Abstract
Background
Few studies have investigated socioeconomic inequalities within cities. Yet, such analyses are particularly important given the increasing international trend to urbanization. Here we investigated area-based socioeconomic inequalities in cancer survival in Hamburg, a port city in the North of Germany (population: 1.84 million people).
Methods
Patients with a diagnosis of colorectal, lung, female breast, and prostate cancer in 2004–2018 (follow-up until 31.12.2018) and registered in the Hamburg cancer registry were included. Area-based socioeconomic deprivation on urban district level was assigned to the patients and grouped in five quintiles. Relative survival in 2014–2018 was calculated using the period approach. Trend analyses between 2004 and 2018 were conducted. Relative excess risks adjusted for age and stage were computed with model-based period analyses.
Findings
For the 73,106 included patients, age-standardized 5-year relative survival in 2014–2018 decreased with increasing deprivation with significant differences between the most and least deprived group of 14·7 (prostate), 10·8 (colorectal), 8·0 (breast), and 2·5 (lung) percent units. Standardization by cancer stage decreased the difference for prostate cancer to 8·5 percent units and for breast cancer to 3·6 percent units but had only a minor effect for colorectal and lung cancer. Similar socioeconomic inequalities were already present in 2004–08.
Interpretation
Strong socioeconomic inequalities in cancer survival were observed in Hamburg, which could be partly explained by differences in the stage distribution. Further research including information on screening participation as well as information on cancer care are important to further understand and finally overcome these inequalities.
Funding
German Cancer Aid.
Abbreviations
- ICD
International classification of diseases
- UICC
union for international cancer control
Research in context.
Evidence before this study
We searched PubMed for articles on the association of socioeconomic deprivation and cancer survival within metropolitan areas. We did not restrict our search by language. The investigation of socioeconomic inequalities within metropolitan areas are important, as the majority of the world's population lives in such areas and there is an increasing trend to urbanization. However, while socioeconomic inequalities in all-cause mortality have been reported for many metropolitan areas, we found only two studies on cancer survival. In these studies from Osaka, patients living in more deprived regions had lower cancer survival.
Added value of this study
We analysed area-based socioeconomic differences in survival after colorectal, lung, female breast, and prostate cancer in Hamburg, the second-largest city in Germany with 1.84 million people. We observed strong socioeconomic inequalities in cancer survival with up to 15% lower five-year relative survival in the most compared to the least deprived district. These differences could be partly explained by stage differences for prostate and breast cancer.
Implications of all the available evidence
Our study shows that strong socioeconomic inequalities are present even within metropolitan areas and deserve further attention. As the survival differences were partly explained by differences in stage distributions, socioeconomic differences in screening participation should be evaluated. Furthermore, studies including information on cancer care are important to further understand and finally overcome these inequalities.
Alt-text: Unlabelled box
1. Introduction
Socioeconomic inequalities in cancer survival are well-known. Studies from many countries and for many cancer sites reported that patients living in more deprived regions have lower cancer survival than patients living in more affluent regions. [1, 2] Studies are mostly based on national deprivation indices that treat cities as one unit. Consequently, regional socioeconomic inequalities within large cities might not be adequately assessed. Yet, analyses on socioeconomic inequalities in cancer survival are particularly interesting, as urban areas are more comparable concerning access to medical care than regions in national analyses, which include urban as well as rural areas. Furthermore, the majority of the world's population lives in urban areas and there is an increasing trend to urbanization. [3]
From studies on all-cause mortality, it is well known that area- as well as individual-based socioeconomic deprivation is associated with higher mortality [4] within cities. Such socioeconomic inequalities were for example reported for the cities Eindhoven, London, Helsinki, Turin, and Madrid [5] and for the cities Osaka (Japan), [6] and Bremen (Germany). [7] In Osaka, area-based socioeconomic deprivation was also shown to be associated with lower cancer survival. [6, 8] For Germany, area-based socioeconomic inequalities in cancer survival have been reported, but in these studies each city was treated as one unit. [9, 10] Here, we analysed for the first time area-based socioeconomic differences in cancer survival in Hamburg, the second-largest city in Germany and the 7th largest city in the European Union. We investigated socioeconomic inequalities in survival after breast, colorectal, lung and prostate cancer and determined whether inequalities can be explained by differences in cancer stage distributions.
2. Methods
2.1. Data source
We used data from the Hamburg Cancer Registry, which covers the population of Hamburg (1.84 million in 2018). Patients at the age of 18 years or older and resident in Hamburg with a primary diagnosis of colorectal (International Classification of Diseases (ICD) 10 C18–20), lung (C34), female breast (C50) or prostate (C61) cancer in 2004–2018 were included in the analyses. Death certificate only cases were excluded. Follow-up was based on population registry data and ended in December 2018.
