Summary
To investigate the feasibility and early efficacy of 3D-printed vertebral body implantation combined with robotic radiosurgery in the treatment of spinal tumors. This study included 14 patients with spinal tumors from December 2017 to June 2018. Before surgery, all patients were subjected to CT scan and 3D data of the corresponding vertebral segments were collected. Titanium alloy formed 3D-printed vertebral body implantation and robotic stereotactic radiotherapy were performed because of the risk of postoperative residual, high risk of recovery, or recurrence after surgery. The main outcomes included the remission of symptoms, vertebral body stability, robotic stereotactic surgical precision, and local tumor control. All patients received complete and successful combination therapy, and all healed primarily without complications. The error of the coverage accuracy for robotic radiosurgery was less than 0.5 mm, and the error of the rotation angle was less than 0.5°. The therapeutic toxicity was limited (mainly in grades 1–2), and adverse events were uncommon. The evaluation of vertebral body stability and histocompatibility for all patients met the postoperative clinical requirements. For patients with post spinal injury, the pain symptoms were reduced or disappeared (93%), and nerve function was improved or even recovered after treatment (100%). During our follow-up period, most tumors were locally well controlled (93%). 3D- printed vertebral body implantation combined with robotic radiosurgery may offer a new treatment of spinal tumors.
Chinese clinical trial registry: ChiCTR-ONN-17013946.
Keywords: 3D-printed technology, spinal tumor, vertebral body implantation, robotic stereotactic radiotherapy
Graphical Abstract
Public Summary
-
•
With the development of 3D printing and robotic radiotherapy technology, the outcome of spinal tumors has been shown to have improving opportunities
-
•
In this study, 14 patients with spinal tumor were treated by 3D printing vertebral body implantation combined with robotic radiotherapy, and the results showed that treatment could achieve requirements of spinal function perfectly and precisely kill the tumor
-
•
3D printing vertebral body implantation combined with robotic stereotactic radiotherapy might be a treatment revolution for spinal tumors
Introduction
The treatment of spinal tumors is difficult. Vertebral body excision plus postoperative radiotherapy is the routine treatment.1 However, since the conventional vertebral implants cannot perfectly meet the mechanical requirements of the spine and do not have sufficient histocompatibility, postoperative spinal instability is a common problem for patients with spinal tumor, especially in patients with multi-segmental lesions. Meanwhile, conventional radiotherapy with suboptimal accuracy may result in a high risk to the normal structures adjacent to spinal lesions, which leads to limitation of radiation dose.2
Due to the disadvantages mentioned above, several serious adverse events may occur during the practice of conventional radiotherapy for patients with destructive spinal lesions. However, with the development of 3D printing and robotic radiotherapy technology, the outcome of spinal tumors could potentially be improved. To our knowledge, our institution—Peking University Third Hospital (Beijing, China)—is the first unit in the world that combined 3D-printed vertebral body implantation and advanced robotic radiosurgery for spinal tumor treatment.3, 4, 5 This report describes the strategies and outcomes of patients who received the advanced combined treatment.
Results
Overall, 14 patients (3 [21.4%] women) aged 19 to 57 years were included in this study. Among the enrolled patients, 13 cases (92.9%) had primary tumors of the spine, and 1 case (7.1%) had metastases of the spine. Of the spinal lesions, 4 cases (28.6%) had postoperative residual, 9 cases (64.3%) were in high risk of recovery, and 1 case (7.1%) had postoperative recurrence. The enrolled cases included one metastasis of leiomyosarcoma, three chordomas, two chondrosarcomas, one rhabdomyosarcoma, two osteosarcomas, one fibroma, three osteoblastomas, and one giant cell tumor of the bone. With regard to tumor location, five were in the cervical spine, six were in the thoracic spine, and three were in the lumbar spine. The radiation dose was 35 or 40 Gy/5 fractions (Gy/5f). Detailed characteristics of each participant are summarized in Table 1.
Table 1.
