Summary
Phototheranostics integrates deep-tissue imaging with phototherapy (containing photothermal therapy and photodynamic therapy), holding great promise in early diagnosis and precision treatment of cancers. Recently, second near-infrared (NIR-II) fluorescence imaging exhibits the merits of high accuracy and specificity, as well as real-time detection. Among the NIR-II fluorophores, organic small molecular fluorophores have shown superior properties in the biocompatibility, variable structure, and tunable emission wavelength than the inorganic NIR-II materials. What's more, some small molecular fluorophores also display excellent cytotoxicity when illuminated with the NIR laser. This review summarizes the progress of small molecular NIR-II fluorophores with different central cores for cancer phototheranostics in the past few years, focusing on the molecular structures and phototheranostic performances. Furthermore, challenges and prospects of future development toward clinical translation are discussed.
Keywords: second near-infrared (NIR-II), small molecular fluorophores, cancer phototheranostics
Graphical Abstract
Public Summary
-
•
Phototheranostics combines diagnostic imaging with phototherapy, showing broad applications in the early diagnosis and precise treatment of tumors
-
•
Small molecular NIR-II fluorophores with good biocompatibility, tunable structure, high imaging quality, and excellent phototoxicity, have shown great potential for cancer phototheranostics
-
•
Small molecular NIR-II fluorophores with different central cores for cancer phototheranostics are summarized, highlighting the design strategies and phototheranostic performances
-
•
Challenges and prospects of future development toward clinical translation are discussed
Introduction
Phototheranostics, which combines diagnostic photo-imaging with light-triggered precision therapy, can simultaneously locate the lesion sites and monitor the therapeutic effects in real time.1, 2, 3 Thus, to achieve efficient and controllable precision treatment, it is necessary to integrate diagnostics with therapeutics.4 Traditional diagnostic modalities, such as computed tomography (CT),5, 6, 7 magnetic resonance imaging (MRI),8, 9, 10 and positron emission tomography (PET),11, 12, 13 face the problems of insufficient sensitivity, low resolution, poor signal-to-background ratio (SBR) and high cost.14,15 As an emerging imaging modality, fluorescence imaging (FLI) has aroused extensive research enthusiasm due to its high sensitivity and excellent specificity.16, 17, 18
The FLI window can be divided into the visible region (400–700 nm), the first near-infrared region (NIR-I, 700–1,000 nm), and the second near-infrared region (NIR-II, 1,000–1,700 nm).19 Though NIR-I FLI has better performance than visible light imaging; shallow tissue penetration hinders its further clinical applications.20 By comparison, NIR-II FLI presents the superiority of high temporal and spatial resolution, excellent signal-to-noise ratio, and deep tissue penetration owing to the reduced tissue absorption and scattering as well as low autofluorescence.21, 22, 23 In 2019, NIR-II FLI was reported for the first time to guide the surgery of liver cancer patients. The intraoperative evaluations confirmed that NIR-II FLI presented higher sensitivity and better lesion recognition ability compared with imaging in visible and NIR-I areas, which opened a new era of the cancer diagnosis.24 At present, cancer treatment strategies, such as surgery,25 radiotherapy,26 and chemotherapy,27 have certain side effects on the human body, which limit their therapeutic effects.28 To achieve efficient, accurate, and noninvasive treatment, a series of diagnosis and treatment-integrated NIR-II fluorophores have been further developed for imaging-mediated phototherapy.29, 30, 31, 32 In the process of imaging-guided photothermal therapy (PTT), NIR-II fluorophores not only have excellent diagnostic capabilities but also exhibit good photothermal conversion efficiency, which can convert light energy into heat to ablate tumors. Meanwhile, as-produced heat can cause thermal expansion of the tumor tissues to generate photoacoustic imaging (PAI) signal.33, 34, 35 Furthermore, some NIR-II fluorophores can also produce singlet oxygen (1O2) or other reactive oxygen species (ROS) under laser irradiation to induce apoptosis or necrosis of tumor cells, which can be applied for imaging-guided photodynamic therapy (PDT) or further to achieve synergistic PDT/PTT.36, 37, 38, 39, 40 These high-performance phototheranostic NIR-II fluorophores show prominent perspectives for fighting cancers.
The currently reported phototheranostic NIR-II fluorophores can be classified into two categories: inorganic materials and organic materials. Inorganic materials, such as carbon nanotubes,41, 42, 43 quantum dots,44, 45, 46 and rare-earth-doped nanoparticles,47, 48, 49 have poor biocompatibility and reproducibility, slow metabolism, and potential long-term biological toxicity, hindering its further clinical development. Although organic conjugated polymers have good biocompatibility, they also have the problems of difficulty in excretion and uncontrollable molecular weight and structure.50, 51, 52, 53, 54, 55 In contrast, organic small molecule fluorophores have attracted more and more attention due to their controllable molecular structure and performance as well as good biosafety, which have a broader clinical application prospect.56, 57, 58, 59, 60 Herein, we attempt to review the recent progress of organic small molecular NIR-II fluorophores for applications in both cancer diagnosis and treatment. First, the design principles and common central cores of NIR-II small molecular fluorophores will be briefly introduced. Then, we will elaborate each type of NIR-II fluorophores in detail, focusing on the molecular structures and phototheranostic performances, and the future challenges and prospects of them are finally discussed.
Design Principles and Common Cores for Small Molecular NIR-II Fluorophores
The mechanism of fluorescence emission can be roughly divided into three steps. First, the ground state (S0) fluorophore absorbs certain photo-energy upon irradiation to jump to the excited state (Sn). Second, the excited state decays to the lowest singlet state (S1) via the processes of internal conversion and vibrational relaxation. Third, the fluorophore at the lowest singlet state S1 returns to the ground state S0 by releasing energy in the form of photon radiation. The outgoing light is named fluorescence. Generally, NIR-II fluorophores are defined as a class of molecules whose fluorescence emission wavelengths locating in the NIR-II biological window. To construct an ideal small molecular NIR-II fluorophore for high-performance phototheranostics, several main factors should be taken into consideration, including stability, fluorescence emission wavelength, brightness, photothermal conversion efficiency and ROS generation efficiency.61, 62, 63, 64
For the NIR-II phototheranostic fluorophores, photo/thermal/chemical-stability is the most basic requirement to be achieved. It is also a guarantee for the smooth expression of various diagnostic and therapeutic properties. It is well known that the longer the wavelength of the light, the deeper the penetration depth, and the lower energy of the photons. Currently, three methods, including the construction of Donor-Acceptor (D-A) motif, the extension of the π-conjugation and quinoidization of the π-system (in some instances), can effectively narrow the highest occupied molecular orbital (HOMO)-lowest unoccupied molecular orbital energy gap (Eg) of the fluorophores. The low Eg will result in a bathochromic shift of their absorbance and fluorescence bands, thereby pushing the fluorescence wavelength to the NIR-II region or even further.65,66
Brightness is a parameter of great importance for small molecular NIR-II fluorophores, which will directly affect the imaging performances, such as resolution, imaging speed, etc. Brightness is determined to be the product of fluorescence quantum yield (QY) and absorbance coefficient (ϵ) at the corresponding excitation wavelength. Regarding fluorescence QY, the molecular aggregation of most organic fluorophores at high concentration will quench or weaken the fluorescence emission, which is called the aggregation-caused quenching (ACQ) effect. Therefore, increasing distortion of the molecular structure to decrease the intermolecular interaction is an effective way to improve the fluorescence QY. Furthermore, an opposite concept of the ACQ process, aggregation-induced emission (AIE), has been proposed.67,68 The fluorophore closely packs to restrict the intramolecular rotations and π-π interactions, switching off the non-radiative transition pathway but switching on the radiative decay pathway. To increase the absorbance coefficient, extension of the π-conjugation with a fused-ring structure is an efficient and practical strategy. However, it can lead to the tendency toward ACQ due to the strong intermolecular π-π interactions. As a result, it is a balance to increase the brightness owing to the paradox between fluorescence QY and absorbance coefficient. Thus, both factors should be optimized simultaneously to achieve high brightness. In addition, to form supramolecular complexes of host-guest or protein-fluorophore is another way to optimize the fluorophore's brightness.69, 70, 71, 72
It is generally believed that S1 molecules have three different energy dissipation pathways, accordingly returning to S0. The fluorescence generation is attributed to the radiative transition of energy from S1 to S0. On the contrary, the non-radiative transition is usually accompanied by the generation of heat. Besides, S1 molecules can also reach the lowest triplet state (T1) through the intersystem crossing (ISC) process and produce phosphorescence in the form of photon radiation. Meanwhile, the transfer of energy (Type II reaction) can generate 1O2, and other ROS can be produced through electron transfer (Type I reaction). In the process of energy dissipation, such three pathways are always in a competitive relationship.73 Therefore, blocking a certain pathway can enhance the corresponding performance of other pathways. However, up to now, there is still a lack of effective methods to regulate the photothermal conversion efficiency and ROS generation efficiency of organic small molecular NIR-II fluorophores.
Water dispersibility is another vital issue to be solved for further biological applications in vivo. However, most organic small molecular NIR-II fluorophores that have been developed until now are hydrophobic ones. And three common strategies are currently employed to address this issue. One is to directly modify the fluorophores with hydrophilic groups (such as PEGylated and sulfonated); the modified molecules not only have good water solubility but also usually show the advantages of easy excretion and good biosafety. Another strategy is to fabricate water-soluble nanoparticles (NPs) via self-assembly with the amphiphilic polymeric matrix. These NPs usually present the characteristics of extended blood circulation time, improved tumor uptake, and boosted drug loading capacity, which are not available in the water-soluble groups-modified molecules. The third is to form a nanosized hydrophilic complex with biomacromolecules such as peptides or proteins.74
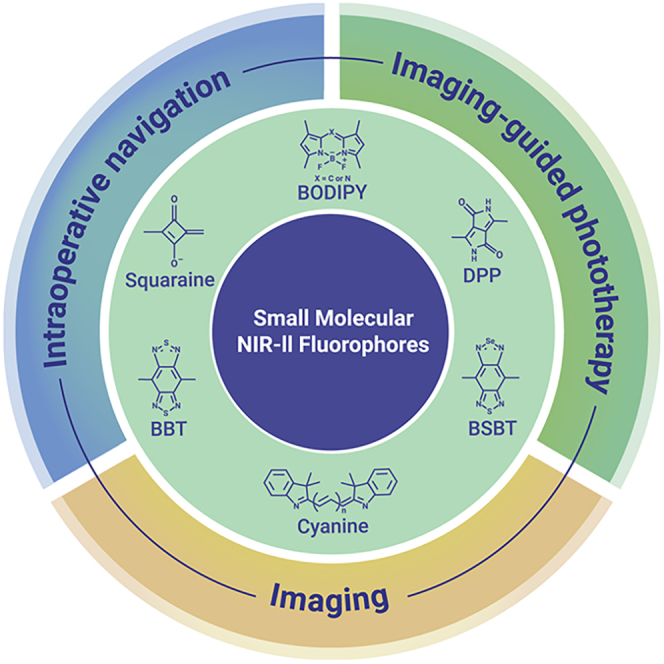
Recently, several types of organic small molecular NIR-II fluorophore have been reported, including Benzobisthiadiazole (BBT), selenadiazolo[3,4-f]benzo[c][1,2,5] thiadiazole (BSBT), cyanine, Dipyrromethene boron difluoride (BODIPY)/aza-BODIPY, Diketopyrrolopyrrole (DPP), and squaraine-based NIR-II fluorophores. Some other NIR-II organic small molecules have also emerged.
NIR-II organic small molecular fluorophores
BBT-based NIR-II fluorophores
The D-A structured fluorophores have attracted considerable research interest owing to their outstanding photophysical properties. As first reported by Ono et al., in 1994, BBT is a 14 π electron system with a hypervalent sulfur atom and one of the most robust electron-withdrawing building blocks for low bandgap compounds construction.75 With the rapid development of organic electronics, BBT-based D-A type molecules have been widely synthesized for organic light-emitting diodes, organic photovoltaics, and biophotonics.76, 77, 78, 79 Recently, a series of BBT-based NIR-II fluorophores have been successfully exploited for cancer imaging and phototherapy (Table 1).80,81
Table 1.
Chemical Structures of Common Types of Organic Small Molecular NIR-II Fluorophores and Some Other NIR-II Organic Small Molecules
 |
 |
 |
 |
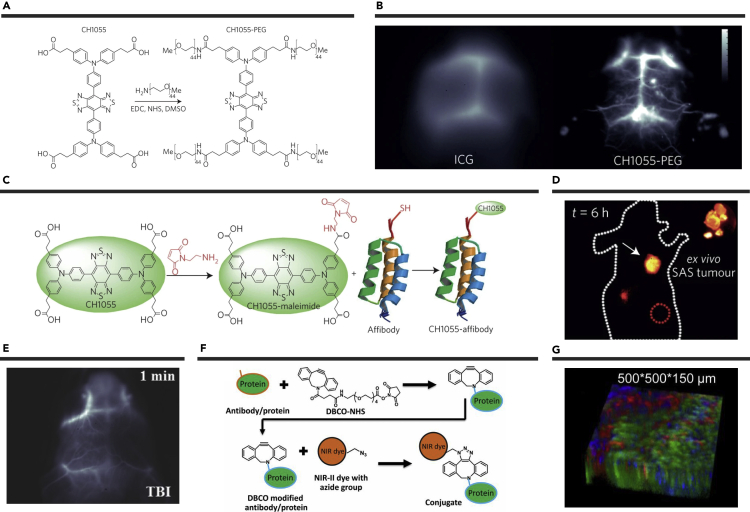
The first BBT-based NIR-II fluorophore CH1055 with a molecular weight of 970 Da only was reported in 2016.82 The electron-rich triphenylamine moiety was used as a donor unit to construct the D-A-D structure. Further PEG-functionalization was performed on the four periphery carboxylic acid groups (-COOH) of CH1055 to prepare CH1055-PEG with improved water solubility and showed rapid renal excretion (∼90% metabolized by kidneys within 24 hr) (Figure 1A, Table 2). The brain vasculature imaging with CH1055-PEG showed a higher SBR than the clinically approved indocyanine green (ICG) (Figure 1B). Moreover, an anti-epidermal growth factor receptor (EGFR) affibody was conjugated onto CH1055 to afford CH1055-EGFR for active tumor-targeting molecular imaging (Figures 1C and 1D). Fluorophore CH1055 not only has great potential for precise imaging-guided tumor treatment but also provides a good platform for the further design and construction of BBT-based NIR-II fluorophores, which brings a new era for the study of NIR-II fluorescence.
Figure 1.
Functional Design and Bioimaging of Small Molecular NIR-II Fluorophores
(A) Synthesis of CH1055-PEG.
(B) Comparison of brain vasculature imaging of ICG in the NIR-I area and CH1055-PEG in the NIR-II area.
(C) Synthetic schematic diagram of CH1055-affibody.
(D) Tumor targeted imaging of CH1055-EGFR. Reproduced with permission from Li et al.4 Copyright 2019 Royal Society of Chemistry.
