Abstract
Background
The emergence of antimicrobial resistance in uropathogens has generated interest in the use of nitrofurantoin in controversial populations, such as in males and those with renal dysfunction. The purpose of this study was to compare the efficacy and safety of nitrofurantoin for the treatment of cystitis in males and females with variable degrees of renal dysfunction.
Methods
A retrospective chart review was conducted in adult patients who received nitrofurantoin for acute cystitis in the outpatient setting. The primary outcome was clinical cure compared between males and females and across various renal function groups (creatinine clearances [CrCl] >60 mL/min, 30–60 mL/min, and <30 mL/min) following nitrofurantoin treatment. The secondary outcome was adverse events.
Results
A total of 446 patients were included, with 278 females and 168 males. The overall clinical cure rate was 86.5% (95% CI, 83.0%–89.4%; n = 386). The clinical cure rate did not vary between genders (odds ratio [OR], 0.6; 95% CI 0.35–1.04; P = .085) or between patients with a CrCl >60 mL/min compared with those with CrCl 30–60 mL/min (OR, 1.01; 95% CI, 0.40–2.44; P = 1). The 1 patient with a CrCl <30 mL/min was not included in the analysis. A history of benign prostatic hyperplasia (OR, 0.5; 95% CI, 0.26–0.99; P = .045) or cirrhosis (OR, 0.21; 95% CI, 0.06–0.82; P = .025) was associated with decreased odds of clinical cure. Adverse events occurred in 2% (n = 9) of patients.
Conclusions
There was no statistically significant difference in clinical cure with nitrofurantoin between genders or various renal functions.
Keywords: cystitis, kidney diseases, nitrofurantoin, renal insufficiency, urinary tract infections
Antimicrobial resistance in uropathogens has generated interest in the use of nitrofurantoin in controversial populations, such as in males and renal dysfunction. Our single-center retrospective study showed no statistically significant difference in clinical cure between genders and various renal functions.
Nitrofurantoin is an ideal antibiotic for the treatment of cystitis due to its activity against gram-negative and gram-positive organisms, high urine concentrations, low resistance rates, and favorable side effect profile. The 2010 Infectious Diseases Society of America (IDSA) guidelines for the treatment of uncomplicated cystitis in females recommends nitrofurantoin as a first-line agent targeting Escherichia coli as the predominate urinary pathogen [1]. However, evidence is lacking for the safety and efficacy of nitrofurantoin in treating cystitis in the male population.
Male urinary tract infections are often complicated by instrumentation, prostate enlargement, urinary stents, or renal or urinary stones, increasing the urinary concentrations required to achieve optimal antimicrobial killing [2, 3]. Therefore, practitioners may be hesitant to use nitrofurantoin for the treatment of cystitis in males. One retrospective study evaluated the safety and efficacy of nitrofurantoin for the treatment of acute cystitis in 485 male veterans at a single VA health care system. An overall clinical cure rate of 77.3% was observed, and the authors identified that renal insufficiency was an independent risk factor for treatment ineffectiveness [2].
In addition, manufacturer labeling recommends avoiding nitrofurantoin in patients with creatinine clearances (CrCls) <60 mL/min [4]. This recommendation was based on studies that demonstrated that nitrofurantoin urinary concentrations in healthy volunteers with CrCls <60 mL/min did not reach adequate levels to be effective against common urinary tract infection (UTI) pathogens after a single dose [5–8].
In 2015, the American Geriatrics Society Beers Criteria Update Expert Panel revised their recommendation for avoiding nitrofurantoin from a CrCl of <60 mL/min to a CrCl of <30 mL/min based on 2 retrospective studies [9]. Geerts et al. compared clinical failure of cystitis treatment and adverse event rates between nitrofurantoin and trimethoprim in females treated in a primary care setting and found no difference in efficacy or safety between patients with an estimated glomerular filtration rate (eGFR) of >50 mL/min/1.73 m2 and those with an eGFR of 30–50 mL/min/1.73 m2. However, an eGFR of 10–29 mL/min/1.73 m2 was associated with higher rates of pulmonary adverse events requiring hospitalization. Notably, only 0.9% of patients in this study had an eGFR <50 mL/min/1.73 m2, and the study only included females [10]. A similar retrospective study by Bains et al., which included predominantly females, found no significant difference in clinical cure or adverse event rates between patients with or without renal impairment [11].
The emergence of antimicrobial resistance in urinary tract pathogens is compelling our providers to use nitrofurantoin not only in males, but also in patients with renal dysfunction. To our knowledge, no previous studies have compared the efficacy and safety of nitrofurantoin for the treatment of acute cystitis between males and females, nor are there previous studies making a similar comparison based on variable renal function in a population with comparable gender proportions. The purpose of this study was to compare the efficacy and safety of nitrofurantoin for the treatment of cystitis between males and females and between patients with and without renal insufficiency.
