Abstract
Background
In the Long-Acting Antiretroviral Treatment Enabling Trial 2 (LATTE-2) phase 2b study, long-acting (LA) injectable cabotegravir + rilpivirine dosed every 8 weeks (Q8W) or every 4 weeks (Q4W) demonstrated comparable efficacy with daily oral antiretroviral therapy (ART) through 96 weeks in ART-naive adults with human immunodeficiency virus type 1 (HIV-1). Here we report efficacy, tolerability, and safety of cabotegravir + rilpivirine LA over approximately 5 years.
Methods
After 20 weeks of oral cabotegravir + abacavir/lamivudine, participants were randomized to cabotegravir + rilpivirine LA Q8W or Q4W or continue oral ART through the 96-week maintenance period. In the extension period through week 256, participants continued their current LA regimen (randomized Q8W/Q4W groups) or switched from oral ART to Q8W or Q4W LA therapy (extension-switch groups). Endpoints assessed included proportion of participants with HIV-1 RNA <50 copies/mL (Snapshot algorithm) and adverse events (AEs).
Results
At week 256, 186 of 230 (81%) participants in randomized Q8W/Q4W groups and 41 of 44 (93%) participants in extension-switch groups had HIV-1 RNA <50 copies/mL. No protocol-defined virologic failures occurred after week 48. Injection wsite reactions infrequently resulted in discontinuation (4 [2%] and 1 [2%] participants in randomized Q8W/Q4W and extension-switch groups, respectively). Three participants in randomized Q8W/Q4W groups experienced drug-related serious AEs, including 1 fatal serious AE (Q4W group); none occurred in extension-switch groups. Of 25 participants with AEs leading to withdrawal, 20 were in the randomized Q4W group; no AE leading to withdrawal occurred in >1 participant.
Conclusions
Cabotegravir + rilpivirine LA exhibited long-term efficacy and tolerability, demonstrating its durability as maintenance therapy for HIV-1 infection.
Clinical Trials Registration. NCT02120352.
Keywords: cabotegravir, integrase strand transfer inhibitor, long-acting, nonnucleoside reverse transcriptase inhibitor, rilpivirine
Although advances in antiretroviral therapy (ART) have made human immunodeficiency virus type 1 (HIV-1) infection a manageable condition, challenges with daily oral therapy, such as pill burden and stigma, may reduce treatment effectiveness and quality of life [1–4]. Long-acting (LA) injectable ART may address these challenges by eliminating daily oral dosing [5].
The first complete LA injectable regimen for HIV-1 treatment consists of cabotegravir, an integrase strand transfer inhibitor, and rilpivirine, a nonnucleoside reverse transcriptase inhibitor [6, 7]. The LATTE phase 2b study demonstrated that 72 weeks of oral cabotegravir + rilpivirine had similar efficacy to 3-drug oral ART and established oral lead-in doses for subsequent LA studies [8]. The LATTE-2 phase 2b study first evaluated efficacy and safety of an LA intramuscular injectable ART regimen in treatment-naive individuals with HIV-1 infection, demonstrating that cabotegravir + rilpivirine LA every 4 weeks (Q4W) or every 8 weeks (Q8W) had comparable efficacy to daily oral ART in maintaining HIV-1 suppression at 32, 48, and 96 weeks [9]. Cabotegravir + rilpivirine LA was generally well tolerated, and participants in LA groups reported high levels of treatment satisfaction [9, 10].
In the phase 3 studies Antiretroviral Therapy as Long Acting Suppression (ATLAS) and First Long-Acting Injectable Regimen (FLAIR), 48 weeks of cabotegravir + rilpivirine LA dosed intramuscularly Q4W was noninferior to daily oral ART for maintaining virologic suppression in adults with HIV-1 infection [11, 12]. Longer-term data in FLAIR demonstrated that cabotegravir + rilpivirine LA remained noninferior to daily oral ART at week 96 [13]. Based on these results, cabotegravir + rilpivirine LA dosed monthly has been approved in the United States, Canada, Europe, and Australia for maintenance of virologic suppression in adults [14–21]. Recently, cabotegravir + rilpivirine LA administered Q8W for 48 weeks demonstrated noninferior efficacy to Q4W dosing in the phase 3b ATLAS-2M study [22], resulting in simultaneous approval of every-2-months dosing in Europe and Australia [18–21].
Chronic use of LA injectables has not been evaluated for HIV-1 treatment, although its use has been well established for contraception and treatment of certain psychiatric disorders [23]. Patient acceptance and tolerance of receiving cabotegravir + rilpivirine LA for >2 years have not been evaluated [13]. Therefore, we report long-term efficacy, safety, and tolerability of cabotegravir + rilpivirine LA dosed Q4W and Q8W over approximately 5 years in LATTE-2.
METHODS
Study Design and Participants
LATTE-2 is a phase 2b, randomized, multicenter, parallel-group, open-label study using an induction-maintenance design conducted at 50 sites in Canada, France, Germany, Spain, and the United States (NCT02120352). Eligible participants were adults aged ≥18 years with HIV-1 RNA ≥1000 copies/mL, CD4+ count ≥200 cells/µL, and ≤10 days of previous ART. Key exclusion criteria were presence of any major resistance-associated mutation, moderate/severe hepatic impairment, clinically relevant hepatitis, chronic hepatitis B infection, history of liver cirrhosis, creatinine clearance <50 mL/minute, laboratory values or electrocardiographic findings of clinical concern, long-term anticoagulation, and presence of HLA-B*5701 plus inability to use an abacavir-containing nucleoside reverse transcriptase inhibitor backbone.
