Abstract
The efficacy of coronavirus disease 2019 (COVID-19) vaccines administered after COVID-19-specific monoclonal antibody is unknown, and “antibody interference” might hinder immune responses leading to vaccine failure. In an institutional review board–approved prospective study, we found that an individual who received mRNA COVID-19 vaccination <40 days after COVID-19-specific monoclonal antibody therapy for symptomatic COVID-19 had similar postvaccine antibody responses to SARS-CoV-2 receptor binding domain (RBD) for 4 important SARS-CoV-2 variants (B.1, B.1.1.7, B.1.351, and P.1) as other participants who were also vaccinated following COVID-19. Vaccination against COVID-19 shortly after COVID-19-specific monoclonal antibody can boost and expand antibody protection, questioning the need to delay vaccination in this setting.
Trial registration.
The St. Jude Tracking of Viral and Host Factors Associated with COVID-19 study; NCT04362995; https://clinicaltrials.gov/ct2/show/NCT04362995.
Keywords: antibody, bamlanivimab, COVID-19, SARS-CoV-2, vaccine failure
Novel vaccines that mimic the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein, an important coronavirus disease 2019 (COVID-19) virulence factor, induce high-titer antibody responses and reduce the risk and severity of infection [1]. Antibodies against the SARS-CoV-2 spike receptor binding domain (RBD) appear to be especially important [2]. Vaccination is recommended even for individuals who have recovered from COVID-19, in part because of improved cross-protection against SARS-CoV-2 variants with RBD mutations [3], particularly currently circulating B.1.351 and P.1 variants [4, 5]. As vaccination uptake increases, it is vitally important to understand factors adversely affecting vaccine protection.
Recent receipt of SARS-CoV-2 RBD-specific monoclonal antibodies is postulated to interfere with vaccine responses by blocking critical epitopes recognized by the immune system—this differs from natural infection alone because these antibodies are not derived from endogenous B-cell stimulation [3]. As antibody half-life is ~18 days, both the US Centers for Disease Control and Prevention (CDC) and the World Health Organization recommend that vaccination be deferred for at least 90 days to avoid “potential interference of the antibody therapy with vaccine-induced immune responses” [3, 6, 7]. Understanding whether monoclonal antibodies interfere with COVID-19 vaccines is critical because they are important tools in protection against severe COVID-19, and vaccination delays could lead to breakthrough infections [6, 8–11].
Here, we report the case of an adult treated with COVID-19-specific monoclonal antibody therapy for COVID-19, then vaccinated with 2 doses of an mRNA COVID-19 vaccine within 40 days.
CASE
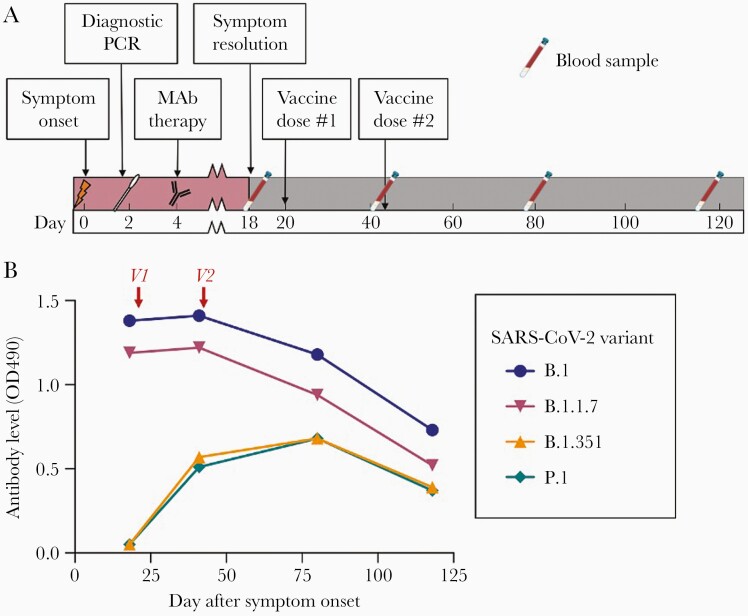
A 66-year-old white male without known immunocompromise presented with loss of smell and taste and was diagnosed with COVID-19 by nasal swab SARS-CoV-2 quantitative reverse transcription polymerase chain reaction (Figure 1A). The virus was found to belong to the B.1.2 strain. Because of age and history of hypertension, he received monoclonal antibody therapy on day 4 of illness (Bamlanivimab, Eli Lilly, Indianapolis, Indiana, USA). Symptoms, including fever, chills, cough, and headache, lasted for 18 days, but hospitalization was not required. He then received 2 doses of BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech, Philadelphia, Pennsylvania, USA) on days 20 and 41 after symptom onset (days 16 and 37 after antibody). As part of a prospective study (The St. Jude Tracking of Viral and Host Factors Associated with COVID-19 study [SJTRC]; NCT04362995), blood samples were collected on days 18, 41, 80, and 118 after symptom onset.
