Abstract
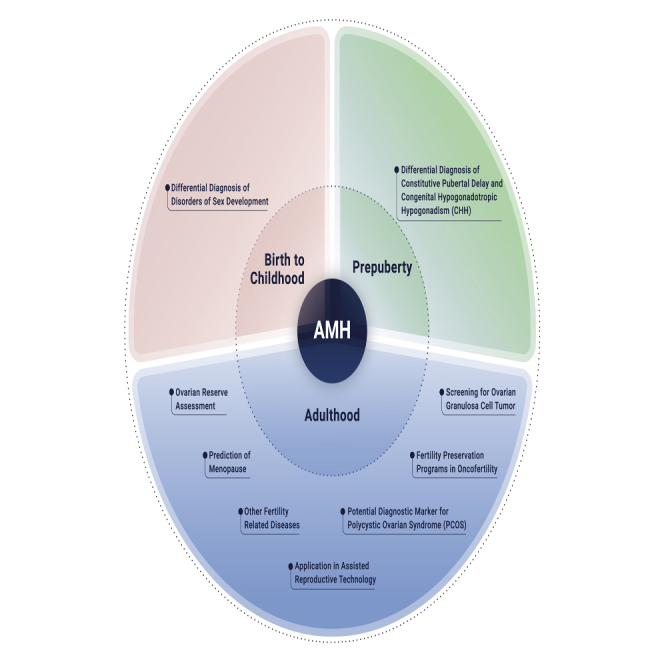
Infertility is one of the most common non-communicable diseases, affecting both men and women equally. Ovarian reserve, the number of primordial follicles in the ovaries is believed to be the most important determinants for female fertility. Anti-Müllerian hormone (AMH) secreted from granulosa cells of growing follicles is recognized as the most important biomarker for ovarian reserve. Ovarian reserve models have been developed using AMH and other hormonal indicators, thus childbearing plans and reproductive choices could be arranged by women. In assisted reproductive technology cycles, measurement of AMH helps to predict ovarian response and guide recombinant follicle-stimulating hormone dosing in women. Serum AMH level is increasingly being recognized as a potential surrogate marker for polycystic ovarian morphology, one of the criteria for diagnosis of polycystic ovarian syndrome. AMH is also secreted by Sertoli cells of testes in men, and AMH measurements in the prediction of surgical sperm recovery rate in men have also been investigated. AMH levels are significantly higher in boys than in girls before puberty. Therefore, serum levels of AMH in combination with testosterone is used for the differential diagnosis of disorders of sex development, anorchia, non-obstructive azoospermia, and persistent Müllerian duct syndrome. Recently, serum AMH measurements have also been used in fertility preservation programs in oncofertility, screening for granulosa cell tumors, and prediction of menopause applications. In this review, we will focus on clinical application of AMH in fertility assessments for healthy men and women, as well as for cancer patients.
Keywords: anti-Müllerian hormone assays, assisted reproductive technology, Sertoli cells, Granulosa cells, ovarian reserve, menopause, PCOS
Graphical Abstract
Public Summary
-
•
Anti-Müllerian hormone (AMH) plays a key role in models assessing ovarian reserve
-
•
AMH is used for the differential diagnosis of disorders of sex development
-
•
AMH provides a molecular marker for related fertility and infertility disorders
-
•
An international standard will aid in the development of various AMH assays
Introduction
Infertility affected 48.5 million couples globally in 2012.1 Recently, many women have tended to delay their childbearing plans in order to pursuit career goals, which may contribute to the high incidence of infertility worldwide. A major determinant of female reproductive potential is ovarian reserve, which is influenced by age, genetics, and environment. The ovarian reserve, the number of primordial follicles in ovarian cortex, is highly heterogeneous, ranging from tens to millions,2 leads to the variation in the age of exhaustion of fertility (menopause) in women. Therefore, assessment of ovarian reserve is of great importance. Recently, ovarian reserve models have been developed, using anti-Müllerian hormone (AMH) and other indicators.3,4 Due to the key role of AMH in ovarian reserve assessment, the physiology and clinical applications of AMH are reviewed here.
In 1947, Jost5 discovered a substance that contributed to the regression of the Müllerian duct during the sexual differentiation of male embryos, denoted AMH. AMH is a member of the transforming growth factor β superfamily,6,7 which has key roles in development and tissue homeostasis, including regulation of development of the male genital tract.8
In females, the secretion of AMH starts around the 36th week of gestation, then reaches a peak around 25 years of age, before declining to undetectable levels during the menopause.9 AMH is secreted by ovarian granulosa cells (GCs) of preantral and small antral follicles.9, 10, 11 One role of AMH in females is to inhibit primordial follicle recruitment12 in a follicle-stimulating hormone (FSH)-independent manner.13,14 In males, the secretion of AMH starts around the eighth week of gestation,15 then declines in the first week after birth, rises rapidly during the first month, peaks at about 6 months of age, declines during childhood, falls to low levels in puberty, and decreases with age after sexual maturation.16, 17, 18 AMH is secreted by immature Sertoli cells (SCs) and provides a valuable molecular marker for these unique cells.19,20
During embryonic development, AMH is associated with regression of the female (Müllerian) reproductive ducts as well as with the development of male reproductive ducts.9 During adulthood, the role of AMH remains inconclusive. However, serum levels of AMH in adult men are similar to those in adult women, and decreasing serum AMH levels with age have been found in both sexes,17,18,21 which indicates its potentially important role in adulthood. An increasing number of studies have shown that abnormal serum levels of AMH might indicate an abnormality of the reproductive system in both sexes.19,22 In this paper, the clinical applications of serum AMH in fertility assessment and other reproductive-related disorders will be reviewed.
Physiology of AMH
Prior to gonadal differentiation, both male and female mammalian embryos have two sets of paired reproductive ducts: the paramesonephric (Müllerian) and mesonephric (Wolffian) ducts.23 In response to AMH and testosterone, which is secreted by immature SCs and Leydig cells respectively in male embryos,24 the mesonephric ducts develop into the epididymides, vasa deferentes and seminal vesicles, while the paramesonephric ducts regress. In the absence of AMH and testosterone, the paramesonephric ducts of female embryos develop into the fallopian tubes, uterus, and proximal vagina, and the mesonephric ducts degenerate.25
Males
In males, AMH is secreted by immature SCs and its circulating concentration before puberty is extremely high compared with women.26,27 As puberty progresses, immature SCs differentiate into mature SCs and AMH levels drop significantly.17,18 In the absence of functional androgen signaling, FSH was reported to be responsible for transcriptional activation of AMH expression through the activation of nuclear factor kappa-B (NF-κB) in mice.19,28 In the presence of functional androgen signaling, testosterone was negatively correlated with AMH expression in both humans and mice,19 possibly via the negative regulation of NF-κB by androgen receptors.29 The sex-determining region Y box 9 (Sox9),30,31 steroidogenic factor-1 (SF1),32,33 and GATA factors34, 35, 36 were also implicated in the transcriptional regulation of AMH; however, their transcriptional activity was less than that of NF-κB in SMAT1 cells, a mouse immortalized immature Sertoli cell line.28 The number of immature SCs produced during the perinatal period ultimately determines the number of germ cells in adult men.37,38 Because AMH is a functional marker of immature SCs, it is possible that higher AMH levels in early life will lead to increased support (by mature SCs) of germ cells in adulthood; thus, AMH levels might be linked to male fertility and infertility.
Females
In females, AMH is secreted by preantral and small antral follicles in the ovary.12,14 It is first secreted by fetal GCs at 36 gestational weeks,9 and the serum AMH level at birth is 1.66 ng/mL. A continuous rise was found to persist to about 10 years of age, peaking in adolescence, and then declining with age after the age of 18 years until menopause in a Chinese cohort.39
AMH acts to provide feedback inhibition of follicle development at two levels. Firstly, it inhibits recruitment of primordial follicles into the growing pool; and secondly, it reduces the sensitivity of antral follicles to FSH,40 which is a feedback mechanism of AMH and FSH after menarche. AMH controls the recruitment mechanism of primordial follicle into primary follicles, to regulate the reproductive life span. A high level of serum AMH during adolescence may serve as the first predictor for natural fertility and reproductive life span.41
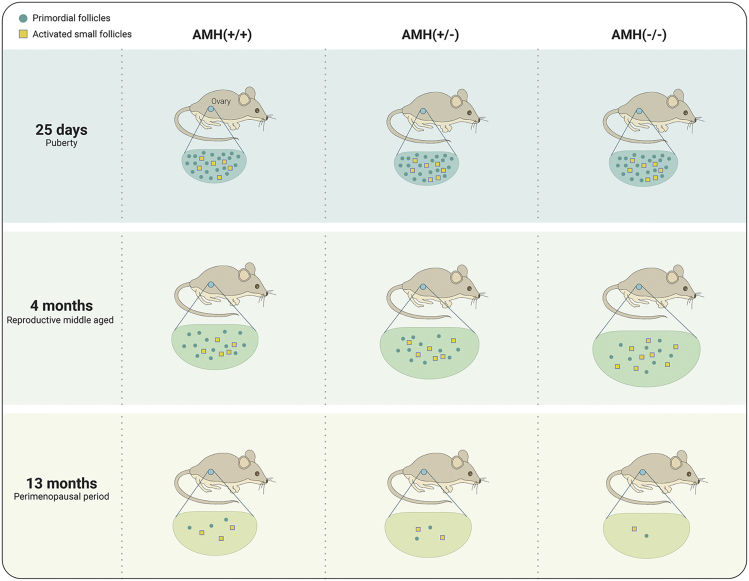
The most accepted role of AMH verified by mouse models is to inhibit the recruitment of primordial (resting) follicles.12,42,43 There is evidence showing that AMH null mice display an increased recruitment of primordial follicles. Nevertheless, these mice do not have proportionally more preovulatory follicles.44 Studies have demonstrated that AMH produced by GCs of the growing follicle suppresses development of the primordial follicles.45,46 In AMH knockout mice, increased FSH-dependent recruitment of small antral follicles and premature exhaustion of the primordial follicle reserve were observed.47 Furthermore, in vitro studies shown the inhibitory effect of recombinant AMH on early human ovarian follicular development in vitro by suppressing the initiation of primordial follicle growth.48 Dynamic changes in murine ovarian follicle numbers and volumes at different reproductive ages in AMH gene null mice and controls are shown in Figure 1, based on data from Durlinger et al.12 The secretion of AMH is independent of gonadotropins,13,49 so the expression of AMH is barely affected by the cyclical fluctuations of gonadotropins (Gn), and its circulating protein level remains relatively stable during the menstrual cycle.50,51
Figure 1.