Baseline of Patient and Treatment Characteristics
| Characteristic | Values |
|---|---|
| Cases | 14 |
| Gender, cases (%) | |
| Male | 11 (79) |
| Female | 3 (21) |
| Age (years) | |
| Range | 19–57 |
| Median | 40.5 |
| Primary or metastases, cases (%) | |
| Primary | 13 (92) |
| Metastases | 1 (8) |
| Lesion site, cases (%) | |
| Cervical vertebra | 5 (36) |
| Thoracic vertebra | 6 (43) |
| Lumbar vertebra | 3 (21) |
| Sacral vertebra | 0 (0) |
| Pathology, cases (%) | |
| Chordoma | 3 (21) |
| Metastasis of leiomyosarcoma | 1 (8) |
| Chondrosarcoma | 2 (14) |
| Rhabdomyosarcoma | 1 (8) |
| Osteosarcoma | 2 (14) |
| Solitary fibroma | 1 (8) |
| Osteoblastoma | 3 (21) |
| Giant cell tumor | 1 (8) |
| Radiotherapy reason, cases (%) | |
| Postoperative residual | 4 (29) |
| High-risk resectiona | 9 (64) |
| Postoperative recurrence | 1 (8) |
| Dose line (%) | |
| Range | 67–80 |
| Median | 72.5 |
| Fraction | |
| Range | 5–5 |
| Median | 5 |
High-risk resection refers to transtumor resection and high risk of cut edge.
3D Printing Technology-Based Surgery
All 14 patients (11 males and 3 females) underwent successful operation, two of whom underwent subtotal vertebrectomy, and the remaining patients underwent total vertebrectomy. Patients were operated through anterior (two patients), posterior (five patients), or anterior and posterior approaches (seven patients) depending on the surgical planning. Most patients had modest bleeding of approximately 1,000 mL (range: 700–4,000; median: 1,100). All patients received postoperative radiotherapy due to postoperative recurrence, transtumor resection path, or positive residual tumor margin, respectively. No postoperative complications occurred (Table 2).
Table 2.
3D Printing Vertebral Body Implantation Operation and Robotic Radiotherapy Treatment
| Cases | Surgical Condition | Operative Approach | Operative Type (Total/Subtotal Vertebrectomy) | Blood Loss (mL) | Operative Complications | Dose Prescription (Gy/5f) | Vertebral Body Positiona |
|---|---|---|---|---|---|---|---|
| 1 | postoperative recurrence | combined | Total | 1,200 | none | 40 | T11-L3 |
| 2 | transtumor resection | posterior | total | 1,400 | none | 35 | T1 |
| 3 | transtumor resection | combined | total | 1,000 | none | 35 | T8-10 |
| 4 | transtumor resection | combined | total | 1,650 | none | 35 | T12-L1 |
| 5 | edge residue | combined | total | 1,600 | none | 35 | T10-12 |
| 6 | edge residue | combined | total | 1,300 | none | 35 | C7-T2 |
| 7 | transtumor resection | combined | total | 2,000 | none | 35 | T10-12 |
| 8 | transtumor resection | combined | total | 700 | none | 35 | C5-6 |
| 9 | transtumor resection | posterior | subtotal | 800 | none | 35 | L3-5 |
| 10 | transtumor resection | posterior | total | 1,000 | none | 35 | L2-3 |
| 11 | transtumor resection | posterior | total | 4,000 | none | 35 | C2-3 |
| 12 | transtumor resection | anterior | total | 800 | none | 35 | L2-4 |
| 13 | edge residue | posterior | subtotal | 1,000 | none | 35 | T6-7 |
| 14 | edge residue | anterior | total | 800 | none | 35 | C2-T1 |
C, cervical vertebra; T, thoracic vertebra; L, lumbar vertebra.
