Summary
Covalent organic frameworks (COFs) are a new type of crystalline porous polymers known for chemical stability, excellent structural regularity, robust framework, and inherent porosity, making them promising materials for capturing various types of pollutants from aqueous solutions. This review thoroughly presents the recent progress and advances of COFs and COF-based materials as superior adsorbents for the efficient removal of toxic heavy metal ions, radionuclides, and organic pollutants. Information about the interaction mechanisms between various pollutants and COF-based materials are summarized from the macroscopic and microscopic standpoints, including batch experiments, theoretical calculations, and advanced spectroscopy analysis. The adsorption properties of various COF-based materials are assessed and compared with other widely used adsorbents. Several commonly used strategies to enhance COF-based materials’ adsorption performance and the relationship between structural property and sorption ability are also discussed. Finally, a summary and perspective on the opportunities and challenges of COFs and COF-based materials are proposed to provide some inspiring information on designing and fabricating COFs and COF-based materials for environmental pollution management.
Keywords: covalent organic frameworks (COFs), heavy metal ions, radionuclides, organic pollutants, interaction mechanism
Graphical Abstract
Public summary
-
•
Covalent organic frameworks (COFs) are a new type of crystalline porous materials known for chemical stability, high specific surface area, and orderly porous channels.With the rapid growth of industrialization, water pollutants remain a serious issue of public health and environmental protection
-
•
COFs as superior adsorbents for the efficient removal of toxic heavy metal ions, radionuclides, and organic pollutants in water is becoming a hot topic
-
•
Information about the interaction mechanisms between various pollutants and COFs materials are summarized.The perspectives and challenges are proposed to provide some useful inspiration for the application of COFs in environmental pollution management
Introduction
Overview of Covalent Organic Framework Materials
The reticular chemistry of linking organic building units by strong covalent bonds to design crystals with extended structures has yielded several new classes of porous materials, and covalent organic frameworks (COFs) have become one of the most widely investigated materials.1 These porous crystalline materials are entirely composed of light elements (i.e., B, C, N, O, and Si) that are linked by strong covalent bonds, such as B-O, C-N, B-N, B-O-Si, C-C, and C-N.2 Organic building units used to make up COFs have general features of rigidity in structures and symmetric multiconnectivity, which are prerequisites for forming regular pore frameworks. In particular, the structures and properties of COFs can be predesigned by carefully selecting the building blocks and their conditions for assembly.2,3 Noteworthy, the reversibility of dynamic covalents, diversity of organic building units, and geometry retention are the key points for the reticular design and synthesis of COFs.3 Covalently crystalline structure endows COFs with outstanding advantages of low density, large surface area, robust thermal stability, permanent porosity, and facile functional design compared with other porous materials (e.g., molecular sieve,4,5 metal-organic frameworks (MOFs),6 zeolite,7 activated carbons (ACs),8,9 porous organic polymers,10 and conjugated microporous polymers.11 Currently, COFs have attracted multidisciplinary interest because of their excellent properties and widespread applications, such as in adsorption,12 storage and separation of gases,13 heterogeneous catalysis,14,15 energy storage,16, 17, 18, 19 photoelectric sensing,20,21 and drug delivery.22, 23, 24
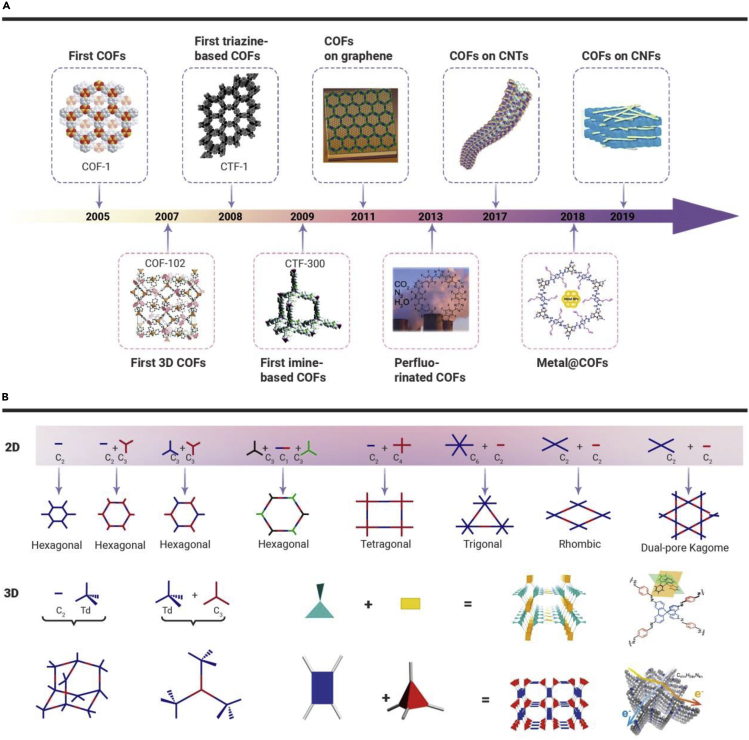
The synthesis of COFs has undergone structural transformation from 2D layers to 3D expanded frameworks, and then to design various functional COF materials (Figure 1A). From the rigid building block dimension standpoint, a combination of rigid building blocks of different geometric shapes determines the COF structures.3 As shown in Figure 1B, combinations, such as 2D-C2 + 2D-C2, 2D-C2 + 2D-C3, 2D-C2 + 2D-C4, and 2D-C3 + 2D-C1 + 2D-C3 can lead to the construction of 2D COFs with designed topology and pore structure. In contrast, selected combinations of 3D-Td + 3D-Td, 3D-Td + 2D-C2, or 3D-Td + 2D-C3 will afford 3D COFs with different crystalline space groups. Besides the successful topology design strategies (ctn, bor, and dia) of 3D COFs, the new pts, ffc, and more topologies are also applied to build 3D COFs.33, 34, 35,38 In 2D COFs, the extended 2D layers are composed of the periodic organic units via covalent bonds, which stack further to form a layered eclipsed structure via π-π interactions.36 Generally, monomers with tetrahedral structure (e.g., building blocks containing sp3 carbon or silane atom) can be used as building units to design the topology structure of 3D COFs. Recently, excellent studies on 3D COFs (SP-3D-COFs and 3D-BMTA-COF) constructed by planar monomers have been reported.34,35 Beyond doubt, 3D COFs possess higher surface area (in some cases >4,000 m2·g−1) and larger total pore volume than 2D COFs. From the viewpoint of chemistry, COFs are functionalized via available design strategy to adjust and control their skeleton and physicochemical properties to meet certain application requirements.36 In a general way, the bottom-up method, postsynthesis strategy, and blending method have been successfully applied for designing various functional COFs, such as COFs on graphene,39 COFs@Fe3O4 with a core-shell structure,25 COFs on carbon nanotubes (CNTs),40 metal@COFs,26 and COFs on CNFs27 (Figure 1A).
Figure 1.
Synthesis of COF-based Materials
(A) Developmental milestones of COF-based materials: single COFs to functionalized COFs.2,25, 26, 27, 28, 29, 30, 31, 32 (B) Sample of topologies accessible through COF synthesis.33, 34, 35, 36, 37
To obtain COFs with higher porosity and structural regularity, rigid p-electron-rich monomers with specific symmetries primarily should be selected as building units to guarantee the complete geometry of the building blocks in COFs.2,37 The π systems of aromatic families have been proved to be suitable building blocks for COFs not only due to their rigid nature and discrete bonding direction of arenes, but also due to the diverse combinations of aromatic systems, which endow COFs with high flexibility.3 Reversible covalent bonds formed by dicyclohexylcarbodiimide (DCC) are the key to form COFs. Unlike conventional covalent bonds controlled by kinetic reactions, DCC can drive reversible covalent bond formation, destruction, and modification.41 Therefore, DCC offers reversible reaction systems with “error-checking” and “proofreading” characteristics, leading to the formation of the most thermodynamically stable structures.3 The exploration of more diverse frameworks by different connectivity leads to the development of more chemical linkages, such as boron-containing linkage, imine linkage, triazine linkage, hydrazone and azine linkages, β-ketoenamine linkage, and other linkages (Figure S1).2,42, 43, 44, 45, 46, 47
Since Yaghi and colleagues reported the first COFs in 2005,28 numerous papers have been published on utilizing these innovative materials in different applications. Low mass density, high thermal stability, permanent porosity, and large specific surface make COFs one of the most widely used materials in environmental pollution management. Compared with traditional adsorbents, COFs not only possess some common features but also many possess special advantages, such as (1) orderly porous channels offer abundant adsorption sites and accelerate the fast diffusion of pollutants; (2) the easily adjustable pore size and shape of COFs provide the possibility for the separation of different contaminants; (3) COFs with strong covalent bonds show high chemical and thermal stability; and (4) low density of COFs implies that they have high adsorption capacity. These advantages, together with the characteristics of predesigned structure, ordered porous structure, and adjustable physicochemical properties, make COFs promising next-generation materials with remarkable high sorption capacities for radioactive/toxic metal ions and organic contaminants.48,49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71,72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89,90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115
Water Decontamination
With the rapid growth of industrialization, water pollutants remain a serious issue of public health and environmental protection. Toxic heavy metal ions (e.g., Hg2+, Pb2+, Cd2+, and Cu2+), radionuclides (e.g., 128Pd, 137Cs, 89Sr, 235U, 79Se, 99Tc, 137Cs, 59Fe, 57Co, 65Zn, 153Eu, and 129I) in nuclear waste, and organic pollutants (e.g., Bisphenol A [BPA], methylene blue [MB], rhodamine B [RhB], CR, Methyl orange [MO], polycyclic aromatic hydrocarbons [PAHs], Sulfamerazine [SMT], and pharmaceutical ibuprofen) in industrial wastewater are the primary pollutants in aqueous solutions that accumulate in living organisms and further create a severe threat to humans and other species through the food chain.
Pollution sources containing heavy metal ions are created during the rapid development of mining, machinery manufacturing, chemical, electronics, instrumentation, and other industries. Heavy metal ions present in surface water are difficult to be degraded into clean and eco-friendly substances, and have the potential to induce acute and chronic toxicity on aquatic biota.116 The highly toxic or carcinogenic heavy metals accumulate in living organisms and further cause great threat for human health. For example, mercury poisoning can cause severe injuries to basic cellular processes, brain tissue, kidney, and liver via its progressive accumulation, and multiple toxicity. Therefore, efficient separation and enrichment of notorious heavy metal ions in aqueous solution is still a necessary but challenging task for environmental pollution treatment.
As a distinguished new energy source, nuclear power, frequently referred to as the “solving the energy crisis,” has recently received attention because of its ability to satisfy basic energy requirements and relieve energy pressures. However, the extensive operation and utilization of nuclear energy undisputedly produces radioactive pollution.117 For example, the harmful effects of U(VI) include inducing leukemia, nervous system disease, cancer, kidney failure, and even death. According to WHO standards, the maximum concentration level of U in normal water is 50 μg·L−1.118 Therefore, accelerating the enrichment of radionuclides in the environment is of considerable significance to public safety and environmental protection.
Organic contaminants are also a significant part of water pollution, and they mostly originate from artificial organic matter, such as organochlorine pesticides, synthetic detergents, and synthetic dyes. Organic pollutants, such as endocrine interferon BPA, organic dyes (such as MB, RhB, CR, and MO), organic pesticides, PAHs and their derivatives, sulfonamides, and tetracyclines, exist in the environment ubiquitously.119 Their discharge into the environment affects not only human beings, but also all living organisms. In particular, persistent organic pollutants (POPs) have the characteristics of high toxicity, long-term residuality, high solubility, and are degradation resistant. Ingestion of small amounts may cause cancer and malformation. Organic pollutants are diversified and complicated, and most of them are poorly degraded totally in a short time by environmental self-purification. Hence, searching for efficient and practical strategies to decontaminate organic pollutants remains a severe challenge.
Environmental Adsorption Techniques
Adsorption technology has been widely used in the purification of environmental pollutants by taking advantage of the high adsorption capacities of adsorbents to remove specific pollutants. Generally, adsorbents are required to possess large specific surface area, high adsorption performance, outstanding selectivity, excellent chemical and thermal stability, are cheap, and have good regeneration and reusability. Adsorption behavior is affected by pollutant properties, pH values, coexisting substances, temperature, contact time, and adsorbent dosage. To our knowledge, adsorption mechanisms include ion exchange, electrostatic adsorption, hydrogen bonding, specific surface bonding, and chelation. Various techniques, such as Fourier transform infrared (FTIR) spectroscopy, X-ray photoelectron spectroscopy (XPS), X-ray absorption fine structure (XAFS) spectroscopy, density functional theory (DFT) calculation, and molecular simulations are commonly applied to unveil internal adsorption mechanism.120 In the section entitled “Mechanism analysis of pollutants removal by COF-based materials,” these techniques are discussed systematically.
Over the past few decades, enormous research about natural and man-made adsorbents, such as clays,121,122 ACs,123 organic resins,124,125 CNTs,126, 127, 128 order mesoporous carbon (OMC),129 carbonaceous materials,130, 131, 132 graphene oxide (GO),133, 134, 135, 136, 137, 138, 139 metal oxides,140,141 layered double hydroxides (LDHs),142, 143, 144, 145 and layered metal sulfides (LMS),146 have been applied in pollution cleanup. However, these materials suffered from some insufficiencies: (1) LDHs and clays exhibit slow sorption kinetics and limited selectivity; (2) ACs and carbonaceous materials possess disadvantages of small pore volumes or pore sizes; (3) GO and CNTs are complicated to synthesize and produce; (4) the poor regeneration and reusability of organic resins; and (5) relatively low chemical and thermal stability of LMS.147 Novel nanomaterials, such as polymer adsorbents,148 2D MXenes,149, 150, 151, 152, 153 nanoscale zero-valent iron (NZVI),154, 155, 156, 157, 158, 159 g-C3N4,120,160, 161, 162, 163, 164 MOFs,165, 166, 167, 168 and COFs,169, 170, 171 are constantly applied to improve their applications in environmental pollution cleanup owing to their superior performance. MOFs and COFs represent porous crystalline materials for network topology. However, compared with MOFs, COFs showed superior structure and performance as adsorbents. COFs have low mass densities, which implies that COFs show higher adsorption capacity when pollutants fully occupy both COFs and MOFs of the same mass. In addition, tailoring and designing the pore structure of COFs is relatively easy, providing more options for selective elimination of target pollutants. In addition, pore-wall engineering can be used to endow COFs with more active adsorptive sites.36
To date, various traditional or advanced techniques, including adsorption, electrocoagulation, chemical precipitation, ultrafiltration, evaporative recovery, solvent extraction, reverse osmosis, photocatalysis, oxidation/reduction, biological treatments, membrane separation, and filtration, have been applied to eliminate pollutants from wastewater. However, most of these methods have some unavoidable disadvantages. For instance, although membrane separation is highly efficient, its low economic viability and high maintenance costs restrict its application in large scale. Precipitation and biological treatments are cost-effective but they are unable to reduce the levels of pollutants below the necessary limits and can produce abundant sludge.172 Photocatalysis generally faces the problems of photoetching, rapid recombination of electron hole pairs, and catalyst ion aggregation.173 Although adsorption technology has the disadvantage that parsing waste liquid is difficult to treat and that the pollutants cannot be degraded completely, adsorption is widely used, owing to its low-cost, simple operation environmental friendliness, simple regeneration, and large-scale application.147,173
The Purpose of This Review
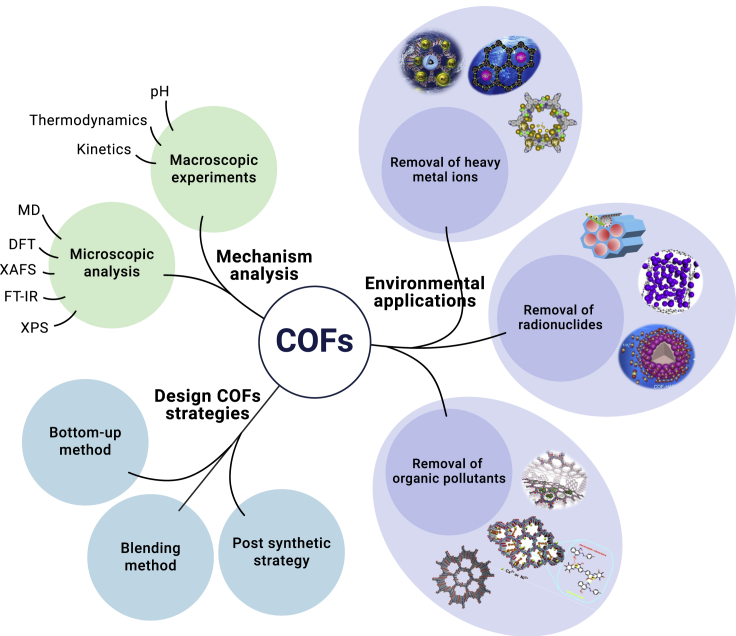
To date, several reviews and monographs have systematically summarized the fundamental theory of COFs and their design synthesis, important structural properties, and applications. However, there are few reviews of COFs in environmental pollution management. A systematic summary about COFs and COF-based materials as adsorbents for the efficient removal of toxic metal ions, radionuclides, and organic pollutants is not available. In this review, we place the emphasis not only on the potential of COFs in practical applications but also on techniques for the characterization of their adsorption behaviors/mechanisms. Various methods for studying adsorption properties, including microscopic spectroscopic analysis and theoretical calculation, are summarized (Scheme 1). We believe that this review, focused on the emerging applications of COF-based materials in environmental pollution management, is of great importance for the future development of environmental pollution regulation and it also provides guiding strategies and clues for the design and synthesis of COF-based materials as high-performance adsorbents.
Scheme 1.
Overview of this Article
The design strategy that has been applied for the functional COFs is firstly introduced, followed by a systematic description of various toxic/radioactive metal ions and the adsorption properties of organic pollutants on COFs and their composites. Finally, the macroscopic/microscopic techniques used to analyze the adsorption mechanism are summarized.
Strategies for Synthesis of COFs and COF-based Materials
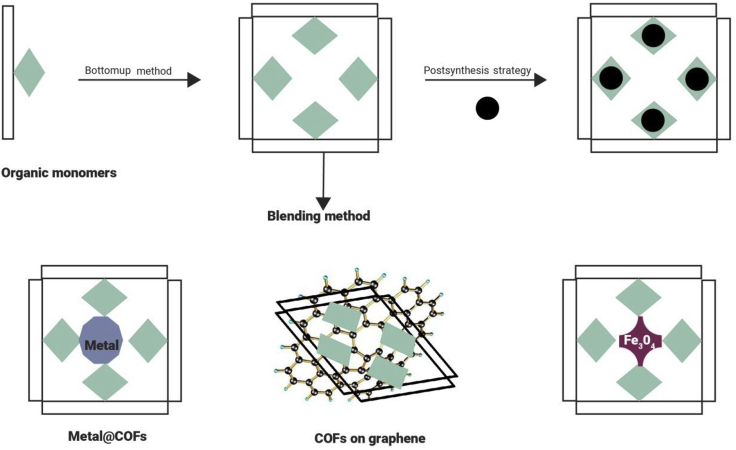
Basically, COF-based materials can be divided into four categories: boron-containing COFs, triazine-based COFs, imine-based COFs, and other COFs.11 For certain applications, functional COFs should be constructed. The adsorption performances of COFs are controlled by their highly ordered framework structures, hydrophilicity, surface area, functionality, and pore size and distribution.147 Like the construction of other functional materials, bottom-up, postmodification, and blending approaches are the general strategies for designing functional COFs (Figure 2).36
Figure 2.
Functional Design Methods for COFs, the General Strategies for Designing Functional COFs Involve Bottom-up, Postmodification, and Blending Approaches
The Bottom-up Method
The bottom-up method is a straightforward but more difficult strategy for constructing functional COFs. It requires the selection of building units directly containing the functional moieties as monomers for COF synthesis. In addition, the functionalization of COFs can be achieved by a slight modification of monomers to COFs. The advantage is the homogeneous distribution of functional groups and the improved hydrothermal or chemical stability of COFs. However, it causes more trouble in maintaining the structural regularity of the functional COFs.174
Designing efficient adsorbents with high abundance active sites is of significance to achieve quick uptake and high capacity for target pollutants. To date, organic building units, containing hydroxyl,175 carboxyl,176,177 phosphate groups,178 amide groups,179 and sulfide functional termini,48 have been successfully used for functional COF synthesis. Oxygen in hydroxyl provides an open chelate site to bind Cd2+,175 amide groups on COF skeletons are active sites for Pb2+,179 and carboxyl groups on COFs significantly facilitate the dye adsorption.177 In an interesting report,178 functional COFs (P-WCA-POFs with [P(O2Ph)3]−) have shown a nonideal performance because the nickel coupling agent blocked the pore opening during the reaction. After washing them with concentrated HCl, the obtained PA-POFs have shown a maximum adsorption capacity of 3,366 mg·g−1 for BPA, which is attributed to the removal of Ni impurities and more pores are created when P-O bonds are broken and ligands are etched away by the acid. This also indicates that COFs with free active functional groups could be used for further postmodification to acquire the best performance.
Postsynthesis Strategy
Postsynthesis strategy is most frequently used to prepare functional COFs. Subsequent modification, such as grafting or structural tailoring, can introduce functional moieties into the COF networks.180 Besides, COF linkages are also transformed via chemical reactions.
The free functional groups in the COF skeleton are used as reaction anchors (e.g., -OH,72,175 -CN181 -C=C-,182,183 and -C≡C-,184,185) and chemical modification is carried out through various reactions, including esterification reaction, “click reaction,” and others. Besides, other chemical reactions, such as N-H bond deprotonation, enolketo tautomerism, and imine-amide transformation of COF linkages, are also adopted for structure modification.36 Postmodified COF-COOCa and COF-COONi are obtained via embedded Ca2+ ions and Ni2+ ions in COF-COOH, respectively, and their adsorption capacity for CR is superior compared with that of COF-COOH, which is attributed to enhanced electrostatic interaction between metal ions and CR.176 The simple treatment of CuP-DMNDA-COF with Fe(III) in acetone has led to the formation of Fe-coordinated COFs (CuP-DMNDA-COF/Fe),186 which has shown ~100% RhB removal, while CuP-DMNDA-COF could only reach 20% RhB removal. The coordination interaction between Fe(III) ions in COF and the carboxy group of RhB plays a significant role in the excellent adsorption capacity of CuP-DMNDA-COF/Fe for RhB.
Postsynthesis strategies require specific anchor points on COFs as sites for later chemical modification. Postfunctionalized COFs exhibited intriguing adsorption performance for specific pollutants, which further signifies that the postsynthesis strategy is a facile, efficient, and practical functionalization strategy. However, the introduction of abrupt functional groups will affect the ordered crystal structure of COFs.
The Blending Method
The blending method could combine the functional materials to COFs to enable the elegant combinations of physicochemical properties, synergistic effects, and multiple functions of individual components. For adsorption, metal, graphene, and magnetic materials have been composited with COFs to increase their stability, selectivity, and adsorption capacity.90,176,187
Shapeless bulk materials are always formed in COFs, leading to an irregular morphology, because it is hard to control their growth rationally. Incorporation of COFs and magnetic nanoparticles has been confirmed as an effective strategy.90 Solid spherical magnetic Fe3O4 cores can offer a center for COF growth and crystallization at all directions equally, which provides a possibility for controllable synthesis of magnetic core-shell COFs. Ag NPs@COF is formed by loading high dispersion of Ag on COFs and exhibits high Hg(II) ion removal efficiency from acidic water, which is interpreted by in situ formation of HgAg alloy.40 In another fascinating study,188 the use of baking soda to produce continuous CO2, transforms 2D COFs into 3D COFs with ordered micropore structures, and synergistically allows different guests to quickly diffuse through the interconnected pore network. Blending COFs with other materials is an effective way to overcome the inherent shortcomings of COFs and accelerate their multidisciplinary applications.
Removal of Toxic Heavy Metals by COF-based Materials
“Heavy metals” refer to metals and metalloids with an atomic density of >4.5 g·cm−3 or more than five times of that of water, which are still toxic at low concentrations.189,190 It will cause great harm to health if the cumulative concentration in the body exceeds the standard limit.191,192 More importantly, unlike organic pollutants, heavy metals are very difficult to degrade into eco-friendly materials.193
Industrial wastewater containing heavy metals is usually acidic, so it requires adsorbents of high stability. Because COFs are connected by numerous strong covalent bonds, they are highly stable in strong acidic and basic conditions. The regular channels and adjustable pore size make it easier for the adsorbed heavy metal ions to diffuse in COFs uniformly and be captured effectively. Therefore, COF-based materials have good applications in removing heavy metals from wastewater.
Table 1 lists the adsorption performance and mechanism of COF-based materials for different heavy metal ions. The functional groups containing O, N, or S atoms have a good adsorption capacity for heavy metal ions. Therefore, through some elaborate designs, the integration of specific functional groups (e.g., -COOH, -NH2, -CN, -SH, -SCH3, and -SO3H) into the framework of COFs will greatly improve the efficiency of metal ion removal. Currently, there are three main methods to synthesize COF-based materials with better adsorption performance: (1) directly synthesize COFs by selecting the building blocks containing specific functional groups; (2) prepare functionalized COFs by postsynthesis modification; and (3) combine COFs with other materials (such as metal nanoparticles and metal oxide). In practical applications, these methods are often combined reasonably.
