Abstract
Background
The incidence of cutaneous malignant melanoma, which is mostly attributable (86%) to UV radiation exposure, has been steadily increasing over the past four decades in predominantly fair-skinned populations. Although public health campaigns are increasing sun-protective behaviour in England, their effect on melanoma incidence is largely unknown. We conducted a retrospective population-based cohort study to examine whether there have been changes in the epidemiology of melanoma in England during the past four decades.
Methods
Individual level data for patients diagnosed with melanoma in England during 1981–2018 were obtained from the Office for National Statistics/Public Health England. Average annual incidence rates were calculated by three age categories (0–34, 35–64, 65+ years), gender and anatomical site during the seven five-year time periods (1981–85 to 2011–15) and the recent three-year period (2016–18). The percentage change in incidence was calculated as change in the average incidence rate from the first (1981–85) to the last time period (2016–18). The Average Annual Percentage Change (AAPC) was estimated using the slope of the linear trend line fitted to the incidence rates by year of diagnosis.
Findings
During the 38-year period (1981–2018), a total of 265,302 cases of melanoma (45.7% males, 54.3% females) were registered in England. The average annual number of cases increased from 837/year in 1981–85 to 6963/year in 2016–18 in males (+732%), and from 1609/year in 1981–85 to 6952/year in 2016–18 in females (+332%). In the young age-group (0–34 years), the average annual incidence rates initially increased from 1981–85 to 2001–05 and then stabilised during the recent period (2006–18). In the middle age group (35–64 years), the rates increased by +332% (AAPC, 10.4%) in males (from 5.6/100,000 in 1981–85 to 24.2/100,000 in 2016–18) and +185% (AAPC, 5.7%) in females (from 10.2/100,000 in 1981–85 to 29.1/100,000 in 2016–18); and in the old age-group (65+ years) the rates increased by +842% (AAPC, 25.7%) in males (from 9.6/100,000 in 1981–85 to 90.4/100,000 in 2016–18) and +381% (AAPC, 11.2%) in females (from 12.5/100,000 in 1981–85 to 60.1/100,000 in 2016–18). The largest increase in incidence in both males and females was observed for melanoma of the trunk (+817%, AAPC, 24.8% in males and +613%, AAPC, 18.3% in females), followed by melanoma of upper limb (+750%, AAPC, 22.9% in males and 518%, AAPC, 15.5% in females).
Interpretation
It appears that the incidence of melanoma among young people in England has stabilised (or levelled off) in recent decades, whereas it continues to increase substantially in older population. These findings suggest that public health campaigns targeted at children/adolescents/parents may be favourably influencing melanoma incidence. The steeper increase in incidence in males is consistent with their relatively greater sun exposure and poor sun-protective behaviour. All the available evidence suggests that the enormous increase in the melanoma of the trunk and upper limb, since the 1980s, is most likely due to increasing trend in intermittent high intensity recreational UV radiation exposure (e.g. sunbathing, holidaying in places with strong sunlight, indoor tanning).
Funding
This work was supported by Brighton and Sussex Medical School (BSMS).
Keywords: Malignant melanoma, Cutaneous melanoma, Skin cancer, Incidence, England
Research in context.
Evidence before this study
We searched PubMed from database inception to 31 August 2020, for articles published in English, with titles that included the search terms (“cutaneous malignant melanoma” or “cutaneous melanoma” or “malignant melanoma” or “melanoma” or “skin cancer”), and titles or abstracts that included the search terms (“incidence” or “incidence rates” or “melanoma incidence” or “incidence of melanoma” or “incidence trends” or “time trends” or “trends in incidence”). We also accessed International Agency for Research on Cancer/World Health Organization databases (GLOBOCAN 2018 and Cancer Incidence in Five Continents) and reports/publications from the Office of National Statistics and Public Health England.
Over the past four decades, the incidence rates of melanoma have been increasing at a much faster pace than any other cancer in the fair-skinned populations in Europe, North America and Oceania. It is estimated that 86% of all melanomas in the UK (i.e. 3.5% of all new cancer cases) are attributable to excessive UV radiation exposure, and the lifetime risk of developing melanoma in the UK population is approximately 1 in 75. Public health campaigns are increasing sun-protective behaviour, but their effect on melanoma incidence is largely unknown, and there is little information on the changing epidemiology of melanoma with regards to age, gender and anatomical site – which has implications for public health policy and prevention efforts.
Added value of this study
To our knowledge, this is the largest population-based study of melanoma in England – including 265,302 cases of melanoma diagnosed over the 38-year period, 1981–2018 – and presents a comprehensive and up-to-date analysis of the latest trends in incidence and changing epidemiology of the disease by age, gender and anatomical site. We have shown, for the first time, that the incidence rate of melanoma among young people in England has stabilised (or levelled off) in recent decades, whereas it continues to increase substantially in older population – in people aged 35–64 years, it has been increasing (on average) each year by 10.4% in males and 5.7% in females; and in people aged 65+ years, it has been increasing (on average) each year by 25.7% in males and 11.2% in females. We have also quantified the enormous increase in the incidence of melanomas of the trunk and upper limb, and the potential benefits of effective public health policy and prevention efforts.
Implications of all the available evidence
The stabilisation of incidence of melanoma in young people emphasise the importance of sustained primary prevention efforts to further improve the UV radiation protection behaviours (i.e. avoidance of excessive sunlight and indoor tanning, appropriate clothing, and application of sunscreens) and secondary prevention activities (i.e. increased surveillance, early detection and prompt treatment of pre-malignant/borderline lesions) to reduce the incidence of, and mortality from, the disease. All the available evidence suggests that the enormous increase in the melanomas of the trunk (+817% in males, +613% in females) and upper limb (+750% in males, +518% in females) since the 1980s in England can be mostly attributable to increasing intermittent high intensity recreational UV radiation exposure due to lifestyle and societal changes (e.g. sunbathing, holidaying in a place with strong sunlight, indoor tanning, proliferation of the budget holiday industry and airlines, increasing trend in travel to sunnier locations and use of sunbeds). Considering that the large majority of melanomas in the UK (86%) and other high risk populations are potentially preventable, we have quantified the potential benefits of effective and sustained primary and secondary prevention efforts to substantially reduce the burden of the disease – in the population and health services.
Alt-text: Unlabelled box
1. Introduction
Over the past four decades, the incidence of cutaneous malignant melanoma (hereafter, melanoma) has been increasing at a faster pace than any other cancer in the fair-skinned populations in Europe, North America and Oceania [1]. Worldwide, melanoma is the 20th most common cancer — there were an estimated 287,723 new cases (1.6% of all cancers) and 60,712 deaths (0.6% of all cancer deaths) from melanoma in the year 2018, and five-year prevalence of 965,623 cases [2]. In Europe, melanoma is the 6th most common cancer — there were an estimated 144,209 new cases (3.4% of all cancers) and 27,147 deaths (1.4% of all cancer deaths) from melanoma in 2018, and a five-year prevalence of 494,111 cases [2]. The highest age-standardised incidence rates (standardised to the world standard population) are observed in Oceania (28.3 per 100,000 population) and the lowest in Asia and Africa (0.48 and 0.51 per 100,000 population, respectively) [2]. In the UK, melanoma is the 5th most common cancer with about 16,200 new cases (4% of all cancers) and 2300 deaths (1.0% of all cancer deaths) in 2017 [3]. About half of the cases are diagnosed at ages 65+ years, and the large majority of cases occur on the trunk in males and on the lower limbs in females [3]. The cumulative risk of developing melanoma by age 74 is 1.34% (1 in 75) in males and 1.35% (1 in 74) in females [1].
