Main Text
This year's Nobel Prize in Physiology or Medicine was awarded to three scientists, each of whom played an integral role in the identification of the hepatitis C virus (HCV) and the characterization of the disease it causes, chronic hepatitis C (CHC). Initially Dr. Harvey Alter, a hematologist at the Clinical Center of the National Institutes of Health in Bethesda, Maryland recognized that there were many cases of post-transfusion hepatitis, unrelated to hepatitis A virus (HAV) or hepatitis B virus (HBV), termed non-A, non-B hepatitis. Dr. Michael Houghton, a microbiologist working at a small biotechnology company, Chiron in Emeryville, California, identified HCV as the causative agent of non-A, non-B hepatitis. After discovery of the HCV, Dr. Charles Rice, at Rockefeller University, New York worked on constructing a replication competent clone of the virus that was later used to demonstrate that the HCV alone was indeed the cause of CHC.
The story of the discovery of the HCV and the development of curative therapy is one of the remarkable success stories of modern medicine. Following the discovery of first, HBV and later, HAV, and the introduction of all-volunteer blood donors, rates of post-transfusion hepatitis fell from 33% to 6%. However, residual cases of post-transfusion hepatitis termed non-A, non-B hepatitis continued to occur and were suspected to be due to a transmissible agent. Dr. Alter along with colleagues conducted seminal chimpanzee inoculation studies, which provided proof that non-A, non-B hepatitis was caused by a transmissible agent.1 He carefully assembled a coded sample set of post-transfusion hepatitis cases that was used to validate the first HCV antibody assay. Dr. Alter also was instrumental in demonstrating that chronic HCV infection could result in liver fibrosis, cirrhosis, and, ultimately, liver cancer.
Dr. Houghton was recruited to Chiron in 1982 to work on identifying the causative agent of non-A, non-B hepatitis. Working with colleagues at Chiron he used multiple approaches to identify a possible infectious agent, but the levels of virus were too low to detect using the techniques they employed. Through a fortunate discussion with a laboratory neighbor, a blind cDNA immunoscreening approach, whereby fragments of nucleic acid from chimpanzees with non-A, non-B hepatitis were cloned and expressed in bacteria, was suggested. Human sera obtained from patients with active non-A, non-B hepatitis were then used to screen a bacterial expression cDNA library to search for a viral antigen. After screening over a million clones, one small clone termed 5-1-1 was obtained, which was shown to be derived from the HCV and was used to identify the remaining viral genome. The isolation of viral cDNA2 led to the rapid development of diagnostic serological and virological tests against HCV. The detection of anti-HCV antibodies and HCV RNA in the blood of infected persons allowed for identification of chronic infection and mass screening of blood products for HCV, which have greatly reduced post-transfusion hepatitis and person-to-person transmission.3
Following the identification of HCV, initial efforts to establish an in vitro culture model system using clinical HCV isolates to infect a variety cultured hepatocytes, including primary human hepatocytes, failed to demonstrate robust virus “replication.”4 Dr. Rice and others discovered that there was an absence of a structured sequence at the 3’ terminus from the initial cloned viral genome.5 Thereafter, Dr. Rice showed that in vitro transcribed viral RNA derived from a clinical isolate (strain H77) when injected into the liver of chimpanzee gave rise to HCV infection and clinical hepatitis, proving that HCV was the agent responsible for non-A, non-B hepatitis.6 However, this viral isolate was still not capable of replicating efficiently in cell culture. To develop a replicon system, Dr. Bartenschlager and colleagues introduced the 5’ untranslated region (UTR), non-structural proteins (NS) NS3-NS5B, and the 3′ UTR from strain Con1 in a selectable bicistronic construct.7 This was the first robust cell-culture model and permitted study of HCV replication and the virus-host interaction. Further knowledge gained on adaptive cell-culture mutations facilitated the expansion of the replicon system to cover almost all genotypes. Later, a unique replicon was generated based on an isolate derived from a case of fulminant hepatitis in a Japanese patient (JFH1).8 JFH1 was exceptional in that it was capable of efficient replication without the need for adaptive mutations. In 2005, the first in vitro HCV infection model (HCVcc) based on JFH1 became available, which allowed studies on the entire viral replication cycle and screening of therapeutic compounds (Figure 1).