A deprivation score for Hamburg (“Sozialindex”) was used as a measure of area-based socioeconomic deprivation on the level of the 104 urban districts in Hamburg. [11] The index is based on official statistics on the unemployment rate, social housing, welfare recipients, house/apartment size, and household income. Two versions were available based on statistics from 2011 to 2017, respectively. We assigned the index to the patients according to the urban district of residence at the time of diagnosis, using the index that is closest to the year of diagnosis (2011 for 2004–2014, 2017 for 2015–2018). Deprivation quintiles were built separately for each version over all patients included in the analyses. One urban district was excluded due to its special location (a tidal island in the North See) and its small sample size. Table A1 shows the minimum, 25th centile, median, 75th centile, and maximum deprivation value for each deprivation quintile. A histogram of deprivation scores is shown in Fig. A1.
Cancer stage was classified following the Union for International Cancer Control (UICC) TNM classification of malignant tumors effective at the time of diagnosis. For patients with information on tumor size and lymph node status but missing information on metastasis status, the absence of metastasis was assumed. Completeness of stage information was overall comparable across deprivation quintiles but different between cancer sites with a completeness of 85%, 80%, 78% and 45% for breast, colorectal, lung and prostate cancer (Table A2). Patients without information on stage and with tumors that cannot be classified according to the UICC stage were excluded from stage analyses, following a complete case approach.
2.2. Statistical analyses
For each cancer site, patient characteristics and stage distributions across deprivation quintiles were explored and tested for differences using the Cochrane-Armitage trend test or simple linear regression. Assumptions of the regression model (normality and homoscedasticity) were visually inspected. Five-year relative survival was the main outcome of the study. It was estimated as the ratio between absolute and expected survival. The latter was estimated using the Ederer II method [12] and life tables for Hamburg stratified by age, gender, and calendar period. Relative survival was estimated using the period approach [13] for 2014–2018 and the cohort approach for 2004–2008 and 2009–2013. Age-standardization was conducted following the International Cancer Survival Standards. [14] For standardization by stage, the stage distribution in Hamburg in 2004–2018 was used. Relative survival differences between deprivation quintiles were tested for statistical significance by model-based period analysis including the factors follow-up time and age (except for age-specific analyses). [15] Besides, models with adjustment for stage were estimated.
2.3. Role of the funding source
The funder had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. All authors had full access to all the data in the study and accept responsibility to submit for publication.
3. Results
After the exclusion of 5 012 (6·4%) DCO cases, data from 73 088 patients remained for the analyses. The proportion of DCO cases was significantly different across deprivation quintiles for colorectal, lung, and prostate cancer, but no systematic pattern across the quintiles was observed (Table 1). The proportion of female patients decreased significantly with increasing deprivation from 51·9% to 46·6% for colorectal and 43·0% to 37·8% for lung cancer. Age at diagnosis was significantly associated with deprivation for colorectal and lung cancer but a gradual association was only observed for lung cancer with decreasing median age from 71 years in the least deprived to 68 years in the most deprived regions. Among colorectal cancer patients, the proportion of patients with colon cancer (ICD-10 C18-C19) significantly decreased from 70·5% in the least deprived to 67·0% in the most deprived region.
Table 1.
Dataset overview by deprivation quintile and cancer site for patients diagnosed in 2004–2018.
| Site | ICD-10 | Characteristic | Total | Deprivation | |||||
|---|---|---|---|---|---|---|---|---|---|
| Least (Q1) | Q2 | Q3 | Q4 | Most (Q5) | P value1 | ||||
| Colorectal | C18–20 | DCO (%) | 7·6 | 8·6 | 6·9 | 8·4 | 7·1 | 7·0 | 0·01 |
| N (%) | 16,480 | 3141 | 3294 | 3446 | 3200 | 3399 | / | ||
| Female (%) | 50·2 | 51·9 | 51·4 | 50·6 | 48·8 | 46·6 | <0·0001 | ||
| Age (Median) | 72·0 | 73·0 | 73·0 | 73·0 | 73·0 | 71·0 | <0·0001 | ||
| Colon (%) | 69·0 | 70·5 | 69·5 | 69·2 | 68·8 | 67·0 | 0·003 | ||
| Lung | C34 | DCO (%) | 10·4 | 12·6 | 10·2 | 11·4 | 9·2 | 9·5 | <0·0001 |
| N (%) | 17,116 | 2398 | 3277 | 3611 | 3422 | 4408 | / | ||
| Female (%) | 39·4 | 43·0 | 40·3 | 39·6 | 37·3 | 37·8 | <0·0001 | ||
| Age (Median) | 69·0 | 71·0 | 69·0 | 69·0 | 69·0 | 68·0 | <0·0001 | ||
| Breast | C50 | DCO (%) | 3·1 | 3·6 | 3·2 | 3·1 | 3·7 | 2·9 | 0·34 |
| N (%) | 22,597 | 5064 | 4742 | 4712 | 4010 | 4051 | / | ||
| Age (Median) | 64·0 | 64·0 | 64·0 | 64·0 | 65·0 | 63·0 | 0.30 | ||
| Prostate | C61 | DCO (%) | 5·0 | 4·0 | 4·4 | 5·8 | 5·6 | 5·5 | 0·0001 |
| N (%) | 16,913 | 4153 | 3141 | 3517 | 3043 | 3059 | / | ||
| Age (Median) | 69·0 | 69·0 | 69·0 | 70·0 | 70·0 | 69·0 | 0·81 | ||
ICD=International classification of disease; DCO=death certificate only.