It is generally believed that melanoma occurs as a result of complex interaction between environmental, genetic and individual host factors [4]. Important risk factors for melanoma include the number of common/atypical nevi, skin phenotype, family history of melanoma, actinic damage, history of sunburns, and excessive exposure to ultraviolet (UV) radiation, particularly in childhood and adolescence [5,6]. Overexposure to UV radiation (or light) from the sun is the main (and potentially preventable) environmental risk factor for melanoma — it has been estimated that 86% of all melanomas in the UK (i.e. 3.5% of all new cancer cases) are attributable to UV radiation exposure [7]. It is generally agreed that this risk is more closely associated with intermittent exposure to high-intensity sunlight (e.g. sunbathing/holidaying in a place with strong sunlight), than to chronic sunlight exposure (e.g. outdoor work/occupation) [4]. At least three systematic reviews/meta-analyses have confirmed this hypothesis and provided summary estimates of risk associated with intermittent and chronic sun exposure [[8], [9], [10]]. There is also emerging molecular evidence that somatic genetic aberrations in melanoma not only vary by histology, but also vary by anatomical site – suggesting that melanomas arising at different anatomical sites may have different causal pathways and that the relationship between sun exposure and melanoma differs by anatomical site and genotype [11]. It has been suggested that the main reason for the increasing incidence could be increased exposure, particularly intense, intermittent exposure, of pale white skin to UV radiation [12]. The latent period from initiation of the carcinogenesis process to clinical presentation is usually more than 10 years [10]. Exposure to artificial sources of UV radiation from indoor tanning beds/lamps is the second most important environmental cause of melanoma [13,14]. In a recent meta-analysis, indoor tanning bed use was associated with a significant increase in the risk of melanoma and the risk increased with number of tanning sessions and with initial usage at a young age (<35 years) [13]. In 2009, the WHO's International Agency for Research on Cancer (IARC) listed UV radiation and indoor tanning beds as Class I carcinogens (in the same class as cigarettes, benzene and asbestos) [14].
While the overall (i.e. all ages combined) incidence rates of melanoma in most fair-skinned populations continue to rise, reports from certain high risk areas such as Australia, New Zealand, USA, western Europe and Scandinavia show that the incidence, especially in young adults, has been either stabilising or declining in recent years – suggesting a cohort effect among recent generations, perhaps due to changes in lifestyle/social behaviour and increasing awareness of skin cancer and its prevention [12,[15], [16], [17]]. In recent years, there have also been significant changes in the epidemiology of melanoma with regards to age, gender and anatomical site in most populations – which have implications for public health policy and prevention efforts.
We conducted a comprehensive and up-to-date analysis of the national cancer registration data for England (population, 56.3 Million) (www.ons.gov.uk/), for the last four decades (1981–2018), to determine the changing epidemiology and trends in age-specific incidence of melanoma – with an objective to inform the development of health education/promotion and primary/secondary prevention initiatives (e.g. school-based programmes), and public health policy/interventions (e.g. indoor tanning regulations/laws) for this largely preventable cancer. It is anticipated that the study will also improve understanding of the aetiology of melanoma and guide commissioning and resource allocation of health/oncology services for patients.
2. Methods
2.1. Data sources
Individual level, national cancer registration data for all patients diagnosed with cutaneous malignant melanoma (International Classification of Diseases (ICD) 9th revision codes: 1720–1729 and ICD-10 codes: C43.0–C43.9) in England, during the 38-year period 1981–2018, were obtained from the Office for National Statistics (ONS) and Public Health England (PHE) Office for Data Release – which is responsible for the collection and collation of registrations from the nine regional population-based cancer registries in England (www.ons.gov.uk/) (www.gov.uk/government/publications/accessing-public-health-england-data/about-the-phe-odr-and-accessing-data). These regional registries collect information on all cancer patients in their geographic area directly from primary/secondary care records, pathology reports and healthcare professionals. Since the early 1970s, cancer registration in England is of a consistently high standard with over 98% completion rates [18]. Items of data obtained included: patient ID number, gender, year of birth, age at diagnosis, calendar year of diagnosis, topography (ICD 9 and 10 codes) and morphology codes (International Classification of Diseases for Oncology, 3rd Edition, ICD-O-3). For the calculation of incidence rates, corresponding annual mid-year national population estimates by age, gender and calendar year (i.e. the denominator data) were obtained from the Population Estimates Unit, ONS. Age-standardised (European standard population) mortality rates of melanoma were obtained from Cancer Research UK [3] and ONS (www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths).
2.2. Statistical analysis
Frequency distribution (N and %) and average annual incidence rates (per 100,000 population) were calculated according to three age categories (young age: 0–34 years, middle age: 35–64 years, and old age: 65+ years), gender and anatomical site during the seven five-year time periods (1981–85 to 2011–15) and the recent three-year period, 2016–18, for which the data were available. For comparison with other European populations, age-standardised incidence rates were calculated using the European Standard Population [3]. The percentage change in incidence (and mortality) rates in each age-group and gender was calculated as change in the average incidence (or mortality) rate from the first (1981–85) to the last time period (2016–18). The Average Annual Percentage Change (AAPC) (i.e. year-on-year increase in incidence (or mortality) during the 38-year study period in each age-group was estimated using the slope of the linear trend line fitted to the incidence (or mortality) rates by year of diagnosis (or death). The incidence (or mortality) rates, calculated for each time period, were regressed against the mid-year of the corresponding time period. For anatomical site specific analysis, melanomas were categorised as occurring on the head and neck (ICD-9 and ICD-10 codes: 1720–1724, C43.0–C43.4), trunk (1725, C43.5), upper limb (1726, C43.6), and lower limb (1727, C43.7). SPSS (IBM SPSS Statistics, version 26) was used for data management and coding, Microsoft Excel (Microsoft Office 2016) was used for the calculation of average annual and age-standardised incidence (and mortality) rates and percentage chance in incidence (and mortality) rates, and SAS (version 9.4) was used for the calculation of AAPC.
3. Results
During the 38-year study period (1981–2018), a total of 265,302 cases of cutaneous malignant melanoma (45.7% males, 54.3% females) were registered in England. The average annual number of new cases increased from 837/year in 1981–85 to 6963/year in 2016–18 in males (an increase of +732%), and from 1609/year in 1981–85 to 6952/year in 2016–18 in females (an increase of +332%).
