Summary
Extreme longevity has evolved multiple times during the evolution of mammals, yet its underlying molecular mechanisms remain largely underexplored. Here, we compared the evolution of 115 aging-related genes in 11 long-lived species and 25 mammals with non-increased lifespan (control group) in the hopes of better understanding the common molecular mechanisms behind longevity. We identified 16 unique positively selected genes and 23 rapidly evolving genes in long-lived species, which included nine genes involved in regulating lifespan through the insulin/IGF-1 signaling (IIS) pathway and 11 genes highly enriched in immune-response-related pathways, suggesting that the IIS pathway and immune response play a particularly important role in exceptional mammalian longevity. Interestingly, 11 genes related to cancer progression, including four positively selected genes and seven genes with convergent amino acid changes, were shared by two or more long-lived lineages, indicating that long-lived mammals might have evolved convergent or similar mechanisms of cancer resistance that extended their lifespan. This suggestion was further corroborated by our identification of 12 robust candidates for longevity-related genes closely related to cancer.
Keywords: mammals, longevity, positive selection, IIS pathway, immune response, cancer resistance
Graphical abstract
Public summary
-
•
Evolution analyses of 115 aging-related genes exploring natural longevity in mammals
-
•
Positively selected genes & rapidly evolved genes enriched in IIS and immune pathways
-
•
Convergent mutations in genes associated with cancer in long-lived species
-
•
Evolution of longevity through cancer resistance in long-lived mammals
Introduction
Extant mammals differ dramatically in their maximum lifespans, ranging from a little over 1 year (e.g., forest shrews, Myosorex varius) to more than 200 years (e.g., bowhead whales, Balaena mysticetus), a difference of more than 100-fold.1 In general, larger species tend to live longer than smaller ones, presumably due to higher intrinsic fitness (i.e., stress resistance) and a lack of apex predators.2 For example, the bowhead whale has an estimated maximum lifespan of 211 years and a body mass of more than 100 tons.3,4 The African elephant (Loxodonta africana), the largest land mammal, weighs more than 6 tons and lives up to 65 years.5 However, some species defy this apparent correlation between large body size and longevity. Brandt's bat (Myotis brandtii) weighs 5–20 g and lives for more than 40 years,6 while the naked mole rat (Heterocephalus glaber) lives for more than 30 years—ten times longer than other similar-sized rodents.7,8 Similar to large long-lived mammals, Brandt's bat and the naked mole rat reduce predation risk through flight/cave-dwelling and a subterranean lifestyle, respectively.9 To allow for cross-species comparisons of longevity, Austad and Fisher introduced the longevity quotient, maximum lifespan corrected for body size.10 Employing this variable, the longevity of many bats and subterranean rodents is striking. Thus, species such as the bowhead whale, African elephant, Brandt's bat, and naked mole rat are well positioned to evolve a longer lifespan.
To achieve longevity, species must evolve better mechanisms to attenuate aging (organismal senescence) and related diseases (e.g., cancer). The current consensus is that aging in diverse species is manifested by distinct hallmarks and that the aging process (and lifespan) can be modulated in various ways—by environmental, genetic, or pharmacological interventions.11 Over the past few decades, numerous aging-related genes have been identified from experiments on model animals (e.g., mouse, fruit fly, and worm).11 However, we do not know whether some of these genes are involved in controlling lifespan variations during the evolution of species. In recent years, aging research has paid more attention to long-lived mammals.12, 13, 14, 15, 16 For example, the small-sized naked mole rat experienced unique coding changes in its HAS2 (Hyaluronan Synthase 2) gene and secretes high-molecular-mass hyaluronan, a polysaccharide that likely mediates early contact inhibition and contributes to cancer resistance.16 A comparative study of liver transcriptomics among mice, naked mole rats, and humans revealed that DNA-repair genes of long-lived species are upregulated compared with those of short-lived mice,17 which agrees with the argument that DNA repair plays a vital role in longevity.11 A similar result was found in long-lived whales: genes linked to DNA repair and cancer resistance were found to be under positive selection and were found to have specific mutations in the bowhead whale and humpback whale (Megaptera novaeangliae).12,13 Importantly, 12–20 copies of the tumor-suppressor gene TP53 were uniquely identified in the genome of elephants, helping to reduce their cancer incidence by increasing their cellular sensitivity to DNA damage.14
It is worth noting that lifespans may be extended by both specific adaptations and shared mechanisms. Better understanding of the latter requires identifying the molecular mechanisms that underlie extended lifespans across mammalian phylogeny, which in turn requires the analysis of aging-related genes shared by long-lived species. In this study, we considered the molecular evolution of aging-associated genes in GenAge, a curated database of genes generated by surveying human disease data (e.g., genes associated with a longer lifespan in a population) and genetic perturbation experiments in animal models.1,18 Making use of 115 aging-related genes and 36 species spanning 14 mammal orders, we searched for the genes or pathways that may contribute to extending lifespan in mammals.
Results
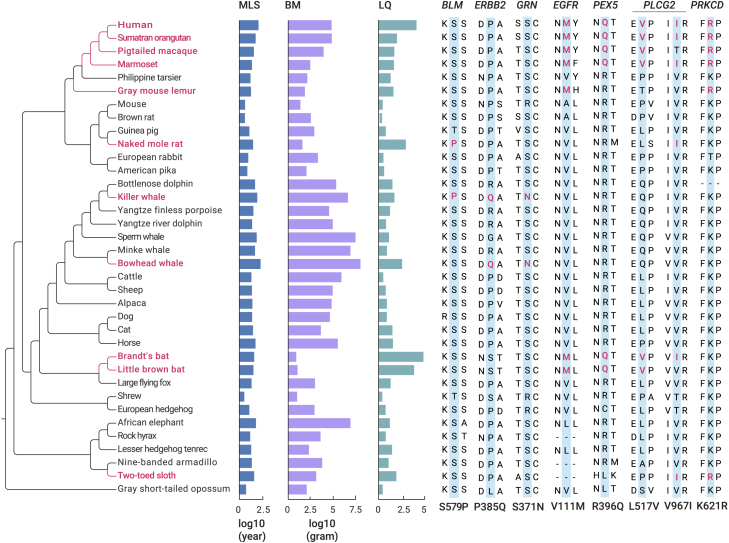
The maximum lifespan and body mass of 987 mammalian species were obtained from the AnAge database.1 We calculated each species' longevity quotient based on the allometric equation for all mammals (see supplemental materials and methods).19 The mean longevity quotient value ± standard deviation (SD) for all mammals was 1 ± 0.57 (Table 1). In our 36-species dataset, 11 species had a longevity quotient value of >1.57 and were classified as long-lived: human (Homo sapiens), Sumatran orangutan (Pongo abelii), pigtailed macaque (Macaca nemestrina), common marmoset (Callithrix jacchus), gray mouse lemur (Microcebus murinus), naked mole rat (H. glaber), bowhead whale (B. mysticetus), killer whale (Orcinus orca), Brandt's bat (M. brandtii), little brown bat (Myotis lucifugus), and Hoffman's two-toed sloth (Choloepus hoffmanni) (Figure 1).
