Abstract
Background
Accurate and timely methods for the diagnosis of histoplasmosis in resource-limited countries are lacking. Histoplasma antigen detection by enzyme immunoassay (EIA) is widely used in the United States (US) but not in resource-limited countries, leading to missed or delayed diagnoses and poor outcomes. Lateral flow assays (LFAs) can be used in this setting.
Methods
Frozen urine specimens were submitted to MiraVista diagnostics for antigen testing from 3 medical centers in endemic areas of the US. They were blinded and tested for the MVista Histoplasma LFA. Patients were classified as controls or cases of histoplasmosis. Cases were divided into proven or probable; pulmonary or disseminated; immunocompetent or immunosuppressed; and mild, moderate, or severe.
Results
Three hundred fifty-two subjects were enrolled, including 66 cases (44 proven, 22 probable) and 286 controls. Most of the cases were immunocompromised (71%), and 46 had disseminated and 20 had pulmonary histoplasmosis. Four cases were mild, 42 moderate, and 20 severe. LFA and EIA were highly concordant (κ = 0.84). Sensitivity and specificity of the LFA were 78.8% and 99.3%, respectively. LFA sensitivity was higher in proven cases (93.2%), patients with disseminated (91.3%), moderate (78.6%), and severe disease (80%), and those with galactomannan levels >1.8 ng/mL (97.8%). Specificity was 99.3% in proven cases, 99.3% in patients with moderate or severe disease, and 96.8% in those with galactomannan levels >1.8 ng/mL. Cross-reactivity was noted with other endemic mycoses.
Conclusions
The MVista Histoplasma LFA meets the need for accurate rapid diagnosis of histoplasmosis in resource-limited countries, especially in patients with high disease burden, potentially reducing morbidity and mortality.
Keywords: antigen, diagnosis, EIA, histoplasmosis, LFA
Histoplasma antigen detection by LFA proved accurate in diagnosing histoplasmosis in a cohort of suspected infection from three highly endemic regions in the United States. This test fulfills the need for accurate rapid diagnosis of histoplasmosis in endemic resource-limited countries.
Histoplasmosis is a major cause of morbidity and mortality for people living with human immunodeficiency virus (HIV) worldwide [1]. While widely available in the United States (US), accurate and timely diagnostic methods continue to be a major barrier in parts of the world with limited resources where histoplasmosis is highly endemic [2, 3]. Histoplasma antigen was detected in urine in 95% of patients with progressive disseminated histoplasmosis and HIV/AIDS in the US using the MVista Histoplasma galactomannan antigen test [4] and was the most sensitive and widely used method for diagnosis [5]. Access to sensitive noninvasive diagnostic tests are needed to improve the outcome of histoplasmosis in resource-limited countries [6, 7]. Enzyme-immunoassay (EIA)–based kits are now available outside the US for the detection of Histoplasma antigen with relatively low sensitivity and diagnostic accuracy [8].
While the MiraVista Quantitative Histoplasma antigen detection EIA is considered to be the most sensitive and accurate test for the diagnosis of disseminated or extensive pulmonary histoplasmosis [2], its widespread use has been limited by logistic barriers such as the requirement of specialized laboratories, expensive equipment, and personnel training in use of the equipment and performance of EIA, potentially leading to delays in result reporting. The EIA uses 96-well microtiter plates that are best suited for large reference laboratories that receive hundreds of specimens daily. Otherwise the microplate would need to be divided to test smaller numbers of specimens, increasing the cost of testing. Consequently, the laboratory may perform the test once or twice weekly, delaying turnaround time.
To address these limitations, MiraVista Diagnostics (Indianapolis, Indiana) has developed a novel Histoplasma galactomannan antigen lateral flow assay (LFA) for detection of Histoplasma antigen in urine, to be used outside the reference laboratory, potentially at the bedside. The purpose of this study was to establish performance characteristics of the MVista LFA using a well-characterized cohort of patients with histoplasmosis and clinically relevant controls from 3 medical centers located in endemic areas of the US.