3.1. Trends in incidence by age and gender
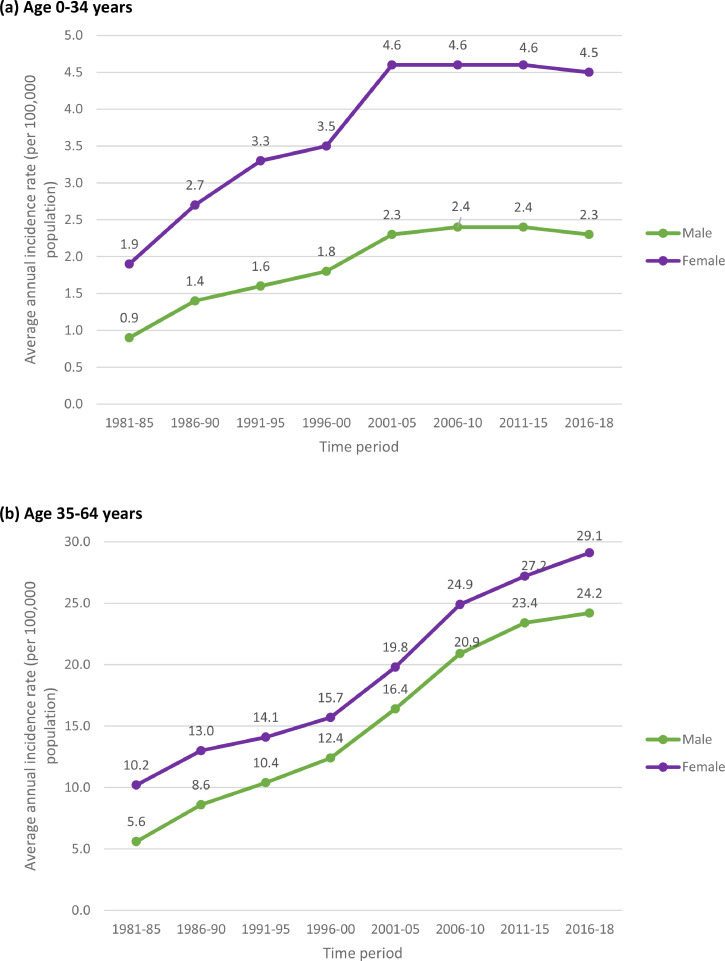
Table 1 and Fig. 1 show the trends in the number of cases and average annual incidence rates (per 100,000 population) of melanoma by age group and gender during the period 1981–2018. In the young age-group (0–34 years), the incidence rates initially increased from 1981–85 to 2001–05 (+156%, AAPC, 7.1% in males and +142%, AAPC, 6.5% in females) and then stabilised during the recent period (2006–18). It is also noteworthy that the rates started out much higher in females in the 1980s, and remained higher in females than males in the young age group throughout the study period. As for the middle and old age-groups, there was a steady and substantial increase in incidence rates during the study period. In the middle age group (35–64 years), the incidence rates increased by +332% (AAPC, 10.4%) in males (from 5.6/100,000 in 1981–85 to 24.2/100,000 in 2016–18) and +185% (AAPC, 5.7%) in females (from 10.2/100,000 in 1981–85 to 29.1/100,000 in 2016–18); and in the old age-group (65+ years) the rates increased by +842% (AAPC, 25.7%) in males (from 9.6/100,000 in 1981–85 to 90.4/100,000 in 2016–18) and +381% (AAPC, 11.2%) in females (from 12.5/100,000 in 1981–85 to 60.1/100,000 in 2016–18). Overall (i.e. for all ages combined), the magnitude of increase was relatively much higher in males compared to females – during the study period, the incidence rates increased by +584% (AAPC, 17.7%) in males (from 3.7/100,000 in 1981–85 to 25.3/100,000 in 2016–18) and +269% (AAPC, 8.0%) in females (from 6.7/100,000 in 1981–85 to 24.7/100,000 in 2016–18), which, as noted above, was essentially due to steadily increasing incidence in people aged ≥35 years. For comparison with other European populations, Table 2 presents the trends in age-standardised (European standard population) incidence rates (per 100,000 population) by gender during the period 1981–2018.
Table 1.
Number of cases and trends in average annual incidence rates (per 100,000 population) of cutaneous malignant melanoma by age-group and gender in England, 1981–2018.
| 1981–85 |
1986–90 |
1991–95 |
1996–00 |
2001–05 |
2006–10 |
2011–15 |
2016–18 |
Total number of casesa | Percentage change in incidence ratesb | AAPCc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | ||||
| Male (years) | |||||||||||||||||||
| 0–34 | 535 | 0.9 | 830 | 1.4 | 959 | 1.6 | 1061 | 1.8 | 1278 | 2.3 | 1383 | 2.4 | 1428 | 2.4 | 851 | 2.3 | 8325 | +156% | 4.8% |
| 35–64 | 2303 | 5.6 | 3573 | 8.6 | 4464 | 10.4 | 5625 | 12.4 | 7983 | 16.4 | 10,670 | 20.9 | 12,093 | 23.4 | 7646 | 24.2 | 54,357 | +332% | 10.4% |
| 65+ | 1348 | 9.6 | 2247 | 15.2 | 3247 | 21.0 | 4660 | 29.1 | 6926 | 41.1 | 11,227 | 62.0 | 16,518 | 79.3 | 12,393 | 90.4 | 58,566 | +842% | 25.7% |
| All ages | 4186 | 3.7 | 6650 | 5.8 | 8670 | 7.4 | 11,346 | 9.5 | 16,187 | 13.2 | 23,280 | 18.3 | 30,039 | 22.6 | 20,890 | 25.3 | 121,248 | +584% | 17.7% |
| Female (years) | |||||||||||||||||||
| 0-34 | 1101 | 1.9 | 1548 | 2.7 | 1872 | 3.3 | 1950 | 3.5 | 2522 | 4.6 | 2597 | 4.6 | 2724 | 4.6 | 1605 | 4.5 | 15,919 | +137% | 4.2% |
| 35-64 | 4257 | 10.2 | 5505 | 13.0 | 6098 | 14.1 | 7247 | 15.7 | 9780 | 19.8 | 12,959 | 24.9 | 14,338 | 27.2 | 9410 | 29.1 | 69,594 | +185% | 5.7% |
| 65+ | 2685 | 12.5 | 3961 | 17.7 | 5007 | 22.0 | 5847 | 25.7 | 7677 | 33.7 | 10,037 | 43.1 | 13,485 | 52.9 | 9842 | 60.1 | 58,541 | +381% | 11.2% |
| All ages | 8043 | 6.7 | 11,014 | 9.0 | 12,977 | 10.5 | 15,044 | 12.0 | 19,979 | 15.7 | 25,593 | 19.4 | 30,547 | 22.3 | 20,857 | 24.7 | 144,054 | +269% | 8.0% |
Total number of cases (n=265,302) diagnosed during the 38-year study period, 1981–2018.
Percentage change in the average annual incidence rates from the first (1981–85) to the last time period (2016–18).
AAPC, Average Annual Percent Change (i.e. year-on-year increase in incidence rates) during the 38-year study period.
Fig. 1.
Trends in average annual incidence rates (per 100,000 population) of cutaneous malignant melanoma by age‐group and gender in England, 1981–2018. (a) Young age: 0–34 years, (b) Middle age: 35–64 years, (c) Old age: 65+ years, (d) All ages combined.
Table 2.
Trends in age-standardised (European standard population) average annual incidence rates (per 100,000 population) of cutaneous malignant melanoma by gender in England, 1981–2018.
| 1981–85 | 1986–90 | 1991–95 | 1996–00 | 2001–05 | 2006–10 | 2011–15 | 2016–18 | Percentage change in incidence ratesa | AAPCb | |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | 4.5 | 7.0 | 8.9 | 11.4 | 15.6 | 21.5 | 25.9 | 28.3 | +529% | 16.3% |
| Female | 7.3 | 9.8 | 11.3 | 12.8 | 16.4 | 20.3 | 23.2 | 25.3 | +247% | 7.4% |
Percentage change in the age-standardised average annual incidence rates from the first (1981–85) to the last time period (2016–18).
AAPC, Average Annual Percent Change (i.e. year-on-year increase in incidence rates) during the 38-year study period.