Therapeutic Accuracy of Robotic Stereotactic Radiotherapy
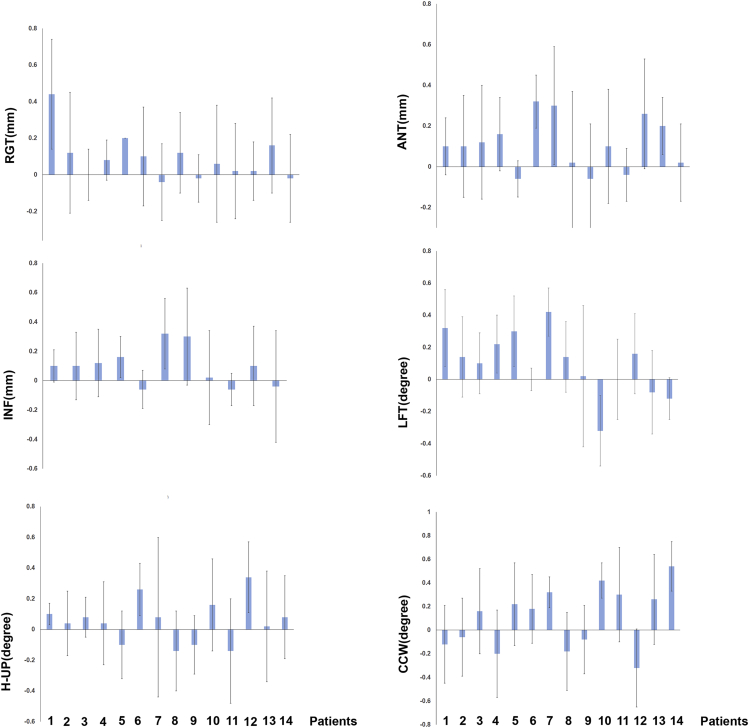
All patients successfully underwent radiotherapy. The total variation for radiation target was 0.07 ± 0.25 mm on the right and left side of the body, 0.10 ± 0.24 mm in the forward and backward directions, −0.03 ± 0.24 mm in the head and foot directions, and 0.09° ± 0.28° on left and right rotatation of the body. H-UP (head up and down) was 0.03° ± 0.27° in the longitudinal direction, and CCW (clockwise and counterclockwise) was 0.08° ± 0.37°. The results showed that the requirements for high-precision spinal tumor surgery could be achieved by robotic stereotactic radiotherapy. The specific treatment process for each patient is shown in the classical case view (Figure 1), and the specific accuracy errors data are listed in Figure 2.
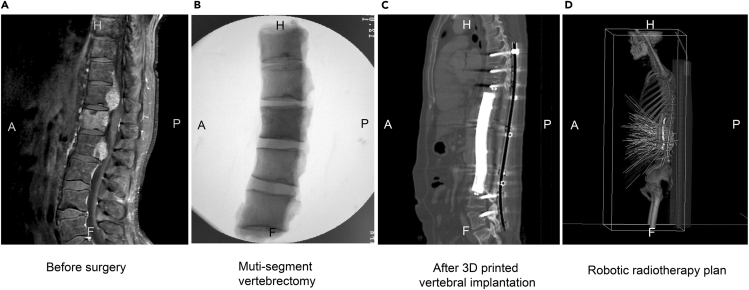
Figure 1.
A Typical Patient Treated with 3D-Printed Vertebral Body Implantation Combined with Robotic Stereotactic Radiotherapy.
(A) Patient with five impaired segments.
(B) The vertebral body resection of the five impaired segments.
(C) Vertebral body implantation.
(D) The plan of stereotactic robotic radiotherapy treatment. A, anterior; P, posterior; H, head; F, foot.
Figure 2.
Therapeutic Accuracy of Robotic Stereotactic Radiotherapy in All Patients.
RGT: left and right move horizontally, LET (+)/RIG (−); ANT: anterior and posterior move vertically, ANT (+)/POS (−); NIF: head and foot direction horizontal movement, NIF (+)/SUP (−); LFT: left and right rotation, R (+)/L (−); H-UP: head up and down, head-up(+)/head-down(−); CCW: clockwise and counterclockwise directions, CW (+)/CCW (−). High-precision treatment of the spinal tumor could be achieved by robotic stereotactic radiotherapy.