(E) Traumatic brain injury imaging of IR-E1. Reproduced with permission from Zhang et al.86 Copyright 2016 Wiley-VCH.
(F) Schematic diagram of IR-FGP and protein binding through click reaction. Reproduced with permission from Wan et al.22 Copyright 2019 Wiley-VCH.
(G) Three-color 3D rendering of nucleus, neuron, and vessel channels obtained with NIR-I/NIR-II confocal microscopy by using IR-FGP. Reproduced with permission from Tu et al.16 Copyright 2019 Elsevier.
Table 2.
BBT- and BSBT-based NIR-II Fluorophores
| Fluorophore | Hydrophilic Modification | Targeted Modification | λAbs/λem (nm) | QYa | Imaging Strategy | Therapeutic Mode | Biological Applications | Ref |
|---|---|---|---|---|---|---|---|---|
| CH1055 | PEGylated | EGFR affibody conjugate | 750/1,055 | 0.03% in water | FLI | / | Vessel, lymph node, and tumor imaging | Antaris et al.82 |
| Q4 | DSPE-mPEG5k | GRPR peptide conjugate | N.A./1,100 | 0.02% in water | FLI | / | Vessel imaging; prostate cancer imaging | Sun et al.83 |
| H1 | DSPE-mPEG5k or PEGylated | mono-c(RGDfk) peptide conjugate | N.A./1,100 | 0.20% in DCM | FLI | / | Vessel and whole-body imaging | Sun et al.84 |
| H3 | PEGylated | / | 760/1,023 | 1.23% in DCM | FLI | / | DMBA-induced carcinoma imaging and imaging-guided surgery | Zeng et al.85 |
| IR-E1 | PEGylated | / | N.A./1,071 | 0.07% in water | FLI | / | Traumatic brain injury imaging | Zhang et al.86 |
| IR-BGP6 | PEGylated | PD-L1 affibody conjugate | 736/1,047 | 0.15% in water | FLI | / | Tumor-targeting imaging | Wan et al.87 |
| IR-FGP | PEGylated | EGFR affibody conjugate | 745/1,050 | 0.19% in water | FLI | / | Brain tissue neuron imaging | Zhu et al.88 |
| IR-FEP | PEGylated | / | 780/1,047 | 0.20% in water | FLI | / | Vessel and tumor imaging | Yang et al.89 |
| IR-FTAP | PEGylated | / | 733/1,048 | 0.53% in water | FLI | / | Hindlimb vessel imaging | Yang et al.90 |
| IR-FP8P | PEGylated | FSH conjugate | 748/1,040 | 0.60% in water | FLI | / | Ovary imaging | Ma et al.91 |
| CH-4T | Sulfonated; FBS | / | 738/1,055 | 0.50%–1.10% in serum | FLI | / | Vessel and lymph node imaging | Antaris et al.92 |
| H2Ha-4T | Sulfonated; FBS | Cetuximab complex | 738/1,024 | 0.01% in water; up to 0.11% in serum when heated | FLI/PTI | PTT | Hindlimb vessel and the lymphatic imaging; imaging-guided sentinel lymph node surgery and colorectal cancer PTT | Zeng et al.93 |
| Q8PNap | PEGylated; FBS | / | N.A. | 0.003% in water; up to 0.0114% in serum when heated | FLI | / | Hindlimb vessel, lymphatic system, and tumor metastasis imaging; imaging-guided tumor and lymph node surgery | Qu et al.94 |
| CQ-4T | Sulfonated; HSA | / | N.A. | 0.104% in PBS; 0.34% in HSA | FLI | / | Monitoring thrombosis, peripheral arterial disease, tumor angiogenesis, and lymphatic drainage; imaging- guided tumor resection and sentinel lymph node biopsy | Li et al.95 |
| p-FE | PS-g-PEG | / | 760/1,010 | 1.65% in water | FLI | / | 3D vessel imaging and tumor imaging | Wan et al.96 |
| TPB-AZO | PS-PEG | / | 690/909 | 1.562% (850–1,500 nm); 0.351% (1,000–1,500 nm) | FLI | / | Determining cardiac cycle and heart rate; tumor detection | Zhang et al.97 |
| SYL | DSPE-mPEG5k | / | 765/976 | N.A. | FLI/PAI | PTT | Tumor imaging and imaging-guided PTT | Zhang et al.99 |
| BPN-BBTD | F127 | / | 700/949 | 0.18% (>1,000 nm) | FLI | PTT | Tumor imaging and imaging-guided PTT, long-term tumor monitoring | Alifu et al.102 |
| TB1 | DSPE-mPEG2k | Decorated with c-RGD | 740/975 | 0.62% | FLI/PAI | / | Brain tumor imaging | Sheng et al.103 |
| BTPPA | DSPE-mPEG2k | / | 761/1,013 | 0.99%; 0.60% (>1,000 nm) |
FLI | / | Hindlimb, cerebral, lymph vessel, and tumor imaging | Wu et al.104 |
| XA1 | F127 | / | 780/1,000 | 1.48% | FLI | / | Hindlimb, cerebral, and tumor imaging | Xu et al.105 |
| HOL2 | DSPE-mPEG5K | / | 710/1,050 | 1.19% (>1,000 nm); 0.016% beyond 1,300 nm; 0.002% beyond 1,550 nm | FLI | / | Vessel and tumor imaging | Li et al.107 |
| HL3 | DPPE-5KPEG | / | 750/1,050 | 1.17% (>1,000 nm); 0.005% in the NIR-IIb | FLI | / | Whole-body, cerebral vessel, and lymph node imaging | Li et al.108 |
| 2TT-Oc26b | DSPE-mPEG2k | / | 700/1,030 | 1.15% (>1,000 nm); 0.012% in the NIR-IIb | FLI | / | Whole-body, cerebral vessel, and intestinal trace imaging | Li et al.109 |
| SY1080 | DSPE-PEG5K | / | 820/1,080 | 0.15% in THF | FLI/PAI | PTT | Tumor imaging and imaging-guided PTT | Zhang et al.110 |
Fluorescence quantum yields of all small molecular NIR-II fluorophores mentioned in this review were recalculated based on IR26 = 0.05% (in 1,2-dichloroethane).
To expand the library of D-A-D scaffold NIR-II fluorophores and push the fluorescence wavelength more red-shifted, a D-π-A-π-D fluorophore Q4 was prepared by Sun et al.83 The thiophene unit was introduced as the π-spacer between triphenylamine (D) and BBT (A) to facilitate intramolecular charge transfer (ICT). However, due to π-π stacking, the fluorescence QY of Q4 was still unsatisfactory. To surmount this problem, another two fluorophores H1 and H3 were prepared by introducing fluorene as the donor unit, or further replacing thiophene with 3,4-ethoxylene dioxythiophene (EDOT) as the π-bridge.84,85 With the installation of alkylated fluorene and EDOT, the intermolecular distance was increased to boost the fluorescence QY.
The D-A-D structured NIR-II fluorophores usually possess a large π-conjugated framework to emit fluorescence in the NIR-II biological window. However, such a large π-system will severely cause intermolecular interactions and aggregation. The interaction between water molecules and organic fluorophores is the main reason for the quenching of the fluorophores' excited states, resulting in a low fluorescence QY in aqueous solutions. To overcome these problems, researchers have put forward the shielding units (S) to flank at the periphery sites to construct the S-D-A-D-S scaffolds. The shielding units can effectively reduce the intermolecular interactions and then protect the excited state molecules from the interference of water molecules.
The first S-D-A-D-S structured NIR-II fluorophore IR-E1 was reported in 2016.86 The BBT acceptor and thiophene/EDOT donor were used to construct the D-A-D structure to endow the fluorophore a low Eg. Meanwhile, EDOT as the π-bridge between the thiophene and BBT can result in molecular distortion and decrease the intermolecular aggregation. The 2,6-alkoxyl-substituted benzene was also applied as the shielding unit to prevent the interaction with water molecules. Furthermore, IR-E1 showed a higher fluorescence QY of 0.07% than CH1055 (0.03%) and was applied for NIR-II fluorescence imaging of cerebrovascular injury in a mouse model with traumatic brain injury (Figure 1E).
Based on IR-E1, NIR-II fluorophore IR-BGP6 was developed by replacing the donor unit with tert(ethylene glycol) (TEG)-substituted thiophene. Due to the introduction of TEG-substituted thiophene, IR-BGP6 obtained good water solubility and rapid renal excretion capacity (∼91% excretion within 10 h). The inherent S-D-A-D-S structure made it exhibit a relatively high fluorescence QY (∼0.15%). IR-BGP6 was further conjugated with the programmed cell death ligand-1 monoclonal antibody (PD-L1 mAb) for targeted imaging of MC38-overexpressed immune checkpoint PD-L1. This work provides a practical way to construct NIR-II fluorophore with rapid renal excretion ability for molecular imaging in vivo.87
In another work, TEG-substituted thiophene was introduced as a donor unit again. In addition to distorting the π-conjugated backbone, TEG-thiophene could also increase the dihedral angle between donor and acceptor in comparison to triphenylamine, EDOT, and thiophene units. The dialkyl chain-substituted fluorene could act as the protecting group to avoid intermolecular interactions. Fluorophore IR-FGP showed an enhanced fluorescence QY of 0.19% in an aqueous solution.88 When coupled with a dibenzocyclooctyne (DBCO)-PEG4-NHS ester connector, the -N3 group on the fluorophore could conjugate a protein or an antibody for 3D-staining of ∼170 μm histological brain tissues sections (Figures 1F and 1G).
Although these efforts have achieved significant progress, there still has much space to improve the fluorescence QY in aqueous solutions. Inspired by IR-E1 and IR-FGP, EDOT and dialkyl fluorene were introduced into the BBTD backbone to develop the NIR-II fluorophores IR-FE/IR-FEP.89 Another two families of fluorophores IR-FT/FTP and IR-BBE/BBEP were prepared as well. IR-FE and IR-FEP presented the fluorescence QY of 3.1% in toluene and 0.2% in the aqueous solution, respectively. Compared with IR-FTP, the fluorescence QY of IR-FEP in aqueous solution was significantly improved by 100-fold. This work has confirmed that both EDOT and the shielding unit of fluorene can improve fluorescence QY in aqueous solution, and EDOT is superior to thiophene for the fluorescence QY enhancement.
Based on IR-FTP, S-D2-D1-A-D1-D2-S structured fluorophore IR-FTAP was prepared by introducing octylthiophene donor as the first donor between the second donor thiophene and BBTD acceptor.90 Thiophene as the second donor could extend the conjugation length, which would cause red-shift but lower fluorescence QY. Compared with the π-bridge units previously studied, such as thiophene, TEG-thiophene, and EDOT, the bulky and hydrophobic octylthiophene group could cause a larger conjugated backbone distortion. Thus, IR-FTAP exhibited an emission peak at 1048 nm and fluorescence QY of 0.53% in aqueous solution. The fluorescence QY of IR-FTAP was higher than all their previously reported water-soluble S-D-A-D-S structured NIR-II fluorophores. Using IR-FTAP, the fast (>25 frames/s) NIR-II imaging with high resolution was realized.
To construct S-D-A-D-S structure is an effective strategy to improve fluorescence QY in aqueous solution. However, the absorption coefficient of these fluorophores is not very high, which restricts their brightness for NIR-II fluorescence imaging. To address this issue, Ma et al. reported another NIR-II fluorophore IR-FP8P by introducing a novel donor unit, dioctyl chains substituted 3,4-propylenedioxy thiophene (PDOT).91 Compared with the octylthiophene of IR-FTAP, PDOT donor had less capability to distort the conjugated backbone. Thus, IR-FP8P exhibited a red-shifted absorption peak and a larger absorption coefficient. Meanwhile, the dioctyl chains could protect the backbone from the attack of water molecules to improve the fluorescence QY. IR-FP8P with an emission peak at 1,040 nm presented a fluorescence QY of 0.6% and a peak absorption coefficient of 1.3 × 104 M−1⋅cm−1 in aqueous solution. Compared with IR-FEP and IR-FTAP, the brightness of IR-FP8P increased 5.7-fold and 3.7-fold, respectively. Owing to the high brightness, IR-FP8P could be applied for video-rate NIR-II imaging at 1,300 nm long-pass filter.
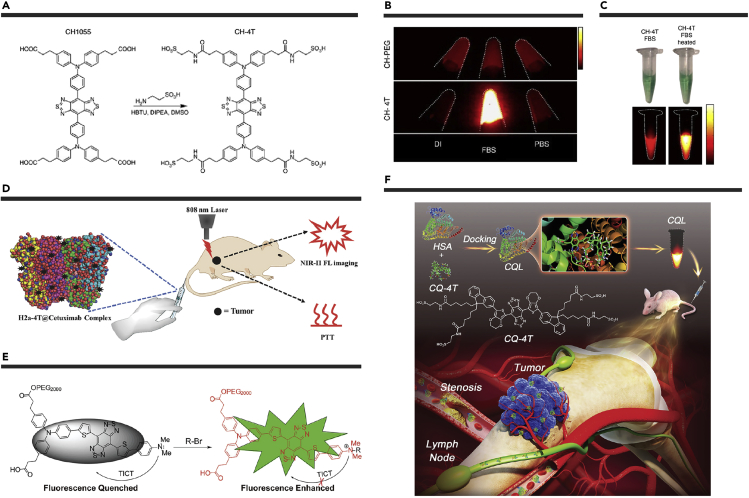
The coating strategy of fluorophore-protein complex formation can also improve the brightness of NIR-II fluorophores. In 2017, Antaris et al. replaced the flanked carboxyl groups of CH1055 with sulfonate to develop a new NIR-II fluorophore CH-4T (Figure 2A).92 Due to the negatively charged sulfonate groups, CH-4T could bind to serum proteins via supramolecular self-assembly. The fluorescence intensity of supramolecular assemblies (QY of ∼0.5%) was dramatically increased by ∼50-fold compared with that of CH-4T in PBS (Figure 2B). Simply heating to 70°C for 10 min, the fluorescence QY of the CH-4T-protein complexes could be boosted from 0.5% to 1.1% (Figure 2C). This may be attributed to the fact that heating could promote the exposure of CH-4T to hydrophobic regions inside the serum protein. Under heating conditions, the brightness of CH-4T/fetal bovine serum (FBS) was significantly improved by 110-fold compared with CH-4T/PBS. CH-4T/FBS enabled the ultrafast NIR-II fluorescence imaging with 50 frames/s and ∼1.5–2 ms exposure times, which was used to resolve cardiac cycles of mice.
Figure 2.
Construction and Biological Applications of Fluorophore-Protein Complexes
(A) Synthesis of CH-4T.
(B) NIR-II fluorescence images of CH-PEG and CH-4T in deioni1zed water, FBS, and PBS.
(C) Photographs and corresponding NIR-II fluorescence images of CH-4T in FBS and heated FBS. Reproduced with permission from Antaris et al.92 Copyright 2017 Springer Nature.
(D) Schematic of H2a-4T@Cetuximab complex used in NIR-II fluorescence imaging and PTT. Reproduced with permission from Zeng et al.93 Copyright 2018 Wiley-VCH.