METHODS
The MVista Histoplasma Lateral Flow Assay
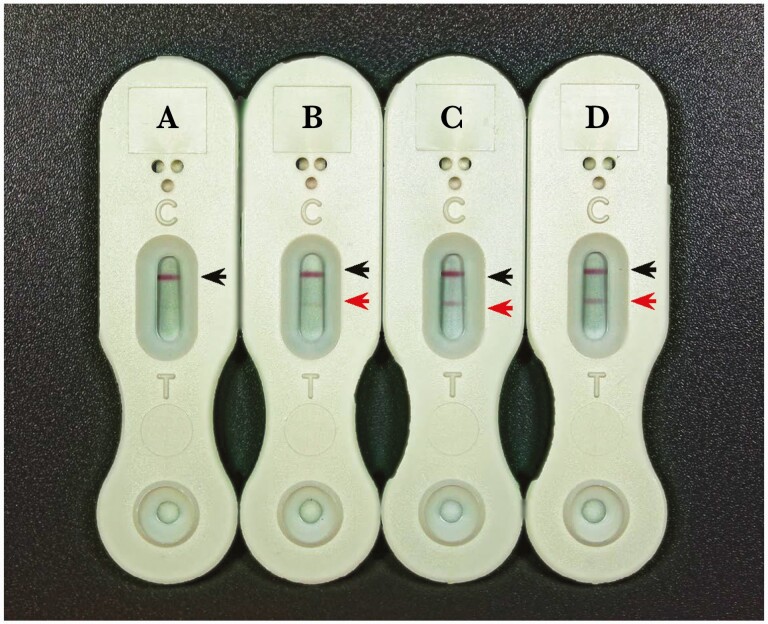
The MVista LFA assay is a qualitative lateral flow–based immunoassay developed and produced by Mira Vista Diagnostics. The assay employs polyclonal antibodies in the direct detection of Histoplasma antigen in urine. A 100-μL urine sample is added to 25 μL of sample diluent using the transfer pipette provided in the kit; 100 μL of the diluted sample is then added to the sample port of the device. As the sample moves through the device, if antigen is present it binds to an anti-Histoplasma detector antibody conjugated to colloidal gold. The Histoplasma-capture antibody, bound to the assay membrane at the Test position of the device reading window, binds the antigen–antibody–colloidal gold detector and yields a visible line (Figure 1). As an assay control, anti-rabbit antibody is bound at the control position of the reading window and binds the capture antibody–colloidal gold complex as it flows across the membrane, to yield a visible line, indicating adequate flow of sample through the test device and therefore indicating a valid test.
Figure 1.
Examples of negative (A) and low (B), intermediate (C), and high (D) positive lateral flow assay results, based on the corresponding antigen level as measured by the enzyme immunoassay. The black arrows point to the positive control line (C) and the red arrow to the detection line (T).
The LFA is read visually by trained laboratory scientists after 30 minutes of incubation at room temperature to determine that the control line is present and whether a test line is visible in the test line window. The control line must be present, or the test is invalid. The test is also invalid if streaking of the gold conjugate obscures reading of the test or control line. Invalid assays are repeated. All devices were examined by 1 of 2 trained examiners for assay validation and determination of sensitivity and specificity.
LFA results that were found to be discordant with diagnosis were resolved by repeat testing in the LFA to eliminate operator errors. If both testers concurred (positive or negative), their result was considered final.
Clinical Trial Design
The study was conducted in collaboration with 3 medical centers located in highly endemic areas of the US: Indiana University Health (Indianapolis, Indiana), University of Kentucky (Lexington, Kentucky), and Vanderbilt University (Nashville, Tennessee). Specimens were submitted to MiraVista clinical services for Histoplasma Antigen EIA testing between 2011 and 2014. All specimens were barcoded, de-identified, and stored at –20°C at the MiraVista biobank and had been freeze-thawed no more than twice. Specimens were then blinded and tested with the MVista Histoplasma LFA for the purpose of this study. Detailed medical records were reviewed by experienced providers at the corresponding medical institutions with the approval of their respective institutional review board and in accordance with their institutional clinical research standards. Data collected included clinical syndrome, final diagnosis, clinical and laboratory basis for the diagnosis, immune status, and severity of illness. A standard questionnaire was used for all the clinical reviews. After the collection of the clinical data, all subjects were de-identified and data were then used for data analysis.
