Abstract
Background
To inform the on-going debate about the use of universal prescriptive versus national intrauterine growth charts, we compared perinatal mortality for small and large-for-gestational-age (SGA/LGA) infants according to international and national charts in Europe.
Methods
We classified singleton births from 33 to 42 weeks of gestation in 2010 and 2014 from 15 countries (N = 1,475,457) as SGA (birthweight <10th percentile) and LGA (>90th percentile) using the international Intergrowth-21st newborn standards and national charts based on the customised charts methodology. We computed sex-adjusted odds ratios (aOR) for stillbirth, neonatal and extended perinatal mortality by this classification using multilevel models.
Findings
SGA and LGA prevalence using national charts were near 10% in all countries, but varied according to international charts with a north to south gradient (3.0% to 10.1% and 24.9% to 8.0%, respectively). Compared with appropriate for gestational age (AGA) infants by both charts, risk of perinatal mortality was increased for SGA by both charts (aOR[95% confidence interval (CI)]=6.1 [5.6–6.7]) and infants reclassified by international charts from SGA to AGA (2.7 [2.3–3.1]), but decreased for those reclassified from AGA to LGA (0.6 [0.4–0.7]). Results were similar for stillbirth and neonatal death.
Interpretation
Using international instead of national charts in Europe could lead to growth restricted infants being reclassified as having normal growth, while infants with low risks of mortality could be reclassified as having excessive growth.
Funding
InfAct Joint Action, CHAFEA Grant n°801,553 and EU/EFPIA Innovative Medicines Initiative 2 Joint Undertaking ConcePTION grant n°821,520. AH received a PhD grant from EHESP.
Keywords: fetal growth, small for gestational age, large for gestational age, fetal growth charts
Research in context.
Evidence before this study
The Intergrowth-21st project published prescriptive international intrauterine and newborn growth charts in 2014, launching a vigorous debate about whether these charts should be used in clinical practice and research for the identification of small and large for gestational age infants (SGA and LGA) or whether local charts should be preferred. To review the papers evaluating these charts we searched PubMed for comparisons of the Intergrowth-21st charts with other local charts published from September 2014 to February 2020, combining the search terms “intergrowth” AND “fetal/intrauterine growth OR fetal/intrauterine growth restriction OR fetal/intrauterine growth retardation OR small for gestational age OR birthweight OR low birthweight OR large for gestational age OR macrosomia OR references OR standards OR growth charts OR growth curves OR biometric measures OR anthropometry”. Results from this literature review show that local or customised charts more accurately described the birthweight distribution and the mortality risks associated with low and high birthweight than the Intergrowth-21st charts in many settings. These studies have been single-country studies and international comparisons of the Intergrowth-21st charts are lacking.
Added value of this study
This study adds to the scientific literature by comparing the Intergrowth-21st newborn charts with national charts customised to each country's population in 15 European countries, making it possible to assess the consequences of using one universal chart versus country specific charts in an international context. The study uses routine population data on birthweight from 1.5 million births in European countries participating in the Euro-Peristat network. We find large differences in the prevalence of both SGA and LGA infants between international and national charts, with a strong north to south gradient when using international charts, demonstrating the major impact of the choice of chart on the comparative assessment of the burden of fetal growth anomalies by country and their relative rankings. Further, we show that births reclassified by the international chart from SGA to appropriate for gestational age (AGA) had over two-fold higher risks of perinatal mortality, whereas births reclassified from AGA to LGA had lower risk.
Implications of all the available evidence
Our results corroborate previous comparative single-country studies evaluating the Intergrowth-21st intrauterine growth charts. They provide further evidence in favor of using national or local growth charts for monitoring growth during pregnancy and at birth and suggest that physiological differences in population anthropometric characteristics should be taken into consideration when constructing growth charts. Moreover, our study sheds new light on the capacity of the Intergrowth-21st charts to identify SGA and LGA infants at risk of fetal and neonatal mortality in a European context; it illustrates limitations at both extremes of the birthweight spectrum in some settings which may create risks of underestimating SGA births and overestimating LGA births. All these elements do not provide support for the use of the Intergrowth-21st international chart for defining SGA and LGA at birth in Europe.
Alt-text: Unlabelled box
1. Introduction
Restricted and excessive growth are severe pregnancy complications associated with short and long-term adverse health outcomes. Fetal growth restriction, defined by insufficient growth in relation to the fetus’ genetic potential [1,2], is associated with risks of stillbirth and neonatal death, major neonatal morbidity, neuro-developmental and metabolic disorders [3], [4], [5]. Excessive growth, a complication of gestational diabetes, is also associated with fetal and neonatal death as well as hypoxic ischaemic encephalopathy, shoulder dystocia and childhood obesity [6], [7], [8], [9], [10]. While restricted and excessive growth are defined in relation to the fetus’ genetic potential, proxies based on weight are used in clinical practice and research. Small-for-gestational-age (SGA) is commonly defined as a birthweight under the 10th percentile and large-for-gestational-age (LGA) as a birthweight over the 90th percentile. While there is a broad consensus on these thresholds [1,10,11], there is an on-going debate about which growth charts should be used and, in particular, whether charts should be universal or specific to national populations.
In line with the World Health Organization charts for children project [12], the Intergrowth-21st project developed intrauterine growth charts based on the assumption that fetal growth is similar across diverse geographical settings as long as nutrition and access to health care are guaranteed and environmental constraints on growth are low [13], [14], [15]. Others claim that the physiological characteristics of each population are essential for defining risk and that national charts are more appropriate [16], [17], [18], [19]. Proponents of national charts point to studies showing the impact of geographic and ethnic origin on birthweight [17], [18], [19], while proponents of using a universal chart argue that population differences are minimal and that international norms are needed to assess deviation from normal growth [20]. This debate is of particular relevance in an international context for studies investigating differences between countries in the prevalence of SGA or LGA births or developing protocols and synthesising evidence across multiple settings.
The objective of this study is to compare the capacity of international neonatal charts, as proposed by the Intergrowth-21st project [13], and national charts customised to each country[21,22] to identify newborns at risk of perinatal mortality in 15 European countries. The European context is of interest given geographically proximate countries with similar standards of living, universal health insurance for pregnant women, but population differences in adult height and weight which may affect fetal size and corresponding thresholds for defining sub-optimal growth [23,24].
2. Methods
This study was undertaken by the Euro-Peristat network to underpin recommendations for selecting growth charts in the ConcePTION project, a European consortium on medications during pregnancy and breastfeeding. The Euro-Peristat network, constituted in 1999, aims to monitor and evaluate the health and care of pregnant women and babies in Europe based on national population data on perinatal health indicators [25,26].
2.1. Data source
The data source is a network study on intrauterine growth conducted in 2016–2017 which included 15 countries (Austria, Belgium, Cyprus, Estonia, Finland, France, Latvia, Lithuania, Luxemburg, Malta, Norway, Poland, Portugal, Scotland and Switzerland). Individual-level information was collected on five variables (birthweight, gestational age at birth, infant sex, vital status at birth (termination of pregnancy, stillbirth, livebirth), and neonatal death before 28 days of life) for all singleton births in the years 2010 and 2014. Data came from birth registers, civil registration systems and routine surveys (see Appendix A). Inclusion criteria, based on Euro-Peristat definitions, were a gestational age of at least 22 weeks of gestation or, if gestational age was missing, birthweight of at least 500 g. Gestational age was requested in complete weeks of gestation (e.g. a birth at 37 weeks and 6 days of gestation was recorded with a gestational age of 37 weeks). The definition of gestational age was the final estimate in the obstetrical records at birth.