(E) The schematic explanation for fluorescence enhancement by introducing quaternary ammonium salts. Reproduced with permission from Qu et al.94 Copyright 2019 Wiley-VCH.
(F) Schematic of CQ-4T/HSA complex for multifunctional biomedical imaging and surgical navigation in the NIR-II area. Reproduced with permission from Li et al.95 Copyright 2019 Wiley-VCH.
Inspired by this work, another sulfonated NIR-II fluorophore H2a-4T was reported by Zeng et al.93 Compared with H2a-4T in water, the fluorescence intensity of H2a-4T@FBS was significantly enhanced by approximately 10-fold at room temperature. The fluorescence QY of H2a-4T@FBS could be boosted to ∼0.11% after heating to 70°C for 10 min. Further, the H2a-4T@Cetuximab complex was developed for antibody-targeted imaging-guided PTT of colon cancer (Figure 2D). In another work, the fluorophore Q8 was modified with PEG2000 to improve water solubility.94 However, the pronounced twisted intramolecular charge transfer (TICT) effect weakened the fluorescence signals. To mitigate the TICT effect, the N, N-dialkylamino unit of Q8P was converted to quaternary ammonium salts (Figure 2E). After mixed with FBS, the fluorescence QY was dramatically enhanced ∼32-fold. Li et al. also reported a sulfonated NIR-II fluorophore CQ-4T and mixed it with human serum albumin (HSA).95 Compared with CQ-4T alone, the complexes of CQ-4T/HSA showed better optical performances and a 6.65-fold enhancement in fluorescence intensity. Because of the excellent brightness and optical properties of CQ-4T/HSA, the in vivo real-time imaging of circulatory system-related disease processes and precise surgery under imaging navigation was achieved (Figure 2F).
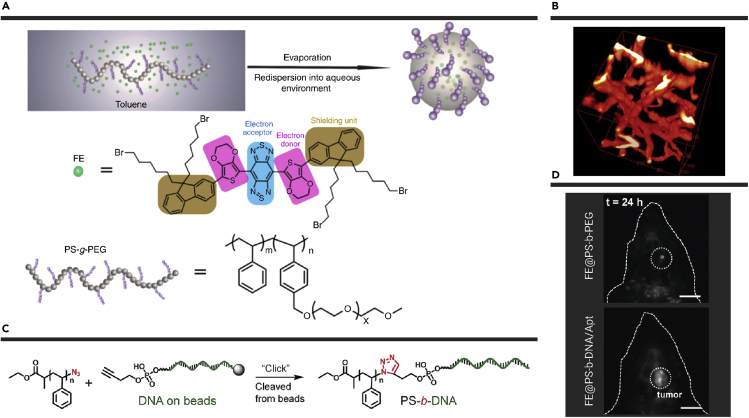
Besides to the serum proteins, Wan et al. also innovatively designed a novel amphiphilic polymer matrix (PS-g-PEG) to increase fluorescence QY in aqueous solutions (Figure 3A).96 The previously reported IR-FE showed a high fluorescence QY of 3.1% in toluene. The hydrophobic interior of PS-g-PEG with a phenyl group could imitate an environment similar to the toluene solvents. Meanwhile, the hydrophilic PEG chains on the polymer matrix could provide good water solubility and biocompatibility. After encapsulating IR-FE with PS-g-PEG, the fluorophore (p-FE) presented an unprecedented high fluorescence QY of ∼1.65% in aqueous solution. This high brightness fluorophore was applied to single-photon-based 3D confocal imaging with an imaging depth up to ∼1.3 mm and a high spatial resolution of sub-10 μm (Figure 3B). Moreover, the fast NIR-II imaging of blood flow in brain vessels was achieved with 2 ms exposure time. This work offers an extremely effective strategy to retain high fluorescence QY in aqueous solutions.
Figure 3.
Construction and Biological Applications of FE-based Nanoparticles
(A) Chemical structures of FE and PS-g-PEG and the formation of p-FE.
(B) 3D reconstruction of vasculatures in brain by using p-FE. Reproduced with permission from Wan et al.96 Copyright 2018 Springer Nature.
(C) Synthesis of PS-b-DNA polymer.
(D) Comparison of mouse brain glioblastoma imaging by using FE@PS-b-PEG and FE@PS-b-DNA/Apt. Scale bars: 0.5 cm. Reproduced with permission from Xiao et al.98 Copyright 2020 Wiley-VCH.
Similar to this, another NIR-II fluorophore TPB-AZO also showed a high fluorescence QY of ∼2.159% in toluene.97 Encapsulated by PS-g-PEG, TPB-AZO presented fluorescence QY of ∼1.562% within 850 to 1,500 nm and ∼0.351% within 1,000–1,500 nm in aqueous solution. As demonstrating the effectiveness of PS-g-PEG encapsulation to impart a non-polar toluene-like circumstance to impede ACQ for fluorescence QY enhancement. TPB-AZO NPs was used for fast imaging (>26 frames/s) of heart rate and cardiac cycle of mice.
Another amphiphilic DNA block copolymer (PS-b-DNA) was reported by Xiao et al. (Figure 3C).98 Through self-assembly, PS-b-DNA could form the micelle structure with many typical features of spherical nucleic acid (SNA). The PS-b-DNA SNA was capable of the blood-brain barrier (BBB)-crossing via cell membrane-bound scavenger receptor-mediated endocytosis. To further increase the accumulation in brain tumor sites, a targeting aptamer was taken to hybridize with DNA in PS-b-DNA. Finally, the nanofluorophore was prepared via loading IR-FE into the hydrophobic core. Compared with IR-FE encapsulated by the normal PS-b-PEG, the fluorescence intensity of this aptamer-modified PS-b-DNA nanofluorophore at the brain glioblastoma site showed a 3.8-fold enhancement (Figure 3D). This innovative work provides an excellent strategy to overcome the obstacles of BBB, opening new insights for the diagnosis and treatment of brain tumors.
Given the advantages of NIR-II fluorescence imaging in disease diagnosis, Zhang et al. developed a multifunctional DD-A-DD structured fluorophore SYL.99 SYL NPs with an average size of ∼120 nm showed an absorption and emission peak at ∼765 nm and ∼976 nm, respectively. Under the 808 nm laser irradiation, SYL NPs presented a photothermal conversion efficiency of ∼21.8% and were used for dual-modal NIR-II fluorescence/photoacoustic imaging-guided PTT for tumors.
At present, several practical strategies have been developed to reduce the aggregations of the fluorophore molecules. Nonetheless, most NIR-II fluorophores still suffer from the severe aggregation-induced quenching (ACQ) effect. Opposite to the ACQ effect, an AIE concept was put forward by Tang et al., in 2001.100 By reducing non-radiative transitions via physically restricting intramolecular rotation, aggregation-induced emissive luminogens (AIEgens) usually present enhanced fluorescence upon aggregates.101 Based on this, some high brightness NIR-II AIE dots have been designed and prepared (Table 1). For example, Tang et al. reported an AIEgen BPN-BBTD by taking N, N-diphenylnaphthalen-1-amine (BPN) as the donor unit.102 Under the 785 nm laser irradiation, the ultra-stable BPN-BBTD dots showed fluorescence QY of ∼0.18% in the NIR-II region and photothermal conversion efficiency of 39.8%. Moreover, BPN-BBTD dots could monitor the tumor progression for a long duration of 32 days, paving the way for the future application of real-time monitor of tumor treatment and metastasis.
Besides, N, N-diphenyl-4-(1,2,2-triphenylvinyl)aniline (DPTPEA) was proposed by Sheng et al. as the Donor unit to design an AIEgen TB1.103 The TB1 dots presented a large absorptivity of 10.2 L g−1·cm−1 at 740 nm, and showed an emission peak at 975 nm with fluorescence QY of 0.62%. Then, c-RGD peptide was further decorated to prepare the tumor-specific TB1-RGD dots. It is applied for targeted NIR-I PAI and NIR-II fluorescence imaging of brain tumors with an SBR of 4.4 and resolution up to 38 μm, indicating an excellent spatiotemporal resolution. Based on TB1, another fluorophore BTPPA was prepared via side-chain engineering.104 The introduction of an octyloxy side-chain impeded the strong intermolecular interaction, not only improving the solubility of BTPPA in organic solvents but also enhancing the fluorescence QY of BTPPA in aggregated status. Comparing to TB1 dots, BTPPA dots showed a higher fluorescence QY of 0.99% in water. Xu et al. reported a novel D-π-A-π-D type AIEgen XA1 with triphenylamine as Donor and tetraphenylethene as the molecular rotor.105 With more twisted molecular conformations to prevent intermolecular π-π interaction, the fluorescence QY of XA1 dots was determined to be 1.48% in aqueous solution, which was the highest among all the NIR-II AIE dots at present.
In the wavelength range of 1,400 to 1,500 nm, water and tissues have strong absorption of NIR light.106 To avoid this, the NIR-II imaging window is further divided into three sub-regions: NIR-IIa’ (1,000–1,300 nm), NIR-IIa (1,300–1,400 nm), and NIR-IIb (1,500–1,700 nm). Recently, several NIR-II AIEgens with both long emission wavelength and high fluorescence QY have been exploited to further suppress photo-scattering and autofluorescence for deeper tissue penetration depth and higher resolution (Table 1).
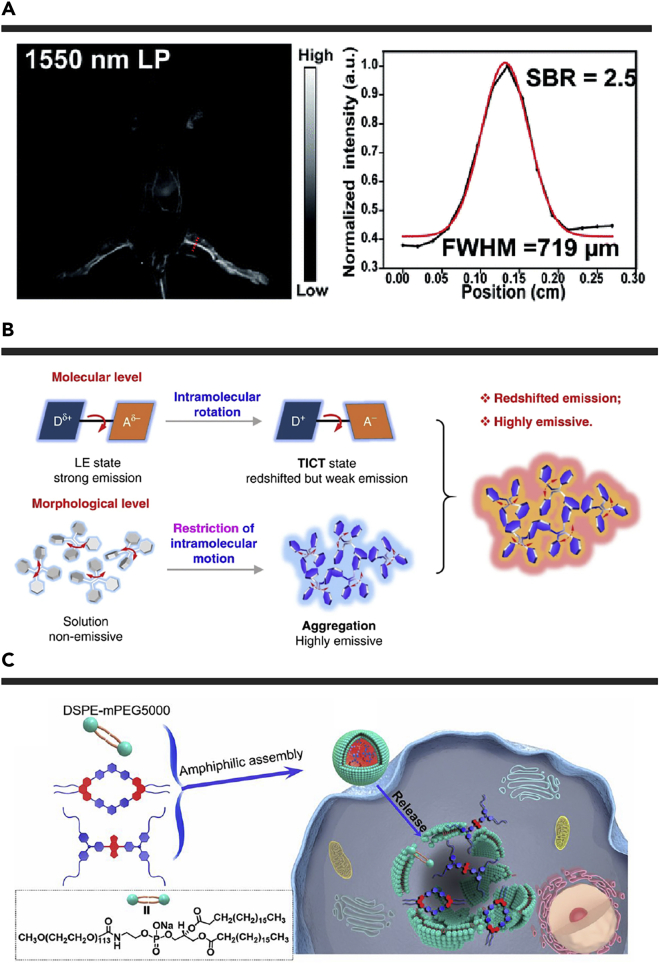
Based on Q4, Li et al. developed a highly twisted AIEgen HQL2 (twist angle of 50.9°) by modifying the dodecyl hydrocarbon chain at different positions of the thiophene spacer.107 HOL2 with higher brightness showed an emission peak at ∼1,050 nm with the emission profile extended to 1,600 nm. The fluorescence QY of HOL2 dots in aqueous solution was determined to be 1.19% in the NIR-II regime, and 0.016% beyond 1,300 nm, 0.002% beyond 1,550 nm. The SBR of fluorescence imaging in the NIR-IIa region was 2.5-fold higher than that in the NIR-IIb region, which was mainly owing to the low fluorescence QY in the NIR-IIb region. To optimize the quality of NIR-IIb fluorescence imaging, another highly twisted NIR-II fluorophore HL3 was prepared with the dihedral angle of 45.5° between acceptor BBT and donor 3,4-bis(hexyloxy)thiophene.108 The HL3 dots showed a high fluorescence QY of 1.17% in the NIR-II region and 0.005% in the NIR-IIb region in aqueous solutions. Comparing to HOL2 dots, the resolution of NIR-IIb fluorescence imaging based on HL3 dots was improved due to the higher fluorescence QY in the NIR-IIb region (Figure 4A).
Figure 4.
Applications of Small Molecular NIR-II Fluorophores in NIR-IIb Imaging and Drug Delivery Monitoring
(A) NIR-IIb fluorescence images of whole mouse blood vessels at 1,550 nm long-pass filters using HL3 dots. Reproduced with permission from Li et al.108 Copyright 2020 Royal Society of Chemistry.
(B) The design strategy of small molecular NIR-IIb fluorophores. Reproduced with permission from Li et al.109 Copyright 2020 Springer Nature.
(C) Schematic of the cellular uptake of self-assembled nanoprobe. Reproduced with permission from Sun et al.111 Copyright 2019 National Academy of Sciences.
Li et al. also elaborated on the design principles of these NIR-IIb AIEgens at the molecular and morphological levels (Figure 4B). Combined with the TICT and AIE effects, another three twisted AIEgens with different flexible hydrocarbon chains on the thiophene unit were developed.109 Among them, 2TT-oC26B dots presented a fluorescence tail extending to 1,600 nm with a fluorescence QY up to 1.15% in the entire NIR-II region and fluorescence QY of 0.012% in the NIR-IIb region. By employing 2TT-oC26B NPs, the NIR-IIb fluorescence imaging displayed a higher imaging resolution and improved SBR. The innovative work provides an extremely effective way for the development of small organic molecules with high brightness and ultra-long emission wavelength.
BSBT-based NIR-II Fluorophores
The selenium-substituted analog of BBT building block, selenadiazolo[3,4-f]benzo[c][1,2,5] thiadiazole (BSBT), is another strong electron-acceptor, which can also be used to construct NIR-II fluorophores through the D-A-D strategy. The heavier selenium (Se) atoms substitute one sulfur atom of BBT acceptor can realize a pronounced red-shift in absorption and emission bands (Table 1).
Zhang et al. reported a multifunctional BSBT-based fluorophore SY1080.110 To solve the blue-shift problem caused by the introduction of the EDOT unit, a selenium atom was introduced to regulate the brightness and emission wavelength. SY1080 showed an emission peak at 1,080 nm, and the NIR-II fluorescence signal was higher than H1 in THF. The fluorescence QY of SY1080 was determined to be 0.15% in THF. Around 820 nm, SY1080 presented a strong fluorescence absorption peak, which suggested it could convert a part of optical energy into heat for cancer therapy. To obtain water solubility and biocompatibility, SY1080 NPs were prepared with a photothermal conversion efficiency of ∼22.3%, and applied for NIR-II fluorescence/photoacoustic imaging-guided PTT of the tumor.