Figure 1.
A, Timeline of events. MAb, COVID-19-specific monoclonal antibody therapy (Bamlanivimab, Eli Lilly, Indianapolis, Indiana, USA); Vaccine, mRNA COVID-19 vaccine (BNT162b2, Pfizer-BioNTech, Philadelphia, Pennsylvania, USA). B, Antibody responses against receptor binding domain for common SARS-CoV-2 variants after infection/monoclonal antibody therapy and COVID-19 vaccine show increases in variant-specific antibodies after vaccination. V1 and V2, administration of first and second doses of mRNA COVID-19 vaccine (BNT162b2 OD490, RBD-specific antibody level measured by ELISA as optical density at 490 nm. Abbreviations: COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; MAb, monoclonal antibody; PCR, polymerase chain reaction; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
METHODS
The SJTRC Study is a prospective, institutional review board–approved, longitudinal cohort study of adult St. Jude employees who provide written informed consent and then provide data about demographics, medical history, and COVID-19. Blood samples are collected at baseline and after SARS-CoV-2 infection or vaccination. Data are managed using the REDCap electronic data capture tools hosted at St. Jude [12, 13]. Vaccination is according to institutional practice.
COVID-19 antibody responses were measured by enzyme-linked immunosorbent assay (ELISA) against RBD for the B.1, B.1.1.7, B.1.351, and P.1 variants, as well as nucleocapsid protein (N) and whole spike protein (S) for B.1, as previously described [14]. Briefly, proteins were diluted to 1.5 µg/mL (RBD), 2 µg/mL (spike), and 1 µg/mL (N protein) in phosphate-buffered saline (PBS) and incubated overnight at 4°C on MaxiSorp 96- or 384-well plates. Plates were blocked with 3% nonfat milk in PBS containing 0.1% Tween-20 (PBST) at room temperature and washed. Plasma samples were diluted 1:50 in 1% milk PBST and incubated on plates for 1.5 hours at room temperature. Plates were washed and incubated with an HRP-conjugated, goat antihuman immunoglobulin G (H + L) secondary (1:10 000 for 384-well and 1:2500 for 96-well plates) for 30 minutes at room temperature. After the final wash, SIGMAFAST OPD solution was added to each well for 8 minutes, then development was stopped with 3N hydrochloric acid. Positive controls included anti-SARS-CoV-2 RBD antibody and known positive plasma (1:50). The negative control was a prepandemic plasma sample (1:50). Optical density (OD) was measured at 490 nm and is reported as a raw value or percent ratio (percentage of sample OD relative to positive control samples).
To evaluate the effect of monoclonal antibody exposure on immunization efficacy, we compared postvaccine antibody responses against RBD for 4 important COVID-19 variants (B.1, B1.1.7, B.1.351, and P.1) between the case participant and control participants who recovered from COVID-19 without antibody therapy and subsequently received a BNT162b2 mRNA COVID-19 vaccine. Similarly, we compared change in variant RBD antibodies between the case participant and control participants who had COVID-19 only and samples collected at similar time points.
RESULTS
In the case participant, the antibody response against the immunologically similar B.1 and B.1.1.7 variant RBDs was high during the early convalescent phase before vaccination (18 days after symptom onset), likely due to residual activity of the monoclonal antibody plus endogenous antibody (Figure 1B). However, antibodies against 2 dissimilar variant RBDs (B.1.351 and P.1) were undetectable (Figure 1B). This is consistent with previous reports of impaired antibody responses to these variants in patients recovering from natural infection and the poor affinity of bamlanivimab for these variants [4].
After each vaccine dose, antibody levels against B.1.351 and P.1 variant RBDs increased, despite little change in antibodies against B.1 and B.1.1.7 variant RBDs (Figure 1B). A significant rise in antibody levels during this time period is not typical of the response to infection alone, suggesting that the change is caused by the vaccine. Specifically, increases in antibodies to these variants of this magnitude were not seen at similar time points (17–21 days vs 22–189 days) in control participants who had experienced COVID-19 but not been vaccinated (Supplementary Figure 1).
