Fluoroquinolones (FQ) are the most frequently reported non-beta-lactam antibiotic allergy.1 The basic FQ structure is a bicyclic skeleton and can be classified by generation, with later generations correlating with broader gram-positive and anaerobic antimicrobial spectrum in particular.1 Interpretation of hypersensitivity reactions to FQ are likely complicated by non-IgE mediated reactions since all FQ are small molecule ligands for the mast cell G-protein coupled receptor MRGPRX2 and have been shown to lead to direct mast cell activation in both in vitro models with the human MRGPRX2 receptor and murine models.2 Further, it is suspected that patients with true IgE-mediated allergy may have selective FQ reactivity rather than generalized positivity on skin testing or oral challenge, as has been described in several cases of lack of complete cross-reactivity between FQ and in particular tolerance of alternative FQ in patients with moxifloxacin hypersensitivity reactions.3 When expert consensus criteria for skin testing have been applied to FQ, utility has been limited by high-false positive rates due to non-specific mast cell activation. Uyttebroek et al. found positive moxifloxacin intradermal testing (IDT) in 10/14 moxifloxacin allergic patients (2 at 0.0016 mg/mL; 2 at 0.016 mg/mL; 6 at 0.16 mg/mL) and in 12/16 moxifloxacin tolerant controls tested (2 at 0.016 mg/mL; 12 at 0.16 mg/mL).4 Non-irritating concentrations of FQ IDT have been proposed, Chang et al. suggesting 0.005 mg/mL (ciprofloxacin, levofloxacin, moxifloxacin).3 Therefore, we present a newly proposed criteria for defining a positive FQ IDT that is founded in an understanding of non-specific mast cell activation and selective FQ reactivity. Further, we examined the safety and outcomes of this new criteria in conjunction with oral challenge (OC) to an index or other FQ in adults who had a history of reacting to one or more FQ, and their subsequent tolerance of future FQ treatment.
Our study presents a retrospective cohort study done under institutional review board (IRB) approved protocols from Vanderbilt University Medical Center (VUMC), IRB #161455. Between May 2015 and October 2019, 163 sequential patients with history-based past immediate, immediate-type, non-severe delayed or unknown reactions to one or more FQ with ongoing avoidance of FQ underwent IDT followed by selective OC in a dedicated outpatient drug allergy clinic at VUMC. Patients with any history of a severe delayed immune mediated reaction, such as Stevens-Johnson syndrome and toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms, acute generalized exanthematous pustulosis, or drug induced nephritis or hepatitis, were excluded. In patients presenting for assessment of FQ allergy with a concurrent history of beta-lactam allergy and/or sulfa antibiotic allergy, de-labeling to beta-lactams and sulfa antibiotics was the priority based on patient need, antimicrobial stewardship by referring infectious diseases physicians, and use of a safe and efficacious strategy to evaluate non-severe delayed reactions to sulfa antibiotics.5 Following successful de-labeling to beta-lactams and sulfa-antibiotics, patients had full assessment of FQ allergy if they had a potential future need. Histamine was performed by skin prick at a concentration of 0.1 mg/mL. IDT to ciprofloxacin, levofloxacin, and moxifloxacin was performed — by European Network on Drug Allergy (ENDA) standardized technique — at concentrations of 0.025 mg/mL and 0.005 mg/mL, followed by single dose OC to the index FQ or other FQ (levofloxacin 250 mg, ciprofloxacin 250 mg, moxifloxacin 200 mg).6 A 200 mg dose for moxifloxacin was used because it is only available as a 400 mg tablet. Oral challenge success was defined by the absence of any symptoms during an observed 2 hour challenge period. Patients were called by phone 24 hours after oral challenge to follow-up on any possible delayed reactions. Oral challenge success resulted in the removal of FQ allergy or revision to confirm tolerance of an alternative FQ and patient education that FQ could now be used in their clinical care as appropriate. We evaluated selected, currently used expert consensus criteria for positive IDT: criteria #1: FQ wheal ≥ saline wheal + 3 mm; criteria #2: FQ wheal ≥ saline wheal + 3 mm and flare present; criteria #3: FQ wheal ≥ 5mm and flare > wheal and compared these to our proposed criteria #4 for positive IDT: specific FQ flare at 0.025 mg/mL ≥ histamine flare, specific FQ flare ≥ 5 mm at 0.005 mg/mL, and no flare ≥ 5 mm for either of the other 2 FQ at 0.005 mg/mL. Criteria #1 is based on the “Drug allergy: an updated practice parameter” criteria.7 Criteria #2 is based on the ENDA criteria.8 Criteria #3 is derived from a currently used interpretation for a positive skin test in penicillin allergy.9 Criteria #4 was developed and refined during routine clinical care in an attempt to codify and apply retrospectively a criteria that encompassed the observation that non-specific wheal without flare frequently occurs to multiple skin tested FQ in patients — that would otherwise be positive by currently accepted skin testing guidelines — who then go on to tolerate FQ oral challenge. Our hypothesis for this observation is that true IgE mediated FQ allergy is uncommon relative to non-IgE mediated reactions and that the majority of FQ reactions are likely a result of an off target, class pharmacologic effect via MRGPRX2 leading to non-IgE mediated mast cell activation. An off target class-wide “pseudoallergy” effect mediated through a pharmacological interaction with MRGPRX2 rather than an IgE mechanism, is similar conceptually to what is observed in reactions associated with non-steroidal anti-inflammatory drugs (NSAIDs). With NSAIDs true IgE-mediated reactions tend to be related to a specific drug (e.g. diclofenac) or shared chemical structure, and “pseudoallergic” reactions are pharmacologically mediated, related to inhibition of cyclooxygenase 1, and associated more broadly across all non-selective NSAIDs and aspirin. Unlike IgE-mediated reactions, which do not have true dose dependency, these pharmacological reactions that result from non-covalent interactions with an off-target receptor do vary based on dose, the kinetics of how the drug is administered (e.g. speed of infusion) and dosing with concurrent medications that have similar properties (e.g. vancomycin, neuromuscular blocking agents and opioids). Study data were collected and managed using REDCap electronic data capture tools hosted at VUMC. Analysis of IDT results was performed with R (R Core Team 2019).
Charts were reviewed for patient demographics, time between index reaction and challenge, index reaction history (immediate within 1 hour, immediate-type within 1 to 36 hours, delayed greater than 36 hours or unknown timing of symptoms from first dose), indication for consult, co-morbidities, nature of initial label (ciprofloxacin, levofloxacin, moxifloxacin, or multiple FQ), total number of drug allergy labels, total number of antibiotic allergy labels, specific antibiotic allergy labels (penicillins, cephalosporins, and sulfa antibiotics), and history of allergy to drugs with implicated non-IgE mechanisms (vancomycin, radiocontrast, opioids, and neuromuscular blocking agents). Like FQ, the drugs reviewed with implicated non-IgE mechanisms can also have IgE-mediated mechanisms. Follow-up assessment to determine tolerance of any subsequent FQ treatments was performed by chart review.
The demographic and clinical characteristics of the 163 patients are described in Table 1. Index reaction history for patients included 40 immediate, 96 immediate-type, 22 non-severe delayed, and 5 unknown. Of the 136 patients with immediate or immediate-type index reactions, 31 patients reported anaphylaxis or multisystem involvement compatible with anaphylaxis but not clearly defined as such on chart review. The labeled FQ for patients included 58 ciprofloxacin-only, 53 levofloxacin-only, 13 moxifloxacin-only, and 39 to ≥ 2 FQ (33 ciprofloxacin labels, 36 levofloxacin labels, and 6 moxifloxacin labels). For these 163 patients, the median total number of antibiotic allergy labels was 5 [IQR 3, 7] and 144/163 (88%) were evaluated for at least one additional antibiotic allergy besides FQ (Table 1). 84/163 (52%) patients reported an allergy to other drugs where a non-IgE mediated mechanism for mast cell degranulation has been reported.