METHODS
Study Design and Population
This was a single-center, retrospective chart review of male and female veterans treated with nitrofurantoin for acute cystitis in an outpatient setting at the Michael E DeBakey Veterans Affairs Medical Center (MEDVAMC) between May 1, 2018, and May 1, 2019. During the study, 94% of all E. coli urinary isolates at our institution were susceptible to nitrofurantoin. Approximately 12% of the E. coli urinary isolates produced extended-spectrum beta-lactamases with 86% susceptibility to nitrofurantoin. Patients included were age 18 years and older and had received an outpatient prescription for nitrofurantoin macrocrystals/monohydrate at an appropriate dose with a duration of 5–14 days for the treatment of acute cystitis. Patients with catheter-associated UTIs were also eligible for inclusion. Patients were excluded if they received nitrofurantoin for asymptomatic bacteriuria, prostatitis, pyelonephritis, chronic suppressive therapy, prophylaxis, or pre/postprocedural. Patients were also excluded if they were treated for an organism with resistance to nitrofurantoin or if they received concomitant antibiotics with nitrofurantoin during treatment for cystitis. The study was approved by the Baylor College of Medicine Institutional Review Board and the Veterans Affairs Office of Research and Development.
Study Definitions
Patients were defined as having acute cystitis if they were documented as having any lower urinary tract symptoms attributed to cystitis or UTI or altered mental status changes with no alternative diagnosis in the Computerized Patient Record System (CPRS). Lower urinary tract symptoms included dysuria, difficulty urinating or change in frequency of urination, new or worsening urinary retention, gross hematuria, suprapubic tenderness, or lower abdominal pain. Patients were excluded if the described symptoms were later attributed to an alternative diagnosis. Pyelonephritis was defined by the presence of systemic symptoms with flank pain and/or costovertebral angle tenderness.
Patients who had at least 1 of the following on urinalysis (UA) were determined to have a positive UA: positive leukocyte esterase, red blood cells >2 per high-powered field (HPF), white blood cells >10 per HPF, or positive for bacteria. Patients with documented symptoms but with a corresponding negative UA and no growth on urine culture were not included in the analysis. However, patients who did not have a documented UA in the chart could still be included.
Study Outcomes
The primary outcome was clinical cure following a treatment course of nitrofurantoin. Rate of clinical cure was compared between males and females and between individuals with CrCl >60 mL/min, 30–60 mL/min, and <30 mL/min. Clinical cure was defined as treatment discontinuation after an appropriate course of nitrofurantoin (between 5 and 14 days) and no other antibiotics initiated or signs/symptoms of a urinary tract infection within 14 days of completion of treatment.
The secondary outcome was having an adverse event related to nitrofurantoin identified during therapy, up to 7 days after completion of therapy. Adverse events evaluated were gastrointestinal distress, headache, peripheral neuropathy, rash, acute pulmonary reaction, elevated liver enzymes, hepatotoxicity, hemolytic reaction, or miscellaneous.
Data Collection
A list of patients who received a prescription for nitrofurantoin within the study period was generated using the Veterans Information Systems and Technology Architecture (VISTA) system. Data collected from the CPRS included age, gender, height, weight, prespecified comorbidities, indication for nitrofurantoin use, urine culture isolate information, presence of a urinary catheter and catheter type (if applicable), antibiotic use during and 14 days following treatment with nitrofurantoin, signs and symptoms suggestive of cystitis, urinalysis, serum creatinine, dialysis mode and schedule (if applicable), and adverse effects potentially related to nitrofurantoin use. CrCl was estimated using the Cockcroft-Gault formula utilizing the most recent serum creatinine, weight, and height values available before initiation of nitrofurantoin. Urine culture data were collected, if available; however, absence of urine culture did not exclude patients from analysis.
Statistical Analysis
Based on previous retrospective studies, the expected incidence of the primary outcome was 80% in patients with CrCl >60 mL/min and 65% in patients with CrCl ≤60 mL/min [2, 11]. Using the Fisher exact test, to detect an expected difference of 15% in the primary outcome with a power of 80% and an alpha level of .05, a sample size of 326 patients was required. This included an expected enrollment of 233 patients with CrCl >60 mL/min and 93 patients with CrCl ≤60 mL/min.
Descriptive statistics were used to summarize demographic and baseline data and reported as frequency with percentage, median with interquartile range (IQR), or mean with SD, as appropriate. Summary statistics were stratified by gender and compared using the Fisher exact, chi-square, Wilcoxon rank-sum, or Student t test as appropriate. Prespecified subgroup analyses compared the primary outcome using the methods described above by dividing patients into subgroups based on gender and CrCl range. Independent logistic regression was used to measure the association between patient characteristics and clinical cure. Characteristics found to have a significant association with clinical cure (P < .05) in bivariate analysis were included in multivariable logistic regression. Odds ratios (ORs) with 95% CIs were reported, and a P value <.05 was used to determine statistical significance. All statistical analyses were performed using JMP, version 15.1 (SAS).