Table 1.
Mean values of MLS (maximum lifespan) and LQ (longevity quotient) computed using 987 species' records from the AnAge database
| na | MLS mean | MLS SDb | MLS limits | LQ mean | LQ SD | LQ limits | |
|---|---|---|---|---|---|---|---|
| Peramelemorphia | 9 | 5.93 | 1.96 | 3.97–7.89 | 0.37 | 0.12 | 0.25–0.49 |
| Monotremata | 3 | 37.77 | 13.77 | 24.00–51.54 | 1.91 | 0.60 | 1.31–2.51 |
| Diprotodontia | 52 | 15.85 | 6.31 | 9.54–22.16 | 0.79 | 0.26 | 0.53–1.05 |
| Dasyuromorphia | 19 | 6.32 | 2.39 | 3.93–8.71 | 0.50 | 0.13 | 0.37–0.63 |
| Primates | 153 | 31.31 | 12.67 | 18.64–43.98 | 1.60 | 0.43 | 1.17–2.03 |
| Scandentia | 5 | 11.76 | 0.53 | 11.23–12.29 | 0.91 | 0.22 | 0.69–1.13 |
| Cetartiodactyla | 177 | 29.61 | 22.76 | 6.85–52.37 | 0.83 | 0.30 | 0.53–1.13 |
| Chiroptera | 88 | 17.62 | 7.77 | 9.85–25.39 | 1.77 | 0.86 | 0.91–2.63 |
| Lagomorpha | 12 | 10.07 | 3.09 | 6.98–13.16 | 0.59 | 0.13 | 0.46–0.72 |
| Eulipotyphla | 17 | 5.40 | 3.51 | 1.89–8.91 | 0.44 | 0.17 | 0.27–0.61 |
| Rodentia | 230 | 9.45 | 5.68 | 3.77–15.13 | 0.68 | 0.33 | 0.35–1.01 |
| Afrotheria | 19 | 24.77 | 24.65 | 0.12–49.42 | 0.94 | 0.45 | 0.49–1.39 |
| Perissodactyla | 15 | 38.16 | 8.49 | 29.67–46.65 | 1.00 | 0.23 | 0.77–1.23 |
| Carnivora | 159 | 20.87 | 8.66 | 12.21–29.53 | 0.94 | 0.27 | 0.67–1.21 |
| Pilosa | 5 | 28.76 | 10.93 | 17.83–39.69 | 1.31 | 0.50 | 0.81–1.81 |
| Cingulata | 8 | 20.86 | 7.74 | 13.12–28.60 | 1.10 | 0.47 | 0.63–1.57 |
| Didelphimorphia | 16 | 4.88 | 1.84 | 3.04–6.72 | 0.38 | 0.15 | 0.23–0.53 |
| Total | 987 | 20.15 | 15.58 | 4.60–35.80 | 1.00 | 0.57 | 0.43–1.57 |
Number of species included in each order.
SD, standard deviation.
Figure 1.
The phylogeny of the mammals used for this study alongside their life-history traits
Long-lived mammals are marked on red on the left-hand side of the figure. Life-history traits, including maximum lifespan (MLS), body mass (BM), and longevity quotient (LQ), are displayed in the middle. Seven cancer-associated genes showed convergent amino acid substitutions within long-lived mammals, which are listed in the right-hand side of the figure (long-lived species-specific amino acid changes are colored red).
Selective pressure test of aging-related genes across mammals
Under lower adult mortality rates, selection will favor gene changes that confer a later maturity and longer lifespan.20,21 To test for divergent evolution patterns between the long-lived and control groups, we performed clade model C, revealing that 20% (23/115) of the genes in the long-lived group were rapidly evolving genes (Table 2). Of these genes, three (INSR, IRS1, and PIK3CB) are associated with the process of signal transduction by insulin receptor kinase and two (ATM [ataxia telangiectasia mutated]and ERCC6) with DNA repair. Moreover, nine genes (BCL2, CDC42, DGAT1, GRN, PIK3CB, PLCG2, STAT5A, STAT5B, and VCP) are involved in the immune process.
Table 2.