Table 1.
Characteristics of patients who underwent fluoroquinolone intradermal testing and/or selective fluoroquinolone oral challenge
| Total N (% total) or Median [IQR] | |
|---|---|
| Total no. of patients | 163 |
| Age | 61 [50, 70] |
| Time since reaction in years (**n=107, with n=56 missing) | 8 [1, 16] |
| Sex | |
| Female | 128 (78.5) |
| Male | 35 (21.5) |
| Race | |
| White | 145 (89.0) |
| Unknown | 11 (6.7) |
| Black | 6 (3.6) |
| Other | 1 (0.7) |
| Index reaction history | |
| Immediate symptoms (< 1 hour) | 40 (24.5) |
| Mild to moderate exanthem | 15 |
| Urticaria | 15 |
| Angioedema | 11 |
| Shortness of breath | 10 |
| Hypotension | 3 |
| Anaphylaxis | 16a |
| Immediate-type symptoms (1 to 36 hours) | 96 (58.9) |
| Mild to moderate exanthem | 37 |
| Urticaria | 29 |
| Angioedema | 20 |
| Shortness of breath | 13 |
| Hypotension | 2 |
| Anaphylaxis | 15a |
| Non-severe delayed symptoms | 22 (13.5) |
| Unknown | 5 (3.1) |
| Indication for Consult | |
| Multi-drug allergy | 137 (84.0) |
| Anticipated need for treatment and/or prophylaxis | 21 (12.9) |
| Infection without other options | 5 (3.1) |
| Total no. drug allergy labels | 7 [5, 11] |
| No. of antibiotic allergy labels | 5 [3, 7] |
| Penicillin allergy | 127 (77.9) |
| Underwent testing | 116 |
| Label removed | 116 |
| Subsequently treated after removal | 28 |
| Label not removed | 0 |
| Cephalosporin allergy | 94 (57.7) |
| Underwent testing | 60 |
| Label removed or revisedb | 59 |
| Subsequently treated after removal | 16 |
| Label not removed | 1 |
| Sulfa antibiotic allergy | 101 (61.9) |
| Underwent testing | 45 |
| Label removed | 44 |
| Subsequently treated after removal | 7 |
| Label not removed | 1 |
| History of allergy to a drug with an implicated non-IgE mechanism? | |
| Yes | 84 (51.5) |
| Opioid | 62 |
| Radiocontrast | 27 |
| Vancomycin | 21 |
| Neuromuscular blocking agent | 1 |
| No | 79 (48.5) |
| Co-morbidities | |
| Hematologic or oncologic malignancy | 53 |
| Diabetes | 41 |
| Recurrent sinusitis | 41 |
| Recurrent UTI | 27 |
| CVID | 8 |
| Cystic fibrosis | 4 |
| MRSA | 4 |
| Pre-solid organ or bone marrow transplant | 4 |
| Solid organ transplant | 4 |
| HIV | 3 |
| Bone marrow transplant | 1 |
| Nature of initial label | |
| Ciprofloxacin-only | 58 (35.6) |
| Levofloxacin-only | 53 (32.5) |
| Moxifloxacin-only | 13 (8.0) |
| Multiple fluoroquinolones | 39 (23.9) |
| Ciprofloxacin label | 33 |
| Levofloxacin label | 36 |
| Moxifloxacin label | 6 |
| Type of challenge (selected/dependent upon index reaction history) | |
| Ciprofloxacin | 50 |
| Levofloxacin | 33 |
| Moxifloxacin | 2 |
Either anaphylaxis or multisystem involvement compatible with anaphylaxis but not clearly defined as such on chart review
Label may have been revised to demonstrate safety for a non-cross reactive R-side chain cephalosporin rather than removal of index label due to presence of positive skin test to the index cephalosporin
Of 163 patients, 96 (59%) were positive by criteria #1, 53 (33%) by criteria #2, 36 (22%) by criteria #3 at either 0.005 mg/mL or 0.025 mg/mL for at least 1 FQ (Table E1 available in this article’s Online Repository at www.jaci-inpractice.org). If the 163 patients were restricted to only the 0.005 mg/mL concentration, 73 (45%) were positive by criteria #1, 19 (12%) by criteria #2, 13 (8%) by criteria #3 for at least 1 FQ. By contrast, only 4/163 (2%) patients had positive IDT by proposed criteria #4. The 4 positive IDT patients by criteria #4 all had an immediate, anaphylactic index reaction history, were IDT positive to their index label FQ (2 moxifloxacin, 1 levofloxacin, and 1 ciprofloxacin), presented for evaluation within 1 year of their index reaction, and did not have any co-existing history of allergy to drugs implicated in non-IgE mediated mast cell activation. Of these 4 patients, 2 underwent and tolerated oral challenge to a skin-test negative FQ and the other 2 did not undergo oral challenge due to time constraints related to penicillin and sulfa antibiotic testing on a single visit and distance constraints to return for oral challenge.