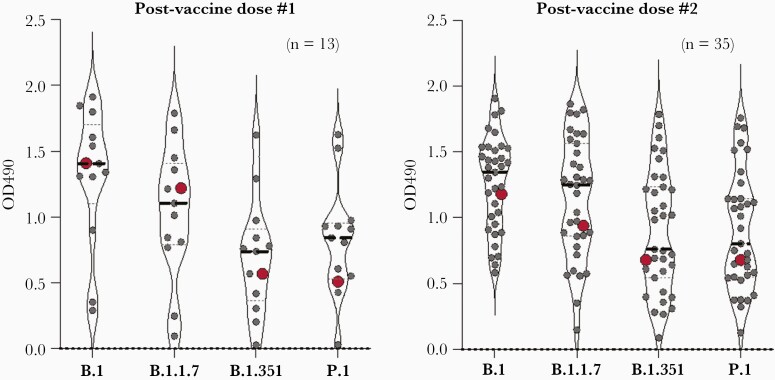
Postvaccine antibody levels against variant RBDs in the case participant were similar to those seen in participants who received 1 (n = 13) (Figure 2A) or 2 doses (n = 35) (Figure 2B) of the same COVID-19 vaccine following COVID-19 but not monoclonal antibody therapy. During the same period, antibody levels against whole B.1 spike and nucleocapsid proteins were also similar to other participants who had COVID-19 and then received the same number of vaccine doses (Supplementary Figure 2).
Figure 2.
Vaccination with COVID-19 vaccine after treatment of COVID-19 with monoclonal antibody is associated with similar RBD antibody responses to vaccination following COVID-19 alone. OD490, RBD antibody levels by ELISA measured as optical density at 490 nm; Red dot, Participant vaccinated with mRNA COVID-19 vaccine following natural infection and monoclonal antibody therapy (Postvaccine dose #1, sample collected 21 days after vaccine and 41 days after symptom onset; Postvaccine dose #2, sample collected 40 days after vaccine and 81 days after symptom onset); Gray dots, participants with vaccination following natural infection only (Postvaccine dose #1, sample collected 14–21 days after vaccine; Postvaccine dose #2, sample collected 23–56 days after vaccine; Dashed line, median. Abbreviations: COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; RBD, receptor binding domain.
DISCUSSION
As COVID-19 vaccination rates increase around the world, it is critical to understand factors that might limit vaccine-associated protection [3]. To our knowledge, this is the first well-characterized individual who received mRNA COVID-19 vaccine in the immediate period after monoclonal antibody therapy. Before vaccination, the participant had evidence of antibody protection against the immunologically similar B.1 (wild-type) and B.1.1.7 (United Kingdom) variants, but not the dissimilar B.1.351 (South Africa) and P.1 (Brazil) variants. Neither bamlanivimab nor natural infection with wild-type virus provides good protection against these variants [4]. Subsequently, despite both vaccine doses being administered within a period where interference might be expected, the patient developed antibodies against all 4 variants that persisted for at least 80 days [6]. This demonstrates that bamlanivimab had minimal interference on the endogenous antibody response to vaccination.
The proposed mechanism of monoclonal antibody interference with COVID-19 vaccines is high levels of RBD antibody blocking critical epitopes recognized by the immune system [3]. Although evidence exists for antibody interference with some live-attenuated virus vaccines (administration of measles vaccine after administration of donor immunoglobulins reduces its immunogenicity by preventing vaccine virus replication [15]), it has not been shown with polysaccharide, killed, mRNA, or protein-adjuvant vaccines.
The generalizability of this case may be limited. Other individuals might have differing immune responses, so a single case demonstrating lack of interference cannot exclude the possibility. Timing of monoclonal antibody administration, viral load, SARS-CoV-2 variant, monoclonal antibody product, or vaccine product might affect outcomes. And, although this participant did have high levels of antibodies against variant RBDs after vaccination, there is no universally accepted threshold for antibody protection, and some RBD-specific antibodies might not provide protection from infection.
CONCLUSIONS
In this case, administration of 2 doses of COVID-19 vaccine within 40 days of monoclonal antibody therapy for COVID-19 was associated with excellent antibody responses to wild-type and variant RBDs, as well as the full-length spike protein, suggesting minimal interference of persistent exogenous monoclonal antibody. These data support the CDC recommendation not to repeat vaccine doses given during the convalescent period in patients who have received COVID-19-specific monoclonal antibody therapy and raise the question of whether any delay is required before vaccination in monoclonal antibody therapy recipients.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors acknowledge the contributions of Tamanna Shamrin, Rishi Kodela, Ashleigh Gowen, Pamela Merritt, staff of St. Jude Children’s Research Hospital, and the SJTRC participants.
