106) | .56 | |
| Log-concentration mean | 10.88 | 10.09 | .70 | |
| Vaginal microbiota with prevalence <20% | ||||
| N. gonorrheae | Presence | 12 (7.6) | 0 (0.0) | .61 |
| C. trachomatis | Presence | 13 (8.2) | 2 (12.5) | .63 |
| M. genitalium | Presence | 7 (4.4) | 0 (0.0) | 1.0 |
| C. albicans | Presence | 8 (5.1) | 2 (12.5) | .23 |
Abbreviations: Ct, cycle threshold; FGS, female genital schistosomiasis; IQR, interquartile range.
aConcentrations are expressed in genome equivalents/mL.
bFor species with >20% prevalence, P values for presence, median (IQR), and log-concentration mean are from the chi-square test, rank-sum test, and t test, respectively. For species with <20% prevalence, P values for presence are from the Fisher exact test.
Ad Hoc Analysis—Clinical Disease Burden
In an ad hoc analysis, participants (n = 16) with a higher FGS burden, defined as ≥2 Schistosoma qPCR–positive genital specimens, had higher prevalence of T. vaginalis compared with FGS-negative participants (P = .01) (Table 4). There was also evidence of a difference in the median concentration and the mean log-concentration of L. iners (both P = .03) compared with FGS-negative women, with lower levels among the higher–FGS burden group (Table 4).
Table 4.
Ad Hoc Analysis of the Presence and Concentration of Vaginal Lactobacilli, Cervicovaginal Microbiota, and Sexually Transmitted Infection in Participants With Schistosoma DNA Detected in ≥2 Genital Specimens Compared With FGS-Negative Participants
| Organism | No. (%) and Concentrationa | FGS Negative (n = 158) | ≥2 PCR FGS (n = 16) | P Valueb |
|---|---|---|---|---|
| L. crispatus | Presence | 49 (31.0) | 7 (43.8) | .30 |
| Median (IQR) | 1.1×107 (2.6×105–7.0×108) | 1.9×106 (7.7×104–2.7×107) | .32 | |
| Log-concentration mean | 16.38 | 14.90 | .34 | |
| L. iners | Presence | 113 (71.5) | 10 (62.5) | .45 |
| Median (IQR) | 2.8×108 (3.9×107–1.6×109) | 5.1×107 (9.0×105–1.1×108) | .03 | |
| Log-concentration mean | 18.98 | 17.00 | .03 | |
| G. vaginalis | Presence | 115 (72.8) | 14 (87.5) | .20 |
| Median (IQR) | 8.1×106 (7.7×105–4.8×107) | 1.6×107 (1.1×106–3.6×108) | .29 | |
| Log-concentration mean | 15.74 | 16.85 | .16 | |
| A. vaginae | Presence | 112 (70.9) | 11 (68.8) | .86 |
| Median (IQR) | 5.8×107 (8.7×106–2.0×108) | 1.6×108 (2.7×107–3.2×108) | .33 | |
| Log-concentration mean | 17.33 | 18.04 | .36 | |
| T. vaginalis | Presence | 34 (21.5) | 8 (50.0) | .01 |
| Median (IQR) | 1.7×105 (56.9–6.3×106) | 5680.0 (2967.5–1.0×106) | .50 | |
| Log-concentration mean | 10.88 | 9.71 | .56 | |
| Vaginal microbiota with prevalence <20% | ||||
| N. gonorrheae | Presence | 12 (7.6) | 0 (0.0) | .61 |
| C. trachomatis | Presence | 13 (8.2) | 2 (12.5) | .63 |
| M. genitalium | Presence | 7 (4.4) | 0 (0.0) | 1.0 |
| C. albicans | Presence | 8 (5.1) | 1 (6.3) | .59 |
Abbreviations: FGS, female genital schistosomiasis; IQR, interquartile range; PCR, polymerase chain reaction.
aConcentrations are expressed in genome equivalents/mL.
bFor species with >20% prevalence, P values for presence, median (IQR), and log-concentration mean are from the chi-square test, rank-sum test and t test, respectively. For species with <20% prevalence, P values for presence are from the Fisher exact test.