3.2. Trends in incidence by anatomical site
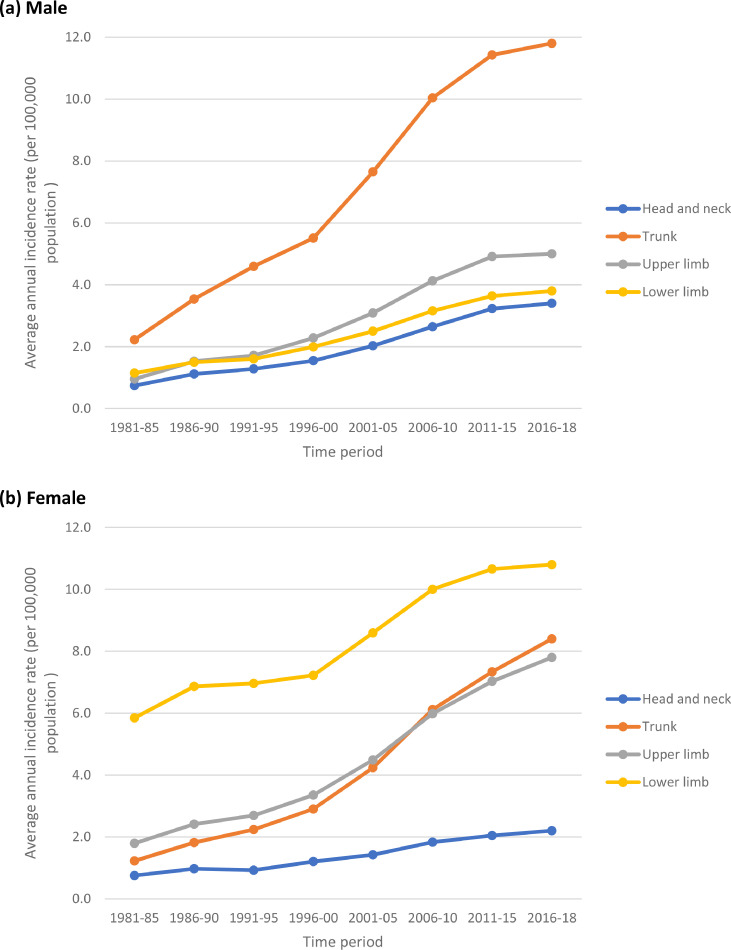
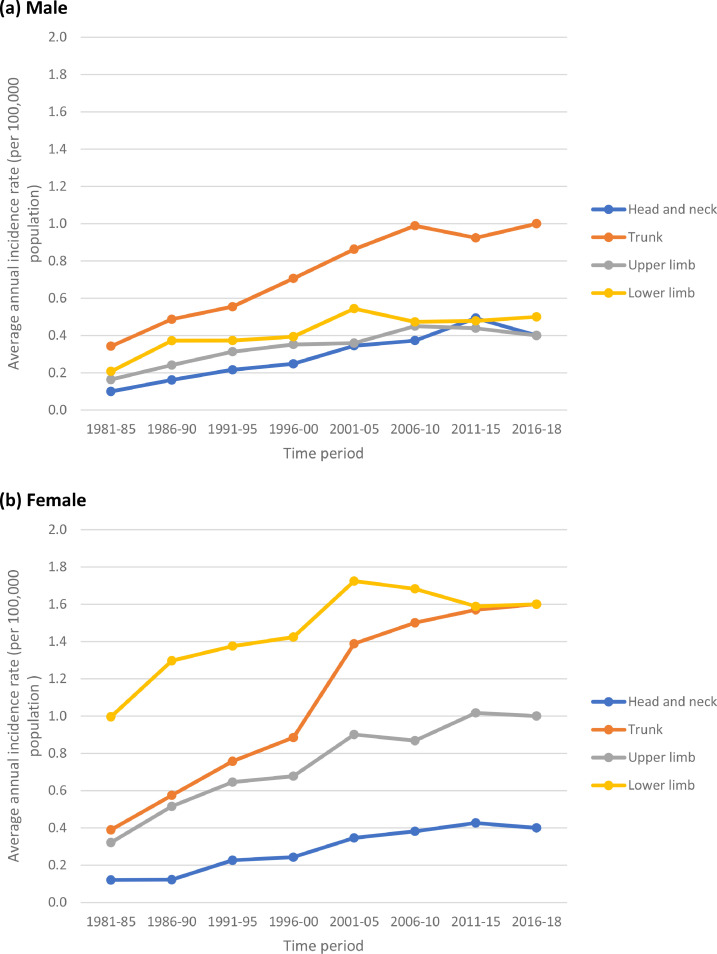
Table 3 and Figs. 2–5 show the trends in the number of cases and average annual incidence rates (per 100,000 population) of melanoma by age-group, gender and anatomical site (i.e. head and neck, trunk, upper limb, and lower limb) during the period 1981–2018. In the young age-group (0–34 years) – and in analogy with the finding in Table 1 – the incidence rates at all anatomical sites initially increased from 1981–85 to 2001–05 and then stabilised during the recent period (2006–18). As for the middle and old age-groups, there was a steady increase in incidence rates at all anatomical sites during the study period.
Fig. 3.
Trends in average annual incidence rates (per 100,000 population) of cutaneous malignant melanoma in persons aged 35–64 years by anatomical site in England, 1981–2018. (a) Male (b) Female.
Fig. 4.
Trends in average annual incidence rates (per 100,000 population) of cutaneous malignant melanoma in persons aged 65+ years by anatomical site in England, 1981–2018. (a) Male (b) Female.
Table 3.
Number of cases and trends in average annual incidence rates (per 100,000 population) of cutaneous malignant melanoma by age-group, gender and anatomical site in England, 1981–2018.
| 1981–85 |
1986–90 |
1991–95 |
1996–00 |
2001–05 |
2006–10 |
2011–15 |
2016–18 |
Total number of casesa | Percentage change in incidence ratesb | AAPCc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | N | Rate | ||||
| Head and neck | |||||||||||||||||||
| Male (years) | |||||||||||||||||||
| 0–34 | 59 | 0.1 | 95 | 0.2 | 127 | 0.2 | 143 | 0.2 | 196 | 0.3 | 216 | 0.4 | 298 | 0.5 | 158 | 0.4 | 1292 | +300% | 10.5% |
| 35–64 | 303 | 0.7 | 465 | 1.1 | 549 | 1.3 | 702 | 1.5 | 986 | 2.0 | 1352 | 2.6 | 1667 | 3.2 | 1067 | 3.4 | 7091 | +386% | 11.7% |
| 65+ | 419 | 3.0 | 729 | 4.9 | 1079 | 7.0 | 1474 | 9.2 | 1240 | 13.3 | 3495 | 19.3 | 5212 | 25.0 | 3549 | 25.9 | 17,197 | +763% | 24.3% |
| All ages | 781 | 0.7 | 1289 | 1.1 | 1755 | 1.5 | 2319 | 1.9 | 2422 | 2.8 | 5063 | 4.0 | 7177 | 5.4 | 4774 | 5.8 | 25,580 | +729% | 22.7% |
| Female (years) | |||||||||||||||||||
| 0–34 | 69 | 0.1 | 70 | 0.1 | 130 | 0.2 | 137 | 0.2 | 192 | 0.3 | 216 | 0.4 | 250 | 0.4 | 138 | 0.4 | 1202 | +300% | 10.4% |
| 35–64 | 313 | 0.8 | 410 | 1.0 | 401 | 0.9 | 555 | 1.2 | 703 | 1.4 | 952 | 1.8 | 1077 | 2.0 | 701 | 2.2 | 5112 | +175% | 5.3% |
| 65+ | 663 | 3.1 | 994 | 4.4 | 1231 | 5.4 | 1362 | 6.0 | 1877 | 8.2 | 2174 | 9.3 | 2897 | 11.4 | 1815 | 11.1 | 13,013 | +258% | 8.2% |
| All ages | 1045 | 0.9 | 1474 | 1.2 | 1762 | 1.4 | 2054 | 1.6 | 2772 | 2.2 | 3342 | 2.5 | 4224 | 3.1 | 2654 | 3.1 | 19,327 | +244% | 7.8% |
| Trunk | |||||||||||||||||||
| Male (years) | |||||||||||||||||||
| 0–34 | 203 | 0.3 | 287 | 0.5 | 326 | 0.6 | 407 | 0.7 | 490 | 0.9 | 573 | 1.0 | 558 | 0.9 | 362 | 1.0 | 3206 | +233% | 6.7% |
| 35–64 | 908 | 2.2 | 1474 | 3.5 | 1967 | 4.6 | 2500 | 5.5 | 3717 | 7.6 | 5128 | 10.0 | 5895 | 11.4 | 3733 | 11.8 | 25,322 | +436% | 13.8% |
| 65+ | 287 | 2.1 | 550 | 3.5 | 823 | 5.3 | 1370 | 8.5 | 2218 | 13.1 | 3949 | 21.8 | 6257 | 30.0 | 4934 | 36.0 | 20,388 | +1614% | 48.7% |
| All ages | 1398 | 1.2 | 2311 | 2.0 | 3116 | 2.7 | 4277 | 3.6 | 6425 | 5.3 | 9650 | 7.6 | 12,710 | 9.6 | 9029 | 11.0 | 48,916 | +817% | 24.8% |
| Female (years) | |||||||||||||||||||
| 0–34 | 223 | 0.4 | 329 | 0.6 | 435 | 0.8 | 499 | 0.9 | 769 | 1.4 | 849 | 1.5 | 920 | 1.6 | 564 | 1.6 | 4588 | +300% | 9.7% |