Most countries provided data for their whole population for the given years, except for France where data come from a national survey including all births during a one-week period in all maternity hospitals in France. France and Poland provided information on stillbirths, but not on neonatal deaths since they weren't collected in the French Perinatal Survey and they couldn't be linked for the Polish data. France and Poland provided data for the year 2010 only, Portugal and Switzerland provided data for the years 2010 and 2013, and Cyprus provided data from 2007 to 2013 to allow for larger sample sizes in this small country.
2.2. Ethical approvals
This study uses a sub-set of Euro-Peristat's core variables, which include no indirect or direct personal identifiers. Data are provided to Euro-Peristat in accordance with each data provider's regulations for data use. The procedures for obtaining and maintaining the Euro-Peristat core indicator database were authorised by the French Advisory Committee on Use of Health Data in Medical Research (N°17–048, 30/03/17) and the French National Commission for Data Protection and Liberties (CNIL, DR.−2019–089, 26/03/19).
2.3. Study population
Among the 1496,321 singleton births in the 15 countries during the study period, we included live births and stillbirths from 33 to 42 weeks of gestation because the Intergrowth-21st newborn charts use these gestational age limits (N = 1477,840). We excluded terminations of pregnancy when it was possible to distinguish them from stillbirths in the dataset (N = 5); this was not possible in Belgium, Cyprus and Luxemburg (in 2010 only). Newborns with undetermined or unknown sex and with missing data on birthweight or gestational age were excluded (N = 2378). Missing data constituted less than 1% of all data, except for Luxembourg (1.8%). The final sample included 1475,457 births from 15 countries with data on stillbirths and 1062,154 births from 13 countries with data on neonatal deaths and stillbirths.
2.4. Outcomes
The study's principal outcomes were stillbirth, neonatal mortality and extended perinatal mortality (stillbirth or neonatal death). Countries have different lower gestational age limits for recording stillbirth [27], but this does not affect births at 33 weeks of GA and over which are registered in all countries. Neonatal death was defined as death before 28 days after a live birth. Rates were calculated per 1000 total births for stillbirth and extended perinatal mortality, and per 1000 live births for neonatal mortality.
2.5. Defining SGA and LGA births
2.5.1. International prescriptive charts
To define SGA and LGA by international charts, we used the Intergrowth-21st standards for newborn weight [13]. These charts are part of a suite of charts developed by the Intergrowth 21st project for monitoring intrauterine growth from a sample of rigorously selected low-risk pregnancies from 8 countries (Brazil, Italy, Oman, UK, USA, China, India, and Kenya) [13,14]. Selection criteria included medical and obstetrical history, socio-demographic and behavioural (nutrition, smoking) characteristics, health service accessibility and current pregnancy complications. The newborn weight chart distinguishes boys and girls and covers births from 33 weeks of gestation up to 42 weeks. Centiles were fitted using fractional polynomials assuming a skew t distribution with four parameters (mean, standard deviation, skewness and kurtosis). Its published values are expressed in exact weeks (specifying the number of weeks and days; for example, 28 exact weeks corresponds to 28+0 days as opposed to 28 completed weeks which covers 28+0 to 28+6 days) [28]. To adapt to our data in completed weeks, we used the midpoint weight for each week.
2.5.2. National descriptive charts based on the customised chart methodology
The national charts were modelled based on the customised chart methodology developed by Gardosi et al. [16]. Customised charts are widely used in the international literature on growth restriction and were adapted by Mikolajczyk et al. [21]. and others[22,29] for use at the country-level. The customised chart's principle is based on the calculation of an individual ideal birthweight at 40 weeks of gestation taking into consideration factors which physiologically affect growth (fetal sex, maternal height, pre-pregnancy weight, parity and ethnicity). To transpose this ideal birthweight to each week of gestation, Hadlock's growth trajectory (expressing estimated fetal weight by gestational age) is used to model individual intrauterine growth trajectories [30]. Assuming a normal distribution, the 10th and 90th percentiles are calculated as a proportion of this individual trajectory using a constant coefficient of variation (calculated as standard deviation over mean of birthweight at 40 weeks of gestation). For our study, in line with previous applications of this model on the country level [21,22], we used each country's mean birthweight and coefficient of variation at 40 weeks of gestation to create national charts for girls and boys separately.
Equations for the national charts 50th percentile (Eqn 1), 10th percentile (Eqn 2) and 90th percentile (Eqn 3) are:
| (1) |
| (2) |
| (3) |
Where: mC is the mean birthweight at 40 weeks of gestation of the country (for boys and girls separately), mH is the mean birthweight at 40 completed weeks of gestation as derived from Hadlock's study sample by Mikolajczyk et al.[21] (3705 g), sC is the standard deviation of birthweight at 40 weeks of gestation of the country (for boys and girls separately) and w is gestational age expressed in exact weeks. The country-specific coefficients for the models are provided in Supplementary Table 1.
2.6. Analysis strategy
First, we compared the prevalence of SGA and LGA infants in each country according to the international and national charts[13,21] and assessed geographic patterns with maps as there are known gradients of birthweight in Europe from north to south [31]. Second, we classified our sample by both charts as: (1) SGA according to both charts; (2) SGA according to the international chart only; (3) SGA according to the national chart only; (4) AGA according to both charts; (5) LGA according to the international chart only; (6) LGA according to the national chart only; and (7) LGA according to both charts. We compared stillbirth, neonatal mortality and extended perinatal mortality rates by this classification and then derived adjusted odds ratios (aOR) using a multi-level logistic regression to take into consideration the clustering of births within countries. We adjusted our model on sex, but not on gestational age since it is an intermediate factor on the pathway between growth restriction and perinatal death (see directed acyclic graph in supplemental Figure 1). However, we carried out sub-group analysis for term births (37 weeks of gestation and over). As our outcomes are rare, odds ratios approximate relative risks.
All analyses were performed using Stata 14.0 [32].
2.7. Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and AH and JZ had final responsibility for the decision to submit for publication.
3. Results
There was a wide range in the number of total births from 7984 in Malta to 398,764 in Poland (Table 1). The overall stillbirth rate was 1.8 per 1000 total births (95% confidence interval (CI): 1.8 to 1.9, 15 countries) with variation from 1.3 stillbirths per 1000 (CI: 1.1 to 1.4) in Portugal to 2.9 per 1000 (CI: 2.4 to 3.4) in Latvia. There were 0.8 neonatal deaths per 1000 live births (CI: 0.7 to 0.8, 13 countries) ranging from 0.2 per 1000 (CI: 0.1 to 0.7) in Luxembourg to 1.5 per 1000 (CI: 1.1 to 1.9) in Latvia. Extended perinatal deaths were 2.5 per 1000 total births (CI: 2.4 to 2.6, 13 countries), with a range from 1.6 (CI: 1.1 to 2.5) in Luxembourg to 4.8 (CI: 3.5 to 6.5) in Malta.