RESULTS
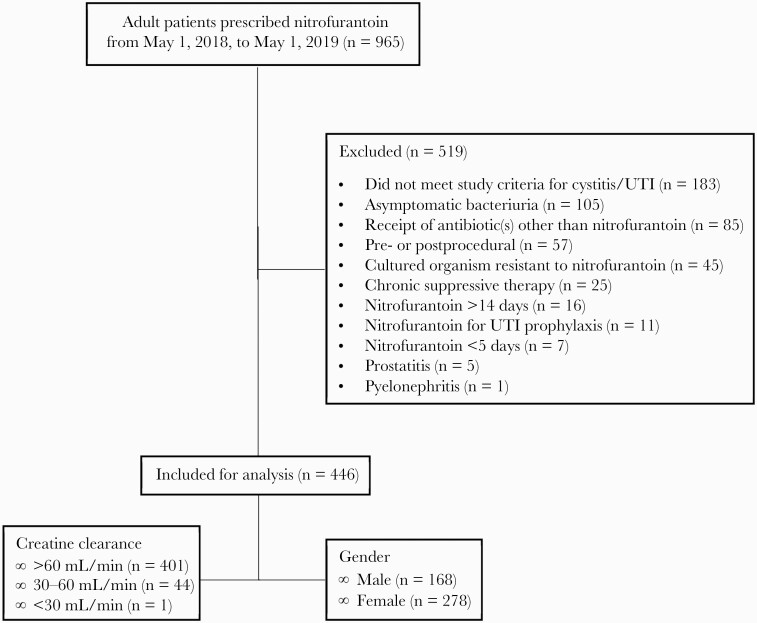
There were 965 patients who were prescribed nitrofurantoin between May 1, 2018, and May 1, 2019. Of these, 446 patients met the inclusion criteria (Figure 1). The most common reasons for exclusion were lack of documented symptoms or diagnostic criteria attributed to acute cystitis or UTI (n = 183), treatment of asymptomatic bacteriuria (n = 105), receipt of antibiotic(s) other than nitrofurantoin during the treatment period (n = 85), and prescribing of nitrofurantoin as a periprocedural antibiotic (n = 45). All patients included in this study received the nitrofurantoin macrocrystals/monohydrate formulation dosed at 100 mg twice a day.
Figure 1.
Flow diagram outlining inclusion and exclusion. Abbreviation: UTI, urinary tract infection.
Baseline Characteristics
Patient baseline characteristics are shown in Table 1. More females (n = 278) than males (n = 168) were included in the study. Males had a greater median (IQR) age (69 [13] vs 48 [24] years; P < .001), lower CrCl (82.2 [37] vs 96.4 [41.2] mL/min; P < .001), and longer treatment duration (8.4 ± 2.4 vs 7.0 ± 1.4 days; P < .001) compared with females. A majority of the patients (89.9%) had a CrCl >60 mL/min (n = 401), followed by 9.9% of patients with a CrCl between 30 and 60 mL/min (n = 44) and 1 patient with a CrCl <30 mL/min. The patient with a CrCl <30 mL/min was excluded from all efficacy analyses. No patients were receiving dialysis. There were more males than females in the group that had a CrCl between 30 and 60 mL/min (16.7% vs 5.8%; P < .001).
Table 1.