Table 1.
The Adsorption Performance of COF-based Materials and Other Promising Materials to Various Heavy Metal Ions, Radionuclides, and Organic Pollutants
| Materials | Target | Adsorption Equilibration Time (min) | Adsorption Capacity (mg g−1) | Adsorption Mechanism | Refs |
|---|---|---|---|---|---|
| COF-LZU8 | Hg(II) | – | 236 | coordination of Hg and S atoms (thioether groups); electrostatic interaction | 49 |
| TAPB-DMTTPA-COF | Hg(II) | 5 | 734 | coordination of Hg and S atoms (thioether groups) | 48 |
| ACOF | Hg(II) | 5 | 175 | interaction between Hg and keto groups (C=O) | 50 |
| U(VI) | 169 | ||||
| TpODH | Hg(II) Cu(II) |
~200 | 1,692 324 |
coordination of Hg and O and N atoms; electrostatic interaction | 71 |
| POFct-1 | Hg(II) Cu(II) |
720 | 167.19 135.60 |
coordination of Hg and O and N atoms | 51 |
| TFPPy-CHYD | Hg(II) | 5 | 758 | coordination of Hg and N atoms (-NH-) | 52 |
| T-COF | Hg(II) | 15 | 1,826 | coordination of Hg and N atoms; electrostatic interaction | 53 |
| COF-S-SH | Hg(II) Hg0 |
10 3 d |
1,350 863 |
coordination of Hg and S atoms (thiol and thioether groups) | 182 |
| COF-S-SH | Hg(II) | <50 | 588.2 | coordination of Hg and S atoms | 54 |
| TPB-DMTP-COF-SH | Hg(II) | 2 | 4,395 | coordination of Hg and O and N and S atoms (triazole and thiol groups) | 184 |
| COF-SO3H [NH4+][COF-SO3−] |
Hg(II) Hg(II) Hg |
10 10 12 h |
1,033 1,299 932.6 |
coordination of Hg and O atoms (SO3−); coordination of Hg and O atoms (SO3−); ion exchange between NH4+ and Hg2+ | 55 |
| Fe3O4/M-COFs | Hg(II) | 80 | 97.65 | coordination of Hg and N atoms | 56 |
| M-COF-SH | Hg(II) | 20 | 383 | coordination of Hg and S atoms (thiol groups) | 57 |
| Ag NPs@COF-LZU1 | Hg(II) | 30 | 113 | interaction between Hg and Ag nanoparticles | 40 |
| COF-TP COF-TE |
~24 h | 140 185.7 |
coordination of Pb and O and N atoms; electrostatic interaction | 58 | |
| COOH@COF | Pb(II) Hg(II) |
5 | 123.8 99.1 |
interaction between Hg/Pb and the carboxyl groups (-COOH) | 179 |
| COF-SH | Pb(II) | – | 239 | coordination of Pb and S atoms; electrostatic interaction | 59 |
| N-enriched COF | Cd(II) | 20 | 396 | coordination of Cd and N atoms; electrostatic interaction | 60 |
| COF-ETTA-2,3-Dha | Cd(II) | 60 | 116 | coordination of Cd and O atoms (ortho-dihydroxy groups) | 175 |
| COF-BTA-DHBZ | Cr(VI) | 12 h | 384 | hydrogen bonds; electrostatic interaction | 61 |
| TpPa-1 Fe3O4@TpPa-1 |
Cr(VI) | 60 | 310.8 245.45 |
hydrogen bonds; electrostatic interaction | 62 |
| COF1 COF2 |
Cr(VI) | – | 462.85 635.06 |
hydrogen bonds; electrostatic interaction | 63 |
| QG-scaffolded COFs | Cu(II) | 40 s | – | coordination of Cu and N atoms | 64 |
| TpPa-NH2@EDTA | Ag(I), Pd(II), Cu(II), Ni(II), Fe(III), Cr(III) | 5 | ~50 | coordination of metal ions and EDTA | 65 |
| TTB-COF | Au(III) | 1 | 560 | coordination of Au and S atoms (thioether groups) | 66 |
| Fe0/COFs | As(III) | – | 135.78 | hydrogen bonds; electrostatic interaction; As(III) is partially oxidized to As(V) | 67 |
| EB-COF:Br | As(V) | <1 h (DI water) 4–5 h (river) |
53.1 (25°C) 27.5 (35°C) 5.1 (45°C) |
hydrogen bonds; electrostatic interaction | 68 |
| Glucan/chitosan (GL/CS) hydrogel | Cu(II) Co(II) Ni(II) Pb(II) Cd(II) |
180 | 342 234 184 395 269 |
coordination of metal ions and O and N atoms | 194 |
| Magnetic graphene oxide (MGO) | Cd(II), As(V) |
– | 234 14 |
coordination of Cd and O atoms; electrostatic interaction; hydrogen bonds | 195 |
| Graphene/polydopamine modified multiwalled carbon nanotubes (MWCNT-PDA/GO) | Cu(II) Pb(II) |
600 | 318.47 350.87 |
coordination of metal ions and O and N atoms; electrostatic interaction | 196 |
| Triamino-anchored monodispersed fibrous silica nanospheres (triamino-KCC-1) | Cr(VI) | ~40 | 317 | electrostatic interaction | 197 |
| Co-Fe2O3 Ni-Fe2O3 Fe2O3 |
Pb(II) | 30 45 |
136.0 97.5 93.9 |
coordination of Pb and O atoms (hydroxyl groups) | 198 |
| Ni/Co-layered double hydroxide (NiCo-LDH) | Cr(VI) | 80 | 99.9 | electrostatic interaction; ion exchange | 199 |
| Nanoscale zero-valent iron (NZVI) | As(III) | <5 h <10 h |
11.52 | complexation, co-precipitation, and reduction | 200 |
| Cd(II) | 48.63 | ||||
| Pb(II) | 85.37 | ||||
| Graphitic-C3N4 (g-C3N4) | Co(II) | 12 h | 137.4 | inner-sphere complexation | 201 |
| Ni(II) | 136.9 | ||||
| Cu(II) | 134.1 | ||||
| Zn(II) | 138.0 | ||||
| Metal-organic framework-101 (MIL-101) iron-doped MIL-101 (Fe-MIL-101) | Pb(II) | 90–120 | 57.96 86.20 |
coordination of Pb and O atoms (hydroxyl groups); electrostatic interaction | 202 |
| ZIF-8 | Pb(II) | 15 (2.0 g/L) | 1,119.80 | coordination of metal ions and N atoms | 203 |
| Cu(II) | 60 (0.5 g/L) | 454.72 | |||
| ZIF-67 | Pb(II) | 120 (0.2 g/L) | 1,348.42 | ||
| Cu(II) | 15 | 617.51 | |||
| COF-TpPa-1 | U(VI) | 300 | 152 | chemisorption | 76 |
| COF-HBI | U(VI) | 30 | 211 | binding carboxyl groups | 204 |
| COF-COOH | U(VI) | 120 | 213.8 | binding carboxyl groups | 204 |
| COF-HAP | U(VI) | 240 | 510 | surface precipitation, complexation, and ion exchange | 80 |
| COF-TpAb-AO | U(VI) | 90 | 408 | coordination with amidoxime groups | 181 |
| o-GS-COF | U(VI) | 20 | 144.2 | coordination with oxime groups | 81 |
| COF-SO3H | U(VI) | 100 | 360 | coordination interaction | 82 |
| [NH4]+[COF-SO3−] | U(VI) | 100 | 851 | coordination interaction and ion exchange | 82 |
| NFeU-AC | U(VI) | 30 | ~20 | surface complexation | 205 |
| CNFs | U(VI) | 240 | 125 | surface precipitation, complexation | 130 |
| MMT@C | U(VI) | 300 | 20.76 | inner-sphere surface complexation | 206 |
| Defective TiO2-x | U(VI) | 120 | 142 | surface complexation | 207 |
| Fe3O4@LDHs | U(VI) | 60 | ~30 | chemisorption | 208 |
| nZVI/C | U(VI) | 540 | 186.9 | surface complexation | 209 |
| hypha/GO aerogel | U(VI) | 30 | 288.4 | coordination with carboxyl and hydroxyl groups | 210 |
| PZS-TPP/CNT/Fe3O4 | U(VI) | 150 | 606 | binding with polyphosphazene group | 211 |
| Fe@ZIF-8 | U(VI) | 720 | 277.7 | chemisorption, surface complexation | 212 |
| azo-MOF | U(VI) | 150 | 200 | coordination with zao and amide units | 213 |
| TpPa-1-COF | Eu(III) | 60 | 1107.6 | chelating coordination | 87 |
| PAM/GO | Eu(III) | <720 | 189.2 | strong complexation | 214 |
| g-C3N4 nanosheets | Eu(III) | <420 | 155 | multilayer adsorption on heterogeneous surfaces | 215 |
| porous Al2O3 | Eu(III) | 25 | 223.37 | hydroxy groups surface complexation | 216 |
| SCU-COF-1 | TcO4− | 1 | 702.4 | electrostatic and van der Waals interactions | 217 |
| DhaTGCl | TcO4− | 5 | 437 | guanidine and hydroxyl groups complexation | 89 |
| MOF- SCU-101 | TcO4− | 10 | 217 | coordination with H bond | 218 |
| UiO-66-NH2 | TcO4− | <720 | 159 | – | 219 |
| Fe3O4@TpBD | BPA | 5 min | 160.6 | hydrogen bonding; π-π interaction | 90 |
| Fe3O4@TpND | BPA | 10 min | 115.0 | hydrogen bonding; surface complexation |
91 |
| TPT-DMBD-COF | MB | 60 min | 45.5 | electrostatic attraction; π-π interaction | 96 |
| TPT-TAPB-COF | RhB | 90 min | 970.0 | homogeneous adsorption | 99 |
| CuP-DMNDA-COF/Fe | RhB | – | 378.0 | surface complexation | 186 |
| COF-COONi | CR | 120 min | 781.3 | electrostatic attraction; hydrogen bonding; π-π interaction | 176 |
| COF-TzDBd | CV | 15 min | 307.7 | electrostatic attraction; π-π interaction; different size values | 177 |
| TPB-DMTP-COF | sulfamerazine | 80 min | 209.0 | C–H···π interaction; electrostatic attraction | 170 |
| Magnetic TPB-DMTP-COF | diclofenac | 50 min | 109.0 | C–H···π interaction; π-π interaction | 169 |
| NCCT | cefotaxime | 8 h | 309.3 | electrostatic attraction; hydrogen bonding; π-π interaction | 108 |
| COF-3 | TPhP | 12 h | 371.2 | hydrogen bonding; π-π interaction; different size values | 112 |
| COFs | 2-nitrophenol | 90 min | 239.9 | π-π interaction; different size values; surface complexation | 110 |
| DhaTab-PBA | catechol | 40 min | 160.0 | π-π interaction; homogeneous adsorption | 113 |
| TpBD-Me2-COF | okadaic acid | 60 min | 279.0 | heterogeneous adsorption | 115 |
Adsorption of Hg(II)
Hg can affect cell function and growth and even lead to cell necrosis by affecting intracellular metabolic pathways. Hg2+ can form stable covalent bonds with functional groups containing O, N, or S. In particular, the strong soft-soft interaction between sulfur derivatives and mercury has a strong affinity for Hg(II). Therefore, COFs and COF-based materials with high O, N, or S contents have a good effect on Hg adsorption.
Pristine COFs
O-, N-, or S-enriched COFs can be directly synthesized by selecting specific building blocks. Ding et al.49 synthesized COF-LZU8 with thioether hydrazone bonding and applied it for Hg2+ removal with high selectivity, sensitivity and adsorption capacity. The rigid π conjugation structure acts as the fluorophore for signal sensing, and the evenly and densely distributed thioether side chains act as ion receptors for capturing Hg2+. Huang et al.48 synthesized an extremely stable TAPB-BMTTPA-COF by integrating the methyl sulfide units onto the edge of the phenyl groups and thus introducing sulfur-containing functional groups onto the COF skeleton. The shortest functional chain of sulfides is introduced, and these methyl sulfides are evenly distributed on the pore wall of COFs with high accessibility. The active sulfur sites could be fully exposed to capture Hg2+ with a saturated adsorption capacity of 734 mg·g−1. Li et al.50 firstly proposed a solvent- and catalyst-free method to synthesize an azine-linked organic framework (ACOF) containing azide bonds. When ACOF adsorbs metal ions, its structure converts from enol to ketone form, which has stronger coordination with Hg(II) (175 mg·g−1). The structural transformation could improve ACOF adsorption performance. Li et al.238 used Tp and flexible alkyl amine (ODH) as building blocks to synthesize a hydrazone-linked COF (TpODH). TpODH has irreversible enol-to-keto tautomerism and intramolecular hydrogen bonds with excellent crystallinity and stability. In addition, the large amount of oxygen and nitrogen in the skeleton of TpODH has strong electrostatic synergy and coordination for metal ions, i.e., Hg2+ (1,692 mg·g−1) and Cu2+ (324 mg·g−1). A 3D porous organic framework (POFct-1) with topological structure has been used to selectively remove Hg2+ and Cu2+,51 and its color changes in the presence of hydrogen ions and visible light. The O-containing and N-containing functional groups of POFct-1 effectively adsorb Hg2+ and Cu2+ with adsorption capacities of 167.19 mg·g−1 (Hg2+) and 135.60 mg·g−1 (Cu2+). Cui et al.52 used pyrene-based ligands (TFPPy) and a carbohydrazide linker (CHYD) to synthesize TFPPy-CHYD directly through a solvothermal route. Abundant secondary amine groups (-NH-) are evenly distributed on the pore wall of TFPPy-CHYD, and they are receptors to detect and remove Hg2+ selectively with a saturated adsorption capacity of 758 mg·g−1. The key advantage of TFPPy-CHYD is that the secondary amine groups could combine with Hg2+ reversibly. A novel stable triazine-based COF (T-COF)53 could effectively adsorb Hg2+ in the solution within 15 min with a saturated adsorption capacity of 1,826 mg·g−1, which is attributed to the soft-soft interaction between N atoms in triazine and Hg2+.
Modified COFs
Preparing functionalized COFs by postsynthesis modification is also an effective way to enhance the adsorption capability for heavy metal ions. Sun et al.182 used 2,5-divinylterephthalaldehyde and 1,3,5-tris(4-aminophenyl)-benzene to synthesize vinyl-functionalized COF (COF-V). Then they modified COF-V with 1,2-ethanedithiol through thiol-ene “click” reaction to prepare COF-S-SH. The modified sulfur species has good accessibility, flexibility and density with strong affinity for soft metal ions, such as Hg2+, Pb2+, and Cu2+. COF-S-SH could effectively remove Hg2+ from aqueous solution and gas phase, with a saturated adsorption capacity of 1,350 mg·g−1 for Hg2+ and 863 mg·g−1 for Hg0. Similarly, Li et al.54 selected a new triamino-monomer (TABPB) as a linker to synthesize a new kind of COF-S-SH, but its saturated adsorption capacity is lower (588.2 mg·g−1 for Hg2+). Meri-Bofi et al.184 synthesized TPB-DMTP-COF-SH by introducing triazole and thiol groups into the skeleton of COF. TPB-DMTP-COF-SH has the highest removal efficiency of Hg2+ (4,395 mg·g−1) because of the strong synergistic chelation of triazole and thiol groups with Hg2+. Tao et al.55 reported a SO3−-anchored COF material ([NH4+] [COF-SO3−]), which could selectively adsorb Hg2+ and Hg0 through ion exchange with NH4+ and then be captured by independent SO3− cells through Hg-O coordination.
COF-based Composites
The performance of COFs can be improved by combining them with other materials. Magnetic COF composites are prepared by combining the COFs with iron oxide nanoparticles or other magnetic materials. On the basis of retaining their original excellent properties, it cannot only improve the adsorption performance but also facilitate solid-liquid separation. Ge et al.56 prepared amino-functionalized Fe3O4-modified melamine-based COFs (M-COF) by a microwave method. The high density of N-containing groups could remove Hg2+ from wastewater selectively. The amino-functionalized Fe3O4 has excellent dispersivity and endows the COF composites with magnetism. Huang et al.57 synthesized thiol-functionalized COFs (M-COF-SH) by using disulfide derivatives as specific building blocks. The thiol functional groups in M-COF-SH have a good chelating ability to Hg2+ within 10 min to achieve equilibration. This subsequent cutting strategy provides a new method for postsynthesis modification. In the report of Wang et al.,40 Ag NPs@COF-LZU1 loaded with Ag nanoparticles was successfully prepared. Due to the large number of N-containing groups on COF skeleton, the stability and catalytic activity of Ag NPs could be promoted. The strong interaction between Ag and Hg could reduce Hg2+ to Hg0 and form an Ag Hg nanoalloy, and thereby could remove Hg2+ in acidic aqueous solution effectively.
Adsorption of Pb(II)
Lead has a huge impact on the nervous system, immune system, and almost all other organ systems of the human body. It is known that O and N atoms have lone pairs of electrons to form coordination bonds with Pb2+. Therefore, negatively charged ligands containing O and N atoms can form stable metal complexes with Pb2+. Carboxyl-functionalized COFs (COOH@COF) prepared by postsynthesis modification have a good adsorption capacity and high selectivity for Hg2+ and Pb2+ with saturated adsorption capacities of 99.1 and 123.9 mg·g−1, respectively, due to the strong synergistic effect of carboxyl groups.58 In particular, the adsorption capacity of COOH@COF remains almost constant after 20 cycles. Li et al.179 synthesized two amide-rich layered COFs (COF-TP and COF-TE) and applied them for Pb2+ removal. Amide groups are considered as the active sites to capture Pb2+ through coordination with amido groups (Figure 3A), with saturated adsorption capacities of 140.0 and 185.7 mg·g−1 for COF-TP and COF-TE, respectively. COF-TE has better adsorption effect due to its fewer aromatic rings, weaker π-π stacking, larger layer spacing, and higher internal diffusion rate of Pb2+ compared with COF-TP. Interestingly, the removal efficiency of Pb2+ by the two materials is still above 95% after 10 cycles. Cao et al.59 prepared a sulfhydryl functionalized COF (COF-SH) by a mild solvothermal solution suspension method. The large amount of sulfur substances distributed in the channels of COF-SH has a strong affinity with Pb2+ (239 mg·g−1) in solution due to the combined action of chelation and electrostatic attraction.
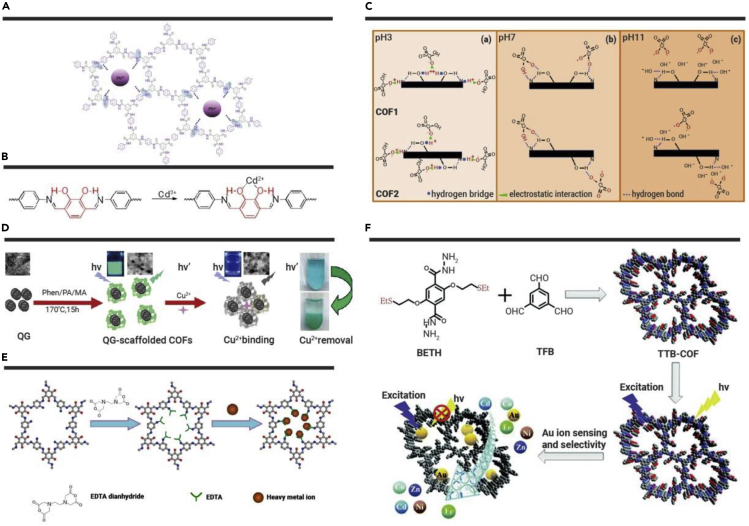
Figure 3.
The Adsorption Mechanism of COF-based Materials for Some Heavy Metal Ions
(A) Multi-coordination of Pb2+ with the amide groups in the structure pore of COF-TP.179 (B) The intramolecular hydrogen bonding and chelation between a cadmium ion and the ortho-dihydroxy unit.175 (C) The adsorption mechanism of Cr(VI) by COF1 and COF2.63 (D) The fabrication of QG-scaffolded COFs with bright green fluorescence, which could be quenched by Cu2+ ions for Cu2+ removal.64 (E) Synthesis of TpPa-NH2@EDTA for the removal of heavy metal ions.65 (F) Synthesis of TTB-COF and its application in selective detection and capture of Au ions.66
Adsorption of Cd(II)
Cadmium can cause anemia, hypertension, neuralgia, osteoporosis, nephritis, and other diseases. The well-known “Itai-Itai disease” is a typical case of chronic cadmium poisoning. Similar to Pb(II), the negatively charged COFs can absorb Cd2+ by electrostatic attraction, or use special functional groups containing O or N as active sites to capture Cd2+ through chelation. Dinari et al.60 synthesized a novel N-enriched COF material through the condensation of triazine and trialdehyde. Due to the high N content and the accessible lone pairs of N electrons, it is easy to coordinate with Cd2+. Moreover, the N-enriched COF would change into an anion form at high pH. The active centers are exposed, and have a strong electrostatic interaction with Cd2+ (396 mg·g−1). Liu et al.175 successfully synthesized a novel heteropore COF (COF-ETTA-2,3-Dha) using a solvothermal method. In the crystal structure, there are triangular micropores and hexagonal mesopores with pore sizes of 5.9 and 26.3 Å, respectively. Due to the introduction of a catechol segment, intramolecular hydrogen bonds in the structure of COF could not only enhance stability but also provide accessible active sites. The ortho-dihydroxy groups could capture Cd2+ from solution by chelation (Figure 3B).
Adsorption of Cr(VI)
Cr(VI) has strong oxidability, which can cause carcinogenesis and teratogenesis, and its toxicity is about 100 times that of Cr(III). Cr(VI) exists in the form of oxyanions (Cr2O72−, HCrO4−, and CrO42−) in water. So, the removal mechanism for metal cations is not applicable to Cr(VI). To improve the adsorption properties of COFs for Cr(VI), it is necessary to introduce some specific functional groups that are easily protonated, such as -OH, -NH2, and C=O, which can bind Cr(VI) effectively by electrostatic attraction or hydrogen bonds. It is worth noting that the adsorption of Cr(VI) is often accompanied by Cr(VI) reduction. Cui et al.61 synthesized a dual-pore COF (COF-BTA-DHBZ) with hydroxyl groups and firstly applied it for Cr(VI) elimination. COF-BTA-DHBZ has triangular micropores and hexagonal mesopores with pore sizes of 12.7 and 22.0 Å, respectively. The density of hydroxyl is higher in triangular micropores, and the adsorbed Cr(VI) is mainly wrapped in triangular micropores. The hydroxyl groups could not only provide the active sites, but also reduce Cr(VI) to Cr(III). COF-BTA-DHBZ has shown fast adsorption kinetics and excellent adsorption capacity (384 mg·g−1). Zhong et al.62 synthesized a novel magnetic COF material (Fe3O4@TpPa-1), and the amino groups of Fe3O4@TpPa-1 are changed into protonated cations (-NH3+) at pH 1.0, which had strong attraction to HCrO4− and CrO42−, so it could easily remove Cr(VI) from the solution. Zhu and colleagues63 synthesized two kinds of COFs (COF1 and COF2) with different hydroxyl distributions. Due to the synergistic effect of electrostatic interaction and intramolecular hydrogen bonds, they could adsorb Cr(VI) effectively (462.85 mg·g−1 for COF1 and 635.06 mg·g−1 for COF2). Interestingly, the adsorption properties of the two materials are different at different Cr(VI) concentrations. At low concentration, the ortho-distribution of hydroxyl groups in COF1 is more conducive to adsorption, so the adsorption performance is better than that of COF2. However, at high concentration, the para-distribution of hydroxyl groups in COF2 could provide more active sites and thereby increases its adsorption performance (Figure 3C).
Adsorption of Other Heavy Metals
In recent years, there also have been a few reports about using COFs to adsorb heavy metals. Cai et al.64 synthesized Q-graphene (QG)-scaffolded COFs (Figure 3D) to detect Cu2+ in solution and blood. QG-scaffolded COFs could not only detect Cu2+ but also remove Cu2+ effectively. Jiang et al.65 synthesized TpPa-NH2@EDTA by using EDTA to modify COF (Figure 3E). TpPa-NH2@EDTA has a good adsorption effect on metal ions, such as soft Lewis acid (Ag+, Pd2+), borderline Lewis acid (Cu2+, Ni2+), and hard Lewis acid (Fe3+, Cr3+), with removal efficiencies of >85% within 5 min. Zhou et al.66 synthesized a fluorescent thioether-functionalized COF (TTB-COF) to preconcentrate Au selectively in low concentrations (Figure 3F) with excellent stability. The strong coordination interaction between Au ions and S atoms in the thioether groups dominates the high Au adsorption (560 mg·g−1). The color of Au/TTB-COF has clearly changed from yellow to brown after treatment with Na2S solution, suggesting the reduction of Au3+ to stable gold nanoparticles. Fe0/TAPB-PDA67 could adsorb As(III) from acidic wastewater in nonferrous smelting industry. The positively charged surface has electrostatic interaction with negatively charged As(III) species (H2AsO3−). When the existing form of As(III) is H3AsO3, it could also form hydrogen bonds with the material. In addition, due to the presence of Fe0, H2O2 and ·OH would be produced in the adsorption process to oxidize As(III) to As(V). Yang et al.68 synthesized EB-COF:Br and applied it to remove As(V) from nearly neutral water. The =N+ sites could produce electrostatic interaction with arsenate anions through the formation of hydrogen bonds with C-C=O groups.