Of the 159 patients with negative IDT by criteria #4, 82/159 (52%) underwent OC and 82/82 (21 immediate, 47 immediate-type, 13 non-severe delayed, and 1 unknown index reaction history; 100%) were de-labeled to their index FQ or an alternative FQ based on lack of an immediate or delayed reaction to single dose FQ (Figure 1).
Figure 1.
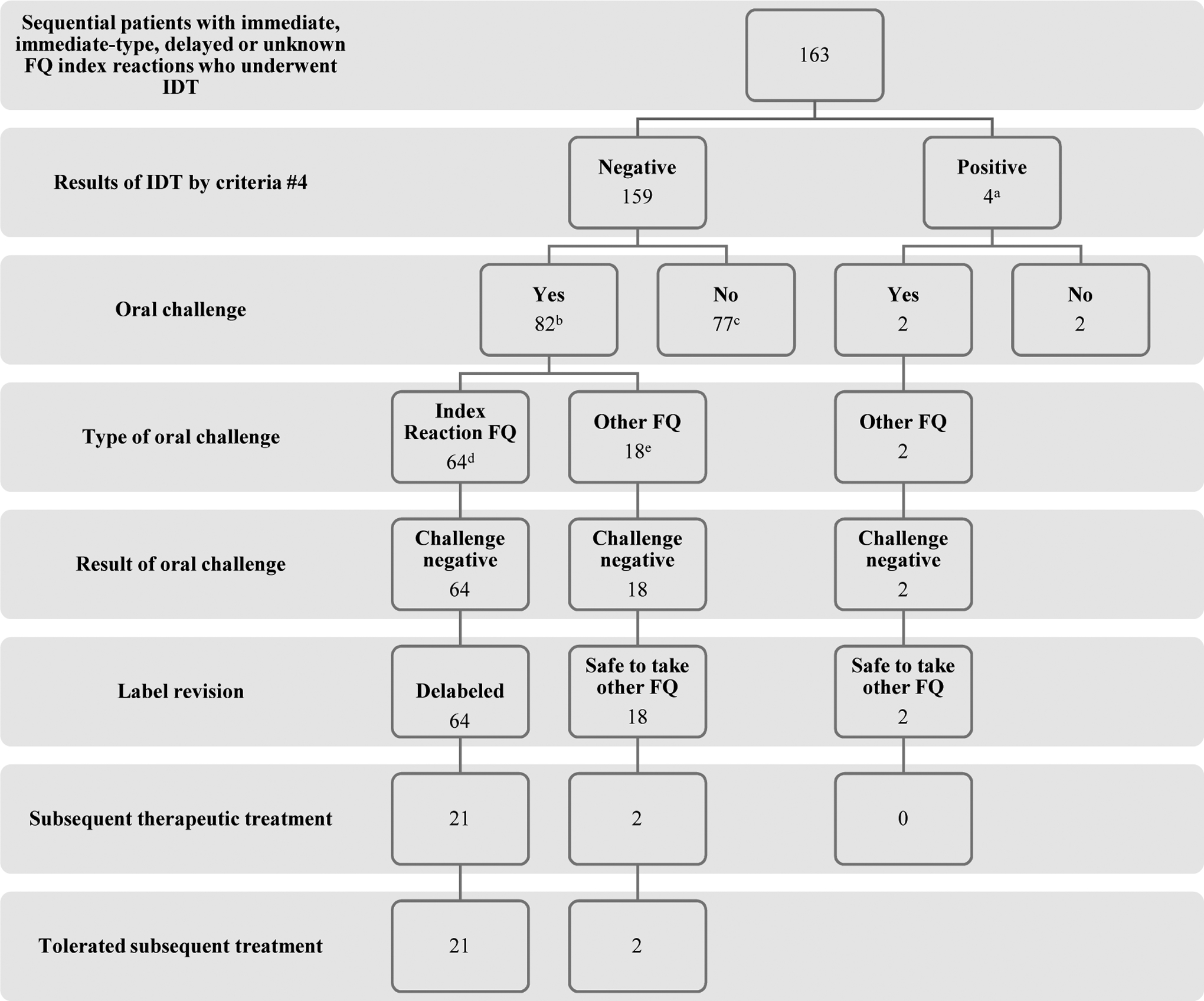
Flowchart of the study. FQ, fluoroquinolone; IDT, intradermal testing
a96/163 (59%) were IDT positive by criteria #1; 53/163 (33%) by criteria #2; and 36/163 (22%) by criteria #3 at either 0.005 mg/L or 0.025 mg/mL for at least 1 FQ
b47/82 (57%) were positive by criteria #1; 27/82 (29%) by criteria #2; and 29/82 (35%) by criteria #3 at either 0.005 mg/L or 0.025 mg/mL for at least 1 FQ. For greater detail on index reaction type, severity, and FQ; oral challenge FQ; subsequent treatment FQ, please see Table E2 (available in this article’s Online Repository at www.jaci-inpractice.org)
cThese 77 patients did not undergo OC as a result of time constraints in clinic due to our prioritized testing of beta-lactam and sulfa allergies first. All of these 77 patients, if they were to return to clinic in the future, would qualify for FQ oral challenge.
d30 were to ciprofloxacin, 17 to levofloxacin, 2 to moxifloxacin and 15 to at least 1 index FQ in patient with allergy history to ≥ 2 FQ.
eThe 18 patients who underwent oral challenge to an alternative FQ did so earlier on in the development of this FQ testing strategy due to a cautious evolution of our routine clinical care. Of these 18 patients, 4 reported an index reaction history of anaphylaxis or multisystem involvement compatible with anaphylaxis and 2 reported an immediate reaction consisting of urticaria.