Toxicity of Robotic Stereotactic Radiotherapy
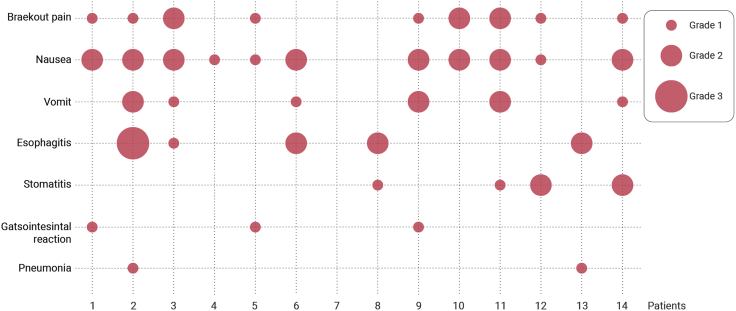
Nausea, vomiting, pain, esophagitis, and bloating were common toxic reactions during the radiotherapy, and were mostly grade 1–2. Only one patient had grade 3 esophagitis (Figure 3), and his symptom was improved after 1 week of treatment.
Figure 3.
Toxicity of Robotic Stereotactic Radiotherapy.
The side effects and grading of each patient. Most of which were in grade 1–2. Only one patient had grade 3 esophagitis.
Tumor Treatment and Local Control
All patients healed well after surgery, their symptoms and signs improved remarkably and their organ function was largely recovered compared with previously. The preoperative pain score of all patients was 5.25 ± 1.89, while the postoperative pain score was 1.83 ± 1.09. The symptoms for all patients were improved significantly (t = 5.86, p = 0.00). Furthermore, all patients with D-grade spinal cord injury before treatment (n = 8) were recovered to E-grade after operation, and one patient with C-grade before was recovered to D-grade after the operation. Vertebral body stability and histocompatibility were not affected by radiosurgery in any cases. The implantation of the artificial vertebral body provided good support for the spine. During the follow-up period, superior local tumor control was observed in 13 patients, while only 1 patient had recurrence after surgery (without performing the radiosurgery after surgery), whose recurrent lesion was successfully controlled by additional radiosurgery (Table 3).
Table 3.
Efficacy of Tumor Treatment and Local Control
| Characteristics | Values |
|---|---|
| Surgical healing, cases (%) | |
| Healing | 14 (100) |
| Not healing | 0 (0) |
| Spinal cord injury | |
| No spinal cord injury before treatment | 5 (36) |
| Spinal cord injury improved after treatment | 9 (64) |
| Spinal cord injury no-improved after treatment | 0 (0) |
| Pain relief, cases (%) | |
| Disappear | 2 (14) |
| Obvious improvement | 11 (79) |
| No change | 1 (7) |
| Vertebral stability, cases (%) | |
| Stable | 14 (100) |
| Unstable | 0 (0) |
| Histocompatibility, cases (%) | |
| Compatible | 14 (100) |
| Incompatible | 0 (0) |
| Tumor control, cases (%) | |
| Control | 13 (93) |
| Uncontrolleda | 1 (7) |
The lesion recurred after surgery and was controlled after radiosurgery.
Discussion
The 3D-printed vertebral body implantation combined with robotic stereotactic radiotherapy achieved gratifying results in the treatment of spinal tumors. The characteristics of spinal tumors and the advantages of combination treatment are critical for patients with spinal tumors to achieve a better clinical effect.6 The load-bearing feature of the spine requires that the substituted implants provide durably firm support and have high stability characteristics. Traditional implants cannot meet these requirements perfectly due to an incomplete match of the artificial implant ends and the original vertebral body. In contrast, the design specifications of the 3D printing artificial vertebral body system fully addressed the orthopedic requirements of the spinal surgical region, which provided an excellent solution for spinal corpectomy and reconstruction, which guaranteed stability of the spine.7
High-intensity radiotherapy is often required postoperatively to address residual tumor and local recurrence. Using a traditional human bone implant is problematic due to the interference of bone growth.8 The 3D-printed vertebral body can be made in a porous structure, which is similar to natural bone tissue,8 that can maintain a high consistency between the titanium alloy implant and the individual anatomical structure. Moreover, the typical porous structure is favorable to the growth of osteoblasts, thereby effectively promoting the fusion of the real bone with the artificial implants, thus ensuring high histocompatibility.