Comparison between COF-based Materials and Other Materials in Removing Heavy Metals
Table 1 also lists some traditional materials for the removal of heavy metals. The adsorption kinetics of clay minerals, carbonaceous materials, and LDHs is slow, and the recyclability is poor. Metal oxides, mesoporous silica, and other materials are unstable under extremely alkaline or acidic conditions. NZVI has some advantages, such as large specific surface area, fast reaction rate, and rich active sites, but NZVI nanoparticles are easy to aggregate and oxidize. Mesoporous carbon nitride (CN) is a type of N-enriched material, which has triazine units in the structure that have a strong interaction with metal ions, but it is mainly used as a photocatalyst in the photocatalytic degradation of organic pollutants. MOFs have the advantages of high porosity, large specific surface area, and unsaturated metal sites. In contrast, COFs not only have the advantages of high porosity, large specific surface area, and stable structure, but also can be used as good modification platforms.
Functional groups, such as active amino, hydroxyl, and carboxyl groups, can be introduced into COFs through reasonable chemical modification. COFs have excellent adsorption performance because of their good adsorption capacity, fast adsorption kinetics, and great recyclability. Therefore, COFs have great application prospect and will remain a research hotspot in the future.
Strategy of Reduction and Immobilization
Due to limitations on the length of this paper, we only give the examples of heavy metal ion removal by adsorption. In fact, it is also a good strategy to reduce and fix heavy metal ions on COFs for heavy metal treatment. The adsorption of heavy metal ions by some COFs is often accompanied by a reduction reaction. For example, the toxicity of Cr(III) is much lower than that of Cr(VI); thus, Cr(VI) reduction to Cr(III) by using COFs has attracted a lot of attention.69,70 The photoconductivity and photocatalytic activity of COFs can be improved by modifying specific groups on the skeleton or combining them with other materials. Therefore, it is a promising research direction to treat heavy metal ions with COFs through reduction and immobilization.
Some studies on the removal of heavy metal ions by COFs are listed here. Generally, the adsorption and removal mechanisms of COFs for heavy metal ions are as follows (1) adsorption to unsaturated coordination sites, (2) adsorption through electrostatic interaction, and (3) adsorption by forming hydrogen bonds. Through some elaborate designs, modifying some special functional groups on the skeleton of COFs will greatly improve their performance.
Although COFs are very promising materials for the removal of heavy metals, there are few studies on the adsorption of some heavy metals (such as Cd, Co, and Ni). Thus, there are still many challenges for the development of COFs in the adsorption of heavy metals: (1) there are few studies on the possible risk assessment of COFs on humans and the environment, (2) the key to improve the adsorption performance of COFs for heavy metal ions is to apply appropriate adsorption mechanisms and select better modified groups, and (3) it is necessary for the commercial availability of COFs to explore a simpler synthesis method, improve the yield, and reduce the preparation cost.
Removal of Radionuclides by COF-based Materials
With the continuous increase of human demand for energy, nuclear energy has gradually been considered as a very important and necessary energy source.220 Although nuclear energy has the advantages of being clean and renewable, the generation of nuclear waste is a tricky question.221 The release of radionuclides into the ecosystem poses a huge threat to the environment and seriously affects the safety of humans and other organisms.222,223 Thus, the effective capture of radionuclides will bring huge gains to humanity.223, 224, 225 Because of the high chemical stability and postsynthesis modification with functional groups,226,227 COFs exhibit promising acid stability that can easily maintain original morphology and chemical property in acid conditions of 3 M, some even in conditions of 8 M.217,228 Up to now, we have simply divided COFs into three groups, namely, single COFs, organic-group-functionalized COFs, and COF-based composites. By introducing functional groups, metal ions, or other materials into COFs, the adsorption performance toward radionuclides will be significantly improved.
Removal of U(VI)
Uranium is an actinide element, which generally exists as UO22+ in radioactive waste.73 Long-time exposure to U(VI) can lead to liver damage and cancer risk.74 US EPA set the acceptable limitation of <30 ppb for uranium levels in drinking water.75 Thus, it is vital to remove U(VI) ions to ensure environmental safety. Recent progress regarding the adsorption of U(VI) on COFs and derivates is summarized in the succeeding subsections.
Pristine COFs
Li et al.76 synthesized a single COF-TpPa-1 using a solvothermal method. The COF-TpPa-1 has shown no significant difference before and after U(VI) adsorption, suggesting its high chemical stability after U(VI) loading. The adsorption of U(VI) onto this single COF-TpPa-1 is dependent on the completion of the chemisorption monolayer. The main adsorption capability results from surface physisorption rather than selective affinity, complexation, and ion exchange. Thus, the amount of uranium ions removed by single COFs is distinctively less than that of functionalized COFs and COF composites (Table 1).
Modified COFs
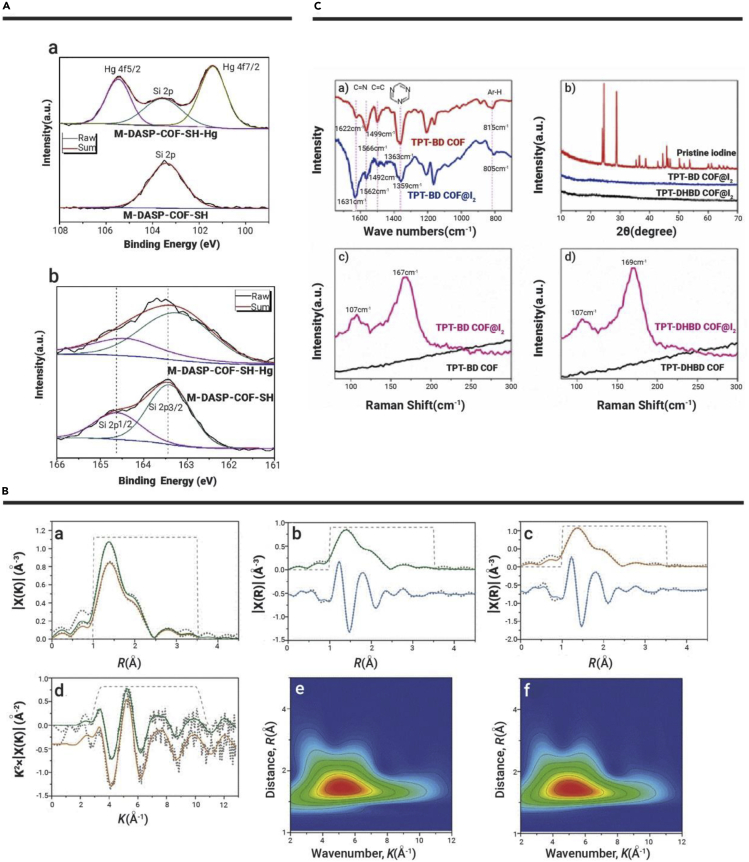
Numerous studies have confirmed that the functionalization of pristine COFs is an effective way to enhance their sorption performance.77, 78, 79 You et al.80 synthesized a COF-HAP composite using a solvothermal method to enhance U(VI) adsorption through the carboxyl groups by surface complexation. The uranyl cation is regarded as a strong Lewis acid with high affinity for hard oxygen donors, including carboxylate and hydroxide. In addition, the hydroxyl apatite in the COF-HAP could ionize partially under acidic conditions, which produces a spot of Ca2+ and PO43− ions to immobilize UO22+ by surface precipitation. The introduction of the amino groups neighboring the amidoxime could alter the electron density of the complex, which could lower the overall charge of uranyl and provide an additional hydrogen bonding site to align uranyl species in a favorable coordination fashion, thereby increasing the affinity toward uranyl. Sun et al.181 synthesized amidoxime-functionalized COFs (Figure 4A) for U(VI) extraction from potable water, well water, river water, and seawater. The U(VI) concentrations in these water samples could be reduced to less than 0.1 ppb with a single treatment by COFs. The ordered pore channels of COFs could facilitate the chelating groups to trap U(VI) ions. In addition, the stacking structure (Figure 4B) highly enhances U(VI) mobility, which again increases the contact opportunity between U(VI) ions and chelating groups. The selectivity of AO groups to U(VI) is attributed to the thermodynamically favorable η2-binding interaction and strong chelating ability of amidoxime groups with U(VI). Based on XPS and FTIR analysis, the binding energy of U 4f5/2 of U@COF-AO (~392.4 eV) is lower than that of UO2(NO3)·6H2O (393.4 eV), and a significant red shift of antisymmetric vibrations of UO22+ for U@COF-AO (~933 cm−1) compared with UO2(NO3)·6H2O (960 cm−1) demonstrated the interaction between U(VI) and AO. The binding energies of N 1s XPS spectra (~400 eV) of GO-COF before and after U(VI) adsorption are almost unchanged, yet a distinct shift of O 1s is observed, revealing the high efficient interaction of O-containing groups rather than N-containing groups.81 Thus, amidoxime- and oximation-modified COFs could serve as potential candidates for U(VI) uptake. The introduction of functional groups enhances the selective affinity of the COF skeleton toward radionuclides but, at the same time, the large cost of synthesis and little amount of production may limit industrial use of factories. Thus, a great effort still needs to be taken to optimize real practical applications.
Figure 4.
Modification of COF Structures
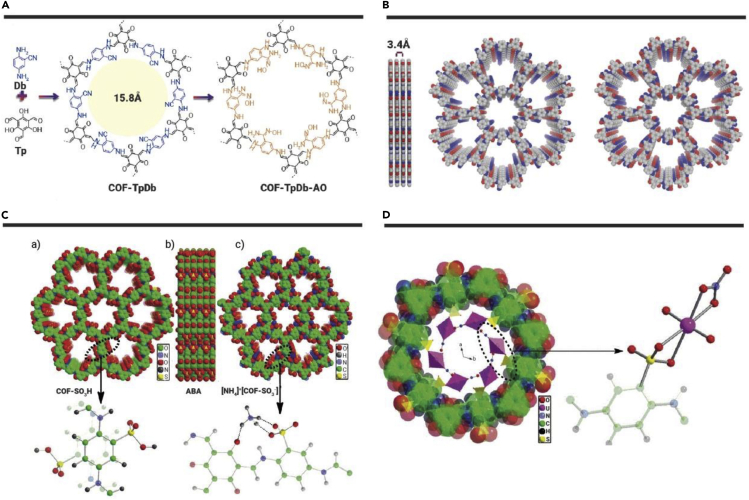
(A) Synthetic scheme of COF-TpDb-AO.
(B–D) (B) Graphic view of the eclipsed AA stacking structure of COF-TpDb and COF-TpDb-AO.181 (C) Space-filling view of the structure of (a and b) COF-SO3H and (c) [NH4]+[COF-SO3−]. The COF-SO3H shows the ABA stacking fashion, which means that the arrangement of -SO3H in the layers (a) and (b) is in the opposite mode rather than the common same-side mode (see the insert); the location of NH4+ and the hydrogen bond interactions between NH4+ and COF skeleton are highlighted (see the insert). (D) View of the DFT optimized uranyl-loaded structure for the [NH4]+[COF-SO3−] samples.82
COF-based Composites
With delicately and rationally designed properties, COFs combined with other materials can achieve considerably high adsorption performance because of synergistic effects. Wen et al.81 prepared oximation-functionalized and graphene-synergized 2D COF (o-GS-COF). The π-π interaction strengthens the interconnection of active functional groups located in the interlayer and intralayer of the lamellar structure. This composite maintains effective adsorption when pH is >2, owing to the comprehensive influences derived from mutual intercalation and the supporting effects of the two participating materials. GO and TDCOF in the composite not only lead to a significant increase of specific surface area of GS-COF but also introduce TDCOF into GO. The adsorption capacity of this composite is improved up to 221.1 mg·g−1 because the o-GO sheets offered additional adsorption sites. FTIR and XPS results show that U(VI) adsorption occurs due to definite coordination interactions. This composite also features better selectivity among 10 coexisting ions (Zn2+, Co2+, Ni2+, Mn2+, Sm3+, Gd3+, La3+, Nd3+, Sr2+, and Ce3+) and excellent acid stability after the treatment of 8 M HNO3 for 12 h. Recently, Xiong et al.82 synthesized COF-SO3H with a combination of 2,4,6-triformylphloroglucinol and 1,2-dichlorobenzene, and then prepared [NH4]+[COF-SO3−] by immersing COF-SO3H in NH3·H2O. The abundant –SO3− units in the pore wall could implement the coordination interaction toward U(VI). The introduced abundant NH4+ groups could serve as a deprotonation and U exchanger and thus could increase U(VI) removal efficiency (421 mg·g−1 for COF-SO3H and 869 mg·g−1 for [NH4]+[COF-SO3−]), which has exceeded many reported adsorbents (Table 1). The ultrahigh adsorption capacity and rapid adsorption kinetics are due to a synergistic effect from the robust and chemically stable porous framework, ion exchange between [NH4]+ and U(VI), as well as strong chemical adsorption sites of abundant freestanding -SO3− units on the pore wall. In the infrared (IR) spectra, new peaks at 926 and 918 cm−1 in the COF-SO3H and [NH4]+[COF-SO3−] samples are assigned to the antisymmetric vibration of uranyl ions, indicating the strong coordination interactions between uranyl ions and -SO3− groups. The adsorption ability in the real nuclear acid wastewater shows highly effective U(VI) adsorption at pH 1 and 8, giving 100% and 98% removal efficiency, respectively. NH4+ cations replace the position of H+ and are adsorbed near sulfonic functional groups by triple hydrogen bonds with two sulfonic oxygen atoms. Uranyl adsorption on [NH4]+[COF-SO3−] was in a way that NH4+ is rapidly displaced by (UO2)NO3+, firstly leading to the release of NH4NO3, and then (UO2)NO3+ is bound by sulfonic units (Figures 4C and 4D).
Although the combined COF composites possess many advantages to separate U(VI), their application is currently limited to laboratory setting. In addition, most combined materials are easy to collapse or hydrolyze due to their weak structural affinity and intensity. Meanwhile, the manufacturing cost is too high. Thus, the real application of COFs and COF-based materials in the separation of U(VI) from wastewater in large scale is still a great challenge.
Removal of Other Radionuclides
Some other radionuclides, such as Eu3+, Th4+, TcO4−, ReO4−, 129I−, Co2+, Cs+, and Sr2+, are also highly hazardous to human health.83, 84, 85, 86 In recent years, COF-based materials have shown a great potential in the preconcentration of radionuclides from radioactive wastewater.
Pristine COFs
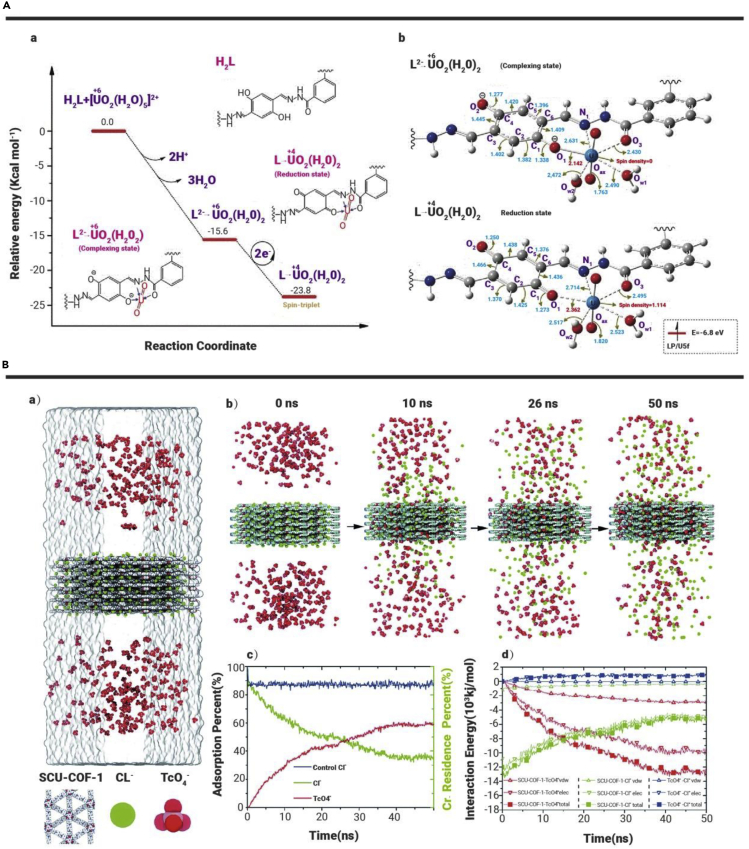
Single COFs have limited variety compared with functionalized COFs and COF-based composites. Their functionality mainly results from different raw material ligands and different pore sizes and pore topologies. For example, TpPa-1-COF is synthesized via Schiff-base reactions for Eu(III) removal from polluted water.87 SEM images and XRD patterns have demonstrated the snow-flake-like morphology, loose-layered structure, and π-π stacking of TpPa-1. Single TpPa-1-COF maintains effective adsorption ability in the range of pH 2–8, which makes it promising for practical application in spent nuclear fuel postprocessing. The Eu3d5/2 spectrum of TpPa-1-Eu can be disintegrated into two peaks at 1,134.65 and 1,136.98 eV, corresponding to -OH···Eu3+ and (≡X)3···Eu0, suggesting good affinity between Eu(III) and TpPa-1-COF. According to the Langmuir model, the maximum adsorption capacity of Eu(III) on TpPa-1-COF is 1,107.63 mg g−1 at pH 6.5 and T = 298 K, which is much higher than that reported for other materials (Table 1). Yin et al.88 synthesized a novel heterospore single COF (SIOC-COF-7) with two different micropores. These unprecedented COFs exist as hollow microspheres and exhibit an extremely high volatile iodine uptake (up to 481 wt%) by encapsulating iodine in the inner cavities and porous shells of the microspheres. Brunauer-Emmett-Teller (BET) surface area is estimated to be 618 m2·g−1 from the isotherm data in the range of P/P0 = 0.01–0.3. The pore size distribution, as reflected by nonlocal DFT, reveals two main pore size distributions at 5.0 and 11.8 Å. The formation of two kinds of channels (diameters of ~5 and ~12 Å) across the shells of the COF spheres cannot only accommodate iodine molecules but also facilitate the diffusion of iodine molecules into the inner cavities of the microspheres. He et al.217 synthesized a radiation-resistant COF (SCU-COF-1), which exhibits ultrahigh acid stability and great resistance toward both high-dose β- and γ-irradiation and 99TcO4− ultrahigh uptake capacity with extremely fast sorption kinetics to reach equilibration within 1 min and good anion-exchange selectivity. The almost unchanged characteristic BET surface area and vibrational peaks in the IR spectra of the samples treated with 3 M HNO3 further underscore the acidic stability. In addition, PXRD discloses that SCU-COF-1 can retain its crystal structure in THF, DMF, H2O, aqueous HNO3 (1 and 3 M), aqueous HCl (1 M), and NaOH (1 M) solutions and after β-irradiation at 200, 400, and 600 kGy for 48 h. In the presence of high concentrations of SO42−, CO32−, and PO43−, SCU-COF-1 still exhibits excellent selectivity with removal percentages from 85% to 99%. The high selectivity is due to the hydrophobicity of the COF skeleton, which provides remarkable affinity for hydrophobic anion with low charge density.
Modified COFs
The capture of 99TcO4− from nuclear waste is extremely desirable for waste disposal and environmental restoration. Da et al.89 synthesized functionalized guanidine-based hydrolytically stable cationic covalent organic nanosheets (i.e., iCON, which resulted from self-exfoliation of COFs by incorporating positively charged building blocks into the intralayer of COFs) named as DhaTGCl for effective uptake of ReO4− (a nonradioactive surrogate of TcO4−). DhaTGCl has shown extremely fast exchange kinetics toward ReO4− with a high uptake capacity of 437 mg·g−1 and prominent distribution coefficient of 5.0 × 105. Meanwhile, DhaTGCl shows an excellent selective capture of ReO4− in the presence of NO3−, CO32−, PO43−, and SO42− in a simulated Hanford LAW Melter Recycle Stream. The weak interaction between chlorine ions and the skeleton contributes to the exchange of ReO4−. Lu et al.72 synthesized a 3D carboxyl-functionalized COF (3D-COOH-COF) for selective extraction of Nd3+ ions. The extraction of Nd3+ is much higher than that of Sr2+ and Fe3+, which could be ascribed to the greater charge density and the larger ionic radius of Nd3+ relative to Sr2+ and Fe3+. IAST results showed that Nd3+ can be isolated from Sr2+ and Fe3+ solutions at high purity, which suggests that the coordination environment and strength of the metal ion framework interactions are very uniform in 3D-COOH-COF. Carbon-13 nuclear magnetic resonance signal of carbons in the carboxyl groups downfield has shifted 2.9 nm, demonstrating the strong interaction between Nd3+ and the carboxyl groups in Nd/3D-COOH-COF. The negative shift of 3.6 eV in the XPS spectrum is evidence of the interaction between Nd3+ and the carboxyl groups in Nd/3D-COOH-COF.
Strategy of Reduction and Immobilization
The oxidized U(VI) is highly hazardous and soluble, while reduced U(IV) poses no evident threat to ecosystem and is easy to separate. Thus, the perniciousness of radioactive ions could also be eliminated by changing the valence state. The reduction of radionuclides mainly takes place in two ways, namely, electric reduction and photocatalytic reduction. However, electric reduction, mostly used in lithium storage,229 hydrogen evolution,230 and CO2 reduction,231 is rarely used for adsorption-reduction of radionuclides.
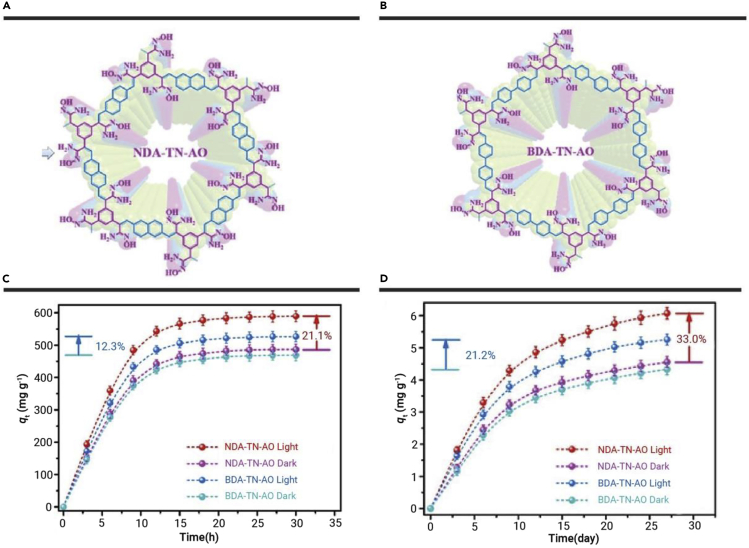
To have an efficient photocatalytic activity, a higher planar extended π-conjugated system is critical to transmit the electrons freely in the π system. Cui et al.232 synthesized the highly planar conjugated naphthalene-based sp2-carbon COFNDA-TN-AO and interrupted π-conjugated COFNDA-TN-AO (Figures 5A and 5B). The ESI and photocurrent response measurements have demonstrated that COFNDA-TN-AO outperforms COFBDA-TN-AO, indicating a much more efficient separation of charge carriers and interfacial charge transfer in the highly planar π-conjugated COFNDA-TN-AO skeleton. The excellent photoelectric effect of COFNDA-TN-AO could effectively release electrons from the skeleton and form a positive surface electric field around the skeleton, which shows a strong electrostatic attraction to [UO2(CO3)3]4− to increase the extraction capacity of uranium. After irradiation by simulated sunlight, the U(VI) adsorption capacity of COFNDA-TN-AO increases from 486.4 to 589.1 mg·g−1 (21.2% increase, Figure 5C), while light irradiation of COFBDA-TN-AO only increases U(VI) adsorption capacity by 12.3%, which could be attributed to the much better photoelectric and photocatalytic activity of COFNDA-TN-AO. XPS has demonstrated the coexistence of U(VI) and U(IV) under simulated sunlight irradiation. Under dark conditions, the adsorption capacities of COFNDA-TN-AO and COFBDA-TN-AO are 4.56 and 4.33 mg·g−1, respectively. The uranium adsorption capacities of COFNDA-TN-AO and COFBDA-TN-AO increase by 33.0% and 21.2%, respectively, after 27 days of light exposure (Figure 5D), which is attributed to the photocatalytic and photoelectric activity.
Figure 5.