Traditional drug chemotherapy is nonspecific and has toxic side effects on normal tissues and cells. To solve this problem, they also designed another integrated diagnostic and therapeutic nano-agent by encapsulating the Pt(II) metallacycle and BSBT-based NIR-II fluorophore SY1100 into DSPE-mPEG5k (Figure 4C).111 Under the tracking of NIR-II FLI, the nano-agent could selectively deliver the Pt(II) metallacycle to the tumor sites via the enhanced permeability and retention (EPR) effect and presented a better anti-tumor activity than cisplatin alone. This strategy of simultaneously loading chemotherapy drugs and imaging molecules into the carrier can not only achieve effective treatment to reduce side effects, but also monitor the size of the tumor in real-time.
Cyanine-based NIR-II Fluorophores
Cyanine fluorophores start with the traditional NIR-I fluorophore ICG, which has been approved by the U.S. Food and Drug Administration (FDA) for clinical applications in humans. With a tail emission extending to the NIR-II region, ICG has been applied to NIR-II fluorescence imaging.112,113 Besides ICG, some other cyanine fluorophores with the primary emission in the NIR-I window, such as IR-12N3, IRDye800CW, IR-783, and IR-820, also have the potential for NIR-II fluorescence imaging (Table 1).114, 115, 116 Although these fluorophores could be employed for NIR-II fluorescence imaging, the NIR-II emission is not very ideal, which limits the further improvement of NIR-II fluorescence imaging quality. Thus, certain cyanine-based NIR-II fluorophores have been exploited by extending π-conjugation or embedding heterocycle substitutions (Table 1).117
The NIR-II polymethine fluorophore Flav7 was first reported in 2017 by introducing the 7-dimethylamino flavylium heterocycle (Table 3).118 Flav7 showed the absorption and emission peaks at 1,026 nm and 1,045 nm, respectively. Under the 808 nm laser irradiation, Flav7 presented a 13-fold higher brightness than that of IR-26 in DCM. This pioneering work opens the door for the development of cyanine-based NIR-II fluorophores. Inspired by the structure of ICG, a polymethine thiopyrylium fluorophore 5H5 was reported by Hong's group in 2018.119 By introducing sulfur atoms into the polymethine backbone, 5H5 showed an absorption peak at 1,069 nm, an emission peak at ∼1,125 nm and a shoulder emission peak at ∼1,260 nm. Moreover, the tail emission of 5H5 extended to 1,400 nm. By using 5H5, NIR-IIa fluorescence imaging under 1,064 nm laser irradiation was readily achieved.
Table 3.
Cyanine-, BODIPY-, DPP-, Squaraine-based NIR-II Fluorophores and the Other NIR-II Organic Small Molecules
| Fluorophore | Hydrophilic Modification | Targeted Modification | λAbs/λem (nm) | QYa | Imaging Strategy | Therapeutic Mode | Biological Applications | Ref |
|---|---|---|---|---|---|---|---|---|
| Flav7 | mPEG-DSPE | / | 1,026/1,045 | 0.53% | FLI | / | Hindlimb imaging | Cosco et al.118 |
| 5H5 | PEGylated | cRGDfk conjugate | 1,069/1,125 | 0.26% | FLI | / | Blood pool, tumorous microvasculature, and tumor imaging | Ding et al.119 |
| FD-1080 | FBS | / | 1,064/1,080 | 0.31%; 5.94% in serum |
FLI | / | Hindlimb vasculature, abdomen, and brain vessel imaging | Li et al.120 |
| FD-1080 J-aggregates | DMPC | / | 1,360/1,370 | 0.0545% | FLI | / | Hindlimb and brain vasculature imaging, dynamic vascular imaging | Sun et al.121 |
| CX-1 | DSPE-mPEG2k | / | 883/920 | 0.66% | FLI | / | Lymphatic imaging, APAP-induced hepatotoxicity detection | Lei et al.122 |
| CX-2 | 981/1,032 | 0.45% | ||||||
| CX-3 | 1,089/1,140 | 0.091% | ||||||
| BTC980 | DOPE-mPEG | / | 932/980 | 0.22% | FLI | / | Lymphatic imaging, ratiometric imaging, and gastric pH quantification | Wang et al.123 |
| BTC982 | 944/982 | 0.30% | ||||||
| BTC1070 | 1,014/1,070 | 0.016% | ||||||
| IRBTP-B/IRBTP-O | DSPE-mPEG2k | / | 575 or 905 />1,000 |
0.10% in methanol | Activatable FLI | / | ONOO− detection, monitoring drug-induced hepatotoxicity | Li et al.125 |
| Hydro-1080/Et-1080 | / | / | 1,039/1,080 | 0.045% | Activatable FLI | / | ·OH detection, detecting hepatotoxicity caused by drug overdose | Feng et al.126 |
| IR-1060/HISSNPs | HA conjugate | / | major peak at 810, shoulder peak at 1,050/1,070 | N.A. | Activatable FLI | / | Cell and tumor imaging | Tang et al.127 |
| QT-RGD | / | cRGD peptides conjugate | 841/1,068 | 0.12% | FLI/PAI/SPECT | PTT | Multimodal imaging-guided PTT of tumor | Zhao et al.129 |
| NJ960 | F127 | / | 651 or 799/960 | N.A. | FLI | / | Hindlimb, vessel, and brain imaging | Bai et al.135 |
| NJ1030 | 668 or 830/1,030 | |||||||
| NJ1060 | 672 or 910/1,060 | |||||||
| SWIR-WAZABY-01 | Ammonium groups onto the boron atom | / | N.A./emission between 720 and 1,200 in mouse serum | 0.25% in plasma | FLI | / | Cell and tumor imaging | Godard et al.136 |
| ZX-NIR/NIRII-HS | mPEG-DSPE | / | N.A./900 | N.A. | Activatable FLI | / | H2S detection, cell and tumor imaging | Xu et al.137 |
| BOD-M-βgal/BOD-M-SH | / | / | N.A. | N.A. | Activatable FLI | / | βgal activatable, cell and tumor imaging | Chen et al.138 |
| WD-CH3 | / | / | 768/960 | N.A. | FLI | / | Tracking the variation of viscosity in diabetes-induced liver injury | Dou et al.139 |
| WD-NO2 | 818/982 | 0.16% in glycerol | ||||||
| WD-OCH3 | 830/1,024 | N.A. | ||||||
| WD-NME2 | 850/1,070 | N.A. | ||||||
| DPP-BDT | DSPE-PEG5k | / | 625/980 | N.A. | FLI/PAI | PTT/PDT | Dual-modal imaging-guided PTT/PDT of tumor | Wang et al.142 |
| DPP-BT | DSPE-PEG-FA | / | 686/1,089 | N.A. | FLI/PAI | PTT/PDT/chemotherapy | Dual-modal imaging-guided PTT/PDT/chemo combination therapy of tumor | Wang et al.143 |
| SQP J-aggregates | F-127 | / | N.A./N.A. 1,100 | N.A. | FLI | PTT | Vessel, brain, and body imaging; imaging-guided PTT | Sun et al.147 |
| SQ1 | DSPE-PEG2000 | Decorated with penta-peptide CREKA | 930/970 | 0.17% | FLI/PAI | PTT | Vessel imaging and imaging-guided PTT of tumor | Yao et al.148 |
| CPTIC-4F | DSPE-mPEG | / | 810 or 876/1,110 | 0.39% (900–1,400 nm); 0.33% (1,000–1,400 nm); 0.015% (1,300–1,400 nm) | FLI | / | Whole-body vessels imaging | Zhu et al.149 |
| NDI-NA J-aggregates | DSPE-mPEG2000 | / | 904/1,020 | 0.21% | FLI | PTT/PDT | Imaging-guided PTT/PDT of tumor | Tang et al.150 |
| IPIC | TPP-PEG-PPG-PEG-TPP | / | N.A. | 0.22% | FLI | PTT/PDT | Imaging-guided mitochondria-targeting PTT/PDT of tumor | Wang et al.151 |
| TSSI | DSPE-mPEG2000 | / | N.A. | N.A. | FLI/PAI/PTI | PTT/PDT | NIR-II FLI/PAI/PTI trimodal imaging-guided PDT/PTT synergistic therapy of tumor | Zhang et al.152 |
| bis-ACP | / | / | 894/1,029 | / | / | / | / | Lavaud et al.153 |
| CSM2 | F-127 | / | 878/N.A. | / | PAI | PTT | imaging-guided NIR-II PTT of tumor | Shao et al.154 |
| Zn4-H2 [Pc(OC12H17)24] | DSPE-PEG2k-OCH3 | / | 1,064/N.A. | / | PAI | PTT | imaging-guided NIR-II PTT of tumor | Pan et al.155 |
| IR-SS | DSPE-PEG | / | 1,060/N.A. | / | PAI | PTT | imaging-guided NIR-II PTT of tumor | Li et al.156 |
Fluorescence quantum yields of all small molecular NIR-II fluorophores mentioned in this review were recalculated based on IR26 = 0.05% (in 1,2-dichloroethane).
In 2018, cyanine fluorophore FD-1080 with heptamethine structure was prepared and presented both NIR-II absorption and fluorescence.120 To improve the water solubility and stability of this fluorophore, the sulfonic and cyclohexene groups were further introduced. FD-1080, with a small molecular mass of 760 Da, was capable of fast kidney metabolism and presented an absorption and emission peak at 1,064 nm and 1,080 nm, respectively. The fluorescence QY of FD-1080 was 0.31% in ethanol. After encapsulation by FBS to form a complex, the fluorescence QY could increase to 5.94%. Compared with the common NIR excitation, the imaging under NIR-II excitation presented a deeper tissue penetration and higher resolution. FD-1080 could not only perform high-performance hindlimb and brain vessel imaging, but also quantify the respiration frequency of mice by dynamic imaging (7.16 frames/s). Furthermore, the J-aggregates were prepared through self-assembly between 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) lipids and FD-1080.121 The FD-1080 J-aggregates presented an absorption and emission peak at 1,360 nm and 1,370 nm, respectively. Using the J-aggregates, the NIR-IIb dynamic imaging of hindlimb vessels and brain was achieved and showed a superior SBR and high resolution.
Besides FD-1080 and its J-aggregates, a panel of novel polymethine dyes CX-1, CX-2, and CX-3 were prepared by extending the π-conjugation length of the backbone.122 With the increase of the π-conjugated C=C units, pronounced red-shift of both absorption and fluorescence was observed. By using CX-1 and CX-3, peroxynitrite (OONO−) could be detected via Förster resonance energy transfer (FRET) process in the acetaminophen (APAP)-induced liver injury model.
To address the problem of solvatochromism-caused quenching in water, Wang et al. also developed another two photo-stable pentamethine cyanine fluorophores, BTC982 and BTC1070, with the anti-quenching behavior.123 By changing the position of the diethylamino (-NEt2) groups on the phenyl ring from the meta-position to the para-position, a pronounced red-shift could be realized. In comparison to IR26 in aqueous solution, BTC1070 and BTC982 showed ∼7-fold and ∼44-fold enhanced brightness, respectively. As the pH changed from 1 to 4, the ratiometric fluorescence of BTC1070 could change from 1,065 nm to 980 nm. By taking BTC1070, the gastric pH assessment at 4 mm depth was realized, and reliable accuracy was comparable to that of the standard pH meter.
At present, most NIR-II fluorophores, whether with passive tumor-targeting or active tumor-targeting behavior, belong to the "always-on" type. This type of "always-on" fluorophores produces fluorescent signals all the time, whether it is in the lesion or accumulated in normal tissues. The "always-on" fluorescent signal will cause a low target-to-background ratio or even false positive, which is a severe problem for biosensing, disease diagnosis, and cancer treatment.124 In this perspective, "turn-on" type fluorophores are in urgent need for both academy and industry. Li et al. developed an ONOO− activatable NIR-II fluorophore IRBTP-B via combining the benzothiopyrylium cyanines backbone with the phenyl borate.125 Under the activation of ONOO−, the NIR-II fluorescence could be lighted up when IRBTP-B converted into another fluorophore IRBTP-O by eliminating the phenyl borate unit (Table 1). Further research showed the high selectivity toward ONOO−, since some other in vivo factors, such as biothiols, ROS, and reactive nitrogen species had almost no effect on this transformation process. In addition, the activated fluorescence signal could be detected at a depth of 5 mm. By using this fluorophore, ONOO− could be effectively and selectively detected to monitor the preclinical APAP-induced liver injury.
Feng et al. reported an ·OH-activatable NIR-II fluorescent probe Hydro-1080.126 Due to the low π-conjugation and non-planarity, Hydro-1080 showed a weak absorption in the visible region and even no fluorescence emission (Table 1). In the presence of ·OH, both π-conjugation and planarity of Hydro-1080 were significantly improved. The activated molecule Et-1080 showed an absorption and emission peak at 1039 nm and 1080 nm in DMSO. The ·OH-activatable probe could accurately and selectively identify the ·OH species. Moreover, the subtle changes of ·OH content in the liver injury model could be tracked under NIR-II fluorescence imaging.
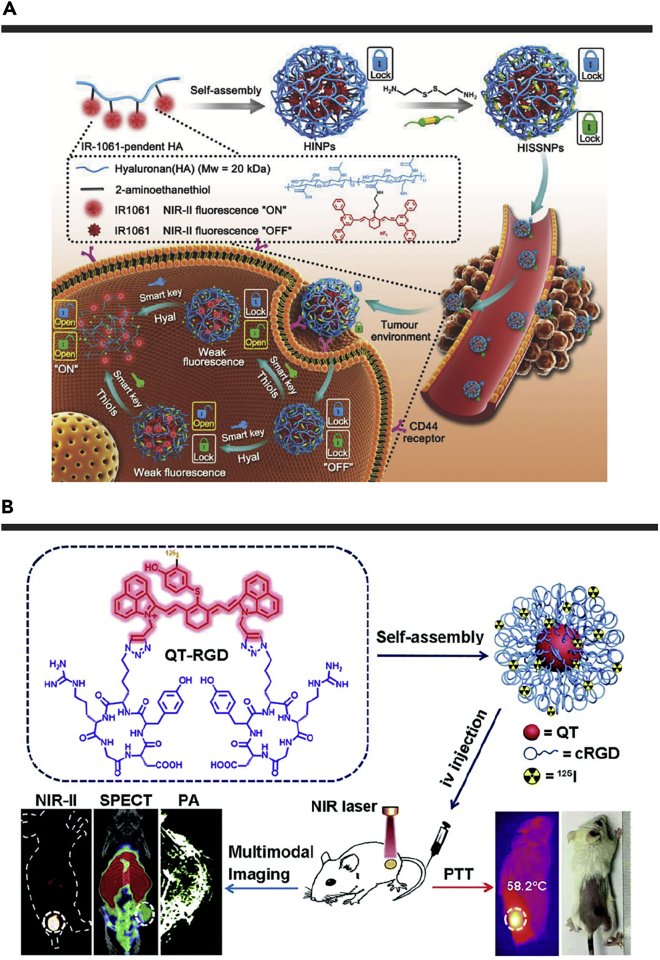
Besides the single pathological parameter activatable probes, Tang et al. reported a dual pathological parameter activatable nanoprobe (Figure 5A).127 First, 2-aminoethanethiol was used to link the hydrophilic hyaluronic acid (HA) and hydrophobic NIR-II fluorophore IR-1061. The single-factor response probe (HINPs) was developed through self-assembly. Then, HA was cross-linked by disulfide bond to form the dual-factor responsive probe HISSNPs. HA could not only specifically bind to glycoprotein on the surface of CD44 overexpressed tumor cells, but cooperate with disulfide to lock IR-1061. The locked fluorophores were in the aggregated states, resulting in the NIR-II fluorescence quenching. In the tumor microenvironment with overexpression of hyaluronidase and thiols, IR-1061 was released on-site to emit its NIR-II fluorescence. In the tumor-bearing mice model, HISSNPs presented a lower background, higher tumor-to-normal tissue ratio (15.4/3.3), as well as a higher tumor-to-liver ratio (5.87/1.1) than HINPs. HISSNPs significantly improved the imaging quality and more accurately distinguished normal tissues from diseased tissues.
