Abstract
Background
Little is known about variations in care and outcomes of patients undergoing surgical repair for type A aortic dissection(TAAD). We aim to investigate decade-long trends in TAAD surgical repair in England.
Methods
Retrospective review of the National Adult Cardiac Surgery Audit, which prospectively collects demographic and peri‑operative information for all major adult cardiac surgery procedures performed in the UK. We identified patients undergoing surgery for TAAD from January 2009-December 2018, reviewed trends in operative frequency, patient demographics, and mortality.
Findings
Over the 10-year period,3,680 TAAD patients underwent surgical repair in England. A doubling in the overall number of operations conducted in England was observed (235 cases in 2009 to 510 in 2018). Number of procedures per hospital per year also doubled(9 in 2009 to 23 in 2018). Overall, in-hospital mortality was 17.4% with a trend toward lower mortality in recent years(23% in 2009 to 14.7% in 2018). There was a significant variation in operative mortality between hospitals and surgeons. We also found that most patients presented towards the middle of the week and during winter.
Interpretation
Surgery is the only treatment for acute TAAD but is associated with high mortality. Prompt diagnosis and referral to a specialist center is paramount. The number of operations conducted in England has doubled in 10 years and the associated survival has improved. Variations exist in service provision with a trend towards better survival in high volume centers.
Funding
British Heart Foundation and NIHR Biomedical Research center(University Hospitals Bristol and Weston NHS Foundation Trust and University of Bristol).
Research in Context.
Evidence before this study
-
•
Acute Type A aortic dissections are a life-threatening condition for which timely surgical repair is the gold standard of treatment despite its inherent risks.
-
•
Detailed analysis of trends in England of mortality and morbidity following surgery have not previously been published
-
•
Outcome-volume relationship between individual surgeon's experience and mortality
Added value of this study
-
•
Significant outcome-volume relationship at center-level as well- decreased mortality, increased complexity of surgery and increased number of operations on very elderly patients
-
•
Lower incidence of cardiovascular compromise at the time of presentation.
-
•
Trend towards increased mortality risk in patients from areas of high deprivation (IMD<5).
-
•
Higher volume of operations over winter. Lower operative volume at weekends.
-
•
Increased mortality at weekends
Implications of all the available evidence
-
•
Establishment of a national network of experienced dedicated aortic surgeons and high-volume centers should be considered to provide a uniform high standard of care
-
•
Further research into referral times, transfer times and initial management of patients between peripheral hospitals and tertiary care providers
-
•
Increased research into provision and outcome of care in areas of high deprivation
Alt-text: Unlabelled box
1. Introduction
According to the Healthcare Safety Investigation Branch (HSIB) data [1], aortic dissection(AD) may occur in around 4.5 per 100,000 of the population per year, equal to around 2500 cases per year in England. AD involving primarily the ascending thoracic aorta (type A) is a life-threatening condition associated with significant risk of mortality. Around 50% of patients with type A AD(TAAD) die before reaching any hospital and 50% die before reaching a specialist center [1], [2], [3], [4], [5]. Prompt surgical repair remains the standard treatment for TAAD [6,7]. Improvement in diagnostic techniques, initial management and increased clinical awareness [8] over the last decade are expected to have increased the number of patients promptly diagnosed and referred to surgery. In addition, recent changes in the provision of services, with subspecialty aortic services having been developed within cardiothoracic centers across the UK, have also contributed to a more-equitable access to surgical treatment and improved outcomes [3,9]. Accurate trends and outcomes of patients who survive long enough to undergo surgical repair in England have yet to be reported. We aim to investigate decade-long trends in TAAD surgical repair in England using national-level data.
2. Methods
The register-based cohort study is part of a research approved by the Health Research Authority (HRA) and Health and Care Research Wales and a waiver for patients’ consent was waived (HCRW) (IRAS ID: 278,171).
2.1. Data extraction and cleaning
A complete extract of prospectively collected data from the National Adult Cardiac Surgery Audit (NACSA) was obtained from the National Institute of Cardiovascular Outcomes Research (NICOR) central cardiac database. Definitions of database variables used for the study are available at https://www.nicor.org.uk/national-cardiac-audit-program/adult-cardiac-surgery-surgery-audit/ . The National Institute for Cardiovascular Outcomes Research (NICOR) registry prospectively collects demographic, as well as peri -operative clinical information, including mortality, for all major adult cardiac surgery procedures performed in the UK. The flow of the data from surgeon-input to analysis has been described elsewhere [10]. Briefly, data entered locally by surgeons are validated at the unit-level by database managers prior to upload via a web-portal to NICOR. At this stage, further validation is performed according to logical rules and missing data reports are generated for primary variables (e.g., EuroSCORE risk factors, patient identifiers and outcome data). The data are then forwarded to an academic healthcare informatics department for data cleaning. The complete data cleaning process has been previously described [10]. Briefly, duplicate records are removed, transcriptional discrepancies re-coded and clinical and temporal conflicts resolved. The data cleaning is performed by the analyst responsible for the governance analysis in collaboration with surgeons and the audit manager. All cleaning is made reproducible by programming a series of scripts, which are updated following each new data extract. At this stage, and prior to analysis, data for the last 3 years are returned to each contributing hospital for local validation, and units update their records in the central registry repository where necessary. Most missing data are resolved during the validation stages of the data transfer from individual centers. Missing and conflicting data for in-hospital mortality status are backfilled and validated via record linkage to the Office for National Statistics (ONS) census database. The overall percentage of missing data for baseline information is very low (1.7%). Missing categorical or dichotomous variable data were imputed with the mode while missing continuous variables data imputed with the median.
For the present analysis, from the NACSA registry we identified patients undergoing surgery for TAAD from January 2009 to December 2018 in England. All procedures included in the present analysis were classified under the heading of urgent (non-elective admission with need for surgery during the same admission), emergency (operation before the next working day) or salvage (patients needing cardiopulmonary resuscitation on route to theater or before anesthetic induction). A set of variables related to clinical presentation and operative data were obtained. We also reported on socioeconomic status using terciles the Index of Multiple Deprivation (IMD), which is obtained by combining information from seven domains to give a summative relative measure of deprivation (0 corresponds to most deprived areas and 10 least deprived). For each patient, surgeon and hospital annual volume relative to the year of surgery was derived. Expected risk was assessed using a validated risk prediction model, the International Registry of Aortic Dissection (IRAD) score [11]. The primary outcome was in-hospital mortality. Other outcomes investigated were postoperative non-fatal cerebrovascular events, need for post-operative dialysis and re-exploration for bleeding.