Table 1.
Total births in the study sample and stillbirth and neonatal mortality rates between 33 and 42 weeks of gestation by country.
| Country | Total births | Stillbirth rate |
Neonatal mortality rate |
Extended perinatal mortality rate |
|||
|---|---|---|---|---|---|---|---|
| N | ‰ total birth [CI 95%] | n | ‰ live births[CI 95%] | n | ‰ total births [CI 95%] | ||
| Austria | 153,410 | 258 | 1.7 | 92 | 0.6 | 350 | 2.3 |
| [1.5; 1.9] | [0.5; 0.7] | [2.1; 2.5] | |||||
| Belgium | 71,988 | 156 | 2.2 | 70 | 1.0 | 226 | 3.1 |
| [1.9; 2.5] | [0.8; 1.2] | [2.8; 3.6] | |||||
| Cyprus | 20,290 | 39 | 1.9 | 18 | 0.9 | 57 | 2.8 |
| [1.4; 2.6] | [0.6; 1.4] | [2.2; 3.6] | |||||
| Estonia | 28,284 | 57 | 2.0 | 24 | 0.9 | 81 | 2.9 |
| [1.6; 2.6] | [0.6; 1.3] | [2.3; 3.6] | |||||
| Finland | 114,610 | 161 | 1.4 | 82 | 0.7 | 243 | 2.1 |
| [1.2; 1.6] | [0.6; 0.9] | [1.9; 2.4] | |||||
| France | 14,539 | 25 | 1.7 | – | – | – | – |
| [1.2; 2.5] | |||||||
| Latvia | 39,166 | 112 | 2.9 | 57 | 1.5 | 169 | 4.3 |
| [2.4; 3.4] | [1.1; 1.9] | [3.7; 5.0] | |||||
| Lithuania | 57 024 | 138 | 2.4 | 84 | 1.5 | 222 | 3.9 |
| [2.0; 2.9] | [1.2; 1.8] | [3.4; 4.4] | |||||
| Luxembourg | 12,854 | 18 | 1.4 | 3 | 0.2 | 21 | 1.6 |
| [0.9; 2.2] | [0.1; 0.7] | [1.1; 2.5] | |||||
| Malta | 7984 | 19 | 2.4 | 19 | 2.4 | 38 | 4.8 |
| [1.5; 3.7] | [1.5; 3.7] | [3.5; 6.5] | |||||
| Norway | 116,603 | 239 | 2.1 | 81 | 0.7 | 320 | 2.7 |
| [1.8; 2.3] | [0.6; 0.9] | [2.5; 3.1] | |||||
| Poland | 398,764 | 826 | 2.1 | – | – | – | – |
| [1.9; 2.2] | |||||||
| Portugal | 177,013 | 225 | 1.3 | 84 | 0.5 | 309 | 1.7 |
| [1.1; 1.4] | [0.4; 0.6] | [1.6; 2.0] | |||||
| Scotland | 107,791 | 230 | 2.2 | 75 | 0.7 | 305 | 2.8 |
| [1.9; 2.4] | [0.6; 0.9] | [2.5; 3.2] | |||||
| Switzerland | 155,137 | 217 | 1.4 | 122 | 0.8 | 339 | 2.2 |
| [1.2; 1.6] | [0.7; 0.9] | [2.0; 2.4] | |||||
| Total | 1,475,457 | 2720 | 1.8 | 811 | 0.8 | 2680 | 2.5 |
| [1.8; 1.9] | [0.7; 0.8] | [2.4; 2.6] | |||||
NOTE: Combined data from the years 2010 and 2014, except Cyprus (2007–2013), Poland and France (2010 only) and Portugal and Switzerland (2010, 2013).
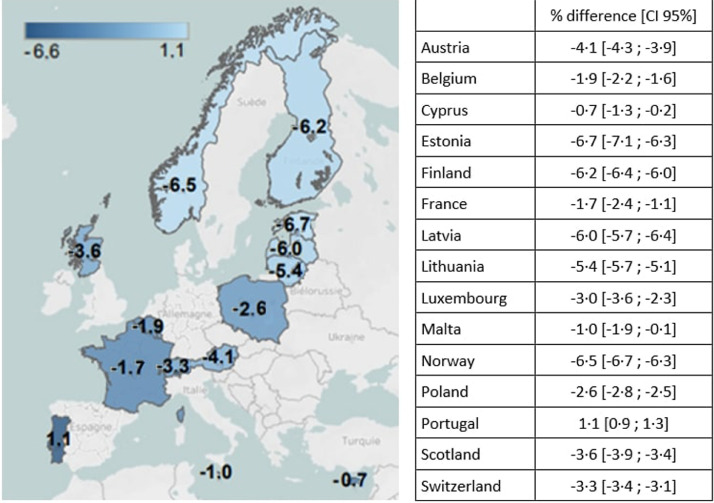
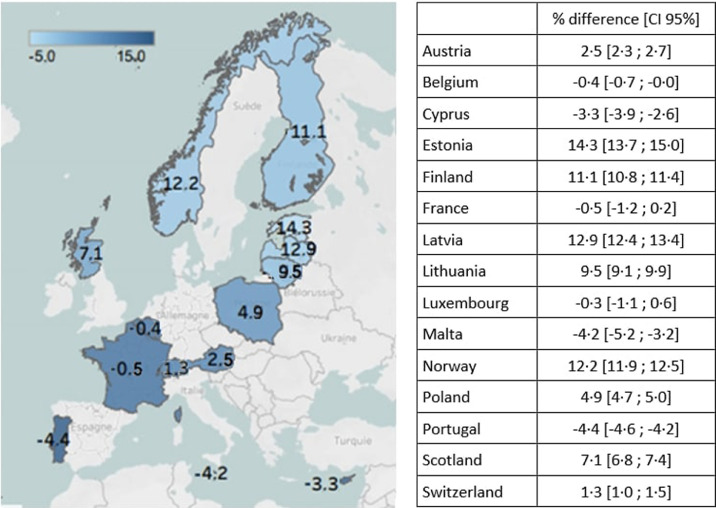
The proportions of SGA and LGA based on national charts were close to the 10% expected values, with a minimum of 8.5% in Cyprus to a maximum of 10.6% in France for SGA, and from 10.3% in Latvia to 14.8% in Malta for LGA (Table 2). However, these proportions varied markedly when using the international charts: from 3.0% in Estonia to 10.1% in Portugal for SGA and from 8.0% in Portugal to 24.9% in Estonia for LGA. Differences in prevalence between the international and national charts were up to −6.7% for SGA prevalence and to 14.3% for LGA prevalence in Estonia. These discrepancies were geographically patterned, with a lower prevalence of SGA in the north and a higher prevalence of SGA in the south, and higher prevalence of LGA in the north and lower prevalence of LGA in the south when using the international chart (Figure 1a and Figure 1b).
Table 2.