Summary of the Role of AMH in AMH Null Mice
In AMH-null female mice 25 days after birth (equivalent to female puberty in humans), the number of primordial follicles was normal, but the numbers of activated follicle were elevated.12 In 4 months old, AMH-null female mice (equivalent to reproductive middle age in humans), the number of primordial follicles was significantly reduced, while the number of activated follicles was significantly higher than normal, together with larger ovarian volume.12 At 13 months old in AMH-null female mice (equivalent to the perimenopausal period in humans), the primordial and activated follicle numbers decreased significantly in AMH-null compared with control mice. The scheme is based on the data from Durlinger et al.12 (https://doi.org/10.1210/endo.140.12.7204).
Although the transcriptional regulation of AMH in women remains mostly unclear, there could be common regulatory mechanisms in both sexes. Because AMH is regulated by NF-κB in males, is it possible that this factor also regulates AMH transcription in females? It is known that NF-κB regulates expression of the androgen, estrogen, glucocorticoid, and progesterone receptors.29 The possible role of NF-κB in the aforementioned recombinant AMH-induced changes to circulating estradiol (E2), progesterone, and testosterone levels needs further investigation. Recent studies using animal models have revealed key underlying mechanisms of AMH actions in females.43,52 Receptors for AMH are found in the hypothalamus, which suggests that AMH might regulate follicular development through the hypothalamic-pituitary-gonadal (HPG) axis, and that an excess of AMH can induce ovulatory disorders. In addition, AMH was found to inhibit the production of E2 by inhibiting the expression of aromatase.42,43,53 Conversely, E2 inhibited AMH transcriptional activation through estrogen receptor-beta (ERβ) in growing follicles.54 Normal oocyte development needs an increase in E2 levels, which could explain the dynamic decrease in AMH levels during ovarian stimulation.55
Development of AMH Assays
Measurements of serum concentrations of AMH have been used along with other measurements, such as for FSH, luteinizing hormone (LH), and E2, for a wide variety of clinical applications.11,22,56, 57, 58 The clinical use of AMH has advantage over other assays because of its relatively stable level across the menstrual cycle.50,59,60 Nevertheless, recent studies have suggested that there is a considerable amount of variation in the level of AMH across cycles in certain women, which implies a risk of misclassification if the variation is beyond 20%.61 In addition to biological variability, there have been concerns regarding differences between assays. The major differences arise from calibrators/standards, antibodies, and assay methodology.62
The first-generation AMH assays came from Diagnostic Systems Laboratory (Webster, United States) and Immunotech (Oxford, UK) as sandwich ELISAs (both assays are out of market now). The Gen II ELISA by Beckman Coulter (Brea, CA) replaced both of these. In 2013, Ansh Labs LLC (Webster, TX) introduced an ultrasensitive AMH assay using a new pair of antibodies. In 2016, two additional immunoassays were introduced by Beckman Coulter (Access) and Roche (Elecsys, Mannheim, Germany) using automated chemiluminescence platforms. These two automated assays use the same antibody pair as the Beckman Coulter Gen II.
In 2016, Li et al.63 compared the four AMH assays from Beckman Coulter (Access), Roche, Ansh Labs, and Beckman Coulter (Gen II ELISA) and showed good correlations between these assays, but significantly different AMH values when the four assays were compared on the same serum samples. Nevertheless, all methods demonstrated excellent discrimination of women with polycystic ovarian syndrome (PCOS) from normal ovulatory controls, with areas under the receiver operating characteristic curve being over 0.9.63 These assays have different analytical performances, such as dynamic range, limit of detection, limit of quantification.
Recently, Ansh Labs introduced a third-generation assay, picoAMH, with more than 10-fold better sensitivity than the previous ultrasensitive AMH assays. This assay was developed to lower the limit of detection to suit the needs for detecting very-low-level AMH in women reaching menopause, in oncofertility studies, and in women with premature ovarian failure. In the United States, the Beckman Coulter Access AMH assay and Roche Elecsys AMH assay were cleared by the Food and Drug Administration (FDA) for use in the assessment of ovarian reserve in women presenting to assisted reproductive technology (ART) clinics. In 2018, the Ansh Labs Menocheck picoAMH assay was cleared by the FDA for use in the determination of menopausal status. Over the past decade, the applications of AMH in clinical practice have been maturing, but standardized AMH tests are still lacking. Internationally standardized assays would provide confidence in cited reference ranges and clinically validated cutoff values. An international standard will support the development of AMH immunoassays that are calibrated to recombinant human AMH.64
Clinical Applications of AMH
Associations between AMH and Fertility in Females
Fertility is defined as the natural capability of a couple to establish a clinical pregnancy.65 Any factor affecting the quantity and quality of oocytes or spermatozoa, as well as factors affecting the process of fertilization and embryo implantation, affect human fertility. Ovarian aging is the most important determinant of female fertility.66 It is established that the age-related decrease in ovarian function is related to the gradual loss of primordial follicles67,68, when follicular atresia takes place.69,70 Follicular atresia is a Gn-independent process71 that starts before birth.
Several lines of evidence derived from clinical and basic science studies collectively support the role of AMH in follicular atresia. By the onset of puberty, 95% (or 1.9 million out of 2 million) of all follicles are lost. AMH is the key regulator that inhibits the default mode of atresia from occurring.71 Studies reported that AMH levels are not stable during childhood, but rise during infancy and continue during the years leading up to puberty, roughly doubling between ages 4 and 8 years and then reaching a plateau during adolescence. Young girls have more primordial follicles activated before puberty, but, because a Gn releasing hormone-dependent feedback loop is not established, the follicles go to atresia.72 The highest rate of recruitment of non-growing follicles takes place between birth and 14 years of age.73 Before entering puberty, around 95% of all primordial follicles undergo atresia,71 which is induced by considerable granulosa cell apoptosis within the follicle.74 One function of AMH is to slow the recruitment of primordial follicles,12,53 so the high level of AMH after puberty might be responsible for the subsequent decreased rate of follicular atresia. The dynamic changes in AMH levels and the numbers of primordial follicles with age are indicated in Figure 2. As we can see, both AMH and primordial follicles decrease with age after sexual maturation, which indicates that AMH is a marker in ovarian aging.
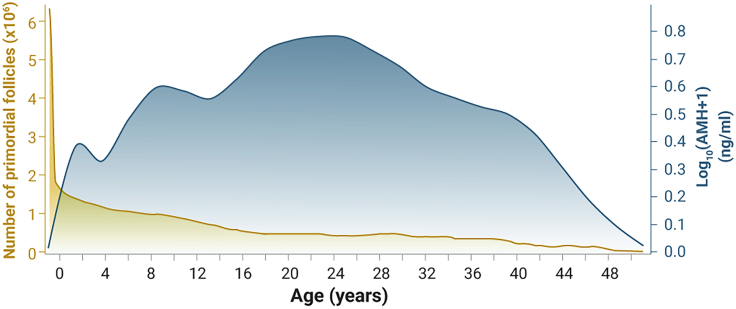
Figure 2.
Dynamic Changes in AMH Levels and Changes in the Numbers of Atretic Follicles with Age
The figure is based on the studies by Faddy et al.75 reporting that the number of primordial follicles was 6–7 million during fetal life (around midgestation), approximately 1–2 million at birth, 300,000–500,000 at the start of puberty, and 1,000 at 51 years of age.75,76 AMH levels are based on the model proposed by Kelsey et al.77 to predict menopause. Yellow represents number of primordial follicles (×106), while blue represents log10(AMH + 1) (ng/mL).
The most accepted predictor for female fertility is ovarian reserve (OR). We have previously established two models, termed AAFA and AFA, for estimating OR. The AAFA model uses four predictors (circulating AMH, the antral follicle count [AFC]; circulating basal FSH, and female age),4 while the AFA model uses just three predictors (circulating AMH, circulating basal FSH, and age).3 The main effects of each variable on the AAFA model were AMH 62.0%, AFC 17.5%, FSH 12.4%, and age 8.1%,4 while the main effects on the AFA model were AMH 85.2%, FSH 6.8%, and age 2.8%.3 Ovarian reserve is ranked according to the predicted probability of a poor ovarian response and is further classified into groups A–D according to OR, from adequate to poor, respectively. We further discovered that the clinical pregnancy and live birth rates for women in group D (with diminished OR) were significantly lower than in groups A and B, which indicates decreased female fertility in the population with a diminished ovarian reserve. The use of serum AMH as a potential marker for female fertility might offer several advantages over traditional markers of OR.4,56,78, 79, 80, 81, 82, 83, 84
Menopause refers to the cessation of menses and the termination of ovarian follicular maturation caused by the reduced production of estrogen and progesterone in the ovary,85 which leads to complete loss of fertility and many other symptoms. Recently, with recognition of the role of AMH and the development of AMH assays, many studies about predicting the time to the final menstrual period (FMP) have been conducted.77,86, 87, 88, 89, 90, 91 Finkelstein et al.86 published an excellent study about the importance of AMH over FSH levels in predicting FMP; however, they included only women in their late reproductive periods. Another study, by Bertone-Johnson et al.,87 included younger women. The blood samples of the participants were collected in 1996–1999, and follow-up was conducted until 2011 to get information about the time to the FMP. Cases of early menopause were women aged less than 45 years and the matched control cases were women who underwent menopause after the age of 45 years, matched 1:1. Matching criteria were based mainly on the women's age at blood sampling (±4 months) and other factors. They discovered significant associations between AMH and the time of menopause, irrespective of smoking habit, adiposity, history of infertility, and menstrual cycle characteristics. However, case control studies are often used to screen risk factors for a certain disease, and such a design means that the time to FMP could not be predicted. In the future, to predict the specific time to FMP in younger women, a cohort study with a large number of women of reproductive age is needed. With the increasing recognition of the role of AMH in ovarian aging and the increasing standardization of AMH kits, the specific time to FMP in younger women should be predictable, which would be beneficial to young women in terms of their plans for childbearing.
Associations between AMH and Fertility in Males
In males, AMH is secreted by SCs19, which share the same origin as GCs before the initiation of sexual differentiation in early gestation.92,93 AMH is a well-known proxy for the number of immature SCs.93 Germ cell numbers in adult testes are closely linked to the numbers of immature SCs produced during perinatal development,37,38 suggesting an important role for AMH in establishing male fertility. Men exhibit declining serum AMH levels with age after sexual maturity,17,18,21 which indicates an age-related reduction in SC function. In addition, the function of SCs declines earlier than that for Leydig cells in aging men,94 which suggests that decreased SC numbers might be an early event of male infertility. Therefore, decreased serum AMH levels could be an early sign of infertility in men.