DISCUSSION
In this study, we describe the association of FGS with the cervicovaginal microbiota, including lactobacilli, Candida spp., markers of a “nonoptimal” cervicovaginal environment, and STI. We did not find evidence that the presence or concentration of key cervicovaginal species was associated with FGS. While FGS was not associated with C. trachomatis, M. genitalium, or N. gonorrhoeae, there was weak evidence of an association of presence of T. vaginalis with FGS, which remained after adjusting for age. This association was also present when participants with FGS and probable FGS were combined and was strengthened in the ad hoc analyses of participants with higher-burden FGS.
We performed 2 ad hoc analyses. High-intensity S. haematobium infection, in the absence of evaluation for FGS, has been associated with higher cervicovaginal alpha diversity [11]. Thus, first we investigated whether Schistosoma DNA concentrations might be associated with the cervicovaginal microbiota in 16 participants with a higher FGS burden, indicated by moderate/high genital Schistosoma DNA concentrations. In this ad hoc analysis, we found that the G. vaginalis log-concentration mean was higher in women with a higher FGS burden. Participants underwent CVL when they were not menstruating, and we have previously described that 66.2% (139/210) of women in this cohort had detectable CVL hemoglobin. Iron sources, like hemoglobin, are often required for bacterial growth [33]. G. vaginalis is well adapted to harvest iron from the environment [34], and higher concentrations of G. vaginalis coincide with menses [33]. Cervical tissue in women with FGS is more vascularized than non-egg-containing tissue, and thus the abnormal cervical vessels and contact bleeding seen in clinical FGS provide a plausible link to increased concentrations of G. vaginalis in high-burden FGS [35].
In a second ad hoc analysis, we examined participants with multiple qPCR-positive genital specimens as a potential proxy marker of high-burden FGS and found that reduced a L. iners concentration (median and log mean) was associated with high-burden FGS. The cervicovaginal microbiota was characterized with 16S rRNA sequencing in Tanzanian women with S. haematobium infection (n = 16). Although power was limited, women with high-intensity S. haematobium infection had reduced abundance of L. iners compared with women with low-intensity infection, albeit without evidence of a difference (P = .39) [11]. Further research is needed to evaluate the relationship between the presence and concentration of L. iners in FGS.
In both ad hoc analyses, we found that presence of T. vaginalis was higher among the participants with a higher FGS burden. Our finding supports results from a small South African study (n = 45) that reported an association between FGS (diagnosed by identification of sandy patches on colposcopy) and presence of T. vaginalis in young women (ages 15–23) [36]. Acquisition of T. vaginalis and S. haematobium may share common risk factors, like age and socioeconomic status [18]. We have previously described higher FGS prevalence among younger age groups [18]. Epidemiologic data from Zambian adolescents, sex workers, and pregnant women (aged 13–45 years) describe a T. vaginalis prevalence between 24.6% and 33.2% [37], consistent with the prevalence in our population (24.9%). While S. haematobium is geographically restricted to Africa and the Middle East, it is associated with poverty [38] and is acquired through contact with cercariae-infested fresh water [1]. T. vaginalis is primarily sexually transmitted, is prevalent worldwide, with the highest prevalence in women from low-income countries [39]. Both S. haematobium [40] and T. vaginalis infection [41, 42] have been reported as risk factors for HIV-1 acquisition, although these associations are not universally reported [43]. Additionally, T. vaginalis may influence HIV-1 transmission. Studies in women not uniformly receiving antiretroviral therapy (ART) with T. vaginalis show a decline in genital HIV-1 shedding after metronidazole therapy [44–46]. However, this decline in cervical HIV-1 shedding was not seen in a study of Kenyan women receiving ART [47]. The highest global T. vaginalis incidence rates have been reported in Africa [39]. Overlapping synergies between T. vaginalis and S. haematobium cervicovaginal pathogenesis may begin with, but are not limited to, disruption of the cervicovaginal epithelium [48]. Both parasites have been associated with characteristic cervicovaginal manifestations [17, 49], the “strawberry cervix” in T. vaginalis [49] and sandy patches, rubbery papules, and abnormal blood vessels in FGS [17]. Breaches in the cervicovaginal epithelium in T. vaginalis infection potentially represent one mechanism of HIV-1 vulnerability in what is likely a multifactorial cascade, including modulating the cervicovaginal immune environment [50–52], disabling innate immunity [51], and disrupting the local microbiota [52]. To disentangle the association between S. haematobium, T. vaginalis, and HIV-1, future studies using sensitive molecular methods for both parasites are needed. Additionally, macrophage polarization can be influenced by the local immune environment, schistosomes, and T. vaginalis [53, 54]. We have previously shown that, compared with FGS-negative women, high-burden FGS is associated with higher concentrations of cervicovaginal Th2 cytokines [32]. A mouse model of urogenital S. haematobium infection suggested that the Th2 immune environment may be associated with delayed pathogen clearance [55]. Thus, further research is needed regarding the interaction between the immune environment and macrophage phenotypes in FGS and their role in potentially influencing T. vaginalis persistence.