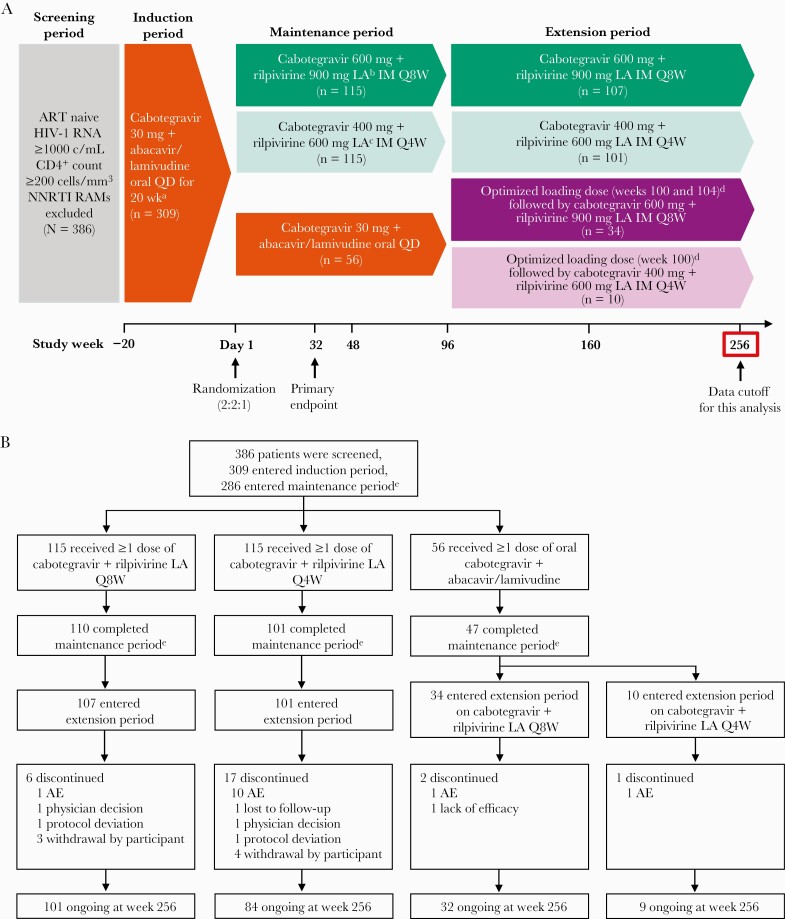
During the induction period, eligible participants received once-daily oral cabotegravir 30 mg + abacavir 600 mg/lamivudine 300 mg for 20 weeks, with once-daily oral rilpivirine 25 mg added for the last 4 weeks (Figure 1A). Participants achieving HIV-1 RNA <50 copies/mL at week –4 who tolerated the regimen were randomized to receive intramuscular injections Q8W (cabotegravir 600 mg + rilpivirine 900 mg LA) or Q4W (cabotegravir 400 mg + rilpivirine 600 mg LA) or continue once-daily oral cabotegravir + abacavir/lamivudine in the 96-week maintenance period. Injections were scheduled with a ±7-day dosing window from the projected dosing date relative to the first injection on day 1. After week 96, participants randomized to LA therapy continued their maintenance period regimen into the extension period. Participants randomized to oral therapy could switch to their choice of LA dosing groups, either Q8W (cabotegravir 600 mg + rilpivirine 900 mg LA) or Q4W (cabotegravir 400 mg + rilpivirine 600 mg LA), in the extension period, starting with an optimized loading dose of cabotegravir 600 mg + rilpivirine 900 mg LA at week 100 and continuing to receive LA therapy for 156 weeks through week 256. Daily oral cabotegravir 30 mg + rilpivirine 25 mg was available for short-term temporary oral therapy in exceptional circumstances to cover planned interruptions in injection dosing with medical monitor approval.
Figure 1.
Study design (A) and participant disposition through week 256 (B). aOral rilpivirine 25 mg once daily was added from week –4 to day 1. bParticipants received a loading dose of cabotegravir 800 mg (two 2-mL injections) + rilpivirine 900 mg (one 3-mL injection) LA on day 1 and a second loading dose of cabotegravir 600 mg (one 3-mL injection) LA at week 4 before starting every-8-week dosing at week 8. cParticipants received a loading dose of cabotegravir 800 mg (two 2-mL injections) + rilpivirine 600 mg (one 2-mL injection) LA on day 1 and started every-4-week dosing at week 4. dOptimized loading doses were cabotegravir 600 mg (one 3-mL injection) + rilpivirine 900 mg (one 3-mL injection) LA. eReasons for discontinuation have been previously reported [9]. Abbreviations: AE, adverse event; ART, antiretroviral therapy; c/mL, copies per milliliter; HIV-1, human immunodeficiency virus type 1; IM, intramuscular; LA, long acting; NNRTI, nonnucleoside reverse transcriptase inhibitor; Q4W, every 4 weeks; Q8W, every 8 weeks; QD, once daily; RAM, resistance-associated mutation.
Patient Consent Statement
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. The study protocol was approved by investigational center ethics committees or institutional review boards of each study site. All participants provided written informed consent.
Study Endpoints and Assessments
Primary endpoints were proportion of participants in the intention-to-treat, maintenance-exposed population with HIV-1 RNA <50 copies/mL at week 32 using the US Food and Drug Administration Snapshot algorithm, proportion of participants with protocol-defined virologic failure (PDVF), and incidence and severity of adverse events (AEs) and laboratory abnormalities. Secondary endpoints included proportion of participants with HIV-1 RNA <50 copies/mL and changes from baseline in CD4+ cell count over time. Adherence to the dosing window around injection visits was also assessed.