List of rapidly evolving genes in long-lived group identified using the clade model C
| Parameter estimates |
||||||||
|---|---|---|---|---|---|---|---|---|
| Gene | -lnLCmC | -lnLM2a_rel | p value | Proportion | ω0 | ω1 | Background ω | Foreground ω |
| ARNTL | 7,351.482 | 7,355.586 | 0.004 | p0 = 0.892; p1 = 0.005; p2 = 0.102 | 0.005 | 1.000 | 0.107 | 0.432 |
| ATM | 51,496.600 | 51,509.852 | 0.000 | p0 = 0.595; p1 = 0.063; p2 = 0.343 | 0.025 | 1.000 | 0.300 | 0.491 |
| BCL2 | 2,542.311 | 2,545.377 | 0.013 | p0 = 0.859; p1 = 0.000; p2 = 0.141 | 0.014 | 1.000 | 0.160 | 0.714 |
| CDC42 | 2,300.064 | 2,303.047 | 0.015 | p0 = 0.066; p1 = 0.000; p2 = 0.934 | 0.094 | 1.000 | 0.000 | 0.039 |
| DGAT1 | 8,002.016 | 8,007.764 | 0.001 | p0 = 0.765; p1 = 0.019; p2 = 0.216 | 0.008 | 1.000 | 0.164 | 0.413 |
| EFEMP1 | 8,311.676 | 8,315.715 | 0.004 | p0 = 0.758; p1 = 0.025; p2 = 0.217 | 0.010 | 1.000 | 0.185 | 0.412 |
| EGR1 | 9,962.475 | 9,964.822 | 0.030 | p0 = 0.717; p1 = 0.003; p2 = 0.280 | 0.005 | 1.000 | 0.127 | 0.205 |
| ERCC6 | 16,782.841 | 16,785.213 | 0.029 | p0 = 0.739; p1 = 0.033; p2 = 0.228 | 0.014 | 1.000 | 0.234 | 0.365 |
| FGF23 | 2,670.472 | 2,673.045 | 0.023 | p0 = 0.690; p1 = 0.023; p2 = 0.287 | 0.022 | 1.000 | 0.194 | 0.463 |
| GHR | 9,743.770 | 9,746.715 | 0.015 | p0 = 0.583; p1 = 0.041; p2 = 0.376 | 0.029 | 1.000 | 0.328 | 0.542 |
| GRN | 12,342.258 | 12,346.864 | 0.002 | p0 = 0.530; p1 = 0.136; p2 = 0.334 | 0.006 | 1.000 | 0.211 | 0.354 |
| HBP1 | 7,950.469 | 7,953.984 | 0.008 | p0 = 0.726; p1 = 0.026; p2 = 0.249 | 0.003 | 1.000 | 0.189 | 0.417 |
| HESX1 | 2,960.034 | 2,965.417 | 0.001 | p0 = 0.541; p1 = 0.112; p2 = 0.347 | 0.011 | 1.000 | 0.210 | 0.771 |
| INSR | 27,632.398 | 27,638.724 | 0.000 | p0 = 0.811; p1 = 0.004; p2 = 0.185 | 0.006 | 1.000 | 0.121 | 0.197 |
| IRS1 | 19,448.998 | 19,451.540 | 0.024 | p0 = 0.834; p1 = 0.015; p2 = 0.151 | 0.007 | 1.000 | 0.157 | 0.244 |
| NCOR1 | 31,049.485 | 31,051.550 | 0.042 | p0 = 0.784; p1 = 0.017; p2 = 0.198 | 0.014 | 1.000 | 0.252 | 0.326 |
| PDGFRB | 22,001.509 | 22,005.606 | 0.004 | p0 = 0.732; p1 = 0.028; p2 = 0.240 | 0.010 | 1.000 | 0.199 | 0.296 |
| PIK3CB | 14,288.132 | 14,292.053 | 0.005 | p0 = 0.777; p1 = 0.008; p2 = 0.214 | 0.007 | 1.000 | 0.185 | 0.345 |
| PLCG2 | 24,791.961 | 24,795.709 | 0.006 | p0 = 0.810; p1 = 0.012; p2 = 0.177 | 0.009 | 1.000 | 0.142 | 0.214 |
| PTPN1 | 5,729.684 | 5,731.999 | 0.031 | p0 = 0.855; p1 = 0.010; p2 = 0.135 | 0.006 | 1.000 | 0.150 | 0.307 |
| STAT5A | 14,464.372 | 14,468.614 | 0.004 | p0 = 0.836; p1 = 0.004; p2 = 0.160 | 0.005 | 1.000 | 0.113 | 0.194 |
| STAT5B | 11,082.355 | 11,121.641 | 0.000 | p0 = 0.079; p1 = 0.000; p2 = 0.920 | 0.174 | 1.000 | 0.005 | 0.051 |
| VCP | 12,614.071 | 12,634.515 | 0.000 | p0 = 0.977; p1 = 0.000; p2 = 0.023 | 0.001 | 1.000 | 0.000 | 1.384 |
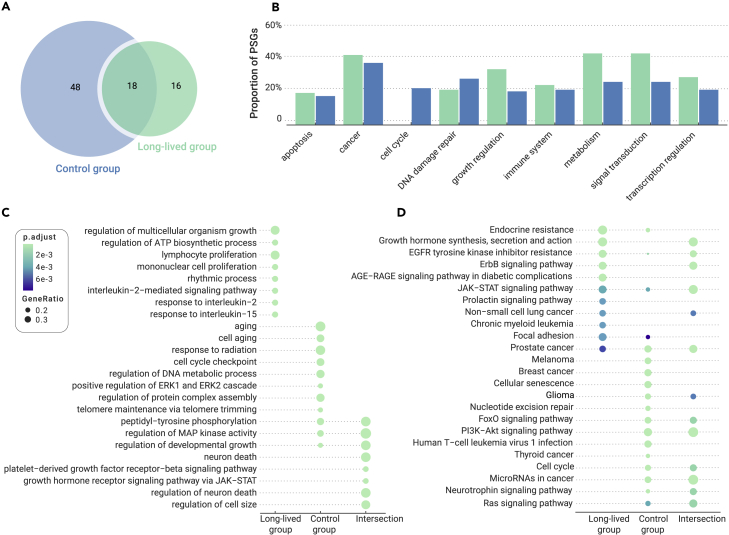
The branch-site model was further used to identify positively selected genes on each branch across the phylogeny. A total of 29.57% (34/115) of the aging-related genes were identified to be under positive selection in the long-lived group after p-value adjustment (Table S3). Of them, 18 genes were also identified in the control groups; however, 16 genes were in at least one of the 11 long-lived lineages (Figure 2A and Table S4). For example, five (CTGF, BCL2, GHRH, DBN1, and ERCC3) and two (CTGF and DBN1) genes were under positive selection along the branches leading to the little brown bat and Brandt's bat, respectively (Table S4). In addition, four positively selected genes were determined in two long-lived species: PDGFRB in the little brown bat and sloth; and CTGF, DBN1, and ABL1 in both the little brown bat and Brandt's bat (i.e., genus Myotis) (Table S3).
Figure 2.
Functional enrichment of positively selected genes in long-lived and control species
(A) Number of positively selected genes (PSGs) identified in the long-lived and control groups.
(B) Proportion of positively selected genes (PSGs) for gene function in the long-lived and control groups.
(C and D) GO and KEGG pathway enrichment of PSGs in long-lived and control groups. Top functional terms of biological process or pathways are shown. Circle sizes are proportional to the number of genes assigned to a pathway, and the color of the circle indicates the adjusted p value for each pathway.