Figure 5.
Fluorescence Activation Process and Multimodal Imaging of Small Molecular NIR-II Fluorophores
(A) Synthesis of “lock-and-key”-controlled tumor-specific imaging nanoparticles (HISSNPs) and its NIR-II fluorescence activation process. Reproduced with permission from Tang et al.127 Copyright 2018 Wiley-VCH.
(B) Self-assembly of QT-RGD and its applications in trimodal imaging and PTT of tumors in vivo. Reproduced with permission from Zhao et al.129 Copyright 2020 Royal Society of Chemistry.
Some integrated diagnostic and therapeutic cyanine-based NIR-II agents have also been developed. The macromolecular PF was prepared via conjugating NIR-II fluorophore Flav7 with an amphiphilic polypeptide.128 Through self-assembly, PF could form the water-soluble NPs by itself. Through the EPR effect and endothelial leakiness, PF NPs could effectively accumulate at the tumor site. Due to the good photothermal property of Flav7, the stable PF NPs presented a photothermal conversion efficiency of 42.3% under the 808-nm laser irradiation. The location, size, and ablation of the tumor could be clearly visualized through NIR-II FLI.
In addition to passive accumulation at the tumor sites via the EPR effect, another NIR-II probe QT-RGD with active tumor-targeting property was prepared by conjugating a125 I-modified NIR-II cyanine fluorophore and two cRGD peptides (Figure 5B).129 Due to the cRGD peptides, the probe could specifically bind to αvβ3 integrin for active tumor-targeting. Under the NIR laser irradiation, the probe was capable of three-mode NIR-II fluorescence imaging, PAI, and single-photon emission computed tomography (SPECT) imaging. Moreover, the good photothermal conversion efficiency (36.5%) enabled it as an excellent photothermal agent to ablate the tumors under 808 nm photoirradiation. This targeted probe integrates multiple imaging methods and phototherapy, which dramatically improves the accuracy of tumor diagnosis and the efficiency of cancer treatment.
BODIPY/aza-BODIPY-based NIR-II Fluorophores
Dipyrromethene boron difluoride (BODIPY)-derived fluorophores are widely used in the fields of NIR-I imaging and phototherapy due to the advantages of excellent chemical/photostability, high molar extinction coefficient, and low toxicity.130, 131, 132, 133 As a typical derivative of BODIPY, Aza-BODIPY has more prolonged absorption and emission wavelengths due to the replacement of the carbon atom at the meso-position by a nitrogen atom, resulting in pronounced red-shift.134 Recently, some NIR-II fluorophores based on BODIPY/Aza-BODIPY have been developed (Table 1).
Bai et al. developed three Aza-BODIPY-based NIR-II fluorophores NJ960, NJ1030, and NJ1060 via D-A-D strategy.135 Corresponding NPs of these novel fluorophores presented a large Stokes shift, excellent brightness, and superior photostability. These new Aza-BODIPY-based NIR-II fluorophores provided an excellent platform to further construct NIR-II fluorescent probes with better water solubility, higher fluorescence QY, and more extended NIR-II emission for clinical applications. Very recently, another water-soluble Aza-BODIPY SWIR-WAZABY-01 with a fluorescence tail into the NIR-II region was reported by Godard et al.136 Although this is the first report of NIR-II water-soluble aza-BODIPY fluorophore, the introduction of ammonium groups caused the blue-shift of the original fluorophore, making the NIR-II emission not perfect.
The "turn-on" type fluorophores have many advantages, such as high target-to-background ratio and excellent specificity. However, the development of such fluorophores is still challenging. Besides the Cyanine-based "turn-on" fluorophores, BODIPY-based "turn-on" NIR-II probes have also been exploited. For example, an H2S-activatable nanoprobe was prepared by encapsulating an H2S-responsive fluorophore ZX-NIR and an H2S-inert fluorophore aza-BOD with DSPE-mPEG and TBNBr.137 The fluorophore ZX-NIR could generate NIR-II fluorescence only when activated by H2S (Table 1). Another fluorophore aza-BOD acted as the internal reference to enable the nanoprobe to emit multiple wavelengths for ratiometric imaging. In vivo studies on animal models demonstrated that such water-soluble, biocompatible, and fast-responsive nanoprobe could accurately image and diagnose colorectal tumors with rich H2S. Another enzyme-activated probe BOD-M-βgal was prepared by employing a self-disappearing unit to link β-galactosidase (β-Gal) residues and BODIPY-based fluorophore (Table 1).138 Under the β-Gal activation, BOD-M-βgal showed an NIR-II emission range from 900 nm to 1,300 nm. The β-Gal-rich ovarian tumors could be quickly and accurately detected upon the NIR-II fluorescence imaging of this probe.
Besides the H2S-activatable and β-Gal-activatable probes, a series of viscosity-activatable NIR-II fluorophores were developed by introducing different substituents onto the BODIPY-based platform.139 Among them, WD-NO2 showed a relatively high fluorescence QY of 0.16% in glycerol, and it was very sensitive to viscosity variations but not sensitive to some other environmental factors, such as pH and polarity (Table 1). With the increase of environmental viscosity, the fluorescence intensity of WD-NO2 presented a 31-fold enhancement. Due to these characteristics, WD-NO2 was used to track the diabetes-induced liver injury process-related viscosity variations in vivo.
DPP-based NIR-II Fluorophores
DPP with planar lactam structure is a common electron-acceptor.140 Due to the existence of multiple reaction sites on the DPP segment, various DPP derivatives have been exploited with the advantages of high fluorescence QY, tunable photophysical properties, excellent thermal/photostability, and low dark toxicity.141 However, DPP-based NIR-II small molecular fluorophores are still very rare (Table 1).
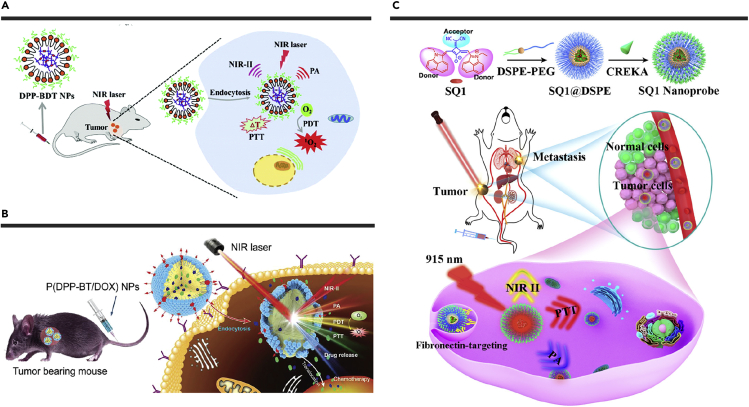
In 2019, Wang et al. reported a multifunctional NIR-II fluorophore DPP-BDT.142 The water-soluble and biocompatible DPP-BDT NPs were prepared via nanoprecipitation and showed good photostability. The maximum absorption and emission peak of DPP-BDT NPs were 625 nm and 980 nm, respectively. Under the 660 nm laser irradiation, DPP-BDT NPs exhibited a photothermal conversion efficiency of 23% and a 1O2 QY of ∼49.3%. In vitro and in vivo studies confirmed that these NPs had a high tumor treatment efficacy, benefiting from the combination of PTT and PDT under NIR-II fluorescence/photoacoustic dual-modal imaging navigation (Figure 6A).
Figure 6.
Integrated Diagnosis and Treatment of Small Molecular NIR-II Fluorophores
(A) Applications of DPP-BDT NPs in NIR-II fluorescence/photoacoustic imaging-guided cancer PDT/PTT. Reproduced with permission from Wang et al.142 Copyright 2019 Royal Society of Chemistry.
(B) Applications of P(DPP-BT/DOX) NPs in NIR-II fluorescence/photoacoustic dual-modal imaging-guided cancer PDT/PTT/Chemotherapy. Reproduced with permission from Wang et al.143 Copyright 2019 Wiley-VCH.
(C) Synthetic schematic diagram of SQ1 nanoprobe for NIR-II fluorescence/photoacoustic dual-modal imaging-guided PTT of metastatic breast cancer. Reproduced with permission from Yao et al.148 Copyright 2020 American Chemical Society.
In the same year, another multifunctional NIR-II fluorophore DPP-BT was reported.143 DPP-BT NPs with an absorption peak at 686 nm and emission peak at 1,089 nm could not only be capable of NIR-II fluorescence/photoacoustic dual-modal imaging but also both had photodynamic and photothermal properties. Under the 730-nm laser irradiation, DPP-BT NPs presented a 1O2 QY of ∼27.3% and high photothermal conversion efficiency of 50%. To further integrate chemotherapy, the single NIR laser triggered phototheranostic platform P(DPP-BT/DOX) NPs was developed via encapsulating DPP-BT, chemo-drug doxorubicin (DOX), and natural phase-change materials with the folic acid-modified amphiphilic polymer matrix and lecithin. Due to the combination of photothermal/photodynamic and chemotherapy, the single laser triggered NPs presented unprecedented anti-tumor performance (Figure 6B). This work provides an innovative way to design and construct multimodal diagnosis and treatment-integrated nanoplatforms for clinical cancer therapy.
Squaraine-based NIR-II Fluorophores
Squaraine dyes are a type of zwitterionic molecules featured with a unique four-membered ring π-conjugated skeleton derived from squaric acid. They are widely employed to construct fluorophores due to their intensive fluorescence and easily tunable bandgap.144 Especially, it can form J-aggregates via nanoprecipitation, which will cause red-shift or brightness enhancement145,146; however, the development of squaraines with long emission is still challenging (Table 1).
In 2018, Sun et al. reported a squaraine-based fluorophore SQP.147 The J-aggregated SQP-NPs(J) were prepared by encapsulating SQP into amphiphilic F127, showing high brightness with an emission peak of ∼1,100 nm. Compared with the H-aggregated SQP-NPs(H), the emission intensity of SQP-NPs(J) presented a 4.8-fold enhancement. SQP-NPs(J) presented a photothermal conversion efficiency of 36% under the LED lamp irradiation and was then used for NIR-II fluorescence imaging-guided PTT of MCF-7 tumor-bearing mice.
Based on the common squaraine, another squaraine-based D-A-D structured fluorophore SQ1 was synthesized by introducing a powerful electron-withdrawing unit, malononitrile, to improve the electron affinity, resulting in a strong ICT and a pronounced red-shift.148 As a result, the fluorescence spectrum of SQ1 reached the NIR-II region, with an absorption and emission peak at 930 nm and 970 nm, respectively. SQ1 NPs were prepared via nanoprecipitation and were then decorated with an active-targeting ligand peptide CREKA to form SQ1 nanoprobe (Figure 6C). After being coated with the amphiphilic polymer, fluorescence QY of SQ1 could be increased from 0.013% to 0.17%, which was attributed to the formation of J-aggregates in the hydrophobic interior of the polymer by SQ1 molecules. With the targeted peptide, the nanoprobe could specifically target fibronectin overexpressed in breast cancer. Under NIR-II fluorescence and photoacoustic dual-modal imaging, breast cancer and lung metastasis could be accurately detected. Under the 915-nm laser irradiation, SQ1 nanoprobe with a photothermal conversion efficiency of 25.6% had hyperthermia effects on tumors.
Other NIR-II Organic Small Molecules
In the past few years, small molecular NIR-II fluorophores have entered a new stage with rapid development. In addition to expanding the library of original fluorophores, it is still necessary to further exploit new types of NIR-II small molecular fluorophores (Table 1). Very recently, a bright fused-ring acceptor based NIR-II molecule CPTIC-4F has been reported.149 CPTIC-4F NPs presented an emission peak at 1,110 nm with a fluorescence QY of 0.39% and a high absorption coefficient of 14.5 × 104 M−1⋅cm−1. Another novel naphthalenediimide-based polycyclic fluorophore NDI-NA could form J-aggregates via nanoprecipitation, which caused a pronounced red-shift in both absorption and emission.150 Besides, NDI-NA NPs presented a photothermal conversion efficiency of 30.8%.
Moreover, a “one-for-all” probe by self-assembly of NIR-II molecule IPIC and triphenylphosphonium (TPP) modified amphiphilic polymer (TPP-PEG-PPG-PEG-TPP) was reported, the TPP group endowed the probe mitochondrial targeting property.151 Under the 808-nm laser irradiation, the probe showed fluorescence QY of 0.22% in aqueous solution and photothermal conversion efficiency of 39.6%. Meanwhile, it had singlet oxygen QY of 2.3%, which was approximately 12-fold higher than that of ICG. Recently, Zhang et al. also reported a “one-for-all” NIR-II AIEgen TSSI through the introduction of a new electron-acceptor of 1,3-bis(dicyanomethylidene)indane moiety for extremely strong ICT.152 With a balance between non-radiative and radiative transitions, TSSI NPs presented a high photothermal conversion efficiency of 46% and acceptable NIR-II fluorescence. Interestingly, TSSI NPs could generate ·OH, which suggested it could be used as a type I photosensitizer for PDT. And TSSI NPs succeeded in NIR-II fluorescence/photoacoustic/photothermal trimodal imaging-guided synergistic PDT/PTT. As brought up new viewpoints in the molecular design of multifunctional NIR-II fluorophores for tumor phototheranostics.
Up to now, many NIR-II fluorophores have been successfully used for phototheranostics; nevertheless, their absorption wavelength is mainly limited to the NIR-I region, which impedes the phototherapeutic efficacy of deep-seated tumors. Recently, several small molecules with NIR-II absorption, such as bis-ACP, CSM2, and Zn4-H2[Pc(OC12H17)24], have been reported to accomplish PAI-guided NIR-II PTT (Table 1).153, 154, 155 Among them, phthalocyanine-derived Zn4-H2[Pc(OC12H17)24] showed a high photothermal conversion efficiency of 58.3% under the 1,064-nm laser irradiation. Though these molecules had been successfully applied for NIR-II PTT, the photothermal conversion efficiency still needed to be improved. Very recently, an NIR-II photothermal agent IR-SS was developed via Se-tailoring strategy.156 By introducing two Se atoms into the BBT acceptor skeleton, a pronounced red-shift in absorption could be observed. IR-SS NPs presented an unprecedented high photothermal conversion efficiency of 77% under the 1,064-nm laser irradiation. This work opens a new insight into the design and development of high-performance NIR-II photothermal agents.