Figure 1.
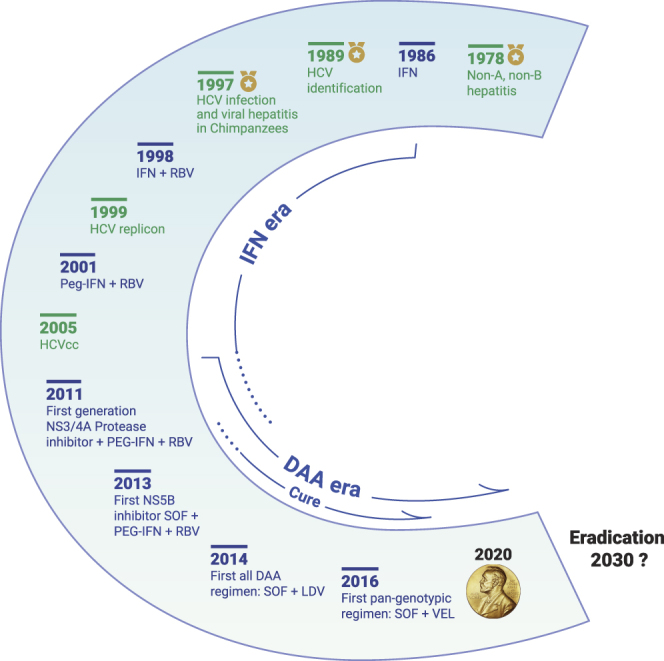
Milestones in HCV Research and Antiviral Treatment Development
Between 2013 and 2017, five NS3/4A protease inhibitors (simeprevir, ritonavir-boosted paritaprevir, grazoprevir, voxilaprevir, glecaprevir), six NS5A inhibitors (ledipasvir, ombitasvir, daclatasvir, elbasvir, velpatasvir, pibrentasvir), and two NS5B polymerase inhibitors (sofosbuvir, dasabuvir) were approved by the US Food and Drug Administration for treatment of chronic HCV infection in adults. Combinations of two or three direct-acting antivirals from different classes for 8 to 24 weeks achieve cure (sustained virological response) in more than 90% of people treated. IFN, interferon; RBV, ribavirin; PEG-IFN, pegylated interferon; HCVcc, cell-culture-derived HCV; SOF, sofosbuvir; LDV, ledipasvir; VEL, velpatasvir.
After the identification of HCV and the recognition that it was a substantial contributor to cirrhosis and hepatocellular carcinoma worldwide,1 the search for curative therapies began in earnest. Interferon (IFN), an antiviral, was one of the first agents to be tested and showed some early promise, but rates of viral eradication were low at less than 10%. Over the next two decades, the addition of ribavirin to IFN and later the development of pegylated forms of IFN greatly improved rates of viral eradication, but still such therapies were only effective in half of the treated patients, and treatment was poorly tolerated because of many adverse effects.9 Better treatment options with greater efficacy and better tolerance were needed. The availability of the replicon system and molecular characterization of key viral proteins, the viral protease (NS3), phosphoprotein (NS5A) and polymerase (NS5B), led to the development of specific inhibitors targeting virus entry and assembly. Now, 30 years after the discovery of HCV, we have several potent, well-tolerated, all-oral agents to treat CHC. Several co-formulated regimens administered for 8–12 weeks results in cure rates in more than 90% of treated patients and covers all genotypes10 (Figure 1).
Chronic HBV and HCV infections account for over 1.4 million deaths from cirrhosis and hepatocellular carcinoma annually. Recognition of this fact together with the availability of sensitive diagnostic tools to identify both infections and effective therapies prompted the World Health Organization in 2016 to propose the “elimination of viral hepatitis as a public health threat” by 2030. However, therapeutics alone will be insufficient to eradicate HCV. Learning from the lessons of smallpox, the only human viral infection successfully eradicated, the development of a vaccine will be crucial to achieving global control of HCV. Current strategies for HCV vaccine development include the production of recombinant proteins, synthetic peptides, DNA vaccines, virus-like particles, and viral vectors expressing various antigens. Although obtaining a vaccine capable of inducing sterilizing immunity will be a difficult task, a partially effective vaccine should be feasible based on the evidence of protective immunity.