| 35–64 | 509 | 1.2 | 767 | 1.8 | 971 | 2.2 | 1338 | 2.9 | 2095 | 4.2 | 3181 | 6.1 | 3863 | 7.3 | 2727 | 8.4 | 15,451 | +600% | 18.3% |
| 65+ | 232 | 1.1 | 326 | 1.5 | 360 | 1.6 | 462 | 2.0 | 680 | 3.0 | 1101 | 4.7 | 1759 | 6.9 | 1528 | 9.3 | 6448 | +745% | 20.7% |
| All ages | 964 | 0.8 | 1422 | 1.2 | 1766 | 1.4 | 2299 | 1.8 | 3544 | 2.8 | 5131 | 3.9 | 6542 | 4.8 | 4819 | 5.7 | 26,487 | +613% | 18.3% |
| Upper limb | |||||||||||||||||||
| Male (years) | |||||||||||||||||||
| 0–34 | 97 | 0.2 | 142 | 0.2 | 184 | 0.3 | 203 | 0.4 | 204 | 0.4 | 261 | 0.5 | 265 | 0.4 | 144 | 0.4 | 1500 | +100% | 3.7% |
| 35–64 | 389 | 1.0 | 636 | 1.5 | 735 | 1.7 | 1035 | 2.3 | 1501 | 3.1 | 2108 | 4.1 | 2535 | 4.9 | 1594 | 5.0 | 10,533 | +400% | 12.8% |
| 65+ | 157 | 1.1 | 280 | 1.9 | 445 | 2.9 | 680 | 4.2 | 1111 | 6.6 | 1943 | 10.7 | 3170 | 15.2 | 2460 | 17.9 | 10,246 | +1527% | 46.0% |
| All ages | 643 | 0.6 | 1058 | 0.9 | 1364 | 1.2 | 1918 | 1.6 | 2819 | 2.3 | 4312 | 3.4 | 5970 | 4.5 | 4198 | 5.1 | 22,279 | +750% | 22.9% |
| Female (years) | |||||||||||||||||||
| 0–34 | 184 | 0.3 | 295 | 0.5 | 371 | 0.6 | 382 | 0.7 | 499 | 0.9 | 491 | 0.9 | 596 | 1.0 | 343 | 1.0 | 3161 | +233% | 6.9% |
| 35–64 | 745 | 1.8 | 1017 | 2.4 | 1168 | 2.7 | 1546 | 3.4 | 2219 | 4.5 | 3112 | 6.0 | 3701 | 7.0 | 2520 | 7.8 | 16,028 | +333% | 10.2% |
| 65+ | 434 | 2.0 | 564 | 2.5 | 821 | 3.6 | 1042 | 4.6 | 1609 | 7.1 | 2423 | 10.4 | 3633 | 14.2 | 2851 | 17.4 | 13,377 | +770% | 22.8% |
| All ages | 1363 | 1.1 | 1876 | 1.5 | 2360 | 1.9 | 2970 | 2.4 | 4327 | 3.4 | 6026 | 4.6 | 7930 | 5.8 | 5714 | 6.8 | 32,566 | +518% | 15.5% |
| Lower limb | |||||||||||||||||||
| Male (years) | |||||||||||||||||||
| 0–34 | 123 | 0.2 | 219 | 0.4 | 219 | 0.4 | 227 | 0.4 | 309 | 0.5 | 274 | 0.5 | 289 | 0.5 | 179 | 0.5 | 1839 | +150% | 3.7% |
| 35–64 | 468 | 1.1 | 624 | 1.5 | 687 | 1.6 | 904 | 2.0 | 1215 | 2.5 | 1612 | 3.2 | 1878 | 3.6 | 1215 | 3.8 | 8603 | +245% | 7.6% |
| 65+ | 294 | 2.1 | 410 | 2.8 | 525 | 3.4 | 614 | 3.8 | 830 | 4.9 | 1270 | 7.0 | 1833 | 8.8 | 1437 | 10.5 | 7213 | +400% | 11.6% |
| All ages | 885 | 0.8 | 1253 | 1.1 | 1431 | 1.2 | 1745 | 1.5 | 2354 | 1.9 | 3156 | 2.5 | 4000 | 3.0 | 2831 | 3.4 | 17,655 | +325% | 9.7% |
| Female (years) | |||||||||||||||||||
| 0–34 | 570 | 1.0 | 742 | 1.3 | 790 | 1.4 | 803 | 1.4 | 955 | 1.7 | 952 | 1.7 | 931 | 1.6 | 572 | 1.6 | 6315 | +60% | 1.7% |
| 35–64 | 2430 | 5.8 | 2895 | 6.9 | 3020 | 7.0 | 3328 | 7.2 | 4250 | 8.6 | 5202 | 10.0 | 5612 | 10.7 | 3474 | 10.8 | 30,211 | +86% | 2.7% |
| 65+ | 1130 | 5.2 | 1673 | 7.5 | 2070 | 9.1 | 2456 | 10.8 | 3001 | 13.2 | 3892 | 16.7 | 5106 | 20.0 | 3636 | 22.2 | 22,964 | +327% | 9.6% |
| All ages | 4130 | 3.4 | 5310 | 4.4 | 5880 | 4.8 | 6587 | 5.3 | 8206 | 6.4 | 10,046 | 7.6 | 11,649 | 8.5 | 7682 | 9.1 | 59,490 | +168% | 5.0% |
Total number of cases (n=265,302) diagnosed during the 38-year study period, 1981–2018. Of the 265,302 cases, data on anatomical site was missing for 13,002 (4.9%) cases.
Percentage change in the average annual incidence rates from the first (1981–85) to the last time period (2016–18).
AAPC, Average Annual Percent Change (i.e. year-on-year increase in incidence rates) during the 38-year study period.
Fig. 2.
Trends in average annual incidence rates (per 100,000 population) of cutaneous malignant melanoma in persons aged 0–34 years by anatomical site in England, 1981–2018. (a) Male (b) Female.
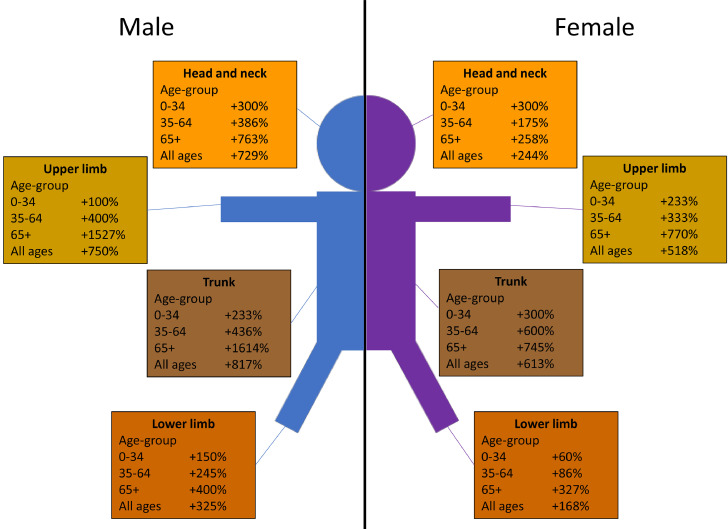
Fig. 5.