The proportion of positively selected genes identified in the long-lived species was larger than that in the control group for genes related to immunity, metabolism, growth regulation, signal transduction, transcription regulation, cancer, and apoptosis, based on the GeneCards description (Figure 2B). In addition, we evaluated the functional enrichment of positively selected genes using gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) annotations. The 16 long-lived group-specific positively selected genes were significantly enriched for immune progress, such as lymphocyte proliferation, response to interleukin, and interleukin-2-mediated signaling pathway (Figure 2C). These genes were also over-represented in several KEGG pathways, including endocrine resistance (i.e., estrogen resistance in breast cancer), focal adhesion, and the AGE-RAGE signaling pathway in diabetic complications (Figure 2D). In contrast, the genes under positive selection in the control group were enriched for DNA repair, the cell cycle, ERK1 and ERK2 signaling, and nucleotide excision repair (Figures 2B–2D and Table S5). In addition, 18 positively selected genes shared by two groups were enriched for the regulation of mitogen-activated protein (MAP) kinase activity and the phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway (Figures 2C and 2D).
Convergent amino acid substitutions between long-lived species
To assess convergent evolution in long-lived species, we first reconstructed ancestral sequences for the internal nodes of the species tree to identify shared amino acid substitutions along lineages leading to extreme longevity based on the JTT-fgenes model. We then found three convergent amino acid changes in the distant species, including one change (BLM: S579P) in the naked mole rat and killer whale, and two substitutions (ERBB2: P385Q and GRN: S371N) in the lineages leading to the bowhead and killer whales (Figure 1). Furthermore, five long-lived group-specific unique amino acid changes were also determined in four genes: EGFR (V111M), PEX5 (R396Q), PLCG2 (L517V, V967I), and PRKCD (K621R) (Figure 1). For example, the long-lived primates and bats (genus Myotis) had three convergent substitutions in EGFR, PEX5, and PLCG2 (Figure 1).
Gene-phenotype coevolution
To assess the relationship between the rate of gene evolution and aging-associated life-history traits, we performed a univariate linear regression analysis of maximum lifespan and two other longevity-associated traits (body mass and longevity quotient) obtained from AnAge. As expected,22 the analyses revealed a significant association: maximum lifespan covaries with body mass (R2 = 0.47, p < 2.17 × 10−6) and longevity quotient (R2 = 0.57, p < 5.19 × 10−8). Multiple linear regression followed by a type I analysis of variance revealed that longevity quotient was the best predictor, accounting for 50% of the maximum lifespan variance (p < 2 × 10−16), whereas body mass accounted for 47% of the remaining variance and the remainder (3%) was residual error (Figure S1).
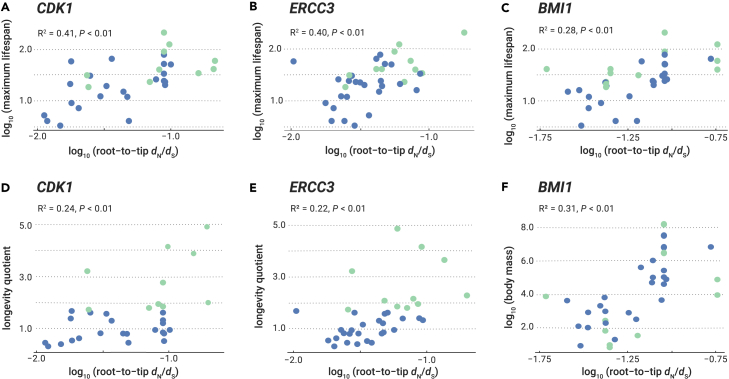
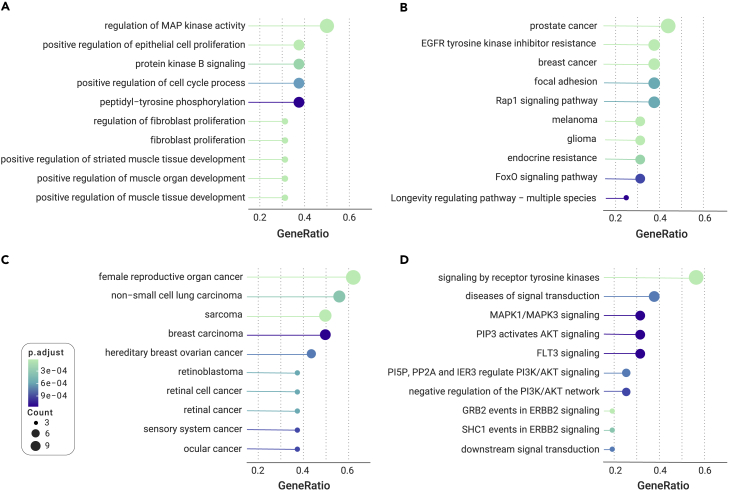
Pagel's λ model, used to assess the phylogenetic signal, showed that phylogeny explained a high proportion of the variance in mammalian maximum lifespan (λ = 0.97), body mass (λ = 0.99), and longevity quotient (λ = 0.97) (Table S6). We next employed the phylogenetic generalized least-squares method to assess correlations between the evolutionary rate of genes (root-to-tip dN/dS) and longevity-associated traits. Phylogenetic generalized least-squares analysis revealed that the evolutionary rates of nine genes (ARNTL, ATM, BMI1, CDK1, CTNNB1, ERCC3, ERCC5, NRG1, and STAT5A) are associated with maximum lifespan (Table S7). Seven genes (BMI1, CTNNB1, E2F1, ERBB2, IGF1, IGF1R, and PDGFB) exhibited an association with body mass, while four (CDK1, ERCC3, HRAS, and INSR) showed an association with longevity quotient (Table S7). These 16 genes associated with one or more longevity-associated phenotypes were regarded as longevity-associated genes. Notably, the evolutionary rates of both CDK1 and ERCC3 showed an association with both maximum lifespan and longevity quotient, while the rate of BMI1 was associated with maximum lifespan and body mass (Figure 3). Interestingly, a negative correlation was found between body mass and the evolutionary rates of two genes, IGF1R and IGF1 (Table S7). Specifically, these 16 longevity-associated genes were particularly enriched in several KEGG pathways, including prostate cancer, breast cancer, and the Rap1 signaling pathway. In addition, the 16 longevity-associated genes were also significantly assigned to GO terms such as regulation of cell-cycle processes and cell aging, disease ontology (DO) terms including female reproductive organ cancer, sarcoma, and hereditary breast ovarian cancer, and Reactome pathways, including signaling by receptor tyrosine kinases and diseases of signal transduction (Figure 4).