Target accuracy of traditional palliative radiotherapy in spinal tumor treatment is also difficult to achieve because of the poor discernment of the exact location of the pain, which is an unsolved critical issue. Stereotactic robotic radiotherapy offers an alternative choice because the location of the spinal tumors can be automatically tracked, detected, and corrected via the spinal tracking system.9,10 The spinal tracking system enables radiation treatment of the whole spine possible, as the radiation shadow conforms exactly with the anatomical form.11 Thus, the spinal lesions can be treated with high-precision and high-intensity radiation by precise locating and targeting using the spinal tracking strategy. Consequently, 3D-printed vertebral body implantation combined with robotic stereotactic radiotherapy fulfills the unique requirements of spinal tumor treatment, and thus provides better therapeutic outcome for patients.
While 3D printed technology and conventional implant surgery are currently applied simultaneously in the treatment of spinal tumors, 3D-printed technology requires long-term follow-up inspection. Also, 3D-printed treatment is not a standard treatment method, and it is used in parallel with conventional vertebral implantation in surgery. Differences in the efficacy of the two methods require long-term follow-up testing.
In conclusion, although our study is limited by the single-center small case series with short-term follow-up, we found that selected patients might benefit from treatment with 3D printing artificial vertebral body implantation combined with robotic stereotactic radiotherapy, which was verified by our preliminary findings. Therefore, our approach is a good innovation in the treatment of spinal tumors and provides valuable information for making clinical treatment decisions.
Materials and Methods
Patients
In our study, the inclusion criteria were: patients with primary or metastatic spinal tumors; patients with spinal instability, vertebral compression, or neurological dysfunction; able to tolerate surgery; and the pathological diagnosis confirmed by fine-needle aspiration biopsy. The exclusion criteria were: patients with multiple metastases with an expected survival time of less than 3 months; patients with small vertebral lesions that could be cured by radiotherapy alone; and inability to tolerate surgery.
Pain was assessed by the visual analog scale,12 local spinal stability was evaluated by the spinal instability neoplastic score (SINS),13,14 and the neurological function of spinal cord injury was calculated using the modified Frankel scale.
This study was approved by the Ethics Committee of the Peking University Third Hospital and completed under its supervision. Informed consent was signed by all participants. Our study was in accordance with all the ethical requirements and followed the reporting guideline for case series.
3D Printing of the Vertebral Body
Before treatment, all patients received a thin-slice computed tomography (CT) scan and the CT data were transmitted to the 3D printing implant manufacturer. Next, 3D graphics were reconstructed by engineers via software analysis. After identifying the location and extent of the spinal lesion, we quantified the structure and destruction of the bone lesion. Subsequently, the artificial vertebral body was designed according to the operation designs of the surgeon. On completion of design, the data were uploaded to the printing computer. Titanium alloy powder was used as raw material, and the electrode wires that can form ultra-high pressure were used as the energy source. The implant was forged layer by layer using the 3D printing technology of Electron Beam Melting (Aikang, Beijing). This implant product has excellent advantages in individual suitability as it was designed and modified in a personalized customization strategy. The mechanical properties and biocompatibility of the above material had been tested and established previously.15
Surgery
The surgical indications included primary malignant and borderline tumors of the spine, or spinal metastases with a spinal SINS13,14 score≥13. The anesthesia method and surgical approach were selected according to the location of the lesion. The diseased vertebral segments were, as a rule, always resected completely for the purpose of tumor excision, spinal cord decompression, and correction of deformity. Attention should be paid to the bilateral spinal nerve roots and the thecal sac behind the vertebral body during surgery. One end of the artificial vertebral body was inserted into the anterior or middle part of the human vertebral segment, and we embedded it into the bone by gently tapping until the other end reached the predetermined position. Fluoroscopy was used to check the implant position. Once the surgeon was satisfied with the position, the artificial vertebral body was rotated toward the intended direction. Finally, the vertebral segments were distracted to restore the original height. Once the height reached a predetermined level, the screws at both ends of the artificial vertebral body were fixed and tightened. For patients with extensive resection, plate fixation was added in case the artificial vertebral body replacement alone was not stable enough. For spinal lesions in which a combined anterior and posterior surgical approach surgery was necessary, pedicle screw fixation was added.16,17
Robotic Stereotactic Radiotherapy
All patients had received IRIS stereotactic robotic radiotherapy (Accuray, CA, USA). Preoperative and postoperative magnetic resonance imaging, CT-based simulation, and image fusion were performed in all patients. The tumor residual, high-risk, or recurrence areas were accurately delineated based on the preoperative and postoperative images, and the gross tumor volume (GTV) or clinical target volume (CTV) were determined according to the patient's operating conditions. The planning target volume was moderately extended based on GTV or CTV.18 Dose fractionations of 35 or 40 Gy/5f were given to the patients based on their treatment needs, and doses to normal organs were as referred to in report TG101.19 The Multiplan 4.6 planning system (Accuray) was used to formulate the treatment plan. Once the plan was completed, validation of the plan was carried out and robotic stereotactic radiotherapy was employed subsequently. The X sight-spine tracking technique and 6D bed systems were used to accurately control the target position and location of the lesion. In the case of radiation center change during the treatment, the machine would automatically recognize and interrupt the treatment until the position was corrected. The robot was able to irradiate simultaneously from more than 3,000 angles, which avoided damage of adjacent organs, especially the spinal cord, and thus achieved precise radiotherapy.