Percentage change in the average annual incidence rates of cutaneous malignant melanoma from 1981–85 to 2016–18, by age group, gender and anatomical site in England, 1981–2018.
In the middle age group (35–64 years), the largest increase was observed in the melanomas of the trunk in both genders (+436%, AAPC, 13.8% in males and +600%, AAPC, 18.3% in females). In the old age group (65+ years), the largest increase was observed in the melanomas of the trunk (+1614%, AAPC, 48.7% in males and +745%, AAPC, 20.7% in females) and upper limb (+1527%, AAPC, 46.0% in males and +770%, AAPC, 22.8% in females). Overall (i.e. for all ages combined) the largest increase in incidence in both males and females was observed for melanoma of the trunk (+817%, AAPC, 24.8% in males and +613%, AAPC, 18.3% in females) followed by melanoma of upper limb (+750%, AAPC, 22.9% in males and 518%, AAPC, 15.5% in females). In analogy with the findings in Table 1, the magnitude of increase was relatively much higher in males compared to females, which was essentially due to steadily increasing incidence in people aged ≥35 years (Table 3 and Figs. 2–5). The anatomical site of the tumour is most likely associated with the pattern of UV radiation exposure [4] – overall, the melanoma occurred most commonly on the trunk in males (42.7%) and on the lower limb in females (43.1%) (Table 3).
3.3. Trends in mortality by gender
For most cancers, trends in mortality reflect trends in incidence and survival (which is the function of the stage at diagnosis and efficacy of treatment). For example, increasing mortality may reflect increasing incidence and stable survival, and decreasing (or stable) mortality may reflect increasing incidence and increasing survival (due to early diagnosis, e.g. by screening, and improved treatment). Table 4 shows the trends in average annual mortality rates (per 100,000 population) of melanoma by gender during the period 1981–2018. In males, the mortality rates increased by +117% (AAPC, 3.9%), from 2.3/100,000 in 1981–85 to 5.0/100,000 in 2016–18 and in females the mortality rates increased by +32% (AAPC, 0.9%), from 2.2/100,000 in 1981–85 to 2.9/100,000 in 2016–18. Compared with the enormous increase in the age-standardised incidence rate of melanoma (+529% in males, +247% in females, Table 2), which is the function of the aetiology of the disease, the mortality data imply that there has been a substantial improvement in survival/prognosis – most likely due to the early diagnosis and improving treatment of the disease over the last four decades.
Table 4.
Trends in age-standardised (European standard population) average annual mortality rates (per 100,000 population) of cutaneous malignant melanoma by gender in England, 1981–2018
| 1981–85 | 1986–90 | 1991–95 | 1996–00 | 2001–05 | 2006–10 | 2011–15 | 2016–18 | Percentage change in mortality ratesa | AAPCb | |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | 2.3 | 2.6 | 3.3 | 3.7 | 4.1 | 4.7 | 5.2 | 5.0 | +117% | 3.9% |
| Female | 2.2 | 2.5 | 2.7 | 2.9 | 2.8 | 3.0 | 3.0 | 2.9 | +32% | 0.9% |
Percentage change in the age-standardised average annual mortality rates from the first (1981–85) to the last time period (2016–18).
AAPC, Average Annual Percent Change (i.e. year-on-year increase in mortality rates) during the 38-year study period.
4. Discussion
This retrospective population-based cohort study included 265,302 cases of cutaneous malignant melanoma diagnosed during the 38-year period (1981–2018), and presents the most comprehensive and up-to-date analysis of the latest trends in the incidence and changing epidemiology of the disease by age, gender and anatomical site in England (population, 56.3 Million). The study showed that overall (i.e. all ages combined), there has been a steady and significant increase in the incidence of melanoma in both genders during the last four decades, which was essentially due to the continually increasing incidence in middle (age 35–64 years) and old ages (65+ years). The steepest increase was observed in males (i.e. more than two-folds as compared to females) and at old ages. On the other hand, the study also showed that in young people (aged 0–34 years) the incidence rates initially increased from 1981–2005 and then stabilised (or levelled off) during the last two decades (2006–18). To our knowledge, this is the first report to determine the stabilisation (or levelling off) of melanoma incidence among young people in England. With regard to the anatomical site, the largest increase in incidence in both males and females was observed for melanoma of the trunk, followed by melanoma of upper limb.
The strength of this study includes a novel analysis of the national population-based cancer registration dataset including 265,302 cases of melanoma, allowing the calculation of accurate incidence rates and the trends in incidence over four decades. While the cancer registration system in England is of a consistently high standard with over 98% completion rates, there is a possibility that information on a small number of cancer cases exclusively diagnosed and treated in private hospitals may be incomplete [18]. This is unlikely to have a material impact as 98–99% of hospital activity in England is funded by the National Health Service (NHS) [18]. All NHS healthcare providers are mandated to submit cancer data to PHE, and this includes NHS-funded activity in private hospitals. The cancer registration system is an opt-out scheme – i.e. PHE can collect data on individuals without their consent, unless they choose to opt-out. This is also unlikely to have a material impact, as the rate of opt-out is <1/10,000 people [18]. Anatomical site was not recorded for 4.9% of the cases throughout the 38-year study period (Table 3). However, the recording of site improved over time and therefore could have some impact on the trends. As the data on anatomical site were recorded under the four broad ICD categories (i.e. head and neck, trunk, upper/lower limb), lack of information on precise sub-sites (e.g. face, ear, anterior/posterior trunk) and laterality prevented further insights into the pattern and causative impact of UV radiation exposure, as it seems likely that sun exposure would lead to a greater increase in lesions on the posterior trunk relative to the chest and abdomen.
Our finding of the overall increasing incidence of melanoma in England during the last four decades is in agreement with reports of increasing incidence in many European countries, North America and Oceania [12,[19], [20], [21]]. On the other hand, a number of studies published during the last ten or so years have reported either stabilisation or a slight decline in incidence in younger age groups in some of these high risk populations, suggesting a birth cohort effect in recent generations [12,[15], [16], [17],21]. Our finding of the stabilisation (or levelling off) of melanoma incidence rates in young people in England is the second such report (after Norway) from Europe [12,22]. The stabilisation or decline in incidence in cohorts (or generations) born since the 1980s is probably due to the public health efforts to recognise skin cancer as a major public health problem and improve sun-protective behaviour, and may be associated with changing UV radiation exposure patterns following a growing awareness of skin cancer and its risk factors (Examples of skin cancer prevention and early diagnosis interventions in England (www.ncin.org.uk/view?rid=2999) (www.sunsmart.org.uk).
For example, multiple public health campaigns in Queensland, Australia, started in the early 1980s, were successful in increasing sun-protective behaviour and were associated with a declining incidence of melanoma, since the mid-to late 1990s, in young people who were exposed to primary prevention and early detection programmes since birth [23]. It is noteworthy that this recent stabilisation of incidence in young people also corresponds with the widespread availability and use of sunscreens (with sun protection factor – SPF) since the 1980s (www.nytimes.com/2010/06/24/fashion/24skinside.html). Considering that the skin of babies and children is much more sensitive to UV radiation than adult skin and damage caused by intermittent exposure to sunlight could lead to the development of skin cancer later in life, most of the primary prevention campaigns are targeted at sun protection during younger years and educating parents to protect their children. In a systematic review of epidemiological studies, exposure to high levels of sunlight in childhood was found to be a strong determinant of melanoma risk [5].