Our study is the first to evaluate the cervicovaginal microbiota and STI in FGS defined by qPCR. This is particularly relevant in a population of sexually active, nonpregnant women in the context of high HIV-1 prevalence. However, there are also some relevant limitations. FGS does not have an accepted diagnostic reference standard, and the sensitivity of cervicovaginal PCR for the detection of Schistosoma DNA is imperfect. Thus, studies using Schistosoma DNA to detect FGS may be subject to misclassification bias. To maximize sensitivity, we used the results of 3 genital specimens. This decision was supported by previous work showing a sensitivity of 80.0% (61.4%–92.3%) with a specificity of 100.0% (99.3%–100.0%) when vaginal and cervical swab PCR were compared with any positive genital PCR (CVL, cervical swab, or vaginal swab) as the reference standard [18]. The study was conducted in an urban location with relatively low S. haematobium prevalence; thus the numbers of FGS cases in the primary and ad hoc analyses were small, limiting precision in the effect sizes. This analysis also included multiple statistical comparisons; thus, we focused on the species that showed a consistent pattern of association across primary and ad hoc analyses, rather than overinterpreting significance testing for any 1 species in isolation. Evidence for these associations in the ad hoc analyses should be viewed as hypothesis-generating. Additionally, the cross-sectional study design limited our ability to assess FGS duration and the long-term impact on the prevalence and concentrations of key species or STIs. There were a number of behavioral and biological factors that were not measured in our study including tobacco use [56], viral STIs (human papillomavirus and herpes simplex virus–2) [56], and intravaginal cleansing practices [57]. As these factors may be associated with the cervicovaginal microbiota, we cannot exclude residual or unmeasured confounding. Genital swabs were self-collected by participants, raising the potential for false-negative genital swabs. In future work, β-globin PCR could be implemented as a positive control to confirm the presence of human DNA [18]. Lastly, we defined FGS by Schistosoma DNA detection on qPCR; however, we cannot exclude that cervicovaginal qPCR detected S. haematobium eggs from a sexual partner’s semen [18].
In conclusion, we report weak evidence of an association between presence of T. vaginalis and FGS, with a consistently stronger association in women with a higher burden FGS infection. Additional research is needed to understand the interactions between S. haematobium and T. vaginalis, especially regarding HIV-1 vulnerability.
Supplementary Material
Acknowledgments
We wish to acknowledge the participants for devoting their time and trust to this study. We would like to recognize the work performed by the BILHIV project supervisor Namakau Chola and the BILHIV community workers Ethel Mwansa, Mwiingana Lukonga, Ruth Mwanza, Mervis Kantukaleza, and Judith Lungu. Our work in Livingstone would not have been possible without support from Clement Mwakamui (Zambart). We gratefully acknowledge Eric A. T. Brienen (LUMC) for performing the genital Schistosoma PCR analysis and Claudia J. de Dood (LUMC) and Pytsje T. Hoekstra (LUMC) for performing the CAA analysis.
Financial support. Dr. A. Bustinduy received funding from the Wellcome Trust (Award 205954/Z/17/Z). Dr. P. Cools received funding from the Research Foundation–Flanders (BOFSTG2019010101) and was financially supported by a Bill and Melinda Gates Foundation Project (OPP1120972). Dr. E. Webb and Professor R. Hayes received funding from MRC Grant Reference MR/K012126/1, and Dr. S. C. Francis received salary from MRC Grant Reference MR/N023692/1. These awards are jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and are also part of the EDCTP2 program supported by the European Union. HPTN 071 (PopART) was supported by the National Institute of Allergy and Infectious Diseases (NIAID) under Cooperative Agreements UM1-AI068619, UM1-AI068617, and UM1-AI068613, with funding from the US President’s Emergency Plan for AIDS Relief (PEPFAR); the International Initiative for Impact Evaluation with support from the Bill and Melinda Gates Foundation; the NIAID, the National Institute on Drug Abuse, and the National Institute of Mental Health, all part of the National Institutes of Health. Professor Eyrun Kjetland was supported by South-Eastern Regional Health Authority, Norway, project #2016055. Bruno Levecke received funding from the Fund for Scientific Research–Flanders (Grant No. 1285316N).