Although early SC injury can cause a transient increase in AMH, such as in prepubertal patients with Klinefelter syndrome (KS)95 and in patients with early-onset varicocele96, over time, severe damage to SCs leads to a decreased serum AMH concentration and impaired fertility. A study of adult men showed that the circulating AMH levels of infertile men were 60% lower than the corresponding control group.97 Non-obstructive azoospermia (NOA), characterized by the absence of spermatozoa in semen samples resulting from impaired spermatogenesis,98 directly causes male infertility, and the concentration of serum AMH in such men decreases more significantly than among those with varicocele.96 An extreme example is in patients with anorchia (loss of fertility), where the AMH concentration is so low that it is undetectable. The above results suggest that, within a certain low concentration range, the lower the serum AMH concentration, the worse the man's fertility.
Fertility problems are frequently encountered in patients affected by disorders of sex development (DSD). AMH, produced by fetal SCs, and testosterone, produced by fetal Leydig cells, are responsible for male genital differentiation.19,24,99 Dysfunction of either one or both of them may result in DSD. Persistent Müllerian duct syndrome (PMDS) is one kind of DSD; the function of SCs in these patients is severely impaired and these patients are always infertile.19 The Leydig cell function of patients with PMDS is generally normal,100 manifested by normal levels of serum testosterone and LH.100,101 Mutations in the AMH gene or its receptor gene (AMRHII) account for 88% of the overall PMDS patients100; moreover, we cannot rule out the possibility of AMH or AMHRII mutations in the remaining 12% of patients with idiopathic PMDS because AMH or AMHRII mutations might be under-detected and the sensitivity and specificity of sequencing can be limited.100 In patients with AMH mutations, very low or undetectable serum AMH levels are detected combined with normal serum inhibin B levels, while, in patients with AMHRII mutations, the serum AMH level is normal but serum inhibin B is undetectable.100,101 To distinguish PMDS from mixed DSD, which is a disorder of both Leydig cells and SCs,102 no external genital ambiguities, especially the lack of hypospadias, are the main clinical features. In individuals with mixed DSD, subnormal levels of both serum AMH and testosterone and external genital malformations were discovered. If a 46 XY karyotype infant has bilateral nonpalpable testes, a serum AMH test is needed to distinguish anorchia from the situation of bilateral abdominal testes to avoid surgical exploration,103 and the use of other hormone tests, including those for FSH, LH, inhibin B, and testosterone.103
Studies on unilateral undescended testes (UDT) revealed that the undescended testes were smaller than the normally descended testes.104,105 Given that the SCs account for 75% of testis mass in boys,106,107 there might be a decline in the number of SCs in cases of UDT. The degree of AMH decrease in boys with UDT (unilateral cryptorchidism) is related to the severity of dysfunction,108, 109, 110 which suggests that impaired function of SCs might be an early event of UDT. Infertility is a long-term concern for such patients,103 with risks of 30% and 54% in UDT and bilateral cryptorchidism, respectively,111, 112, 113, 114, 115 linked to the duration of testicular exposure to abdominal temperature. Risks of future infertility of 75%–100% were found in boys with bilateral undescended testes in whom no germ cells were found on biopsy.116 In conclusion, the degree of male infertility caused by UDT is related to the severity of injury to SCs, manifested by the degree of abnormal declines in AMH levels.
AMH in ART for Both Sexes
In Females
For women, the most established use of AMH analysis is predicting ovarian response (the number of mature oocytes) during ovarian stimulation.11,22 AMH cutoff values are recommended by the European Society of Human Reproduction and Embryology (ESHRE)117 and the Patient-Oriented Strategies Encompassing Individualized Oocyte Number (POSEIDON) group,118 to individualize strategies for ovarian stimulation. Recent data have suggested that individualized treatment considering AMH levels contributed to a reduced cost during ART treatment.119 AMH provides one of the key indicators for individualized counseling and ovarian stimulation. However, there is debate concerning the suitability of the ESHRE and POSEIDON recommendations, and a better model for directing individualized ovarian stimulation is needed in the future.
AMH is highly correlated with AFC, and it is considered to be the best predictive marker for ovarian hyper- or hypo-responses.10,56,120,121 However, although AMH and AFC are strongly linked, with a correlation coefficient of 0.73 reported by Fanchin et al.,122 they are not always in a linear relationship. For example, we have established an AAFA model for assessing OR using a poor response of fewer than five oocytes retrieved as the outcome variable, and then ranking the OR according to the predicted probability of a poor ovarian response.4 We divided the population into 16 subgroups in the order of OR from good to bad. Subgroups 1–3 and 14–16 accounted for the majority, with AMH and AFC levels high or low at the same time, but there were a few groups with an intermediate OR, with high AMH and low AFC (accounting for 14.8%), or low AMH and high AFC (accounting for 4.9%). Another example is that, in patients with hypogonadotropic hypogonadism, the AFC is extremely low because of the extremely low level of FSH, but such young patients still have sufficient OR, normal AMH levels, and good pregnancy outcomes when receiving ovarian stimulation. The explanation might be that AMH and AFC respond to different stages of follicular development. AMH is mostly secreted by preantral and small antral follicles, which reflects the Gn-independent phase of follicular development,13 while the AFC is based on follicles of more than 2 mm in diameter, which reflects the early phase of Gn-dependent follicular development. However, when the OR is depleted to a certain threshold, both AMH and AFC will be affected and decrease to near zero.
AMH cutoff points have been used frequently to estimate the OR and predict ovarian responses. However, its sensitivity and specificity are suboptimal.123,124 Recently, dynamic AMH changes during ovarian stimulation were found to be associated with oocyte yield and pregnancy outcomes,55 which suggests that the dynamic changes in AMH levels combined with other indicators might provide another choice for predicting ART response, oocyte quality, and pregnancy outcomes. The exact mechanism for the reduced AMH levels during ovarian stimulation remains unclear. A reasonable explanation for the dynamic decrease in AMH levels during ovarian stimulation is that AMH is produced by secondary, preantral, and small antral follicles in the early phase of stimulation and declines as these follicles are recruited into dominant growing follicle cohorts.125 However, it is unclear whether this decreased AMH level during ovarian stimulation is caused by a decrease in the total number of activated small follicles in response to stimulation or by a decrease in AMH secretion at the single-follicle level. Furthermore, FSH controls the expression of AMH via oocyte-derived factors, such as GDF9 and BMP15, by negative feedback loop.126 In addition, why is the dynamic decrease in AMH level during ovarian stimulation related to good pregnancy outcome? It has been well documented that AMH contributes to the inhibition of E2 production by suppression of aromatase expression,42,43,53 and, in return, E2 inhibits the transcriptional activation of AMH via ERβ in growing follicles.54 Thus, it is possible that a sufficient rise in E2 levels during ovarian stimulation needs a decrease in the AMH level. Likewise, a sufficient increase in E2 during pregnancy may be required for a dynamic decrease in AMH levels. One study reported that pregnancies lacking a decline in AMH levels had a higher risk of preterm birth and might require interventional therapies, such as supplemental E2 and progesterone.127
In Males
Although AMH analysis has been applied widely in ART-related clinical practice for women, AMH has not been routinely used in the diagnosis and treatment of male infertility. In men, the most promising application appears to be the differential diagnosis of NOA from obstructive azoospermia (OA).19 Furthermore, serum AMH tests might also contribute to a differential diagnosis between anorchidism and cryptorchidism.101,128,129 The rationale is similar to that for the differential diagnosis of NOA and OA; that is, AMH is secreted by SCs. If AMH is particularly low and unable to support the steroidogenic function of Leydig cells, the diagnosis is NOA or anorchia; otherwise, OA or cryptorchidism is to be suspected.19,130
Because AMH is secreted by immature SCs,19 and spermatogenesis requires functional SCs, some studies have explored using AMH levels to predict the sperm recovery rate (SRR) of testicular sperm extraction (TESE) or microdissection TESE (MD-TESE) for patients with NOA.131, 132, 133, 134 However, the utility of AMH analysis has been inconclusive in this regard. One core issue might be that it remains unknown to what extent a decline in AMH reflects the complete loss of SC support for spermatogenesis. A multivariate mathematic model combining AMH, inhibin B, testosterone, and other clinical characteristics could be promising for predicting the SRR during TESE/MD-TESE procedures.
AMH and PCOS-like Phenotypes
In Females
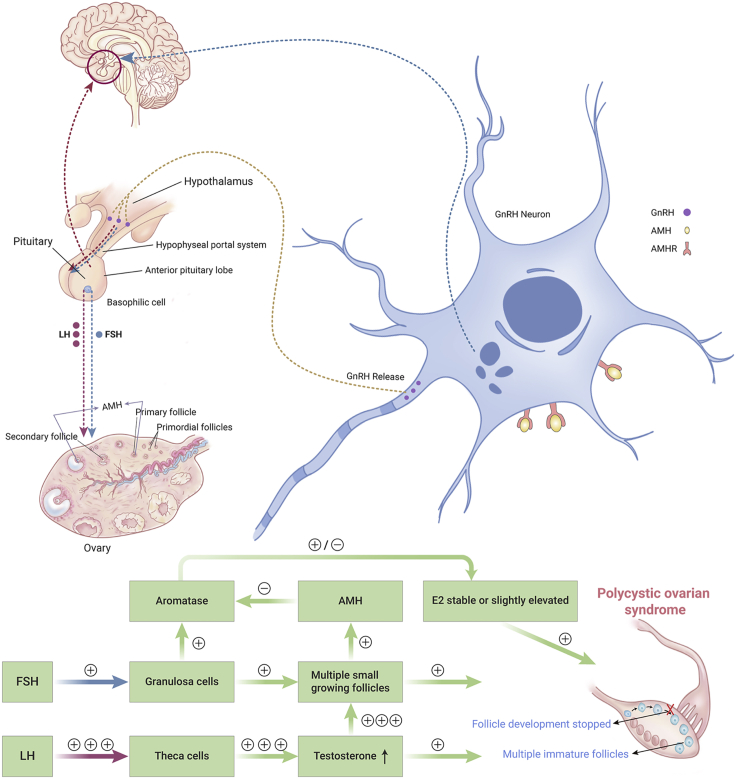
PCOS is a common endocrine disorder affecting approximately 6%–20% of women of reproductive age.135 However, the etiology of PCOS remains complicated because of its heterogeneous characteristics, which include disruption to endocrine, metabolic, psychological, or reproductive functions. Hyperandrogenism can contribute to the pathophysiology of PCOS, supported by the appearance of PCOS-like phenotypes induced by different androgens in animal models,136,137 but how excessive androgens are produced remains largely unknown. It has been proposed that excessive androgen levels can be induced by insulin resistance and hyperinsulinemia,138,139 or by the disturbed regulation of kisspeptin.140, 141, 142, 143, 144 However, this hypothesis was not supported by animal models, because no PCOS-like phenotype was induced by manipulating insulin or kisspeptin levels in animal models.43,145 In 2016, Giacobini and coworkers found that the AMH receptor was expressed in hypothalamic Gn-releasing hormone-producing neurons and AMH was involved in regulating the HPG axis.52 They proposed that AMH might be responsible for the excessive androgens in patients with PCOS.52 A model of AMH-induced activation of the HPG axis in PCOS patients is shown in Figure 3. We believe that this research was a breakthrough for the diagnosis and treatment of PCOS.43,52
Figure 3.