Plasma HIV-1 RNA was quantified using the Abbott Real-Time HIV-1 assay (Abbott Molecular, Des Plaines, Illinois). Protocol-defined virologic failure was characterized as having 2 consecutive HIV-1 RNA measurements ≥200 copies/mL. Safety and tolerability were assessed by monitoring AEs, including injection site reactions (ISRs), laboratory assessments, vital signs, and electrocardiograms.
The intention-to-treat, maintenance-exposed population consisted of participants initially randomized to and receiving ≥1 dose of LA therapy during the maintenance period. The extension-switch population included participants randomized to oral therapy during the maintenance period who switched to and received ≥1 dose of the optimized LA regimen in the extension period. Data are reported from the maintenance plus extension periods for the randomized Q8W/Q4W groups and from the extension period only for the extension-switch groups.
RESULTS
Study Population
The first participant was screened on 28 April 2014, and the last participant’s week 256 visit occurred on 5 December 2019. Baseline characteristics were balanced among participants in the randomized Q8W/Q4W and extension-switch groups (Table 1).
Table 1.
Baseline Demographics
| Characteristic | ITT, Maintenance-Exposed, Randomized Population | Extension-Switch Population | ||
|---|---|---|---|---|
| Q8W IM | Q4W IM | Q8W IM | Q4W IM | |
| (n = 115) | (n = 115) | (n = 34) | (n = 10) | |
| Age, y, median (range) | 34 (20–64) | 36 (19–62) | 36 (19–56) | 41 (21–56) |
| Reported gender, No. (%) | ||||
| Female | 8 (7) | 6 (5) | 6 (18) | 2 (20) |
| Male | 107 (93) | 109 (95) | 28 (82) | 8 (80) |
| BMI, kg/m2, median (range) | 24 (19–35) | 24 (17–37) | 24 (20–62) | 24 (19–30) |
| Race, No. (%) | ||||
| White | 93 (81) | 94 (82) | 24 (71) | 6 (60) |
| Black/African American | 17 (15) | 12 (10) | 10 (29) | 3 (30) |
| American Indian/Alaska Native | 0 | 8 (7) | 0 | 1 (10) |
| Other | 5 (4) | 1 (<1) | 0 | 0 |
| Baseline CD4+ cell count, cells/µL, median (IQR) | 449 (343–618)a | 499 (359–624)a | 988 (747–1093)b | 754 (644–962)b |
| Baseline HIV-1 RNA, log10 copies/mL, median (IQR) | 4.42 (4.05–4.80)a | 4.46 (4.00–4.97)a | 1.59 (1.59–1.59)b,c | 1.59 (1.59–1.59)b,c |
Abbreviations: BMI, body mass index; HIV-1, human immunodeficiency virus type 1; IM, intramuscular; IQR, interquartile range; ITT, intention to treat; Q4W, every 4 weeks; Q8W, every 8 weeks.
aBaseline is at week –20.
bExtension baseline is at week 100.
cAll participants had HIV-1 RNA <50 copies/mL at extension baseline.
Of 230 participants in the randomized Q8W/Q4W groups, 90% entered the extension period, and 80% were ongoing at week 256 (Figure 1B). In the extension period, 6 and 17 participants in the randomized Q8W and Q4W groups, respectively, withdrew; 11 participants (5%) withdrew because of AEs, 10 of whom were in the randomized Q4W group. Of 44 participants who switched to LA therapy in the extension period, 93% were ongoing at week 256. Three participants in the extension-switch groups withdrew, with 2 (5%; 1 from each group) withdrawing because of AEs.
Efficacy
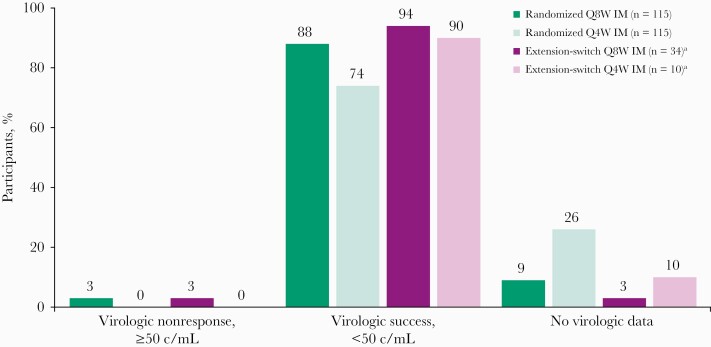
At week 256, 88% (n = 101) and 74% (n = 85) of participants in the randomized Q8W and Q4W groups, respectively, maintained HIV-1 RNA <50 copies/mL (Figure 2; Table 2). After 156 weeks of LA therapy in the extension period, 94% (n = 32) and 90% (n = 9) of participants in the extension-switch Q8W and Q4W groups, respectively, maintained virologic suppression. Five participants had HIV-1 RNA ≥50 copies/mL at week 256 (n = 4 in the randomized Q8W group, n = 1 in the extension-switch Q8W group). A higher proportion of participants had no virologic data in the assessment window at week 256 in the randomized Q4W group (26%) vs the randomized Q8W group (9%), driven by more discontinuations due to AEs (n = 15) or death (n = 3) and other nonvirologic reasons, including participant withdrawal (n = 6), protocol deviation (n = 3), loss to follow-up (n = 1), and physician decision (n = 1) in the randomized Q4W group. One participant from each extension-switch group had no virologic data at week 256, both of whom discontinued due to AEs before week 256.
Figure 2.