Figure 3.
Root-to-tip dN/dS values of genes with significant correlation with three life-history traits
Scatterplots of significant relationships between log10 (maximum lifespan) (A–C), log10 (body mass) (F), longevity quotient (D and E), and root-to-tip dN/dS values. Green and blue points represent long-lived and control species, respectively.
Figure 4.
Pathway enrichment of genes with significant correlation with longevity-associated traits
Enriched (A) GO terms, (B) KEGG pathways, (C) DO terms, and (D) Reactome pathways of genes correlate with the longevity-associated traits (i.e., maximum lifespan, longevity quotient, and body mass). Only the top ten terms are shown. Circle sizes are proportional to the number of genes assigned to a pathway, and the color of the circle indicates the adjusted p value for each pathway.
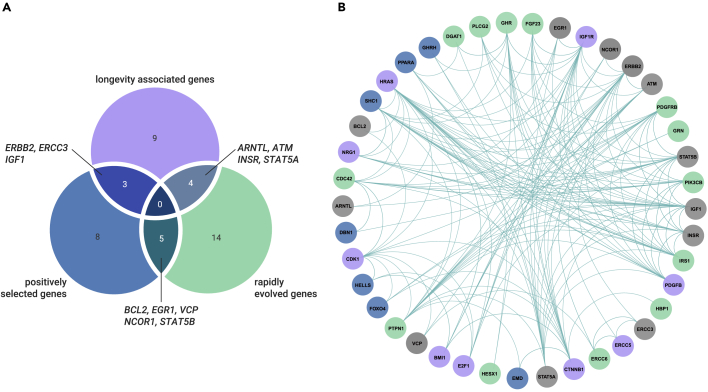
Overlap among different datasets
Our results revealed 23 rapidly evolving genes, 16 positively selected genes, and 16 longevity-associated genes in the long-lived group. There was some overlap among the three types of genes: five genes (BCL2, EGR1, NCOR1, STAT5B, and VCP) were identified as both positively selected and rapidly evolving genes, four (ARNTL, ATM, INSR, and STAT5A) were both rapidly evolving and longevity-associated genes, and three (ERBB2, ERCC3, and IGF1) were both positively selected and longevity-associated genes (Figure 5A). Importantly, these overlapping genes were involved in DNA repair (ERCC3 and ATM), immune processes (BCL2, STAT5A, STAT5B, and VCP), and the insulin/IGF-1 signaling (IIS) pathway (IGF1 and INSR), which are essential for inhibiting tumorigenesis or longevity. Therefore, these 12 genes can be considered robust candidates of longevity-related genes. We further used the protein-protein interactions database STRING (http://www.string-db.org) to explore the interactions among the rapidly evolving genes, positively selected genes, and longevity-associated genes, and found that all these genes interacted with each other (p < 1.0 × 10−16, Figure 5B). Specifically, the top genes with relatively high degrees of connectivity (≥10 degrees) were involved in the IIS pathway: GHR (11), IRS1 (10), PTPN1 (11), and SHC1 (13). In addition, three genes related to DNA repair interacted with each other: ERCC3 (2), ERCC5 (2), and ERCC6 (3).
Figure 5.
Overview of 12 robust longevity-associated genes
(A) Venn diagram of overlaps among positively selected genes, longevity-associated genes, and rapidly evolving genes.
(B) Protein-protein interaction network generated using STRING. Nodes for positively selected genes, longevity-associated genes, rapidly evolving genes, and overlap genes are colored blue, purple, green, and gray, respectively. Lines between each node indicate inferred/experimentally demonstrated biological associations.
Discussion
Long lifespan evolved multiple times during the evolution of mammals. The last decade has seen an explosion in the number of genome assemblies and amount of genomic data from several long-lived mammals, and these have revealed shared and lineage-specific changes that facilitate a long lifespan by enhancing homeostasis throughout life. Sometimes this involves the changes directly resisting tumor development or progression, as is the case for the duplication of the tumor-suppressor gene TP53 in elephants (12–20 copies) and FBXO31 (Forkhead box protein 31) in Brandt's bat (57 copies).14,15 In this study, we examined the evolution of a set of 115 genes, designated “aging-associated” genes in the GenAge database,1,18 spanning 36 mammals in 14 orders.
The IIS pathway and immune genes contribute to extending longevity
Our results identified 16 positively selected genes and 23 rapidly evolving genes in the long-lived species, which included nine genes (growth hormone receptor [GHR], GHRH, IGF1, IRS1, INSR, SHC1, PIK3CB, PTPN1, and FOXO4 [Forkhead box protein 4]) involved in the IIS pathway, a key lifespan regulatory pathway.23 Multiple genetic manipulations that attenuate signaling intensity at different levels of the IIS pathway extend the lifespan of mice.24, 25, 26 For example, previous studies showed that lower IGF1 levels and GHRH knockout in mice can extend their lifespan.26 Mice with an adipose-specific knockout of INSR live 18% longer than those without the knockout.25 In addition, mice heterozygous for IGF1R knockout live 26% longer than wild-type mice.27 Interestingly, consistent with our findings, a number of genes in the IIS pathway were found to have unique sequence and expression changes in long-lived species. For example, unique amino acid deletion or replacement in the GHR was identified in the small-body-size and long-lived bat species.15 Interestingly, previous studies have revealed that mutations or deficiencies of the GHR result in human Laron-type dwarfism and increased resistance to cancer in humans and mice.28, 29, 30 In addition, the expression of insulin receptor (INSR) protein, which regulates energy metabolism by activating the insulin signaling pathway, was recently reported to be positively correlated with longevity across mammals.31 Taken together, genes involved in the IIS pathway were identified to be under accelerated evolution or positive selection in the long-lived lineages, which may be contributing to extending lifespan in mammals.