The other 77/159 patients with negative IDT by criteria by #4 did not undergo OC as a result of time constraints in clinic due to our prioritized testing of beta-lactam and sulfa allergies first. All of these 77 patients — if they were to return to clinic in the future — would qualify for OC to FQ. All patients who had an immediate or perceived high future need for a FQ underwent FQ ingestion challenge. No delayed challenge reactions or delayed positive skin tests were reported.
There were 68 patients with an immediate or immediate-type reaction history who underwent OC, 15 of whom reported anaphylaxis or multisystem involvement compatible with anaphylaxis but not clearly defined as such on chart review. Of the 64 patients who underwent OC to their index FQ, 30 were to ciprofloxacin, 17 to levofloxacin, 2 to moxifloxacin, and 15 to at least 1 index FQ in patients with multiple FQ allergy history (Table E2, available in this article’s Online Repository at www.jaci-inpractice.org). There were 18 additional patients who underwent OC to an alternative FQ earlier on in the development of this FQ testing strategy due to a cautious evolution of our routine clinical care toward direct challenge with the implicated agent. Of these 18 patients, 4 reported an index reaction history of anaphylaxis or multisystem involvement compatible with anaphylaxis and 2 reported an immediate reaction consisting of urticaria. Of these 82 patients who underwent OC, 47/82 (57%) would have been deemed positive by criteria #1, 27/82 (29%) by criteria #2, and 29/82 (35%) by criteria #3 at either 0.005 mg/mL or 0.025 mg/mL for at least 1 FQ. Of the 82 patients with a negative OC, 23 (28%) patients were subsequently treated with a multiple dose therapeutic FQ course (12/23 to an index FQ; 7 levofloxacin, 5 ciprofloxacin), and all were tolerated uneventfully (Table E2, available in this article’s Online Repository at www.jaci-inpractice.org).