Baseline Characteristics
| Baseline Characteristics | Total | Male | Female | P Value (Male vs Female) |
|---|---|---|---|---|
| (n = 446) | (n = 168) | (n = 278) | ||
| Age, median (IQR), y | 58 (26.5) | 69 (13.0) | 48 (24.0) | <.001* |
| CrCl, median (IQR), mL/min | 91.4 (40.5) | 82.2 (37.0) | 96.4 (41.2) | <.001* |
| Treatment duration, mean ± SD, d | 7.5 ± 1.9 | 8.4 ± 2.4 | 7.0 ± 1.4 | <.001* |
| 5 d, No. (%) | 59 (13.2) | 21 (12.4) | 38 (13.7) | |
| 7 d, No. (%) | 291 (65.3) | 75 (44.6) | 216 (77.7) | |
| 10 d, No. (%) | 78 (17.5) | 58 (34.5) | 20 (7.2) | |
| 12 d, No. (%) | 1 (0.22) | 1 (0.6) | 0 (0) | |
| 14 d, No. (%) | 17 (3.8) | 13 (7.7) | 4 (1.4) | |
| CrCl range, No. (%) | .001* | |||
| >60 mL/min | 401 (89.9) | 139 (82.7) | 262 (94.2) | |
| 30–60 mL/min | 44 (9.9) | 28 (16.7) | 16 (5.8) | |
| <30 mL/min | 1 (0.2) | 1 (0.6) | 0 (0) | |
| Urinary catheter use, No. (%) | 25 (5.6) | 24 (14.3) | 1 (0.4) | <.001* |
| Foley | 17 (3.8) | 16 (9.5) | 1 (0.4) | |
| Condom | 1 (0.2) | 1 (0.6) | 0 (0) | |
| Intermittent/straight | 6 (1.3) | 6 (3.6) | 0 (0) | |
| Suprapubic | 1 (0.2) | 1 (0.6) | 0 (0) | |
| Urinalysis, No. (%) | 393 (88.1) | 158 (94) | 235 (84.5) | |
| Positivea | 366 (93.1) | 151 (95.5) | 215 (91.5) | .189 |
| Negativea | 27 (6.9) | 7 (4.4) | 20 (8.5) | |
| Urine culture results, No. (%) | ||||
| Culture attained | 348 (78) | 134 (79.8) | 214 (77) | |
| Culture growthb | 289 (83) | 112 (83.5) | 177 (82.7) | .359 |
| Culture organism, No. (%)b | – | |||
| E. coli (total) | 170 (48.8) | 74 (55.2) | 96 (44.9) | |
| E. coli (ESBL-producing) | 16 (4.6) | 14 (10.4) | 2 (0.9) | |
| Streptococcus sp. | 16 (4.6) | 6 (4.5) | 10 (4.7) | |
| Staphylococcus sp. | 10 (2.9) | 4 (3.0) | 6 (2.8) | |
| Enterococcus sp. | 12 (3.4) | 9 (6.7) | 3 (1.4) | |
| Klebsiella sp. | 14 (4.0) | 5 (3.7) | 9 (4.2) | |
| Proteus sp. | 1 (0.3) | 0 (0) | 1 (0.5) | |
| Citrobacter sp. | 3 (0.9) | 0 (0) | 3 (1.4) | |
| Other sp.c | 58 (16.7) | 19 (14.2) | 39 (18.2) | |
| Comorbidities, No. (%) | ||||
| Spinal cord injury | 6 (1.3) | 4 (2.4) | 2 (0.7) | |
| BPH | 94 (21.1) | 94 (56.0) | – | |
| Structural urinary obstruction | 4 (0.9) | 3 (1.8) | 1 (0.4) | |
| Active cancer and/or cancer treatment | 19 (4.3) | 13 (7.7) | 6 (2.2) | |
| Receipt of agent known to cause immunosuppression | 17 (3.8) | 6 (3.6) | 11 (4.0) | |
| Autoimmune disorder | 15 (3.4) | 2 (1.2) | 13 (4.7) | |
| Active prostate cancer w/in 12 mo | 8 (1.8) | 8 (4.8) | – | |
| COPD or asthma | 39 (8.7) | 14 (8.3) | 25 (9.0) | |
| Diabetes | 116 (26.0) | 73 (43.5) | 43 (15.5) | |
| Liver disease (noncirrhotic) | 19 (4.3) | 4 (2.4) | 15 (5.4) | |
| Liver disease (cirrhotic) | 10 (2.2) | 6 (3.6) | 4 (1.4) | |
| Chronic heart failure | 16 (3.6) | 14 (8.3) | 2 (0.7) |
Abbreviations: BPH, benign prostatic hyperplasia; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; ESBL, extended-spectrum beta-lactamase; IQR, interquartile range.
*P < .05, statistically significant.
aNo. (%) calculated from total number of patients who had a urinalysis.
bNo. (%) calculated from total number of patients who had a urine culture.
cIncludes gram-positive organisms, lactose-negative/positive gram-negative rods, Candida sp., Lactobacillus sp., Aerococcus urinae, Gardnerella vaginallis.
Approximately 5.6% (n = 25) of the study population had a catheter, with a majority (68%, n = 17) utilizing a Foley catheter. Males also had higher rates of urinary catheter use compared with females (14.3% vs 0.4%; P < .001).
A urinalysis was obtained from 88.1% (n = 393) of the patients, of whom 93.1% (n = 366) had a positive UA. A urine culture was acquired from 78% (n = 348) of the total patients, of whom 83% (n = 289) grew at least 1 organism. The most common organisms documented were E. coli (48.8%), Streptococcus sp. (4.6%), Staphylococcus sp. (2.9%), and Enterococcus sp. (2.9%).