Potential conflicts of interest. The authors report no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Amy S. Sturt—conceptualization, data curation, formal analysis, BILHIV project administration, visualization, original manuscript preparation, manuscript editing and revision. Emily L. Webb—conceptualization, data curation, formal analysis, supervision, visualization, original manuscript preparation, manuscript editing and revision. Lisa Himschoot—investigation, manuscript editing and revision. Comfort R. Phiri—BILHIV project administration, writing–review and editing. Maina Mudenda—investigation, writing–review and editing. Joyce Mapani—investigation, writing–review and editing. Eyrun F. Kjetland—investigation, writing–review and editing. Tobias Mweene—investigation, writing–review and editing. Bruno Levecke—investigation, resources, writing–review and editing. Govert J. van Dam—investigation, writing–review and editing. Paul L. A. M. Corstjens—investigation, writing–review and editing. Helen Ayles—resources, writing–review and editing. Richard J. Hayes—supervision, resources, writing–review and editing. Lisette van Lieshout—investigation, writing–review and editing. Isaiah Hansingo—resources, supervision, writing–review and editing. Suzanna C. Francis—conceptualization, supervision, original manuscript preparation, manuscript editing and revision. Piet Cools—conceptualization, resources, investigation, original manuscript preparation, manuscript editing and revision. Amaya L. Bustinduy—conceptualization, funding acquisition, supervision, original manuscript preparation, manuscript editing and revision.
References
- 1.Sturt AS, Webb EL, Francis SC, et al. Beyond the barrier: female genital schistosomiasis as a potential risk factor for HIV-1 acquisition. Acta Trop 2020; 209:105524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kjetland EF, Ndhlovu PD, Gomo E, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS 2006; 20:593–600. [DOI] [PubMed] [Google Scholar]
- 3.Boris S, Barbés C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect 2000; 2:543–6. [DOI] [PubMed] [Google Scholar]
- 4.McKinnon LR, Achilles SL, Bradshaw CS, et al. The evolving facets of bacterial vaginosis: implications for HIV transmission. AIDS Res Hum Retroviruses 2019; 35:219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Wijgert J, Jespers V. The global health impact of vaginal dysbiosis. Res Microbiol 2017; 168:859–64. [DOI] [PubMed] [Google Scholar]
- 6.Torrone EA, Morrison CS, Chen PL, et al. ; STIMA Working Group. Prevalence of sexually transmitted infections and bacterial vaginosis among women in Sub-Saharan Africa: an individual participant data meta-analysis of 18 HIV prevention studies. PLoS Med 2018; 15:e1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med 1995; 333:1737–42. [DOI] [PubMed] [Google Scholar]
- 8.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385:430–40. [DOI] [PubMed] [Google Scholar]
- 9.Atashili J, Poole C, Ndumbe PM, et al. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 2008; 22:1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jespers V, Crucitti T, Menten J, et al. ; Vaginal Biomarkers Study Group. Prevalence and correlates of bacterial vaginosis in different sub-populations of women in Sub-Saharan Africa: a cross-sectional study. PLoS One 2014; 9:e109670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bullington BW, Lee MH, Mlingi J, et al. Cervicovaginal bacterial communities in reproductive-aged Tanzanian women with Schistosoma mansoni, Schistosoma haematobium, or without schistosome infection. ISME J 2021; 15:1539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gadoth A, Mvumbi G, Hoff NA, et al. Urogenital schistosomiasis and sexually transmitted coinfections among pregnant women in a schistosome-endemic region of the Democratic Republic of Congo. Am J Trop Med Hyg 2019; 101:828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leutscher PD, Ramarokoto CE, Hoffmann S, et al. Coexistence of urogenital schistosomiasis and sexually transmitted infection in women and men living in an area where Schistosoma haematobium is endemic. Clin Infect Dis 2008; 47:775–82. [DOI] [PubMed] [Google Scholar]
- 14.Yirenya-Tawiah D, Annang TN, Apea-Kubi KA, et al. Chlamydia trachomatis and Neisseria gonorrhoeae prevalence among women of reproductive age living in urogenital schistosomiasis endemic area in Ghana. BMC Res Notes 2014; 7:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poggensee G, Kiwelu I, Saria M, et al. Schistosomiasis of the lower reproductive tract without egg excretion in urine. Am J Trop Med Hyg 1998; 59:782–3. [DOI] [PubMed] [Google Scholar]