With regard to gender, we observed a much steeper overall increase in the incidence rates among males in the middle and old age-groups and all the anatomical sites. Recent studies have suggested that some of this disparity may be explained by relatively higher sun exposure and lower knowledge/awareness of skin cancer and compliance with sun protection measures in men [[24], [25], [26]]. It is generally agreed that alongside sun exposure patterns, individual factors and response to exposure to UV radiation may also be important [6]. It has also been suggested that this disparity may be due to biological differences in skin damage and repair mechanism between males and females – the male skin is probably more prone to the damage caused by UV radiation, and female skin is relatively better at repairing the damage caused by UV radiation [24].
There has been a consistent debate that the role of sunlight in causing melanoma differs according to the anatomical site. It has been hypothesised that melanomas at different sites are associated with distinct pattern of sun exposure. In a case-control study from Australia, melanomas of the head and neck were associated with chronic patterns of sun exposure (i.e. high levels of total sun exposure during adulthood; outdoor work/occupation), whereas melanomas of the trunk were associated with intermittent patterns of exposure (i.e. lower levels of sun exposure overall, but higher levels of recreational exposure on the chest and back) [11]. The authors noted that their findings were consistent with other studies that reported that outdoor workers had a relative excess of head and neck melanomas. In our study, the most common site of melanoma was trunk in males (42.7%) and lower limb in females (43.1%), and the largest increase in incidence in both genders was observed for melanoma of the trunk followed by melanoma of upper limb – suggesting differing patterns of exposure between males and females and changes in exposure over the last four decades. Considering the findings of Whiteman et al. 2006 and others [11,27,28], it appears that a large proportion of the observed increase in melanoma of the trunk and upper limb in our study can be attributable to the intermittent high intensity recreational exposure, most likely due to lifestyle changes in recent decades (e.g. sunbathing or holidaying in a place with strong sunlight, use of indoor tanning beds/lamps, travelling to sunnier locations and use of sunbeds) [[29], [30], [31], [32], [33]]. For example, due to the heightened demand in recent years, many UK-based holiday companies now offer holidaymakers a chance to book a ‘prime’ sunbed spot (according to the location, intensity, and duration of sunlight) in advance [34]. This situation, often dubbed by the media as ‘Sunbed wars’, has become more complex in the midst of the COVID-19 pandemic due to the new social distancing rules at holiday resorts [35]. Our findings are consistent with these observations – the more chronically exposed (i.e. due to outdoor work/occupation) anatomical sites, i.e. head and neck, showed relatively lower increases in incidence compared to the intermittently exposed sites, i.e. trunk and upper limb, suggesting an increase in intermittent high intensity recreational exposure during the past decades.
The rapidly increasing overall incidence (i.e. all ages and both genders combined) of melanoma over the past four decades in the fair-skinned populations in Europe, North America and Oceania is largely attributable to the lifestyle changes in these decades with increasing trend in intermittent high intensity recreational UV radiation exposure [9,11]. Some of this increase is also believed to be due to increased surveillance and early detection [36]. On the other hand, the most plausible explanations for the stabilisation or slight decline in incidence rates in recent decades in young people, in some of the high risk countries, are growing awareness of the dangers of excessive sunbathing, improving sun-protective behaviour, use of sunscreen and primary and secondary prevention (i.e. early detection and excision of pigmented lesions) programmes. Simulation studies have shown that interventions aimed at protecting people during outdoor recreational activities/outdoor occupational work against the harmful effects of UV radiation exposure could lead to substantial reduction in melanoma incidence [37].
5. Conclusions and clinical and public health policy implications
In conclusion, this comprehensive and most up-to-date population-based cohort study of the age- gender- and anatomical site-specific trends in the incidence of cutaneous malignant melanoma in England showed that overall (i.e. all ages combined) there has been a substantial increase in the incidence of the disease in both genders during the last four decades – which was essentially due to the steadily increasing incidence in middle and old ages, with the steepest increase in males and older people. The study also demonstrated, for the first time, the stabilisation (or levelling off) of incidence in young people during the last two decades. This stabilisation of incidence in young people (i.e. in recent birth cohorts) is encouraging, and emphasise the importance of continued and sustained primary prevention efforts, particularly targeting children, adolescents and their parents, to further improve the UV radiation protection behaviours (i.e. avoidance of excessive exposure to sunlight, indoor tanning beds/lamps and sunbeds, appropriate clothing, and application of sunscreens) and secondary prevention activities (i.e. increased surveillance, early detection and prompt treatment of pre-malignant/borderline lesions) to reduce the incidence of, and mortality from, the disease. All the available evidence suggests that this enormous increase in the incidence of melanomas of the trunk (+817% in males, +613% in females) and upper limb (+750% in males, +518% in females) observed during the past four decades in England can be mostly attributable to increasing trend in intermittent high intensity recreational UV radiation exposure due to lifestyle and societal changes in these decades (e.g. sunbathing/holidaying in a place with strong sunlight, use of indoor tanning beds/lamps, proliferation of the budget holiday industry and airlines, increasing trend in travel to sunnier locations and use of sunbeds). Considering that 86% of all melanomas in the fair-skinned populations are attributable to UV radiation exposure and are potentially preventable (i.e. majority of the 35,902 cases out of the 41,747 new cases diagnosed in England during 2016–18), this study has quantified the potential benefits of effective and sustained primary and secondary prevention efforts to substantially reduce the burden of the disease (in the population and health services) – thereby making melanoma as one of the most preventable forms of cancer on a global scale.
Funding
This work was supported by Brighton and Sussex Medical School (BSMS).
Role of the funding source
The funder had no role in the study design, data collection, data analysis, interpretation, and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author contributions
AM conceived and designed the study. PB, IR, JS and AM conducted the literature search. PB, PWJ RJQM and AM designed and conducted the statistical analysis. AM wrote the manuscript and responded to the Editors’ and Reviewers’ comments. BA and RJQM critically reviewed the manuscript for intellectual content. AM guided and supervised the overall work.
Declaration of Interests
The authors declare no conflict of interest.
Acknowledgement
We thank the National Cancer Registration and Analysis Service (NCRAS), Public Health England Office of Data Release and Office for National Statistics (ONS) for providing the cancer registration and population data.
References
- 1.Bray F, Colombet M, Mery L, Piñeros M, Znaor A, Zanetti R, Ferlay J, editors. Cancer incidence in five continents, Vol. XI. International Agency for Research on Cancer; Lyon: 2017. https://ci5.iarc.fr Available from: accessed (Accessed 28 August 2020) [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I. International Agency for Research on Cancer/World Health Organization; Lyon: 2018. Global and regional estimates of the incidence and mortality for 38 cancers: GLOBOCAN 2018.https://gco.iarc.fr (Accessed 28 August 2020) [Google Scholar]
- 3.Cancer Research UK: Cancer Statistics for the UK (www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk (Accessed 28 August 2020).