2.2. Statistical analysis
Categorical variables were summarized as counts and percentages. Continuous variables were summarized as median and standard deviation (or median and interquartile range in case of non-normal distribution). Comparison of variables distribution across the years was performed by means of χ2 test, ANOVA and Kruskal Wallis test.
For the primary analysis, baseline characteristics operative data and outcomes were stratified by year of surgery to identify relative changes over a decade period. Outcomes were further stratified by age categories and socioeconomic neighborhood status. To investigate the effect of hospital and surgeon, outcomes were presented by terciles of annual hospital and surgeon volume and the variance due to individual hospital and surgeon was analyzed using risk-adjusted funnel plot. Finally, we analyzed possible chronological patterns (i.e., year of surgery weekday and month) in presentation and overall mortality.
Analysis of hospital and surgeon effect: to investigate whether variation in mortality can be attributed to individual hospital or surgeon performance, risk adjusted funnel plots were generated. The IRAD score was used as expected risk. Each hospital and surgeons were then displayed as a scatter point showing the individual risk adjusted mortality calculated as observed mortality rate/predicted mortality rate multiplied by the national average. Upper and lower control limits were calculated at 3 standard deviations (corresponding to 99.8% confidence intervals(CIs)) from the mean, using the exact binomial method described by Spiegelhalter [12]. An upper warning limit (calculated similarly to 95% CIs) was also calculated at 2 standard deviation from the mean.
Time series analysis: Generalized Additive Model (GAM) was implemented to analyze the effect of weekday, month and year on the number of dissection and observed mortality. Cyclic spline term was used for months and p-spline term was used for weekday and years. Number of knots used were 7,12,10 for weekdays, months and years. The effect of each term on outcomes was assessed the significance of smooth terms.
Statistical analysis was performed using R version 4.0.0.
Role of the funding source: The sponsor did not influence the study design nor analysis of the data. They funded the data acquisition and analysis and support the submission of this article for publication.
3. Results
3.1. Number of procedures per years
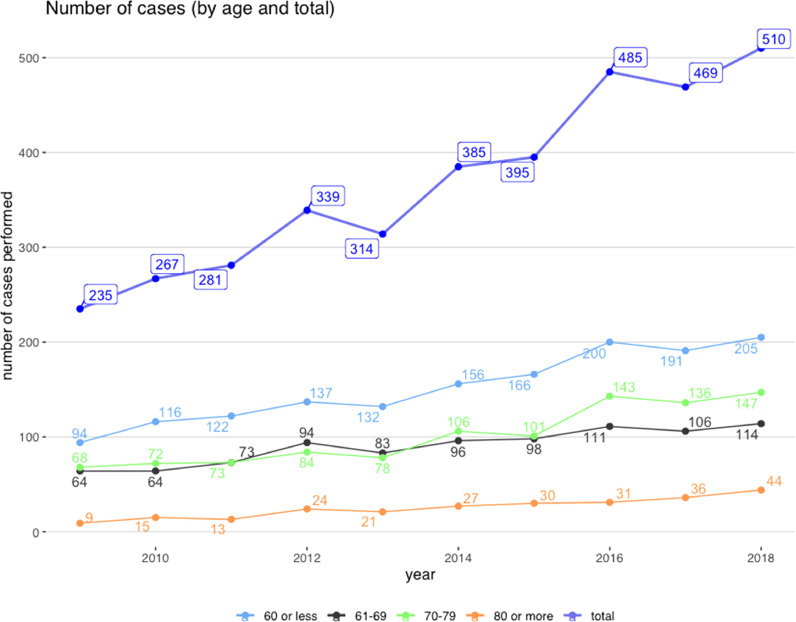
Overview of patients undergoing TAAD repair in England during the study period is reported in Table 1. Over the 10-year analysis period, 3680 cases of TAAD were operated on in England. There has been a steady doubling in the overall number of operations performed in that time, from 235 in 2009 to 510 in 2018 and this was observed across all age categories. (Fig. 1A). As a consequence, the mean number of procedures per surgeon and per center per year, increased from` from 9 cases in 2009 to 21 in 2018.
Table 1.
Annual variations.
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | p | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 235 | 267 | 281 | 339 | 314 | 385 | 395 | 485 | 469 | 510 | ||
| Age (mean (SD)) | 61.56 (13.80) | 61.00 (13.79) | 61.43 (13.35) | 61.86 (13.57) | 61.12 (13.74) | 61.65 (14.47) | 61.75 (14.19) | 62.45 (13.52) | 62.27 (13.97) | 62.73 (14.15) | 0.772 | |
| Age Category (%) | 0.852 | |||||||||||
| 60 or less | 94 (40.0) | 116 (43.4) | 122 (43.4) | 137 (40.4) | 132 (42.0) | 156 (40.5) | 166 (42.0) | 200 (41.2) | 191 (40.7) | 205 (40.2) | ||
| 61–69 | 64 (27.2) | 64 (24.0) | 73 (26.0) | 94 (27.7) | 83 (26.4) | 96 (24.9) | 98 (24.8) | 111 (22.9) | 106 (22.6) | 114 (22.4) | ||
| 70–79 | 68 (28.9) | 72 (27.0) | 73 (26.0) | 84 (24.8) | 78 (24.8) | 106 (27.5) | 101 (25.6) | 143 (29.5) | 136 (29.0) | 147 (28.8) | ||
| 80 or more | 9 (3.8) | 15 (5.6) | 13 (4.6) | 24 (7.1) | 21 (6.7) | 27 (7.0) | 30 (7.6) | 31 (6.4) | 36 (7.7) | 44 (8.6) | ||