Subjects from the study centers with urine samples submitted for testing at MiraVista were enrolled in the study. Subjects with compatible clinical findings and positive culture or cytopathology/histopathology from respiratory secretions or extrapulmonary sites demonstrating organisms consistent with Histoplasma were classified as proven cases. Subjects with compatible clinical findings with positive tests for Histoplasma antigen or anti-Histoplasma antibody by immunodiffusion or complement fixation, with negative or unavailable cultures and/or pathology were classified as probable cases [4]. All cases were first-time diagnosed, and none were tested while on antifungal therapy following prior diagnosis. Cases of histoplasmosis were further classified as progressive disseminated histoplasmosis (PDH) or pulmonary histoplasmosis. PDH was defined as the presence of clinical, laboratory, or imaging evidence of extrapulmonary involvement. The diagnosis of pulmonary histoplasmosis required respiratory symptoms and pulmonary radiographs and/or computed tomography that demonstrated infiltrates and/or mediastinal lymphadenopathy, in the absence of evidence for PDH [4]. Subjects with compatible clinical findings who were diagnosed with other illnesses were classified as controls, irrespective of antigen or antibody results. Histoplasmosis was classified as severe if patients required treatment in an intensive care unit, moderately severe if hospitalization was required, and mild if hospitalization was not required. [4]
The de-identified data were used for comparison of antigen results by LFA and EIA, and used for determination of sensitivity, specificity, and accuracy of the LFA using the final clinical-pathological diagnosis as the gold standard comparator.
Nonfungal Controls
Specimens were tested from patients in whom endemic mycoses were not diagnosed and fungal cultures, histopathology, or cytopathology were negative.
Controls With Other Endemic Mycoses
Specimens were available from patients with other endemic mycoses that had been described in prior publications or unpublished studies. All specimens had been stored at –20°C at MiraVista Diagnostics. These included specimens from 27 patients with confirmed blastomycosis [4], 20 patients with confirmed coccidioidomycosis [9], 7 patients with confirmed paracoccidioidomycosis and 18 patients with confirmed talaromycosis that were not previously reported [10], and unpublished studies of 34 patients with HIV/AIDS and talaromycosis from northern Thailand.
Statistical Analysis
Cases were stratified based on the clinical syndrome, severity of illness, and underlying immune status. The proportions of patients with positive results were compared using the χ 2 test. Cohen κ analysis was used to assess the measure of agreement between the EIA and LFA test of antigen detection [11]. Student t test was used for pairwise comparisons of mean antigen levels among the different clinical syndrome groups. To calculate positive and negative predictive values as well as test accuracy, an overall prevalence of 7.1% was used. This was based on the annual average percentage of total positive tests among submitted samples from the study sites during the study period. An overall significance level of .05 was used for all comparisons. SPSS version 27 software (IBM, Armonk, New York) was used for the statistical analysis.
RESULTS
Examples of high, moderate, low, and negative results are shown in Figure 1.
Assay Validation
Within-laboratory precision was determined by testing 4 urine samples—a negative, moderate, low, and high positive control—tested in duplicate over 10 runs, with 2 runs per day for 5 days. 100% of samples were correctly interpreted.
Within-run repeatability was determined by testing 20 replicates of a low positive urine and 20 replicates of a negative urine once; 100% were correctly interpreted.
Analytical sensitivity was determined by testing urine samples containing 0.6–2.2 ng/mL of Histoplasma antigen. Forty replicates were tested in each of 3 lots of lateral flow devices. The lowest concentration at which at least 95% of the replicates were interpreted as positive was selected as the assay limit of detection (LoD) for each lot. The LoD was 1.8 ng/mL for all 3 lots.
Clinical Study
A total of 352 subjects were enrolled, including 66 cases and 286 controls as determined by the clinical review of medical records. Among the cases, 44 were proven and 22 were probable. Twenty had pulmonary and 46 disseminated histoplasmosis. Four patients were classified as mild, 42 as moderate, and 20 as severe. Forty-seven patients were immunocompromised and 19 were immunocompetent (Table 1).
Table 1.