- 4.Whiteman D, Green A. In: Skin cancer – a world-wide perspective. Dummer R., editor. Springer-Verlag; Berlin Heidelberg: 2011. Epidemiology of malignant melanoma. [DOI] [Google Scholar]
- 5.Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12(1):69–82. doi: 10.1023/a:1008980919928. [DOI] [PubMed] [Google Scholar]
- 6.Gandini S, Sera F, Cattaruzza MS. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41(14):2040–2059. doi: 10.1016/j.ejca.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Parkin D., Mesher D., Sasieni P. 13. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105:S66–S69. doi: 10.1038/bjc.2011.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelemans PJ, Rampen FH, Ruiter DJ, Verbeek AL. An addition to the controversy on sunlight exposure and melanoma risk: a meta-analytical approach. J Clin Epidemiol. 1995;48(11):1331–1342. doi: 10.1016/0895-4356(95)00032-1. [DOI] [PubMed] [Google Scholar]
- 9.Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73(2):198–203. doi: 10.1002/(sici)1097-0215(19971009)73:2<198:aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Gandini S, Sera F, Cattaruzza MS. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41(1):45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Whiteman DC, Stickley M, Watt P, Hughes MC, Davis MB, Green AC. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol. 2006;24(19):3172–3177. doi: 10.1200/JCO.2006.06.1325. [DOI] [PubMed] [Google Scholar]
- 12.Erdmann F, Lortet-Tieulent J, Schüz J. International trends in the incidence of malignant melanoma 1953-2008 – are recent generations at higher or lower risk? Int J Cancer. 2013;132(2):385–400. doi: 10.1002/ijc.27616. [DOI] [PubMed] [Google Scholar]
- 13.Boniol M, Autier P, Boyle P, Gandini S. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757. doi: 10.1136/bmj.e4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Ghissassi F, Baan R, Straif K. A review of human carcinogens – part D: radiation. Lancet Oncol. 2009;10(8):751–752. doi: 10.1016/s1470-2045(09)70213-x. [DOI] [PubMed] [Google Scholar]
- 15.de Vries E, Bray FI, Coebergh JW, Parkin DM. Changing epidemiology of malignant cutaneous melanoma in Europe 1953-1997: rising trends in incidence and mortality but recent stabilizations in western Europe and decreases in Scandinavia. Int J Cancer. 2003;107(1):119–126. doi: 10.1002/ijc.11360. [DOI] [PubMed] [Google Scholar]
- 16.Arnold M, Holterhues C, Hollestein LM. Trends in incidence and predictions of cutaneous melanoma across Europe up to 2015. J Eur Acad Dermatol Venereol. 2014;28(9):1170–1178. doi: 10.1111/jdv.12236. [DOI] [PubMed] [Google Scholar]
- 17.Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016;136(6):1161–1171. doi: 10.1016/j.jid.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 18.Henson KE, Elliss-Brookes L, Coupland VH, Payne E, Vernon S, Rous B, Rashbass J. Data resource profile: national cancer registration dataset in England. Int J Epidemiol. 2020;49(1) doi: 10.1093/ije/dyz076. Feb 116-16h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downing A, Newton-Bishop JA, Forman D. Recent trends in cutaneous malignant melanoma in the Yorkshire region of England; incidence, mortality and survival in relation to stage of disease, 1993-2003. Br J Cancer. 2006;95(1):91–95. doi: 10.1038/sj.bjc.6603216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacKie RM, Bray C, Vestey J. Melanoma incidence and mortality in Scotland 1979-2003. Br J Cancer. 2007;96(11):1772–1777. doi: 10.1038/sj.bjc.6603801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulson KG, Gupta D, Kim TS. Age-specific incidence of Melanoma in the United States. JAMA Dermatol. 2020;156(1):57–64. doi: 10.1001/jamadermatol.2019.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aitken JF, Youlden DR, Baade PD, Soyer HP, Green AC, Smithers BM. Generational shift in melanoma incidence and mortality in Queensland, Australia, 1995-2014. Int J Cancer. 2018;142(8):1528–1535. doi: 10.1002/ijc.31141. Apr 15. [DOI] [PubMed] [Google Scholar]
- 23.Iannacone MR, Green AC. Towards skin cancer prevention and early detection: evolution of skin cancer awareness campaigns in Australia. Melanoma Manag. 2014;1(1):75–84. doi: 10.2217/mmt.14.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanton WR, Janda M, Baade PD, Anderson P. Primary prevention of skin cancer: a review of sun protection in Australia and internationally. Health Promot Int. 2004;19(3):369–378. doi: 10.1093/heapro/dah310. [DOI] [PubMed] [Google Scholar]
- 25.Buller DB, Cokkinides V, Hall HI. Prevalence of sunburn, sun protection, and indoor tanning behaviors among Americans: review from national surveys and case studies of 3 states. J Am Acad Dermatol. 2011;65(1):S114–S123. doi: 10.1016/j.jaad.2011.05.033. 5 Suppl. [DOI] [PubMed] [Google Scholar]
- 26.American Academy of Dermatology (2016). “Survey: Men's skin cancer knowledge lags behinds women's.” (News release, April 28, 2016). https://www.aad.org/public/diseases/skin-cancer/types/common/melanoma/men-50 (accessed 27 December 2020).
- 27.Bulliard J-I, Cox B. Cutaneous malignant melanoma in New Zealand: trends by anatomical site, 1969–1993. Int J Epidemiol. 2000;29(3):416–423. doi: 10.1093/ije/29.3.416. June. [DOI] [PubMed] [Google Scholar]
- 28.Wallingford SC, Alston RD, Birch JM, Green AC. Increases in invasive melanoma in England, 1979-2006, by anatomical site. Br J Dermatol. 2011;165(4):859–864. doi: 10.1111/j.1365-2133.2011.10434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bentham G, Aase A. Incidence of malignant melanoma of the skin in Norway, 1955-1989: associations with solar ultraviolet radiation, income and holidays abroad. Int J Epidemiol. 1996;25(6):1132–1138. doi: 10.1093/ije/25.6.1132. DecPMID: 9027516. [DOI] [PubMed] [Google Scholar]
- 30.de Vries E, Coebergh JW. Cutaneous malignant melanoma in Europe. Eur J Cancer. 2004;40(16):2355–2366. doi: 10.1016/j.ejca.2004.06.003. Nov. [DOI] [PubMed] [Google Scholar]
- 31.de Haas ER, Nijsten T, de Vries E. Population education in preventing skin cancer: from childhood to adulthood. J Drugs Dermatol. 2010;9(2):112–116. Feb. [PubMed] [Google Scholar]
- 32.Rodvall Y, Wahlgren CF, Wiklund K. Future reduction of cutaneous malignant melanoma due to improved sun protection habits and decreased common melanocytic nevi density among Swedish children?: A follow-up from 2002 to 2012. Eur J Cancer. 2019;118:149–155. doi: 10.1016/j.ejca.2019.06.016. SepEpub 2019 Jul 23. [DOI] [PubMed] [Google Scholar]
- 33.Agredano YZ, Chan JL, Kimball RC, Kimball AB. Accessibility to air travel correlates strongly with increasing melanoma incidence. Melanoma Res. 2006;16(1):77–81. doi: 10.1097/01.cmr.0000195696.50390.23. Feb. [DOI] [PubMed] [Google Scholar]
- 34.The Guardian, 29 January 2018. https://www.theguardian.com/business/2018/jan/29/towel-war-thomas-cook-sunbed-booking (accessed 27 December 2020).
- 35.Mirror, 22 May 2020. https://www.mirror.co.uk/travel/news/sunbed-wars-erupt-spain-greece-22058573 (accessed 27 December 2020).
- 36.Bataille V, de Vries E. Melanoma – Part 1: epidemiology, risk factors, and prevention. BMJ. 2008;337:a2249. doi: 10.1136/bmj.a2249. Nov 20PMID: 19022841. [DOI] [PubMed] [Google Scholar]
- 37.de Vries E, Arnold M, Altsitsiadis E. Potential impact of interventions resulting in reduced exposure to ultraviolet (UV) radiation (UVA and UVB) on skin cancer incidence in four European countries, 2010-2050. Br J Dermatol. 2012;167(2):53–62. doi: 10.1111/j.1365-2133.2012.11087.x. Suppl. [DOI] [PubMed] [Google Scholar]