| Female (%) | 68 (28.9) | 86 (32.2) | 89 (31.7) | 116 (34.2) | 109 (34.7) | 107 (27.8) | 134 (33.9) | 164 (33.8) | 174 (37.1) | 171 (33.5) | 0.255 | |
| Known Marfans (%) | 2 (0.9) | 2 (0.7) | 7 (2.5) | 15 (4.4) | 9 (2.9) | 17 (4.4) | 12 (3.0) | 12 (2.5) | 14 (3.0) | 13 (2.5) | 0.092 | |
| COPD (%) | 21 (8.9) | 26 (9.7) | 30 (10.7) | 35 (10.3) | 33 (10.5) | 51 (13.2) | 25 (6.3) | 30 (6.2) | 40 (8.5) | 14 (2.7) | <0.001 | |
| Smoking status (%) | 0.914 | |||||||||||
| Never smoked | 114 (48.5) | 133 (49.8) | 133 (47.3) | 179 (52.8) | 162 (51.6) | 208 (54.0) | 206 (52.2) | 266 (54.8) | 247 (52.7) | 250 (49.0) | ||
| Ex-smoker | 79 (33.6) | 95 (35.6) | 103 (36.7) | 104 (30.7) | 99 (31.5) | 118 (30.6) | 124 (31.4) | 147 (30.3) | 148 (31.6) | 177 (34.7) | ||
| Current smoker | 42 (17.9) | 39 (14.6) | 45 (16.0) | 56 (16.5) | 53 (16.9) | 59 (15.3) | 65 (16.5) | 72 (14.8) | 74 (15.8) | 83 (16.3) | ||
| Absent paedal pulses | 54 (23.0) | 63 (23.6) | 69 (24.6) | 57 (16.8) | 56 (17.8) | 74 (19.2) | 71 (18.0) | 86 (17.7) | 113 (24.1) | 88 (17.3) | 0.016 | |
| Hypertension (%) | 160 (68.1) | 186 (69.7) | 203 (72.2) | 224 (66.1) | 196 (62.4) | 252 (65.5) | 250 (63.3) | 334 (68.9) | 327 (69.7) | 351 (68.8) | 0.150 | |
| Diabetic Status (%) | 0.095 | |||||||||||
| Not Diabetic | 226 (96.2) | 257 (96.3) | 268 (95.4) | 327 (96.5) | 298 (94.9) | 375 (97.4) | 374 (94.7) | 459 (94.6) | 440 (93.8) | 469 (92.0) | ||
| Diet | 1 (0.4) | 3 (1.1) | 4 (1.4) | 1 (0.3) | 2 (0.6) | 2 (0.5) | 2 (0.5) | 9 (1.9) | 7 (1.5) | 10 (2.0) | ||
| Oral therapy | 8 (3.4) | 6 (2.2) | 7 (2.5) | 10 (2.9) | 11 (3.5) | 6 (1.6) | 13 (3.3) | 17 (3.5) | 19 (4.1) | 24 (4.7) | ||
| Insulin | 0 (0.0) | 1 (0.4) | 2 (0.7) | 1 (0.3) | 3 (1.0) | 2 (0.5) | 6 (1.5) | 0 (0.0) | 3 (0.6) | 7 (1.4) | ||
| Pre-op CVA (%) | 17 (7.2) | 22 (8.2) | 15 (5.3) | 13 (3.8) | 20 (6.4) | 19 (4.9) | 20 (5.1) | 19 (3.9) | 35 (7.5) | 30 (5.9) | 0.159 | |
| LVEF (%) | 0.496 | |||||||||||
| good(>=50%) | 174 (74.0) | 212 (79.4) | 222 (79.0) | 269 (79.4) | 248 (79.0) | 310 (80.5) | 327 (82.8) | 385 (79.4) | 368 (78.5) | 416 (81.6) | ||
| moderate(30–49%) | 54 (23.0) | 45 (16.9) | 49 (17.4) | 64 (18.9) | 51 (16.2) | 61 (15.8) | 57 (14.4) | 88 (18.1) | 87 (18.6) | 80 (15.7) | ||
| poor(<30%) | 7 (3.0) | 10 (3.7) | 10 (3.6) | 6 (1.8) | 15 (4.8) | 14 (3.6) | 11 (2.8) | 12 (2.5) | 14 (3.0) | 14 (2.7) | ||
| Baseline Creatinine>200 mmol/L (%) | 11 (4.7) | 13 (4.9) | 22 (7.8) | 13 (3.8) | 13 (4.1) | 19 (4.9) | 10 (2.5) | 14 (2.9) | 16 (3.4) | 18 (3.5) | 0.053 | |
| Previous cardiac surgery (%) | 22 (9.4) | 16 (6.0) | 24 (8.5) | 21 (6.2) | 18 (5.7) | 34 (8.8) | 18 (4.6) | 24 (4.9) | 28 (6.0) | 27 (5.3) | 0.091 | |
| Acute Kidney Injury (%) | 2 (0.9) | 5 (1.9) | 9 (3.2) | 13 (3.8) | 10 (3.2) | 14 (3.6) | 12 (3.0) | 11 (2.3) | 16 (3.4) | 15 (2.9) | 0.574 | |
| Preop Ventilation (%) | 14 (6.0) | 16 (6.0) | 10 (3.6) | 16 (4.7) | 19 (6.1) | 16 (4.2) | 17 (4.3) | 18 (3.7) | 28 (6.0) | 21 (4.1) | 0.602 | |
| VF/VT (%) | 1 (0.4) | 1 (0.4) | 3 (1.1) | 2 (0.6) | 2 (0.6) | 1 (0.3) | 1 (0.3) | 1 (0.2) | 0 (0.0) | 4 (0.8) | 0.559 | |
| Ongoing Chest Pain(%) | 36 (15.3) | 34 (12.7) | 33 (11.7) | 38 (11.2) | 33 (10.5) | 44 (11.4) | 38 (9.6) | 39 (8.0) | 33 (7.0) | 14 (2.7) | <0.001 | |
| Ischaemic ECG Changes (%) | 8 (3.4) | 8 (3.0) | 9 (3.2) | 7 (2.1) | 17 (5.4) | 9 (2.3) | 11 (2.8) | 15 (3.1) | 11 (2.3) | 13 (2.5) | 0.408 | |
| Cardiogenic Shock (%) | 39 (16.6) | 41 (15.4) | 43 (15.3) | 49 (14.5) | 59 (18.8) | 54 (14.0) | 58 (14.7) | 73 (15.1) | 74 (15.8) | 65 (12.7) | 0.673 | |
| TIA = 1 (%) | 3 (1.3) | 11 (4.1) | 16 (5.7) | 16 (4.7) | 10 (3.2) | 15 (3.9) | 12 (3.0) | 20 (4.1) | 29 (6.2) | 16 (3.1) | 0.082 | |
| Bicuspid Aortic Valve (%) | 6 (2.6) | 9 (3.4) | 5 (1.8) | 6 (1.8) | 7 (2.2) | 5 (1.3) | 11 (2.8) | 18 (3.7) | 10 (2.1) | 10 (2.0) | 0.471 | |
| Aortic Insufficiency (%) | 73 (31.1) | 79 (29.6) | 82 (29.2) | 90 (26.5) | 92 (29.3) | 97 (25.2) | 126 (31.9) | 147 (30.3) | 156 (33.3) | 160 (31.4) | 0.350 | |