Prevalence of small and large for gestational age births in European countries according to international and national charts.
| Country | Total births (N) | International |
National |
||||
|---|---|---|---|---|---|---|---|
| SGA (%) | AGA (%) | LGA (%) | SGA (%) | AGA (%) | LGA (%) | ||
| Austria | 153,410 | 5.9 | 80.0 | 14.1 | 10.0 | 78.4 | 11.6 |
| Belgium | 71,988 | 8.2 | 80.1 | 11.7 | 10.1 | 77.8 | 12.1 |
| Cyprus | 20,290 | 7.7 | 81.5 | 10.8 | 8.5 | 77.4 | 14.1 |
| Estonia | 28,284 | 3.0 | 72.1 | 24.9 | 9.7 | 79.7 | 10.6 |
| Finland | 114,610 | 3.9 | 73.0 | 23.0 | 10.1 | 78 | 11.9 |
| France | 14,539 | 8.8 | 80.5 | 10.7 | 10.6 | 78.3 | 11.2 |
| Latvia | 39,166 | 4.0 | 72.8 | 23.2 | 10.1 | 79.6 | 10.3 |
| Lithuania | 57 024 | 4.4 | 75.2 | 20.5 | 9.7 | 79.3 | 11.1 |
| Luxembourg | 12,854 | 6.5 | 81.0 | 12.5 | 9.4 | 77.8 | 12.8 |
| Malta | 7984 | 8.6 | 80.8 | 10.6 | 9.6 | 75.6 | 14.8 |
| Norway | 116,603 | 3.9 | 72.8 | 23.3 | 10.4 | 78.5 | 11.1 |
| Poland | 398,764 | 6.7 | 77.0 | 16.3 | 9.3 | 79.3 | 11.4 |
| Portugal | 177,013 | 10.1 | 81.9 | 8.0 | 9.0 | 78.5 | 12.4 |
| Scotland | 107,791 | 6.5 | 75.1 | 18.4 | 10.1 | 78.6 | 11.3 |
| Switzerland | 155,137 | 6.4 | 80.4 | 13.2 | 9.6 | 78.5 | 11.9 |
| Total | 1475 457 | 6.4 | 77.5 | 16.1 | 9.7 | 78.7 | 10.7 |
Fig. 1a.
Difference in prevalence of SGA between international and national birthweight charts This map shows the geographic pattern of differences in SGA prevalence depending on the use of international compared to national birthweight charts, with the lightest blue color denoting countries where differences between the charts are most pronounced. Differences are largest in the north of Europe where international charts give lower SGA prevalence than the national charts.
Fig. 1b.
Difference in prevalence of LGA between international and national birthweight charts This map shows the geographic pattern of differences in LGA prevalence depending on use of international compared to national birthweight charts, with the lightest blue color denoting countries where the differences between the charts are most pronounced. Differences are largest in the north of Europe, where international charts give higher LGA prevalence than the national charts.
As shown in Table 3, 6.3% of infants in the overall sample were SGA according to both charts, 3.4% were SGA by national charts but AGA by the international charts, 73.2% were AGA by both charts, 5.4% were considered LGA by international charts but AGA by national charts and 10.7% were LGA according to both charts. Very few births were SGA according to international charts but AGA by national charts (0.2%) and AGA by international charts but LGA by national charts (0.9%); most of these births occurred in Portugal (99.4% and 56.2% respectively). Supplementary Table 2 provides these distributions by country. Infants considered AGA by both charts had mortality rates of 1.3, 0.6 and 1.9 per 1000 for stillbirth, neonatal death and perinatal death, respectively. These rates were highest for infants who were SGA according to both charts (8.5, 3.0 and 10.9 per 1000), followed by those SGA by national charts only (4.3, 1.4 and 5.6 per 1000). They were lowest for infants considered LGA by international charts only (0.7, 0.4 and 1.2 per 1000). These patterns were similar among term births.
Table 3.
Risk of stillbirth, neonatal and perinatal death by birthweight (BW) classification for all births ≥ 33 weeks and term births.
| Stillbirth | All births1 | Stillbirths | Adjusted model2 | All term births1 | Term stillbirths | Adjusted model2 |
|---|---|---|---|---|---|---|
| N (%) | n (rate per 1000) | aOR [95% CI] | N (%) | n (rate per 1000)) | aOR [95% CI] | |
| SGA both | 92 559 (6.3) | 791 (8.5) | 6.7 [6.1; 7.3] | 85 035 (6.0) | 434 (5.1) | 5.9 [5.3; 6.6] |
| SGA international only | 2 188 (0.2) | 2 (0.9) | 1.1 [0.3; 4.3] | 2 163 (0.2) | 2 (0.9) | 1.8 [0.4; 7.3] |
| SGA national only | 50 245 (3.4) | 218 (4.3) | 3.1 [2.7; 3.6] | 45 744 (3.3) | 101 (2.2) | 2.2 [1.8; 2.8] |
| AGA both | 1 079 324 (73.2) | 1424 (1.3) | Reference | 1 033 397 (73.4) | 927 (0.9) | Reference |
| LGA national only | 13 885 (0.9) | 31 (2.2) | 2.2 [1.5; 3.1] | 10 745 (0.8) | 5 (0.5) | 0.8 [0.3; 1.8] |
| LGA international only | 79 251 (5.4) | 58 (0.7) | 0.5 [0.4; 0.6] | 79 216 (5.6) | 58 (0.7) | 0.7 [0.5; 0.9] |
| LGA both | 158 005 (10.7) | 196 (1.2) | 0.9 [0.8; 1.1] | 151 267 (10.8) | 148 (1.0) | 1.1 [0.9; 1.3] |
| Female | 716 647 (48.6) | 1 321 (1.8) | Reference | 685 846 (48.7) | 819 (1.2) | Reference |
| Male | 758 810 (51.4) | 1 399 (1.8) | 1.0 [0.9; 1.1] | 721 721 (51.3) | 856 (1.1) | 1.0 [0.9; 1.1] |
| Variance at country level | 0.06 [0.03; 0.14] | 0.08 [0.03; 0.20] | ||||
| Neonatal death | Live births1,3 | Neonatal deaths | Adjusted model2 | Term live births1 | Term neonatal deaths | Adjusted model2 |
|---|---|---|---|---|---|---|
| N (%) | n (rate per 1000) | aOR [95% CI] | N (%) | n (rate per 1000) | aOR [95% CI] | |
| SGA both | 64 082 (6.0) | 194 (3.0) | 5.4 [4.6; 6.4] | 58 881 (5.8) | 131 (2.2) | 5.0 [4.1; 6.1] |
| SGA international only | 2 186 (0.2) | 1 (0.5) | 1.3 [0.2; 9.6] | 2 161 (0.2) | 1 (0.5) | 1.5 [0.2; 11.2] |
| SGA national only | 39 271 (3.7) | 56 (1.4) | 2.2 [1.7; 2.9] | 35 850 (3.6) | 32 (0.9) | 1.7 [1.2; 2.5] |
| AGA both | 771 619 (72.8) | 452 (0.6) | Reference | 738 348 (73.0) | 343 (0.5) | Reference |
| LGA national only | 12 596 (1.2) | 9 (0.7) | 1.6 [0.8; 3.0] | 10 211 (1.0) | 1 (0.1) | 0.3 [0.0; 1.9] |
| LGA international only | 58 485 (5.5) | 22 (0.4) | 0.5 [0.4; 0.8] | 58 450 (5.8) | 22 (0.4) | 0.7 [0.5; 1.1] |
| LGA both | 112 046 (10.6) | 77 (0.7) | 1.1 [0.9; 1.5] | 107 365 (10.6) | 52 (0.5) | 1.0 [0.8; 1.4] |
| Female | 516 953 (48.8) | 372 (0.7) | Reference | 494 524 (48.9) | 272 (0.6) | Reference |
| Male | 543 332 (51.2) | 439 (0.8) | 1.1 [1.0; 1.3] | 516 742 (51.1) | 310 (0.6) | 1.1 [0.9; 1.3] |
| Variance at country level | 0.19 [0.07; 0.49] | 0.16 [0.06; 0.42] |
| Perinatal death | All births1,3 | Perinatal deaths | Adjusted model2 | All term births1 | Term perinatal deaths | Adjusted model2 |
|---|---|---|---|---|---|---|
| N (%) | n (rate per 1000)) | aOR[95% CI] | N (%) | n (rate per 1000) | aOR [95% CI] | |
| SGA both | 64 590 (6.1) | 702 (10.9) | 6.1 [5.6; 6.7] | 59 161 (5.8) | 411 (6.9) | 5.5 [4.9; 6.2] |
| SGA international only | 2 188 (0.2) | 3 (1.4) | 1.1 [0.4; 3.6] | 2 163 (0.2) | 3 (1.4) | 1.7 [0.6; 5.4] |