U(VI) Adsorption by Modified COF-based Materials
(A) Fully planar π-conjugated BDATN-AO. (B) Interrupted π-conjugated NDA-TN-AO. (C) Uranium extraction capacity. (D) Adsorption of uranium from seawater.232
Comparison between COF-based Materials and Other Adsorbents in Removing Radionuclides
Apart from the above-mentioned COF-based materials, many other COF-based materials have been applied for the adsorption of radionuclides (Table 1). Complexation and coordination are the main mechanisms of U(VI) sorption onto pristine COFs, owing to their highly ordered structures. For modified COFs and COF-based materials, coordination with the functional groups is an alternative interaction mechanism. The highest adsorption capacity for U(VI) has been reported to be 851 mg·g−1 for [NH4]+[COF-SO3−].82 To the authors’ knowledge, the most selective COF-based adsorbent for U(VI) reported to date is o-GS-COF.81 The COF with the highest radiation stability reported is SCU-COF-1, which has been confirmed to maintain its physicochemical property even under irradiation of 600 kGy β-rays and 600 kGy γ-rays,217 enough to meet the need of applications in radioactive waste management. As shown in Table 1, several COF-based materials have shown superior adsorption capacities, which are much higher than those of other widely used materials.
COF-based Materials for Organic Pollutants Elimination
Organic pollutants extensively exist in industry and agriculture production, arousing great attention.233, 234, 235 In this section, the elimination of serval categories of pollutants by novel COF-based materials is summarized and analyzed. Also, different removal strategies, such as magnetic solid-phase extraction, solid-phase microextraction, membrane filtration, and interfacial adsorption are mentioned and described.
Removal of Bisphenol A
Over the last decades, endocrine-disrupting chemicals have been detected in various natural aqueous solutions due to their extensive applications in the fields of polycarbonate plastics, epikotes, flame retardants, and pesticides among others. BPA is a typical endocrine disruptor, which can result in hormone disorder, heart disease, and abnormal liver function in humans even in ultralow concentrations.236 Here, the adsorption behaviors of BPA by different kinds of COF-based materials are summarized and discussed.
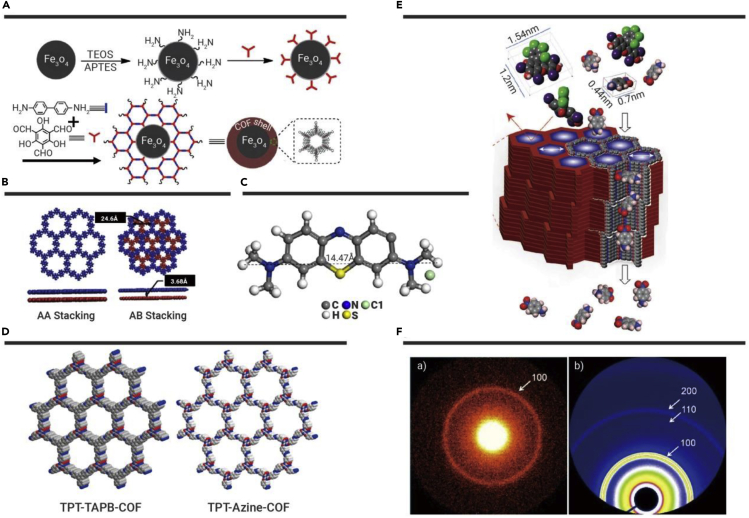
Magnetic COF-based materials are widely investigated due to their practicability and efficiency with regard to magnetic separation. Li et al.90 reported a core-shell Fe3O4@TpBD with an inner core of super-paramagnetic Fe3O4 and a TpBD shell. Amino groups are introduced on the surface of Fe3O4 for pregrafting of the TpBD monomer (Figure 6A). The adsorption of BPA on Fe3O4@TpBD has shown equilibrium within 5 min with a maximum sorption capacity of 160.6 mg·g−1. Hydrogen bonding and a π-π interaction between the benzene ring and BPA are the main removal mechanisms. Recently, our group91 investigated BPA adsorption by MSPE technology via macroscopic experiments, spectroscopic analyses, and theoretical calculation. The high tolerance of ionic-strength variation indicates that the removal of BPA is controlled by inner-surface complexation. PXRD has demonstrated that the arrangement of TpND belongs to staggered AB stacking rather than the eclipsed AA pattern and the crystal structure is due to a hexagonal space group with lattice parameters (Figure 6B). BPA can be adsorbed onto a TpND shell due to the existence of hydrogen bonds with phenolic aldehyde groups. Besides, TpND could provide sufficient binding sites and trap BPA molecules.
Figure 6.
Characterization of COF-based Materials
(A) Illustration of the in situ growth strategy for the fabrication of core-shell Fe3O4@TpBD nanospheres.90 (B) Experimental PXRD pattern for TpND and calculated PXRD patterns for AA stacking and AB stacking.91 (C) Modeling illustration of an MB molecule.96 (D) Top views of TPT-TAPB-COF and TPT-Azine-COF99. (E) Schematic of the molecular sieving mechanism of the dye through TpBD.101 (F) 2D SAXS and synchrotron SAXS profiles of PC-COF.103
For the practical utilization of COF material, Liu et al.92 shaped azine-linked COF powder into a hierarchically porous monolith via ring-opening polymerization. The surface functional hydrazine and aldehyde groups, crystallinity, and micropore of COF powder have remained well after integration into the monolith. Flow tests of BPA elimination by serial monoliths have illustrated that the BPA removal efficiency of M28 reached ~97%, with the cumulative pollutant volume achieved at 18 mL, and the monoliths are well regenerated with flowing methanol. The high capacities and rapid regeneration rates of COF monoliths are attributed to the π-π and hydrogen bond interactions.
Removal of Organic Dyes
More than 100,000 organic dyes have been utilized by the textile, printing, leather tanning, and plastic industries.93 Most of the industrial waste effluent is discharged into the hydrosphere without proper management. The organic molecules and their complex characteristics and structural diversities potentially threaten the natural environment and human health due to their esthetic consequences, xenobiotic properties, toxicological properties, and other health-risk factors.94 Based on the charge of the chromophore groups, various organic dyes can be classified into anionic, cationic, and nonionic dyes. In the following sections, we primarily summarize four typical organic dyes (MB, RhB, CR, and MO) and their sorption by COF-based materials.
Elimination of MB
MB is one of the most common cationic organic dyes applied in paper making and cellular staining. Although MB is not considered the most toxic and pathogenic organic pollutant, negative symptoms, such as increase in heart rate, vomiting, and obnubilation, are observed in humans after intake of low concentrations.95 To uptake MB from water solutions, TPT-DMBD-COF has been utilized.96 The well-fitted intraparticle diffusion model revealed a two-stage adsorption reaction: film diffusion and intraparticle diffusion. Moreover, electrostatic attraction, π-π stacking of aromatic rings, and differences in pore size between MB (Figure 6C) and COF have been confirmed to be the uptake mechanism. Zhu et al.97 compared the adsorption processes of MB on task-specific TS-COF-1 and TS-COF-2. Based on the nonlocal DFT and pore size distribution, the pore size of TS-COF-1 was concentrated at 31 Å, which was sufficient to accommodate the transport of MB molecule (dimensions of 13.4 × 5.0 × 4.2 Å). According to Monte Carlo simulations, the saturation capacities of MB on TS-COF-1 and TS-COF-2 were computed to be ~1,200 and ~445 mg·g−1, which were consistent with the isothermal experimental data (~1,691 and 377 mg·g−1). Macroscopic experiments and in silico simulations indicated that the spatial effect of MB molecules and the essential pore size of COFs should be considered simultaneously in further elimination of organic dyes by COF-based materials.
Elimination of RhB
RhB, a typical fluorochrome, is widely utilized in food additives, cosmetics, and fluorescence probes. However, the carcinogenicity of RhB was reported by IARC, which revealed its potential threats.98 Two types of COFs (TPT-azine-COF and TPT-TAPB-COF, Figure 6D) with a heteroatom-rich linker TPT-CHO were applied for RhB removal.99 The high RhB removal (725 mg·g−1 for TPT-azine-COF and 970 mg·g−1 for TPT-TAPB-COF) were in accordance with the BET surface areas (957 m2·g−1 for TPT-TAPB-COF and 1,020 m2·g−1 for TPT-azine-COF). Higher removal capacities of RhB demonstrated the excellent selectivity and regeneration capability of TPT-azine-COF and TPT-TAPB-COF.
CuP-DMNDA-COF, a novel imine-linked porphyrin COF, was synthesized with the construction of CuTAPP and DMNDA.186 CuP-DMNDA-COF was modified with FeCl3 in acetone to form CuP-DMNDA-COF/Fe. The enhanced adsorption performance of RhB on CuP-DMNDA-COF/Fe revealed that the removal process was affected by the coordinate interactions between Fe(III) ions in the COF and the carboxy group of RhB. Thermodynamics and the van't Hoff equation were used to investigate the adsorption process, and the results (positive ΔH and ΔS, negative ΔG) illustrated a spontaneous and endothermic process.
Elimination of CR
CR, a typical anionic dye, is extensively used in the paper making industry and as an acid-base indicator and biological dye.100 The complicated chemical structure, high solubility, and carcinogenicity of CR demonstrate its high priority to be eliminated from the hydrosphere. To date, serial COF-based materials have been utilized for CR removal. A novel COF-COOH was synthesized through polymerization of Tp and DBA.176 Afterward, modified ions (Ca2+/Ni2+) were further introduced to form COF-COOCa and COF-COONi. In the FTIR spectra, the new peaks at 1,574 and 1,224 cm−1 corresponding to C=C and C-N bonds demonstrate the formation of a keto structure in COF-COOH. The numerous hydrogen bonding sites and π-conjugated structure of COF-COOH contributed to good capture efficiency for CR. The adsorption capacities of CR on COF-COOCa and COF-COONi were calculated to be 704.2 and 781.3 mg·g−1 respectively, which were attributed to the enhanced electrostatic interaction of metal ion sites with CR.
A porous and crystalline COF membrane was fabricated by the methodology of baking organic linkers in the presence of PTSA and water.101 A filter adsorption study for the decontamination of CR (MW = 696.6, dimension ≈0.75 × 1.9 nm) was conducted in comparison with Rose Bengal (MW = 1,017.6, ≈1.2 × 1.54 nm), MB (MW = 319.8, ≈0.75 × 1.52 nm), and nitroaniline (MW = 138, ≈0.44 × 0.7 nm). Compared with other pollutants, the lower rejection rate (80%) for CR removal was correlated to its bigger pore size than that of COM. The sieving mechanism (Figure 6E) shows that the difference between the pollutant dimension and COF pore sizes is the crucial mechanism besides hydrogen bonding and π-π interactions.
Elimination of MO
MO has been typically utilized as an indicator for acid-base titration, biological coloring, and textile printing. The acute toxicity and mutagenicity of MO have been previously confirmed.102 Yu et al.103 synthesized a polycationic 2D COF (PC-COF) using TAPB and BFBP2+·2Cl− and applied it for the removal of MO. In the 2D SAXS profile and synchrotron profile (Figure 6F), the sharp scattering peak matching with the 100 facet and the synchrotron profile assigning to the 100, 110, and 200 facets indicated interlayer stacking in PC-COF despite the electrostatic repulsion between BIPY substances. Adsorption experiments showed a positive performance (85.1%) under strong solvation of dye molecules in aqueous solution. The main driving force was the electrostatic attraction between bipyridinium moieties of PC-COF and the anionic moieties of MO.
Elimination of Other Dyes
The decontamination of triphenylmethane dyes (CV and BG) by carboxyl COF-TzDBd was studied by Yan's group.177 Although the adsorption processes of CV and BG onto COF-TzDBd displayed good resistance under an NaCl concentration of 0.2 mol·L−1, the adsorptive capacities sharply reduced when the concentration was above 0.2 mol·L−1, indicating that the electrostatic interaction was one of the removal mechanisms for triphenylmethane dye uptake. The synergistic effects of the π-π interaction from the conjugated aromatic structure of COF-TzDBd were further proved by an obvious red shift of characteristic peaks based on UV-vis spectroscopic analyses. In addition, the molecular sizes of cationic CV and BG were favorable for the capture by TzDBd with a pore size of ~40 Å and anionic surface.
To date, direct fast scarlet 4BS was applied widely as the advanced substitute of Congo red. Afshari and Dinari104 reported an N-enriched T-COF for efficient adsorption of DFS-4BS from textile water. The N-enriched T-COF could adsorb DFS-4BS molecules for approximately 8.5 times of its own weight within 100 min. Interestingly, a smartphone colorimeter system was constructed with accessible equipment for accuracy comparison during the sorption of DFS-4BS. As shown in Figure 7A, the phone colorimeter system was structured with an LED lamp, quartz cell, and smartphone with Color Grab software 3.6.1. In this work, the dynamic range of the colorimetry system was limited to 70 mg·L−1, and an excellent concordance between spectrometer and phone colorimeter results was achieved in the removal of dye from real textile wastewater. The results further demonstrated that the proposed smartphone colorimeter system is a potential replacement for spectrometry.
Figure 7.
COF Foam Simulation and Application
(A) The smartphone colorimeter system for DFS-4BS concentration estimation.104 (B) Schematic illustration of COF foam synthetization and 3D volume rendered X-ray-computed images of the foam matrix and disordered macropores.181 (C) Space-filled model of ordered micropores of 2D crystal TpPa.188 (D) Graphical illustration of diclofenac and sulfamethazine elimination process by the MSPE strategy.169 (E) The fabricated exhibition and antibiotic removal reaction of NCCT.108
Recently, Karak et al.188 reported an in situ gas-phase foaming technique to induce disordered foam in ordered COF micropores. A schematic illustration of COF foams is depicted in Figures 7B and 7C. Various amines were reacted with PTSA, and then the generation of CO2 contributed to the formation of COF foams. Among the serial COF foams synthesized with different amines, TpPa-2 foam manifested excellent removal capacities and high rates (within 10 s) for the elimination of various organic dyes from water solutions. All elimination efficiencies for Acid Fuchsin, Rose Bengal, iodine, MB, and RhB were over ~96%, and the pseudo-second-order rate constants were 1.4, 30.3, 4.3, 11.8, and 7.4 g·mg−1·min−1 correspondingly. The diffusion through macropores and remaining adsorption inside micropores and mesopores were regarded as the main interaction mechanisms.
Overall, the molecular size of organic pollutants and predesigned pore diameter are extremely important in adsorptive applications of the COFs. In addition, the introduction of additives could enhance the electrostatic interaction between COFs and pollutants, which could significantly improve the removal performance of COF-based materials.
Removal of Pharmaceuticals
Over the decades, the rapid development of pharmaceutical industries and increasing consumption of pharmaceuticals for personal health unavoidably lead to the improper discharge of PPCPs into the aqueous environment.105 The toxicity, oncogenicity, teratogenicity, and mutagenicity of pharmaceuticals, such as ibuprofen, diclofenac, sulfamethazine, and ampicillin, severely threaten the ecosystem and humans.106 The crucial mechanisms for pharmaceutical decontamination have been reported to be electrostatic attraction, H bonding, π-π interaction, and surface complexation between pollutants and COFs.
Sulfamerazine, a typical sulfonamide used as a bacteriostat and as an anti-infective, is commonly utilized in clinical practice. However, its potential threats to the ecological system have aroused extensive concern. Zhuang et al.170 fabricated TPB-DMTP-COF for efficient elimination of sulfamerazine from aqueous solutions. Its highly crystalline structure, significant specific surface area (2,115 m2·g−1), and large channel (~3.3 nm) are favorable for considerable capacity (209 mg·g−1), fast reaction equilibrium (in 80 min), and good cyclability for sulfamerazine adsorption. Distinct pH-dependent sorption, zeta potential charge, and dissociation constants indicate electrostatic interaction as a vital mechanism for sulfamerazine elimination. Moreover, DFT calculations demonstrate that pollutant molecules could be adsorbed on the pore sites of COFs predominantly through the C-H···π interaction.
Ibuprofen, which is commonly selected as a lipophilic pharmaceutical contaminant, is frequently detected in effluent. Mellah et al.107 synthesized TpBD-(CF3)2 and applied it for ibuprofen removal. Equilibrium was reached within 60 min. High removal amounts at neutral conditions (~119 mg·g−1) and pH 2 (~150 mg·g−1) revealed the strong resistance of COFs under harsh circumstances. In contrast, a significant decrease in removal rate under alkaline conditions illustrated repulsions against the ionic carboxylic acid moiety on the COF surface. The adsorption comparison among ibuprofen, acetaminophen, and ampicillin indicates the potential selectivity of TpBD-(CF3)2 in the decontamination of lipophilic pharmaceutical contaminants.
TPB and DMTA were utilized as monomers to fabricate the precursor TPB-DMTP-COF and then an Fe element was introduced to form a magnetic COF. MSPE technology was used to uptake pharmaceutical contaminants from water solutions, such as diclofenac (109 mg·g−1) and sulfamethazine (113.2 mg·g−1).169 The specific surface area and pore size of magnetic COF were computed to be 2,245 m2·g−1 and 2.5 nm, respectively. DFT calculation was conducted to probe the removal mechanism, and the results illustrated that the adsorption of pharmaceutical contaminants was dominated by the π-π interaction and the C-H···π interaction, while H bonding was not observed. A graphical illustration of the removal process using the MSPE route is depicted in Figure 7D.
Although pharmaceutical antibiotics are widely used, they are identified as persistent contaminants due to their potential toxicity and due to the presence of bacteria resistant to them. Tetracycline and cefotaxime are frequently detected from aquatic environments and are difficult to eliminate. Li et al.108 reported a novel COF-based material named as “NCCT” to eliminate pharmaceutical antibiotics using the MSPE method. A brief fabricated illustration and removal reaction of NCCT are depicted in Figure 7E. The synergic research in the distribution of TC and pH-dependent results in chemicals has demonstrated that the driving force of adsorption is controlled by electrostatic attraction and surface complexation. According to FTIR analyses, cation exchange between the protonated amino groups and π electrons on NCCT is involved in the adsorption process of TC. Further spectroscopic studies have confirmed the existence of the condensation reaction, electrostatic effect, H bonding, and π-π interaction. The high removal capacities of TC (388.52 mg·g−1) and CTX (309.26 mg·g−1) have further implied the potential application of NCCT on the management of antibiotic contaminants in wastewater.
Removal of Aromatic Compounds
Compounds with benzene ring structure (mostly) and aromaticity are classified as aromatic compounds, which are confirmed to be stable, toxic, and nonbiodegradable. Typical aromatic compounds include phenolates, chloroarenes, nitrobenzenes, aromatic ethers, terephthalate esters, and related compounds.109
Solid-phase microextraction technology has been used to remove phenols. Adsorption of six nitroaromatic compounds (NACs) (2-nitrophenol, 3-nitrophenol, 4-nitrophenol, 2,4-dinitrotoluene, 1-fluoro-2-nitrobenzene, and 2,4-difluoronitrobenzene) on porous COF110 has shown fast dynamic performances (all NACs achieved equilibrium within 60 min) and experimental elimination amounts of NACs (239.88, 159.43, 158.25, 137.99, 98.50, and 78.63 mg·g−1). For application simulation, the recoveries of six NACs were in the range of 84.0%–112.3% in lake water, wastewater, and tap water samples. The extraction mechanism of NACs has indicated the size selectivity of COF for single-benzene ring compounds instead of multiple-benzene ring compounds, and that NACs with ortho groups possess better interaction with COF. Overall, the π-π stacking effect, hydrogen bonding, size difference, and functional group preference between COF and NACs are dominant forces. Wang et al.111 reported a solid-phase microextraction approach to remove seven phenols (phenol, 4-chlorophenol, 4-bromophenol, 2,4-dichlorophenol, 2,3-dichlorophenol, 3,4-dichlorophenol, and 2,3,6-trichlorophenol) from wolfberry, Robinia, and Codonopsis honey samples. Selectivity experiments among phenols, PAHs, and n-alkanes have indicated that π-π stacking interactions, π conjugation of aromatic groups, and hydrophobic interaction have dominated the capture reactions between SNW-1-coated fiber and contaminants.
Triphenyl phosphate, a typical aryl-organophosphorus flame retardant, is widely applied in ignition prevention for electronics equipment, plastics, and woods. Wang et al.112 synthesized COF-1, COF-2, and COF-3 with increasing pore size by Schiff-base reactions and enol-to-keto tautomerization between TFP and linkers PDA, BD, and DT, respectively. All COFs have exhibited high stabilities under 350°C and harsh acid conditions (8 M HCl for 6 days). The maximum removal capacities of TPhP on COFs have been computed to be 86.1, 387.2, and 371.2 mg·g−1, revealing that the proper pore size of COFs is favorable for adsorption reactions.
Catechol (pyrocatechol or 1,2-dihydroxybenzene) is utilized as a fungicide, a pesticide, a perfume, and an electroplating additive. The group of Yan113 reported a phenylboronic acid-functionalized COF (DhaTab-PBA) for efficient elimination of catechol. S 2p XPS spectra of DhaTab-PBA and organosulfur compounds have been observed due to the introduction of 4-mercaptophenylboronic acid. A negative enthalpy change has demonstrated excellent adsorption properties of DhaTab-PBA (160.0 mg·g−1). After five cycles of adsorption-desorption reactions, an inconspicuous decrease of adsorption capacities has revealed the good reusability of DhaTab-PBA. The selective study has indicated that the synergistic effect between the π-π interaction and phenylboronic acid moiety leads to a highly efficiency removal of catechol by DhaTab-PBA.
Elimination of Other Organic Pollutants
PFAS, such as PFOA and GenX, are utilized in fluoropolymer industries, as stain repellents, and as aqueous film-forming foams. Dichtel's group114 reported a hexagonal imine-linked 2D COF loaded with serial concentrations of amine functional groups (X%[NH2]-COFs) and azides (X%[N3]-COFs), which has exhibited high affinity toward anionic PFAS. Nonlocal DFT analyses have represented narrow pore size distributions (~2.6 nm), and that the pore sizes have increased along with the reduction of azide and amine (X) concentrations. The results demonstrated the formation of 2D imine-linked COFs with amine loadings (1%–100% terephthaldehyde monomers). Compared with X%[N3]-COF, higher GenX binding capacities have been obtained by X%[NH2]-COF (240 mg·g−1 for 20%[NH2]-COF). The amines in COF have produced beneficial microenvironments for GenX adsorption and a maximum of two GenX molecules could be bound under a sparsely distributed network.
Marine phycotoxin okadaic acid, the most extensive cause of diarrheic shellfish poisoning, could attribute to harmful algal blooms, which has caused great economic losses to seafood aquaculture. However, only a ow concentration of the toxin could be detected during an okadaic acid (OA) outbreak because most toxins are reserved in producer microalgae. Salonen et al.115 utilized TpBD-Me2-COF to eliminate OA from synthetic seawater under 19°C, and the reaction achieved equilibrium in 1 h, with a maximum adsorption capacity of 279 mg·g−1, which is much higher than that of previous studies. In addition, OA could be desorbed from TpBD-Me2-COF efficiently and reused for three cycles, revealing the potential for in situ concentration of OA with the use of TpBD-Me2-COF.
Overall, various organic matters are classified into EDCs, aromatic chemicals, dyes, and pharmaceutical pollutants. The adsorption performances by COF-based materials are exhaustively summarized and tabulated in Table 1. The abundant active sites, surface functional groups, high BET surface areas, opposite charges, and appropriate pore size distributions of COFs and modified materials contributed to the high capacities and efficiencies of contaminant elimination. Moreover, the sorption processes are mostly dominated by electrostatic attraction, π-π interaction, and hydrogen bond interaction, and some of the mechanisms are ascribed to surface complexation, molecular sieving, and functional group selectivity.
Mechanism Analysis of Removal of Pollutants by COF-based Materials
Investigating the mechanism of action of pollutants is predominant in the explanation of the removal process, which is vitally important to determine the reaction conditions of adsorption, desorption, and regeneration. Interaction mechanisms are mostly performed with the use of comprehensive experimental observations, spectroscopic analyses, and theoretical calculations. Adsorption performances are highly affected not only by multiple weak intermolecular interactions (e.g., hydrophobicity, hydrophilicity, electrostatic interactions, hydrogen bonding, and van der Waals [vdW] interactions) but also by intrinsic characteristics (e.g., surface charge, pore size, shape, electronic states, crystallinity, surface-to-mass ratio, decorated functional groups, and surface wrapping in the biological medium) of various adsorbents.237 Therefore, multiple mechanisms may simultaneously control pollutant adsorption, and the exact removal mechanism could be understood via macroscopic experiments and microscopic analysis.
Macroscopic Experiments
Typically, the adsorption mechanism may be ascribed to diverse types of interactions, which could be determined based on the reaction conditions. Therefore, investigating environmental conditions, such as pH, reaction time, and temperature, is useful to understand the removal mechanism. Typical kinetic and thermodynamic models are summarized in Table S1.