Clinical Cure
The results of the clinical cure analysis are shown in Table 2. The overall clinical cure rate was 86.5% (95% CI, 83.0%–89.4%; n = 386). The odds of clinical cure did not vary between males and females (OR, 0.60; 95% CI, 0.35–1.04; P = .085), nor did it vary between patients whose CrCl was >60 mL/min and those whose CrCl was 30–60 mL/min (OR, 1.01; 95% CI, 0.40–2.44; P = 1). The group with CrCl <30 mL/min was excluded from analysis as it consisted of only 1 patient.
Table 2.
Rate of Clinical Cure by Gender and by Renal Function
| Clinical Cure, No. Clinical Cure/Total (%) | ||||
|---|---|---|---|---|
| Male | Female | OR (95% CI) | P Value | |
| Total | 139/168 (82.7) | 247/278 (88.9) | 0.60 (0.35–1.04) | .085 |
| CrCl >60 mL/min | 113/139 (81.3) | 234/262 (89.3) | 0.52 (0.29–0.92) | .031* |
| CrCl 30–60 mL/min | 25/28 (89.3) | 13/16 (81.3) | 1.92 (0.34–11.11) | .652 |
| Clinical Cure, No. Clinical Cure/Total (%) | ||||
| CrCl >60 mL/min | CrCl 30–60 mL/min | OR (95% CI) | P Value | |
| Total | 347/401 (86.5) | 38/44 (86.4) | 1.01 (0.40–2.44) | 1 |
| Male | 113/139 (81.3) | 25/28 (89.3) | 0.52 (0.15–1.85) | .417 |
| Female | 234/262 (89.3) | 13/16 (81.3) | 1.93 (0.52–7.19) | .401 |
Abbreviations: CrCl, creatinine clearance; OR, odds ratio.
*P < .05, statistically significant.
Subgroup analyses were conducted for each comparison based on subdivisions of gender and CrCl range. Among those with a CrCl >60 mL/min, the odds of clinical cure was lower in males than in females (OR, 0.52; 95% CI, 0.29–0.92; P = .031) (Table 2). The odds of clinical cure did not vary based on CrCl for either gender (Table 2).
Table 3 details the results of the independent and multivariable logistic regression analysis investigating the association between various patient-specific characteristics and clinical cure. According to independent regressions, the odds of clinical cure did not vary based on age (P = .381), CrCl (P = .797), gender (P = .07), or having a CrCl of 30–60 mL/min compared with >60 mL/min (P = .975). Various comorbidities were assessed in this analysis, and history of benign prostatic hyperplasia (BPH; OR, 0.39; 95% CI, 0.22–0.70; P = .002) or cirrhosis (OR, 0.15; 95% CI, 0.04–0.53; P = .003) was associated with decreased odds of clinical cure. The use of a Foley catheter was also associated with decreased odds of clinical cure (OR, 0.2; 95% CI, 0.07–0.54; P = .002). History of BPH, cirrhosis, and Foley catheter use were all analyzed via multivariable logistical regression analysis, but only BPH (adjusted OR, 0.50; 95% CI, 0.26–0.99; P = .045) and cirrhosis (adjusted OR, 0.21; 95% CI, 0.06–0.82; P = .025) retained a significant association with decreased odds of clinical cure.
Table 3.
Independent and Multivariable Regression Analysis of Clinical Cure
| Bivariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Characteristic | OR of Clinical Cure (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
| Age, y | 1.01 (0.99–1.02)a | .381 | ||
| CrCl, mL/min | 1.00 (0.99–1.01)a | .797 | ||
| Treatment duration, d | 1.07 (0.94–1.22)a | .303 | ||
| Male | 0.60 (0.35–1.03) | .070 | ||
| CrCl range 30–60 mL/min | 1.01 (0.41–2.51) | .975 | ||
| Urinary catheter | ||||
| No catheter | 4.06 (1.70–9.69) | .002* | ||
| Foley catheter | 0.20 (0.07–0.54) | .002* | 0.41 (0.13–1.31) | .135 |
| Non-Foley catheter | 0.41 (0.08–2.10) | .289 | ||
| Complicated cystitis | 0.72 (0.42–1.25) | .242 | ||
| Obstructing renal calculi | 0.15 (0.01–2.50) | .188 | ||
| Spinal cord injury | 0.78 (0.09–6.83) | .826 | ||
| History of BPH | 0.39 (0.22–0.70) | .002* | 0.50 (0.26–0.99) | .045* |
| Structural urinary obstruction | 0.46 (0.05–4.54) | .510 | ||
| Active cancer and/or active cancer treatment | 0.57 (0.18–1.77) | .329 | ||
| Receipt of agent known to cause immunosuppressionb | 0.71 (0.20–2.57) | .610 | ||
| Autoimmune disorder | 0.61 (0.17–2.24) | .460 | ||
| Active prostate cancer within 12 mo | 0.46 (0.09–2.33) | .348 | ||
| COPD or asthma | 0.69 (0.29–1.63) | .395 | ||
| Diabetes | 0.72 (0.40–1.30) | .275 | ||
| Liver disease | ||||
| No liver disease | 1.75 (0.68–4.50) | .243 | ||
| Noncirrhotic | 2.68 (0.35–20.52) | .341 | ||
| Cirrhotic | 0.15 (0.04–0.53) | .003* | 0.21 (0.06–0.82) | .025* |
| Chronic heart failure | 0.67 (0.18–2.41) | .535 |
Abbreviations: BPH, benign prostatic hyperplasia; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance.