P value for trend across deprivation quintiles. For N, no test was performed as the assignment of deprivation quintiles was based on the number of cancer patients.
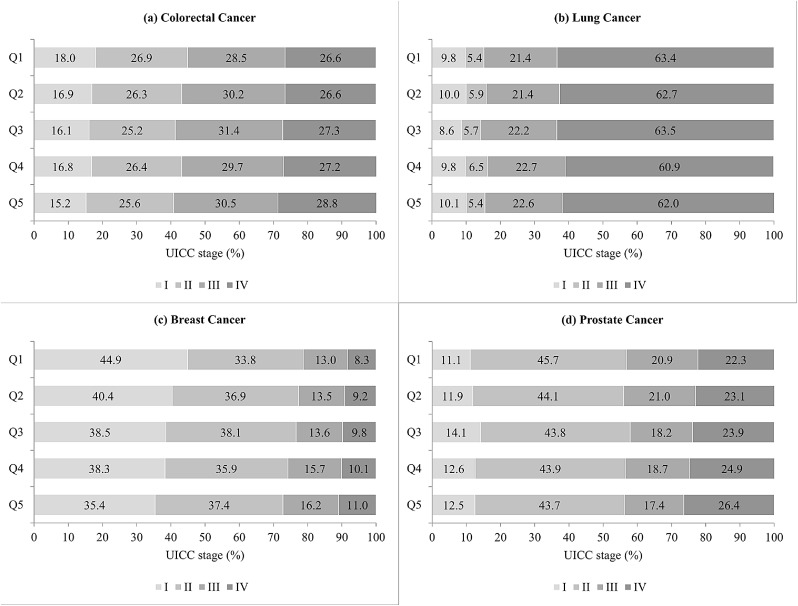
Stage was significantly associated with deprivation in breast (p-value <0.0001) and prostate cancer patients (p-value 0.02; Fig. 1). The proportion of stage I breast cancers decreased from 44·8% in the least deprived to 35·4% in the most deprived regions. The proportion of metastatic prostate cancers increased with increasing deprivation from 22·3 to 26·1%· No significant differences were observed for colorectal (p-value 0·13) and lung cancer (p-value 0·52). However, for colorectal cancer, a tendency to a more favourable stage distribution in less deprived regions was observed.
Fig. 1.
Stage distribution of cancer patients diagnosed in 2004–2018 by socioeconomic deprivation quintile (Q1 – least deprived; Q5 – most deprived).
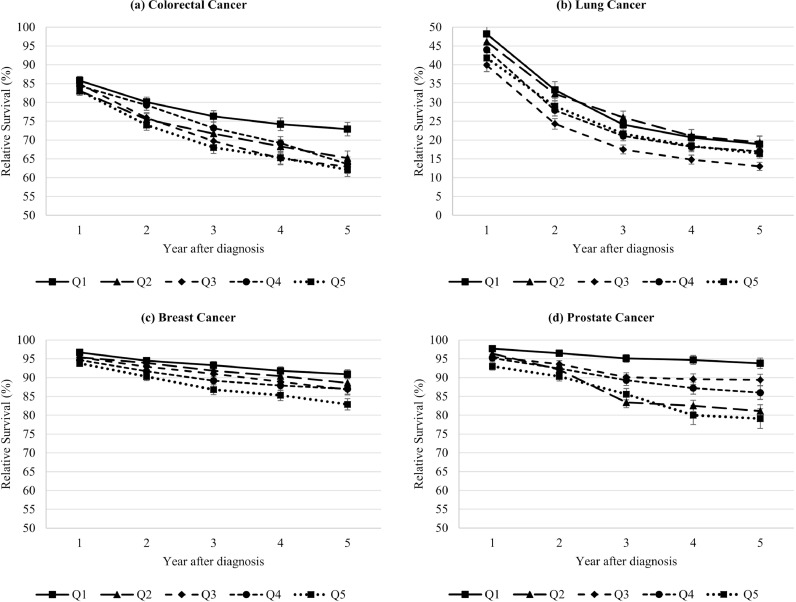
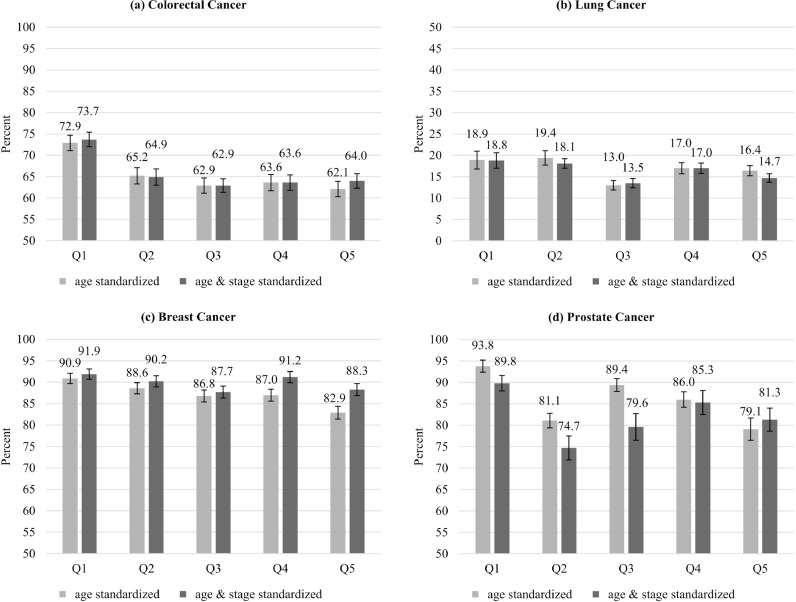
For colorectal, breast, and prostate cancer, differences across deprivation quintiles increased over time after diagnosis (Fig. 2). For all cancer sites, we observed a tendency to higher 5-year relative survival in the least deprived regions with differences of 10·8 (colorectal), 2·5 (lung), 8·0 (breast) and 14·7% units (prostate) between the most and least deprived regions (Fig. 3). Model-based analyses showed significant relative excess risks of death for the most compared to the least deprived regions of 35% (corresponding to an estimate of 1.35; confidence interval: 1·14–1·60; colorectal), 10% (1.10 (1·00–1·22); lung), 99% (1.99 (1·50–2·63), breast), and 202% (3.02 (1·95–4·69); prostate). While overall a gradual trend across deprivation quintiles was observed, lung cancer patients in quintile 3 and prostate cancer patients in quintile 2 showed a noticeable lower survival.
Fig. 2.
One- to five-year age-standardized relative survival (and standard error) in 2014–2018 by socioeconomic deprivation quintile (Q1 – least deprived; Q5 – most deprived).
Fig. 3.
Five-year age-standardized relative survival (and standard error) in 2014–2018 by deprivation quintile with/without standardization for stage (Q1 – least deprived; Q5 – most deprived).