| Arch Involvement (%) | 55 (23.4) | 53 (19.9) | 43 (15.3) | 61 (18.0) | 65 (20.7) | 81 (21.0) | 98 (24.8) | 123 (25.4) | 136 (29.0) | 143 (28.0) | <0.001 | |
| Interposition Graft | 188 (80.0) | 203 (76.0) | 171 (60.9) | 228 (67.3) | 221 (70.4) | 301 (78.2) | 283 (71.6) | 361 (74.4) | 341 (72.7) | 376 (73.7) | ||
| Aortic Arch Replacement | 6 (2.6) | 8 (3.0) | 23 (8.2) | 16 (4.7) | 4 (1.3) | 4 (1.0) | 6 (1.5) | 12 (2.5) | 12 (2.6) | 16 (3.1) | ||
| Root Replacement | 41 (17.4) | 56 (21.0) | 87 (31.0) | 95 (28.0) | 89 (28.3) | 80 (20.8) | 106 (26.8) | 112 (23.1) | 116 (24.7) | 118 (23.1) | ||
| Concomitant CABG (%) | 32 (13.6) | 42 (15.7) | 37 (13.2) | 44 (13.0) | 37 (11.8) | 40 (10.4) | 48 (12.2) | 60 (12.4) | 58 (12.4) | 50 (9.8) | 0.536 | |
| Annual operative volume per surgeon (mean (SD)) | 3.11 (2.01) | 3.13 (2.06) | 3.90 (2.69) | 3.11 (1.82) | 4.29 (2.91) | 4.59 (2.38) | 4.78 (2.60) | 4.79 (2.55) | 5.52 (4.21) | 5.90 (4.18) | <0.001 | |
| Annual operative volume (mean (SD)) | 9.94 (3.98) | 12.03 (5.89) | 13.33 (6.29) | 17.11 (9.63) | 16.17 (8.38) | 18.13 (8.71) | 18.88 (8.20) | 21.32 (9.11) | 21.95 (10.81) | 23.31 (10.98) | <0.001 | |
| Indices of Multiple Deprivation Tercile (%) | 0.083 | |||||||||||
| [1,4] | 86 (37.2) | 93 (36.0) | 85 (31.5) | 107 (32.6) | 94 (31.3) | 105 (29.1) | 125 (33.4) | 174 (37.3) | 165 (36.7) | 154 (31.0) | ||
| (4,8] | 104 (45.0) | 95 (36.8) | 124 (45.9) | 135 (41.2) | 141 (47.0) | 172 (47.6) | 155 (41.4) | 204 (43.8) | 185 (41.1) | 228 (46.0) | ||
| (8,10] | 41 (17.7) | 70 (27.1) | 61 (22.6) | 86 (26.2) | 65 (21.7) | 84 (23.3) | 94 (25.1) | 88 (18.9) | 100 (22.2) | 114 (23.0) | ||
| IRAD score (mean (SD)) | 0.14 (0.12) | 0.13 (0.11) | 0.15 (0.14) | 0.13 (0.10) | 0.13 (0.12) | 0.13 (0.10) | 0.12 (0.10) | 0.13 (0.12) | 0.13 (0.11) | 0.12 (0.10) | 0.04 | |
| Death (%) | 54 (23.0) | 45 (16.9) | 58 (20.6) | 57 (16.8) | 48 (15.3) | 71 (18.4) | 67 (17.0) | 87 (17.9) | 80 (17.1) | 75 (14.7) | 0.257 | |
| Post-op Non-fatal CVA (%) | 18 (7.7) | 30 (11.2) | 30 (10.7) | 28 (8.3) | 31 (9.9) | 28 (7.3) | 25 (6.3) | 28 (5.8) | 70 (14.9) | 50 (9.8) | <0.001 | |
| Post-op dialysis (%) | 34 (16.3) | 37 (15.7) | 45 (17.9) | 52 (16.5) | 42 (14.0) | 45 (12.4) | 53 (14.9) | 50 (11.6) | 79 (18.2) | 73 (15.8) | 0.198 | |
| Deep Sternal Wound Infection (%) | 0 (0.0) | 0 (0.0) | 2 (1.4) | 1 (0.4) | 4 (1.8) | 7 (2.6) | 4 (1.5) | 4 (1.4) | 4 (1.2) | 1 (0.3) | 0.446 | |
| Re-exploration (%) | 39 (17.4) | 25 (10.3) | 37 (14.5) | 44 (14.6) | 45 (16.1) | 41 (12.0) | 37 (10.3) | 33 (7.7) | 45 (10.8) | 32 (7.5) | <0.001 |
SD-standard deviation;COPD-chronic obstructive pulmonary disease;CVA-cerebrovascular accident;LVEF-left ventricular ejection fraction;VT/VT-ventricular fibrillation/ventricular tachycardiac;TIA-transient ischaemic attack;CABG-codonary artery bypass graft;IRAD-International Registry of Acute Aortic Dissection.
Fig. 1.
Number of type A aortic dissection repairs by year and age group.
3.2. Clinical presentation and outcomes
Although mean age at presentation did not change significantly during the study period, there was some increase in the number of operations on octogenarians(Table 1 and Fig. 1A). Approximately one third of those operated on were female without significant change across the years,. Pre-operative diagnosis of Marfan's syndrome, a form of connective tissue disorder that is linked to a risk of TAAD, has more than doubled– 0.8% in 2009 to 2.5% in 2018. There was a trend towards a reduction in the number of patients presenting with transient neurological deficit, ongoing chest pain, acute kidney injury, absent paedal pulses or in cardiogenic shock (Table 1). The overall risk (International Registry of Aortic Dissection(IRAD) score) showed a marginally significant reduction in recent years. Notably, patients were more likely to undergo a more extensive repair with an increased number of patients undergoing full aortic root replacement.