AMH Stimulates the HPG Axis in Females with Polycystic Ovarian Syndrome
Here, plus (+) means stimulation, while minus (–) means inhibition.
Women with PCOS have elevated serum AMH levels and later age at menopause compared to women without PCOS.146 In the PCOS women, the high AMH level inhibits the recruitment of primary follicles from the primordial pool and fewer growing follicles but more 2–6-mm non-growing follicles.147 These 2–6-mm follicles produce highest amount of AMH per follicles. The AMH produced by the pool of non-growing follicles acts as a negative paracrine feedback signal on neighboring primordial follicle initiation.71
If AMH represents a significant driving force for PCOS, AMH could provide a potential indicator for its diagnosis, although AMH as a single-index diagnosis for PCOS remains to be established. We believe that a single indicator alone has its drawbacks. For example, AMH levels decrease with age, so older women with PCOS will have lower AMH levels despite exhibiting PCOS symptoms. Thus, we believe that a mathematical model combining AMH and age and other predictors, such as body mass index, might be required for the future diagnosis of PCOS. Age-stratified thresholds for AMH have been reported for the diagnosis of PCOS,148 and AMH is predicted to replace AFC as a diagnostic indicator for this syndrome.149
In Males
It remains unknown whether a PCOS equivalent is present in males. It has been reported that male relatives of women with PCOS exhibit hormonal and metabolic abnormalities, with an increased incidence of early-onset (<35 years old) androgenetic alopecia and an increased prevalence of type II diabetes mellitus and cardiovascular disease,150,151 which collectively suggest the existence of a male equivalent for PCOS via the inheritance of susceptibility genes. Although similar clinical features of PCOS observed in women have been found in male subjects with male PCOS equivalence syndrome, the exact mechanism of the hormone and metabolic background of these patients has not been clarified.152 As AMH has been identified to be the driving force for elevated LH, hyperandrogenemia, and ovulatory disorders of PCOS,153 whether AMH could also be used for predicting or diagnosing the PCOS equivalent in men, to apply early lifestyle interventions to manage the progression of this condition, is worth investigating in the future.
AMH in Chemotherapy
In Females
Because of chemotherapeutic damage to follicles, chemotherapy often impairs future reproductive potential, especially OR.154,155 As mentioned above, AMH is produced by preantral and small antral follicles in the human ovary12,14 and acts in an FSH-independent manner.13,14 Patients whose AMH levels were higher before chemotherapy maintained better menstrual cycles after treatment, and they had a higher probability to achieve pregnancy.156,157 Therefore, for patients receiving chemotherapy, it is important to assess their OR function regularly. We have established two OR models termed AAFA4 and AFA;3 and these could be of great importance for the survivors of chemotherapy to assess their OR, predict reproductive life span, and arrange their childbearing planning, and might be useful clinically.
In Males
Can an AMH-related assessment of fertility before or after chemotherapy be applied to men? For example, could we use dynamic changes to serum AMH levels pre and post chemotherapy to evaluate testicular damage? AMH levels were reported to rise shortly after chemotherapy.158 Other studies have reported a transient increase in AMH levels after testicular injury. For example, AMH levels were elevated in prepubertal and pubertal boys with varicocele96 and were significantly decreased in subfertile men with severe varicoceles.97 In addition, boys with KS (characterized by accelerated germ cell depletion from puberty)19 exhibit a delay in the puberty-related decline of AMH, followed by a quick reduction of AMH, inhibin B, and testosterone levels in adulthood.19,95 These examples indicate a compensatory increase in SC function in the early onset of testicular injury and the long-term decrease in SC function caused by severe testicular damage. Thus, it is possible that AMH might not be a good marker for evaluating testicular injury shortly after chemotherapy but could provide a suitable marker for evaluating long-term damage.
AMH in Ovarian GC and Testicular SC Tumors
SCs and GCs have the same developmental origin during embryogenesis,93 so SC tumors (SCTs) and GC tumors (GCTs) share common gene expression patterns. As described above, AMH is a marker for ovarian GCs of immature follicles22 and for immature testicular SCs.19 Tumor cells are typically undifferentiated, so AMH would be predicted to be highly expressed in both GCTs and SCTs. Indeed, AMH was overexpressed in GCTs,159 and was not found in other types of gonadal or nongonadal tumors.160 A combination of AMH and inhibin B treatment was shown to increase the accuracy of differentially diagnosing GCTs from epithelial ovarian carcinomas and endometriomas.161
AMH could be used as a potential marker for male SCTs; however, human testicular SCTs are very rare and account for only 0.4%–1.5% of testicular tumors.162 Therefore, current research on SCTs is mostly in the stage of animal experimentation. Thus, canine AMH levels were higher in an SCT group (22 ng/mL) compared with a normal control group (10 ng/mL), while AMH levels with other types of testicular tumor ranged between these two groups.163,164 More investigations are needed to determine the diagnostic threshold of AMH for SCTs in humans.
AMH in the Differential Diagnosis of Constitutive Pubertal Delay and Congenital Hypogonadotropic Hypogonadism in Prepubertal Boys
Delayed sexual maturation in prepubertal boys is the common clinical characteristics of boys with constitutive pubertal delay or congenital hypogonadotropic hypogonadism (HH). As is known, the clinical value of serum Gn and testosterone is limited, because of their low serum levels in both conditions. Thus, AMH as a marker of immature SCs is of great potential importance in the differential diagnosis between constitutive pubertal delay and congenital HH.16,165 Decreased numbers of SCs were reported in patients with congenital HH, accompanied by low levels of serum AMH.166, 167, 168 However, in boys with constitutive pubertal delay, SC function is normal, so the serum AMH levels are also normal.169
Summary and Prospects
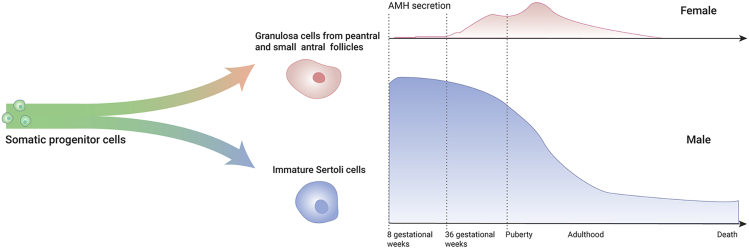
AMH is secreted by immature SCs in men19,20 and GCs of small growing follicles in women,9, 10, 11 where it has important roles in regulating genital tract development and function.6,7 In male embryos, the production of AMH starts in the eighth gestational week and is responsible for regression of the Müllerian ducts.15 In female embryos, AMH is not expressed until the 36th week of gestation, when the primordial follicle pool is fully differentiated.9 The production of AMH in both men and women is shown in Figure 4, which is based on data from several studies.9,15,17,39
Figure 4.
Production of AMH in Both Sexes
The production and serum level of AMH throughout the life span in both male and female humans.
AMH levels vary considerably between boys and girls, and this marked sex difference in AMH levels lasts until puberty. Therefore, serum levels of AMH are often used for the diagnosis of DSDs.19 After sexual maturity, AMH levels become similar in men and women, and an age-related decline in AMH levels is found in both sexes.17,18,21 As shown in Figure 2, when the primordial follicle pool is depleted at menopause, circulating AMH becomes undetectable. Therefore, AMH is often used to assess fertility and predict ovarian aging. As an indicator of OR, AMH is also often used to predict the ovarian response and to guide recombinant FSH dose during ovarian stimulation in ART.10,170 AMH levels are positively correlated with the number of small growing follicles in women and immature SCs in men, so AMH might serve as a diagnostic marker for GCTs and SCTs. Serum AMH levels have also been used for predicting and evaluating ovarian damage before and after chemotherapy.
Women with PCOS have elevated serum AMH levels and later age at menopause compared with women without PCOS.146 In a mice model, excess AMH was reported to be involved in regulating the HPG axis,52 and excess AMH leads to hyperandrogenemia and anovulation,43 which may contribute to the onset of PCOS. Thus, AMH is being increasingly recognized as a marker for the diagnosis of this disorder, although a specific cutoff value needs further improvement. The potential application of AMH in cases of male androgenetic alopecia could be another future direction for AMH-related studies in men, because androgenetic alopecia is potentially the equivalent of PCOS.
Studies on the clinical application of AMH in adult men are limited. However, based on the homology between SCs in men and GCs in women, it has been suggested that AMH might provide a valuable indicator for several male infertility-related disorders. For example, the absence of AMH in adult men indicates the absence of functional testicular tissue and could provide a differential diagnosis between NOA and OA in men or for the differential diagnosis between anorchia and cryptorchidism in boys.
Acknowledgments
This study was supported by the National Natural Science Foundation of China for Distinguished Young Scholars (grant no. 81925013); the Major National R&D Projects of China (grant no. 2017ZX09304012-012); the National Key Research and Development Program of China (grant no. 2018YFC1002104, 2018YFC1002106); National Natural Science Foundation of China (grant no. 81771650); the Capital Health Research and Development of Special Project (grant no. 2018-1-4091); Program for the Innovative Research Team of Yunnan, China (grant no. 2017HC009). We thank James Cummins, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author Contributions
H.X., M.Z and H.Z. contributed equally to writing the manuscript. K.A., L.W, and R.L. helped writing the manuscript. J.Q. supervised, organized and revised the manuscript. All authors read and approved the final manuscript.
Declaration of Interests
The authors declare no competing interests.