Background pH Value
Generally, the states of adsorbents, species of adsorbates, and their interaction are greatly influenced by solution pH values.96,170 The effects of solution pH on the adsorption process are mainly as follows (1) influencing the species distribution of pollutants, (2) altering the functional groups by protonation/deprotonation, and (3) changing the surface charge of adsorbents.76,87 For example, the adsorption process of U(VI) is strongly dependent on pH because of the different U(V) species at different pH conditions.76 UO22+ is the main species at pH < 3; polymerized hydrolysis ions, such as UO2(OH)+, (UO2)2(OH)22+, and (UO2)3(OH)5+, become the dominant species at pH > 3; and U(VI) directly becomes a precipitate (schoepite) at pH > 8.80 In terms of Hg(II), Hg2+ exists at pH < 3, while Hg2+, Hg(OH)+, and Hg(OH)2 are dominant states at pH 3–6. Insoluble Hg(OH)2 can be formed at pH > 6.58 Most BPA would not dissociate and exist in molecular form at pH < 9. However, BPA are deprotonated into anionic BPA (HBPA− and BPA2−) at pH ≥ 9.62
The surface charge of adsorbents is also dependent upon solution pH, which dramatically influences the adsorption ability of COF-based materials. At values less than pHzpc, the adsorbent surface is protonated, and increasing the positive charge will lead to a higher electrostatic interaction between adsorbent and anionic adsorbate. On the contrary, the surface charge of adsorbent turns to negative at pH > pHzpc, and leads to electrostatic repulsion between adsorbent and anionic pollutants.104 Taking TpPa-1 as an example, the amino groups of TpPa-1 and Fe3O4@TpPa-1 can be protonated to positive (-NH3+) at pH 1.0, which have a strong attraction to negatively charged chromium species (HCrO4− and Cr2O42−). Therefore, chromium anions could be easily removed from aqueous solution via electrostatic attraction.62 The removal of Cd(II) on N-enriched COF is negligible in mildly acidic conditions because the active sites of adsorbency are occupied through protonation by the nitrogen groups. By increasing the solution pH, an anionic form of COF is formed, so active centers of adsorbent are accessible for Cd(II) removal.60 Above all, the electrostatic interaction plays a vital role in the adsorption process.
Thermodynamic Analyses
Based on Brownian movement theory, the adsorption process of pollutants toward COF-based materials can be directly influenced by the temperature.58,238 Several isotherm models, such as the Langmuir, Freundlich, Dubinin-Radushkevich (D-R), and Temkin models, have been applied to reveal the interaction. The Langmuir and Freundlich models are extensively utilized for adsorption simulation. The applicability of isotherms is compared by recognizing the correlation coefficients and error analysis.60,71 The suitable model can accurately depict these adsorption processes and evaluate the bonding properties of COF-based materials.110 Assuming that there is no interaction between adsorbates, and that all the sorption sites on the solid surface are the same and distributed uniformly, then a uniform monolayer adsorption can be confirmed by the Langmuir model. Supposing that the adsorbate arrives at the nonuniform surface of adsorbents from the liquid phase and that the adsorbent has different adsorption sites on the surface, thus leading to different adsorption energies and unevenly distributed surface adsorption heat, then the uneven multilayer adsorption is in accordance with the Freundlich model.76,80 In brief, the Langmuir equation assumes homogeneous surface monolayer coverage, and the Freundlich model is an empirical multilayer adsorption model.169,178 The D-R isotherm has been studied to determine the nature of the adsorption (physical or chemical), which is related to the sorption mean free energy (E, kJ/mol).87 The dimensionless parameter RL is derived from the equation of KL, and the value demonstrates the acceptable adsorption for 0 > RL > 1, unfavorable for RL > 1, linear for RL = 1.0, and irreversible for RL = 0.87 The 1/n value of the Freundlich constant refers to the favorability of the adsorption process. An n > 1 implies a favorable adsorption process, while n = 1 displays an irreversible adsorption.239 For ED (kJ/mol) (D-R isotherm), ED < 8 (kJ/mol) suggests physical adsorption, while ED = 8–20 (kJ/mol) demonstrates chemical adsorption. Also, bT (the Temkin constant) is related to the heat of adsorption, and bT < 20 kJ/mol suggests a good consistency of superior physical adsorption.60
The Langmuir model has proved to be more suitable than the Freundlich model for describing the adsorption process of U(VI) on COF-TpPa-1,76 Hg(II) on TPB-DMTP-COF-SH,184 and six types of NACs on novel porous COFs.110 The adsorption of these pollutants toward COF adsorbents is dependent on the completion of the chemisorption monolayer and likely homogeneously dispersed on the porous scaffold. From the Langmuir equation, the maximum adsorption capacities of COF-TpPa-1 and TPB-DMTP-COF-SH are calculated to be 168.1 mg·g−1 for U(VI),76 and 4,395 mg·g−1 for Hg(II), respectively.184 The adsorption isotherm of BPA on PA-POF-2 is better fitted with the Freundlich model as compared with the Langmuir model, suggesting that multilayer adsorption, rather than monolayer adsorption, controlled the elimination process. The KF value (1,276 mg·L−1) and 1/n (0.4185) obtained from the Freundlich model indicate the large adsorption capacity of BPA.178 Sips model, which takes into account more comprehensive conditions, is more advanced than the Langmuir and Freundlich models to describe the adsorption process. The Sips model is a combination of both models and can be simplified into the Langmuir or Freundlich models under specific conditions.169 Both TCS and TCC have similar adsorption processes on Fe3O4@COFs. The Freundlich model is well fitted in the low-concentration region, while the Langmuir model is more appropriate at high concentration levels. This phenomenon might be multilayer adsorption through the interactions of space embedding effect, vdW forces, and benzene ring π-π stacking at low-concentration levels, and monolayer adsorption through strong π-π stacking between TCS or TCC and Fe3O4@COFs at high concentration levels.237 The synthesized N-enriched COF exhibits excellent performance in Cd(II) removal with high capacity (396 mg·g−1) by the Langmuir model. More importantly, bT (<20 kJ·mol−1) is in good agreement with superior physical adsorption. Moreover, Ed has been measured experimentally to be 1.4145 kJ·mol−1, indicating that the major mechanism is physical adsorption.60 Therefore, using isotherm models can confirm the good adsorption capacity of COF-based materials, and that the adsorption process can be put forward based on the experimental data.
To evaluate the adsorption thermodynamics, Gibbs free energy (ΔG0, kJ/mol), changes in standard values of enthalpy (ΔH0, kJ/mol) and entropy (ΔS0, kJ/(mol·K)) are generally calculated from temperature-dependent adsorption isotherms.62,240 For example, the removal of U(VI) by COF-TpPa-1 has shown that the adsorption process is endothermic, shows an increase in freedom, and is spontaneous with positive ΔH0 (48.605 kJ/mol), positive ΔS0 (221.77 J/(mol·k)), and negative ΔG0 (−17.515, −19.733, and −21.950 kJ/mol at 298.15, 308.15, and 318.15 K, respectively). Besides, ΔG0 decreases with increasing temperature, indicating an increased reaction at high temperature.76 As for Cr(VI)/BPA adsorption on TpPa-1, the thermodynamic study has confirmed its spontaneous, endothermic, and chemisorption processes. The negative ΔG0 value demonstrates the thermodynamic feasibility as well as a spontaneous adsorption process. Positive ΔH0 indicates an endothermic process, implying that chemisorption is the principal reaction mechanism. In addition, positive ΔS0 values not only reflect the affinity between adsorbents and adsorbates, but also improve the level of disorder at the solid-liquid interfaces.62 The adsorption of BPAF and BPA on Fe3O4@TpBD is confirmed to be a thermodynamically spontaneous process with both negative ΔH0 and ΔS0.40 TzDBd also exhibited good affinity for CV and BG. The carboxyl group in TzDBd forms H bonds with water molecules, resulting in a higher state of ordering with a lower entropy value. When adsorption reaction occurs, CV and BG molecules replace water molecules due to electrostatic interaction. As a result, more water molecules are released and the hydration shell is disrupted, leading to an increase of enthalpy.240
Above all, investigating the temperature effect on the adsorption process is vital to assess the application scope of COF-based materials. Different surface adsorption models have been used to determine the adsorption types and properties between adsorbents and adsorbates. Adsorption capacities of COF-based materials are also calculated from the models. Adsorption thermodynamics can further confirm the extent and driving force of the adsorption process, and different parameters can reflect the affinity between adsorbents and adsorbates.
Adsorption Kinetics Analyses
Adsorption kinetics are mainly utilized to investigate the diffusion mechanism, adsorption control step, and influencing factors of adsorbed velocity.87,237 To further investigate the kinetic adsorption process, the pseudo-first-order kinetic model, the pseudo-second-order kinetic model, the intraparticle diffusion model, and the Elovich model are widely used.80 The correlation coefficient (R2) is used to check the validity of each model.60,76
Generally, the adsorption rate of adsorbate on a porous adsorbent mainly depends on intraparticle diffusion. The intraparticle diffusion model is applied to fit the adsorption kinetic data to clarify the diffusion process.112 The simulation of TPhP adsorption on COFs of three different pore sizes (COF1<COF2<COF3) by the intraparticle diffusion model implies that intraparticle diffusion is the rate-controlling step for COF1 and COF2, while the model fails to fit TPhP adsorption on COF3. Therefore, TPhP could more easily diffuse into a larger pore size of COFs.112 For the adsorption of Hg(II) over DAPS50-COF-SH, the rate-controlling step is controlled by chemisorption, intraparticle diffusion, and external diffusion according to the intraparticle diffusion and film diffusion models.57 The intraparticle diffusion model can also provide relevant information at different stages of the adsorption process.96 In addition, it is adopted to simulate dynamics data of Cr(VI)/BPA on Fe3O4@TpPa-1, and two phases with varying rates of adsorption have been observed. The first phase indicates instantaneous external surface adsorption or boundary layer diffusion, with the second phase corresponding to the slower internal-particle diffusion indicating that Cr(VI)/BPA diffuse into mesoporous of TpPa-1, which serves as a rate-determining step. Beyond that, the second-phase simulation curve did not pass through the origin, suggesting a chemical complex reaction or a chemical oxidation-reduction process.62
The pseudo-first-order model that describes that the adsorption of the adsorbate from the solution to the solid surface is controlled by the diffusion step, which is based on the assumption that the adsorption rate is controlled by a chemical process (electrostatic interaction, sharing of electrons, and ion exchange) or strong surface complexation.87,96 The pseudo-second-order model simulation of U(VI)/Eu(III) on TpPa-1 demonstrates that chemisorption or strong surface complexation govern adsorption processes.87 The pseudo-second-order model simulation suggests the rate-limiting factor of NAC adsorption to COFs.110 The pseudo-second-order kinetic model is more satisfactory in illustrating the adsorption of CV and BG on TzDBd, which also confirms the electrostatic interactions between the cationic dye molecules and the anionic carboxyl group of TzDBd.240 The adsorption of TCS and TCC onto Fe3O4@COFs is both better fitted by the pseudo-second-order model, and the process could be divided into two steps: a diffusion process via vdW forces dominates the first step in the initial 10 min, while the second step is dominated by special intermolecular π-π stacking interactions between benzene rings of pollutants and Fe3O4@COFs and the space-embedding effect of TCS and TCC on the Fe3O4@COFs mesoporous surface over 10 min.237
The Elovich model can reveal the irregularities of adsorption data that might be ignored by other kinetic models.80 The Elovich model simulation of Pb(II) kinetics adsorption on COF-TP and COF-TE has shown to be the best fitting, suggesting a heterogeneous process of Pb(II) diffusion into the lamellar COF materials. The fitting parameter α from the Elovich model is an initial rate parameter, and COF-TE (454.1 mg·g−1·h−1) presents a higher α value than COF-TP (356.3 mg·g−1·h−1), indicating that Pb(II) has a higher diffusion rate toward COF-TE. Weaker and less tight π-π stacking and larger interlayer spacing in COF-TE account for favorable diffusion and adsorption.179
Therefore, research on adsorption kinetics is essential to understand the mechanism of the adsorption process. According to different kinetic models, the rate-controlling steps and adsorption diffusion mechanism can be estimated using chemisorption, strong surface complexation, intraparticle diffusion, and film diffusion.
Microscopic Analysis
Besides macroscopic analysis, various characterization technologies not only determine the surface properties of COF-based materials but also help us to understand the reaction pathway and mechanism via analysis of elemental species and surface functional groups before and after reactions.177,241,242
XPS Analysis
XPS can provide the surface composition of atoms and determine the valence of elements on the surface of materials.177,242 For example, in the XPS spectrum of Hg(II)-loaded M-DAPS50-COF-SH, the peaks at 101.4 and 105.4 eV are assigned to Hg 4f7/2 and Hg 4f5/2, confirming the successful adsorption of Hg(II) via chemical bonding (Figure 8A(a)). Compared with the Hg-loaded sample, the corresponding binding energy of S 2p in M-DAPS50-COF-SH shows a slight shift toward a higher energy (~0.4 eV) (Figure 8A(b)), demonstrating the involvement of S functional groups during Hg(II) adsorption.57 In addition, the 2P XPS spectra of S before and after Hg adsorption showed a movement of 0.3 eV.184 Therefore, the thiol and triazole functional groups in COF-based materials are confirmed to be the determining factor for the enhancement of Hg(II) adsorption. High-resolution Cr 2p spectrum undoubtedly confirms the successful adsorption of Cr(VI) on TpPa-1. Moreover, the peak of Cr 2p3/2 is deconvoluted into two components, which suggests the reduction of Cr(VI) to Cr(III). According to the inconspicuous fluctuation of molar percentage of Cr(III) after the introduction of Fe3O4, the reduction of Cr(VI) is mainly caused by electron donor groups of TpPa-1, with the aid of π electrons.62 The spectra of U 4f and Eu 3d confirm that U(VI) and Eu(III) are successfully adsorbed onto TpPa-1.87 Above all, applying XPS spectroscopy can identify the different species of the metals before and after adsorption. It can concluded that the removal of metal ions is not only by adsorption on the typical structures of the COFs but also by reduction with the functional group of the COF materials.
Figure 8.
Advanced Spectroscopy Analysis of Interaction Mechanism
(A) XPS spectra of Hg 4f (a) and S 2p (b) of M-DAPS50-COF-SH and Hg-loading samples.57 (B) (a) Fourier transform of U LIII-edge EXAFS spectra of POP-TpDb-AO (green line) and COF-TpDb-AO (orange line) in R-space, (b) Fourier transform of U LIII-edge EXAFS spectrum of COF-TpDb-AO in R-space. The magnitude of Fourier transform is fitted by a green line; the real component is fitted with a blue line. (c) Fourier transform of U LIII-edge EXAFS spectrum of POP-TpDb-AO in R-space. The magnitude of Fourier transform is fitted by an orange line; the real component is fitted with a blue line. (d) Accompanying k2-weighted χ(k) data and fit for POP and COF (green and orange lines, respectively). (e) Wavelet transform analysis of COF-TpDb-AO and (f) POP-TpDb-AO, demonstrating the extreme similarity between datasets. In all panels, gray dashes denote the fit window.181 (C) (a) FTIR spectra of TPT-BD COF and TPT-BD COF@I2, (b) Raman spectra of TPT-BD COF, and (c) TPT-DHBD COF before and after volatile iodine adsorption. (d) PXRD patterns of the pristine iodine and iodine-loaded COFs.243
FTIR Analysis
The FTIR technique involves profound investigation into the molecular structures and surface functional groups of adsorbents, which gives direct evidence of the bonding information in adsorption.80
FTIR characterization has been used to track the interaction between COF-PDAN-AO and U(VI) species. A new vibration peak at 920 cm−1 belonging to O=U=O confirms the successful adsorption of U(VI) on COF-PDAN-AO.244 Noticeable changes occur on the FTIR spectra of COF-Tz-OH after Pb(II) adsorption. The shifting of the stretching vibration band of the hydroxyl group and the adsorption bands of the triazine unit indicate that the adsorption process involves the interaction between Pb(II) and the surface hydroxyl and triazine moieties of COF-Tz-OH.224 In the FTIR spectra of DAPS50-COF-SH before and after Hg(II) loading, a new peak at 1,388 cm−1, which is attributed to the S-Hg stretching is clearly observed, suggesting the chemical bond between S and Hg.57 In the FTIR spectrum of TpBD-(CF3)2 after ibuprofen adsorption, the presence of the pharmaceutical is evident due to the appearance of C=O stretching of the ibuprofen carboxylic acid moiety at ~1,720 cm−1. The slight blue shift of free ibuprofen indicates the importance of hydrophobic interactions in pharmaceutical adsorption.107 The corresponding peaks in the FTIR spectra can confirm the presence of a functional group and its variation after adsorption. Therefore, the interaction between pollutants and COF-based materials, such as hydrophobic interactions, and electrostatic interactions, can be clearly explained.
XAFS Analysis
XAFS spectroscopy, including XANES and EXAFS, is useful for determining the binding environment and microstructure of metal ions.245,246 XANES spectroscopy provides persuasive evidence for the district oxidation-reduction state of metal ions by directly observing the energy shift of the absorption edge.247 EXAFS spectroscopy has been considered as a useful analytical technique for measuring the microstructures from the coordination numbers (N), bond distance (R), and Debye Waller factor (s2).143,247
Despite its widespread use in the fields of geochemistry and material science, XAFS has a limited application in COF research to characterize the adsorption on COF materials.242 To gain more insight into the coordination environment of mercury in the three thiol- or thioether-functionalized COF materials, Sun et al.182 collected the EXAFS spectra at the Hg LIII-absorption edge (12.284 keV). In contrast to previous EXAFS data, no Hg-O contributions were observed. This reveals that each Hg is bound exclusively by two S rather than through -Hg-O-Hg- layering or formation of terminal Hg-OH species, and confirms the adsorption of Hg by the COF materials rather than precipitation as HgO. The U LIII-absorption edge (17.166 keV) further shows the coordination environment of U(VI) in both COF-TpDb-AO and POP-TpDb-AO (Figure 8B).181 Inspection of the data suggests that the U(VI) coordination environment is similar between the two porous frameworks, and that reasonable fits are obtained from applying the same uranyl-benzamidoxime η2 binding motif model to both COF-TpDb-AO and POP-TpDb-AO (Figure 8B(a, b)). The successful simultaneous fitting of the EXAFS dataset with the same structure model, in conjunction with affording refined parameters within experimental uncertainty of each other, demonstrates a common uranium-binding model. In addition, the wavelet transform is applied for a common U-binding between both POP and COF materials (Figure 8B(c–f)). In contrast to the commonly used Fourier transform, the wavelet transform provides information regarding the localization of different EXAFS signal components in both k- and R-spaces simultaneously. This approach affords superior discrimination of EXAFS contributions from different species, as the phase and backscattering amplitude from different elements are emphasized in different regions of the k-space. The similarity between wavelet transforms for POP and COF indicates identical U-binding environments. However, compared with the randomly populated form in the POPs, the COF-based adsorbents are observed with improved performance, which suggested that the well-oriented chelating groups on the COFs are more ready to bind with U species. The difference is also confirmed by the above IR and XPS results. The computation results also predict that the binding angle of amidoxime groups and uranyl ions affects the overall energy of the resultant complex. Thus, one can conclude that the structural differences in the adsorbent framework are responsible for the divergent performance in adsorption. Applying XAFS spectroscopy can further confirm the ways of bonding and environments between pollutants and COF-based materials, which are essential to understand the interaction.
Combining Multi-technique Analysis
Besides the above-mentioned methods, other techniques, such as XPS, Raman, FTIR, UV-vis, and PXRD can reveal the mechanism on one side, while combining two or more techniques can explain the reaction process in more detail.
The adsorption mechanism of Cu(II) on TpODH has been explored by high-resolution FTIR and XPS analyses. In the FTIR spectrum, the band at 3,373 cm−1 weakened and shifted to 3,320 cm−1, and the bands at 1,540 and 1,237 cm−1 became broader and shifted to 1,519 and 1,227 cm−1, respectively. The shift of these peaks might reflect the electrostatic and ion exchange interactions between metallic ions and the functional group (-NH) of the material. The reduction in the intensity of FTIR signals demonstrates that coordination interactions between Cu(II) and amide, amino, and carbonyl groups have occurred. In the high-resolution XPS spectrum of TpODH-Cu, the binding energies at 932.5 and 952.2 eV, which correspond to the peaks of Cu 2p3/2 and Cu 2p1/2, indicate the interactions between Cu(II) and functional groups (-NH, -C=O). N 1s and O 1s XPS spectra of TpODH and TpODH-Cu further confirm the adsorption via electrostatic interaction and coordination interaction.238 The mechanism of I2 enrichment on COFs has been preliminarily studied by combing FTIR spectra, Raman spectra, and PXRD patterns. In FTIR spectra, the characteristic peak position of the materials changes significantly before and after the adsorption. The C=C and C-H bonds of the phenyl ring in TPT-BD COF have shifted from 1,499 and 815 cm−1 to 1,492 and 805 cm−1, respectively, and the C=N bonds of the triazine ring at 1,566 and 1,363 cm−1 have shifted to 1,562 and 1,359 cm−1, respectively. The imine linkages change markedly from 1,622 to 1,631 cm−1, indicating that iodine adsorption could simultaneously occur at imine linkage, triazine ring, and phenyl ring in the materials (Figure 8C(a)). Raman spectroscopy has been used to detect the species of iodine in TPT-BD COF and TPT-DHBD COF (Figure 8C(b,c)), and the strongest peak at ~167 cm−1 is confirmed to be the signature peak of I5− (consistent with I5− in the V or L configuration). The generation of I5− might occur through a charge transfer interaction between iodine guest molecules and the electron-rich TPT-DHBDX COFs. In addition, diffraction peaks of TPT-BD COF@I2 and TPT-DHBD COF@I2 in PXRD patterns did not show any characteristic peaks belonging to molecular iodine under saturated adsorption, suggesting the transformation of iodine as well (Figure 8C(d)). Based on these results, the high nitrogen content and abundant electron-rich π-conjugated system of TPT-DHBDX COFs lead to its reversible and ultrahigh adsorption capacities for volatile iodine.243
EDS can show the presence of significant amounts of captured adsorbates and their homogeneous distribution on COF-based materials.181 The evident bathochromic change of the characteristic peaks in UV-vis spectra can confirm the interaction during the adsorption process.240 IR spectroscopy and Raman spectroscopy are vibrational spectra, and can identify the functional groups.177 Therefore, to trace the interaction between the adsorbents and pollutants, multiple characterization methods can be performed together as a complement with each other.
Theoretical Calculations
Theoretical calculations are also urgently needed to probe the intrinsic adsorption behavior and establish structure property relationships.143 Such theoretical calculation are useful to elucidate the interaction mechanism, which is extremely difficult to achieved from experimental results.91 Combining structural information, such as bond length, bond angle, and binding energy, as well as the system charge density, underlying mechanisms can be successfully identified by theoretical calculation.143 The structural features of robust skeleton, high surface area, open mesopore channel, and density nitrogen sites of Ttba-TPDA-COF with RhB are evaluated by combining experimental results and DFT calculations.224
DFT can calculate the behavior of materials based on quantum mechanics.170 The most important advantage of DFT calculation is the simulation of the interaction processes and probing the species and microstructures at molecular levels under very complicated conditions. For example, DFT is utilized to explore and analyze the selective removal of uranyl ions by Redox-COF1. The species involved in the calculation and their relative free energy profiles are shown in Figure 9A(a). The relative free energy profiles of Redox-COF1 decreased from 0 to −15.6 kcal/mol after the removal of two protons and coordination with UO22+ to form a complex state. Next, two electrons of the complex state are transferred from oxygen to uranium to form a reduction state, and the process further lowers the relative free energy from −15.6 to −23.8 kcal/mol, indicating the reduction of U(VI) to U(IV) by Redox-COF1. The spin density of U changes from 0 to 1.114, which corresponds to 5f orbital occupancy (the lower right corner of Figure 9A(b)), suggesting that the electrons transfer from the O atom to U 5f orbitals. After electron transfer, bond lengths of C1-C2, C3-C4, C4-C5, and C1-C6 become longer, while those of C2-C3, C5-C6, C1-O1, and C4-O2 become shorter. The results are well consistent with the fact that hydroquinone is oxidized by uranyl ions to form a benzoquinone structure.248 Wang et al.40 simulated Hg interaction with Ag NPs@COF-LZU1. Comparing the calculated adsorption energies (using PW91/BS-II) for Hg(II) adsorption to different pure sites of the substrate, the highest adsorption energy is for the top sites of Ag NPs in COF-LZU1. In addition, the adsorption energies for Hg on COF-LZU1 are also very much large (using M06-2X/BS-I). These results suggest that the chemical adsorption between Hg and Ag NPs and physical adsorption with COF-LZU1 play roles simultaneously. The charge distribution analysis indicate the electron density of Hg atoms has increased, while that of Ag NPs has decreased, indicating that electrons are transferred from COF and Ag NPs to Hg atoms during the adsorption process. Therefore, a micro-redox reaction occurred on Ag NP surface (proton donors) during Hg (proton acceptors) adsorption from water. The COF material does not only serve as a supporting matrix but also as an electron donor during Ag Hg nanoalloy formation.40 In general, π-π interaction and H bonding interaction are found to contribute to organic pollutant adsorption onto COFs. Wei et al.91 used the DFT calculation to study the adsorption of BPA to TpND and found that BPA is preferentially captured by TpND phenolic aldehyde groups via the hydrogen bonds. Comparing the potential adsorbing sites, including the amine group (N1), the phenolic aldehyde groups (O1 and O2), and the benzene ring, they found that BPA molecules are prone to bind with TpND phenolic aldehyde groups with high adsorption energy. After calculating the density difference of the cluster, the main electron transfer occurs between the oxygen atoms (O1 and O2) in TpND phenolic aldehyde groups and the BPA hydrogen atoms (H1 and H2), suggesting a hydrogen bonding interaction during the adsorption process.91 The C-H···π interaction also plays an important role in the adsorption process of organic pollutants. Zhuang et al.169 simulated the partial mechanism of the adsorption between SMT/DCF and magnetic COFs, and found that the π-π interaction is the dominant interlayer interaction for the formation of the layered stacking structure, and that the C-H···π interaction contributes to the adsorption of SMT/DCF onto COFs. Zhuang et al.170 also proposed that the C-H···π interaction derived from the T-shaped or edge-to-face modes should be responsible for SMT adsorption by COFs according to the DFT calculation, which is also used to clarify the complicated relationship between pore size and sorption capacity. The DFT calculation of TPhP adsorption on three COFs with different pore sizes112 has proven that TPhP molecules could be easily diffused into COF2 and COF3 with larger pore sizes, while they are prevented from entering the pore structure of COF1 due to its relatively small pore size, resulting in an extremely low sorption capacity of TPhP on COF1. Although TPhP molecules could diffuse into the pore of COF3, the larger pore size could decrease the affinity of the pore structure for TPhP molecules and increase the tendency of TPhP molecules to escape outwards. The affinity is the result of multiple adsorption interactions, including the hydrophobic interaction and the π-π interaction, which occur between the aromatic groups on TPhP and COFs. According to the statistical results of annealing configurations, almost all SMT is trapped in the pore site rather than top site of the COFs. Although SMT molecules could be adsorbed on the top site by π-π interaction, available top adsorption sites are rather limited because of the AA stacking mode. The symmetry of regular hexagon pores and its extending channels account for the optimal symmetric adsorption site distribution on COF pore walls.170
Figure 9.