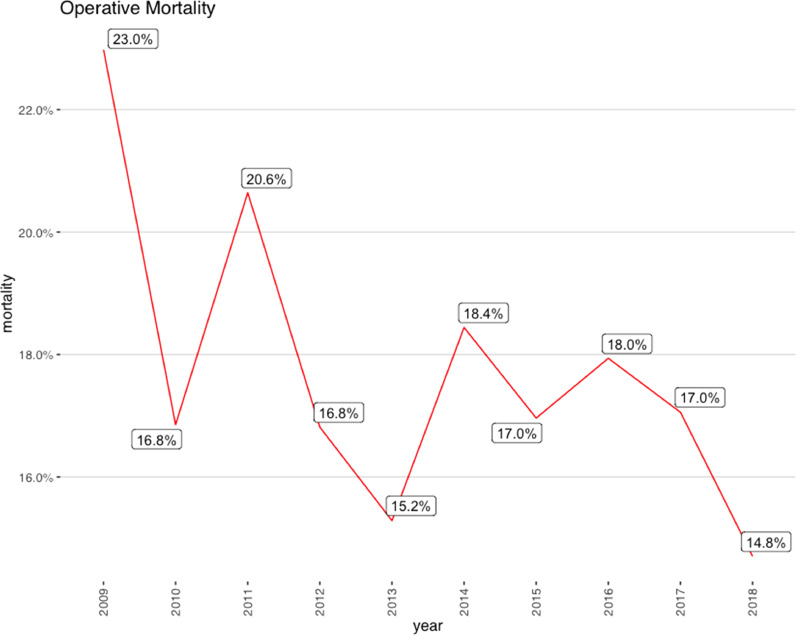
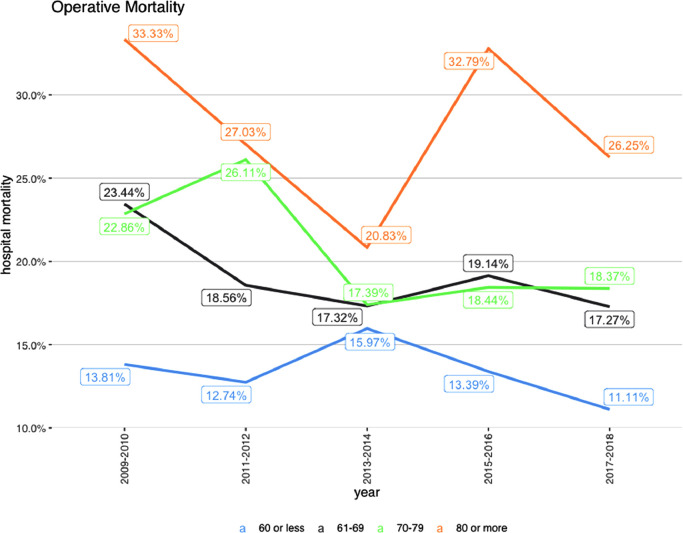
Overall, in-hospital mortality was 17.4% with a trend toward lower mortality in the most recent years (23% in 2009 to 14.7% in 2018)(Table 1 and Fig. 2A). The rate of post-operative stroke has remained unchanged (9–10%),as did the need for dialysis and incidence of sternal wound infections. A decrease in the need for post-operative re-exploration for bleeding was observed over time, from 17.4% to 7.5% of cases. Mortality was significantly higher among octogenarians and on average remained unchanged (Table 2 and Fig. 2B). We also found that mortality was higher in patients from most deprived socioeconomic neighborhoods(Table 3).
Fig. 2.
Mortlaity:a) By year.b) By age.
Table 2.
Post-op Outcomes by Age.
| 60 or less | 61–69 | 70–79 | 80 or more | p | |
|---|---|---|---|---|---|
| N | 1519 | 903 | 1008 | 250 | |
| IRAD score (mean (SD)) | 0.11 (0.11) | 0.11 (0.10) | 0.16 (0.11) | 0.17 (0.12) | <0.001 |
| Death (%) | 201 (13.2) | 170 (18.8) | 202 (20.0) | 69 (27.6) | <0.001 |
| Non-fatal CVA (%) | 121 (8.0) | 86 (9.5) | 115 (11.4) | 16 (6.4) | 0.011 |
| Dialysis(%) | 201 (14.4) | 137 (16.8) | 137 (15.0) | 35 (15.2) | 0.520 |
| Deep Sternal Wound Infection (%) | 9 (1.0) | 9 (1.7) | 4 (0.7) | 5 (3.3) | 0.047 |
| Re-exploration (%) | 165 (12.1) | 102 (12.7) | 92 (10.3) | 19 (8.7) | 0.194 |
IRAD-International Registry of Acute Aortic Dissection;SD-standard deviation;CVA-cerebrovascular accident.
Table 3.
Post-op Outcomes by Indices of Multiple Deprivation category.
| [1,4] | (4,8] | (8,10] | p | |
|---|---|---|---|---|
| N | 1188 | 1543 | 803 | |
| IRAD score (mean (SD)) | 0.13 (0.12) | 0.13 (0.11) | 0.13 (0.11) | 0.136 |
| Death (%) | 230 (19.4) | 243 (15.7) | 141 (17.6) | 0.047 |
| Non-fatal CVA (%) | 122 (10.3) | 133 (8.6) | 71 (8.8) | 0.307 |
| Dialysis(%) | 184 (16.8) | 193 (13.9) | 111 (15.2) | 0.133 |
| Deep Sternal Wound Infection (%) | 8 (1.1) | 11 (1.3) | 6 (1.3) | 0.953 |
| Re-exploration (%) | 137 (12.9) | 146 (10.7) | 86 (11.9) | 0.253 |
IRAD-International Registry of Acute Aortic Dissection;SD-standard deviation;CVA-cerebrovascular accident.