Conclusion and Outlook
Herein, we have systematically reviewed the recent advances in the preparation and application of organic small molecular NIR-II fluorophores and classified them based on the electron-withdrawing cores. NIR-II small molecular fluorophores with the advantages of good biocompatibility, structural adjustment, and excellent photophysical properties have been widely used in the biomedical field, such as bioimaging, surgical navigation, and imaging-guided phototherapy. Due to the high resolution, good SBR, and excellent lesion recognition ability, NIR-II FLI can accurately and real-time visualize various physiological and pathological processes. Significantly, the tumors can be removed more thoroughly and precisely by NIR-II FLI-guided surgery. The integration of NIR-II FLI and phototherapy has greatly further improved the therapeutic efficiency and accuracy of cancer treatment.
In addition to the existing success, the future development and application of NIR-II small molecular fluorophores still face some challenges in the following aspects.
Improving NIR-II Fluorescence Imaging Quality
Improving imaging quality usually revolves around the optimization of the fluorescence wavelength, fluorescence QY, and fluorescence brightness. At present, most strategies focus on improving the fluorescence QY to improve brightness, such as the construction of S-D-A-D-S structure or self-assembly with serum. The absorption coefficient is also the other factor for brightness optimization. Exploiting fluorophore molecules with both high fluorescence QY and the high absorption coefficient is still a challenge. Recently, NIR-IIa and NIR-IIb fluorescence imaging have greatly improved the imaging depth. However, fluorophores with such characteristics are still in their infancy. Thus, the development of fluorophores with great stability, high brightness, and long absorption/emission wavelength is still an important research direction in the future.
Exploiting Targeted, Activatable, and Multifunctional NIR-II Fluorophores
Currently, most NIR-II fluorophores passively accumulate at the tumor site via the EPR effect. However, this passive targeting way often results in a certain accumulation of fluorophores in normal tissues, which will cause poor target-to-background ratio or even false positive. Thus, the development of active-targeting and activatable probes is of great significance to accurately distinguish diseased tissues from normal tissues. To improve the efficiency of tumor treatment, it is also urgent to develop various probes with integrated diagnostic and therapeutic performance. At present, most multifunctional probes are used for imaging-guided PTT of tumors, and chemotherapy is still the main way for clinical cancer treatment. Therefore, the combination of NIR-II fluorescence imaging with photodynamic therapy, chemotherapy, or vascular-disruption therapy is of great importance to further improve the efficiency of cancer treatment. In our point of view, this is also a potential research direction with considerable research value.
Overcoming the BBB
The diagnosis and treatment of brain tumors have always been a difficult problem in biomedicine. This is mainly because the dense BBB can effectively block the penetration of contrast agents or probes. However, in current reports, NIR-II small molecular probes that can effectively cross the BBB are relatively rare. Thus, the development of NIR-II probes with BBB-crossing capability is of great significance for the diagnosis and treatment of brain tumors.
Accelerating Clinical Translation
Based on the FDA-approved ICG, NIR-II fluorescence imaging has been used in clinical surgical navigation. However, low fluorescence QY and poor fluorescence intensity of the traditional NIR-I fluorophore in the NIR-II region limit the clinical performance of NIR-II fluorescence imaging. The rapid excretion and nontoxic features of ICG are the key to its clinical translation. Unfortunately, none of the currently developed NIR-II fluorophores can well balance various properties to meet the requirement of the FDA. That is the main reason why no NIR-II fluorophore has been approved by the FDA for clinical application, as is also a huge challenge in the future. Accelerating the clinical translation of NIR-II fluorophores still has a long way to go.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (NNSFC) (61525402, 61775095), Natural Science Foundation of Jiangsu Province (BK20200092), Jiangsu Province Policy Guidance Plan (BZ2019014), Six talent peak innovation team in Jiangsu Province (TD-SWYY-009), and “Taishan scholars” construction special fund of Shandong Province.
Author Contributions
H.D., J.S., F.G., and X.D. contributed to the design and writing of this manuscript. Q.S. and W.W. gave suggestions of conceptual ideas and language improvements for the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published Online: January 18, 2021
Contributor Information
Jinjun Shao, Email: iamjjshao@njtech.edu.cn.
Fan Gao, Email: 003335@nuist.edu.cn.
Xiaochen Dong, Email: iamxcdong@njtech.edu.cn.
References
- 1.Yang Z., Chen X. Semiconducting perylene diimide nanostructure: multifunctional phototheranostic nanoplatform. Acc. Chem. Res. 2019;52:1245–1254. doi: 10.1021/acs.accounts.9b00064. [DOI] [PubMed] [Google Scholar]
- 2.Chen C., Ou H., Liu R., et al. Regulating the photophysical property of organic/polymer optical agents for promoted cancer phototheranostics. Adv. Mater. 2020;32:1806331. doi: 10.1002/adma.201806331. [DOI] [PubMed] [Google Scholar]
- 3.Li J., Zhen X., Lyu Y., et al. Cell membrane coated semiconducting polymer nanoparticles for enhanced multimodal cancer phototheranostics. ACS. Nano. 2018;12:8520–8530. doi: 10.1021/acsnano.8b04066. [DOI] [PubMed] [Google Scholar]
- 4.Li J., Pu K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 2019;48:38–71. doi: 10.1039/c8cs00001h. [DOI] [PubMed] [Google Scholar]
- 5.Saba L., Sanfilippo R., Montisci R., et al. Carotid artery wall thickness: comparison between sonography and multi-detector row CT angiography. Neuroradiology. 2010;52:75–82. doi: 10.1007/s00234-009-0589-5. [DOI] [PubMed] [Google Scholar]
- 6.Takami Y., Norikane T., Yamamoto Y., et al. A preliminary study of relationship among the degree of internal carotid artery stenosis, wall shear stress on MR angiography and 18F-FDG uptake on PET/CT. J. Nucl. Cardiol. 2020 doi: 10.1007/s12350-020-02300-3. [DOI] [PubMed] [Google Scholar]
- 7.Wang D., Li Z., Zheng X., et al. Head and neck CT angiography to assess the internal carotid artery stealing pathway. BMC. Neurol. 2020;20:334. doi: 10.1186/s12883-020-01915-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pichler B.J., Kolb A., Nagele T., et al. PET/MRI: paving the way for the next generation of clinical multimodality imaging applications. J. Nucl. Med. 2010;51:333–336. doi: 10.2967/jnumed.109.061853. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Garcia-Gabilondo M., Grayston A., et al. PLGA protein nanocarriers with tailor-made fluorescence/MRI/PET imaging modalities. Nanoscale. 2020;12:4988–5002. doi: 10.1039/c9nr10620k. [DOI] [PubMed] [Google Scholar]
- 10.Rischpler C., Siebermair J., Kessler L., et al. Cardiac PET/MRI: current clinical status and future perspectives. Semin. Nucl. Med. 2020;50:260–269. doi: 10.1053/j.semnuclmed.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Bezukladova S., Tuisku J., Matilainen M., et al. Insights into disseminated MS brain pathology with multimodal diffusion tensor and PET imaging. Neurol. Neuroimmunol. Neuroinflamm. 2020;7:691. doi: 10.1212/NXI.0000000000000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ming Y., Wu N., Qian T., et al. Progress and future trends in PET/CT and PET/MRI molecular imaging approaches for breast cancer. Front. Oncol. 2020;10:1301. doi: 10.3389/fonc.2020.01301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimitrakopoulou-Strauss A., Pan L., Sachpekidis C. Kinetic modeling and parametric imaging with dynamic PET for oncological applications: general considerations, current clinical applications, and future perspectives. Eur. J. Nucl. Med. Mol. Imaging. 2020 doi: 10.1007/s00259-020-04843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miao Q., Pu K. Organic semiconducting agents for deep-tissue molecular imaging: second near-infrared fluorescence, self-luminescence, and photoacoustics. Adv. Mater. 2018;30:1801778. doi: 10.1002/adma.201801778. [DOI] [PubMed] [Google Scholar]
- 15.Huang J., Xie C., Zhang X., et al. Renal-clearable molecular semiconductor for second near-infrared fluorescence imaging of kidney dysfunction. Angew. Chem. Int. Ed. 2019;58:15120. doi: 10.1002/anie.201909560. [DOI] [PubMed] [Google Scholar]
- 16.Tu L., Xu Y., Ouyang Q., et al. Recent advances on small-molecule fluorophores with emission beyond 1000 nm for better molecular imaging in vivo. Chin. Chem. Lett. 2019;30:1731–1737. [Google Scholar]
- 17.Zhang F., Lei Z. Molecular engineering of NIR-II fluorophores for improved biomedical detection. Angew. Chem. Int. Ed. 2020 doi: 10.1002/anie.202007040. [DOI] [PubMed] [Google Scholar]
- 18.Li C., Chen G., Zhang Y., et al. Advanced fluorescence imaging technology in the near-infrared-II window for biomedical applications. J. Am. Chem. Soc. 2020;142:14789–14804. doi: 10.1021/jacs.0c07022. [DOI] [PubMed] [Google Scholar]
- 19.Zhu S., Yung B.C., Chandra S., et al. Near-infrared-II (NIR-II) bioimaging via off-peak NIR-I fluorescence emission. Theranostics. 2018;8:4141–4151. doi: 10.7150/thno.27995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao J., Zhu B., Zheng K., et al. Recent progress in NIR-II contrast agent for biological imaging. Front. Bioeng. Biotechnol. 2019;7:487. doi: 10.3389/fbioe.2019.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J., Pu K. Activatable molecular probes for second near-infrared fluorescence, chemiluminescence, and photoacoustic imaging. Angew. Chem. Int. Ed. 2020;59:11717. doi: 10.1002/anie.202001783. [DOI] [PubMed] [Google Scholar]
- 22.Wan H., Du H., Wang F., et al. Molecular imaging in the second near-infrared window. Adv. Funct. Mater. 2019;29:1900566. doi: 10.1002/adfm.201900566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He S., Song J., Qu J., et al. Crucial breakthrough of second near-infrared biological window fluorophores: design and synthesis toward multimodal imaging and theranostics. Chem. Soc. Rev. 2018;47:4258–4278. doi: 10.1039/c8cs00234g. [DOI] [PubMed] [Google Scholar]
- 24.Hu Z., Fang C., Li B., et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng. 2020;4:259–271. doi: 10.1038/s41551-019-0494-0. [DOI] [PubMed] [Google Scholar]
- 25.Wang C., Wang Z., Zhao T., et al. Optical molecular imaging for tumor detection and image-guided surgery. Biomaterials. 2018;157:62–75. doi: 10.1016/j.biomaterials.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorolla M.A., Parisi E., Sorolla A. Determinants of sensitivity to radiotherapy in endometrial cancer. Cancers. 2020;12:1906. doi: 10.3390/cancers12071906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun B., Luo C., Cui W., et al. Chemotherapy agent-unsaturated fatty acid prodrugs and prodrug-nanoplatforms for cancer chemotherapy. J. Control Release. 2017;264:145–159. doi: 10.1016/j.jconrel.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 28.De Ruysscher D., Niedermann G., Burnet N.G., et al. Radiotherapy toxicity. Nat. Rev. Dis. Primers. 2019;5:13. doi: 10.1038/s41572-019-0064-5. [DOI] [PubMed] [Google Scholar]
- 29.Cai Y., Wei Z., Song C., et al. Optical nano-agents in the second near-infrared window for biomedical applications. Chem. Soc. Rev. 2019;48:22–37. doi: 10.1039/c8cs00494c. [DOI] [PubMed] [Google Scholar]
- 30.Yang N., Xiao W., Song X., et al. Recent advances in tumor microenvironment hydrogen peroxide-responsive materials for cancer photodynamic therapy. Nano-micro Lett. 2020;12:15. doi: 10.1007/s40820-019-0347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Z., Fan T., An J., et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020;49:8065–8087. doi: 10.1039/d0cs00215a. [DOI] [PubMed] [Google Scholar]
- 32.Liang P., Huang X., Wang Y., et al. Tumor-microenvironment-responsive nanoconjugate for synergistic antivascular activity and phototherapy. ACS. Nano. 2018;12:11446–11457. doi: 10.1021/acsnano.8b06478. [DOI] [PubMed] [Google Scholar]
- 33.Teng L., Song G., Liu Y., et al. Nitric oxide-activated "dual-key-one-lock" nanoprobe for in vivo molecular imaging and high-specificity cancer therapy. J. Am. Chem. Soc. 2019;141:13572–13581. doi: 10.1021/jacs.9b05901. [DOI] [PubMed] [Google Scholar]
- 34.Zou J., Zhu J., Yang Z., et al. A phototheranostic strategy to continuously deliver singlet oxygen in the dark and hypoxic tumor microenvironment. Angew. Chem. Int. Ed. 2020;59:8833–8838. doi: 10.1002/anie.201914384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia J., Liu G., Xu W., et al. Fine-tuning the homometallic interface of Au-on-Au nanorods and their photothermal therapy in the NIR-II window. Angew. Chem. Int. Ed. 2020;59:14443–14448. doi: 10.1002/anie.202000474. [DOI] [PubMed] [Google Scholar]
- 36.Cai Y., Si W., Huang W., et al. Organic dye based nanoparticles for cancer phototheranostics. Small. 2018;14:1704247. doi: 10.1002/smll.201704247. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y.Y., Liu Y.C., Sun H., et al. Type I photodynamic therapy by organic-inorganic hybrid materials: from strategies to applications. Coord. Chem. Rev. 2019;395:46–62. [Google Scholar]
- 38.Li X., Kwon N., Guo T., et al. Innovative strategies for hypoxic-tumor photodynamic therapy. Angew. Chem. Int. Ed. 2018;57:11522. doi: 10.1002/anie.201805138. [DOI] [PubMed] [Google Scholar]