*P < .05, statistically significant.
aPer unit change.
bDuring the previous 6 months.
Safety Outcome
Adverse events occurred in 2% (n = 9) of patients and are presented in Table 4. Five patients experienced gastrointestinal distress, leading to nitrofurantoin discontinuation in 1 patient; 2 patients experienced minor rashes, both leading to discontinuation; and there were 2 cases of elevated liver enzymes with no discontinuation. The odds of having an adverse event did not vary based on gender or CrCl range.
Table 4.
Rate of Adverse Events by Gender and Renal Function
| Adverse Events, No. AE/Total (%) | OR (95% CI) | P Value | |
|---|---|---|---|
| Male | Female | ||
| 4/168 (2.4) | 5/278 (1.8) | 1.33 (0.35–5.00) | .734 |
| CrCl >60 mL/min | CrCl 30–60 mL/min | ||
| 7/401 (1.8) | 2/44 (4.6) | 0.37 (0.075–1.85) | .221 |
Abbreviations: AE, adverse events; CrCl, creatinine clearance; OR, odds ratio.
DISCUSSION
The emergence of antimicrobial resistance in uropathogens has increased consideration of nitrofurantoin in controversial populations, such as in males and in those with renal dysfunction. Previous studies have demonstrated mixed results regarding the efficacy of nitrofurantoin in renal insufficiency, and studies that did not demonstrate a difference in efficacy based on renal function were conducted in primarily females [10, 11]. This study compared the efficacy and safety of nitrofurantoin for the treatment of acute cystitis between males and females and between patients with and without renal insufficiency in a veteran population.
In our study population, we found that age, CrCl, and the rate of catheter use differed significantly between males and females. Males tended to be older, have lower renal function, use urinary catheters more frequently, and were prescribed longer courses of nitrofurantoin compared with females. Despite this, no significant differences in the rate of clinical cure were found between genders or between patients with or without renal insufficiency. To meet 80% power, a sample size of 326 patients was required, with an expected enrollment of 233 patients with a CrCl >60 mL/min and 93 patients with a CrCl ≤60 mL/min. However, enrollment of individuals with a CrCl ≤60 mL/min was lower than anticipated (n = 45). Therefore, our finding that efficacy did not vary based on renal function should be interpreted as hypothesis-generating due to the small sample size for the group with renal impairment. Also, a power calculation was not performed for the male and female comparison groups; therefore, we cannot comment on the probability of a type II error for this comparison.
The overall clinical cure rate (86.5%) from this study is comparable to previously reported cure rates with nitrofurantoin treatment for acute cystitis ranging from 79% to 92% [12]. Previous studies have evaluated the efficacy of nitrofurantoin in mixed study populations, but patients were predominantly female, making it difficult to ascertain efficacy in males [10–12]. To our knowledge, this is the first study that has compared clinical results between males and females in comparable gender proportions.
The subgroup analysis revealed that clinical cure in males was lower compared with females with a CrCl >60 mL/min. This may be explained by the fact that male urinary infections are often complicated by instrumentation, prostate enlargement, and renal or urinary stones. However, this subgroup analysis was considered an exploratory outcome, and this result should be interpreted with caution, as the primary comparison of overall clinical cure in males vs females did not demonstrate a significant difference.
In this study, a history of cirrhosis or BPH was associated with lower odds of clinical cure. BPH is associated with urinary retention, renal insufficiency, the development of gross hematuria, bladder calculi, urinary incontinence, and recurrent UTIs [13, 14]. Furthermore, it is known that BPH is one of the most common causes of urinary tract obstruction and stasis predisposing to UTIs, with UTIs being more common in patients with BPH who are unable to empty their bladder completely. A review article suggests that treatment of UTIs in the presence of a urinary tract obstruction requires not only requires effective antimicrobial therapy, but also appropriate urological intervention to remove the obstruction and repair the function of the urinary tract to prevent recurrent UTIs [15].