Observation and Follow-up
Local lesions were routinely reexamined 3 months after the operation and 2 months after the radiosurgery. The follow-up examination interval was determined based on the lesion type and general condition, but at least three times in the first year and two times per year thereafter. Follow-up included magnetic resonance imaging and CT of the spine, bone scan, and spine X-ray with front and lateral positions. The main outcomes included symptom remission, vertebral body stability, material histocompatibility, robotic stereotactic surgical precision, limit of therapeutic toxicity, and local tumor control. Statistical analysis of improvement of patient's symptoms before and after treatment was performed with SPSS 23.0 (IBM). Statistical significance was based on paired sample t test with a threshold of p < 0.05.
Acknowledgments
This research was funded by key clinical projects of Peking University Third Hospital (Peking University talent introduction fund, BYSY2017030).
Declaration of Interests
The authors declare no competing interests.
Published: August 28, 2020
Contributor Information
Hongqing Zhuang, Email: hongqingzhuang@163.com.
Zhongjun Liu, Email: 1763180506@bjmu.edu.cn.
References
- 1.Parthiban J.K., Rudrappa S., Prahlad S.T., Govindasamy R. Evolution of surgical techniques in the management of vertebral body tumours and the current status. Neurol. India. 2018;66:1254–1269. doi: 10.4103/0028-3886.241398. [DOI] [PubMed] [Google Scholar]
- 2.Huang L., Djemil T., Zhuang T., Andrews M., Chao S.T., Suh J.H., Xia P. Treatment plan quality and delivery accuracy assessments on 3 IMRT delivery methods of stereotactic body radiotherapy for spine tumors. Med. Dosim. 2019;44:11–14. doi: 10.1016/j.meddos.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Wardak Z., Bland R., Ahn C., Xie X.J., Chason D., Morrill K., Stehel E., Nedzi L., Ding C., Medin P., et al. A phase II clinical trial of SAbR followed by immediate vertebroplasty for spine metastases. Int. J. Radiat. Oncol. Biol. Phys. 2019;104:83–89. doi: 10.1016/j.ijrobp.2019.01.072. [DOI] [PubMed] [Google Scholar]
- 4.Blanck O., Wang L., Baus W., Grimm J., Lacornerie T., Nilsson J., Luchkovskyi S., Cano I.P., Shou Z., Ayadi M., et al. Inverse treatment planning for spinal robotic radiosurgery: an international multi-institutional benchmark trial. J. Appl. Clin. Med. Phys. 2016;17:313–330. doi: 10.1120/jacmp.v17i3.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim N., Lee H., Kim J.S., Baek J.G., Lee C.G., Chang S.K., Koom W.S. Clinical outcomes of multileaf collimator-based CyberKnife for spine stereotactic body radiation therapy. Br. J. Radiol. 2017;90:20170523. doi: 10.1259/bjr.20170523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi D., Bilsky M., Fehlings M., Fisher C., Gokaslan Z. Spine oncology—metastatic spine tumors. Neurosurgery. 2017;80:S131–S137. doi: 10.1093/neuros/nyw084. [DOI] [PubMed] [Google Scholar]
- 7.Provaggi E., Leong J.J.H., Kalaskar D.M. Applications of 3D printing in the management of severe spinal conditions. Proc. Inst. Mech. Eng. H. 2017;231:471–486. doi: 10.1177/0954411916667761. [DOI] [PubMed] [Google Scholar]