Performance Characteristics of the Lateral Flow Assay and Enzyme Immunoassay Histoplasma Antigen in the Various Patient Groups
| Category (No, [%]) | Positive LFA, No. (%) | Positive EIA, No. (%) | EIA, ng/mL, Mean ± SD | P Valuea |
|---|---|---|---|---|
| Cases (66 [18.75%]) | 52 (78.88) | 63 (95.50) | 17.38 ± 17.61 | <.001 |
| Controls (286 [81.25%]) | 2 (0.70) | 1 (0.35) | 0.00 ± 0.04 | |
| Proven (44 [66.67%]) | 41 (93.18) | 41 (93.18) | 22.83 ± 17.72 | <.001 |
| Probable (22 [33.33%]) | 11 (50) | 22 (100) | 6.49 ± 11.43 | |
| Pulmonary (20 [30.30%]) | 10 (50) | 18 (90) | 1.54 ± 1.57 | <.001 |
| Disseminated (46 [69.70%]) | 42 (91.30) | 45 (97.83) | 24.27 ± 16.94 | |
| Immunocompetent (19 [28.79%]) | 8 (42.11) | 17 (89.47) | 1.34 ± 1.43 | <.001 |
| Immunocompromised (47 [71.21%]) | 44 (93.62) | 46 (97.87) | 23.87 ± 16.98 | |
| Mild (4 [6.06%]) | 3 (75) | 4 (100) | 11.35 ± 18.50 | .064 |
| Moderate (42 [63.64%]) | 33 (78.57) | 39 (92.86) | 14.34 ± 16.64 | |
| Severe (20 [30.30%]) | 16 (80) | 20 (100) | 24.98 ± 17.92 |
Abbreviations: EIA, enzyme immunoassay; LFA, lateral flow assay; SD, standard deviation.
aP values comparing antigen levels as measured by the EIA quantitative assay using t test or analysis of variance.
Histoplasma antigen levels were significantly higher in the proven (22.83 ± 17.72 ng/mL) than probable (6.49 ± 11.43 ng/mL) cases, as measured by the quantitative EIA assay (P < .001). Similarly, antigen levels were significantly higher in patients with disseminated (24.27 ± 16.94 ng/mL) than pulmonary (1.54 ± 1.57 ng/mL) histoplasmosis (P < .001) and in those who were immunocompromised (23.87 ± 16.98 ng/mL) than immunocompetent (1.34 ± 1.43 ng/mL) (P < .001). Antigen levels tended to be higher in patients with severe disease (24.98 ± 17.92 ng/mL), compared to those with moderate (14.34 ± 16.64 ng/mL) and mild (11.35 ± 18.50 ng/mL) disease (P = .064). The sensitivity and specificity of the EIA assay were 95.46% and 99.65%, respectively (κ = 0.96 [95% confidence interval {CI}, .92–1.00]; P < .001).
Overall, the sensitivity and specificity of the LFA assay were 78.79% and 99.31%, respectively (κ = 0.84 [95% CI, .76–.92]; P < .001). LFA negative and positive predictive values and test accuracy were calculated using a calculated prevalence of 7.1% of histoplasmosis in a larger representation of the study population (Table 2). LFA was significantly less sensitive than EIA (P = .009). LFA’s sensitivity was significantly higher in those with proven (93.18%) than probable (50%) disease (κ = 0.738 [95% CI, .65–.82]; P < .001), with disseminated (91.3%) than pulmonary histoplasmosis (50%) (κ = 0.45 [95% CI, .21–.69]; P < .001), and in immunocompromised (93.62%) than immunocompetent (42.1%) patients (κ = 0.56 [95% CI, .33–.79]; P < .001). Sensitivity of LFA was comparable among patients with severe (80%) and moderate (78.6%) disease compared with mild (75%) disease (κ = 0.002 [95% CI, –.12 to .13]; P = .976).
Table 2.