To our knowledge, this study is the first to identify a correlation between treatment failure with nitrofurantoin for acute cystitis in patients with BPH. In comparison, a previous retrospective study evaluating nitrofurantoin, pivmecillinam, or trimethoprim for treatment of cystitis in males found that the presence of BPH was not associated with a difference in rates of a new antibiotic prescription requirement or relapse of infection within 3 months following an initial treatment course. Efficacy outcomes were also similar between patients with a history of BPH receiving nitrofurantoin and pivmecillinam [16]. We did not identify any other publications reporting outcomes regarding the efficacy of nitrofurantoin vs other antibiotics in individuals with BPH.
It is known that cirrhosis is associated with immune dysfunction, increased risk of bacterial infections, and worse outcomes associated with infections. UTIs have been reported as the second most common bacterial infection in patients with cirrhosis, with a frequency about twice that of matched controls [17]. We did not identify any studies evaluating the efficacy of nitrofurantoin for the treatment of UTIs in patients with cirrhosis, nor is there a known physiological mechanism for decreased efficacy of nitrofurantoin in patients with liver disease. However, its use is contraindicated in patients with a history of nitrofurantoin-induced cholestatic jaundice or hepatic dysfunction [4, 18, 19].
Clinical cure was assessed at a follow-up of 14 days to decrease the likelihood that patients were experiencing a reinfection or recurrence of infection vs true failure of treatment. Unfortunately, we did not assess whether patients had a history of urinary retention, incomplete bladder emptying, or recurrent UTIs, which could have helped to further refine contributing factors to treatment failure.
Adverse events were infrequent (2%) and did not vary based on gender or CrCl, but were comparable to previous observational studies [2, 10, 12]. Our rates are lower than the 5%–16% cited across randomized controlled studies [12]. This is partially explained by the higher risk of reporting bias with retrospective studies vs randomized trials.
In addition to the limitations previously addressed, the retrospective nature of our study means that it is associated with a high risk of reporting bias due to lack of documentation of signs and symptoms of UTI, adverse events, return of UTI symptoms, alternative diagnoses for lower urinary symptoms, early discontinuation of therapy by patients, and lack of follow-up. Per chart review, we could assess whether a patient was prescribed nitrofurantoin, but we could not assess completion of treatment. Another major limitation is that 22% of patients did not have an associated urine culture; therefore, these patients were being treated empirically. As this study was conducted in an outpatient setting, providers may not always order a urine culture, or patients did not always provide urine samples for cultures as ordered. However, all patients included in our analysis without a urine culture had a positive UA with associated urinary symptoms. Additionally, organisms identified in cultures within our study aligned with previous results from a large epidemiological study of 40 618 community-acquired UTIs. Overall, culture results from Laupland and colleagues revealed similar distributions compared with our study of E. coli (74.2% vs 48.8%), Klebsiella spp. (7.1% vs 4%), Enterococcus spp. (5.3% vs 3.4%), Streptococcus spp. (3.7% vs 4.6%), and Staphylococcus spp. (2.1% vs 2.9%) [20]. Also, culture susceptibility testing at our facility did not always include testing for all relevant antibiotics. For example, susceptibility testing for nitrofurantoin is not routinely performed for Enterococcus sp., Staphylococcus sp., or Streptococcus sp. Thus, we cannot exclude the possibility of other resistant organisms contributing to treatment failure. The timing of acquisition of urine culture relative to the initiation of nitrofurantoin was not recorded. Therefore, we cannot exclude the possibility of patients receiving 1 or more doses of nitrofurantoin before acquisition of urine culture, which may explain the obtained urine cultures that had no growth (17%; n = 59). Additionally, the calculated CrCl utilized was dependent on the frequency of laboratory and vital assessment, and thus may not always reflect the true renal function of patients at the time of treatment.
Regarding the strengths of this study, we assessed patients with a wide age range and balanced gender proportions, which resulted in a more diverse population than assessed in previous similar studies. Also, the Department of Veterans Affairs is an integrated health care system that uses a common electronic health record across all facilities. Investigations in a VA population therefore offer the advantage of a presumably high rate of follow-up and thus relatively complete documentation within the available health record.
CONCLUSIONS
There was no statistically significant difference in clinical cure with nitrofurantoin between genders and various renal impairments. Subgroup analysis revealed lower efficacy in males vs females in patients with CrCl >60 mL/min. In addition, history of cirrhosis or BPH was associated with decreased efficacy. This study adds to the growing body of literature suggesting that renal dysfunction with a CrCl of 30–60 mL/min may not carry the risk of treatment failure and adverse effects previously associated with nitrofurantoin, but larger randomized trials are needed to confirm these results.
Acknowledgments
We would like to thank Kristen Staggers, MS, and Barbara W. Trautner, MD, PhD, for their contributions to the statistical analysis and manuscript review. Kristen Staggers is a biostatistician at the Baylor College of Medicine, and Dr. Trautner is an infectious diseases clinician-investigator at the Baylor College of Medicine and the Michael E. DeBakey Veterans Affairs Medical Center.