Interpretation of hypersensitivity skin testing to FQ is likely complicated by non-IgE mediated reactions secondary to direct FQ mast cell activation via the G-protein coupled receptor MRGPRX2 that has been demonstrated by the mouse homolog MRGPRB2 and in vitro studies with the human receptor MRGPRX2.2 We observed that from 13/163 (8%) to 73/163 (45%) patients in our cohort had skin testing that would have been deemed as a positive test under current, expert consensus criteria at the 0.005 mg/mL concentration for interpretation of immediate IDT. Under our proposed criteria, however, only 4 (2%) patients had a FQ specific positive IDT, and all 4 were specific to the single drug that was implicated in the original reaction. Importantly, there were no cases of patients with positive oral challenge reactions who had an IDT negative on criteria #4 that were positive on criteria #1, #2, or #3. 82 FQ IDT negative patients by our proposed criteria were able to tolerate challenge without any observed reactions, including from 27/82 (29%) to 47/82 (57%) who would have been deemed as having positive IDT under current, expert consensus criteria. A limitation of our retrospective cohort design is that we were not able to capture the rate of minor, non-allergic symptoms during oral challenge that self-resolved without treatment. Our lack of observation of any significant reactions on oral challenge may be the result of: challenge dose not high enough to elucidate non-IgE mediated reactions; inclusion of patients with index reactions inconsistent with an immediate reaction (22 non-severe delayed, 5 unknown); long time interval since index reaction (median 8 years). While moxifloxacin is associated with the majority of anaphylaxis to FQ, it is not as commonly used in the United States as ciprofloxacin and levofloxacin and only accounted for 19/199 (10%) FQ labels in our study.1 Our results need to be validated in a separate and ideally multicenter population sample using skin testing and oral challenges to strengthen the case for using these modified criteria.
In our population of patients with a potential immediate FQ allergy, we have started to characterize two phenotypes. The most prevalent of these is patients who likely have non-IgE mediated mast cell activation who tolerate challenges to FQ and are commonly labeled with reactions to drugs that also cause non-IgE mediated mast cell activation. Much less common are patients with a clinical presentation consistent with anaphylaxis with true IgE-mediated reactions to FQ whose reactions are typically selective for a particular FQ and do not have multiple drug allergy labels, and in particular drug allergy labels that include other drugs likely to associate with non-IgE mediated mast cell activation such as vancomycin and opioids. In the typical outpatient allergy clinical setting, we believe that our proposed criteria may be able to help differentiate cases of non-IgE mediated mast cell activation prevalently associated with FQ and not a contraindication to future treatment, from the much less common and typically FQ selective IgE-mediated cases where the FQ should be avoided. Hence, we can precisely target patients with IgE versus non-IgE mediated FQ allergy who would have drug-specific, dose-independent skin test positivity at the 0.005 mg/mL concentration. Furthermore, our results support the safety of the strategy of using a single rather than a graded challenge and using a lower dose (200 to 250 mg) of a FQ for OC that would be adequate to rule out a true IgE-mediated reaction, but low enough not to provoke a dose related reaction consistent with non-IgE mediated mast cell activation. Use of the lower therapeutic range of FQ in drug challenge is in keeping with other delabeling studies for antibiotic allergy.5, 9 It is, however, still possible for patients with a negative FQ oral challenge to experience a non-IgE mediated reaction in the future — as such reactions are stochastic to dose. We acknowledge that a higher evidence base is needed to support this but the practice of administering antihistamines to improve tolerance of non-IgE mediated reactions associated with FQ has biological plausibility, and we have recommended that patients take scheduled antihistamines. Of 23 patients that were exposed to future treatment courses of FQ, 15 (65%) were taking antihistamines throughout the course, and we postulate that this was beneficial in their tolerance. With regards to evaluating patients with a non-severe delayed reaction history, a negative single dose challenge to a FQ may not entirely exclude a potential delayed reaction as it may take several doses to reappear, particularly with a less recent reaction, or may not be provoked with a 200 to 250 mg dose.