Virologic outcomes at week 256 by United States Food and Drug Administration Snapshot algorithm. aParticipants in extension-switch groups switched to cabotegravir + rilpivirine long-acting at week 100. Abbreviations: c/mL, copies per milliliter; IM, intramuscular; Q4W, every 4 weeks; Q8W, every 8 weeks.
Table 2.
Virologic Outcomes at Week 256 by United States Food and Drug Administration Snapshot Algorithm
| Outcome | ITT, Maintenance-Exposed, Randomized Population | Extension-Switch Population | ||
|---|---|---|---|---|
| Q8W IM | Q4W IM | Q8W IM | Q4W IM | |
| (n = 115) | (n = 115) | (n = 34) | (n = 10) | |
| HIV-1 RNA <50 copies/mL | 101 (88) | 85 (74) | 32 (94) | 9 (90) |
| HIV-1 RNA ≥50 copies/mL | 4 (3) | 0 | 1 (3) | 0 |
| Discontinued for lack of efficacy | 1 (<1) | 0 | 1 (3) | 0 |
| Discontinued for other reasons | 3 (3)a | 0 | … | … |
| No virologic data | 10 (9) | 30 (26) | 1 (3) | 1 (10) |
| Discontinued due to AE or deathb | 2 (2)c | 18 (16)d,e | 1 (3)f | 1 (10)g |
| Discontinued for other reasons | 8 (7) | 11 (10) | … | … |
| Missing data during window but on study | 0 | 1 (<1) | … | … |
Data are presented as No. (%).
Abbreviations: AE, adverse event; HIV-1, human immunodeficiency virus type 1; IM, intramuscular; ITT, intention to treat; Q4W, every 4 weeks; Q8W, every 8 weeks.
aIncludes consent withdrawal due to injection intolerability.
bParticipants could have ≥1 AE leading to withdrawal.
cChills, injection site pain, injection site pruritus, and pain (n = 1); hepatitis C virus infection (n = 1).
dThree deaths occurred due to epilepsy (unrelated to study treatment), toxicity to various agents (unrelated to study treatment), and myocardial infarction (drug related).
eAcute kidney injury, adjustment disorder with depressed mood, coronary artery disease, deep venous thrombosis, drug abuse, eosinophilic granulomatosis with polyangiitis, epilepsy, fatigue, hepatitis C virus infection, hypoesthesia, injection site nodule, injection site pain, lymphadenopathy, mesenteric vein thrombosis, metabolic acidosis, motor neuron disease, muscular weakness, myocardial infarction, portal vein thrombosis, prolonged QT interval, psychotic disorder, rash, respiratory tract infection, rhabdomyolysis, sinus tachycardia, splenic vein thrombosis, suicide attempt, and toxicity to various agents.
fBack pain, conjunctival hyperemia, erythema, and papular urticaria.
gInjection site pain.
No participant in any group met PDVF criteria after week 48. Three participants (Q8W group, n = 2; oral treatment group, n = 1) met PDVF criteria through week 48 (Supplementary Materials) [9].
At week 256, mean CD4+ cell counts increased from week –20 by 326 (standard deviation [SD], 218) cells/µL in the randomized Q8W group (n = 102) and 396 (SD, 294) cells/µL in the randomized Q4W group (n = 85). Mean CD4+ cell count from week 100 to week 256 increased by 211 (SD, 318) cells/µL in the extension-switch Q4W group (n = 9) and decreased by 14 (SD, 319) cells/µL in the extension-switch Q8W group (n = 32).
Adherence
In the randomized Q8W/Q4W groups, 96% of 9803 expected injection visits through week 256 occurred within the ±7-day dosing window (Supplementary Figure 1). Through week 256, 208 (90%) participants demonstrated ≥90% adherence to injection visits within the ±7-day dosing window; 125 (54%) were 100% adherent. Of 23 (<1%) missed injection visits, 20 were planned interruptions in injection dosing and covered with oral cabotegravir + rilpivirine therapy between injection visits; temporary oral therapy was used for 3 injection visits in the Q8W group (n = 2) and 17 in the Q4W group (n = 6). Two participants in the Q4W group had ≥2 injection visits covered by oral dosing because of travel or prolonged psychiatric hospitalization. All participants who used temporary oral dosing to manage planned interruptions in injection dosing maintained HIV-1 RNA <50 copies/mL upon resuming LA therapy and at week 256.
Safety
All participants in each group experienced ≥1 AE through week 256 (Table 3). Excluding ISRs, the most common AE was nasopharyngitis in the randomized Q8W/Q4W (45%) and extension-switch (25%) groups. Most participants in the randomized Q8W/Q4W (67%) and extension-switch (77%) groups reported AEs of maximum grade 1/2. The most common non-ISR, drug-related AEs in the randomized Q8W/Q4W groups were pyrexia (7%), back pain (3%), and fatigue (3%). No non-ISR, drug-related AE occurred in >1 participant in the extension-switch groups. Through week 256, 3 (3%) and 20 (17%) participants in the randomized Q8W and Q4W groups, respectively, had AEs leading to withdrawal, with 2 (2%) and 8 (7%) participants, respectively, reporting AEs leading to withdrawal that were treatment related. AEs leading to withdrawal reported in >1 participant in the randomized Q8W/Q4W groups were injection site pain (n = 2 in the Q8W group, n = 1 in the Q4W group) and hepatitis C virus infection (n = 1 in each group). Four non-drug-related thrombotic AEs leading to withdrawal were reported in 2 participants in the randomized Q4W group, 3 of which were abdominal and occurred in the same participant. Of 17 participants in the randomized Q4W group who withdrew because of AEs and had available follow-up viral load data, 16 maintained HIV-1 RNA <50 copies/mL at each follow-up assessment, 9 of whom switched to an oral ART regimen after discontinuing LA therapy. The remaining participant had an HIV-1 RNA measurement of 164 copies/mL 11 days after discontinuation and achieved HIV-1 RNA <50 copies/mL 1 month later; the posttreatment ART regimen of this participant was not available. One participant in each extension-switch group had drug-related AEs leading to withdrawal.