| SGA national only | 39 436 (3.7) | 221 (5.6) | 2.7 [2.3; 3.1] | 35 932 (3.6) | 114 (3.2) | 2.1 [1.7; 2.6] |
| AGA both | 772 613 (72.7) | 1446 (1.9) | Reference | 738 987 (73.0) | 982 (1.3) | Reference |
| LGA national only | 12 618 (1.2) | 31 (2.5) | 1.7 [1.2; 2.4] | 10 215 (1.0) | 5 (0.5) | 0.5 [0.2; 1.2] |
| LGA international only | 12 618 (5.5) | 72 (1.2) | 0.6 [0.4; 0.7] | 58 500 (5.8) | 72 (1.2) | 0.8 [0.6; 1.0] |
| LGA both | 112 174 (10.6) | 205 (1.8) | 1.0 [0.8; 1.1] | 107 461 (10.6) | 148 (1.4) | 1.0 [0.8; 1.2] |
| Female | 517 853 (48.8) | 1 272 (2.5) | Reference | 495 080 (48.9) | 828 (1.7) | Reference |
| Male | 544 301 (51.2) | 1 408 (2.6) | 1.1 [1.0; 1.2] | 517 339 (51.1) | 907 (1.8) | 1.1 [1.0; 1.2] |
| Variance at country level | 0.09 [0.04; 0.22] | 0.09 [0.04; 0.21] |
NOTE: (1) ≥ 33 to ≤42 completed weeks of gestation (2) Model adjusted on fetal sex and with a supplementary level for the country (3) Births with data on perinatal death.
In mixed effects models adjusted for sex, infants classified as SGA by both charts faced highest mortality risks (aOR for perinatal death: 6.1 [5.6; 6.7]) compared to infants who were AGA according to both charts. Infants considered SGA by the national chart but AGA by the international chart also had increased risks of mortality (aOR for perinatal death: 2.7 [2.3; 3.1]). Being classified as LGA by the international chart only was associated with lower risks (aOR for perinatal death: 0.6 [0.4; 0.7]). Finally, being LGA according to both charts was not associated with an increased risk of mortality. Models for stillbirths and neonatal mortality yielded similar results, as did analyses restricted to term births.
4. Discussion
4.1. Main findings
Our results showed marked discordance between international and national charts for identifying SGA and LGA infants in European countries. Using national charts led to about 10% of infants being classified as SGA and LGA in all the countries, as expected. In contrast, applying international charts led to wide between-country variation from 3.0% to 10.1% for SGA and from 8.0% to 24.9% for LGA, following a geographic pattern of higher SGA prevalence in the south and higher LGA prevalence in the north. Compared to infants considered AGA by both charts, those reclassified from SGA to AGA using the international charts were at 2.7 (2.3 to 3.1) increased risk of perinatal death, whereas those reclassified from AGA to LGA using the international chart were at reduced risk 0.6 (0.4 to 0.7). Very few infants were reclassified from AGA to SGA using the international charts. Taken together, these results do not provide support for the use of international birthweight charts in Europe.
4.2. Interpretation
Intergrowth-21st international charts for intrauterine growth monitoring were published in 2014, but their application in daily practice is an on-going debate. Multiple single-country studies have compared Intergrowth-21st's newborn charts with national charts. Similar to our results, local birthweight charts[33], [34], [35] as well as Gardosi's customised model[36,37] have found that using Intergrowth-21st yielded a lower prevalence of SGA and a higher prevalence of LGA than national or customised charts. We add to this literature by showing that the differences in the prevalence of SGA and LGA when using international charts varied greatly between European countries and followed a geographic gradient from north to south. Our results support the position that population anthropometric characteristics should be considered in growth monitoring [8,[37], [38], [39]].
Our results also corroborate studies comparing mortality risks using international versus local or customised charts. Francis et al. found that being SGA by customised charts alone led to higher risks of stillbirth and adverse neonatal outcomes [36], and a Canadian study showed that detection rates for their composite mortality and morbidity outcome were higher among newborns considered SGA according to their local chart than among SGA according to Intergrowth-21st [33]. A Swedish study revealed that the risk of perinatal mortality was significantly increased up to the 35th percentile of the Intergrowth-21st chart but only up to the 15th percentile of their local chart [37]. We found that infants classified as SGA according to national charts, but considered AGA by the international chart, had an over two-fold increased risk of perinatal death when compared to those AGA by both charts. Since the national charts’ tenth percentile was higher than the international chart for all countries except Portugal, and mortality decreases linearly with weight percentile to an optimum which has been shown to be higher than the mean [8], an elevated risk in this group could be expected. However, the magnitude of the increased risk is of concern given the proportion and unequal geographic distribution of reclassified infants: 3.4% of the overall sample and over 6% in Estonia and Norway. Ideally, we would compare the performance of the charts in terms of sensitivity and specificity, however there is no consensual gold-standard as all current definitions of fetal growth restriction include at least one criterion based on a weight percentile defined in relation to a growth chart [2,[40], [41], [42]].
Infants reclassified as LGA according to the international chart had significantly lower risks of mortality than those AGA by both charts and represented about 10% or more of the births in the Nordic and Baltic countries. Infants considered LGA by both charts were not at higher risk for any of the outcomes compared to AGA infants according to both charts. This result differed from what was expected but may be explained by the fact that the association between excessive growth and mortality or morbidity has previously been investigated using absolute weights, over 4000 or 4500 gs, rather than percentiles[7,43]. Using a higher percentile cutoff, such as the 97th, may be more appropriate for capturing the mortality risks associated with LGA and should be explored in further studies. Our models also confirmed the well-documented increased risk of neonatal mortality among boys; risk of stillbirth did not differ which is in line with some recent studies showing no sex differences in overall stillbirth rates [44].