Standardization for stage decreased the difference between the least and most deprived regions from 8·0 to 3·6% units in breast and 14·7 to 8·5% units in prostate cancer patients, with non-significant relative excess risks of 22% (1.22 (0·95–1·55)) and 10% (1.10 (0·79–1·52)), respectively (Fig. 3). For colorectal cancer patients, stage standardization had only a minor effect (10·8 to 9·7% units) and the difference remained significant (1·20 (1·00–1·43)). For lung cancer patients, stage-standardization increased the absolute difference slightly from 2·5 to 4·5% units but did not affect the relative excess risk (1·09 (0·98–1·21)).
In all subgroups by age, gender, and, for colorectal cancer, site, 5-year relative survival was lower in the most compared to the least deprived regions, although differences were not always significant (Table 2). No systematic relationship between the association strength and age was observed across cancer sites. Deprivation-associated survival differences were comparable in women and men. Among colorectal cancer patients, deprivation-associated survival differences were observed for colon and rectal cancer patients, but they were statistically significant for colon cancer patients only. In general, adjusting for stage at diagnosis decreased survival differences in almost all subgroups.
Table 2.
Five-year relative survival (standard error) in 2014–2018 by deprivation quintile, cancer site and subgroups.
| Site | Subgroup | Deprivation | Relative Excess Risk1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Least (Q1) | Q2 | Q3 | Q4 | Most (Q5) | Q5-Q1 | Adjusted for: Age |
Adjusted for: Age+Stage |
||
| Colorectal | Colon2 | 74·2 (2·1) | 64·9 (2·3) | 63·9 (2·2) | 66 (2·3) | 63·7 (2·2) | −10·5 | 1·38 (1·12–1·71) | 1·16 (0·93–1·44) |
| Rectum [2] | 68·7 (3·1) | 64·4 (3·3) | 61·0 (3·1) | 57·6 (3·3) | 60·1 (3·0) | −8·6 | 1·24 (0·94–1·65) | 1·31 (0·97–1·78) | |
| 15–64 years | 77 (2·7) | 67·3 (3·0) | 68·3 (2·9) | 69·2 (3·1) | 68·5 (2·7) | −8·5 | 1·46 (1·05–2·02) | 1·43 (1·00–2·04) | |
| 65–74 years | 74·2 (3·2) | 64·2 (3·2) | 61·1 (3·0) | 60·3 (3·3) | 59·5 (3·2) | −14·7 | 1·65 (1·19–2·28) | 1·25 (0·89–1·75) | |
| 75+ years | 65·1 (3·6) | 63·6 (3·7) | 55·6 (3·3) | 57·8 (3·5) | 55·5 (3·5) | −9·6 | 1·11 (0·87–1·42) | 1·05 (0·81–1·35) | |
| Female2 | 70·8 (1·7) | 63·2 (1·8) | 61·0 (1·7) | 61·6 (1·8) | 60·1 (1·7) | −10·7 | 1·38 (1·08–1·78) | 1·19 (0·91–1·54) | |