Table 3.
Summary of Adverse Events Through Week 256
| Preferred Term | ITT, Maintenance-Exposed, Randomized Population | Extension-Switch Population | ||
|---|---|---|---|---|
| Q8W IM | Q4W IM | Q8W IM | Q4W IM | |
| (n = 115) | (n = 115) | (n = 34) | (n = 10) | |
| Any AE | 115 (100) | 115 (100) | 34 (100) | 10 (100) |
| AE, excluding ISRa | ||||
| Nasopharyngitis | 50 (43) | 53 (46) | 6 (18) | 5 (50) |
| Diarrhea | 35 (30) | 30 (26) | 3 (9) | 2 (20) |
| Headache | 29 (25) | 26 (23) | 3 (9) | 0 |
| Influenza | 20 (17) | 26 (23) | 7 (21) | 3 (30) |
| Back pain | 20 (17) | 26 (23) | 5 (15) | 3 (30) |
| Syphilis | 32 (28) | 22 (19) | 2 (6) | 2 (20) |
| Upper respiratory tract infection | 28 (24) | 26 (23) | 5 (15) | 1 (10) |
| Gastroenteritis | 26 (23) | 22 (19) | 7 (21) | 0 |
| Grade ≥3 AE | 39 (34) | 38 (33) | 7 (21) | 3 (30) |
| Excluding ISR | 31 (27) | 35 (30) | 4 (12) | 2 (20) |
| Drug related, excluding ISR | 4 (3) | 7 (6) | 0 | 0 |
| SAE | 25 (22) | 27 (23) | 6 (18) | 1 (10) |
| Excluding ISR | 25 (22) | 27 (23) | 6 (18) | 1 (10) |
| Drug related | 1 (<1)b | 2 (2)c | 0 | 0 |
| Fatal SAE | 0 | 3 (3)d | 0 | 0 |
| AE leading to withdrawale | 3 (3) | 20 (17) | 1 (3) | 1 (10) |
| Excluding ISR | 2 (2)f | 18 (16)g | 1 (3)h | 0 |
| Drug related | 2 (2) | 8 (7) | 1 (3) | 1 (10)i |
Data are presented as No. (%).
Abbreviations: AE, adverse event; IM, intramuscular; ISR, injection site reaction; ITT, intention to treat; Q4W, every 4 weeks; Q8W, every 8 weeks; SAE, serious adverse event.
aAEs reported in >20% of participants in a treatment group.
bDelusion and depression.
cChest pain and abdominal pain, dyspnea, flushing, and myocardial infarction (n = 1 participant each).
dEpilepsy (unrelated to study treatment), toxicity to various agents (unrelated to study treatment), and myocardial infarction (drug related) in 1 participant each.
eParticipants could have >1 AE leading to withdrawal.
fChills (drug related), hepatitis C virus infection, and pain (drug related).
gAcute kidney injury, coronary artery disease, deep venous thrombosis, drug abuse, eosinophilic granulomatosis with polyangiitis, epilepsy, fatigue, hepatitis C virus infection, hypoesthesia, lymphadenopathy, mesenteric vein thrombosis, metabolic acidosis, motor neuron disease, muscular weakness, portal vein thrombosis, respiratory tract infection, rhabdomyolysis, splenic vein thrombosis, squamous cell carcinoma of the lung, suicide attempt, and toxicity to various agents (all not drug related). Abdominal pain, adjustment disorder with depressed mood, chest pain, dyspnea, flushing, myocardial infarction, prolonged QT interval, psychotic disorder, rash, and sinus tachycardia (all drug related).
hBack pain, conjunctival hyperemia, erythema, and papular urticaria (all drug related).
iInjection site pain.
ISRs occurred in 38% of 21 179 total injections (mean injections per participant, 47 [range, 3–71]) administered in the randomized Q8W/Q4W groups and 26% of 2319 total injections (mean injections per participant, 27 [range, 5–45]) administered in the extension-switch groups (Table 4). Injection site pain was the most common ISR and most frequently reported overall AE in each group. Incidence of ISRs reduced over time in each group, remaining relatively stable from weeks 96 to 256 in the randomized Q8W and Q4W groups (Supplementary Figure 2). Most ISRs were grade 1/2. Median ISR duration was 2–3 days for each group. Five participants had ISRs leading to study withdrawal: 2 (2%) participants in the randomized Q8W group at weeks 8 and 24, 2 (2%) in the randomized Q4W group at weeks 208 and 224, and 1 (10%) in the extension-switch Q4W group at week 160. No fatal or serious ISRs were reported.
Table 4.