Diagnostic Attributes of MVista Histoplasma Lateral Flow Assay
| Measure | Total Cases | Proven Histo. | Probable Histo. | Pulmonary Histo. | Disseminated Histo. | Moderate and Severe | EIA Antigen >1.8 ng/mL | Immunocompromised |
|---|---|---|---|---|---|---|---|---|
| Sensitivity, % | 78.79 | 93.18 | 50.00 | 50.00 | 91.30 | 79.03 | 97.78 | 93.62 |
| Specificity, % | 99.31 | 99.30 | 99.30 | 99.30 | 99.30 | 99.30 | 96.74 | 99.30 |
| NPV, %a | 98.39 | 99.48 | 96.29 | 96.29 | 99.34 | 99.77 | 99.83 | 99.51 |
| PPV, %a | 89.72 | 91.05 | 84.52 | 84.52 | 90.88 | 91.38 | 69.63 | 91.10 |
| Accuracy, %a | 97.85 | 98.86 | 95.80 | 95.80 | 98.73 | 99.14 | 96.81 | 98.90 |
Abbreviations: EIA, enzyme immunoassay; Histo., Histoplasmosis; NPV, negative predictive value; PPV, positive predictive value.
aCalculated using a prevalence of 7.1% in the study population.
EIA and LFA antigen tests were positive in 64 (18.2%) and 54 (15.3%) urine specimens, respectively. There was a strong level of agreement between the EIA and LFA results (κ = 0.84 [95% CI, .76–.91]; P < .001). Antigen levels were significantly higher in the samples with positive LFA (21.05 ± 17.47 ng/mL) than those with negative LFA reading (0.04 ± 0.23 ng/mL) (P < .001). To validate the lower LoD of 1.8 ng/mL as determined to be the lower LoD, we divided our cohort of patients according to this cutoff. The positive and negative agreement of LFA and EIA were 97.78% and 96.74%, respectively (κ = 0.871 [95% CI, .80–.95]; P < .001).
Cross-Reactivity in Other Endemic Mycoses
Among patients with talaromycosis in whom Histoplasma antigen EIA was positive, the LFA was positive in 39 of 52 (75%) (Table 3); among those with paracoccidioidomycosis in whom the Histoplasma antigen EIA was positive, the LFA was positive in 6 of 7 (85.7%). Twenty-two of 27 (81.5%) patients with blastomycosis who had positive Blastomyces antigen EIA results had positive LFA results. The LFA was positive in 6 of 20 (30%) patients with coccidioidomycosis who had positive Coccidioides EIA antigen results (Table 3).
Table 3.
Cross-Reactivity in Controls With Other Endemic Fungal Infections
| Type of Infection | Cross-Reactivity |
|---|---|
| Blastomycosis (n = 27) | 81.5% |
| Paracoccidioidomycosis (n = 7) | 85.7% |
| Talaromycosis (n = 52) | 75% |
| Coccidioidomycosis (n = 20) | 30% |
DISCUSSION
The MVista Histoplasma galactomannan antigen LFA offers the first rapid test for diagnosis of histoplasmosis and is simple to perform. The test is read visually, no equipment is needed, pretreatment of the urine is unnecessary, and minimal training in required to perform and interpret the results. The test can be performed outside the laboratory and results can be determined within 30 minutes of collection, making it optimal to use in resource-limited parts of the world that are endemic to histoplasmosis and in a dire need for a rapid diagnostic test, especially in patients with high burden and severe disease [12]. Given its simplicity and accuracy, the test also has the potential to be used at the point of care. As few as 1 specimen and as many as 25 can be accurately tested at 1 time by a single technician.
Validation studies determine the LoD to be 1.8 ng/mL for the LFA compared to 0.2 ng/mL for the EIA. Within-laboratory precision and within-run repeatability were 100%. Strong agreement was found between the EIA and LFA when applied to a diverse and relatively large cohort of consecutive unbiased subjects from highly endemic areas of the US. Overall, LFA was less sensitive than EIA (78.79% vs 95.46%; P = .009) in this cohort of patients. This translates into 11 of 66 (16.67%) false-negative tests by LFA.