We derived national charts based on the customised model, which uses Hadlock's fetal growth model. This approach has been previously used to derive country-specific charts[16,21,22] and allowed for consistency across countries and provided proportions of SGA and LGA births in line with expectations. However, it differed from the methodology used in the Intergrowth project and from other birthweight charts. Differences in these charts occur primarily at preterm gestations because birthweight charts include preterm infants with abnormal growth and therefore preterm percentiles are generally lower [45,46]. Differences in the classification of preterm births do not explain our findings, however, as our results were similar when the sample was restricted to term births only. Results from other studies comparing Intergrowth with national curves have been similar for both types of national charts [33,47].
In our observational study of birthweight, we can only measure the differences between international and national newborn charts for identifying births facing higher risks of perinatal mortality. However, our study is in line with research on charts of ultrasound measures (in particular, abdominal circumference) or estimated fetal weight, showing a lower proportion of fetuses with growth parameters under the tenth percentile as well as lower sensitivity of the Intergrowth 21st charts for identifying growth restricted fetuses during pregnancy compared to local or customised charts [47], [48], [49]. The population used to build the Intergrowth 21st charts are the same for the fetal and the newborn charts, and therefore concerns about this reference population apply more broadly. Antenatal screening using charts that are not adapted to the population could lead to failure to identify SGA fetuses and insufficient monitoring of high risk pregnancies, while over-identification of LGA fetuses could increase iatrogenic interventions, parental stress and healthcare costs [50]. Accurate identification of fetuses and newborns at risk is vital to enable appropriate antenatal monitoring and interventions that prevent stillbirth and neonatal morbidity[51], [52], [53] and to guide management after birth.
4.3. Strengths and limitations
This study's strengths are its use of population data from a diverse sample of countries, enabling assessment of the consequences of using international charts on comparisons of sub-optimal growth in Europe. By cumulating data from many countries and over several years, we were able to attain a sample sufficient for investigating fetal and neonatal mortality which are rare events. Limitations are the absence of data on other environmental and maternal characteristics which influence growth. More research is warranted on the factors that influence birthweight in Europe, including the cultural and environmental context (diet or pollutants, for example), physiological characteristics (maternal and paternal height, genetic factors) and risk factors for sub-optimal growth (maternal smoking, maternal obesity and underweight, older maternal age, social disadvantage) to assess their relevance for antenatal and neonatal growth monitoring. Data were from 2010 to 2014, but birthweight as an indicator is stable over time [26,54], the current rate of change in perinatal mortality in Europe is low[26] and the question of whether universal charts should be applied is not time-bound. Because data come from diverse routine sources, we were not able to clearly assess methods for determining gestational age, although countries in Europe all provide early prenatal care, with widespread use of dating ultrasounds [55]. Finally, although we had large samples from a geographically diverse sample, we were not able to study mortality risks stratified at the country-level because the number of deaths was too small in some countries.
5. Conclusion
Our results do not provide support for the use of the Intergrowth 21st international charts for defining SGA and LGA at birth in Europe as this could lead to the underestimation of infants with SGA and overestimation of LGA in some countries. Their use for comparative surveillance and research is also problematic as differences in SGA and LGA prevalence between countries were influenced strongly by population anthropometric characteristics and cannot be interpreted as reflecting variations in perinatal health risks.
Funding statement
The Euro-Peristat network receives funding from the European Commission as part of the InfAct (Information for Action) Joint Action (Consumers, Health, Agriculture and Food Executive Agency (CHAFEA) Grant n° 801,553). This work was conducted as part of the ConcePTION project which has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 821,520. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation program and EFPIA. Alice HOCQUETTE was supported by a PhD grant from EHESP. While the research leading to these Results was conducted as part of the ConcePTION consortium, this paper only reflects the personal views of the stated authors.
Authors’ contribution
AH and JZ had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses.
Study concept and design: JZ, AH
Data acquisition and interpretation AH, MD, KS, RW, KK, SB, TR, TK, LS, AL, IZ, SA, JK, HB, MG, JI, BB, MG, JZ
Drafting of the manuscript: AH, JZ, KS, RW, KK, SB, TR
Critical revision of the manuscript for important intellectual content and approval of final version of the manuscript: AH, MD, KS, RW, KK, SB, TR, TK, LS, AL, IZ, SA, JK, HB, MG, JI, BB, MG, JZ
Data management and statistical analysis AH, JZ, MD
Data sharing statement
Individual participant data will not be available to others. Data on birthweight percentiles modeled for each participating country are available upon request from the authors.
Declaration of interest
No conflict of interest to disclose.
Acknowledgements
We would like to thank C. Barbez for assistance with the maps.
Footnotes
Supplementary material associated with this article can be found in the online version at doi: https://doi.org/10.1016/j.lanepe.2021.100167.
Appendix. Supplementary materials
References
- 1.Vayssière C., Sentilhes L., Ego A., Bernard C., Cambourieu D., Flamant C. Fetal growth restriction and intra-uterine growth restriction: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians. Eur J Obstet Gynecol Reprod Biol. 2015 Oct;193:10–18. doi: 10.1016/j.ejogrb.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 2.ACOG Practice Bulletin No. 204: fetal growth restriction. Obstet Gynecol. 2019;133(2):e97–109. doi: 10.1097/AOG.0000000000003070. [DOI] [PubMed] [Google Scholar]
- 3.Flenady V., Koopmans L., Middleton P., Frøen J.F., Smith G.C., Gibbons K. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011 Apr 16;377(9774):1331–1340. doi: 10.1016/S0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]