Computational Simulation
(A) (a) The species involved in the calculation and the relative free energy profiles (in kcal/mol) for the complexing and redox reaction between RedoxCOF1 and uranyl ions obtained by the DFT calculations. (b) The optimized structures of the stationary points for adsorption structure of the complexing state and the reduction state. Furthermore, the spin density of uranium atom and important bond lengths (Å) are also shown in this figure.248 (B) MD simulations on the adsorption of 99TcO4− anions into SCU-COF-1. (a) The initial configuration for MD simulations. (b) A time-series of snapshots to show the adsorbing dynamics of 99TcO4− anions into SCU-COF-1. The blue framework is the simulation box edge. (c) Time evolution of the adsorption rate of 99TcO4− anions (red) and the residence rate of Cl− anions in SCU-COF-1 (green); as well as the residence rate of Cl-anions in SCU-COF-1 in pure water as the control (blue curve). (d) The nonbonded interaction energies (including electrostatic, vdW, and their sum) of SCU-COF-99TcO4−, SCU-COF-Cl−, and 99TcO4−-Cl− as the function of simulation time.217
Molecular dynamics (MD) simulations are a comprehensive technique to simulate properties of molecular structures and explore dynamic interactions through the calculation of interaction energy and driving forces.145 He et al.217 used MD simulations to explore 99TcO4− adsorption dynamics to SCU-COF-1 and the underlying uptake mechanism (Figure 9B(a)). In the process, many Cl− anions that originally dwelt in the SCU-COF-1 pores are desorbed from the solid structure. To quantitatively describe the dynamics process, the adsorption kinetics of 99TcO4− from solution environment (red curve in Figure 9B(c)) and the residence kinetic of original Cl− in SCU-COF-1 (green curve in Figure 9B(c)), along with the interaction time, were calculated. As shown in Figure 9B(c), after ~26 ns, the amount of 99TcO4− ions entering SCU-COF-1 exceeds the amount of Cl− ions that still remain in SCU-COF-1. However, another control simulation in pure water demonstrates that nearly 87.2% of Cl− ions could be retained in SCU-COF-1 in the absence of 99TcO4− (blue curve in Figure 9B(c)). The simulation results suggest that the adsorption of 99TcO4− ions has initiated and furthered the anion exchange. To uncover the driving forces in the anion-exchange processes, time evolution of the nonbonded interaction energies of COF-Cl−, COF-99TcO4−, and 99TcO4−-Cl− were calculated, and they were further deconvoluted into the contributions from the vdW and electrostatic interactions as well (Figure 9B(d)). During the main 99TcO4− adsorption processes along with desorbing Cl− ions, the nonbonded interaction energy between SCU-COF-1 and 99TcO4− ions sharply decreased by ~13,000 kJ/mol, of which the electrostatic part was ~10,000 kJ/mol, about 3.3-fold of the vdW part (~3,000 kJ/mol). The nonbonded interaction energy between Cl− ions and SCU-COF-1 increased by ~8,000 kJ/mol, with the electrostatic part being ~7,400 kJ/mol and the vdW part ~600 kJ/mol. Meanwhile, the nonbonded interaction energy between 99TcO4− and Cl− ions increased by ~850 kJ/mol. The electrostatic part increased by 900 kJ/mol (repulsive), whereas the vdW part decreased by ~50 kJ/mol (attractive). The strong direct nonbonded interaction between SCU-COF-1 and 99TcO4− ions is intensively derived in this vigorous 99TcO4− adsorption process. Moreover, the direct electrostatic repulsive interaction between Cl− and 99TcO4− further promotes Cl− desorption. Thereby, the anion-exchange process could be revealed as: the strong binding free energy between SCU-COF-1 and 99TcO4− led to the successful substitution of Cl− by taking over the anion binding sites in SCU-COF-1 as contact time progresses. On the other hand, the occupation of 99TcO4− significantly weakened the direct interactions between Cl− and SCU-COF-1 and gradually excluded Cl−.217 MD simulations were also carried out to investigate BPA movement from the external solvent to the interior porous structure of TpND. Three BPA of different molecular numbers (Num 5, 10, and 20) were randomly settled in the box under the favorable adsorption conditions. For Num 5, the BPA molecules could easily pass through the surface and then be trapped in the interior of the TpND. For Num 10, a portion of BPA molecules are aggregated into the chain-type cluster and intercalated between the inner layers. With a continuous increase of the number of BPA molecules (Num 20), a mass of them aggregates into a big cluster and embed on the surface of TpND. Therefore, the TpND porous structure also plays significant roles in the adsorption process. The BPA molecules are trapped in the TpND porous structure, and the aggregating effect plays a vital role in the adsorption process at a high concentration of BPA.91
Above all, the theoretical calculations can confirm the results from experimental results at molecular level. From the DFT calculation, the adsorption binding sites and the reduction of some radionuclides/heavy metals can be simulated clearly. In addition, DFT can also reveal the complicated relationship between pore size and sorption capacity. MD simulations can further study the movement of adsorbates in solution and uncover the driving force in adsorption. The role of the porous structure in adsorption, ion exchange and electrostatic interactions is also confirmed by MD simulation. Therefore, theoretical calculation can play a primary role in the mechanism study.
Conclusions and Perspectives
The orderly porous structure of the COFs provides a high surface area and abundant porous channels, which are conducive to adsorption and fast diffusion of pollutants. These properties endow the COFs with the potential to be excellent adsorbents. To obtain COF-based materials with superior properties, specific functional groups are introduced into the COFs to enhance their interaction with the target pollutants. COF-based materials containing N, O, and S functional groups have higher adsorption capacity than single COFs due to electrostatic interaction, coordination, and chelation with metal ions.48,49,51,53,71 In addition, the configuration transformation of functional groups (i.e., the structure would convert from the enol form to the ketone form) can improve the adsorption performance.50 Combining the functional materials with COFs could enable an elegant combination of physical and chemical properties, synergistic effects, and multiple functions of individual components. Furthermore, the pore structure (e.g., pore size and shape) of COFs can be easily tailored by various methods, offering abundant options for the selective isolation of different pollutants. The hydrophobic skeleton structures of COFs with tailorable pore size also endow COFs with high adsorption performance toward toxic organic molecules via molecular sieving, π-π interaction, and H bonding among others. In addition, the pore-wall engineering of COFs is of great importance with regard to enriching them with more active adsorption sites.
We provide a comprehensive review of recent works on COFs and COF-based materials to remove toxic heavy metal ions, radionuclides, and organic pollutants. We elaborate the analyses of adsorption performance and approaches for mechanism investigations, including adsorption kinetics, adsorption thermodynamics, advanced spectroscopy techniques, and theoretical calculations at the molecular level. The adsorption performance of COFs and their composites with other traditional and advanced nanomaterials are compared. The outstanding adsorption capacities of COFs and COF-based composites mainly resulted from interactions between target ions and functional binding groups on COFs. The low density, permanent porosity, high specific surface area, highly ordered porous structure, and abundant porous channels also work together, which accelerates the diffusion rate of pollutants into the inner structures. Grafting appropriate functional groups onto COFs or blending them with other functional materials to tune their structures and properties have been proven to be efficient strategies for increasing their selectivity and adsorption capacity for targeted contaminants. The great potential of COFs and COF-based materials in environmental remediation has been gradually noted. COF-based catalysts can also be utilized in photocatalysis, redox reaction for pollutant degradation, or metal ion reduction. Various types of COF-based catalysts, including metal-free, heterometal, and hybrid ones, have been fabricated to increase their catalytic activities. However, study on these aspects is still at the initial stage and few studies have been reported.
While some intriguing progress and achievements have been achieved, the study of COFs and COF-based materials in environmental applications is still in its infancy. Several challenges in future development are discussed and some suggestions are proposed as follows: (1) to date, construction of functional COFs with desired performance, such as high adsorption capacity, strong forehead stability, and excellent ductility, still remains a challenging task. Therefore, more efforts should be devoted in the methods of synthesis of COFs and COF-based materials with excellent properties. (2) The disadvantages of high cost of synthesis, intricate operation, and low yield of COFs similarly hinder their further application in the field of environment decontamination. Thus, exploring green, simple, low-cost, and straightforward approaches is also a challenge of great urgency. (3) In general, COFs exhibit excellent removal effects toward a single target pollutant, but, in actual water bodies, there are often complex conditions in which multiple pollutants coexist. Therefore, a study on selective adsorption of COF materials is of more crucial practical value and should be further explored. (4) A large number of studies on the removal of environmental pollutants by COFs and COF-based materials have been reported, but the relationship between the structure, performance, and mechanism of COFs remains uncertain. The calculation of DFT, in situ XRD, XPS, EXAFS, and other advanced spectral techniques are highly recommended to detect the reaction process to distinguish reactive intermediates from the active sites.
The application of COFs and COF-based materials still presents many challenges in the efficient elimination of pollutants in real applications in large scale, such as the reusability, price, selectivity under complicated conditions, and separation from wastewater. Such problems will be resolved with the development of technology in the future. We believe that COFs and COF-based materials will have huge prospect in environmental pollution management.
Acknowledgments
Financial support from NSFC (21836001), National Key Research and Development Program of China (2017YFA0207002 and 2018YFC1900105), Science Challenge Project (TZ2016004), and Beijing Outstanding Young Scientist Program were greatly appreciated.
Declaration of interests
The authors declare that they have no conflicts of interest.
Published Online: January 4, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xinn.2021.100076.
Contributor Information
Baowei Hu, Email: hbw@usx.edu.cn.
Hui Yang, Email: h.yang@ncepu.edu.cn.
Xiangke Wang, Email: xkwang@ncepu.edu.cn.
Supplemental Information
References
- 1.Waller P.J., Gandara F., Yaghi O.M. Chemistry of covalent organic framework. Acc. Chem. Res. 2015;48:3053–3063. doi: 10.1021/acs.accounts.5b00369. [DOI] [PubMed] [Google Scholar]
- 2.Pyles D.A., Crowe J.W., Baldwin L.A., et al. Synthesis of benzobisoxazole-linked two-dimensional covalent organic frameworks and their carbon dioxide capture properties. ACS. Macro. Lett. 2016;5:1055–1058. doi: 10.1021/acsmacrolett.6b00486. [DOI] [PubMed] [Google Scholar]
- 3.Feng X., Ding X.S., Jiang D.L. Covalent organic frameworks. Chem. Soc. Rev. 2012;41:6010–6022. doi: 10.1039/c2cs35157a. [DOI] [PubMed] [Google Scholar]
- 4.Costa J.A.S., de Jesus R.A., da Silva C.M.P., et al. Efficient adsorption of a mixture of polycyclic aromatic hydrocarbons (PAHs) by Si-MCM-41 mesoporous molecular sieve. Powder Technol. 2017;308:434–441. [Google Scholar]
- 5.Yang Z.Y., Wang D.C., Meng Z.Y., et al. Adsorption separation of CH4/N-2 on modified coal-based carbon molecular sieve. Sep. Purif. Technol. 2019;218:130–137. [Google Scholar]
- 6.Furukawa H., ordova K.E., 'Keeffe M., et al. The chemistry and applications of metal-organic frameworks. Science. 2013;341:974. doi: 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- 7.Wibowo E., Rokhmat M., Sutisna K., et al. Reduction of seawater salinity by natural zeolite (clinoptilolite): adsorption isotherms, thermodynamics and kinetics. Desalination. 2017;409:146–156. [Google Scholar]
- 8.Demiral H., Gungor C. Adsorption of copper(II) from aqueous solutions on activated carbon prepared from grape bagasse. J. Clean. Prod. 2016;124:103–113. [Google Scholar]
- 9.Mohan D., Pittman C.U. Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006;137:762–811. doi: 10.1016/j.jhazmat.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 10.Pi Y.H., Li X.Y., Xia Q.B., et al. Adsorptive and photocatalytic removal of persistent organic pollutants (POPs) in water by metal-organic frameworks (MOFs) Chem. Eng. J. 2018;337:351–371. [Google Scholar]
- 11.Wu J.M., Zhou J.K., Wang W.A., et al. Progress in synthesis and application of covalent organic frameworks. Ekoloji. 2019;28:4369–4378. [Google Scholar]
- 12.Ashourirad B., Sekizkardes A.K., Altarawneh S., et al. Exceptional gas adsorption properties by nitrogen-doped porous carbons derived from benzimidazole-linked polymers. Chem. Mater. 2015;27:1349–1358. [Google Scholar]
- 13.Gao Q., Bai L.Y., Zhang X.J., et al. Synthesis of microporous nitrogen-rich covalent-organic framework and its application in CO2 capture. Chin. J. Chem. 2015;33:90–94. [Google Scholar]
- 14.Thote J., Aiyappa H.B., Deshpande A., et al. A covalent organic framework-cadmium sulfide hybrid as a prototype photocatalyst for visible-light-driven hydrogen production. Chem Eur. J. 2014;20:15961–15965. doi: 10.1002/chem.201403800. [DOI] [PubMed] [Google Scholar]
- 15.Pachfule P., Kandambeth S., Diaz D.D., et al. Highly stable covalent organic framework-Au nanoparticles hybrids for enhanced activity for nitrophenol reduction. Chem. Commun. (Camb.) 2014;50:3169–3172. doi: 10.1039/c3cc49176e. [DOI] [PubMed] [Google Scholar]
- 16.Stegbauer L., Schwinghammer K., Lotsch B.V. A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem. Sci. 2014;5:2789–2793. [Google Scholar]
- 17.Bhanja P., Bhunia K., Das S.K., et al. New triazine-based covalent organic framework for high-performance capacitive energy storage. ChemSusChem. 2017;10:921–929. doi: 10.1002/cssc.201601571. [DOI] [PubMed] [Google Scholar]
- 18.Vyas V.S., Haase F., Stegbauer L., et al. A tunable azine covalent organic framework platform for visible light-induced hydrogen generation. Nat. Commun. 2015;6:8508. doi: 10.1038/ncomms9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu F., Jin S.B., Zhong H., et al. Electrochemically active, crystalline, mesoporous covalent organic frameworks on carbon nanotubes for synergistic lithium-ion battery energy storage. Sci. Rep. 2015;5:8225. doi: 10.1038/srep08225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaleeswaran D., Vishnoi P., Murugavel R. [3+3] Imine and beta-ketoenamine tethered fluorescent covalent-organic frameworks for CO2 uptake and nitroaromatic sensing. J. Mater. Chem. 2015;3:7159–7171. [Google Scholar]
- 21.Dalapati S., Jin S.B., Gao J., et al. An azine-linked covalent organic framework. J. Am. Chem. Soc. 2013;13:17310–17313. doi: 10.1021/ja4103293. [DOI] [PubMed] [Google Scholar]
- 22.Bai L.Y., Phua S.Z.F., Lim W.Q., et al. Nanoscale covalent organic frameworks as smart carriers for drug delivery. Chem. Commun. (Camb.) 2016;52:4128–4131. doi: 10.1039/c6cc00853d. [DOI] [PubMed] [Google Scholar]
- 23.Zhang G.Y., Li X.L., Liao Q.B., et al. Water-dispersible PEG-curcumin/amine-functionalized covalent organic framework nanocomposites as smart carriers for in vivo drug delivery. Nat. Commun. 2018;9:2785. doi: 10.1038/s41467-018-04910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu M.X., Yang Y.W. Applications of covalent organic frameworks (COFs): from gas storage and separation to drug delivery. Chin. Chem. Lett. 2017;28:1135–1143. [Google Scholar]
- 25.Colson J.W., Mann J.A., DeBlase C.R., et al. Patterned growth of oriented 2D covalent organic framework thin films on single-layer graphene. J. Polym. Sci. Pol. Chem. 2015;53:378–384. [Google Scholar]
- 26.Sun B., Liu J., Cao A.M., Song W.G., et al. Interfacial synthesis of ordered and stable covalent organic frameworks on amino-functionalized carbon nanotubes with enhanced electrochemical performance. Chem. Commun. (Camb.) 2017;53:6303–6306. doi: 10.1039/c7cc01902e. [DOI] [PubMed] [Google Scholar]
- 27.Yao B.J., Li J.T., Huang N., et al. Pd NP-loaded and covalently cross-linked COF membrane microreactor for aqueous CBs dechlorination at room temperature. ACS Appl. Mater. Interfaces. 2018;10:20448–20457. doi: 10.1021/acsami.8b04022. [DOI] [PubMed] [Google Scholar]
- 28.Cote A.P., Benin A.I., Ockwig N.W., et al. Porous, crystalline, covalent organic frameworks. Science. 2005;310:1166–1170. doi: 10.1126/science.1120411. [DOI] [PubMed] [Google Scholar]
- 29.El-Kaderi H.M., Hunt J.R., Mendoza-Cortes J.L., et al. Designed synthesis of 3D covalent organic frameworks. Science. 2007;316:268–272. doi: 10.1126/science.1139915. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn P., Antonietti M., Thomas A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 2008;47:3450–3453. doi: 10.1002/anie.200705710. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y.F., Yao K.X., Teng B.Y., et al. A perfluorinated covalent triazine-based framework for highly selective and water-tolerant CO2 capture. Energ. Environ. Sci. 2013;6:3684–3692. [Google Scholar]
- 32.Yang H., Yang L.X., Wang H.J., et al. Covalent organic framework membranes through a mixed-dimensional assembly for molecular separations. Nat. Commun. 2019;10:2101. doi: 10.1038/s41467-019-10157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gui B., Lin G.Q., Ding H.M., et al. Three-dimensional covalent organic frameworks: from topology design to applications. Acc. Chem. Res. 2020;53:2225–2234. doi: 10.1021/acs.accounts.0c00357. [DOI] [PubMed] [Google Scholar]
- 34.Gao C., Li J., Yin S., Sun J.L., et al. Twist building blocks from planar to tetrahedral for the synthesis of covalent organic frameworks. J. Am. Chem. Soc. 2020;142:3718–3723. doi: 10.1021/jacs.9b13824. [DOI] [PubMed] [Google Scholar]
- 35.Wu C.Y., Liu Y.M., Liu H., et al. Highly conjugated three-dimensional covalent organic frameworks based on spirobifluorene for perovskite solar cell enhancement. J. Am. Chem. Soc. 2018;140:10016–10024. doi: 10.1021/jacs.8b06291. [DOI] [PubMed] [Google Scholar]
- 36.Wang J.L., Zhuang S.T. Covalent organic frameworks (COFs) for environmental applications. Coordin. Chem. Rev. 2019;400:213046. [Google Scholar]
- 37.Haug W.K., Moscarello E.M., Wolfson E.R., et al. The luminescent and photophysical properties of covalent organic frameworks. Chem. Soc. Rev. 2020;49:839–864. doi: 10.1039/c9cs00807a. [DOI] [PubMed] [Google Scholar]
- 38.Lan Y.S., Han X.H., Tong M.M., et al. Materials genomics methods for high-throughput construction of COFs and targeted synthesis. Nat. Commun. 2018;9:5274. doi: 10.1038/s41467-018-07720-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z.G., Zhang B.F., Yu H., et al. Microporous polyimide networks with large surface areas and their hydrogen storage properties. Chem. Commun. (Camb.) 2010;46:7730–7732. doi: 10.1039/c0cc02489a. [DOI] [PubMed] [Google Scholar]
- 40.Wang L.L., Xu H.M., Qiu Y.X., et al. Utilization of Ag nanoparticles anchored in covalent organic frameworks for mercury removal from acidic waste water. J. Hazard. Mater. 2020;389:121824. doi: 10.1016/j.jhazmat.2019.121824. [DOI] [PubMed] [Google Scholar]
- 41.Xu S.Q., Zhan T.G., Wen Q., et al. Diversity of covalent organic frameworks (COFs): a 2D COF containing two kinds of triangular micropores of different sizes. Acs Macro Lett. 2020;5:99–102. [Google Scholar]
- 42.Uribe-Romo F.J., Hunt J.R., Furukawa H., et al. A crystalline imine-linked 3-D porous covalent organic framework. J. Am. Chem. Soc. 2009;131:4570. doi: 10.1021/ja8096256. [DOI] [PubMed] [Google Scholar]
- 43.Waller P.J., AlFaraj Y.S., Diercks C.S., et al. Conversion of imine to oxazole and thiazole linkages in covalent organic frameworks. J. Am. Chem. Soc. 2018;40:9099–9103. doi: 10.1021/jacs.8b05830. [DOI] [PubMed] [Google Scholar]
- 44.Zhuang X.D., Zhao W.X., Zhang F., et al. A two-dimensional conjugated polymer framework with fully sp(2)-bonded carbon skeleton. Polym. Chem. 2016;7:4176–4181. [Google Scholar]
- 45.Zhao C.F., Diercks C.S., Zhu C.H., et al. Urea-linked covalent organic frameworks. J. Am. Chem. Soc. 2018;140:16438–16441. doi: 10.1021/jacs.8b10612. [DOI] [PubMed] [Google Scholar]
- 46.Chandra S., Kandambeth S., Biswal B.P., et al. Chemically stable multilayered covalent organic nanosheets from covalent organic frameworks via mechanical delamination. J. Am. Chem. Soc. 2013;135:17853–17861. doi: 10.1021/ja408121p. [DOI] [PubMed] [Google Scholar]
- 47.Wang H., Wang H., Wang Z.W., et al. Covalent organic framework photocatalysts: structures and applications. Chem. Soc. Rev. 2020;49:4135–4165. doi: 10.1039/d0cs00278j. [DOI] [PubMed] [Google Scholar]
- 48.Huang N., Zhai L.P., Xu H., et al. Stable covalent organic frameworks for exceptional mercury removal from aqueous solutions. J. Am. Chem. Soc. 2017;139:2428–2434. doi: 10.1021/jacs.6b12328. [DOI] [PubMed] [Google Scholar]
- 49.Ding S.Y., Dong M., Wang Y.W., et al. Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury(II) J. Am. Chem. Soc. 2016;138:3031–3037. doi: 10.1021/jacs.5b10754. [DOI] [PubMed] [Google Scholar]
- 50.Li X., Qi Y., Yue G.Z., et al. Solvent- and catalyst-free synthesis of an azine-linked covalent organic framework and the induced tautomerization in the adsorption of U(VI) and Hg(II) Green. Chem. 2019;21:649–657. [Google Scholar]
- 51.Li W.T., Zhuang Y.T., Wang J.Y., et al. A three-dimensional porous organic framework for highly selective capture of mercury and copper ions. ACS Appl. Polym. Mater. 2019;1:2797–2806. [Google Scholar]