Event-Level Summary of Injection Site Reactions Through Week 256
| Outcome | ITT, Maintenance-Exposed, Randomized Population | Extension-Switch Population | ||
|---|---|---|---|---|
| Q8W IM (n = 115) |
Q4W IM (n = 115) |
Q8W IM (n = 34) |
Q4W IM (n = 10) |
|
| Injection, No. | 7673 | 13 506 | 1503 | 816 |
| ISR event, No. | 3373 | 4702 | 429 | 182 |
| Grade, No. (% of ISR events) | ||||
| 1 | 2772 (82) | 4151 (88) | 358 (83) | 143 (79) |
| 2 | 571 (17) | 527 (11) | 64 (15) | 36 (20) |
| 3 | 24 (<1) | 22 (<1) | 7 (2) | 3 (2) |
| 4 | 0 | 0 | 0 | 0 |
| Most common ISR event, No. (% of ISR events) | ||||
| Pain | 2265 (67) | 2936 (62) | 368 (86) | 166 (91) |
| Nodule | 238 (7) | 557 (12) | 26 (6) | 13 (7) |
| Pruritis | 230 (7) | 222 (5) | 8 (2) | 0 |
| Swelling | 200 (6) | 248 (5) | 9 (2) | 2 (1) |
| Duration, d | ||||
| ≤7 | 2964 (88) | 3992 (85) | 399 (93) | 167 (92) |
| 8–14 | 263 (8) | 300 (6) | 12 (3) | 9 (5) |
| ≥15 | 137 (4) | 398 (8) | 17 (4) | 5 (3) |
| Median, d | 3.0 | 3.0 | 3.0 | 2.0 |
| Participants with ISR leading to withdrawal, No. (% of participants)a | 2 (2)b | 2 (2)c | 0 | 1 (10)d |
Abbreviations: IM, intramuscular; ISR, injection site reaction; ITT, intention to treat; Q4W, every 4 weeks; Q8W, every 8 weeks.
aParticipants could have >1 ISR event leading to withdrawal.
bInjection site pain (n = 2), injection site induration (n = 1), injection site pruritis (n = 1), and injection site swelling (n = 1).
cInjection site nodule (n = 1) and injection site pain (n = 1).
dInjection site pain.
Serious AEs (SAEs) occurred in 52 (23%) and 7 (16%) participants in the randomized Q8W/Q4W and extension-switch groups, respectively (Table 3). The most frequently reported SAEs in the randomized Q8W/Q4W groups were acute kidney injury and suicide attempt; each SAE was not treatment related and occurred in 2 participants in the Q8W group and 1 participant in the Q4W group. All SAEs in the extension-switch groups occurred in 1 participant each, except for pneumonia (n = 2 in the Q8W group). No new AEs of clinical concern were reported during the extension period, except for 1 participant in the randomized Q4W group. This individual experienced SAEs of abdominal pain, chest pain, dyspnea, and flushing immediately after rilpivirine injection and before cabotegravir injection at week 256; all events resolved on the same day of onset and were considered by the investigator to be related to rilpivirine, so cabotegravir and rilpivirine were discontinued. Three participants died in the randomized Q4W group. One death occurred at week 30 in the maintenance period due to epilepsy, which was not related to study treatment. A second death occurred at week 223 (between week 220 and 224 study visits) of the extension period due to coronary atherosclerosis secondary to drug toxicity (possibly due to cocaine use) and was not related to study treatment. A third death due to myocardial infarction at week 139 of the extension period was considered by the investigator to be potentially study drug related.
Grade 3/4 treatment-emergent laboratory abnormalities of increased creatine kinase and lipase levels were reported in 13% and 8% of participants in the randomized Q8W/Q4W groups, respectively. Grade 3/4 laboratory abnormalities of increased total cholesterol and low-density lipoprotein cholesterol were each reported in 2 and 1 participants in the extension-switch Q8W and Q4W groups, respectively. Other grade 3/4 laboratory abnormalities were reported in ≤5% of participants in the randomized Q8W/Q4W and extension-switch groups. No clinically significant changes from baseline in electrocardiograms or vital signs were observed.
DISCUSSION
Long-acting therapy is a new option in HIV-1 therapeutics. This is the first report describing >2-year efficacy, safety, tolerability, and durability of cabotegravir + rilpivirine LA. Over approximately 5 years, cabotegravir + rilpivirine LA Q8W and Q4W demonstrated antiviral efficacy in virologically suppressed participants, with none meeting PDVF criteria after week 48. Cabotegravir + rilpivirine LA was well tolerated for both dosing regimens after approximately 5 years of treatment, consistent with the safety profile observed during the maintenance period [9]. Adherence to injection visits was high through 5 years of treatment, with high treatment satisfaction reported with Q8W and Q4W dosing regimens at weeks 48 and 96 [9].
After approximately 5 years of treatment, 81% of participants randomized to LA therapy maintained virologic suppression. The lower rate of virologic suppression observed for the randomized Q4W (74%) vs Q8W (88%) group resulted from an increased proportion of participants with no virologic data at week 256 in the Q4W group, driven by discontinuations due to AEs or other nonvirologic reasons. Few discontinuations were related to ISRs. After switching to and receiving LA therapy for approximately 3 years, 93% of participants in the extension-switch groups maintained virologic suppression. No participants in any group met PDVF criteria after week 48. Through week 48, only 2 of 230 (<1%) participants who received LA therapy met PDVF criteria, both of whom were in the Q8W group [9]. The sustained virologic efficacy and rare occurrence of PDVFs over approximately 5 years in LATTE-2 demonstrate durability of cabotegravir + rilpivirine LA dosed Q4W and Q8W.
Additional support for the durability of cabotegravir + rilpivirine was provided by long-term results from LATTE [24]. After 5.5 years taking oral cabotegravir + rilpivirine, 8 (5%) participants met PDVF criteria, 5 of whom were initially taking the 10-mg cabotegravir dose. After completing LATTE, 90 participants entered the Oral (PO) to Long Acting (LA) Rollover (POLAR) study and received cabotegravir + rilpivirine LA every 2 months; no participants had HIV-1 RNA ≥50 copies/mL or met PDVF criteria after 1 year [25]. Three (1%) participants who received cabotegravir + rilpivirine LA through 96 weeks in FLAIR had confirmed virologic failure; none occurred after week 48 [11, 13]. Therefore, daily oral and monthly and every-2-months LA regimens of cabotegravir + rilpivirine demonstrate efficacy and durability as maintenance therapy for HIV-1 infection.