| Male2 | 75·4 (1·8) | 67·5 (2·0) | 64·9 (1·8) | 65·6 (1·9) | 63·9 (1·8) | −11·5 | 1·31 (1·04–1·65) | 1·20 (0·94–1·53) | |
| Lung | 15–64 years | 22·5 (3·0) | 24·7 (2·5) | 17·0 (1·9) | 21·1 (2·2) | 19·1 (1·8) | −3·4 | 1·10 (0·91–1·33) | 1·04 (0·85–1·27) |
| 65–74 years | 20·4 (2·5) | 16·7 (2·0) | 15·0 (1·8) | 15·8 (1·9) | 16·7 (1·7) | −3·7 | 1·19 (1·01–1·40) | 1·29 (1·08–1·54) | |
| 75+ years | 10·5 (2·2) | 8·4 (1·8) | 5·2 (1·2) | 14·9 (2·3) | 9·8 (1·8) | −0·7 | 1·03 (0·87–1·21) | 0·97 (0·81–1·16) | |
| Female2 | 18·4 (2·1) | 19·0 (1·6) | 12·7 (1·1) | 16·5 (1·2) | 15·9 (1·2) | −2·5 | 1·07 (0·92–1·24) | 1·12 (0·95–1·31) | |
| Male2 | 19·4 (2·1) | 19·8 (1·7) | 13·3 (1·1) | 17·4 (1·3) | 16·7 (1·2) | −2·7 | 1·11 (0·98–1·28) | 1·06 (0·91–1·22) | |
| Breast | 15–64 years | 93·7 (1·0) | 92·4 (1·1) | 90·0 (1·2) | 89·5 (1·3) | 88·5 (1·3) | −5·2 | 1·90 (1·29–2·80) | 1·17 (0·80–1·70) |
| 65–74 years | 95·4 (1·7) | 90·3 (2·0) | 89·4 (2·2) | 91·5 (2·3) | 84·5 (2·6) | −10·9 | 3·47 (1·60–7·54) | 1·51 (0·92–2·47) | |
| 75+ years | 82·6 (3·5) | 81·6 (3·8) | 80·4 (3·7) | 80·0 (3·7) | 73·6 (3·9) | −9·0 | 1·57 (0·95–2·57) | 1·09 (0·70–1·68) | |
| Prostate | 15–64 years | 98·7 (1·6) | 95·2 (2·2) | 94·7 (2·1) | 90·3 (2·6) | 90·1 (2·3) | −8·6 | 5·44 (1·07–27·58) | 1·33 (0·58–3·04) |
| 65–74 years | 97·6 (1·8) | 94·0 (2·2) | 93·9 (2·2) | 90·0 (2·5) | 87·7 (2·5) | −9·9 | 5·77 (1·45–22·94) | 1·65 (0·85–3·20) | |
| 75+ years | 82·6 (4·2) | 71·3 (4·6) | 75·5 (3·9) | 73·1 (4·6) | 69·7 (4·6) | −12·9 | 2·15 (1·33–3·48) | 0·90 (0·60–1·36) | |
Relative excess risk and 95% confidence interval for Q5 versus Q1. Significant relative excess risks are printed in bold.
Age-standardized.
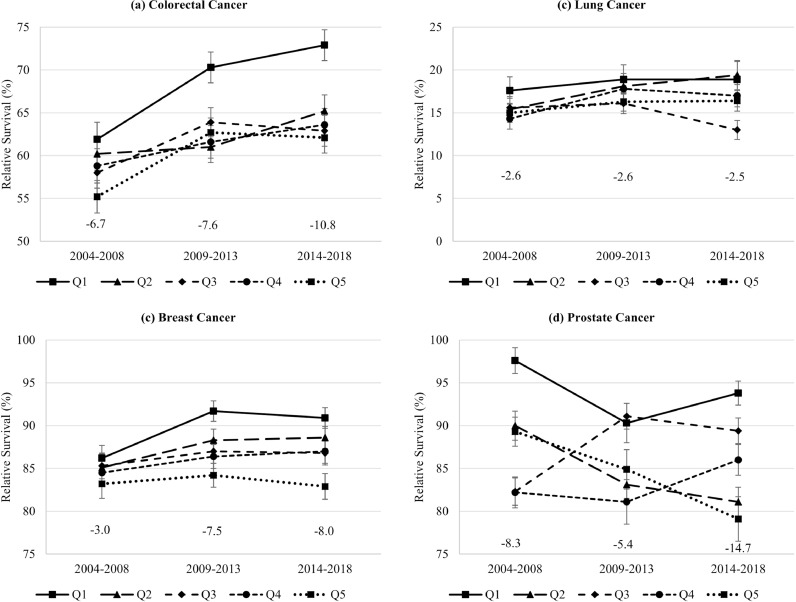
For colorectal and breast cancer patients, the survival difference between the most and least deprived regions increased between 2004 and 08 and 2014–18 from 6·7 to 10·8% units and 3·0 to 8·0% units, respectively (Fig. 4). For lung cancer patients, it remained stable at about 2·6% units. For prostate cancer patients, survival unexpectedly decreased strongly over time in the most deprived group and quintile 2. As a result, the difference between the most and least deprived regions increased from 8·3 to 14·7% units. Trends over time were less obvious after standardization for stage (Table A3).