- 52.Cui W.R., Jiang W., Zhang C.R., et al. Regenerable carbohydrazide-linked fluorescent covalent organic frameworks for ultrasensitive detection and removal of mercury. Acs. Sustain. Chem. Eng. 2020;8:445–451. [Google Scholar]
- 53.Afshari M., Dinari M., Zargoosh K., et al. Novel triazine-based covalent organic framework as a superadsorbent for the removal of mercury(II) from aqueous solutions. Ind. Eng. Chem. Res. 2020;59:9116–9126. [Google Scholar]
- 54.Li Y.F., Hu T.L., Chen R., et al. Novel thiol-functionalized covalent organic framework as adsorbent for simultaneous removal of BTEX and mercury (II) from water. Chem. Eng. J. 2020;398:125566. [Google Scholar]
- 55.Tao Y., Xiong X.H., Xiong J.B., et al. High-performance removal of mercury ions (II) and mercury vapor by SO3-anchored covalent organic framework. J. Solid State Chem. 2020;282:121126. [Google Scholar]
- 56.Ge J.L., Xiao J.D., Liu L.L., et al. Facile microwave-assisted production of Fe3O4 decorated porous melamine-based covalent organic framework for highly selective removal of Hg2+ J. Porous. Mater. 2016;23:791–800. [Google Scholar]
- 57.Huang L.J., Shen R.J., Liu R.Q., et al. Thiol-functionalized magnetic covalent organic frameworks by a cutting strategy for efficient removal of Hg2+ from water. J. Hazard. Mater. 2020;392:122320. doi: 10.1016/j.jhazmat.2020.122320. [DOI] [PubMed] [Google Scholar]
- 58.Lu X.F., Ji W.H., Yuan L., et al. Preparation of carboxy-functionalized covalent organic framework for efficient removal of Hg2+ and Pb2+ from water. Ind. Eng. Chem. Res. 2019;58:17660–17667. [Google Scholar]
- 59.Cao Y., Hu X., Zhu C.Q., et al. Sulfhydryl functionalized covalent organic framework as an efficient adsorbent for selective Pb(II) removal. Colloid. Surf. A. 2020;600:125004. [Google Scholar]
- 60.Dinari M., Hatami M. Novel N-riched crystalline covalent organic framework as a highly porous adsorbent for effective cadmium removal. J. Environ. Chem. Eng. 2019;7:102907. [Google Scholar]
- 61.Cui F.Z., Liang R.R., Qi Q.Y., et al. Efficient removal of Cr(VI) from aqueous solutions by a dual-pore covalent organic framework. Adv. Sustain. Syst. 2019;3:1800150. [Google Scholar]
- 62.Zhong X., Lu Z.P., Liang W., et al. The magnetic covalent organic framework as a platform for high-performance extraction of Cr(VI) and bisphenol a from aqueous solution. J. Hazard. Mater. 2020;393:122353. doi: 10.1016/j.jhazmat.2020.122353. [DOI] [PubMed] [Google Scholar]
- 63.Cao Y., Hu X., Zhu C.Q., et al. Highly efficient and selective removal of Cr(VI) by covalent organic frameworks: structure, performance and mechanism. Colloid. Surf. A. 2020;600:124910. [Google Scholar]
- 64.Cai Y.Y., Jiang Y., Feng L.P., et al. Q-Graphene-scaffolded covalent organic frameworks as fluorescent probes and sorbents for the fluorimetry and removal of copper ions. Anal. Chim. Acta. 2019;1057:88–97. doi: 10.1016/j.aca.2018.12.054. [DOI] [PubMed] [Google Scholar]
- 65.Jiang Y.Z., Liu C.Y., Huang A.S. EDTA-functionalized covalent organic framework for the removal of heavy-metal ions. ACS Appl. Mater. Interfaces. 2019;11:32186–32191. doi: 10.1021/acsami.9b11850. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Z.M., Zhong W.F., Cui K.X., et al. A covalent organic framework bearing thioether pendant arms for selective detection and recovery of Au from ultra-low concentration aqueous solution. Chem. Commun. (Camb.) 2018;54:9977–9980. doi: 10.1039/c8cc05369c. [DOI] [PubMed] [Google Scholar]
- 67.Liu X.S., Xu H.M., Wang L.L., et al. Surface nano-traps of Fe0/COFs for arsenic(III) depth removal from wastewater in non-ferrous smelting industry. Chem. Eng. J. 2020;381:122559. [Google Scholar]
- 68.Yang C.H., Chang J.S., Lee D.J. Covalent organic framework EB-COF:Br as adsorbent for phosphorus (V) or arsenic (V) removal from nearly neutral waters. Chemosphere. 2020;253:126736. doi: 10.1016/j.chemosphere.2020.126736. [DOI] [PubMed] [Google Scholar]
- 69.Chen W.B., Yang Z.F., Xie Z., et al. Benzothiadiazole functionalized D-A type covalent organic frameworks for effective photocatalytic reduction of aqueous chromium(VI) J. Mater. Chem. A. 2019;7:998–1004. [Google Scholar]
- 70.Xue K.H., He R., Yang T.L., et al. MOF-based In2S3-X2S3 (X=Bi; Sb)@TFPT-COFs hybrid materials for enhanced photocatalytic performance under visible light. Appl. Surf. Sci. 2019;493:41–54. [Google Scholar]
- 71.Firoozi M., Rafiee Z., Dashtian K. New MOF/COF hybrid as a robust adsorbent for simultaneous removal of auramine O and rhodamine B dyes. ACS Omega. 2020;5:9420–9428. doi: 10.1021/acsomega.0c00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu Q.Y., Ma Y.C., Li H., et al. Postsynthetic functionalization of three-dimensional covalent organic frameworks for selective extraction of lanthanide ions. Angew. Chem. Int. Ed. 2018;57:6042–6048. doi: 10.1002/anie.201712246. [DOI] [PubMed] [Google Scholar]
- 73.Liu W., Zhang L., Chen F., et al. Efficiency and mechanism of adsorption of low-concentration uranium from water by a new chitosan/aluminum sludge composite aerogel. Dalton Trans. 2020;49:85643. doi: 10.1039/c9dt04670d. [DOI] [PubMed] [Google Scholar]
- 74.Schnug E., Lottermoser B.G. Fertilizer-derived uranium and its threat to human health. Environ. Sci. Technol. 2013;47:2433–2434. doi: 10.1021/es4002357. [DOI] [PubMed] [Google Scholar]
- 75.Casagrande M.F.S., Moreira C.A., Targa D.A. Study of generation and underground flow of acid mine drainage in waste rock pile in an uranium mine using electrical resistivity tomography. Pure Appl. Geophys. 2020;177:703–721. [Google Scholar]
- 76.Li Z.D., Zhang H.Q., Xiong X.H., et al. U(VI) adsorption onto covalent organic frameworks-TpPa-1. J. Solid State Chem. 2019;277:484–492. [Google Scholar]
- 77.Li X., Yadav P., Loh K.P. Function-oriented synthesis of two-dimensional (2D) covalent organic frameworks—from 3D solids to 2D sheets. Chem. Soc. Rev. 2020;49:85964. doi: 10.1039/d0cs00236d. [DOI] [PubMed] [Google Scholar]
- 78.Kim G., Yang J., Nakashima N., et al. Highly microporous nitrogen-doped carbon synthesized from azine-linked covalent organic framework and its supercapacitor function. Chem. Eur. J. 2017;6:85632. doi: 10.1002/chem.201702805. [DOI] [PubMed] [Google Scholar]
- 79.Jiang D., Chen X., Geng K., et al. Covalent organic frameworks: chemical approaches to designer structures and built-in functions. Angew. Chem. Int. Ed. 2020;6:89632. doi: 10.1002/anie.201904291. [DOI] [PubMed] [Google Scholar]
- 80.You Z., Zhang N., Guan Q., et al. High sorption capacity of U(VI) by COF-based material doping hydroxyapatite microspheres: kinetic, equilibrium and mechanism investigation. J. Inorg. Organomet. Polym. Mater. 2019;30:1966–1979. [Google Scholar]
- 81.Wen R., Li Y., Zhang M.C., et al. Graphene-synergized 2D covalent organic framework for adsorption: a mutual promotion strategy to achieve stabilization and functionalization simultaneously. J. Hazard. Mater. 2018;358:273–285. doi: 10.1016/j.jhazmat.2018.06.059. [DOI] [PubMed] [Google Scholar]
- 82.Xiong X.H., Yu Z.W., Gong L., et al. Ammoniating covalent organic framework (COF) for high-performance and selective extraction of toxic and radioactive uranium ions. Adv. Sci. 2019;6:1900547–1900565. doi: 10.1002/advs.201900547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cai Y., Yuan F., Wang X., et al. Synthesis of core-shell structured Fe3O4@carboxymethyl cellulose magnetic composite for highly efficient removal of Eu(III) Cellulose. 2016;24:1–16. [Google Scholar]
- 84.Neeway J.J., Asmussen R.M., Lawter A.R., et al. Removal of TcO4-from representative nuclear waste streams with layered potassium metal sulfide materials. Chem. Mater. 2016;6:86536. [Google Scholar]
- 85.Falciglia P.P., Gagliano E., Brancato V., et al. Microwave based regenerating permeable reactive barriers (MW-PRBs): proof of concept and application for Cs removal. Chemosphere. 2020;251:126582. doi: 10.1016/j.chemosphere.2020.126582. [DOI] [PubMed] [Google Scholar]
- 86.Singh R., Shitiz K., Singh A. Immobilization of bacterial cells in hydrogels prepared by gamma irradiation for bioremoval of strontium ions. Water Air Soil Pollut. 2020;231:86956. [Google Scholar]
- 87.Zhong X., Liang W., Lu Z., et al. Highly efficient enrichment mechanism of U(VI) and Eu(III) by covalent organic frameworks with intramolecular hydrogen-bonding from solutions. Appl. Surf. Sci. 2020;504:144403. [Google Scholar]
- 88.Yin Z.J., Xu S.Q., Zhan T.G., et al. Ultrahigh volatile iodine uptake by hollow microspheres formed from a heteropore covalent organic framework. Chem. Commun. (Camb.) 2017;53:7266–7269. doi: 10.1039/c7cc01045a. [DOI] [PubMed] [Google Scholar]
- 89.Da H.J., Yang C.X., Yan X.P. Cationic covalent organic nanosheets for rapid and selective capture of perrhenate: an analogue of radioactive pertechnetate from aqueous solution. Environ. Sci. Technol. 2019;53:5212–5220. doi: 10.1021/acs.est.8b06244. [DOI] [PubMed] [Google Scholar]
- 90.Li Y., Yang C.X., Yan X.P. Controllable preparation of core-shell magnetic covalent-organic framework nanospheres for efficient adsorption and removal of bisphenols in aqueous solution. Chem. Commun. (Camb.) 2017;53:2511–2514. doi: 10.1039/c6cc10188g. [DOI] [PubMed] [Google Scholar]
- 91.Wei D.L., Li J.C., Chen Z.S., et al. Understanding bisphenol-A adsorption in magnetic modified covalent organic frameworks: experiments coupled with DFT calculations. J. Mol. Liq. 2020;301:112431. [Google Scholar]
- 92.Liu Z., Wang H., Ou J., et al. Construction of hierarchically porous monoliths from covalent organic frameworks (COFs) and their application for bisphenol A removal. J. Hazard. Mater. 2018;355:145–153. doi: 10.1016/j.jhazmat.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 93.Gupta V.K., Kumar R., Nayak A., et al. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv. Colloid. Interf. Sci. 2013;193:24–34. doi: 10.1016/j.cis.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 94.Yagub M.T., Sen T.K., Afroze S., et al. Dye and its removal from aqueous solution by adsorption: a review. Adv. Colloid. Interf. Sci. 2014;209:172–184. doi: 10.1016/j.cis.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 95.Rafatullah M., Sulaiman O., Hashim R., et al. Adsorption of methylene blue on low-cost adsorbents: a review. J. Hazard. Mater. 2010;177:70–80. doi: 10.1016/j.jhazmat.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 96.Huo J., Luo B., Chen Y. Crystalline covalent organic frameworks from triazine nodes as porous adsorbents for dye pollutants. ACS Omega. 2019;4:22504–22513. doi: 10.1021/acsomega.9b03176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu X., An S.H., Liu Y., et al. Efficient removal of organic dye pollutants using covalent organic frameworks. Aiche J. 2017;63:3470–3478. [Google Scholar]
- 98.Su X.M., Li X.Y., Li J.J., et al. Synthesis and characterization of core-shell magnetic molecularly imprinted polymers for solid-phase extraction and determination of rhodamine B in food. Food Chem. 2015;171:292–297. doi: 10.1016/j.foodchem.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 99.Li Y., Chen W., Hao W., et al. Covalent organic frameworks constructed from flexible building blocks with high adsorption capacity for pollutants. ACS. Appl. Nano. Mater. 2018;1:4756–4761. [Google Scholar]
- 100.Raval N.P., Shah P.U., Shah N.K. Adsorptive amputation of hazardous azo dye Congo red from wastewater: a critical review. Environ. Sci. Pollut. Res. Int. 2016;23:14810–14853. doi: 10.1007/s11356-016-6970-0. [DOI] [PubMed] [Google Scholar]
- 101.Kandambeth S., Biswal B.P., Chaudhari H.D., et al. Selective molecular sieving in self-standing porous covalent-organic-framework membranes. Adv. Mater. 2017;29:1603945. doi: 10.1002/adma.201603945. [DOI] [PubMed] [Google Scholar]
- 102.Haque E., Jun J.W., Jhung S.H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235) J. Hazard. Mater. 2011;185:507–511. doi: 10.1016/j.jhazmat.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 103.Yu S.B., Lyu H., Tian J., et al. A polycationic covalent organic framework: a robust adsorbent for anionic dye pollutants. Polym. Chem. 2016;7:3392–3397. [Google Scholar]
- 104.Afshari M., Dinari M. Synthesis of new imine-linked covalent organic framework as high efficient absorbent and monitoring the removal of direct fast scarlet 4BS textile dye based on mobile phone colorimetric platform. J. Hazard. Mater. 2020;385:121514. doi: 10.1016/j.jhazmat.2019.121514. [DOI] [PubMed] [Google Scholar]
- 105.Rivera U.J., Sánchez P.M., Ferro G.M.Á., et al. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere. 2013;93:1268–1287. doi: 10.1016/j.chemosphere.2013.07.059. [DOI] [PubMed] [Google Scholar]
- 106.Wang J., Wang S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: a review. J. Environ. Manage. 2016;182:620–640. doi: 10.1016/j.jenvman.2016.07.049. [DOI] [PubMed] [Google Scholar]
- 107.Mellah A., Fernandes S.P.S., Rodriguez R., et al. Adsorption of pharmaceutical pollutants from water using covalent organic frameworks. Chem. A. Eur. J. 2018;24:10601–10605. doi: 10.1002/chem.201801649. [DOI] [PubMed] [Google Scholar]
- 108.Li Z.H., Liu Y.Y., Zou S.Y., et al. Removal and adsorption mechanism of tetracycline and cefotaxime contaminants in water by NiFe2O4-COF-chitosan-terephthalaldehyde nanocomposites film. Chem. Eng. J. 2020;382:123008. [Google Scholar]
- 109.Lamichhane S., Krishna K.C.B., Sarukkalige R. Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: a review. Chemosphere. 2016;148:336–353. doi: 10.1016/j.chemosphere.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 110.Gao M.J., Fu Q.F., Wang M., et al. Facile synthesis of porous covalent organic frameworks for the effective extraction of nitroaromatic compounds from water samples. Anal. Chim. Acta. 2019;1084:21–32. doi: 10.1016/j.aca.2019.07.071. [DOI] [PubMed] [Google Scholar]
- 111.Wang W., Wang J., Zhang S., et al. A novel Schiff base network-1 nanocomposite coated fiber for solid-phase microextraction of phenols from honey samples. Talanta. 2016;161:22–30. doi: 10.1016/j.talanta.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 112.Wang W., Deng S.B., Ren L., et al. Stable covalent organic frameworks as efficient adsorbents for high and selective removal of an aryl-organophosphorus flame retardant from water. ACS Appl. Mater. Interfaces. 2018;10:30265–30272. doi: 10.1021/acsami.8b06229. [DOI] [PubMed] [Google Scholar]
- 113.Ji S.L., Qian H.L., Yang C.X., et al. Thiol-ene click synthesis of phenylboronic acid-functionalized covalent organic framework for selective catechol removal from aqueous medium. ACS Appl. Mater. Interfaces. 2019;11:46219–46225. doi: 10.1021/acsami.9b17324. [DOI] [PubMed] [Google Scholar]
- 114.Ji W., Xiao L.L., Ling Y.H., et al. Removal of GenX and perfluorinated alkyl substances from water by amine-functionalized covalent organic frameworks. J. Am. Chem. Soc. 2018;140:12677–12681. doi: 10.1021/jacs.8b06958. [DOI] [PubMed] [Google Scholar]
- 115.Salonen L.M., Pinela S.R., Fernandes S.P.S., et al. Adsorption of marine phycotoxin okadaic acid on a covalent organic framework. J. Chromatogr. A. 2017;1525:17–22. doi: 10.1016/j.chroma.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 116.Feng M.B., Zhang P., Zhou H.C., et al. Water-stable metal-organic frameworks for aqueous removal of heavy metals and radionuclides: a review. Chemosphere. 2018;209:783–800. doi: 10.1016/j.chemosphere.2018.06.114. [DOI] [PubMed] [Google Scholar]
- 117.Imam E.A., El-Sayed I.E., Mahfouz M.G., et al. Synthesis of alpha-aminophosphonate functionalized chitosan sorbents: effect of methyl vs phenyl group on uranium sorption. Chem. Eng. J. 2018;352:1022–1034. [Google Scholar]
- 118.Hu Y.Z., Wang X.X., Zou Y.D., et al. Superior sorption capacities of Ca-Ti and Ca-Al bimetallic oxides for U(VI) from aqueous solutions. Chem. Eng. J. 2017;316:419–428. [Google Scholar]
- 119.Liu X.L., Ma R., Zhuang L., et al. Recent developments of doped g-C3N4 photocatalysts for the degradation of organic pollutants. Crit. Rev. Environ. Sci. Technol. 2020 doi: 10.1080/10643389.2020.1734433. [DOI] [Google Scholar]
- 120.Wu Y.H., Pang H.W., Liu Y., et al. Environmental remediation of heavy metal ions by novel-nanomaterials: a review. Environ. Pollut. 2019;246:608–620. doi: 10.1016/j.envpol.2018.12.076. [DOI] [PubMed] [Google Scholar]
- 121.Zhao G.X., Zhang H.X., Fan Q.H., et al. Sorption of copper(II) onto super-adsorbent of bentonite-polyacrylamide composites. J. Hazard. Mater. 2010;173:661–668. doi: 10.1016/j.jhazmat.2009.08.135. [DOI] [PubMed] [Google Scholar]
- 122.Yang S.T., Sheng G.D., Tan X.L., et al. Determination of Ni(II) uptake mechanisms on mordenite surfaces: a combined macroscopic and microscopic approach. Geochim. Cosmochim. Acta. 2011;75:6520–6534. [Google Scholar]
- 123.Ren X.M., Li J.X., Tan X.L., et al. Comparative study of graphene oxide, activated carbon and carbon nanotubes as adsorbents for copper decontamination. Dalton Trans. 2013;42:5266–5274. doi: 10.1039/c3dt32969k. [DOI] [PubMed] [Google Scholar]
- 124.Bhatti A.A., Memon S., Memon N. Dichromate extraction by calix[4]arene appended amberlite XAD-4 resin. Sep. Sci. Technol. 2014;49:664–672. [Google Scholar]
- 125.Zheng Q.X., Li Z.L., Miao X.X., et al. Preparation and characterization of novel organic chelating resin and its application in recovery of Zn(II) from aqueous solutions. Appl. Organomet. Chem. 2017;31:e3546. [Google Scholar]
- 126.Li J., Chen C.L., Zhang S.W., et al. Surface functional groups and defects on carbon nanotubes affect adsorption-desorption hysteresis of metal cations and oxoanions in water. Environ. Sci. Nano. 2014;1:488–495. [Google Scholar]
- 127.Wang X.X., Yang S.B., Shi W.Q., et al. Different interaction mechanisms of Eu(III) and Am-243(III) with carbon nanotubes studied by batch, spectroscopy technique and theoretical calculation. Environ. Sci. Technol. 2015;49:11721–11728. doi: 10.1021/acs.est.5b02679. [DOI] [PubMed] [Google Scholar]
- 128.Wang X.K., Chen C.L., Hu W.P., et al. Sorption of 243Am(III) to multiwall carbon nanotubes. Environ. Sci. Technol. 2005;39:2856–2860. doi: 10.1021/es048287d. [DOI] [PubMed] [Google Scholar]
- 129.Zou Y.D., Cao X.H., Luo X.P., et al. Recycle of U(VI) from aqueous solution by situ phosphorylation mesoporous carbon. J. Radioanal. Nucl. Chem. 2015;306:515–525. [Google Scholar]
- 130.Sun Y.B., Wu Z.Y., Wang X.X., et al. Macroscopic and microscopic investigation of U(VI) and Eu(III) adsorption on carbonaceous nanofibers. Environ. Sci. Technol. 2016;50:4459–4467. doi: 10.1021/acs.est.6b00058. [DOI] [PubMed] [Google Scholar]
- 131.Cheng W.C., Ding C.C., Wang X.X., et al. Competitive sorption of As(V) and Cr(VI) on carbonaceous nanofibers. Chem. Eng. J. 2016;293:311–318. [Google Scholar]
- 132.Ding C.C., Cheng W.C., Wang X.X., et al. Competitive sorption of Pb(II), Cu(II) and Ni(II) on carbonaceous nanofibers: a spectroscopic and modeling approach. J. Hazard. Mater. 2016;313:253–261. doi: 10.1016/j.jhazmat.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 133.Zhao G.X., Li J.X., Ren X.M., et al. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol. 2011;45:10454–10462. doi: 10.1021/es203439v. [DOI] [PubMed] [Google Scholar]
- 134.Li J., Zhang S.W., Chen C.L., et al. Removal of Cu(II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles. ACS Appl. Mater. Interfaces. 2012;4:4991–5000. doi: 10.1021/am301358b. [DOI] [PubMed] [Google Scholar]
- 135.Liu X.L., Ma R., Wang X.X., et al. Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: a review. Environ. Pollut. 2019;252:62–73. doi: 10.1016/j.envpol.2019.05.050. [DOI] [PubMed] [Google Scholar]
- 136.Sun Y.B., Shao D.D., Chen C.L., et al. Highly efficient enrichment of radionuclides on graphene oxide-supported polyaniline. Environ. Sci. Technol. 2013;47:9904–9910. doi: 10.1021/es401174n. [DOI] [PubMed] [Google Scholar]
- 137.Guo R., Jiao T.F., Li R.F., et al. Sandwiched Fe3O4/carboxylate graphene oxide nanostructures constructed by layer-by-layer assembly for highly efficient and magnetically recyclable dye removal. ACS. Sustain. Chem. Eng. 2018;6:1279–1288. [Google Scholar]
- 138.Xing R.R., Wang W., Jiao T.F., et al. Bioinspired polydopamine sheathed nanofibers containing carboxylate graphene oxide nanosheet for high-efficient dyes scavenger. ACS. Sustain. Chem. Eng. 2017;5:4948–4956. [Google Scholar]
- 139.Liu J.M., Zhu K., Jiao T.F., et al. Preparation of graphene oxide-polymer composite hydrogels via thiol-ene photopolymerization as efficient dye adsorbents for wastewater treatment. Colloid. Surf. A. 2017;529:668–676. [Google Scholar]
- 140.Tan X.L., Fan Q.H., Wang X.K., et al. Eu(III) sorption to TiO2 (Anatase and Rutile): batch, XPS, and EXAFS studies. Environ. Sci. Technol. 2009;43:3115–3121. doi: 10.1021/es803431c. [DOI] [PubMed] [Google Scholar]
- 141.Yang S.T., Sheng G.D., Montavon G., et al. Investigation of Eu(III) immobilization on gamma-Al2O3 surfaces by combining batch technique and EXAFS analyses: role of contact time and humic acid. Geochim. Cosmochim. Acta. 2013;121:84–104. [Google Scholar]
- 142.Li J., Fan Q.H., Wu Y.J., et al. Magnetic polydopamine decorated with Mg-Al LDH nanoflakes as a novel bio-based adsorbent for simultaneous removal of potentially toxic metals and anionic dyes. J. Mater. Chem. A. 2016;4:1737–1746. [Google Scholar]
- 143.Wen T., Wu X.L., Tan X.L., et al. One-pot synthesis of water-swellable Mg-Al layered double hydroxides and graphene oxide nanocomposites for efficient removal of As(V) from aqueous solutions. ACS Appl. Mater. Interfaces. 2013;5:3304–3311. doi: 10.1021/am4003556. [DOI] [PubMed] [Google Scholar]
- 144.Wu X.L., Tan X.L., Yang S.T., et al. Coexistence of adsorption and coagulation processes of both arsenate and NOM from contaminated groundwater by nanocrystallined Mg/Al layered double hydroxides. Water Res. 2013;47:4159–4168. doi: 10.1016/j.watres.2012.11.056. [DOI] [PubMed] [Google Scholar]
- 145.Gu P.C., Zhang S., Li X., et al. Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ. Pollut. 2018;240:493–505. doi: 10.1016/j.envpol.2018.04.136. [DOI] [PubMed] [Google Scholar]
- 146.Manos M.J., Kanatzidis M.G. Metal sulfide ion exchangers: superior sorbents for the capture of toxic and nuclear waste-related metal ions. Chem. Sci. 2016;7:4804–4824. doi: 10.1039/c6sc01039c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li J., Wang X.X., Zhao G.X., et al. Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018;47:2322–2356. doi: 10.1039/c7cs00543a. [DOI] [PubMed] [Google Scholar]