The overall safety profile at week 256 was generally consistent with data observed from LATTE-2 at week 96 and from the ATLAS, FLAIR, and ATLAS-2M phase 3 studies [9, 11, 12, 22]. Although ISRs were frequent, most were mild/moderate, of short duration, and decreased over time. Through approximately 5 years of LA therapy with 21 179 total injections, 4 (2%) participants from the randomized Q8W/Q4W groups withdrew due to ISRs, 2 of whom discontinued after ≤24 weeks. In approximately 3 years of LA therapy and 2319 total injections in the extension-switch groups, 1 (2%) participant withdrew due to an ISR after 60 weeks. The proportion of participants who withdrew due to AEs cumulatively through week 256 was higher in the randomized Q4W (17%) vs Q8W group (3%), a trend observed cumulatively through weeks 48 (6% vs <1%) and 96 (7% vs 2%) [9]. This trend did not occur in ATLAS-2M, with 2% of participants each in Q8W and Q4W groups withdrawing due to AEs through week 48 [22]. No specific trend of AEs leading to study withdrawal was observed in the randomized Q4W group, with most AEs leading to withdrawal considered unrelated to study treatment and each AE reported in 1 participant. Therefore, the increased rate of withdrawals due to AEs in the randomized Q4W group does not appear to be attributed to the dosing regimen. Overall, these results support the long-term safety of cabotegravir + rilpivirine LA with Q8W and Q4W dosing regimens.
Adherence to injection visits was high in LATTE-2, with 96% of injections occurring within the ±7-day dosing window. Most interruptions in scheduled injection dosing were planned, and temporary oral therapy with cabotegravir + rilpivirine was used to cover the period between injections. Each participant using oral dosing for planned treatment interruptions maintained virologic suppression when LA injections were resumed and through week 256, demonstrating the flexibility of this strategy. Strict patient adherence to scheduled injection visits may occasionally not be feasible in real-world situations (eg, holidays, prolonged travel, unforeseen life event, clinic closure); therefore, availability of oral dosing between injection visits provides a practical solution for managing planned LA treatment interruptions and maintaining treatment effectiveness.
This study had limitations. Sample sizes were small, particularly for the extension-switch groups. Participants were predominately male, and baseline CD4+ cell count was restricted to ≥200 cells/µL, potentially limiting generalizability. Results from the maintenance period of LATTE-2 informed the selection of optimized LA dosing regimens for the phase 3 clinical program; thus, the cabotegravir + rilpivirine LA loading doses administered to randomized Q8W/Q4W groups differed from optimized loading doses used for the extension-switch groups, in phase 3 clinical studies, and the approved regimen [11, 12, 14, 16, 18, 19, 22].
Long-term data from LATTE-2 show high rates of efficacy and acceptable safety for cabotegravir + rilpivirine LA in virologically suppressed adults with HIV-1 infection. These results demonstrate durability and tolerability of intramuscular cabotegravir + rilpivirine LA injections administered monthly or every 2 months as maintenance therapy and an alternative to daily oral ART.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Figure 1. Adherence to injection visits in participants from randomized Q8W and Q4W IM groups through week 256. Injection timelines were calculated by actual injection visit date minus the projected dosing visit date from day 1. Abbreviations: IM, intramuscular; Q4W, every 4 weeks; Q8W, every 8 weeks.
Supplementary Figure 2. Proportion of participants reporting ISRs over time in (A) randomized Q8W/Q4W groups through week 256 and (B) extension-switch groups from weeks 100 to 256. Data represent incidence of onset of ISR-related adverse events relative to the most recent IM injection visit. Abbreviations: IM, intramuscular; ISR, injection site reaction; Q4W, every 4 weeks; Q8W, every 8 weeks. aNumbers of participants receiving IM injection in randomized Q8W and Q4W groups are listed for Q8W visits starting at day 1. bNumbers of participants receiving IM injection are listed at week 100 and for Q8W visits starting at week 104.
Notes
Acknowledgments. Medical writing and editorial assistance were provided under the direction of the authors by Megan Schmidt, PhD, and Sherri Damlo, ELS, and funded by ViiV Healthcare.
Data sharing. Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Financial support. This work was supported by ViiV Healthcare and Janssen Research and Development.