3.3. Hospital and surgeon effect
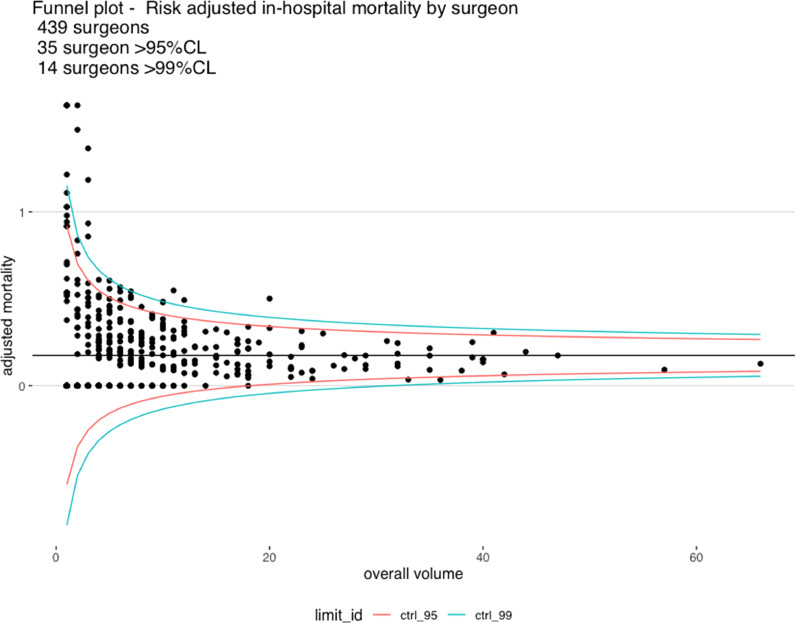
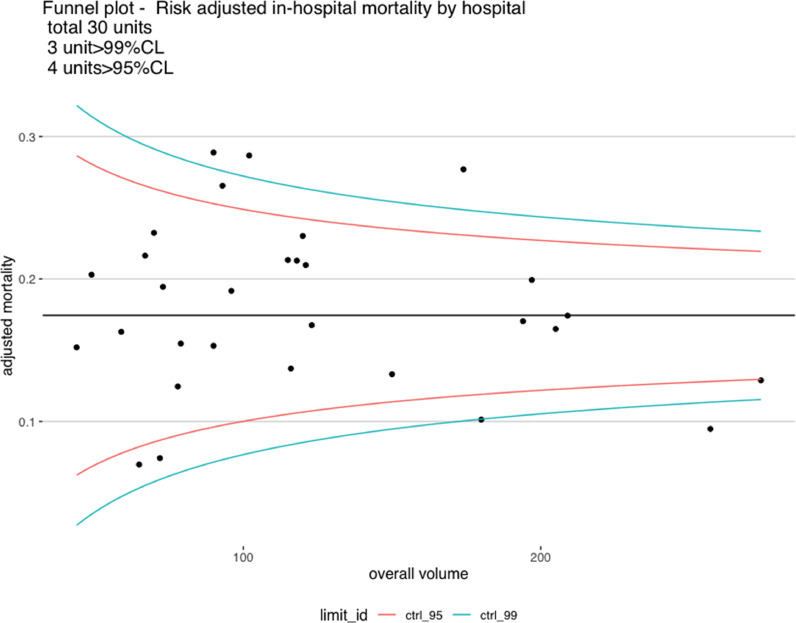
When outcomes were stratified by annual surgeon (Table 4) and center volume (Table 5), we found evidence of improved outcomes for high volume surgeons ( ≥6 cases per year) and high-volume centers (≥22 cases per year). Forest plot analysis showed that a total of fourteen surgeons out of 439 underperformed compared to the national average(mortality above the upper control limit) (Fig. 3A). No surgeon was found to outperform compared to the national average (i.e. below the lower control limit). Three centers out of thirty underperformed compared to the national average(above the upper control limit) andtwo were low-volume centers (<100 cases in 10 years). One unit was outperformed compared to the national average (below the lower control limit) and was a high-volume center.
Table 4.
Post-op Outcomes by Annual Operative Volume per Surgeon.
| [1,3] | (3,5] | (5,20] | p | |
|---|---|---|---|---|
| N | 1653 | 912 | 1115 | |
| IRAD score (mean (SD)) | 0.13 (0.12) | 0.13 (0.11) | 0.12 (0.10) | 0.188 |
| Death (%) | 338 (20.4) | 157 (17.2) | 147 (13.2) | <0.001 |
| Non-fatal CVA (%) | 159 (9.6) | 81 (8.9) | 98 (8.8) | 0.711 |
| Post-op dialysis (%) | 224 (15.2) | 137 (16.6) | 149 (14.2) | 0.344 |
| Deep Sternal Wound Infection(%) | 13 (1.4) | 8 (1.5) | 6 (0.9) | 0.612 |
| Re-exploration(%) | 183 (12.5) | 91 (10.8) | 104 (10.7) | 0.306 |
IRAD-International Registry of Acute Aortic Dissection;SD-standard deviation;CVA-cerebrovascular accident.
Table 5.
Post-op Outcomes by Annual Operative Volume per Center.
| [1,13] | (13,21] | (21,44] | p | |
|---|---|---|---|---|
| N | 1343 | 1157 | 1180 | |
| IRAD score (mean (SD)) | 0.13 (0.12) | 0.13 (0.10) | 0.12 (0.11) | 0.095 |
| Death (%) | 257 (19.1) | 214 (18.5) | 171 (14.5) | 0.005 |
| Non-fatal CVA (%) | 102 (7.6) | 106 (9.2) | 130 (11.0) | 0.012 |
| Post-op dialysis (%) | 167 (13.7) | 214 (19.9) | 129 (12.2) | <0.001 |
| Deep Sternal Wound Infection(%) | 12 (1.5) | 10 (1.3) | 5 (0.9) | 0.629 |
| Re-exploration(%) | 164 (13.7) | 126 (12.2) | 88 (8.5) | <0.001 |
IRAD-International Registry of Acute Aortic Dissection;SD-standard deviation;CVA-cerebrovascular accident.
Fig. 3.
Funnel plot of risk-adjusted mortality:a) By total operative volume per surgeon.b) By total operative volume per center.
3.4. Chronobiological patterns
Time analysis on number of TAAD operated on showed a marked monthly variations with a high incidence in winter (October to December) and a decreased incidence in the Summer (June/July) (P<0.001)(Supplementary Figure 1). There also appeared to be a mid-week increase in the incidence in operations and a decrease on Saturday and Sunday (P<0.001). Time analysis confirmed a progressive reduction in mortality over the years (P<0.001) but a significant trend towards higher mortality at weekend (P<0.001) with no effect related to months (P = 0.45).