Our results also revealed that five positively selected genes (BCL2, VCP, SHC1, EGR1, and STAT5B) and nine rapidly evolving genes (BCL2, CDC42, DGAT1, GRN, PIK3CB, PLCG2, STAT5A, STAT5B, and VCP) identified in the long-lived species were highly enriched in immune-associated pathways, including lymphocyte proliferation, leukocyte proliferation, and the interleukin-2-mediated signaling pathway. For instance, in peripheral immune cells, PLCG2 has been implicated in the signaling pathways downstream of the B cell receptor and is thought to modulate the functions of macrophages, neutrophils, and natural killer cells through the Fc receptor.32 As is well known, the immune system is often under strong selective pressure and has important implications for aging and disease resistance.33 Similarly, previous studies identified immune-response genes to be under positive selection, expanded, and upregulated in long-lived bats, blind mole rats, and naked mole rats.34 Importantly, the expression of immune-response genes in the liver across 33 mammalian species was positively related to maximum lifespan.35
In addition, comparative genomic analysis of the short-lived African turquoise killifish and exceptionally long-lived mammals revealed that some aging and longevity candidates—such as CREBBP, CGNL1, and IGF1R—were under positive selection in both short- and long-lived species, suggesting that the same gene could underlie the evolution of both exceptionally extended and shortened lifespans.36 Similarly, 18 aging-related genes were detected to be under positive selection in both long-lived and control groups. These genes were significantly enriched in the PI3K-Akt signaling pathway, which is critical to the cell-cycle process and is associated with cellular quiescence, proliferation, cancer, and longevity.37
Genes related to cancer progression exhibit molecular convergence in long-lived species
Convergent phenotypic evolution provides unique opportunities for studying how genomes encode phenotypes. Convergence was observed at different molecular levels, such as amino acid substitutions, the same positively selected genes, and convergent shifts in amino acid preference.38 The present study revealed that four positively selected genes (CTGF, DBN1, ABL1, and PDGFRB) related to longevity were uniquely shared by long-lived lineages. Three of these (CTGF, DBN1, and ABL1) were examined in the long-lived little brown bat and Brandt's bat (genus Myotis). ABL1 (ABL proto-oncogene 1 non-receptor tyrosine kinase) is an oncogene that encodes a protein tyrosine kinase involved in various cellular processes, including cell division and DNA repair.39 PDGFRB (platelet-derived growth factor receptor β) was determined to be under positive selection in the little brown bat and Hoffman's two-toed sloth. Previous studies showed that PDGFRB stimulates cell proliferation and tumor migration through an array of signaling pathways, such as MAP kinases, PI3K, and STAT (signal transducers and activators of transcription).40
In addition, three convergent amino acid substitutions in three genes (GRN, ERBB2, and BLM) were identified in the long-lived group. These genes are associated with cancer incidence and DNA repair. For example, GRN (granulin, a growth factor)-knockout mice exhibited decreased survival—with less than 50% of animals living more than 2 years—and signs of cellular aging.41 ERBB2, commonly referred to as HER2, was overexpressed in 20%–30% of invasive breast carcinomas.
Moreover, five specific amino acid changes in four genes (EGFR, PEX5, PLCG2, and PRKCD) were observed in long-lived species. Among them, EGFR was associated with tumorigenesis, and PEX5, PLCG2, and PRKCD were associated with immune processes. Thus, convergent signatures in more than 11 genes related to cancer progression—four positively selected genes and seven genes with convergent amino acid changes—were found in two or more long-lived lineages, suggesting that long-lived mammals might have evolved convergent or similar mechanisms for cancer resistance in response to increased longevity.
Evolution of longevity through cancer resistance
The risk of cancer is a major challenge for increasing lifespan in mammals. Previous studies have shown that long-lived mammals have evolved specific mechanisms to protect themselves from cancer invasion. For instance, the two longest-living subterranean rodent species, the naked mole rat and blind mole rat, were found to resist cancer by secreting high-molecular-mass hyaluronan to mediate early contact inhibition and by using interferon secretion to induce cell death, respectively.42,43
Most notably, the tumor-suppressing TP53 gene might function differently in blind mole rats and another group of long-lived species, elephants. It was found that an amino acid change in the p53 protein of blind mole rats (R174K in human) favors cell-cycle arrest over apoptosis to adapt to the rat's hypoxic subterranean environment.44 However, massive expansion of the many copies of TP53 identified in elephants was suggested to increase cellular sensitivity to DNA damage by triggering p53-dependent apoptosis, which leads to efficient removal of mutant cells.14
Previous studies have also shown that many genes related to cancer control (including DNA damage and repair, immune response, and tumor suppression) evolved under positive selection, duplication, and amino acid changes in several long-lived lineages, suggesting that they share a mechanism. Positive selection of the pro-apoptotic gene FOXO3 and tumor-suppressor gene PRDM1 (positive regulatory domain I), and the specific mutation of the DNA-repair enzymes ERCC1 (excision repair cross-complementation group 1) was identified in long-lived bowhead and humpback whales;12,13 on the other hand, in blind mole rats and microbats, inflammation-regulation-related genes (e.g., Ifnb1, Mx1, and c-REL) showed positive selection, and gene families involved in immune response underwent gene expansion.34
In our study, 12 robust candidates for longevity-related genes identified in the long-lived lineages were involved in DNA repair (ERCC3 and ATM), immune processes (BCL2, STAT5A, STAT5B, and VCP), and the IIS pathway (IGF1 and INSR). Interestingly, 8 of these 12 candidates are known cancer genes according to the COSMIC v9245 and TSGene 2.046 databases: five tumor-suppressor genes (ATM, EGR1, IGF1, STAT5A, and NOCR1); two oncogenes (BCL2 and ERBB2); and STAT5B, which is classified as both a tumor-suppressor gene and an oncogene. For example, EGR1 (early growth response 1), detected to be under positive selection in the long-lived Sumatran orangutan, upregulates the expression of TP53 to induce apoptosis in cancer cells.47 STAT5B (signal transducer and activator of transcription 5B), identified to be under positive selection and rapid evolution in the long-lived lineages, has been shown to activate STAT5, which is associated with the suppression of antitumor immunity and an increase in the proliferation, invasion, and survival of tumor cells.48 ATM is a key DNA-damage response gene that commonly mutates in cancer; it functions as a regulator of a wide variety of downstream proteins, including the tumor-suppressor proteins TP53 and BRCA1.49 Similarly, ATM was also identified to be under positive selection in the genus Myotis.50 As mentioned above, a number of genes involved in cancer-related pathways have evolved via the same or different evolutionary pathways in individual or multiple long-lived lineages, suggesting that cancer resistance could be achieved through lineage-specific adaptations or common mechanisms to extend lifespan. Of course, functional experiments are needed to test whether the candidate cancer-related genes have higher cancer-resistance activity in the long-lived mammals compared with short-lived counterparts; such experiments are important in part because they may provide new strategies to extend the lifespan of humans.