Fig. 4.
Trends in five-year age-standardized relative survival (and standard error) by socioeconomic deprivation quintile and difference between least (Q1) and most deprived (Q5) regions.
4. Discussion
Our study disclosed strong socioeconomic inequalities in relative survival after cancer in Hamburg. Compared to patients living in the least deprived city districts, patients living in the most deprived districts had a lower relative survival with differences up to 14% units. These differences were observed in men and women and across all age groups. Among breast and prostate cancer patients, cancer stage was strongly associated with deprivation with a less favourable stage distribution in more deprived areas. Consequently, accounting for these differences mostly explained the observed differences in cancer survival for these cancer sites. Socioeconomic inequalities were already present in 2004–08 and there was no indication for decreasing inequalities over time.
Socioeconomic inequalities in cancer survival in Germany have already been reported in previous studies. [9, 10] However, in these studies, deprivation was assigned on the level of districts or municipalities and, consequently, cities were either excluded or assigned a single score for all city districts. Compared to the German study from 2014, the difference between the most and least deprived group was larger for prostate (14·3 versus 3·6% units), breast cancer (8·3 versus 3·1% units), and colorectal cancer (11·0 versus 4·9% units) in the current study. However, results are not directly comparable, as the previous study accounted for deprivation-associated differences in all-cause mortality, which play a particularly important role for elderly patients, cancer sites with better prognosis and long-term survival. Another difference was the finer resolution of the deprivation score used in the current study, which could lead to larger effect estimates. [16, 17] However, in a previous German study, which did not account for differences in all-cause mortality and used a small-area socioeconomic deprivation measure, the difference in 5-year survival for colorectal cancer was also lower (4·8% units). [10] It has been hypothesized that socioeconomic inequalities per se tend to be larger in urban areas due to the concentration of poor population in marginalized neighborhoods. [18] However, studies with comparable study designs are needed to explore differences of socioeconomic inequalities in urban and rural areas.
Several studies on cancer mortality reported higher cancer mortality in more deprived urban areas, [5, 6] but differences in cancer mortality are not only caused by differences in cancer survival but also by differences in cancer incidence, which have been assessed and identified in previous studies from Germany. [19] In two studies on cancer survival in Osaka, cancer patients living in more deprived areas had lower survival. [6, 8] However, the first study only investigated total cancer and, thus, survival differences might at least partly be explained by differences in cancer case-mix across deprivation groups caused by differences in cancer incidence. The other study solely focused on cervical and endometrial cancer survival. Thus, there is a lack of evidence on inequalities in cancer survival within cities or urban areas.
To be able to implement health policy programs to overcome socioeconomic inequalities in cancer survival, it is necessary to understand the underlying causes. Hypothesized reasons for inequalities were differences in tumor characteristics or stage, in cancer treatment, lifestyle factors, comorbidity, or insurance status. [20] In our study, we found a more favourable stage distribution in breast and prostate cancer patients living in less deprived areas. Potential underlying reasons for this finding are differences in health awareness [21] or screening uptake. At the time of the study, opportunistic self-paying screening for prostate cancer was offered in Germany. [22] Organized mammography screening has been implemented in Hamburg in 2008. [23] There is evidence for higher screening uptake among people with better area-level or individual socioeconomic status. [24] Thus, differences in screening uptake might explain the observed more favourable stage distribution in breast and prostate cancer patients in least deprived areas as well as the reduction of the socioeconomic inequalities in survival after adjustment for stage. However, in contrast to the results for prostate and breast cancer, we only observed a non-significant tendency to a better stage distribution in less deprived areas for colorectal cancer. Opportunistic screening for colorectal cancer, which is paid by the statutory health insurance, was available at the time of the study. Thus, results might differ by cancer site. Nonetheless, one starting point to decrease socioeconomic inequalities in cancer survival might be programs targeted to increase screening participation in more deprived city districts. Offering an organised screening program instead of opportunistic screening might reduce socioeconomic inequalities [25] but results are inconclusive. [26] Further studies in this direction are urgently needed.
A further aspect that needs to be considered when interpreting socioeconomic inequalities in stage distributions and cancer survival is that higher screening participation rates may lead to a more favourable stage distribution due to earlier detection of tumors but also due to overdiagnosis. [27, 28] Overdiagnosis could lead to higher survival estimates in patient groups with higher screening uptake without a real benefit for the patient. Both effectiveness of cancer screening and the potential for overdiagnosis differ between cancer sites. [27, 29, 30] As a consequence, a more favourable stage distribution and longer survival rates cannot directly be translated to a benefit for the patient. It is important to additionally investigate incidence and mortality estimates, which should be addressed along with the survival estimates in future studies.