The diagnostic accuracy of LFA was particularly favorable in patient with high-burden disease (96.8%) as determined by antigen levels >1.8 ng/mL, moderate to severe disease (99.1%), in those with disseminated infection (98.7%), and immunocompromised patients (98.9%). Sensitivity of the LFA was 93.2% in patients with proven histoplasmosis, comparable to that of the well-known and widely used EIA assay [4]. Sensitivity in probable cases (50%), however, was significantly lower than EIA (100%). Sensitivity is likely to be greater in resource-limited countries where patients usually present later in the course of the illness with more severe disease and greater fungal burden [12].
The MVista Histoplasma LFA was used in a recently published multicenter prospective study of people living with HIV with suspected histoplasmosis in Mexico. The test was performed at a central laboratory. The sensitivity, specificity, and accuracy of the assay were 90.4%, 92.3%, and 91.8%, respectively, for proven disseminated histoplasmosis. A high level of agreement (κ = 0.85) was also noted between this LFA and another EIA-based test [13]. Despite the different geographic location and patient population, these results are very much like ours. An earlier study comparing visual to automated reading of the MVista Histoplasma LFA in people with HIV with proven disseminated histoplasmosis in Colombia showed a similarly high sensitivity (96%) and specificity (90%), with a high degree of agreement between the 2 reading methods (κ = 0.90) [14]. In addition to histoplasmosis, LFA has also been used effectively for the rapid diagnosis of cryptococcosis in a recent large implementation study in Central America [15].
Cross-reactivity in paracoccidioidomycosis and talaromycosis is nearly complete. Paracoccidioidomycosis is endemic in parts of the Americas [3] and talaromycosis is endemic in parts of southeast Asia, other Asian countries, India, and elsewhere [16]. Talaromycosis also occurs among immunocompromised patients with conditions other than HIV/AIDS [17]. Accordingly, the Histoplasma antigen LFA may aid in the diagnosis of paracoccidioidomycosis and talaromycosis in resource-limited countries and potentially aid in the decision to initiate appropriate antifungal therapy in a timely fashion and likely improve outcomes.
Cross-reactions occurred in 75% of patients with blastomycosis and 30% with coccidioidomycosis. Prior studies using the EIA reported >90% cross-reactivity in patients with blastomycosis [18]. This apparent lower level of cross-reactivity might be due to the lower sensitivity of the LFA rather than a truly higher analytical specificity.
Disseminated histoplasmosis is also common in patients with other immunocompromising conditions or who are receiving immunosuppressive medications. And antigen detection is a common method for diagnosis in these groups [4]. The LFA should also be useful for diagnosis of histoplasmosis in patients with other immunosuppressive conditions in resource-limited countries.
Limitations
This study was conducted in highly endemic areas of the US, not in resource-limited countries. Sensitivity may be higher in resource-limited countries because patients usually present with more severe disease characterized by high fungal burden. For example, in patients with HIV/AIDS, mortality in the US in 51 patients treated with 2 weeks of liposomal amphotericin B followed by itraconazole was 2% compared to 45% at 30 days after initiation of antifungal therapy in French Guiana [6]. The LFA should be evaluated in immunocompromised patients in resource-limited countries where histoplasmosis is common.
To overcome the lower sensitivity of the LFA for pulmonary, mild to moderate, and nonimmunocompromised-host histoplasmosis, one might consider either using the EIA-based assay with higher sensitivity or, if that is not available, combining the LFA test with serologic testing to improve the diagnostic yield as was previously shown with the EIA-based antigen detection test [19]. The latter strategy will need to be tested in the appropriate patient population. Another limitation of the LFA-based antigen detection is the lack of quantification and therefore inability to assess severity of illness and follow disease progression while on therapy, as is the case with the EIA-based antigen detection test [4, 20].
In summary, the MVista Histoplasma antigen LFA can provide rapid results in point-of-care settings by personnel who have been trained and exhibit proficiency in performing the test. The test is expected to be most useful in immunocompromised patients including those with HIV/AIDS and other immunosuppressive or inflammatory disorders treated with immunosuppressive medications. The test also may be useful as an aid to diagnosis of talaromycosis and paracoccidioidomycosis, also common in some resource-limited countries.
Notes
Patient consent statement. The design of the work was approved by local ethical committees of the participating institutions (Indiana University, Indianapolis; University of Kentucky, Lexington; and Vanderbilt University, Nashville, Tennessee). The study did not include factors necessitating patient consent.