Conclusion
The striking variability in lifespans across the mammalian phylogeny provides an ideal dataset to investigate the evolution of extended lifespan (longevity) and aging. Using mammalian comparative genomics, we juxtaposed 11 long-lived species with 25 shorter-lived counterparts. Our findings support our hypothesis that the IIS pathway and immune regulation play a particularly important role in exceptional mammalian longevity. Eleven cancer-related genes were found to have convergent signatures in the long-lived species, indicating functional convergence or similar anticancer mechanisms in response to increased longevity in animals. Importantly, we identified 12 robust candidates for longevity-related genes that were closely related to cancer, which corroborated the notion that long-lived mammals have evolved effective anticancer mechanisms to extend their lifespan. Together, these findings provide insights into how evolution reversibly adjusts lifespan and presents candidate genes and pathways for further experimental exploration.
Materials and methods
See supplemental information for details.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NSFC, grant nos. 32070409, 31772448 to S.X., 31872219 to W.R.), the Key Project of the NSFC (grant nos. 32030011, 31630071 to G.Y.), National Key Programme of Research and Development of China, Ministry of Science and Technology (grant no. 2016YFC0503200 to G.Y. and S.X.), the Priority Academic Program Development of Jiangsu Higher Education Institutions to G.Y. and S.X., and the Qinglan project of Jiangsu Province to S.X. These funding bodies played no role in study design, data collection, analysis, interpretation of data, and writing the manuscript. We are particularly grateful to Dr. Yan-bo Sun (Yunnan University, Kunming, Yunnan, China) for the suggestion of data analysis. Many thanks are also given to Zepeng Zhang, Simin Chai, Yuan Mu, and Weijian Guo for support and discussions.
Author contributions
S.X. and G.Y. designed the study. Z.Y. was responsible for data collection and analysis. S.X. and Z.Y. drafted the manuscript. I.S., S.X., and G.Y. revised the manuscript. M.Y. participated in data collection. D.S. contributed to data analysis. R.T. and W.R. assisted with manuscript editing. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published Online: April 28, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xinn.2021.100108.
Contributor Information
Guang Yang, Email: gyang@njnu.edu.cn.
Shixia Xu, Email: xushixia78@163.com.
Lead contact website
Supplemental information
References
- 1.Tacutu R., Thornton D., Johnson E., et al. Human aging genomic resources: new and updated databases. Nucleic Acids Res. 2018;46:D1083–D1090. doi: 10.1093/nar/gkx1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tollis M., Boddy A.M., Maley C.C. Peto’s Paradox: how has evolution solved the problem of cancer prevention? BMC Biol. 2017;15:1–5. doi: 10.1186/s12915-017-0401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John C., Bockstoce J.R. Two historical weapon fragments as an aid to estimating the longevity and movements of bowhead whales. Polar Biol. 2008;31:751–754. [Google Scholar]
- 4.George J.C., Bada J., Zeh J., et al. Age and growth estimates of bowhead whales (Balaena mysticetus) via aspartic acid racemization. Can. J. Zool. 1999;77:571–580. [Google Scholar]
- 5.Wiese R.J., Willis K. Calculation of longevity and life expectancy in captive elephants. Zoo Biol. 2004;23:365–373. [Google Scholar]
- 6.Podlutsky A.J., Khritankov A.M., Ovodov N.D., Austad S.N. A new field record for bat longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:1366–1368. doi: 10.1093/gerona/60.11.1366. [DOI] [PubMed] [Google Scholar]
- 7.Kim E.B., Fang X., Fushan A.A., et al. Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature. 2011;479:223–227. doi: 10.1038/nature10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J. Comp. Physiol. B. 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- 9.Holmes D.J., Austad S.N. Fly now, die later: life-history correlates of gliding and flying in mammals. J. Mammal. 1994;75:224–226. [Google Scholar]
- 10.Austad S. Methusaleh's Zoo: how nature provides us with clues for extending human health span. J. Comp. Pathol. 2010;142:S10–S21. doi: 10.1016/j.jcpa.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Otín C., Blasco M.A., Partridge L., et al. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tollis M., Robbins J., Webb A.E., et al. Return to the sea, get huge, beat cancer: an analysis of cetacean genomes including an assembly for the humpback whale (Megaptera novaeangliae) Mol. Biol. Evol. 2019;36:1746–1763. doi: 10.1093/molbev/msz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keane M., Semeiks J., Webb A.E., et al. Insights into the evolution of longevity from the bowhead whale genome. Cell Rep. 2015;10:112–122. doi: 10.1016/j.celrep.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sulak M., Fong L., Mika K., et al. TP53 copy number expansion is associated with the evolution of increased body size and an enhanced DNA damage response in elephants. eLife. 2016;5:e11994. doi: 10.7554/eLife.11994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seim I., Fang X., Xiong Z., et al. Genome analysis reveals insights into physiology and longevity of the Brandt’s bat Myotis brandtii. Nat. Commun. 2013;4:1–8. doi: 10.1038/ncomms3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian X., Azpurua J., Hine C., et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013;499:346–349. doi: 10.1038/nature12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacRae S.L., Croken M.M., Calder R., et al. DNA repair in species with extreme lifespan differences. Aging (Albany NY) 2015;7:1171. doi: 10.18632/aging.100866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Magalhães J.P., Toussaint O. GenAge: a genomic and proteomic network map of human aging. FEBS Lett. 2004;571:243–247. doi: 10.1016/j.febslet.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 19.De Magalhães J.P., Costa J., Church G.M. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:149–160. doi: 10.1093/gerona/62.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charnov E.L. Oxford University Press; 1993. Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology. [Google Scholar]