- 39.Chen D., Wang Z., Dai H., et al. Boosting O2•− photogeneration via promoting intersystem-crossing and electron-donating efficiency of aza-BODIPY-based nanoplatforms for hypoxic-tumor photodynamic therapy. Small. Methods. 2020;4:2000013. [Google Scholar]
- 40.Chen D., Yu Q., Huang X., et al. A highly-efficient Type I photosensitizer with robust vascular-disruption activity for hypoxic-and-metastatic tumor specific photodynamic therapy. Small. 2020;16:2001059. doi: 10.1002/smll.202001059. [DOI] [PubMed] [Google Scholar]
- 41.Welsher K., Sherlock S.P., Dai H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc. Natl. Acad. Sci. U S A. 2011;108:8943–8948. doi: 10.1073/pnas.1014501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandal A.K., Wu X., Ferreira J.S., et al. Fluorescent sp(3) defect-tailored carbon nanotubes enable NIR-II single particle imaging in live brain slices at ultra-low excitation doses. Sci. Rep. 2020;10:5286. doi: 10.1038/s41598-020-62201-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong G., Lee J.C., Robinson J.T., et al. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nat. Med. 2012;18:1841–1846. doi: 10.1038/nm.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J.J., Lin Y., Zhou H., et al. Cell membrane-camouflaged NIR-II fluorescent Ag2Te quantum dots-based nanobioprobes for enhanced in vivo homotypic tumor imaging. Adv. Healthc. Mater. 2019;8:1900341. doi: 10.1002/adhm.201900341. [DOI] [PubMed] [Google Scholar]
- 45.He H., Lin Y., Tian Z.Q., et al. Ultrasmall Pb: Ag2S quantum dots with uniform particle size and bright tunable fluorescence in the NIR-II window. Small. 2018;14:1703296. doi: 10.1002/smll.201703296. [DOI] [PubMed] [Google Scholar]
- 46.Zebibula A., Alifu N., Xia L., et al. Ultrastable and biocompatible NIR-II quantum dots for functional bioimaging. Adv. Funct. Mater. 2018;28:1703451. [Google Scholar]
- 47.Zhong Y., Ma Z., Wang F., et al. In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-IIb rare-earth nanoparticles. Nat. Biotechnol. 2019;37:1322–1331. doi: 10.1038/s41587-019-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X., He S., Ding B., et al. Cancer cell membrane-coated rare earth doped nanoparticles for tumor surgery navigation in NIR-II imaging window. Chem. Eng. J. 2020;385:123959. [Google Scholar]
- 49.Li X., Jiang M., Li Y., et al. 808nm laser-triggered NIR-II emissive rare-earth nanoprobes for small tumor detection and blood vessel imaging. Mater. Sci. Eng. C. 2019;100:260–268. doi: 10.1016/j.msec.2019.02.106. [DOI] [PubMed] [Google Scholar]
- 50.Liu S., Ou H., Li Y., et al. Planar and twisted molecular structure leads to the high brightness of semiconducting polymer nanoparticles for NIR-IIa fluorescence imaging. J. Am. Chem. Soc. 2020;142:15146–15156. doi: 10.1021/jacs.0c07193. [DOI] [PubMed] [Google Scholar]
- 51.Jiang Y., Upputuri P.K., Xie C., et al. Metabolizable semiconducting polymer nanoparticles for second near-infrared photoacoustic imaging. Adv. Mater. 2019;31:1808166. doi: 10.1002/adma.201808166. [DOI] [PubMed] [Google Scholar]
- 52.Liu G., Zhu J., Guo H., et al. Mo2C-derived polyoxometalate for NIR-II photoacoustic imaging-guided chemodynamic/photothermal synergistic therapy. Angew. Chem. Int. Ed. 2019;58:18641–18646. doi: 10.1002/anie.201910815. [DOI] [PubMed] [Google Scholar]
- 53.Wen G., Li X., Zhang Y., et al. Effective phototheranostics of brain tumor assisted by near-infrared-II light-responsive semiconducting polymer nanoparticles. ACS. Appl. Mater. Interfaces. 2020;12:33492–33499. doi: 10.1021/acsami.0c08562. [DOI] [PubMed] [Google Scholar]
- 54.Yang Y., Fan X., Li L., et al. Semiconducting polymer nanoparticles as theranostic system for near-Infrared-II fluorescence imaging and photothermal therapy under safe laser fluence. ACS. Nano. 2020;14:2509–2521. doi: 10.1021/acsnano.0c00043. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y., Liu J., Chen D., et al. Fluorination enhances NIR-II fluorescence of polymer dots for quantitative brain tumor imaging. Angew. Chem. Int. Ed. 2020;59:21049. doi: 10.1002/anie.202007886. [DOI] [PubMed] [Google Scholar]
- 56.Li J., Liu Y., Xu Y., et al. Recent advances in the development of NIR-II organic emitters for biomedicine. Coord. Chem. Rev. 2020;415:213318. [Google Scholar]
- 57.Shao J., Guo X., Shi N., et al. Acenaphthylene-imide based small molecules/TiO2 bilayer as electron-transporting layer for solution-processing efficient perovskite solar cells. Sci. China Mater. 2018;62:497–507. [Google Scholar]
- 58.Wang W., Yang Q., Du Y., et al. Metabolic labeling of peptidoglycan with NIR-II dye enables in vivo imaging of gut microbiota. Angew. Chem. Int. Ed. 2020;59:2628. doi: 10.1002/anie.201910555. [DOI] [PubMed] [Google Scholar]
- 59.Li L., Dong X., Li J., et al. A short review on NIR-II organic small molecule dyes. Dyes Pigm. 2020;183:108756. [Google Scholar]
- 60.Zhang X., An L., Tian Q., et al. Tumor microenvironment-activated NIR-II reagents for tumor imaging and therapy. J. Mater. Chem. B. 2020;8:4738–4747. doi: 10.1039/d0tb00030b. [DOI] [PubMed] [Google Scholar]
- 61.Fang Y., Shang J., Liu D., et al. Design, synthesis, and application of a small molecular NIR-II fluorophore with maximal emission beyond 1200 nm. J. Am. Chem. Soc. 2020;142:15271–15275. doi: 10.1021/jacs.0c08187. [DOI] [PubMed] [Google Scholar]
- 62.Li Y., Hu D., Sheng Z., et al. Self-assembled AIEgen nanoparticles for multiscale NIR-II vascular imaging. Biomaterials. 2020;264:120365. doi: 10.1016/j.biomaterials.2020.120365. [DOI] [PubMed] [Google Scholar]
- 63.Liu M.H., Zhang Z., Yang Y.C., et al. Polymethine-based semiconducting polymer dots with narrow-band emission and absorption/emission maxima at NIR-II for bioimaging. Angew. Chem. Int. Ed. 2020 doi: 10.1002/anie.202011914. [DOI] [PubMed] [Google Scholar]
- 64.Qi J., Alifu N., Zebibula A., et al. Highly stable and bright AIE dots for NIR-II deciphering of living rats. Nano Today. 2020;34:100893. [Google Scholar]
- 65.Shao J., Wang G., Wang K., et al. Direct arylation polycondensation for efficient synthesis of narrow-bandgap alternating D-A copolymers consisting of naphthalene diimide as an acceptor. Polym. Chem. 2015;6:6836–6844. [Google Scholar]
- 66.Ren T.B., Wang Z.Y., Xiang Z., et al. A general strategy for development of activatable NIR-II fluorescent probes for in vivo high-contrast bioimaging. Angew. Chem. Int. Ed. 2020 doi: 10.1002/anie.202009986. [DOI] [PubMed] [Google Scholar]
- 67.Xu W., Wang D., Tang B.Z. NIR-II AIEgens: a win-win integration towards bioapplications. Angew. Chem. Int. Ed. 2020 doi: 10.1002/anie.202005899. [DOI] [PubMed] [Google Scholar]
- 68.Xie Y., Li Z. Approaching aggregated state chemistry accelerated by aggregation-induced emission. Natl. Sci. Rev. 2020 doi: 10.1093/nsr/nwaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gangemi C.M.A., Puglisi R., Pappalardo A., et al. Supramolecular complexes for nanomedicine. Bioorg. Med. Chem. Lett. 2018;28:3290–3301. doi: 10.1016/j.bmcl.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 70.Song X., Zhu W., Ge X., et al. Engineering the protein corona structure on gold nanoclusters enables red-shifted emissions in the second near-infrared window for gastrointestinal imaging. Angew. Chem. Int. Ed. 2020 doi: 10.1002/anie.202010870. [DOI] [PubMed] [Google Scholar]
- 71.Wu Y., Wang C., Guo J., et al. An RGD modified water-soluble fluorophore probe for in vivo NIR-II imaging of thrombosis. Biomater. Sci. 2020;8:4438–4446. doi: 10.1039/d0bm00729c. [DOI] [PubMed] [Google Scholar]
- 72.Song X., Zhu W., Ge X., et al. A new class of NIR-II gold nanoclusters based protein biolabels for in vivo tumor-targeted imaging. Angew. Chem. Int. Ed. 2020 doi: 10.1002/anie.202010870. [DOI] [PubMed] [Google Scholar]
- 73.Feng G., Zhang G.Q., Ding D. Design of superior phototheranostic agents guided by jablonski diagrams. Chem. Soc. Rev. 2020;49:8179–8234. doi: 10.1039/d0cs00671h. [DOI] [PubMed] [Google Scholar]
- 74.Wang W., Ma Z., Zhu S., et al. Molecular cancer imaging in the second near-infrared window using a renal-excreted NIR-II fluorophore-peptide probe. Adv. Mater. 2018;30:1800106. doi: 10.1002/adma.201800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ono K., Tanaka S., Yamashita Y. Benzobis(thiadiazole)s containing hypervalent sulfur atoms: novel heterocycles with high electron affinity and short intermolecular contacts between heteroatoms. Angew. Chem. Int. Ed. 1994;33:1977–1979. [Google Scholar]
- 76.Qian G., Dai B., Luo M., et al. Band gap tunable, donor-acceptor-donor charge-transfer heteroquinoid-based chromophores: near infrared photoluminescence and electroluminescence. Chem. Mater. 2008;20:6208–6216. [Google Scholar]
- 77.Wang B., Feng G., Seifrid M., et al. Antibacterial narrow-band-gap conjugated oligoelectrolytes with high photothermal conversion efficiency. Angew. Chem. Int. Ed. 2016;56:16063–16066. doi: 10.1002/anie.201709887. [DOI] [PubMed] [Google Scholar]
- 78.Yuen J.D., Wang M., Fan J., et al. Importance of unpaired electrons in organic electronics. J. Polym. Sci. A Polym. Chem. 2015;53:287–293. [Google Scholar]
- 79.Liu Y., Phan H., Herng T.S., et al. Toward benzobis(thiadiazole)-based diradicaloids. Chem. Asian J. 2017;12:2177–2182. doi: 10.1002/asia.201700732. [DOI] [PubMed] [Google Scholar]
- 80.Ye F., Chen W., Pan Y., et al. Benzobisthiadiazoles: from structure to function. Dyes Pigm. 2019;171:107746. [Google Scholar]
- 81.Zhou B., Hu Z., Jiang Y., et al. Theoretical exploitation of acceptors based on benzobis(thiadiazole) and derivatives for organic NIR-II fluorophores. Phys. Chem. Chem. Phys. 2018;20:19759–19767. doi: 10.1039/c8cp03135e. [DOI] [PubMed] [Google Scholar]
- 82.Antaris A.L., Chen H., Cheng K., et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 2016;15:235–242. doi: 10.1038/nmat4476. [DOI] [PubMed] [Google Scholar]
- 83.Sun Y., Qu C., Chen H., et al. Novel benzo-bis(1,2,5-thiadiazole) fluorophores for in vivo NIR-II imaging of cancer. Chem. Sci. 2016;7:6203–6207. doi: 10.1039/c6sc01561a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun Y., Ding M., Zeng X., et al. Novel bright-emission small-molecule NIR-II fluorophores for in vivo tumor imaging and image-guided surgery. Chem. Sci. 2017;8:3489–3493. doi: 10.1039/c7sc00251c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeng X., Xue L., Chen D., et al. A bright NIR-II fluorescent probe for breast carcinoma imaging and image-guided surgery. Chem. Commun. 2019;55:14287–14290. doi: 10.1039/c9cc07694h. [DOI] [PubMed] [Google Scholar]
- 86.Zhang X.D., Wang H., Antaris A.L., et al. Traumatic brain injury imaging in the second near-infrared window with a molecular fluorophore. Adv. Mater. 2016;28:6872–6879. doi: 10.1002/adma.201600706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wan H., Ma H., Zhu S., et al. Developing a bright NIR-II fluorophore with fast renal excretion and its application in molecular imaging of immune checkpoint PD-L1. Adv. Funct. Mater. 2018;28:1804956. doi: 10.1002/adfm.201804956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu S., Yang Q., Antaris A.L., et al. Molecular imaging of biological systems with a clickable dye in the broad 800- to 1,700-nm near-infrared window. Proc. Natl. Acad. Sci. U S A. 2019;114:962–967. doi: 10.1073/pnas.1617990114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Q., Ma Z., Wang H., et al. Rational design of molecular fluorophores for biological imaging in the NIR-II window. Adv. Mater. 2017;29:1605497. doi: 10.1002/adma.201605497. [DOI] [PubMed] [Google Scholar]
- 90.Yang Q., Hu Z., Zhu S., et al. Donor engineering for NIR-II molecular fluorophores with enhanced fluorescent performance. J. Am. Chem. Soc. 2018;140:1715–1724. doi: 10.1021/jacs.7b10334. [DOI] [PubMed] [Google Scholar]
- 91.Ma H., Liu C., Hu Z., et al. Propylenedioxy thiophene donor to achieve NIR-II molecular fluorophores with enhanced brightness. Chem. Mater. 2020;32:2061–2069. [Google Scholar]
- 92.Antaris A.L., Chen H., Diao S., et al. A high quantum yield molecule-protein complex fluorophore for near-infrared II imaging. Nat. Commun. 2017;8:15269. doi: 10.1038/ncomms15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zeng X., Xiao Y., Lin J., et al. Near-infrared II dye-protein complex for biomedical imaging and imaging-guided photothermal therapy. Adv. Healthc. Mater. 2018;7:1800589. doi: 10.1002/adhm.201800589. [DOI] [PubMed] [Google Scholar]
- 94.Qu C., Xiao Y., Zhou H., et al. Quaternary ammonium salt based NIR-II probes for in vivo imaging. Adv. Opt. Mater. 2019;7:1900229. doi: 10.1002/adom.201900229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li D., Qu C., Liu Q., et al. Monitoring the real-time circulatory system-related physiological and pathological processes in vivo using a multifunctional NIR-II probe. Adv. Funct. Mater. 2019;30:1906343. [Google Scholar]
- 96.Wan H., Yue J., Zhu S., et al. A bright organic NIR-II nanofluorophore for three-dimensional imaging into biological tissues. Nat. Commun. 2018;9:1171. doi: 10.1038/s41467-018-03505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang L., Liu C., Zhou S., et al. Improving quantum yield of a NIR-II dye by phenylazo group. Adv. Healthc. Mater. 2019;9:1901470. doi: 10.1002/adhm.201901470. [DOI] [PubMed] [Google Scholar]
- 98.Xiao F., Lin L., Chao Z., et al. Organic spherical nucleic acids for the transport of a NIR-II-emitting dye across the blood-brain barrier. Angew. Chem. Int. Ed. 2020;159:9702–9710. doi: 10.1002/anie.202002312. [DOI] [PubMed] [Google Scholar]