- 4.Gardosi J., Madurasinghe V., Williams M., Malik A., Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ. 2013 Jan 24;346:f108. doi: 10.1136/bmj.f108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baschat A.A., Viscardi R.M., Hussey-Gardner B., Hashmi N., Harman C. Infant neurodevelopment following fetal growth restriction: relationship with antepartum surveillance parameters. Ultrasound Obstet Gynecol. 2009 Jan;33(1):44–50. doi: 10.1002/uog.6286. [DOI] [PubMed] [Google Scholar]
- 6.Beta J., Khan N., Khalil A., Fiolna M., Ramadan G., Akolekar R. Maternal and neonatal complications of fetal macrosomia: systematic review and meta-analysis. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2019 Sep;54(3):308–318. doi: 10.1002/uog.20279. [DOI] [PubMed] [Google Scholar]
- 7.Wang D., Zhu L., Zhang S., Wu X., Wang X., Lv Q. Predictive macrosomia birthweight thresholds for adverse maternal and neonatal outcomes. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2016 Dec;29(23):3745–3750. doi: 10.3109/14767058.2016.1147549. [DOI] [PubMed] [Google Scholar]
- 8.Graafmans W.C., Richardus J.H., Borsboom G.J.J.M., Bakketeig L., Langhoff-Roos J., Bergsjø P. Birth weight and perinatal mortality: a comparison of “optimal” birth weight in seven Western European countries. Epidemiol Camb Mass. 2002 Sep;13(5):569–574. doi: 10.1097/00001648-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Sparano S., Ahrens W., De Henauw S., Marild S., Molnar D., Moreno L.A. Being macrosomic at birth is an independent predictor of overweight in children: results from the IDEFICS study. Matern Child Health J. 2013 Oct;17(8):1373–1381. doi: 10.1007/s10995-012-1136-2. [DOI] [PubMed] [Google Scholar]
- 10.Committee on Practice Bulletins—Obstetrics. Macrosomia: ACOG Practice Bulletin, Number 216. Obstet Gynecol. 2020;135(1):e18–e35. doi: 10.1097/AOG.0000000000003606. [DOI] [PubMed] [Google Scholar]
- 11.McCowan L.M., Figueras F., Anderson N.H. Evidence-based national guidelines for the management of suspected fetal growth restriction: comparison, consensus, and controversy. Am J Obstet Gynecol. 2018 Feb;218(2S):S855–S868. doi: 10.1016/j.ajog.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 12.de Onis M., Garza C., Onyango A.W., Rolland-Cachera M.-.F. le Comité de nutrition de la Société française de pédiatrie. [WHO growth standards for infants and young children] Arch Pediatr Organe Off Soc Francaise Pediatr. 2009 Jan;16(1):47–53. doi: 10.1016/j.arcped.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Villar J., Ismail L.C., Victora C.G., Ohuma E.O., Bertino E., Altman D.G. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. The Lancet. 2014 Sep 6;384(9946):857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 14.Stirnemann J., Villar J., Salomon L.J., Ohuma E., Ruyan P., Altman D.G. International estimated fetal weight standards of the INTERGROWTH-21st Project. Ultrasound Obstet Gynecol. 2017 Apr;49(4):478–486. doi: 10.1002/uog.17347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papageorghiou A.T., Ohuma E.O., Altman D.G., Todros T., Cheikh Ismail L., Lambert A. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet Lond Engl. 2014 Sep 6;384(9946):869–879. doi: 10.1016/S0140-6736(14)61490-2. [DOI] [PubMed] [Google Scholar]
- 16.Gardosi J., Mongelli M., Wilcox M., Chang A. An adjustable fetal weight standard. Ultrasound Obstet Gynecol. 1995 Sep;6(3):168–174. doi: 10.1046/j.1469-0705.1995.06030168.x. [DOI] [PubMed] [Google Scholar]
- 17.Gardosi J. Fetal growth and ethnic variation. Lancet Diabetes Endocrinol. 2014 Oct;2(10):773–774. doi: 10.1016/S2213-8587(14)70188-3. [DOI] [PubMed] [Google Scholar]
- 18.Albert P.S., Grantz K.L. Fetal growth and ethnic variation. Lancet Diabetes Endocrinol. 2014 Oct;2(10):773. doi: 10.1016/S2213-8587(14)70186-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander G.R., Kogan M.D., Himes J.H., Mor J.M., Goldenberg R. Racial differences in birthweight for gestational age and infant mortality in extremely-low-risk US populations. Paediatr Perinat Epidemiol. 1999 Apr;13(2):205–217. doi: 10.1046/j.1365-3016.1999.00174.x. [DOI] [PubMed] [Google Scholar]
- 20.Villar J., Papageorghiou A.T., Pang R., Ohuma E.O., Cheikh Ismail L., Barros F.C. The likeness of fetal growth and newborn size across non-isolated populations in the INTERGROWTH-21st Project: the Fetal Growth Longitudinal Study and Newborn Cross-Sectional Study. Lancet Diabetes Endocrinol. 2014 Oct;2(10):781–792. doi: 10.1016/S2213-8587(14)70121-4. [DOI] [PubMed] [Google Scholar]
- 21.Mikolajczyk R.T., Zhang J., Betran A.P., Souza J.P., Mori R., Gülmezoglu A.M. A global reference for fetal-weight and birthweight percentiles. Lancet Lond Engl. 2011 May 28;377(9780):1855–1861. doi: 10.1016/S0140-6736(11)60364-4. [DOI] [PubMed] [Google Scholar]
- 22.Zeitlin J., Bonamy A.-.K.E., Piedvache A., Cuttini M., Barros H., Van Reempts P. Variation in term birthweight across European countries affects the prevalence of small for gestational age among very preterm infants. Acta Paediatr Oslo Nor 1992. 2017 Sep;106(9):1447–1455. doi: 10.1111/apa.13899. [DOI] [PubMed] [Google Scholar]
- 23.Robinson M.R., Hemani G., Medina-Gomez C., Mezzavilla M., Esko T., Shakhbazov K. Population genetic differentiation of height and body mass index across Europe. Nat Genet. 2015 Nov;47(11):1357–1362. doi: 10.1038/ng.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NCD Risk Factor Collaboration (NCD-RisC) A century of trends in adult human height. Elife. 2016;26:5. doi: 10.7554/eLife.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeitlin J., Mortensen L., Cuttini M., Lack N., Nijhuis J., Haidinger G. Declines in stillbirth and neonatal mortality rates in Europe between 2004 and 2010: results from the Euro-Peristat project. J Epidemiol Community Health. 2016 Jun;70(6):609–615. doi: 10.1136/jech-2015-207013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeitlin J., Alexander S., Barros H., Blondel B., Delnord M., Durox M. Perinatal health monitoring through a European lens: eight lessons from the Euro-Peristat report on 2015 births. BJOG Int J Obstet Gynaecol. 2019;126(13):1518–1522. doi: 10.1111/1471-0528.15857. [DOI] [PubMed] [Google Scholar]
- 27.Smith L.K., Hindori-Mohangoo A.D., Delnord M., Durox M., Szamotulska K., Macfarlane A. Quantifying the burden of stillbirths before 28 weeks of completed gestational age in high-income countries: a population-based study of 19 European countries. Lancet Lond Engl. 2018;392(10158):1639–1646. doi: 10.1016/S0140-6736(18)31651-9. 03. [DOI] [PubMed] [Google Scholar]
- 28.Zeitlin J., Monier I. Clarification of INTERGROWTH-21st newborn birthweight standards. Lancet Lond Engl. 2018;391(10134):1995–1996. doi: 10.1016/S0140-6736(18)30292-7. 19. [DOI] [PubMed] [Google Scholar]
- 29.Ego A., Prunet C., Lebreton E., Blondel B., Kaminski M., Goffinet F. Courbes de croissance in utero ajustées et non ajustées adaptées à la population française. I — Méthodes de construction. J Gynecol Obstet Biol Reprod. 2015 Aug 25;45(2):165–176. doi: 10.1016/j.jgyn.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Hadlock F.P., Harrist R.B., Martinez-Poyer J. In utero analysis of fetal growth a sonographic weight standard. Radiology. 1991 Oct;181(1):129–133. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 31.Lack N., Blondel B., Mohangoo A.D., Sakkeus L., Cans C., Bouvier-Colle M.H. Reporting of perinatal health indicators for international comparisons–enhancing the appearance of geographical plots. Eur J Public Health. 2013 Dec;23(6):957–963. doi: 10.1093/eurpub/cks176. [DOI] [PubMed] [Google Scholar]