4. Discussion
We presented a decade long report on over 3600 patients operated on for type A aortic dissection in England from 2009 to 2018. In this time we noted an almost doubling of the total number of operations performed as well as the number of operations performed per surgeon and per center. Surprisingly we found a small trend towards a reduction in the overall risk profile in recent years (IRAD score) due to less patients undergoing surgery in critical conditions. There was also an increase in complexity of operations performed and a slight increase in the number of patients with known connective tissue disorders undergoing surgery for TAAD.. The net result was a decrease in mortality from 23% to 15% with no increase in the incidence of other complications, notably the rate of strokes. We speculate that the reason for less patients operated on in a critical condition is a combination of increased awareness in peripheral hospitals, increased rapidity of radiological diagnosis and importantly the institution of appropriate analgesia and antihypertensives treatments in the emergency department. There was also a decrease in the incidence of re-explorations post-surgery which may be attributed to improved experience, pharmaceutical management haemostasias and post-operative blood pressure optimization.
Some consider advanced age a contraindication to surgery for TAAD. Although the present analysis has confirmed a higher mortality rate in octogenarians, the observed mortality is not prohibitive and lower than that reported in previous series [13], thus suggesting that surgery should not be considered prohibitive or denied to this group solely based on age and case by case selection should be sought.
In the present analysis, we found a trend towards lower mortality in patients from better socioeconomic neighborhoods. The reasons for this observation are likely to multi-factorial. Patients from most deprived areas may not be able to access healthcare as swiftly as others, be more unstable by the time of presentation or have a greater burden of comorbidities. However, other factors including complex interaction between patients and healthcare providers may contribute to this association and further research in this field is warranted.
Previous studies have shown a relationship between surgeons’ operative volumes [14] and outcomes in TAAD repair as well as between center operative volumes and outcome in all thoracic aortic disease in American and Nordic populations [15], [16], [17], [18], [19]. The present analysis confirms an outcome-volume relationship for both surgeons and centers. This is likely to be related to increased surgeon and team experience and improved management. 3 out of 30 units were found to perform below average. Two of them were low volume centers but surprisingly one was at intermediate volume. This seems to suggest that sporadically factors other than volume can determine poor performance in individual units which may require in-depth review of practices.
We finally investigated variation in presentation and mortality related to time of the year. We noted that there were significantly more cases presenting in winter than over summer. This could possibly be linked with tendency towards increased hypertension in the winter months [20], [21], [22]. There was also a decreased tendency for patients to present over the weekend. Although the exact cause of this is unknown, but it is possible that patient related factors may contribute (i.e. patients less likely to access healthcare facilities over weekend). Knowledge of these variations may be beneficial when planning policy and staffing decisions in tertiary referral centers.
We also found an increased mortality among patients operated at weekend. Relation between weekend surgery and increased mortality in other types of surgery has been previously described [23]. It is possible that the availability of surgeons and other members of the team specialized in aortic surgery can be reduced at weekends and this can translate into poorer outcomes. It has been suggested national outcomes could be improved by the establishment of a network of centers and surgeons (e.g. aortic rota) in order to provide a highly specialized service for all patients referred for TAAD [4,15,24].
5. Limitations
Generally, it is a weakness of registries that not all details can be collected, including patients who were deemed to not be suitable for surgery and their differences compared to those who were operated on. There is also no information on those that were managed intervenitonally or conservatively, predominantly type B aortic dissections. The dataset utilized also does not contain any details of timings from diagnosis to surgery including transfer time to tertiary care centers. We also have no information on variation of postoperative management including transfusion or medications used. Finally we had to extrapolate some of the variables for the calculation of the expected risk as a few risk factors in the IRAD score were not consistent with the original data entry.
6. Conclusions
TAAD remains a lethal condition for which surgery is the only treatment option. Prompt diagnosis and referral to a specialist center is paramount. The number of operations conducted in England has doubled in the last 10 years and the associated mortality has decreased. Increased surgeon and center experience due to more operations per center or surgeon per year is likely to be related to the observed improvement . Establishment of a national network of experienced dedicated aortic surgeons and high-volume centers should be considered to provide a uniform high standard of care for patients with TAAD.
Authors’ contributions
All authors have made a significant contribution to the final article. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. Individual contributions are:
-
•
Umberto Benedetto: conceptualisation, data curation, formal analysis, funding acquisition, methodology, project administration, visualization, writing – original draft, and writing– review & editing
-
•
Shubhra Sinha: conceptualisation, data curation, project administration, validation, writing – original draft, and writing– review & editing
-
•
Arnaldo Dimagli: validation, visualization and writing– review & editing
-
•
Graham Cooper, Giovanni Mariscalco, Rakesh Uppal, Narain Moorjani, George Krasopoulos, Amit Kaura, Mark Field, Uday Trivedi, Simon Kendall, Gianni D Angelini, Enoch F Akowuah and Geoffrey Tsang: conceptualisation, methodology, supervision and writing– review & editing
All authors confirm that they accept responsibility to submit for publication.
Data sharing statement
-
•
Will individual participant data be available (including data dictionaries)? On contacting the authors
-
•
What data in particular will be shared? Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices).
-
•
What other documents will be available? N/A
-
•
When will data be available (start and end dates)? Immediately following publication; no end date
-
•
With whom? Investigators whose proposed use of the data has been approved by the relevant review committees
-
•
For what types of analyses? To achieve aims in the approved proposal
-
•
By what mechanism will data be made available? Proposals should be directed to the corresponding author; to gain access, data requestors may need to sign a data access agreement
Declaration of interests
Authors have no conflict of interests to declare.
Acknowledgement/Funding
This study was supported by the British Heart Foundation and the NIHR Biomedical Research center at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100131.