In addition to the authors listed on the byline, the SJTRC investigative team includes: Ericka Kirkpatrick Roubidoux,1 Pamela Freiden,1 Tomi Mori,6 Diego R. Hijano,1 Hana Hakim,1 David C. Brice,3 Ashley Castellaw,3 Florian Krammer,7 David E. Wittman,8 Jason Hodges,4 Ronald H. Dallas,1 Valerie Cortez,1 Ana Vazquez-Pagan,1 Resha Bajracharya,3 Brandi L. Clark,3 Lee-Ann Van de Velde,3 Walid Awad,3 Taylor L. Wilson,3 Allison M. Kirk,3 Randall T. Hayden,9 James Hoffman,10 Jamie Russell-Bell,1 and James Sparks5
1Department of Infectious Diseases, St Jude Children’s Research Hospital, Memphis, Tennessee, USA, 2Department of Microbiology, Immunology and Biochemistry, University of Tennessee Health Science Center, Memphis, Tennessee, USA, 3Department of Immunology, St Jude Children’s Research Hospital, Memphis, Tennessee, USA, 4Department of Hematology, St Jude Children’s Research Hospital, Memphis, Tennessee, USA, 5Department of Global Pediatric Medicine, St Jude Children’s Research Hospital, Memphis, Tennessee, USA, 6Department of Biostatistics, St Jude Children’s Research Hospital, Memphis, Tennessee, USA, 7Department of Microbiology, Icahn School of Medicine at Mount Sinai, New York, New York, USA, 8Office of Quality and Patient Care St Jude Children’s Research Hospital, Memphis, Tennessee, USA, 9Department of Pathology, St Jude Children’s Research Hospital, Memphis, Tennessee, USA, and 10Department of Pharmaceutical Sciences, St Jude Children’s Research Hospital, Memphis, Tennessee, USA
Financial support. This study was supported by the American Lebanese Syrian Associated Charities and St. Jude Children’s Research Hospital, the NIAID for the St. Jude Center of Excellence for Influenza Research and Surveillance (CEIRS contract HHSN27220140006C) and NIAID Collaborative Influenza Vaccine Innovation Centers (CIVIC) contract 75N93019C00052, NIAID grant 3U01AI144616–02S1 to P.T., M.A.M., R.J.W., and S.S.C., NIAID grant R01AI128756 to E.I.T., and by the University of Tennessee Research Foundation. J.H.E. is supported by the American Society of Hematology Scholar Award. The sponsors had no role in design or conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Potential conflicts of interest. All authors declare that they have no conflicts of interest relevant to the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data access, responsibility, and analysis. J.W. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data sharing. Data are not available due to the identifiable nature of the participant.
Contributor Information
SJTRC Investigative Team:
Ericka Kirkpatrick Roubidoux, Pamela Freiden, Tomi Mori, Diego R Hijano, Hana Hakim, David C Brice, Ashley Castellaw, Florian Krammer, David E Wittman, Jason Hodges, Ronald H Dallas, Valerie Cortez, Ana Vazquez-Pagan, Resha Bajracharya, Brandi L Clark, Lee-Ann Van de Velde, Walid Awad, Taylor L Wilson, Allison M Kirk, Randall T Hayden, James Hoffman, Jamie Russell-Bell, and James Sparks
References
- 1.Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet 2020; 396:1595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020; 584:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States. Available at: https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html. Accessed 4 September 2021.
- 4.Hoffmann M, Arora P, Groß R, et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell 2021; 184:2384-2393.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidal SJ, Collier AY, Yu J, et al. Correlates of neutralization against SARS-CoV-2 variants of concern by early pandemic sera. J Virol 2021; 95:e0040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. Fact sheet for health care providers; emergency use authorization (EUA) of bamlanivimab. Available at: https://www.fda.gov/media/143603/download. Accessed 30 November 2020.
- 7.World Health Organization. Interim recommendations for use of the Pfizer–BioNTech COVID-19 vaccine, BNT162b2, under Emergency Use Listing. Available at: https://apps.who.int/iris/bitstream/handle/10665/338484/WHO-2019-nCoV-vaccines-SAGE_recommendation-BNT162b2-2021.1-eng.pdf. Accessed 19 May 20121.
- 8.Food and Drug Administration. Fact sheet for health care providers; Emergency Use Authorization (EUA) of casirivimab and imdevimab. Available at: https://www.fda.gov/media/143892/download. Accessed 30 November 2020.
- 9.Food and Drug Administration. Fact sheet for health care providers; Emergency Use Authorization (EUA) of bamlanivimab and etesevimab. Available at: https://www.fda.gov/media/145802/download. Accessed 27 April 2021.
- 10.Infectious Diseases Society of America. IDSA guidelines on the treatment and management of patients with COVID-19. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed 27 April 2021.
- 11.Regeneron Pharmaceuticals Inc. Phase 3 prevention trial showed 81% reduced risk of symptomatic SARS-CoV-2 infections with subcutaneous administration of REGEN-COV™ (casirivimab with imdevimab). Available at: https://investor.regeneron.com/news-releases/news-release-details/phase-3-prevention-trial-showed-81-reduced-risk-symptomatic-sars. Accessed 12 April 2021.
- 12.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 2020; 26:1033–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siber GR, Werner BG, Halsey NA, et al. Interference of immune globulin with measles and rubella immunization. J Pediatr 1993; 122:204–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.