- 8.Kim M.J., Lee S.R., Lee M.Y., Sohn J.W., Yun H.G., Choi J.Y., Jeon S.W., Suh T.S. Characterization of 3D printing techniques: toward patient specific quality assurance spine-shaped phantom for stereotactic body radiation therapy. PLoS One. 2017;12:e0176227. doi: 10.1371/journal.pone.0176227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fürweger C., Drexler C., Muacevic A., Wowra B., de Klerck E.C., Hoogeman M.S. CyberKnife robotic spinal radiosurgery in prone position: dosimetric advantage due to posterior radiation access? J. Appl. Clin. Med. Phys. 2014;15:4427. doi: 10.1120/jacmp.v15i4.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalash R., Glaser S.M., Flickinger J.C., Burton S., Heron D.E., Gerszten P.C., Engh J.A., Amankulor N.M., Vargo J.A. Stereotactic body radiation therapy for benign spine tumors: is dose de-escalation appropriate? J. Neurosurg. Spine. 2018;29:220–225. doi: 10.3171/2017.12.SPINE17920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ju Z., Wang J., Zhang H., Du L., Xu W., Wang X., Ge R., Li J., Zheng Q., Li J. Dose fall-off during the treatment of thoracic spine metastasis with CyberKnife stereotactic body radiation therapy (SBRT) Bosn J. Basic Med. Sci. 2020;20:131–139. doi: 10.17305/bjbms.2018.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berghmans J.M., Poley M.J., van der Ende J., Weber F., Van de Velde M., Adriaenssens P.8, Himpe D., Verhulst F.C., Utens E. A Visual Analog Scale to assess anxiety in children during anesthesia induction (VAS-I): results supporting its validity in a sample of day care surgery patients. Paediatr. Anaesth. 2017;27:955–961. doi: 10.1111/pan.13206. [DOI] [PubMed] [Google Scholar]
- 13.Versteeg A.L., Verlaan J.J., Sahgal A., Mendel E., Quraishi N.A., Fourney D.R., Fisher C.G. The spinal instability neoplastic score: impact on oncologic decision-making. Spine (Phila Pa 1976) 2016;41(Suppl 20):S231–S237. doi: 10.1097/BRS.0000000000001822. [DOI] [PubMed] [Google Scholar]
- 14.Hussain I., Barzilai O., Reiner A.S., DiStefano N., McLaughlin L., Ogilvie S., Bilsky M., Laufer I. Patient-reported outcomes after surgical stabilization of spinal tumors: symptom-based validation of the Spinal Instability Neoplastic Score (SINS) and surgery. Spine J. 2018;18:261–267. doi: 10.1016/j.spinee.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen Y., Xun S., Haoye M., Baichuan S., Peng C., Xuejian L., Kaihong Z., Xuan Y., Jiang P., Shibi L. 3D printed porous ceramic scaffolds for bone tissue engineering: a review. Biomater. Sci. 2017;5:1690–1698. doi: 10.1039/c7bm00315c. [DOI] [PubMed] [Google Scholar]
- 16.Regev G.J., Salame K., Keynan O., Lidar Z. Resection of benign vertebral tumors by minimally invasive techniques. Spine J. 2015;15:2396–2403. doi: 10.1016/j.spinee.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Jakubovic R., Ruschin M., Tseng C.L., Pejovic-Milic A., Sahgal A., Yang V.X.D. Surgical resection with radiation treatment planning of spinal tumors. Neurosurgery. 2019;84:1242–1250. doi: 10.1093/neuros/nyy176. [DOI] [PubMed] [Google Scholar]
- 18.James J., Swanson C., Lynch B., Wang B., Dunlap N.E. Quantification of planning target volume margin when using a robotic radiosurgery system to treat lung tumors with spine tracking. Pract. Radiat. Oncol. 2015;5:e337–e343. doi: 10.1016/j.prro.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Benedict S.H., Yenice K.M., Followill D., Galvin J.M., Hinson W., Kavanagh B., Keall P., Lovelock M., Meeks S., Papiez L., et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med. Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]