Published Online: February 8, 2021
Lead Contact Website
References
- 1.Mascarenhas M.N., Flaxman S.R., Boerma T., et al. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daan N.M., Fauser B.C. Menopause prediction and potential implications. Maturitas. 2015;82:257–265. doi: 10.1016/j.maturitas.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Xu H., Shi L., Feng G., et al. An ovarian reserve assessment model based on anti-mullerian hormone levels, follicle-stimulating hormone levels, and age: retrospective cohort study. J. Med. Internet Res. 2020;22:e19096. doi: 10.2196/19096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H., Feng G., Wang H., et al. A novel mathematical model of true ovarian reserve assessment based on predicted probability of poor ovarian response: a retrospective cohort study. J. Assist Reprod. Genet. 2020;37:963–972. doi: 10.1007/s10815-020-01700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jost A. The age factor in the castration of male rabbit fetuses. Proc. Soc. Exp. Biol. Med. 1947;66:302–303. doi: 10.3181/00379727-66-16071. [DOI] [PubMed] [Google Scholar]
- 6.Pangas S.A. Regulation of the ovarian reserve by members of the transforming growth factor beta family. Mol. Reprod. Dev. 2012;79:666–679. doi: 10.1002/mrd.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monsivais D., Matzuk M.M., Pangas S.A., et al. The TGF-beta family in the reproductive tract. Cold Spring Harb. Perspect. Biol. 2017;9:a022251. doi: 10.1101/cshperspect.a022251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran D., Muesy-Dessole N., Josso N., et al. Anti-Mullerian hormone is a functional marker of foetal Sertoli cells. Nature. 1977;269:411–412. doi: 10.1038/269411a0. [DOI] [PubMed] [Google Scholar]
- 9.Rajpert-De Meyts E., Jorgensen N., Graem N., et al. Expression of anti-Mullerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J. Clin. Endocrinol. Metab. 1999;84:3836–3844. doi: 10.1210/jcem.84.10.6047. [DOI] [PubMed] [Google Scholar]
- 10.Broer S.L., Dolleman M., Opmeer B.C., et al. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum. Reprod. Update. 2011;17:46–54. doi: 10.1093/humupd/dmq034. [DOI] [PubMed] [Google Scholar]
- 11.La Marca A., Sunkara S.K. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum. Reprod. Update. 2014;20:124–140. doi: 10.1093/humupd/dmt037. [DOI] [PubMed] [Google Scholar]
- 12.Durlinger A.L., Kramer P., Karels B., et al. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 13.Fanchin R., De Pawn K., Taieb J., et al. Lack of AMH response to EFORT suggests that AMH production is gonadotropin-independent in adult women. Fertil. Steril. 2005;84:S424. [Google Scholar]
- 14.La Marca A., Volpe A. Anti-Mullerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin. Endocrinol. (Oxf) 2006;64:603–610. doi: 10.1111/j.1365-2265.2006.02533.x. [DOI] [PubMed] [Google Scholar]
- 15.Josso N., Lamarre I., Picard J.Y., et al. Anti-Mullerian hormone in early human development. Early Hum. Dev. 1993;33:91–99. doi: 10.1016/0378-3782(93)90204-8. [DOI] [PubMed] [Google Scholar]
- 16.Matuszczak E., Hermanowicz A., Komarowska M., Debek W. Serum AMH in physiology and pathology of male gonads. Int. J. Endocrinol. 2013;2013:128907. doi: 10.1155/2013/128907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aksglaede L., Sorensen K., Boas M., et al. Changes in anti-mullerian hormone (AMH) throughout the life span: a population-based study of 1027 healthy males from birth (cord blood) to the age of 69 years. J. Clin. Endocrinol. Metab. 2010;95:5357–5364. doi: 10.1210/jc.2010-1207. [DOI] [PubMed] [Google Scholar]
- 18.Chong Y.H., Dennis N.A., Connolly M.J., et al. Elderly men have low levels of anti-mullerian hormone and inhibin B, but with high interpersonal variation: a cross-sectional study of the Sertoli cell hormones in 615 community-dwelling men. PLoS One. 2013;8:e70967. doi: 10.1371/journal.pone.0070967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H.Y., Zhang H.X., Xiao Z., et al. Regulation of anti-Mullerian hormone (AMH) in males and the associations of serum AMH with the disorders of male fertility. Asian J. Androl. 2019;21:109–114. doi: 10.4103/aja.aja_83_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AlAttar L., Noel K., Dutertre M., et al. Hormonal and cellular regulation of Sertoli cell anti-Mullerian hormone production in the postnatal mouse. J. Clin. Invest. 1997;100:1335–1343. doi: 10.1172/JCI119653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramezani Tehrani F., Mansournia M.A., Solaymani-Dodaran M., et al. Serum variations of anti-mullerian hormone and total testosterone with aging in healthy adult Iranian men: a population-based study. PLoS One. 2017;12:e0179634. doi: 10.1371/journal.pone.0179634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewailly D., Andersen C.Y., Balen A., et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum. Reprod. Update. 2014;20:370–385. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 23.Sobel V., Zhu Y.S., Imperato-McGinley J., et al. Fetal hormones and sexual differentiation. Obstet. Gynecol. Clin. North Am. 2004;31:837. doi: 10.1016/j.ogc.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Jost A. Problems of fetal endocrinology - the gonadal and hypophyseal hormones. Recent Prog. Horm. Res. 1953;8:379–418. [Google Scholar]
- 25.Sajjad Y. Development of the genital ducts and external genitalia in the early human embryo. J. Obstet. Gynaecol. Res. 2010;36:929–937. doi: 10.1111/j.1447-0756.2010.01272.x. [DOI] [PubMed] [Google Scholar]
- 26.Petersen C., Soder O. The Sertoli cell - a hormonal target and "super" nurse for germ cells that determines testicular size. Horm. Res. 2006;66:153–161. doi: 10.1159/000094142. [DOI] [PubMed] [Google Scholar]
- 27.Nistal M., Jimenez F., Paniagua R., et al. Sertoli-cell types in the Sertoli-cell-only syndrome - relationships between sertoli-cell morphology and etiology. Histopathology. 1990;16:173–180. doi: 10.1111/j.1365-2559.1990.tb01086.x. [DOI] [PubMed] [Google Scholar]
- 28.Lukas-Croisier C., Lasala C., Nicaud J., et al. Follicle-stimulating hormone increases testicular anti-Mullerian hormone (AMH) production through Sertoli cell proliferation and a nonclassical cyclic adenosine 5 ’-monophosphate-mediated activation of the AMH gene. Mol. Endocrinol. 2003;17:550–561. doi: 10.1210/me.2002-0186. [DOI] [PubMed] [Google Scholar]
- 29.McKay L.I., Cidlowski J.A. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr. Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 30.De Santa Barbara P., Bonneaud N., Boizet B., et al. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Mullerian hormone gene. Mol. Cell Biol. 1998;18:6653–6665. doi: 10.1128/mcb.18.11.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arango N.A., Lovell-Badge R., Behringer R.R., et al. Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell. 1999;99:409–419. doi: 10.1016/s0092-8674(00)81527-5. [DOI] [PubMed] [Google Scholar]
- 32.Shen W.H., Moore C.C.D., Ikeda Y., et al. Nuclear receptor steroidogenic factor-1 regulates the mullerian-inhibiting substance gene - a link to the sex determination cascade. Cell. 1994;77:651–661. doi: 10.1016/0092-8674(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 33.Giuili G., Shen W.H., Ingraham H.A., et al. The nuclear receptor SF-1 mediates sexually dimorphic expression of Mullerian inhibiting substance, in vivo. Development. 1997;124:1799–1807. doi: 10.1242/dev.124.9.1799. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe K., Clarke T.R., Lane A.H., et al. Endogenous expression of Mullerian inhibiting substance in early postnatal rat Sertoli cells requires multiple steroidogenic factor-1 and GATA-4-binding sites. Proc. Natl. Acad. Sci. U S A. 2000;97:1624–1629. doi: 10.1073/pnas.97.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tremblay J.J., Viger R.S. Transcription factor GATA-4 enhances Mullerian inhibiting substance gene transcription through a direct interaction with the nuclear receptor SF-1. Mol. Endocrinol. 1999;13:1388–1401. doi: 10.1210/mend.13.8.0330. [DOI] [PubMed] [Google Scholar]
- 36.Beau C., Rauch M., Joulin V., et al. GATA-1 is a potential repressor of anti-Mullerian hormone expression during the establishment of puberty in the mouse. Mol. Reprod. Dev. 2000;56:124–138. doi: 10.1002/(SICI)1098-2795(200006)56:2<124::AID-MRD2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 37.Griswold M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998;9:411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 38.Orth J.M., Gunsalus G.L., Lamperti A.A., et al. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology. 1988;122:787–794. doi: 10.1210/endo-122-3-787. [DOI] [PubMed] [Google Scholar]
- 39.Cui L., Qin Y., Gao X., et al. Antimullerian hormone: correlation with age and androgenic and metabolic factors in women from birth to postmenopause. Fertil. Steril. 2016;105:481–485.e1. doi: 10.1016/j.fertnstert.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 40.Broekmans F.J., Visser J.A., Laven J.S., et al. Anti-Mullerian hormone and ovarian dysfunction. Trends Endocrinol. Metab. 2008;19:340–347. doi: 10.1016/j.tem.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Broer S.L., Broekmans F.J., Laven J.S., et al. Anti-Mullerian hormone: ovarian reserve testing and its potential clinical implications. Hum. Reprod. Update. 2014;20:688–701. doi: 10.1093/humupd/dmu020. [DOI] [PubMed] [Google Scholar]
- 42.Hayes E., Kushnir V., Ma X.T., et al. Intra-cellular mechanism of anti-Mullerian hormone (AMH) in regulation of follicular development. Mol. Cell Enocrinol. 2016;433:56–65. doi: 10.1016/j.mce.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 43.Tata B., Mimouni N.E.H., Barbotin A.L., et al. Elevated prenatal anti-Mullerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat. Med. 2018;24:834. doi: 10.1038/s41591-018-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Visser J.A., Durlinger A.L., Peters I.J., et al. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Mullerian hormone null mice. Endocrinology. 2007;148:2301–2308. doi: 10.1210/en.2006-1265. [DOI] [PubMed] [Google Scholar]