- 148.Zhao G.X., Huang X.B., Tang Z.W., et al. Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: a review. Polym. Chem. 2018;9:3562–3582. [Google Scholar]
- 149.Wang H.H., Cui H.Z., Song X.J., et al. Facile synthesis of heterojunction of MXenes/TiO2 nanoparticles towards enhanced hexavalent chromium removal. J. Colloid Interface Sci. 2020;561:46–57. doi: 10.1016/j.jcis.2019.11.120. [DOI] [PubMed] [Google Scholar]
- 150.Gan D.F., Huang Q., Dou J.B., et al. Bioinspired functionalization of MXenes (Ti3C2Tx) with amino acids for efficient removal of heavy metal ions. Appl. Surf. Sci. 2020;504:144603. [Google Scholar]
- 151.Jun B.M., Kim S., Heo J., et al. Review of MXenes as new nanomaterials for energy storage/delivery and selected environmental applications. Nano. Res. 2019;12:471–487. [Google Scholar]
- 152.Zhang Y.J., Wang L., Zhang N.N., et al. Adsorptive environmental applications of MXene nanomaterials: a review. Rsc. Adv. 2018;8:19895–19905. doi: 10.1039/c8ra03077d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guo X., Zhang X.T., Zhao S.J., et al. High adsorption capacity of heavy metals on two-dimensional MXenes: an ab initio study with molecular dynamics simulation. Phys. Chem. Chem. Phys. 2016;18:228–233. doi: 10.1039/c5cp06078h. [DOI] [PubMed] [Google Scholar]
- 154.Zhang T.T., Yang Y.L., Gao J.F., et al. Synergistic degradation of chloramphenicol by ultrasound-enhanced nanoscale zero-valent iron/persulfate treatment. Sep. Purif. Technol. 2020;240:116575. [Google Scholar]
- 155.Li J., Fana M.J., Li M., et al. Cr(VI) removal from groundwater using double surfactant-modified nanoscale zero-valent iron (NZVI): effects of materials in different status. Sci. Total. Environ. 2020;717:137112. doi: 10.1016/j.scitotenv.2020.137112. [DOI] [PubMed] [Google Scholar]
- 156.Wu J.X., Wang B., Cagnetta G., et al. Nanoscale zero valent iron-activated persulfate coupled with Fenton oxidation process for typical pharmaceuticals and personal care products degradation. Sep. Purif. Technol. 2020;239:116534. [Google Scholar]
- 157.Zhang Y.T., Jiao X.Q., Liu N., et al. Enhanced removal of aqueous Cr(VI) by a green synthesized nanoscale zero-valent iron supported on oak wood biochar. Chemosphere. 2020;245:125542. doi: 10.1016/j.chemosphere.2019.125542. [DOI] [PubMed] [Google Scholar]
- 158.Zhang X., Cao X.Q., Li G., et al. Preparation of novel ALRCs/NZVI composite and its removal of Cr(VI) from aqueous. Int. J. Environ. Res. 2020;14:123–133. [Google Scholar]
- 159.Lye J.W.P., Saman N., Noor A.M.M., et al. Application of nanoscale zero-valent iron-loaded natural zeolite for tetracycline removal process. Chem. Eng. Technol. 2020;43:1285–1296. [Google Scholar]
- 160.Yi F.T., Ma J.Q., Lin C.W., et al. Insights into the enhanced adsorption/photocatalysis mechanism of a Bi4O5Br2/g-C3N4 nanosheet. J. Alloy. Compd. 2020;821:153557. [Google Scholar]
- 161.Sahoo S.K., Padhiari S., Biswal S.K., et al. Fe3O4 nanoparticles functionalized GO/g-C3N4 nanocomposite: an efficient magnetic nanoadsorbent for adsorptive removal of organic pollutants. Mater. Chem. Phys. 2020;244:122710. [Google Scholar]
- 162.Asadi F., Goharshadi E.K., Sadeghinia M. Highly efficient solar-catalytic degradation of reactive black 5 dye using mesoporous plasmonic Ag/g-C3N4 nanocomposites. ChemistrySelect. 2020;5:2735–2745. [Google Scholar]
- 163.He H.B., Luo Z.Z., Yu C.L. Diatomite-anchored g-C3N4 nanosheets for selective removal of organic dyes. J. Alloy. Compd. 2020;816:152652. [Google Scholar]
- 164.Feng Z.M., Huang C.H., Guo Y.H., et al. Graphitic carbon nitride derivative with large mesopores as sorbent for solid-phase microextraction of polycyclic aromatic hydrocarbons. Talanta. 2020;209:120541. doi: 10.1016/j.talanta.2019.120541. [DOI] [PubMed] [Google Scholar]
- 165.Fan W.W., Liu X.L., Cheng Y., et al. Two-dimensional metal-organic framework nanobelts for selective Fe3+ removal from aqueous solution with high adsorption capacity. Sep. Purif. Technol. 2020;239:116559. [Google Scholar]
- 166.Sun S.W., Yang Z.H., Cao J., et al. Copper-doped ZIF-8 with high adsorption performance for removal of tetracycline from aqueous solution. J. Solid State Chem. 2020;285:121219. [Google Scholar]
- 167.Wu Y.H., Li B.Y., Wang X.X., et al. Magnetic metal-organic frameworks (Fe3O4@ZIF-8) composites for U(VI) and Eu(III) elimination: simultaneously achieve favorable stability and functionality. Chem. Eng. J. 2019;378:122105. [Google Scholar]
- 168.Wu Y.H., Li B.Y., Wang X.X., et al. Synthesis of rod-like metal-organic framework (MOF-5) nanomaterial for efficient removal of U(VI): batch experiments and spectroscopy study. Sci. Bull. 2018;63:831–839. doi: 10.1016/j.scib.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 169.Zhuang S.T., Chen R., Liu Y., et al. Magnetic COFs for the adsorptive removal of diclofenac and sulfamethazine from aqueous solution: adsorption kinetics, isotherms study and DFT calculation. J. Hazard. Mater. 2020;385:121596. doi: 10.1016/j.jhazmat.2019.121596. [DOI] [PubMed] [Google Scholar]
- 170.Zhuang S.T., Liu Y., Wang J.L. Covalent organic frameworks as efficient adsorbent for sulfamerazine removal from aqueous solution. J. Hazard. Mater. 2020;383:121126. doi: 10.1016/j.jhazmat.2019.121126. [DOI] [PubMed] [Google Scholar]
- 171.Sharma R.K., Yadav P., Yadav M., et al. Recent development of covalent organic frameworks (COFs): synthesis and catalytic (organic-electro-photo) applications. Mater. Horiz. 2020;7:411–445. [Google Scholar]
- 172.Narayani M., Shetty K.V. Chromium-resistant bacteria and their environmental condition for hexavalent chromium removal: a review. Crit. Rev. Environ. Sci. Technol. 2013;43:955–1009. [Google Scholar]
- 173.Zhang S., Gu P.C., Ma R., et al. Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: a critical review. Catal. Today. 2019;335:65–77. [Google Scholar]
- 174.Ding S.Y., Wang W. Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 2013;42:548–568. doi: 10.1039/c2cs35072f. [DOI] [PubMed] [Google Scholar]
- 175.Liu N., Shi L.F., Han X.H., et al. A heteropore covalent organic framework for adsorptive removal of Cd(II) from aqueous solutions with high efficiency. Chin. Chem. Lett. 2020;31:386–390. [Google Scholar]
- 176.Yue J.Y., Wang L., Ma Y., et al. Metal ion-assisted carboxyl-containing covalent organic frameworks for the efficient removal of Congo red. Dalton Trans. 2019;48:17763–17769. doi: 10.1039/c9dt04175c. [DOI] [PubMed] [Google Scholar]
- 177.Li L.Y., Hu J.W., Shi X.D., et al. Nanoscale zero-valent metals: a review of synthesis, characterization, and applications to environmental remediation. Environ. Sci. Pollut. Res. Int. 2016;23:17880–17900. doi: 10.1007/s11356-016-6626-0. [DOI] [PubMed] [Google Scholar]
- 178.Liu Y.Q., Landskron K. Anionic porous organic frameworks as advanced functional adsorbents for CO2 and organic micropollutants in water. J. Mater. Chem. A. 2017;5:23523–23529. [Google Scholar]
- 179.Li G.L., Ye J.R., Fang Q.L., et al. Amide-based covalent organic frameworks materials for efficient and recyclable removal of heavy metal lead(II) Chem. Eng. J. 2019;370:822–830. [Google Scholar]
- 180.Wang Z.Q., Cohen S.M. Postsynthetic modification of metal-organic frameworks. Chem. Soc. Rev. 2009;38:1315–1329. doi: 10.1039/b802258p. [DOI] [PubMed] [Google Scholar]
- 181.Sun Q., Aguila B., Earl L.D., et al. Covalent organic frameworks as a decorating platform for utilization and affinity enhancement of chelating sites for radionuclide sequestration. Adv. Mater. 2018;30:1705479. doi: 10.1002/adma.201705479. [DOI] [PubMed] [Google Scholar]
- 182.Sun Q., Aguila B., Perman J., et al. Postsynthetically modified covalent organic frameworks for efficient and effective mercury removal. J. Am. Chem. Soc. 2017;139:2786–2793. doi: 10.1021/jacs.6b12885. [DOI] [PubMed] [Google Scholar]
- 183.Bai C.Y., Zhang M.C., Li B., et al. Modifiable diyne-based covalent organic framework: a versatile platform for in situ multipurpose functionalization. Rsc. Adv. 2016;6:39150–39158. [Google Scholar]
- 184.Meri-Bofi L., Royuela S., Zamora F., et al. Thiol grafted imine-based covalent organic frameworks for water remediation through selective removal of Hg(II) J. Mater. Chem. A. 2017;5:17973–17981. [Google Scholar]
- 185.Xu H., Chen X., Gao J., et al. Catalytic covalent organic frameworks via pore surface engineering. Chem. Commun. (Camb.) 2014;50:1292–1294. doi: 10.1039/c3cc48813f. [DOI] [PubMed] [Google Scholar]
- 186.Hou Y.X., Zhang X.M., Wang C.M., et al. Novel imine-linked porphyrin covalent organic frameworks with good adsorption removing properties of RhB. New J. Chem. 2017;41:6145–6151. [Google Scholar]
- 187.Colson J.W., Woll A.R., Mukherjee A., et al. Oriented 2D covalent organic framework thin films on single-layer graphene. Science. 2011;332:228–231. doi: 10.1126/science.1202747. [DOI] [PubMed] [Google Scholar]
- 188.Karak S., Dey K., Torris A., et al. Inducing disorder in order: hierarchically porous covalent organic framework nanostructures for rapid removal of persistent organic pollutants. J. Am. Chem. Soc. 2019;141:7572–7581. doi: 10.1021/jacs.9b02706. [DOI] [PubMed] [Google Scholar]
- 189.Hawkes J.S. Heavy metals. J. Chem. Educ. 1997;74:1369–1374. [Google Scholar]
- 190.Duruibe J.O., Ogwuegbu M.O.C., Egwurugwu J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007;2:112–118. [Google Scholar]
- 191.Kurniawan T.A., Chan G.Y.S., Lo W.H., et al. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006;118:83–98. [Google Scholar]
- 192.Azimi A., Azari A., Rezakazemi M., et al. Removal of heavy metals from industrial wastewaters: a review. Chembioeng Rev. 2017;4:37–59. [Google Scholar]
- 193.Lv S.W., Liu J.M., Wang Z.H., et al. Recent advances on porous organic frameworks for the adsorptive removal of hazardous materials. J. Environ. Sci. 2019;80:169–185. doi: 10.1016/j.jes.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 194.Jiang C.L., Wang X.H., Wang G.H., et al. Adsorption performance of a polysaccharide composite hydrogel based on crosslinked glucan/chitosan for heavy metal ions. Compos. B Eng. 2019;169:45–54. [Google Scholar]
- 195.Huang D., Wu J., Wang L., et al. Novel insight into adsorption and co-adsorption of heavy metal ions and an organic pollutant by magnetic graphene nanomaterials in water. Chem. Eng. J. 2019;358:1399–1409. [Google Scholar]
- 196.Zhan W.W., Gao L., Fu X., et al. Green synthesis of amino-functionalized carbon nanotube-graphene hybrid aerogels for high performance heavy metal ions removal. Appl. Surf. Sci. 2019;467:1122–1133. [Google Scholar]
- 197.Zarei F., Marjani A., Joshaghani A.H. Triamino-anchored KCC-1: a novel and promising adsorbent for fast and highly effective aqueous Cr-VI removal. J. Environ. Chem. Eng. 2019;7:103309. [Google Scholar]
- 198.Chen W.Q., Lu Z.H., Xiao B.H., et al. Enhanced removal of lead ions from aqueous solution by iron oxide nanomaterials with cobalt and nickel doping. J. Clean. Prod. 2019;211:1250–1258. [Google Scholar]
- 199.Hu H.J., Liu J.Y., Xu Z.H., et al. Hierarchical porous Ni/Co-LDH hollow dodecahedron with excellent adsorption property for Congo red and Cr(VI) ions. Appl. Surf. Sci. 2019;478:981–990. [Google Scholar]
- 200.Li Z.T., Wang L., Meng J., et al. Zeolite-supported nanoscale zero-valent iron: new findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil. J. Hazard. Mater. 2018;344:1–11. doi: 10.1016/j.jhazmat.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 201.Liao Q., Pan W., Zou D.S., et al. Using of g-C3N4 nanosheets for the highly efficient scavenging of heavy metals at environmental relevant concentrations. J. Mol. Liq. 2018;261:32–40. [Google Scholar]
- 202.Thanh H.T.M., Phuong T.T.T., Hang P.T.L., et al. Comparative study of Pb(II) adsorption onto MIL-101 and Fe-MIL-101 from aqueous solutions. J. Environ. Chem. Eng. 2018;6:4093–4102. [Google Scholar]
- 203.Huang Y., Zeng X.F., Guo L.L., et al. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018;194:462–469. [Google Scholar]
- 204.Li J., Yang X.D., Bai C.Y., et al. A novel benzimidazole-functionalized 2D COF material: synthesis and application as a selective solid-phase extractant for separation of uranium. J. Colloid Interface Sci. 2015;437:211–218. doi: 10.1016/j.jcis.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 205.Mahmoud M.E., Khalifa M.A., El Wakeel Y.M., et al. Engineered nano-magnetic iron oxide-urea-activated carbon nanolayer sorbent for potential removal of uranium(VI) from aqueous solution. J. Nucl. Mater. 2017;487:13–22. [Google Scholar]
- 206.Zhang R., Chen C., Li J., et al. Preparation of montmorillonite@carbon composite and its application for U(VI) removal from aqueous solution. Appl. Surf. Sci. 2015;349:129–137. [Google Scholar]
- 207.Song S.A., Huang S.Y., Zhang R., et al. Simultaneous removal of U(VI) and humic acid on defective TiO2−x investigated by batch and spectroscopy techniques. Chem. Eng. J. 2017;325:576–587. [Google Scholar]
- 208.Yan L.G., Yang K., Shan R.R., et al. Kinetic, isotherm and thermodynamic investigations of phosphate adsorption onto core-shell Fe3O4@LDHs composites with easy magnetic separation assistance. J. Colloid Interface Sci. 2015;448:508–516. doi: 10.1016/j.jcis.2015.02.048. [DOI] [PubMed] [Google Scholar]
- 209.Liu H.B., Li M.X., Chen T.H., et al. New synthesis of nZVI/C composites as an efficient adsorbent for the uptake of U(VI) from aqueous solutions. Environ. Sci. Technol. 2017;51:9227–9234. doi: 10.1021/acs.est.7b02431. [DOI] [PubMed] [Google Scholar]
- 210.Li Y., Li L.Y., Chen T., et al. Bioassembly of fungal hypha/graphene oxide aerogel as high performance adsorbents for U(VI) removal. Chem. Eng. J. 2018;347:407–414. [Google Scholar]
- 211.Liu Y., Zhao Z., Yuan D., et al. Fast and high amount of U(VI) uptake by functional magnetic carbon nanotubes with phosphate group. Ind. Eng. Chem. Res. 2018;57:14551–14560. [Google Scholar]
- 212.Zhang X., Liu Y., Jiao Y., et al. Enhanced selectively removal uranyl ions from aqueous solution by Fe@ZIF-8. Microporous Mesoporous Mater. 2019;277:52–59. [Google Scholar]
- 213.Yang L.X., Feng X.F., Yin W.H., et al. Metal-organic framework containing both azo and amide groups for effective U(VI) removal. J. Solid State Chem. 2018;265:148–154. [Google Scholar]
- 214.Song W., Wang X., Wang Q., et al. Plasma-induced grafting of polyacrylamide on graphene oxide nanosheets for simultaneous removal of radionuclides. Phys. Chem. Chem. Phys. 2015;17:398–406. doi: 10.1039/c4cp04289a. [DOI] [PubMed] [Google Scholar]
- 215.Liao Q., Zou D.S., Pan W., et al. Highly efficient capture of Eu(III), La(III), Nd(III), Th(IV) from aqueous solutions using g-C3N4 nanosheets. J. Mol. Liq. 2018;252:351–361. [Google Scholar]
- 216.Huang S.Y., Pang H.W., Li L., et al. Unexpected ultrafast and high adsorption of U(VI) and Eu(III) from solution using porous Al2O3 microspheres derived from MIL-53. Chem. Eng. J. 2018;353:157–166. [Google Scholar]
- 217.He L.W., Liu S.T., Long C. Mechanism unravelling for ultrafast and selective 99TcO4− Uptake by a radiation-resistant cationic covalent organic framework: a combined radiological experiment and molecular dynamics simulation study. Chem. Sci. 2019;10:4293–4305. doi: 10.1039/c9sc00172g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhu L., Sheng D.P., Xu C., et al. Identifying the recognition site for selective trapping of 99TcO4– in a hydrolytically stable and radiation resistant cationic metal-organic framework. J. Am. Chem. Soc. 2017;139:14873–14876. doi: 10.1021/jacs.7b08632. [DOI] [PubMed] [Google Scholar]
- 219.Banerjee D., Xu W.Q., Nie Z.M., et al. Zirconium-based metal-organic framework for removal of perrhenate from water. Inorg. Chem. 2016;55:8241–8253. doi: 10.1021/acs.inorgchem.6b01004. [DOI] [PubMed] [Google Scholar]
- 220.Cheng R., Kang M., Zhuang S. Adsorption of Sr(II) from water by mercerized bacterial cellulose membrane modified with EDTA. J. Hazard. Mater. 2019;364:645–653. doi: 10.1016/j.jhazmat.2018.10.083. [DOI] [PubMed] [Google Scholar]
- 221.Basu H., Pimple M.V., Saha S. TiO2 microsphere impregnated alginate: a novel hybrid sorbent for uranium removal from aquatic bodies. New J. Chem. 2020;44:85325. [Google Scholar]
- 222.Wang X., Dai X., Shi C., et al. A 3,2-hydroxypyridinone-based decorporation agent that removes uranium from bones in vivo. Nat. Commun. 2019;10:85632. doi: 10.1038/s41467-019-10276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Panda A., Yanqin Y., Venkateswarlu S., et al. Highly durable covalent organic framework for the simultaneous ultrasensitive detection and removal of noxious Hg2+ Micropor. Mesopor. Ma. 2020;6:523–536. [Google Scholar]
- 224.Xu T., An S., Peng C., et al. Construction of large-pore crystalline covalent organic framework as high-performance adsorbent for rhodamine B dye removal. Ind. Eng. Chem. Res. 2020;6:86954. [Google Scholar]
- 225.Rahmanian O., Falsafin M., Dinari M. High surface area benzimidazole based porous covalent organic framework for removal of methylene blue from aqueous solutions. Polym. Int. 2020;69:712–718. doi: 10.1002/pi.6007. [DOI] [Google Scholar]
- 226.Ding H., Mal A., Wang C. Tailored covalent organic frameworks by post-synthetic modification. Mater. Chem. Front. 2020;4:113–127. [Google Scholar]
- 227.Jiang C.L., Wang X.H., Wang G.H., et al. Amino bearing core-shell structured magnetic covalent organic framework nanospheres: preparation, postsynthetic modification with phenylboronic acid and enrichment of monoamine neurotransmitters in human urine. Anal. Chim. Acta. 2020;1093:61–74. doi: 10.1016/j.aca.2019.09.078. [DOI] [PubMed] [Google Scholar]
- 228.Li H., Li H., Dai Q., et al. Hydrolytic stability of boronate ester-linked covalent organic frameworks. Adv. Theor. Simul. 2018;6:1700015. [Google Scholar]
- 229.Lei Z.D., Yang Q.S., Xu Y., et al. Boosting lithium storage in covalent organic framework via activation of 14-electron redox chemistry. Nat. Commun. 2018;9:576. doi: 10.1038/s41467-018-02889-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Liu Q., Fang Q., Chu W.S., et al. Electron-doped 1T-MoS2 via interface engineering for enhanced electrocatalytic hydrogen evolution. Chem. Mater. 2017;29:4738–4744. [Google Scholar]
- 231.Zhang W.J., Hu Y., Ma L.B., et al. Progress and perspective of electrocatalytic CO2 reduction for renewable carbonaceous fuels and chemicals. Adv. Sci. 2018;5:1700275–1700286. doi: 10.1002/advs.201700275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cui W.R., Li F.F., Xu R.H., et al. Regenerable covalent organic frameworks for photo-enhanced uranium adsorption from seawater. Angew. Chem. Int. Ed. 2020;6:75078. doi: 10.1002/anie.202007895. [DOI] [PubMed] [Google Scholar]
- 233.Aksu Z. Application of biosorption for the removal of organic pollutants: a review. Process. Biochem. 2005;40:997–1026. [Google Scholar]
- 234.Das R., Vecitis C.D., Schulze A., et al. Recent advances in nanomaterials for water protection and monitoring. Chem. Soc. Rev. 2017;46:6946–7020. doi: 10.1039/c6cs00921b. [DOI] [PubMed] [Google Scholar]
- 235.Gibson L.T. Mesosilica materials and organic pollutant adsorption. Part B. Removal from aqueous solution. Chem. Soc. Rev. 2014;43:5173–5182. doi: 10.1039/c3cs60095e. [DOI] [PubMed] [Google Scholar]
- 236.Lin K., Liu W., Gan J. Oxidative removal of bisphenol A by manganese dioxide: efficacy, products, and pathways. Environ. Sci. Technol. 2009;43:3860–3864. doi: 10.1021/es900235f. [DOI] [PubMed] [Google Scholar]
- 237.Li Y.X., Zhang H., Chen Y., et al. Core-shell structured magnetic covalent organic framework nanocomposites for triclosan and triclocarban adsorption. ACS Appl. Mater. Interfaces. 2019;11:22492–22500. doi: 10.1021/acsami.9b06953. [DOI] [PubMed] [Google Scholar]
- 238.Li Y., Wang C., Ma S.J., et al. Fabrication of hydrazone-linked covalent organic frameworks using alkyl amine as building block for high adsorption capacity of metal ions. ACS Appl. Mater. Interfaces. 2019;11:11706–11714. doi: 10.1021/acsami.8b18502. [DOI] [PubMed] [Google Scholar]
- 239.Arica T.A., Ayas E., Arica M.Y. Magnetic MCM-41 silica particles grafted with poly(glycidylmethacrylate) brush: modification and application for removal of direct dyes. Micropor Mesopor Mat. 2017;243:164–175. [Google Scholar]
- 240.Li Y., Yang C.X., Qian H.L., et al. Carboxyl-functionalized covalent organic frameworks for the adsorption and removal of triphenylmethane dyes. ACS Appl. Nano Mater. 2019;2:7290–7298. [Google Scholar]
- 241.Yu S.J., Wang X.X., Pang H.W., et al. Boron nitride-based materials for the removal of pollutants from aqueous solutions: a review. Chem. Eng. J. 2018;333:343–360. [Google Scholar]
- 242.Bae S., Collins R.N., Waite T.D., et al. Advances in surface passivation of nanoscale zerovalent iron: a critical review. Environ. Sci. Technol. 2018;52:12010–12025. doi: 10.1021/acs.est.8b01734. [DOI] [PubMed] [Google Scholar]
- 243.Guo X.H., Tian Y., Zhang M.C., et al. Mechanistic insight into hydrogen-bond-controlled crystallinity and adsorption property of covalent organic frameworks from flexible building blocks. Chem. Mater. 2018;30:2299–2308. [Google Scholar]
- 244.Li F.F., Cui W.R., Jiang W., et al. Stable sp2 carbon-conjugated covalent organic framework for detection and efficient adsorption of uranium from radioactive wastewater. J. Hazard. Mater. 2020;392:122333. doi: 10.1016/j.jhazmat.2020.122333. [DOI] [PubMed] [Google Scholar]
- 245.Hu J., Tan X.L., Ren X.M., et al. Effect of humic acid on nickel(II) sorption to Ca-montmorillonite by batch and EXAFS techniques study. Dalton Trans. 2012;41:10803–10810. doi: 10.1039/c2dt31057k. [DOI] [PubMed] [Google Scholar]
- 246.Ren X.M., Yang S.T., Hu F.C., et al. Microscopic level investigation of Ni(II) sorption on Na-rectorite by EXAFS technique combined with statistical F-tests. J. Hazard. Mater. 2013;252:2–10. doi: 10.1016/j.jhazmat.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 247.Chen C.C., McKimmy E.J., Pinnavaia T.J., et al. XAS study of mercury(II) ions trapped in mercaptan-functionalized mesostructured silicate with a wormhole framework structure. Environ. Sci. Technol. 2004;38:4758–4762. doi: 10.1021/es049689o. [DOI] [PubMed] [Google Scholar]
- 248.Li Y., Guo X.H., Li X.F., et al. Redox-active two-dimensional covalent organic frameworks (COFs) for selective reductive separation of valence-variable, redox-sensitive and long-lived radionuclides. Angew. Chem. Int. Ed. 2020;59:4168–4175. doi: 10.1002/anie.201916360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.