Potential conflicts of interest. M. M., S. G., and L. J. W. are employees of MiraVista Diagnostics, the provider of the enzyme immunoassays and lateral flow assays. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Azar MM, Loyd JL, Relich RF, et al. Current concepts in the epidemiology, diagnosis, and management of histoplasmosis syndromes. Semin Respir Crit Care Med 2020; 41:13–30. [DOI] [PubMed] [Google Scholar]
- 2.Hage CA, Carmona EM, Epelbaum O, et al. Microbiological laboratory testing in the diagnosis of fungal infections in pulmonary and critical care practice. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2019; 200:535–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombo AL, Tobón A, Restrepo A, et al. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 2011; 49:785–98. [DOI] [PubMed] [Google Scholar]
- 4.Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 2011; 53:448–54. [DOI] [PubMed] [Google Scholar]
- 5.Azar MM, Hage CA. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin Chest Med 2017; 38:403–15. [DOI] [PubMed] [Google Scholar]
- 6.Adenis A, Nacher M, Hanf M, et al. HIV-associated histoplasmosis early mortality and incidence trends: from neglect to priority. PLoS Negl Trop Dis 2014; 8:e3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caceres DH, Zuluaga A, Arango-Bustamante K, et al. Implementation of a training course increased the diagnosis of histoplasmosis in Colombia. Am J Trop Med Hyg 2015; 93:662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falci DR, Hoffmann ER, Paskulin DD, Pasqualotto AC. Progressive disseminated histoplasmosis: a systematic review on the performance of non-culture-based diagnostic tests. Braz J Infect Dis 2017; 21:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kassis C, Durkin M, Holbrook E, et al. Advances in diagnosis of progressive pulmonary and disseminated coccidioidomycosis. Clin Infect Dis 2021; 72:968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheat J, Wheat H, Connolly P, et al. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin Infect Dis 1997; 24:1169–71. [DOI] [PubMed] [Google Scholar]
- 11.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012; 22:276–82. [PMC free article] [PubMed] [Google Scholar]
- 12.Nacher M, Adenis A, Mc Donald S, et al. Disseminated histoplasmosis in HIV-infected patients in South America: a neglected killer continues on its rampage. PLoS Negl Trop Dis 2013; 7:e2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez-Gamboa A, Niembro-Ortega MD, Torres-González P, et al. Diagnostic accuracy of antigen detection in urine and molecular assays testing in different clinical samples for the diagnosis of progressive disseminated histoplasmosis in patients living with HIV/AIDS: a prospective multicenter study in Mexico. PLoS Negl Trop Dis 2021; 15:e0009215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cáceres DH, Gómez BL, Tobón AM, et al. Evaluation of a Histoplasma antigen lateral flow assay for the rapid diagnosis of progressive disseminated histoplasmosis in Colombian patients with AIDS. Mycoses 2020; 63:139–44. [DOI] [PubMed] [Google Scholar]
- 15.Caceres DH, Arauz AB, Flores C, et al. Implementation of rapid diagnostics assays for detection of histoplasmosis and cryptococcosis in Central American people living with HIV [manuscript published online ahead of print 9 May 2021]. Mycoses 2021. doi:10.1111/myc.13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao C, Xi L, Chaturvedi V. Talaromycosis (penicilliosis) due to Talaromyces (Penicillium) marneffei: insights into the clinical trends of a major fungal disease 60 years after the discovery of the pathogen. Mycopathologia 2019; 184:709–20. [DOI] [PubMed] [Google Scholar]
- 17.Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect 2016; 5:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheat LJ, Freifeld AG, Kleiman MB, et al. , Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–25. [DOI] [PubMed] [Google Scholar]
- 19.Richer SM, Smedema ML, Durkin MM, et al. Improved diagnosis of acute pulmonary histoplasmosis by combining antigen and antibody detection. Clin Infect Dis 2016; 62:896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hage CA, Kirsch EJ, Stump TE, et al. Histoplasma antigen clearance during treatment of histoplasmosis in patients with AIDS determined by a quantitative antigen enzyme immunoassay. Clin Vaccine Immunol 2011; 18:661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]