- 45.Gruijters M.J., Visser J.A., Durlinger A.L., Themmen A.P. Anti-Mullerian hormone and its role in ovarian function. Mol. Cell Endocrinol. 2003;211:85–90. doi: 10.1016/j.mce.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 46.Weenen C., Laven J.S., Von Bergh A.R., et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol. Hum. Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 47.Durlinger A.L., Gruijters M.J., Kramer P., et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 48.Carlsson I.B., Scott J.E., Visser J.A., et al. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum. Reprod. 2006;21:2223–2227. doi: 10.1093/humrep/del165. [DOI] [PubMed] [Google Scholar]
- 49.Dewailly D., Robin G., Peigne M., et al. Interactions between androgens, FSH, anti-Mullerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update. 2016;22:709–724. doi: 10.1093/humupd/dmw027. [DOI] [PubMed] [Google Scholar]
- 50.La Marca A., Stabile G., Artenisio A.C., Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum. Reprod. 2006;21:3103–3107. doi: 10.1093/humrep/del291. [DOI] [PubMed] [Google Scholar]
- 51.Cook C.L., Siow Y., Taylor S., Fallat M.E. Serum mullerian-inhibiting substance levels during normal menstrual cycles. Fertil. Steril. 2000;73:859–861. doi: 10.1016/s0015-0282(99)00639-1. [DOI] [PubMed] [Google Scholar]
- 52.Cimino I., Casoni F., Liu X.H., et al. Novel role for anti-Mullerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat. Commun. 2016;7:10055. doi: 10.1038/ncomms10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kano M., Sosulski A.E., Zhang L.H., et al. AMH/MIS as a contraceptive that protects the ovarian reserve during chemotherapy. Proc. Natl. Acad. Sci. U S A. 2017;114:E1688–E1697. doi: 10.1073/pnas.1620729114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grynberg M., Pierre A., Rey R., et al. Differential regulation of ovarian anti-mullerian hormone (AMH) by estradiol through alpha- and beta-estrogen receptors. J. Clin. Endocrinol. Metab. 2012;97:E1649–E1657. doi: 10.1210/jc.2011-3133. [DOI] [PubMed] [Google Scholar]
- 55.Styer A.K., Gaskins A.J., Brady P.C., et al. Dynamic antimullerian hormone levels during controlled ovarian hyperstimulation predict in vitro fertilization response and pregnancy outcomes. Fertil. Steril. 2015;104:1153. doi: 10.1016/j.fertnstert.2015.07.1161. [DOI] [PubMed] [Google Scholar]
- 56.Xu H., Zeng L., Yang R., et al. Retrospective cohort study: AMH is the best ovarian reserve markers in predicting ovarian response but has unfavorable value in predicting clinical pregnancy in GnRH antagonist protocol. Arch. Gynecol. Obstet. 2017;295:763–770. doi: 10.1007/s00404-016-4274-8. [DOI] [PubMed] [Google Scholar]
- 57.Kim C., Slaughter J.C., Wang E.T., et al. Anti-Mullerian hormone, follicle stimulating hormone, antral follicle count, and risk of menopause within 5 years. Maturitas. 2017;102:18–25. doi: 10.1016/j.maturitas.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kotanidis L., Nikolettos K., Petousis S., et al. The use of serum anti-Mullerian hormone (AMH) levels and antral follicle count (AFC) to predict the number of oocytes collected and availability of embryos for cryopreservation in IVF. J. Endocrinol. Invest. 2016;39:1459–1464. doi: 10.1007/s40618-016-0521-x. [DOI] [PubMed] [Google Scholar]
- 59.Streuli I., Fraisse T., Pillet C., et al. Serum antimullerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil. Steril. 2008;90:395–400. doi: 10.1016/j.fertnstert.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 60.Hadlow N., Longhurst K., McClements A., et al. Variation in antimullerian hormone concentration during the menstrual cycle may change the clinical classification of the ovarian response. Fertil. Steril. 2013;99:1791–1797. doi: 10.1016/j.fertnstert.2013.01.132. [DOI] [PubMed] [Google Scholar]
- 61.Bungum L., Tagevi J., Jokubkiene L., et al. The impact of the biological variability or assay performance on AMH measurements: a prospective cohort study with AMH tested on three analytical assay-platforms. Front Endocrinol. (Lausanne) 2018;9:603. doi: 10.3389/fendo.2018.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magnusson A., Olerod G., Thurin-Kjellberg A., Bergh C. The correlation between AMH assays differs depending on actual AMH levels. Hum. Reprod. Open. 2017;2017:hox026. doi: 10.1093/hropen/hox026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li H.W., Wong B.P., Ip W.K., et al. Comparative evaluation of three new commercial immunoassays for anti-Mullerian hormone measurement. Hum. Reprod. 2016;31:2796–2802. doi: 10.1093/humrep/dew248. [DOI] [PubMed] [Google Scholar]
- 64.Ferguson J.M., Pepin D., Duru C., et al. Towards international standardization of immunoassays for Mullerian inhibiting substance/anti-Mullerian hormone. Reprod. Biomed. Online. 2018;37:631–640. doi: 10.1016/j.rbmo.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zegers-Hochschild F., Adamson G.D., Dyer S., et al. The international glossary on infertility and fertility care, 2017. Fertil. Steril. 2017;108:393–406. doi: 10.1016/j.fertnstert.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 66.Tatone C., Amicarelli F. The aging ovary–the poor granulosa cells. Fertil. Steril. 2013;99:12–17. doi: 10.1016/j.fertnstert.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 67.de Bruin J.P., Dorland M., Spek E.R., et al. Age-related changes in the ultrastructure of the resting follicle pool in human ovaries. Biol. Reprod. 2004;70:419–424. doi: 10.1095/biolreprod.103.015784. [DOI] [PubMed] [Google Scholar]
- 68.Broekmans F.J., Soules M.R., Fauser B.C. Ovarian aging: mechanisms and clinical consequences. Endocr. Rev. 2009;30:465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 69.Depalo R., Nappi L., Loverro G., et al. Evidence of apoptosis in human primordial and primary follicles. Hum. Reprod. 2003;18:2678–2682. doi: 10.1093/humrep/deg507. [DOI] [PubMed] [Google Scholar]
- 70.Glamoclija V., Vilovic K., Saraga-Babic M., et al. Apoptosis and active caspase-3 expression in human granulosa cells. Fertil. Steril. 2005;83:426–431. doi: 10.1016/j.fertnstert.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 71.Seifer D.B., Merhi Z. Is AMH a regulator of follicular atresia? J. Assist Reprod. Genet. 2014;31:1403–1407. doi: 10.1007/s10815-014-0328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lashen H., Dunger D.B., Ness A., Ong K.K. Peripubertal changes in circulating antimullerian hormone levels in girls. Fertil. Steril. 2013;99:2071–2075. doi: 10.1016/j.fertnstert.2013.01.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wallace W.H., Kelsey T.W. Human ovarian reserve from conception to the menopause. PLoS One. 2010;5:e8772. doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Regan S.L.P., Knight P.G., Yovich J.L., et al. Granulosa cell apoptosis in the ovarian follicle–A changing view. Front Endocrinol. (Lausanne) 2018;9:61. doi: 10.3389/fendo.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faddy M.J., Gosden R.G., Gougeon A., et al. Accelerated disappearance of ovarian follicles in midlife - implications for forecasting menopause. Hum. Reprod. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 76.American College of Obstetricians and Gynecologists Committee opinion no. 618: ovarian reserve testing. Obstet. Gynecol. 2015;125:268–273. doi: 10.1097/01.AOG.0000459864.68372.ec. [DOI] [PubMed] [Google Scholar]
- 77.Kelsey T.W., Wright P., Nelson S.M., et al. A validated model of serum anti-mullerian hormone from conception to menopause. PLoS One. 2011;6:e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Practice Committee of the American Society for Reproductive Medicine Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil. Steril. 2015;103:e9–e17. doi: 10.1016/j.fertnstert.2014.12.093. [DOI] [PubMed] [Google Scholar]
- 79.Sunkara S.K., Rittenberg V., Raine-Fenning N., et al. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum. Reprod. 2011;26:1768–1774. doi: 10.1093/humrep/der106. [DOI] [PubMed] [Google Scholar]
- 80.Sunkara S.K., La Marca A., Seed P.T., Khalaf Y. Increased risk of preterm birth and low birthweight with very high number of oocytes following IVF: an analysis of 65 868 singleton live birth outcomes. Hum. Reprod. 2015;30:1473–1480. doi: 10.1093/humrep/dev076. [DOI] [PubMed] [Google Scholar]
- 81.Ubaldi F., Vaiarelli A., D’Anna R., Rienzi L. Management of poor responders in IVF: is there anything new? Biomed. Res. Int. 2014;2014:352098. doi: 10.1155/2014/352098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steward R.G., Lan L., Shah A.A., et al. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil. Steril. 2014;101:967–973. doi: 10.1016/j.fertnstert.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 83.Lukaszuk K., Liss J., Kunicki M., et al. Anti-Mullerian hormone (AMH) is a strong predictor of live birth in women undergoing assisted reproductive technology. Reprod. Biol. 2014;14:176–181. doi: 10.1016/j.repbio.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 84.Tal R., Seifer D.B., Wantman E., et al. Antimullerian hormone as a predictor of live birth following assisted reproduction: an analysis of 85,062 fresh and thawed cycles from the Society for Assisted Reproductive Technology Clinic Outcome Reporting System database for 2012-2013. Fertil. Steril. 2018;109:258–265. doi: 10.1016/j.fertnstert.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 85.Nelson H.D. Menopause. Lancet. 2008;371:760–770. doi: 10.1016/S0140-6736(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 86.Finkelstein J.S., Lee H., Karlamangla A., et al. Anti-Mullerian hormone and impending menopause in late reproductive age: the study of women’s health across the nation. J. Clin. Endocrinol. Metab. 2020;105:e1862–e1871. doi: 10.1210/clinem/dgz283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bertone-Johnson E.R., Manson J.E., Purdue-Smithe A.C., et al. Anti-Mullerian hormone levels and incidence of early natural menopause in a prospective study. Hum. Reprod. 2018;33:1175–1182. doi: 10.1093/humrep/dey077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Depmann M., Eijkemans M.J., Broer S.L., et al. Does anti-Mullerian hormone predict menopause in the general population? Results of a prospective ongoing cohort study. Hum. Reprod. 2016;31:1579–1587. doi: 10.1093/humrep/dew112. [DOI] [PubMed] [Google Scholar]