While we mostly observed decreasing survival rates with increasing deprivation, some results are not in line with this pattern. For prostate cancer, 5-year relative survival was noticeably low in the second least deprived group (Q2) for patients older than 74 years at diagnosis, and we observed strong divergent trends over time across deprivation groups. For lung cancer, the middle deprivation group (Q3) had noticeably lower survival than all other deprivation groups for men and women and for all age groups. These patterns should be explored further in future studies using additional regional information sources.
Limitations of the study need to be considered in the interpretation of our results. We could not account for deprivation-associated differences in non-cancer mortality and were not able to investigate cancer care, comorbidity, and lifestyle factors. However, investigations on cancer care will become possible in the future through the national clinical cancer registration in Germany. Migration status was not investigated in our study, although it might be associated with deprivation, [31] screening participation [32, 33] and cancer mortality. [34] The same applies to health literacy. Deprivation quintiles could only be assigned once to the patient for the entire follow-up period. Thus, we were not able to account for moves to other city districts or outside of Hamburg after diagnosis. We had a large proportion of patients with missing information on stage, especially for prostate cancer. We observed that patients with and without information on stage had different survival estimates, which indicates that stage was not missing at random. However, completeness of stage did not depend on deprivation and, thus, excluding patients with missing information should not have led to a pronounced bias in the analyses on the association between deprivation and survival.
Despite these limitations, our study shows strong socioeconomic differences in cancer survival in Hamburg. For breast and prostate cancer, these differences decreased or even disappeared after accounting for the more favourable stage distribution in less deprived areas. Further research including information on screening participation as well as information on cancer care are important to further understand and finally overcome these inequalities.
Author Contributions
Lina Jansen: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing - original draft. Cynthia Erb and Alice Nennecke: Conceptualization, Data curation, Investigation, Methodology, Validation, Resources, Writing - review & editing. Isabelle Finke: Data curation, Investigation, Methodology, Project administration, Validation, Software, Writing - review & editing. Ron Pritzkuleit and Bernd Holleczek: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Writing - review & editing. Hermann Brenner: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
Acknowledgment
This research is supported by the German Cancer Aid (grant number 70112090). The funder had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. All authors had full access to all the data in the study and accept responsibility to submit for publication.
Members of the German Cancer Survival Working Group: Cancer Registry of Bavaria / Regional Center Erlangen-Nürnberg, Clinical Cancer Registry Dresden, Clinical Cancer Registry Thuringia, Regional Center Erfurt, Cancer Registry of Bavaria / Regional Center Regensburg, Cancer Registry of Bavaria, Cancer Registry of Bremen, Cancer Registry of Berlin and the New Federal States, Hamburg Cancer Registry, Cancer Registry of Lower Saxony, Saarland Cancer Registry, Cancer Registry of Rhineland-Palatinate, Cancer Registry of North Rhine-Westphalia, Cancer Registry of Schleswig-Holstein, Cancer Registry of Baden-Württemberg, Institute of Health Economics and Health Care Management / Helmholtz Zentrum München, and German Cancer Research Center.
We would like to thank Werner Maier and Lars Schwettmann (Institute of Health Economics and Health Care Management, Helmholtz Zentrum München – German Research Center for Environmental Health), and Michael Gerken (Cancer Registry of Bavaria / Regional Center Regensburg) for their constructive input to the manuscript.
Data sharing
The data analysed in this study was obtained from the Hamburg Cancer Registry. Restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Hamburg cancer registry.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100063.
Appendix. Supplementary materials
References
- 1.Aarts M.J., Lemmens V.E., Louwman M.W., Kunst A.E., Coebergh J.W. Socioeconomic status and changing inequalities in colorectal cancer? A review of the associations with risk, treatment and outcome. Eur J Cancer. 2010;46(15):2681–2695. doi: 10.1016/j.ejca.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 2.Finke I., Behrens G., Weisser L., Brenner H., Jansen L. Socioeconomic differences and lung cancer survival-systematic review and meta-analysis. Front Oncol. 2018;8:536. doi: 10.3389/fonc.2018.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United Nations, Department of Economic and Social Affairs, Population Division. United Nations. New York: 2015. World Urbanization Prospects. The 2014 revision; 1p pp.