In conclusion, others have previously questioned the utility of skin testing as a diagnostic modality for immediate reactions associated with FQ because of the high degree of non-IgE mediated mast cell activation that has impaired the ability to interpret skin testing by currently used criteria. Our data is reassuring in suggesting that most patients with non-anaphylactic immediate histories such as urticaria will tolerate single dose 200 to 250 mg challenge with a FQ and further tolerate therapeutic courses of FQ. Similar to other drugs when skin testing utility is unproven or not available to a clinician in patients who do not report a history of anaphylaxis, single or graded oral challenge to FQ may be safely applied in the outpatient setting. For patients whose histories are consistent with anaphylaxis, we propose that a distinct set of criteria for FQ skin test positivity are needed and that these criteria can further help identify patients who should be excluded from a specific FQ but may tolerate an alternative one. We demonstrate a set of criteria that appear to safely identify patients who are eligible for challenge, many of whom would otherwise be excluded by current skin testing criteria.
Supplementary Material
Clinical Implications:
For patients who report fluoroquinolone allergy, we suggest specific restrictive intradermal skin test criteria are useful to identify those with true anaphylaxis as well as those where drug challenge can be safely applied.
Sources of Funding:
Dr. Stone receives funding from AHRQ/PCORI 1K12HS026395-01.
Dr. Phillips receives funding from the National Institutes of Health (1P50GM115305-01, R21AI139021, R34AI136815, 1 R01 HG010863-01) and the National Health and Medical Research Foundation of Australia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
IRB: This study was done under IRB approved protocols from Vanderbilt University Medical Center, Vanderbilt IRB #161455.
Conflicts of Interest: The authors declare that they have no relevant conflicts of interest
References
- 1.Doña I, Moreno E, Pérez-Sánchez N, Andreu I, Hernández Fernandez de Rojas D, Torres MJ. Update on Quinolone Allergy. Current allergy and asthma reports 2017; 17:56. [DOI] [PubMed] [Google Scholar]
- 2.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015; 519:237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang B, Knowles SR, Weber E. Immediate hypersensitivity to moxifloxacin with tolerance to ciprofloxacin: report of three cases and review of the literature. Ann Pharmacother 2010; 44:740–5. [DOI] [PubMed] [Google Scholar]
- 4.Uyttebroek AP, Sabato V, Bridts CH, De Clerck LS, Ebo DG. Moxifloxacin hypersensitivity: Uselessness of skin testing. The journal of allergy and clinical immunology. In practice2015; 3:443–5. [DOI] [PubMed] [Google Scholar]
- 5.Krantz MS, Stone CA, Abreo A, Phillips EJ. Oral Challenge with Trimethoprim-Sulfamethoxazole in Patients with “Sulfa” Antibiotic Allergy. J Allergy Clin Immunol Pract 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbaud A, Weinborn M, Garvey LH, Testi S, Kvedariene V, Bavbek S, et al. Intradermal Tests With Drugs: An Approach to Standardization. Front Med (Lausanne) 2020; 7:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010; 105:259–73. [DOI] [PubMed] [Google Scholar]
- 8.Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, et al. Skin test concentrations for systemically administered drugs -- an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 2013; 68:702–12. [DOI] [PubMed] [Google Scholar]
- 9.Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and Management of Penicillin Allergy: A Review. JAMA 2019; 321:188–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