Potential conflicts of interest. G. H. R. S. conducts clinical trials funded by Merck, Gilead Sciences, and GlaxoSmithKline/ViiV Healthcare. W. K. H. conducts clinical trials funded by Merck, Janssen Pharmaceutica, Gilead Sciences, and GlaxoSmithKline/ViiV Healthcare, with funds going to his institution. D. P. has received research grants and/or honoraria for advisory board and/or conference participation from ViiV Healthcare, Gilead Sciences, Janssen Pharmaceutica, and Merck Sharp & Dohme (MSD). M. D. M. M. has received personal fees and nonfinancial support from ViiV Healthcare, Janssen, and MSD. H. J. has received honoraria for presentations and scientific consulting from Gilead Sciences, MSD, Janssen-Cilag, ViiV Healthcare, and Theratechnologies. M.-A. K.-J. has received reimbursement costs related to the study protocol from Hôpital Delafontaine; meeting/travel fees from Janssen, MSD, and Overcome; and personal fees from MSD, Gilead Sciences, and ViiV Healthcare. M. L. M.-R. has received personal fees from ViiV Healthcare, Janssen, Gilead Sciences, and MSD. H.-J. S. has received personal fees from ViiV Healthcare, Gilead Sciences, Janssen-Cilag, MSD, and Theratechnologies and has received other fees from GlaxoSmithKline. Y. Y. has served as a board member for and received consultant fees from AbbVie, Bristol-Myers Squibb, Gilead Sciences, MSD, Johnson & Johnson, Pfizer, and ViiV Healthcare. G. J. R. has received grants from Gilead Sciences, ViiV Healthcare, TaiMed Biologics, and Insmed. K. C. S., C. C. M., M. H. S. C., K. Y. S., and W. R. S. are employees of ViiV Healthcare and may hold stock in GlaxoSmithKline. F. Z. is an employee of and may hold stock in GlaxoSmithKline. K. V. and R. V. S.-R. are employees of Janssen Pharmaceutica and may hold stock in Johnson & Johnson. D. A. M. was an employee of ViiV Healthcare at the time the study was conducted and may hold stock in GlaxoSmithKline. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCES
- 1.Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med 2012; 2:a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc 2013; 16:18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis 2006; 194:11–9. [DOI] [PubMed] [Google Scholar]
- 4.de Los Rios P, Okoli C, Castellanos E, et al. Physical, emotional, and psychosocial challenges associated with daily dosing of HIV medications and their impact on indicators of quality of life: findings from the Positive Perspectives Study. AIDS Behav 2021; 25:961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margolis DA, Boffito M. Long-acting antiviral agents for HIV treatment. Curr Opin HIV AIDS 2015; 10:246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trezza C, Ford SL, Spreen W, et al. Formulation and pharmacology of long-acting cabotegravir. Curr Opin HIV AIDS 2015; 10:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams PE, Crauwels HM, Basstanie ED. Formulation and pharmacology of long-acting rilpivirine. Curr Opin HIV AIDS 2015; 10:233–8. [DOI] [PubMed] [Google Scholar]
- 8.Margolis DA, Brinson CC, Smith GHR, et al. ; LAI116482 Study Team. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis 2015; 15:1145–55. [DOI] [PubMed] [Google Scholar]
- 9.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017; 390:1499–510. [DOI] [PubMed] [Google Scholar]
- 10.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Safety, efficacy and durability of long-acting cabotegravir and rilpivirine as two-drug IM maintenance therapy for HIV-1 infection: LATTE-2 week 160 results [poster]. In: HIV Drug Therapy Glasgow, Glasgow, UK; 2018. [Google Scholar]
- 11.Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020; 382:1124–35. [DOI] [PubMed] [Google Scholar]
- 12.Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382:1112–23. [DOI] [PubMed] [Google Scholar]
- 13.Orkin C, Oka S, Philibert P, et al. Long-acting cabotegravir plus rilpivirine for treatment in adults with HIV-1 infection: 96-week results of the randomised, open-label, phase 3 FLAIR study. Lancet HIV 2021; 8:e185–96. [DOI] [PubMed] [Google Scholar]
- 14.ViiV Healthcare. Cabenuva [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2021. [Google Scholar]
- 15.ViiV Healthcare. ViiV Healthcare announces FDA approval of Cabenuva (cabotegravir, rilpivirine), the first and only complete long-acting regimen for HIV treatment.https://viivhealthcare.com/en-us/us-news/us-articles/2021/viiv-healthcare-announces-fda-approval-of-cabenuva--cabotegravir/. Accessed 25 March 2021.
- 16.ViiV Healthcare. Cabenuva [package insert]. Laval, Quebec: ViiV Healthcare; 2020. [Google Scholar]
- 17.ViiV Healthcare. ViiV Healthcare announces first global regulatory approval of Cabenuva; the first complete, long-acting, regimen for the treatment of HIV.https://viivhealthcare.com/en-gb/media/press-releases/2020/march/viiv-healthcare-announces-first-global-regulatory-approval-of-ca/. Accessed 25 March 2021.
- 18.ViiV Healthcare BV. Vocabria [summary of product characteristics]. Amersfoort, Netherlands: ViiV Healthcare BV; 2020. [Google Scholar]
- 19.Janssen Pharmaceutica NV. Rekambys [summary of product characteristics]. Beerse, Belgium: Janssen Pharmaceutica NV; 2020. [Google Scholar]
- 20.ViiV Healthcare. ViiV Healthcare announces the marketing authorisation of the first complete long-acting injectable HIV treatment in Europe.https://viivhealthcare.com/en-gb/media/press-releases/2020/december/viiv-healthcare-announces-the-marketing-authorisation/. Accessed 25 March 2021.
- 21.ViiV Healthcare. Cabenuva [product information]. Abbotsford, Victoria, Australia: ViiV Healthcare; 2021. [Google Scholar]
- 22.Overton ET, Richmond G, Rizzardini G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet 2020; 396:1994–2005. [DOI] [PubMed] [Google Scholar]
- 23.Spreen WR, Margolis DA, Pottage JC Jr. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS 2013; 8:565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margolis DA, Sutton KC, De Vente J, et al. Long-term efficacy, safety, and durability of CAB and RPV as two-drug oral maintenance therapy—LATTE week 312 results [poster]. In: IDWeek, Washington, DC; 2019. [Google Scholar]
- 25.Mills A, Richmond GJ, Newman C, et al. Antiviral activity and safety of long-acting cabotegravir plus long-acting rilpivirine administered every 2 months in HIV-positive participants: results from the POLAR study [poster]. In: IDWeek (Virtual); 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.