- 21.De Magalhães J.P., Church G.M. Analyses of human-chimpanzee orthologous gene pairs to explore evolutionary hypotheses of aging. Mech. Aging Dev. 2007;128:355–364. doi: 10.1016/j.mad.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Magalhães J.P., Costa J. A database of vertebrate longevity records and their relation to other life-history traits. J. Evol. Biol. 2009;22:1770–1774. doi: 10.1111/j.1420-9101.2009.01783.x. [DOI] [PubMed] [Google Scholar]
- 23.Kenyon C. The first long-lived mutants: discovery of the insulin/IGF-1 pathway for aging. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:9–16. doi: 10.1098/rstb.2010.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selman C., Lingard S., Choudhury A.I., et al. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- 25.Blüher M., Kahn B.B., Kahn C.R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 26.Shimokawa I., Higami Y., Utsuyama M., et al. Life span extension by reduction in growth hormone-insulin-like growth factor-1 axis in a transgenic rat model. Am. J. Pathol. 2002;160:2259–2265. doi: 10.1016/S0002-9440(10)61173-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holzenberger M., Dupont J., Ducos B., et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 28.David A., Hwa V., Metherell L.A., et al. Evidence for a continuum of genetic, phenotypic, and biochemical abnormalities in children with growth hormone insensitivity. Endocr. Rev. 2011;32:472–497. doi: 10.1210/er.2010-0023. [DOI] [PubMed] [Google Scholar]
- 29.Ikeno Y., Hubbard G.B., Lee S., et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J. Gerontol. A. Biol. Sci. Med. Sci. 2009;64:522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guevara-Aguirre J., Balasubramanian P., Guevara-Aguirre M., et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma S., Upneja A., Galecki A., et al. Cell culture-based profiling across mammals reveals DNA repair and metabolism as determinants of species longevity. eLife. 2016;5:e19130. doi: 10.7554/eLife.19130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D., Feng J., Wen R., et al. Phospholipase Cγ2 is essential in the functions of B cell and several Fc receptors. Immunity. 2000;13:25–35. doi: 10.1016/s1074-7613(00)00005-4. [DOI] [PubMed] [Google Scholar]
- 33.Barreiro L.B., Quintana-Murci L. From evolutionary genetics to human immunology: how selection shapes host defence genes. Nat. Rev. Genet. 2010;11:17–30. doi: 10.1038/nrg2698. [DOI] [PubMed] [Google Scholar]
- 34.Ma S., Gladyshev V.N. Molecular signatures of longevity: insights from cross-species comparative studies. Semin. Cell Dev. Biol. 2017;70:190–203. doi: 10.1016/j.semcdb.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fushan A.A., Turanov A.A., Lee S.G., et al. Gene expression defines natural changes in mammalian lifespan. Aging Cell. 2015;14:352–365. doi: 10.1111/acel.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valenzano D.R., Benayoun B.A., Singh P.P., et al. The African turquoise killifish genome provides insights into evolution and genetic architecture of lifespan. Cell. 2015;163:1539–1554. doi: 10.1016/j.cell.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie Y., Shi X., Sheng K., et al. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia. Mol. Med. Rep. 2019;19:783–791. doi: 10.3892/mmr.2018.9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hao Y., Qu Y., Song G., Lei F. Genomic insights into the adaptive convergent evolution. Curr. Genomics. 2019;20:81–89. doi: 10.2174/1389202920666190313162702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khatri A., Wang J., Pendergast A.M. Multifunctional Abl kinases in health and disease. J. Cell. Sci. 2016;129:9–16. doi: 10.1242/jcs.175521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Östman A., Heldin C.H. PDGF receptors as targets in tumor treatment. Adv. Cancer Res. 2007;97:247–274. doi: 10.1016/S0065-230X(06)97011-0. [DOI] [PubMed] [Google Scholar]
- 41.Wils H., Kleinberger G., Pereson S., et al. Cellular aging, increased mortality and FTLD-TDP-associated neuropathology in progranulin knockout mice. J. Pathol. 2012;228:67–76. doi: 10.1002/path.4043. [DOI] [PubMed] [Google Scholar]
- 42.Gorbunova V., Hine C., Tian X., et al. Cancer resistance in the blind mole rat is mediated by concerted necrotic cell death mechanism. Proc. Natl. Acad. Sci. U S A. 2012;109:19392–19396. doi: 10.1073/pnas.1217211109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delaney M., Nagy L., Kinsel M., Treuting P. Spontaneous histologic lesions of the adult naked mole rat (Heterocephalus glaber): a retrospective survey of lesions in a zoo population. Vet. Pathol. 2013;50:607–621. doi: 10.1177/0300985812471543. [DOI] [PubMed] [Google Scholar]
- 44.Ashur-Fabian O., Avivi A., Trakhtenbrot L., et al. Evolution of p53 in hypoxia-stressed Spalax mimics human tumor mutation. Proc. Natl. Acad. Sci. U S A. 2004;101:12236–12241. doi: 10.1073/pnas.0404998101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tate J.G., Bamford S., Jubb H.C., et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47:D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao M., Kim P., Mitra R., et al. TSGene 2.0: an updated literature-based knowledgebase for tumor suppressor genes. Nucleic Acids Res. 2016;44:D1023–D1031. doi: 10.1093/nar/gkv1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu J., Baron V., Mercola D., et al. A network of p73, p53 and Egr1 is required for efficient apoptosis in tumor cells. Cell Death Differ. 2007;14:436–446. doi: 10.1038/sj.cdd.4402029. [DOI] [PubMed] [Google Scholar]
- 48.Rani A., Murphy J.J. STAT5 in cancer and immunity. J. Interferon Cytokine Res. 2016;36:226–237. doi: 10.1089/jir.2015.0054. [DOI] [PubMed] [Google Scholar]
- 49.Weber A.M., Ryan A.J. ATM and ATR as therapeutic targets in cancer. Pharmacol. Ther. 2015;149:124–138. doi: 10.1016/j.pharmthera.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 50.Foley N.M., Hughes G.M., et al. Growing old, yet staying young: the role of telomeres in bats’ exceptional longevity. Sci. Adv. 2018;4:eaao0926. doi: 10.1126/sciadv.aao0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.