- 16.Renaud G, Devidas A, Develoux M, et al. Prevalence of vaginal schistosomiasis caused by Schistosoma haematobium in an endemic village in Niger. Trans R Soc Trop Med Hyg 1989; 83:797. [DOI] [PubMed] [Google Scholar]
- 17.Kjetland EF, Ndhlovu PD, Mduluza T, et al. Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg 2005; 72:311–9. [PubMed] [Google Scholar]
- 18.Sturt AS, Webb EL, Phiri CR, et al. Genital self-sampling compared with cervicovaginal lavage for the diagnosis of female genital schistosomiasis in Zambian women: the BILHIV study. PLoS Negl Trop Dis 2020; 14:e0008337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downs JA, Mguta C, Kaatano GM, et al. Urogenital schistosomiasis in women of reproductive age in Tanzania’s Lake Victoria region. Am J Trop Med Hyg 2011; 84:364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pillay P, van Lieshout L, Taylor M, et al. Cervical cytology as a diagnostic tool for female genital schistosomiasis: correlation to cervical atypia and Schistosoma polymerase chain reaction. Cytojournal 2016; 13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kjetland EF, Hove RJ, Gomo E, et al. Schistosomiasis PCR in vaginal lavage as an indicator of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg 2009; 81:1050–5. [DOI] [PubMed] [Google Scholar]
- 22.Galappaththi-Arachchige HN, Holmen S, Koukounari A, et al. Evaluating diagnostic indicators of urogenital Schistosoma haematobium infection in young women: a cross sectional study in rural South Africa. PLoS One 2018; 13:e0191459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Randrianasolo BS, Jourdan PM, Ravoniarimbinina P, et al. Gynecological manifestations, histopathological findings, and Schistosoma-specific polymerase chain reaction results among women with Schistosoma haematobium infection: a cross-sectional study in Madagascar. J Infect Dis 2015; 212:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes RJ, Donnell D, Floyd S, et al. ; HPTN 071 (PopART) Study Team. Effect of universal testing and treatment on HIV incidence - HPTN 071 (PopART). N Engl J Med 2019; 381:207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Female Genital Schistosomiasis: A Pocket Atlas for Clinical Health-Care Professionals.World Health Organization; 2015. Available at: http://www.who.int/iris/handle/10665/180863. Accessed April 30, 2021. [Google Scholar]
- 26.Zambian Ministry of Health. Guidelines for the Etiological and Clinical Management of Sexually Transmitted Infections in Zambia. Zambian Ministry of Health; 2017.
- 27.Corstjens PL, De Dood CJ, Kornelis D, et al. Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology 2014; 141:1841–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corstjens PLAM, de Dood CJ, Knopp S, et al. Circulating anodic antigen (CAA): a highly sensitive diagnostic biomarker to detect active Schistosoma infections—improvement and use during SCORE. Am J Trop Med Hyg 2020; 103:50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obeng BB, Aryeetey YA, de Dood CJ, et al. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann Trop Med Parasitol 2008; 102:625–33. [DOI] [PubMed] [Google Scholar]
- 30.Pillay P, Taylor M, Zulu SG, et al. Real-time polymerase chain reaction for detection of Schistosoma DNA in small-volume urine samples reflects focal distribution of urogenital schistosomiasis in primary school girls in KwaZulu Natal, South Africa. Am J Trop Med Hyg 2014; 90:546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jespers V, van de Wijgert J, Cools P, et al. ; Vaginal Biomarkers Study Group. The significance of Lactobacillus crispatus and L. vaginalis for vaginal health and the negative effect of recent sex: a cross-sectional descriptive study across groups of African women. BMC Infect Dis 2015; 15:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sturt AS, Webb EL, Patterson C, et al. Cervicovaginal immune activation in Zambian women with female genital schistosomiasis. Front Immunol 2021; 12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One 2010; 5:e10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarosik GP, Land CB, Duhon P, et al. Acquisition of iron by Gardnerella vaginalis. Infect Immun 1998; 66:5041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jourdan PM, Roald B, Poggensee G, et al. Increased vascularity in cervicovaginal mucosa with Schistosoma haematobium infection. PLoS Negl Trop Dis 2011; 5:e1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleppa E, Ramsuran V, Zulu S, et al. Effect of female genital schistosomiasis and anti-schistosomal treatment on monocytes, CD4+ T-cells and CCR5 expression in the female genital tract. PLoS One 2014; 9:e98593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crucitti T, Jespers V, Mulenga C, et al. Trichomonas vaginalis is highly prevalent in adolescent girls, pregnant women, and commercial sex workers in Ndola, Zambia. Sex Transm Dis 2010; 37:223–7. [DOI] [PubMed] [Google Scholar]