- 99.Zhang R., Xu Y., Zhang Y., et al. Rational design of a multifunctional molecular dye for dual-modal NIR-II/photoacoustic imaging and photothermal therapy. Chem. Sci. 2019;10:8348–8353. doi: 10.1039/c9sc03504d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang H., Zhao Z., Turley A.T., et al. Aggregate science: from structures to properties. Adv. Mater. 2020;32:2001457. doi: 10.1002/adma.202001457. [DOI] [PubMed] [Google Scholar]
- 101.Liu S., Li Y., Kwok R.T.K., et al. Structural and process controls of AIEgens for NIR-II theranostics. Chem. Sci. 2020 doi: 10.1039/D0SC02911D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alifu N., Zebibula A., Qi J., et al. Single-molecular near-infrared-II theranostic systems: ultrastable aggregation-induced emission nanoparticles for long-term tracing and efficient photothermal therapy. ACS. Nano. 2018;12:11282–11293. doi: 10.1021/acsnano.8b05937. [DOI] [PubMed] [Google Scholar]
- 103.Sheng Z., Guo B., Hu D., et al. Bright aggregation-induced-emission dots for targeted synergetic NIR-II fluorescence and NIR-I photoacoustic imaging of orthotopic brain tumors. Adv. Mater. 2019;30:1800766. doi: 10.1002/adma.201800766. [DOI] [PubMed] [Google Scholar]
- 104.Wu W., Yang Y.Q., Yang Y., et al. An organic NIR-II nanofluorophore with aggregation-induced emission characteristics for in vivo fluorescence imaging. Int. J. Nanomedicine. 2019;14:3571–3582. doi: 10.2147/IJN.S198587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu P., Kang F., Yang W., et al. Molecular engineering of a high quantum yield NIR-II molecular fluorophore with aggregation-induced emission (AIE) characteristics for in vivo imaging. Nanoscale. 2020;12:5084–5090. doi: 10.1039/c9nr09999a. [DOI] [PubMed] [Google Scholar]
- 106.Diao S., Blackburn J.L., Hong G., et al. Fluorescence imaging in vivo at wavelengths beyond 1500 nm. Angew. Chem. Int. Ed. 2019;54:14758–14762. doi: 10.1002/anie.201507473. [DOI] [PubMed] [Google Scholar]
- 107.Li Q., Ding Q., Li Y., et al. Novel small-molecule fluorophores for in vivo NIR-IIa and NIR-IIb imaging. Chem. Commun. 2020;56:3289–3292. doi: 10.1039/c9cc09865h. [DOI] [PubMed] [Google Scholar]
- 108.Li Y., Liu Y., Li Q., et al. Novel NIR-II organic fluorophores for bioimaging beyond 1550 nm. Chem. Sci. 2019;11:2621–2626. [Google Scholar]
- 109.Li Y., Cai Z., Liu S., et al. Design of AIEgens for near-infrared IIb imaging through structural modulation at molecular and morphological levels. Nat. Commun. 2019;11:1255. doi: 10.1038/s41467-020-15095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang R., Wang Z., Xu L., et al. Rational design of a multifunctional molecular dye with single dose and laser for efficiency NIR-II fluorescence/photoacoustic imaging guided photothermal therapy. Anal. Chem. 2019;91:12476–12483. doi: 10.1021/acs.analchem.9b03152. [DOI] [PubMed] [Google Scholar]
- 111.Sun Y., Ding F., Zhou Z., et al. Rhomboidal Pt(II) metallacycle-based NIR-II theranostic nanoprobe for tumor diagnosis and image-guided therapy. Proc. Natl. Acad. Sci. U S A. 2019;116:1968–1973. doi: 10.1073/pnas.1817021116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Starosolski Z., Bhavane R., Ghaghada K.B., et al. Indocyanine green fluorescence in second near-infrared (NIR-II) window. PLoS One. 2017;12:0187563. doi: 10.1371/journal.pone.0187563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cai Z., Zhu L., Wang M., et al. NIR-II fluorescence microscopic imaging of cortical vasculature in non-human primates. Theranostics. 2020;10:4265–4276. doi: 10.7150/thno.43533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tian R.A.O., Zeng Q.A.O., Zhu S.A.O., et al. Albumin-chaperoned cyanine dye yields superbright NIR-II fluorophore with enhanced pharmacokinetics. Sci. Adv. 2019;5:eaaw0672. doi: 10.1126/sciadv.aaw0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhu S., Hu Z., Tian R., et al. Repurposing cyanine NIR-I dyes accelerates clinical translation of near-infrared-II (NIR-II) bioimaging. Adv. Mater. 2018;30:4141–4151. doi: 10.1002/adma.201802546. [DOI] [PubMed] [Google Scholar]
- 116.Feng Z., Yu X., Jiang M., et al. Excretable IR-820 for in vivo NIR-II fluorescence cerebrovascular imaging and photothermal therapy of subcutaneous tumor. Theranostics. 2019;9:5706–5719. doi: 10.7150/thno.31332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li B., Zhao M., Zhang F. Rational design of near-infrared-II organic molecular dyes for bioimaging and biosensing. ACS. Mater. Lett. 2020;2:905–917. [Google Scholar]
- 118.Cosco E.D., Caram J.R., Bruns O.T., et al. Flavylium polymethine fluorophores for near- and shortwave infrared imaging. Angew. Chem. Int. Ed. 2017;56:13126–13129. doi: 10.1002/anie.201706974. [DOI] [PubMed] [Google Scholar]
- 119.Ding B., Xiao Y., Zhou H., et al. Polymethine thiopyrylium fluorophores with absorption beyond 1000 nm for biological imaging in the second near-infrared subwindow. J. Med. Chem. 2019;62:2049–2059. doi: 10.1021/acs.jmedchem.8b01682. [DOI] [PubMed] [Google Scholar]
- 120.Li B., Lu L., Zhao M., et al. An efficient 1064 nm NIR-II excitation fluorescent molecular dye for deep-tissue high-resolution dynamic bioimaging. Angew. Chem. Int. Ed. 2018;57:7483–7487. doi: 10.1002/anie.201801226. [DOI] [PubMed] [Google Scholar]
- 121.Sun C., Li B., Zhao M., et al. J-aggregates of cyanine dye for NIR-II in vivo dynamic vascular imaging beyond 1500 nm. J. Am. Chem. Soc. 2019;141:19221–19225. doi: 10.1021/jacs.9b10043. [DOI] [PubMed] [Google Scholar]
- 122.Lei Z., Sun C., Pei P., et al. Stable, wavelength-tunable fluorescent dyes in the NIR-II region for in vivo high-contrast bioimaging and multiplexed biosensing. Angew. Chem. Int. Ed. 2019;58:8166–8171. doi: 10.1002/anie.201904182. [DOI] [PubMed] [Google Scholar]
- 123.Wang S., Fan Y., Li D., et al. Anti-quenching NIR-II molecular fluorophores for in vivo high-contrast imaging and pH sensing. Nat. Commun. 2019;10:1058. doi: 10.1038/s41467-019-09043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen C., Tian R., Zeng Y., et al. Activatable fluorescence probes for "turn-on" and ratiometric biosensing and bioimaging: from NIR-I to NIR-II. Bioconjug. Chem. 2020;31:276–292. doi: 10.1021/acs.bioconjchem.9b00734. [DOI] [PubMed] [Google Scholar]
- 125.Li D., Wang S., Lei Z., et al. Peroxynitrite activatable NIR-II fluorescent molecular probe for drug-induced hepatotoxicity monitoring. Anal. Chem. 2019;91:4771–4779. doi: 10.1021/acs.analchem.9b00317. [DOI] [PubMed] [Google Scholar]
- 126.Feng W., Zhang Y., Li Z., et al. Lighting up NIR-II fluorescence in vivo: an activable probe for noninvasive hydroxyl radical imaging. Anal. Chem. 2019;91:15757–15762. doi: 10.1021/acs.analchem.9b04002. [DOI] [PubMed] [Google Scholar]
- 127.Tang Y., Li Y., Hu X., et al. "Dual lock-and-key"-controlled nanoprobes for ultrahigh specific fluorescence imaging in the second near-infrared window. Adv. Mater. 2018;30:1801140. doi: 10.1002/adma.201801140. [DOI] [PubMed] [Google Scholar]
- 128.Li T., Li C., Ruan Z., et al. Polypeptide-conjugated second near-infrared organic fluorophore for image-guided photothermal therapy. ACS. Nano. 2019;13:3691–3702. doi: 10.1021/acsnano.9b00452. [DOI] [PubMed] [Google Scholar]
- 129.Zhao M., Ding J., Mao Q., et al. A novel αvβ3 integrin-targeted NIR-II nanoprobe for multimodal imaging-guided photothermal therapy of tumors in vivo. Nanoscale. 2020;12:6953–6958. doi: 10.1039/c9nr10720g. [DOI] [PubMed] [Google Scholar]
- 130.Boens N., Verbelen B., Ortiz M.J., et al. Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coord. Chem. Rev. 2019;399:213024. [Google Scholar]
- 131.Nguyen V.N., Yim Y., Kim S., et al. Molecular design of highly efficient heavy-atom-free triplet BODIPY derivatives for photodynamic therapy and bioimaging. Angew. Chem. Int. Ed. 2020;59:8957–8962. doi: 10.1002/anie.202002843. [DOI] [PubMed] [Google Scholar]
- 132.Qi S., Kwon N., Yim Y., et al. Fine-tuning the electronic structure of heavy-atom-free BODIPY photosensitizers for fluorescence imaging and mitochondria-targeted photodynamic therapy. Chem. Sci. 2020;11:6479–6484. doi: 10.1039/d0sc01171a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Z., Huang L., Yan Y., et al. Elucidation of the intersystem crossing mechanism in a helical BODIPY for low-dose photodynamic therapy. Angew. Chem. Int. Ed. 2020;59:16114. doi: 10.1002/anie.202005269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen D., Zhong Z., Ma Q., et al. Aza-BODIPY-based nanomedicines in cancer phototheranostics. ACS. Appl. Mater. Interfaces. 2020;12:26914–26925. doi: 10.1021/acsami.0c05021. [DOI] [PubMed] [Google Scholar]
- 135.Bai L., Sun P., Liu Y., et al. Novel aza-BODIPY based small molecular NIR-II fluorophores for in vivo imaging. Chem. Commun. 2019;55:10920–10923. doi: 10.1039/c9cc03378e. [DOI] [PubMed] [Google Scholar]
- 136.Godard A., Kalot G., Pliquett J., et al. Water-soluble aza-BODIPYs: biocompatible organic dyes for high contrast in vivo NIR-II imaging. Bioconjug. Chem. 2020;31:1088–1092. doi: 10.1021/acs.bioconjchem.0c00175. [DOI] [PubMed] [Google Scholar]
- 137.Xu G., Yan Q., Lv X., et al. Imaging of colorectal cancers using activatable nanoprobes with second near-infrared window emission. Angew. Chem. Int. Ed. 2018;57:3626. doi: 10.1002/anie.201712528. [DOI] [PubMed] [Google Scholar]
- 138.Chen J.A., Pan H., Wang Z., et al. Imaging of ovarian cancers using enzyme activatable probes with second near-infrared window emission. Chem. Commun. 2020;56:2731–2734. doi: 10.1039/c9cc09158k. [DOI] [PubMed] [Google Scholar]
- 139.Dou K., Huang W., Xiang Y., et al. Design of activatable NIR-II molecular probe for in vivo elucidation of disease-related viscosity variations. Anal. Chem. 2020;92:4177–4181. doi: 10.1021/acs.analchem.0c00634. [DOI] [PubMed] [Google Scholar]
- 140.Liang P., Tang Q., Cai Y., et al. Self-quenched ferrocenyl diketopyrrolopyrrole organic nanoparticles with amplifying photothermal effect for cancer therapy. Chem. Sci. 2017;8:7457–7463. doi: 10.1039/c7sc03351f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang X., Wang L., Tang H., et al. Diketopyrrolopyrrole: an emerging phototherapy agent in fighting cancer. Dyes Pigm. 2020;181:108599. [Google Scholar]
- 142.Wang Q., Xia B., Xu J., et al. Biocompatible small organic molecule phototheranostics for NIR-II fluorescence/photoacoustic imaging and simultaneous photodynamic/photothermal combination therapy. Mater. Chem. Front. 2019;3:650–655. [Google Scholar]
- 143.Wang Q., Dai Y., Xu J., et al. All-in-one phototheranostics: single laser triggers NIR-II fluorescence/photoacoustic imaging guided photothermal/photodynamic/chemo combination therapy. Adv. Funct. Mater. 2019;29:1901480. [Google Scholar]
- 144.Ilina K., MacCuaig W.M., Laramie M., et al. Squaraine dyes: molecular design for different applications and remaining challenges. Bioconjug. Chem. 2020;31:194–213. doi: 10.1021/acs.bioconjchem.9b00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li J., Lv B., Yan D., et al. Tunable self-assembled micro/nanostructures of carboxyl-functionalized squarylium cyanine for ammonia sensing. Adv. Funct. Mater. 2015;25:7442–7449. [Google Scholar]
- 146.Shen C.A., Wurthner F. NIR-emitting squaraine J-aggregate nanosheets. Chem. Commun. 2020;56:9878–9881. doi: 10.1039/d0cc03686b. [DOI] [PubMed] [Google Scholar]
- 147.Sun P., Wu Q., Sun X., et al. J-aggregate squaraine nanoparticles with bright NIR-II fluorescence for imaging guided photothermal therapy. Chem. Commun. 2018;54:13395–13398. doi: 10.1039/c8cc08096h. [DOI] [PubMed] [Google Scholar]
- 148.Yao D., Wang Y., Zou R., et al. Molecular engineered squaraine nanoprobe for NIR-II/photoacoustic imaging and photothermal therapy of metastatic breast cancer. ACS Appl. Mater. Interfaces. 2020;12:4276–4284. doi: 10.1021/acsami.9b20147. [DOI] [PubMed] [Google Scholar]
- 149.Zhu X., Liu C., Hu Z., et al. High brightness NIR-II nanofluorophores based on fused-ring acceptor molecules. Nano Res. 2020;13:2570–2575. [Google Scholar]
- 150.Tang C., Song C., Wei Z., et al. Polycyclic naphthalenediimide-based nanoparticles for NIR-II fluorescence imaging guided phototherapy. Sci. China Chem. 2020;63:946–956. [Google Scholar]
- 151.Wang Q., Xu J., Geng R., et al. High performance one-for-all phototheranostics: NIR-II fluorescence imaging guided mitochondria-targeting phototherapy with a single-dose injection and 808 nm laser irradiation. Biomaterials. 2020;231:119671. doi: 10.1016/j.biomaterials.2019.119671. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Z., Xu W., Kang M., et al. An all-round athlete on the track of phototheranostics: Subtly regulating the balance between radiative and nonradiative decays for multimodal imaging-guided synergistic therapy. Adv. Mater. 2020;32:2003210. doi: 10.1002/adma.202003210. [DOI] [PubMed] [Google Scholar]
- 153.Lavaud L., Azarias C., Canard G., et al. Fused bis-azacalixphyrin that reaches NIR-II absorptions. Chem. Commun. 2020;56:896–899. doi: 10.1039/c9cc09021e. [DOI] [PubMed] [Google Scholar]
- 154.Shao W., Wei Q., Wang S., et al. Molecular engineering of D-A-D conjugated small molecule nanoparticles for high performance NIR-II photothermal therapy. Mater. Horiz. 2020;7:1379–1386. [Google Scholar]
- 155.Pan H., Li S., Kan J.L., et al. A cruciform phthalocyanine pentad-based NIR-II photothermal agent for highly efficient tumor ablation. Chem. Sci. 2019;10:8246–8252. doi: 10.1039/c9sc02674f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li S., Deng Q., Zhang Y., et al. Rational design of conjugated small molecules for superior photothermal theranostics in the NIR-II biowindow. Adv. Mater. 2020;32:2001146. doi: 10.1002/adma.202001146. [DOI] [PubMed] [Google Scholar]