Appendix. Supplementary materials
References
- 1.Mahase E. Half of patients with acute aortic dissection in England die before reaching a specialist centre. BMJ. 2020;364:m304. doi: 10.1136/bmj.m304. [DOI] [PubMed] [Google Scholar]
- 2.Braverman A.C. Acute aortic dissection: clinician update. Circulation. 2010;122(2):184–188. doi: 10.1161/CIRCULATIONAHA.110.958975. [DOI] [PubMed] [Google Scholar]
- 3.Evangelista A., Isselbacher E.M., Bossone E., Gleason T.G., Di Eusanio M., Sechtem U. Insights from the international registry of acute aortic dissection: a 20-year experience of collaborative clinical research. Circulation. 2018;137(17):1846–1860. doi: 10.1161/CIRCULATIONAHA.117.031264. [DOI] [PubMed] [Google Scholar]
- 4.Richens D., Briggs T. Cardiothoracic surgery girft programme national specialty report girft is delivered in partnership with the royal national orthopaedic hospital NHS trust and NHS improvement. 2018;(March). Available from: http://gettingitrightfirsttime.co.uk/wp-content/uploads/2018/04/GIRFT-Cardiothoracic-Report-1.pdf
- 5.Howard D.P.J., Banerjee A., Fairhead J.F., Perkins J., Silver L.E., Rothwell P.M. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation. 2013 May;127(20):2031–2037. doi: 10.1161/CIRCULATIONAHA.112.000483. 2013/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erbel R., Aboyans V., Boileau C., Bossone E., Di Bartolomeo R., Eggebrecht H. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 7.Hiratzka L.F., Bakris G.L., Beckman J.A., Bersin R.M., Carr V.F., Casey D.E. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. J Am Coll Cardiol. 2010;55(14):e27–129. doi: 10.1016/j.jacc.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Bossone E., Gorla R., LaBounty T.M., Suzuki T., Gilon D., Strauss C. Presenting systolic blood pressure and outcomes in patients with acute aortic dissection. J Am Coll Cardiol. 2018;71(13):1432–1440. doi: 10.1016/j.jacc.2018.01.064. [DOI] [PubMed] [Google Scholar]
- 9.Krüger T., Weigang E., Hoffmann I., Blettner M., Aebert H. Cerebral protection during surgery for acute aortic dissection type A: results of the German registry for acute aortic dissection type a (GERAADA) Circulation. 2011;124(4):434–443. doi: 10.1161/CIRCULATIONAHA.110.009282. [DOI] [PubMed] [Google Scholar]
- 10.Hickey G.L., Grant S.W., Cosgriff R., Dimarakis I., Pagano D., Kappetein A.P. Clinical registries: governance, management, analysis and applications. Eur J Cardio-Thoracic Surg Off J Eur Assoc Cardio-Thoracic Surg. 2013 Oct;44(4):605–614. doi: 10.1093/ejcts/ezt018. [DOI] [PubMed] [Google Scholar]
- 11.Booher A.M., Isselbacher E.M., Nienaber C.A., Trimarchi S., Evangelista A., Montgomery D.G. The IRAD classification system for characterizing survival after aortic dissection. Am J Med [Internet] 2013;126(8):730.e19–730.e24. doi: 10.1016/j.amjmed.2013.01.020. Available from: [DOI] [PubMed] [Google Scholar]
- 12.Spiegelhalter D.J. Funnel plots for comparing institutional performance. Stat Med. 2005 Apr;24(8):1185–1202. doi: 10.1002/sim.1970. [DOI] [PubMed] [Google Scholar]
- 13.Hsu M.E., Chou A.H., Cheng Y.T., Lee H.A., Liu K.S., Chen D.Y. Outcomes of acute aortic dissection surgery in octogenarians. J Am Heart Assoc. 2020;9(18):1–9. doi: 10.1161/JAHA.120.017147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bashir M., Harky A., Fok M., Shaw M., Hickey G.L., Grant S.W. Acute type A aortic dissection in the United Kingdom: surgeon volume-outcome relation. J Thorac Cardiovasc Surg [Internet] 2017;154(2):398–406.e1. doi: 10.1016/j.jtcvs.2017.02.015. Available from: [DOI] [PubMed] [Google Scholar]
- 15.Bottle A., Mariscalco G., Shaw M.A., Benedetto U., Saratzis A., Mariani S. Unwarranted variation in the quality of care for patients with diseases of the thoracic aorta. J Am Heart Assoc. 2017;6(3):1–51. doi: 10.1161/JAHA.116.004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chikwe J., Cavallaro P., Itagaki S., Seigerman M., Diluozzo G., Adams D.H. National outcomes in acute aortic dissection: influence of surgeon and institutional volume on operative mortality. Ann Thorac Surg [Internet] 2013;95(5):1563–1569. doi: 10.1016/j.athoracsur.2013.02.039. Available from: [DOI] [PubMed] [Google Scholar]
- 17.Iribarne A., Milner R., Merlo A.E., Singh A., Saunders C.R., Russo M.J. Outcomes following emergent open repair for thoracic aortic dissection are improved at higher volume centers. J Card Surg. 2015;30(1):74–79. doi: 10.1111/jocs.12470. [DOI] [PubMed] [Google Scholar]
- 18.Geirsson A., Ahlsson A., Franco-Cereceda A., Fuglsang S., Gunn J., Hansson E.C. Hospital volumes and later year of operation correlates with better outcomes in acute Type A aortic dissection. Eur J Cardiothorac Surg. 2018;53(1):276–281. doi: 10.1093/ejcts/ezx231. [DOI] [PubMed] [Google Scholar]
- 19.Dobaria V., Kwon O.J., Hadaya J., Sanaiha Y., Sareh S., Aguayo E. Impact of center volume on outcomes of surgical repair for type A acute aortic dissections. Surg (United States) [Internet] 2020;168(1):185–192. doi: 10.1016/j.surg.2020.04.007. Available from: [DOI] [PubMed] [Google Scholar]
- 20.Deshmukh A., Pant S., Kumar G., Murugiah K., Mehta J. Seasonal Variation in Hypertensive Emergency Hospitalization. J Clin Hypertens. 2012;14(4):269–270. doi: 10.1111/j.1751-7176.2012.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fares A. Winter Hypertension : potential mechanisms Introduction: int J Health Sci (Qassim) 2013;7(2):210–219. doi: 10.12816/0006044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takagi H., Ando T., Umemoto T. (ALICE [All-Literature Investigation of Cardiovascular Evidence] Group). Meta-analysis of seasonal incidence of aortic dissection. Am J Cardiol [Internet] 2017;120(4):700–707. doi: 10.1016/j.amjcard.2017.05.040. Available from: [DOI] [PubMed] [Google Scholar]
- 23.McCallum I.J.D., McLean R.C., Dixon S., O'Loughlin P. Retrospective analysis of 30-day mortality for emergency general surgery admissions evaluating the weekend effect. Br J Surg. 2016 doi: 10.1002/bjs.10261. [DOI] [PubMed] [Google Scholar]
- 24.Goldstone A.B., Chiu P., Baiocchi M., Lingala B., Lee J., Rigdon J. Interfacility transfer of medicare beneficiaries with acute Type A aortic dissection and regionalization of care in the United States. Circulation. 2019;140(15):1239–1250. doi: 10.1161/CIRCULATIONAHA.118.038867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.