- 32.StataCorp . StataCorp LP; College Station, TX: 2015. Stata Statistical Software: Release 14. [Google Scholar]
- 33.Liu S., Metcalfe A., León J.A., Sauve R., Kramer M.S., Joseph K.S. Evaluation of the INTERGROWTH-21st project newborn standard for use in Canada. PLoS ONE. 2017;12(3) doi: 10.1371/journal.pone.0172910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poon L.C.Y., Tan M.Y., Yerlikaya G., Syngelaki A., Nicolaides K.H. Birth weight in live births and stillbirths. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2016 Nov;48(5):602–606. doi: 10.1002/uog.17287. [DOI] [PubMed] [Google Scholar]
- 35.Choi S.K.Y., Gordon A., Hilder L., Henry A., Hyett J.A., Brew B.K. Performance of six birthweight and estimated fetal weight standards for predicting adverse perinatal outcomes: a 10-year nationwide population-based study. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2020 Jul 16 [Google Scholar]
- 36.Francis A., Hugh O., Gardosi J. Customized vs INTERGROWTH-21st standards for the assessment of birthweight and stillbirth risk at term. Am J Obstet Gynecol. 2018;218(2S):S692–S699. doi: 10.1016/j.ajog.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Vieira M.C., Relph S., Persson M., Seed P.T., Pasupathy D. Determination of birth-weight centile thresholds associated with adverse perinatal outcomes using population, customised, and Intergrowth charts: a Swedish population-based cohort study. PLoS Med. 2019;16(9) doi: 10.1371/journal.pmed.1002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buck louis G.M., Grewal J., Albert P.S., Sciscione A., Wing D.A., Grobman W.A. Racial/Ethnic Standards for Fetal Growth, the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2015 Oct;213(4):449.e1–449.e41. doi: 10.1016/j.ajog.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiserud T., Piaggio G., Carroli G., Widmer M., Carvalho J., Neerup Jensen L. The World Health Organization fetal growth charts: a multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight. PLoS Med [Internet] 2017 Jan 24;14(1) doi: 10.1371/journal.pmed.1002220. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5261648/ [cited 2019 Dec 18]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordijn S.J., Beune I.M., Thilaganathan B., Papageorghiou A., Baschat A.A., Baker P.N. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2016 Sep;48(3):333–339. doi: 10.1002/uog.15884. [DOI] [PubMed] [Google Scholar]
- 41.Beune I.M., Bloomfield F.H., Ganzevoort W., Embleton N.D., Rozance P.J., van Wassenaer-Leemhuis A.G. Consensus BASED DEFINITION OF GROWTH RESTRICTION IN THE NEWBORN. J Pediatr. 2018 May;196:71–76. doi: 10.1016/j.jpeds.2017.12.059. e1. [DOI] [PubMed] [Google Scholar]
- 42.Collège National des Gynécologues et Obstétriciens Français Recommandations pour la pratique clinique - Le retard de croissance intra-utérin. J Gynecol Obstet Biol Reprod. 2013;42:1018–1025. doi: 10.1016/j.jgyn.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 43.Beta J., Khan N., Fiolna M., Khalil A., Ramadan G., Akolekar R. Maternal and neonatal complications of fetal macrosomia: cohort study. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2019 Sep;54(3):319–325. doi: 10.1002/uog.20278. [DOI] [PubMed] [Google Scholar]
- 44.Voskamp B.J., Peelen M.J.C.S., Ravelli A.C.J., van der Lee R., Mol B.W.J., Pajkrt E. Association between fetal sex, birthweight percentile and adverse pregnancy outcome. Acta Obstet Gynecol Scand. 2020;99(1):48–58. doi: 10.1111/aogs.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ego A., Prunet C., Blondel B., Kaminski M., Goffinet F., Zeitlin J. [Customized and non-customized French intrauterine growth curves. II - Comparison with existing curves and benefits of customization] J Gynecol Obstet Biol Reprod (Paris) 2016 Feb;45(2):165–176. doi: 10.1016/j.jgyn.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Zaw W., Gagnon R., da Silva O. The risks of adverse neonatal outcome among preterm small for gestational age infants according to neonatal versus fetal growth standards. Pediatrics. 2003 Jun;111(6 Pt 1):1273–1277. doi: 10.1542/peds.111.6.1273. [DOI] [PubMed] [Google Scholar]
- 47.Nwabuobi C., Odibo L., Camisasca-Lopina H., Leavitt K., Tuuli M., Odibo A.O. Comparing INTERGROWTH-21st Century and Hadlock growth standards to predict small for gestational age and short-term neonatal outcomes. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2020 Jun;33(11):1906–1912. doi: 10.1080/14767058.2018.1533945. [DOI] [PubMed] [Google Scholar]
- 48.Sovio U., Smith G.C.S. Comparison of estimated fetal weight percentiles near term for predicting extremes of birthweight percentile. Am J Obstet Gynecol. 2020 Aug 21 doi: 10.1016/j.ajog.2020.08.054. [DOI] [PubMed] [Google Scholar]
- 49.Hua X., Shen M., Reddy U.M., Louis G.B., Souza J.P., Gülmezoglu A.M. Comparison of the INTERGROWTH-21st, National Institute of Child Health and Human Development, and WHO fetal growth standards. Int J Gynecol Obstet. 2018;143(2):156–163. doi: 10.1002/ijgo.12637. [DOI] [PubMed] [Google Scholar]
- 50.Melamed N., Yogev Y., Meizner I., Mashiach R., Ben-Haroush A. Sonographic prediction of fetal macrosomia: the consequences of false diagnosis. J Ultrasound Med Off J Am Inst Ultrasound Med. 2010 Feb;29(2):225–230. doi: 10.7863/jum.2010.29.2.225. [DOI] [PubMed] [Google Scholar]
- 51.Boers K.E., Vijgen S.M.C., Bijlenga D., van der Post J a.M, Bekedam D.J., Kwee A. Induction versus expectant monitoring for intrauterine growth restriction at term: randomised equivalence trial (DIGITAT) BMJ. 2010 Dec 21;341:c7087. doi: 10.1136/bmj.c7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ganzevoort W., Mensing Van Charante N., Thilaganathan B., Prefumo F., Arabin B., Bilardo C.M. How to monitor pregnancies complicated by fetal growth restriction and delivery before 32 weeks: post-hoc analysis of TRUFFLE study. Ultrasound Obstet Gynecol. 2017 Jun;49(6):769–777. doi: 10.1002/uog.17433. [DOI] [PubMed] [Google Scholar]
- 53.Ego A., Monier I., Skaare K., Zeitlin J. Antenatal detection of fetal growth restriction and stillbirth risk: a population-based case-control study. Ultrasound Obstet Gynecol. 2019 Jul 31 doi: 10.1002/uog.20414. [DOI] [PubMed] [Google Scholar]
- 54.Schneider E.B. Fetal health stagnation: have health conditions in utero improved in the United States and Western and Northern Europe over the past 150 years? Soc Sci Med 1982. 2017 Apr;179:18–26. doi: 10.1016/j.socscimed.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 55.Zeitlin J., Mohangoo A.D., Delnord M., Cuttini M., EURO-PERISTAT Scientific Committee The second European Perinatal Health Report: documenting changes over 6 years in the health of mothers and babies in Europe. J Epidemiol Community Health. 2013 Dec 1;67(12):983–985. doi: 10.1136/jech-2013-203291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.