The remarkable success in conquering HCV can be attributed not only to the key contributions from this year's recipients of the Nobel Prize in Physiology or Medicine but also to the joint efforts of numerous basic and clinical researchers, the pharmaceutical industry, the patient communities, and governmental and private health organizations. As a result of this collaborative effort, HCV is now the only chronic viral infection that can be cleared in the history of human infectious disease. While we should celebrate the award of the Nobel Prize to HCV researchers, this should not signal that the HCV story is over. Rather, we should view the award of the Nobel Prize to Drs. Alter, Houghton, and Rice as an inspiration to tackle many of the remaining challenges in the field. To healthcare providers and policy makers this involves how to identify asymptomatic HCV carriers, how to link them to care and provide treatment, how to make treatment more financially accessible, and how to prevent re-infection after cure in the absence of a vaccine. To scientists this involves how to improve the regimens for difficult-to-treat patients (patients with HCV genotype 3 infection, patients with cirrhosis, or patients who have failed a direct-acting antiviral regimen) and development of an HCV vaccine. As all the DAAs are relatively new on the market, whether resistant mutations will occur still needs to be carefully monitored. As much as we have learned about the HCV life cycle, its genetic heterogeneity, its dependence on host factors, and interactions with host immunity, there remain many scientific questions lacking clear answers, alongside the need to develop novel viral- and host-targeted antivirals. Of particular importance, an immunocompetent, small animal model is urgently needed for vaccine development.
Alongside the great success of HCV treatment we should not forget about HBV, another hepatotrophic virus that can lead to cirrhosis and liver cancer and is an even larger threat to global public health. Although an effective HBV vaccine is available, finding curative treatment for the ~290 million persons with chronic infection will be a challenge. What can we learn from the efforts of this year's winners of the Nobel Prize for Physiology or Medicine? We ascertain that success will require a combination of scientific expertise, persistence, and a small amount of luck. With these elements a functional cure for HBV should be possible. With further success against HCV and HBV, we should be on the road to achieving the World Health Organization goal to eliminate viral hepatitis as public health threat by 2030.
Contributor Information
Xiaoming Cheng, Email: xiaoming.cheng@nih.gov.
Marc G. Ghany, Email: marcg@bldg10.niddk.nih.gov.
References
- 1.Alter H.J., Purcell R.H., Holland P.V., et al. Transmissible agent in non-A, non-B hepatitis. Lancet. 1978;1:459–463. doi: 10.1016/s0140-6736(78)90131-9. [DOI] [PubMed] [Google Scholar]
- 2.Choo Q.L., Kuo G., Weiner A.J., et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 3.Gretch D.R. Diagnostic tests for hepatitis C. Hepatology. 1997;26:43S–47S. doi: 10.1002/hep.510260708. [DOI] [PubMed] [Google Scholar]
- 4.Lohmann V., Bartenschlager R. On the history of hepatitis C virus cell culture systems. J. Med. Chem. 2014;57:1627–1642. doi: 10.1021/jm401401n. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T., Kato N., Cho M.J., et al. A novel sequence found at the 3' terminus of hepatitis C virus genome. Biochem. Biophys. Res. Commun. 1995;215:744–749. doi: 10.1006/bbrc.1995.2526. [DOI] [PubMed] [Google Scholar]
- 6.Kolykhalov A.A., Agapov E.V., Blight K.J., et al. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science. 1997;277:570–574. doi: 10.1126/science.277.5325.570. [DOI] [PubMed] [Google Scholar]
- 7.Lohmann V., Korner F., Koch J., et al. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 8.Kato T., Date T., Miyamoto M., et al. Efficient replication of the genotype 2a hepatitis C virus subgenomic replicon. Gastroenterology. 2003;125:1808–1817. doi: 10.1053/j.gastro.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Feld J.J., Hoofnagle J.H. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 10.Vermehren J., Park J.S., Jacobson I.M., et al. Challenges and perspectives of direct antivirals for the treatment of hepatitis C virus infection. J. Hepatol. 2018;69:1178–1187. doi: 10.1016/j.jhep.2018.07.002. [DOI] [PubMed] [Google Scholar]