- 89.Tehrani F.R., Shakeri N., Solaymani-Dodaran M., Azizi F. Predicting age at menopause from serum antimullerian hormone concentration. Menopause. 2011;18:766–770. doi: 10.1097/gme.0b013e318205e2ac. [DOI] [PubMed] [Google Scholar]
- 90.Freeman E.W., Sammel M.D., Lin H., Gracia C.R. Anti-Mullerian hormone as a predictor of time to menopause in late reproductive age women. J. Clin. Endocrinol. Metab. 2012;97:1673–1680. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Rooij I.A.J., Broekmans F.J.M., Scheffer G.J., et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil. Steril. 2005;83:979–987. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 92.Rotgers E., Jorgensen A., Yao H.H.C., et al. At the crossroads of fate-somatic cell lineage specification in the fetal gonad. Endocr. Rev. 2018;39:739–759. doi: 10.1210/er.2018-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stevant I., Kuehne F., Greenfield A., et al. Dissecting cell lineage specification and sex fate determination in gonadal somatic cells using single-cell transcriptomics. Cell Rep. 2019;26:3272. doi: 10.1016/j.celrep.2019.02.069. [DOI] [PubMed] [Google Scholar]
- 94.Haji M., Tanaka S., Nishi Y., et al. Sertoli-cell function declines earlier than Leydig-cell function in aging Japanese men. Maturitas. 1994;18:143–153. doi: 10.1016/0378-5122(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 95.Aksglaede L., Christiansen P., Sorensen K., et al. Serum concentrations of anti-Mullerian hormone (AMH) in 95 patients with Klinefelter syndrome with or without cryptorchidism. Acta Paediatr. 2011;100:839–845. doi: 10.1111/j.1651-2227.2011.02148.x. [DOI] [PubMed] [Google Scholar]
- 96.Trigo R.V., Bergada I., Rey R., et al. Altered serum profile of inhibin B, Pro-alphaC and anti-Mullerian hormone in prepubertal and pubertal boys with varicocele. Clin. Endocrinol. (Oxf) 2004;60:758–764. doi: 10.1111/j.1365-2265.2004.02051.x. [DOI] [PubMed] [Google Scholar]
- 97.Goulis D.G., Iliadou P.K., Tsametis C., et al. Serum anti-Mullerian hormone levels differentiate control from subfertile men but not men with different causes of subfertility. Gynecol. Endocrinol. 2008;24:158–160. doi: 10.1080/09513590701672314. [DOI] [PubMed] [Google Scholar]
- 98.Klami R., Mankonen H., Perheentupa A. Successful microdissection testicular sperm extraction for men with non-obstructive azoospermia. Reprod. Biol. 2018;18:137–142. doi: 10.1016/j.repbio.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 99.Zirkin B.R., Papadopoulos V. Leydig cells: formation, function, and regulation. Biol. Reprod. 2018;99:101–111. doi: 10.1093/biolre/ioy059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Picard J.Y., Cate R.L., Racine C., Josso N. The persistent mullerian duct syndrome: an update based upon a personal experience of 157 cases. Sex Dev. 2017;11:109–125. doi: 10.1159/000475516. [DOI] [PubMed] [Google Scholar]
- 101.Johansen M.L., Hagen C.P., Johannsen T.H., et al. Anti-Mullerian hormone and its clinical use in pediatrics with special emphasis on disorders of sex development. Int. J. Endocrinol. 2013;2013:198698. doi: 10.1155/2013/198698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rey R.A., Grinspon R.P. Normal male sexual differentiation and aetiology of disorders of sex development. Best Pract. Res. Clin. Endocrinol. Metab. 2011;25:221–238. doi: 10.1016/j.beem.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 103.Kolon T.F., Granholm T., Nordenskjold A., Ritzen E.M. Evaluation and treatment of cryptorchidism: AUA guideline. J. Urol. 2014;192:337–345. doi: 10.1016/j.juro.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 104.Kollin C., Granholm T., Nordenskjold A., Ritzen E.M. Growth of spontaneously descended and surgically treated testes during early childhood. Pediatrics. 2014;131:e1174–e1180. doi: 10.1542/peds.2012-2902. [DOI] [PubMed] [Google Scholar]
- 105.van der Plas E.M., Zijp G.W., Froeling F.M., et al. Long-term testicular volume after orchiopexy at diagnosis of acquired undescended testis. J. Urol. 2013;190:257–262. doi: 10.1016/j.juro.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 106.Young J., Chanson P., Salenave S., et al. Testicular anti-mullerian hormone secretion is stimulated by recombinant human FSH in patients with congenital hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 2005;90:724–728. doi: 10.1210/jc.2004-0542. [DOI] [PubMed] [Google Scholar]
- 107.Nistal M., Abaurrea M.A., Paniagua R. Morphological and histometric study on the human Sertoli cell from birth to the onset of puberty. J. Anat. 1982;134:351–363. [PMC free article] [PubMed] [Google Scholar]
- 108.Matuszczak E., Hermanowicz A., Debek W., et al. Serum AMH concentration as a marker evaluating gonadal function in boys operated on for unilateral cryptorchidism between 1st and 4th year of life. Endocrine. 2012;41:334–337. doi: 10.1007/s12020-011-9551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Demircan M., Akinci A., Mutus M. The effects of orchiopexy on serum anti-Mullerian hormone levels in unilateral cryptorchid infants. Pediatr. Surg. Int. 2006;22:271–273. doi: 10.1007/s00383-006-1646-3. [DOI] [PubMed] [Google Scholar]
- 110.Guibourdenche J., Lucidarme N., Chevenne D., et al. Anti-Mullerian hormone levels in serum from human foetuses and children: pattern and clinical interest. Mol. Cell Endocrinol. 2003;211:55–63. doi: 10.1016/j.mce.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 111.Cortes D. Cryptorchidism--aspects of pathogenesis, histology and treatment. Scand. J. Urol. Nephrol. Suppl. 1998;196:1–54. [PubMed] [Google Scholar]
- 112.Engeler D.S., Hosli P.O., John H., et al. Early orchiopexy: prepubertal intratubular germ cell neoplasia and fertility outcome. Urology. 2000;56:144–148. doi: 10.1016/s0090-4295(00)00560-4. [DOI] [PubMed] [Google Scholar]
- 113.Lee P.A., O’Leary L.A., Songer N.J., et al. Paternity after unilateral cryptorchidism: a controlled study. Pediatrics. 1996;98:676–679. [PubMed] [Google Scholar]
- 114.Thorup J., McLachlan R., Cortes D., et al. What is new in cryptorchidism and hypospadias--a critical review on the testicular dysgenesis hypothesis. J. Pediatr. Surg. 2010;45:2074–2086. doi: 10.1016/j.jpedsurg.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 115.Lee P.A., Coughlin M.T. Fertility after bilateral cryptorchidism. Evaluation by paternity, hormone, and semen data. Horm. Res. 2001;55:28–32. doi: 10.1159/000049960. [DOI] [PubMed] [Google Scholar]
- 116.Cortes D., Thorup J.M., Beck B.L. Quantitative histology of germ cells in the undescended testes of human fetuses, neonates and infants. J. Urol. 1995;154:1188–1192. [PubMed] [Google Scholar]
- 117.Ferraretti A.P., La Marca A., Fauser B.C., et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum. Reprod. 2011;26:1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 118.Alviggi C., Andersen C.Y., Buehler K., et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil. Steril. 2016;105:1452–1453. doi: 10.1016/j.fertnstert.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 119.Andersen A.N., Nelson S.M., Fauser B.C., et al. Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil. Steril. 2017;107:387. doi: 10.1016/j.fertnstert.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 120.Buyuk E., Seifer D.B., Younger J., et al. Random anti-Mullerian hormone (AMH) is a predictor of ovarian response in women with elevated baseline early follicular follicle-stimulating hormone levels. Fertil. Steril. 2011;95:2369–2372. doi: 10.1016/j.fertnstert.2011.03.071. [DOI] [PubMed] [Google Scholar]
- 121.Nardo L.G., Gelbaya T.A., Wilkinson H., et al. Circulating basal anti-Mullerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil. Steril. 2009;92:1586–1593. doi: 10.1016/j.fertnstert.2008.08.127. [DOI] [PubMed] [Google Scholar]
- 122.Fanchin R., Schonauer L.M., Righini C., et al. Serum anti-Mullerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum. Reprod. 2003;18:323–327. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 123.La Marca A., Argento C., Sighinolfi G., et al. Possibilities and limits of ovarian reserve testing in ART. Curr. Pharm. Biotechnol. 2012;13:398–408. doi: 10.2174/138920112799361972. [DOI] [PubMed] [Google Scholar]
- 124.Practice Committee of the American Society for Reproductive Medicine Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil. Steril. 2012;98:1407–1415. doi: 10.1016/j.fertnstert.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 125.Bottcher B., Tsybulyak I., Grubinger T., et al. Dynamics of anti-Mullerian hormone during controlled ovarian stimulation. Gynecol. Endocrinol. 2014;30:121–125. doi: 10.3109/09513590.2013.860124. [DOI] [PubMed] [Google Scholar]
- 126.Roy S., Gandra D., Seger C., et al. Oocyte-derived factors (GDF9 and BMP15) and FSH regulate AMH expression via modulation of H3K27AC in granulosa cells. Endocrinology. 2018;159:3433–3445. doi: 10.1210/en.2018-00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stegmann B.J., Santillan M., Leader B., et al. Changes in antimullerian hormone levels in early pregnancy are associated with preterm birth. Fertil. Steril. 2015;104:347. doi: 10.1016/j.fertnstert.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pastuszak A.W., Lipshultz L.I. AUA guideline on the diagnosis and treatment of cryptorchidism. J. Urol. 2014;192:346–349. doi: 10.1016/j.juro.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 129.Josso N., Rey R., Picard J.Y., et al. Testicular anti-mullerian hormone: clinical applications in DSD. Semin. Reprod. Med. 2012;30:364–373. doi: 10.1055/s-0032-1324719. [DOI] [PubMed] [Google Scholar]