Potential conflicts of interest. All authors report no conflicts of interest related to this study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. E.W. conceived and designed the study, collected the data, performed the statistical analysis, and wrote the manuscript. S.S., C.A., and C.P. conceived and designed the study, helped revise the manuscript, and conducted study supervision. All authors contributed to the manuscript and its review.
Patient consent. The study was reviewed by the Institutional Review Boards (IRBs) at the Baylor College of Medicine and the Michael E. DeBakey Veterans Affairs Medical Center Research & Development Program and designated as “exempt.” The IRBs approved a waiver of HIPAA authorization to access protected health information.
References
- 1.Gupta K, Hooton TM, Naber KG, et al. ; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52:e103–20. [DOI] [PubMed] [Google Scholar]
- 2.Ingalsbe ML, Wojciechowski AL, Smith KA, Mergenhagen KA. Effectiveness and safety of nitrofurantoin in outpatient male veterans. Ther Adv Urol 2015; 7:186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez JM, Coyle EA. Urinary tract infections and prostatitis. In: DiPiro JT, Yee GC, Posey L, Haines ST, Nolin TD, Ellingrod V, eds. Pharmacotherapy: A Pathophysiologic Approach. 11th ed. McGraw-Hill, 2020. Available at: http://accesspharmacy.mhmedical.com.ezproxy.lib.utexas.edu/content.aspx?bookid=2577§ionid=219307238. Accessed 5 August 2019. [Google Scholar]
- 4.Macrobid (nitrofurantoin). Prescribing information. Almatica Pharma; 2018. [Google Scholar]
- 5.Sachs J, Geer T, Noell P, Kunin CM. Effect of renal function on urinary recovery of orally administered nitrofurantoin. N Engl J Med 1968; 278:1032–5. [DOI] [PubMed] [Google Scholar]
- 6.Schlegel JU, Goff JB, O’Dell RM. Bacteriuria and chronic renal disease. Trans Am Assoc Genitourin Surg 1967; 59:32–6. [PubMed] [Google Scholar]
- 7.Lippman RW, Wrobel CJ, Rees R, Hoyt R. A theory concerning recurrence of urinary infection: prolonged administration of nitrofurantoin for prevention. J Urol 1958; 80:77–81. [DOI] [PubMed] [Google Scholar]
- 8.Felts JH, Hayes DM, Gergen JA, Toole JF. Neural, hematologic and bacteriologic effects of nitrofurantoin in renal insufficiency. Am J Med 1971; 51:331–9. [DOI] [PubMed] [Google Scholar]
- 9.American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–46. [DOI] [PubMed] [Google Scholar]
- 10.Geerts AF, Eppenga WL, Heerdink R, et al. Ineffectiveness and adverse events of nitrofurantoin in women with urinary tract infection and renal impairment in primary care. Eur J Clin Pharmacol 2013; 69:1701–7. [DOI] [PubMed] [Google Scholar]
- 11.Bains A, Buna D, Hoag NA. A retrospective review assessing the efficacy and safety of nitrofurantoin in renal impairment. Can Pharm J 2009; 142:248–52. [Google Scholar]
- 12.Huttner A, Verhaegh EM, Harbarth S, et al. Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother 2015; 70:2456–64. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen SJ, Girman CJ, Lieber MM. Natural history of benign prostatic hyperplasia. Urology 2001; 58:5–16; discussion 16. [DOI] [PubMed] [Google Scholar]
- 14.McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185: 1793–803. [DOI] [PubMed] [Google Scholar]
- 15.Heyns CF. Urinary tract infection associated with conditions causing urinary tract obstruction and stasis, excluding urolithiasis and neuropathic bladder. World J Urol 2012; 30:77–83. [DOI] [PubMed] [Google Scholar]
- 16.Montelin H, Forsman KJ, Tängdén T. Retrospective evaluation of nitrofurantoin and pivmecillinam for the treatment of lower urinary tract infections in men. PLoS One 2019; 14:e0211098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol 2011; 9:727–38. [DOI] [PubMed] [Google Scholar]
- 18.Conklin JD. The pharmacokinetics of nitrofurantoin and its related bioavailability. Antibiot Chemother (1971) 1978; 25:233–52. [DOI] [PubMed] [Google Scholar]
- 19.Wijma RA, Huttner A, Koch BCP, et al. Review of the pharmacokinetic properties of nitrofurantoin and nitroxoline. J Antimicrob Chemother 2018; 73: 2916–26. [DOI] [PubMed] [Google Scholar]
- 20.Laupland KB, Ross T, Pitout JD, et al. Community-onset urinary tract infections: a population-based assessment. Infection 2007; 35:150–3. [DOI] [PubMed] [Google Scholar]