- 38.Hotez PJ, Kamath A. Neglected tropical diseases in Sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis 2009; 3:e412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ 2019; 97:548-62P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wall KM, Kilembe W, Vwalika B, et al. Schistosomiasis is associated with incident HIV transmission and death in Zambia. PLoS Negl Trop Dis 2018; 12:e0006902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mavedzenge SN, Pol BV, Cheng H, et al. Epidemiological synergy of Trichomonas vaginalis and HIV in Zimbabwean and South African women. Sex Transm Dis 2010; 37:460–6. [DOI] [PubMed] [Google Scholar]
- 42.Masha SC, Cools P, Sanders EJ, et al. Trichomonas vaginalis and HIV infection acquisition: a systematic review and meta-analysis. Sex Transm Infect 2019; 95:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bochner AF, Baeten JM, Secor WE, et al. Associations between schistosomiasis and HIV-1 acquisition risk in four prospective cohorts: a nested case-control analysis. J Int AIDS Soc 2020; 23:e25534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang CC, McClelland RS, Reilly M, et al. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J Infect Dis 2001; 183:1017–22. [DOI] [PubMed] [Google Scholar]
- 45.Kissinger P, Amedee A, Clark RA, et al. Trichomonas vaginalis treatment reduces vaginal HIV-1 shedding. Sex Transm Dis 2009; 36:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson BL, Firnhaber C, Liu T, et al. Effect of trichomoniasis therapy on genital HIV viral burden among African women. Sex Transm Dis 2012; 39:638–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masese LN, Graham SM, Gitau R, et al. A prospective study of vaginal trichomoniasis and HIV-1 shedding in women on antiretroviral therapy. BMC Infect Dis 2011; 11:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guenthner PC, Secor WE, Dezzutti CS. Trichomonas vaginalis-induced epithelial monolayer disruption and human immunodeficiency virus type 1 (HIV-1) replication: implications for the sexual transmission of HIV-1. Infect Immun 2005; 73:4155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kissinger P. Trichomonas vaginalis: a review of epidemiologic, clinical and treatment issues. BMC Infect Dis 2015; 15:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thurman AR, Doncel GF. Innate immunity and inflammatory response to Trichomonas vaginalis and bacterial vaginosis: relationship to HIV acquisition. Am J Reprod Immunol 2011; 65:89–98. [DOI] [PubMed] [Google Scholar]
- 51.Mercer F, Diala FG, Chen YP, et al. Leukocyte lysis and cytokine induction by the human sexually transmitted parasite Trichomonas vaginalis. PLoS Negl Trop Dis 2016; 10:e0004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fichorova RN, Buck OR, Yamamoto HS, et al. The villain team-up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sex Transm Infect 2013; 89:460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortes-Selva D, Fairfax K. Schistosome and intestinal helminth modulation of macrophage immunometabolism. Immunology 2021; 162:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han IH, Song HO, Ryu JS. IL-6 produced by prostate epithelial cells stimulated with Trichomonas vaginalis promotes proliferation of prostate cancer cells by inducing M2 polarization of THP-1-derived macrophages. PLoS Negl Trop Dis 2020; 14:e0008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsieh YJ, Fu CL, Hsieh MH. Helminth-induced interleukin-4 abrogates invariant natural killer T cell activation-associated clearance of bacterial infection. Infect Immun 2014; 82:2087–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cherpes TL, Hillier SL, Meyn LA, et al. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis 2008; 35:78–83. [DOI] [PubMed] [Google Scholar]
- 57.Low N, Chersich MF, Schmidlin K, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med 2011; 8:e1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.