- 130.Esteves S.C., Miyaoka R., Agarwal A. An update on the clinical assessment of the infertile male (vol 66, pg 691, 2011) Clinics. 2012;67:203. doi: 10.1590/S1807-59322011000400026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alfano M., Ventimiglia E., Locatelli I., et al. Anti-Mullerian hormone-to-testosterone ratio is predictive of positive sperm retrieval in men with idiopathic non-obstructive azoospermia. J. Urol. 2018;199:E796–E797. doi: 10.1038/s41598-017-17420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Toulis K.A., Iliadou P.K., Venetis C.A., et al. Inhibin B and anti-Mullerian hormone as markers of persistent spermatogenesis in men with non-obstructive azoospermia: a meta-analysis of diagnostic accuracy studies. Hum. Reprod. Update. 2010;16:713–724. doi: 10.1093/humupd/dmq024. [DOI] [PubMed] [Google Scholar]
- 133.Mitchell V., Boitrelle F., Pigny P., et al. Seminal plasma levels of anti-Mullerian hormone and inhibin B are not predictive of testicular sperm retrieval in nonobstructive azoospermia: a study of 139 men. Fertil. Steril. 2010;94:2147–2150. doi: 10.1016/j.fertnstert.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 134.Duvilla E., Lejeune H., Trombert-Paviot B., et al. Significance of inhibin B and anti-Mullerian hormone in seminal plasma: a preliminary study. Fertil. Steril. 2008;89:444–448. doi: 10.1016/j.fertnstert.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 135.March W.A., Moore V.M., Willson K.J., et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2010;25:544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 136.Padmanabhan V., Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids. 2013;78:734–740. doi: 10.1016/j.steroids.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Paixao L., Ramos R.B., Lavarda A., et al. Animal models of hyperandrogenism and ovarian morphology changes as features of polycystic ovary syndrome: a systematic review. Reprod. Biol. Endocrine. 2017;15:12. doi: 10.1186/s12958-017-0231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pappalardo M.A., Russo G.T., Pedone A., et al. Very high frequency of the polymorphism for the insulin receptor substrate 1 (IRS-1) at codon 972 (glycine972arginine) in southern Italian women with polycystic ovary syndrome. Horm. Metab. Res. 2010;42:575–584. doi: 10.1055/s-0030-1249020. [DOI] [PubMed] [Google Scholar]
- 139.Pappalardo M.A., Vita R., Di Bari F., et al. Gly972Arg of IRS-1 and Lys121Gln of PC-1 polymorphisms act in opposite way in polycystic ovary syndrome. J. Endocrinol. Invest. 2017;40:367–376. doi: 10.1007/s40618-016-0569-7. [DOI] [PubMed] [Google Scholar]
- 140.Albalawi F.S., Daghestani M.H., Daghestani M.H., et al. rs4889 polymorphism in KISS1 gene, its effect on polycystic ovary syndrome development and anthropometric and hormonal parameters in Saudi women. J. Biomed. Sci. 2018;25:50. doi: 10.1186/s12929-018-0452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Katulski K., Podfigurna A., Czyzyk A., et al. Kisspeptin and LH pulsatile temporal coupling in PCOS patients. Endocrine. 2018;61:149–157. doi: 10.1007/s12020-018-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Osuka S., Iwase A., Nakahara T., et al. Kisspeptin in the hypothalamus of 2 rat models of polycystic ovary syndrome. Endocrinology. 2017;158:367–377. doi: 10.1210/en.2016-1333. [DOI] [PubMed] [Google Scholar]
- 143.Brown R.E., Wilkinson D.A., Imran S.A., et al. Hypothalamic kiss1 mRNA and kisspeptin immunoreactivity are reduced in a rat model of polycystic ovary syndrome (PCOS) Brain Res. 2012;1467:1–9. doi: 10.1016/j.brainres.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 144.Cernea M., Padmanabhan V., Goodman R.L., et al. Prenatal testosterone treatment leads to changes in the morphology of KNDy neurons, their inputs, and projections to GnRH cells in female sheep. Endocrinology. 2015;156:3277–3291. doi: 10.1210/en.2014-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Osuka S., Nakanishi N., Murase T., et al. Animal models of polycystic ovary syndrome: a review of hormone-induced rodent models focused on hypothalamus-pituitary-ovary axis and neuropeptides. Reprod. Med. Biol. 2019;18:151–160. doi: 10.1002/rmb2.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tehrani F.R., Solaymani-Dodaran M., Hedayati M., Azizi F. Is polycystic ovary syndrome an exception for reproductive aging? Hum. Reprod. 2010;25:1775–1781. doi: 10.1093/humrep/deq088. [DOI] [PubMed] [Google Scholar]
- 147.Webber L.J., Stubbs S., Stark J., et al. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–1021. doi: 10.1016/s0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- 148.Dewailly D. Age-stratified thresholds of anti-Mullerian hormone improve prediction of polycystic ovary syndrome over a population-based threshold. Clin. Endocrinol. 2017;87:649–650. doi: 10.1111/cen.13479. [DOI] [PubMed] [Google Scholar]
- 149.Eilertsen T.B., Vanky E., Carlsen S.M. Anti-Mullerian hormone in the diagnosis of polycystic ovary syndrome: can morphologic description be replaced? Hum. Reprod. 2012;27:2494–2502. doi: 10.1093/humrep/des213. [DOI] [PubMed] [Google Scholar]
- 150.Cannarella R., Condorelli R., Mongioi L.M., et al. Does a male polycystic ovarian syndrome equivalent exist? J. Endocrinol. Invest. 2018;41:49–57. doi: 10.1007/s40618-017-0728-5. [DOI] [PubMed] [Google Scholar]
- 151.Sanke S., Chander R., Jain A., et al. A comparison of the hormonal profile of early androgenetic alopecia in men with the phenotypic equivalent of polycystic ovarian syndrome in women. JAMA Dermatol. 2016;152:986–991. doi: 10.1001/jamadermatol.2016.1776. [DOI] [PubMed] [Google Scholar]
- 152.Di Guardo F., Ciotta L., Monteleone M., Palumbo M. Male equivalent polycystic ovarian syndrome: hormonal, metabolic, and clinical aspects. Int. J. Fertil. Steril. 2020;14:79–83. doi: 10.22074/ijfs.2020.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Risal S., Pei Y., Lu H., et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat. Med. 2019;25:1894–1904. doi: 10.1038/s41591-019-0666-1. [DOI] [PubMed] [Google Scholar]
- 154.Anderson R.A., Mitchell R.T., Kelsey T.W., et al. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol. 2015;3:556–567. doi: 10.1016/S2213-8587(15)00039-X. [DOI] [PubMed] [Google Scholar]
- 155.Byrne J., Fears T.R., Gail M.H., et al. Early menopause in long-term survivors of cancer during adolescence. Am. J. Obstet. Gynecol. 1992;166:788–793. doi: 10.1016/0002-9378(92)91335-8. [DOI] [PubMed] [Google Scholar]
- 156.Iwase A., Sugita A., Hirokawa W., et al. Anti-Mullerian hormone as a marker of ovarian reserve following chemotherapy in patients with gestational trophoblastic neoplasia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;167:194–198. doi: 10.1016/j.ejogrb.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 157.Anderson R.A., Cameron D.A. Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J. Clin. Endocrinol. Metab. 2011;96:1336–1343. doi: 10.1210/jc.2010-2582. [DOI] [PubMed] [Google Scholar]
- 158.Levi M., Hasky N., Stemmer S.M., et al. Anti-Mullerian hormone is a marker for chemotherapy-induced testicular toxicity. Endocrinology. 2015;156:3818–3827. doi: 10.1210/en.2015-1310. [DOI] [PubMed] [Google Scholar]
- 159.Farkkila A., Koskela S., Bryk S., et al. The clinical utility of serum anti-Mullerian hormone in the follow-up of ovarian adult-type granulosa cell tumors–A comparative study with inhibin B. Int. J. Cancer. 2015;137:1661–1671. doi: 10.1002/ijc.29532. [DOI] [PubMed] [Google Scholar]
- 160.Rey R., Sabourin J.C., Venara M., et al. Anti-Mullerian hormone is a specific marker of Sertoli- and granulosa-cell origin in gonadal tumors. Hum. Pathol. 2000;31:1202–1208. doi: 10.1053/hupa.2000.18498. [DOI] [PubMed] [Google Scholar]
- 161.Haltia U.M., Hallamaa M., Tapper J., et al. Roles of human epididymis protein 4, carbohydrate antigen 125, inhibin B and anti-Mullerian hormone in the differential diagnosis and follow-up of ovarian granulosa cell tumors. Gynecol. Oncol. 2017;144:83–89. doi: 10.1016/j.ygyno.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 162.Werther M., Schmelz H.U., Schwerer M., Sparwasser C. Sclerosing Sertoli cell tumor of the testis - a tumor rare tumor - case report and review of the literature on the subtypes of Sertoli-cell. Urologe A. 2007;46:1551. doi: 10.1007/s00120-007-1556-6. [DOI] [PubMed] [Google Scholar]
- 163.Holst B.S., Dreimanis U. Anti-Mullerian hormone: a potentially useful biomarker for the diagnosis of canine Sertoli cell tumours. BMC Vet. Res. 2015;11:166. doi: 10.1186/s12917-015-0487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Banco B., Veronesi M.C., Giudice C., et al. Immunohistochemical evaluation of the expression of anti-mullerian hormone in mature, immature and neoplastic canine sertoli cells. J. Comp. Pathol. 2012;146:18–23. doi: 10.1016/j.jcpa.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 165.Edelsztein N.Y., Grinspon R.P., Schteingart H.F., Rey R.A. Anti-Mullerian hormone as a marker of steroid and gonadotropin action in the testis of children and adolescents with disorders of the gonadal axis. Int. J. Pediatr. Endocrinol. 2016;2016:20. doi: 10.1186/s13633-016-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hero M., Tommiska J., Vaaralahti K., et al. Circulating antimullerian hormone levels in boys decline during early puberty and correlate with inhibin B. Fertil. Steril. 2012;97:1242–1247. doi: 10.1016/j.fertnstert.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 167.Rey R.A., Grinspon R.P., Gottlieb S., et al. Male hypogonadism: an extended classification based on a developmental, endocrine physiology-based approach. Andrology. 2013;1:3–16. doi: 10.1111/j.2047-2927.2012.00008.x. [DOI] [PubMed] [Google Scholar]
- 168.Coutant R., Biette-Demeneix E., Bouvattier C., et al. Baseline inhibin B and anti-Mullerian hormone measurements for diagnosis of hypogonadotropic hypogonadism (HH) in boys with delayed puberty. J. Clin. Endocrinol. Metab. 2010;95:5225–5232. doi: 10.1210/jc.2010-1535. [DOI] [PubMed] [Google Scholar]
- 169.Adan L., Lechevalier P., Couto-Silva A.C., et al. Plasma inhibin B and antimullerian hormone concentrations in boys: discriminating between congenital hypogonadotropic hypogonadism and constitutional pubertal delay. Med. Sci. Monit. 2010;16 CR511–517. [PubMed] [Google Scholar]
- 170.Broer S.L., van Disseldorp J., Broeze K.A., et al. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum. Reprod. Update. 2013;19:26–36. doi: 10.1093/humupd/dms041. [DOI] [PubMed] [Google Scholar]