- 4.Pickett K.E., Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. J Epidemiol Community Health. 2001;55(2):111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Lenthe F.J., Borrell L.N., Costa G. Neighbourhood unemployment and all cause mortality: a comparison of six countries. J Epidemiol Community Health. 2005;59(3):231–237. doi: 10.1136/jech.2004.022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueda K., Tsukuma H., Ajiki W., Oshima A. Socioeconomic factors and cancer incidence, mortality, and survival in a metropolitan area of Japan: a cross-sectional ecological study. Cancer Sci. 2005;96(10):684–688. doi: 10.1111/j.1349-7006.2005.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eberle A., Luttmann S., Foraita R., Pohlabeln H. Socioeconomic inequalities in cancer incidence and mortality—a spatial analysis in Bremen, Germany. J Public Health Bangkok. 2010;18(3):227–235. [Google Scholar]
- 8.Ueda K., Kawachi I., Tsukuma H. Cervical and corpus cancer survival disparities by socioeconomic status in a metropolitan area of Japan. Cancer Sci. 2006;97(4):283–291. doi: 10.1111/j.1349-7006.2006.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen L., Eberle A., Emrich K. Socioeconomic deprivation and cancer survival in Germany: an ecological analysis in 200 districts in Germany. Int J Cancer. 2014;134(12):2951–2960. doi: 10.1002/ijc.28624. [DOI] [PubMed] [Google Scholar]
- 10.Jansen L., Behrens G., Finke I. Area-based socioeconomic inequalities in colorectal cancer survival in Germany: investigation based on population-based clinical cancer registration. Front Oncol. 2020;10:857. doi: 10.3389/fonc.2020.00857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FHH-BGV: Hebammenversorgung in Hamburg. Kurzbericht zur Gesundheit. Hamburg 2018, Page 50 ff.
- 12.Ederer F., Heise H. National Cancer Institute; Bethesda, MD: 1959. Instructions to IBM 650 Programmers in Processing Survival Computations. Methodological note no. 10. End Results Evaluation Section. [Google Scholar]
- 13.Brenner H., Gefeller O., Hakulinen T. Period analysis for 'up-to-date' cancer survival data: theory, empirical evaluation, computational realisation and applications. Eur J Cancer. 2004;40(3):326–335. doi: 10.1016/j.ejca.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Corazziari I., Quinn M., Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40(15):2307–2316. doi: 10.1016/j.ejca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Brenner H., Hakulinen T. Up-to-date and precise estimates of cancer patient survival: model-based period analysis. Am J Epidemiol. 2006;164(7):689–696. doi: 10.1093/aje/kwj243. [DOI] [PubMed] [Google Scholar]
- 16.Tervonen H.E., Morrell S., Aranda S. The impact of geographic unit of analysis on socioeconomic inequalities in cancer survival and distant summary stage - a population-based study. Aust N Z J Public Health. 2017;41(2):130–136. doi: 10.1111/1753-6405.12608. [DOI] [PubMed] [Google Scholar]
- 17.Krieger N., Chen J.T., Waterman P.D., Soobader M.J., Subramanian S.V., Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter? The public health disparities geocoding project. Am J Epidemiol. 2002;156(5):471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 18.Nolasco A., Moncho J., Quesada J.A. Trends in socioeconomic inequalities in preventable mortality in urban areas of 33 Spanish cities, 1996-2007 (MEDEA project) Int J Equity Health. 2015;14:33. doi: 10.1186/s12939-015-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoebel J., Kroll L.E., Fiebig J. Socioeconomic inequalities in total and site-specific cancer incidence in Germany: a population-based registry study. Front Oncol. 2018;8:402. doi: 10.3389/fonc.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woods L.M., Rachet B., Coleman M.P. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17(1):5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 21.Schülein S., Taylor K.J., Schriefer D., Blettner M., Klug S.J. Participation in preventive health check-ups among 19,351 women in Germany. Prev Med Rep. 2017;6:23–26. doi: 10.1016/j.pmedr.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starker A., Buttmann-Schweiger N., Krause L., Barnes B., Kraywinkel K., Holmberg C. Cancer screening in Germany: availability and participation. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018;61(12):1491–1499. doi: 10.1007/s00103-018-2842-8. [DOI] [PubMed] [Google Scholar]
- 23.Malek D., Kaab-Sanyal V. Implementation of the German mammography screening program (German MSP) and first results for initial examinations, 2005-2009. Breast Care Basel. 2016;11(3):183–187. doi: 10.1159/000446359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pruitt S.L., Shim M.J., Mullen P.D., Vernon S.W., Amick B.C., 3rd Association of area socioeconomic status and breast, cervical, and colorectal cancer screening: a systematic review. Cancer Epidemiol Biomark Prev. 2009;18(10):2579–2599. doi: 10.1158/1055-9965.EPI-09-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Relecom A., Arzel B., Perneger T. Effect of an organised screening program on socioeconomic inequalities in mammography practice, knowledge and attitudes. Int J Equity Health. 2018;17(1):95. doi: 10.1186/s12939-018-0811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wardle J., von Wagner C., Kralj-Hans I. Effects of evidence-based strategies to reduce the socioeconomic gradient of uptake in the English NHS bowel cancer screening programme (ASCEND): four cluster-randomised controlled trials. Lancet. 2016;387(10020):751–759. doi: 10.1016/S0140-6736(15)01154-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.International Agency for Research on Cancer . Vol. 15. IARC; Lyon: 2016. Breast cancer screening. (IARC Handbook of Cancer Prevention). [Google Scholar]
- 28.Loeb S., Bjurlin M.A., Nicholson J. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65(6):1046–1055. doi: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.International Agency for Research on Cancer . Vol. 16. IARC; Lyon: 2018. Colorectal cancer screening. (IARC Handbook of Cancer Prevention). [Google Scholar]
- 30.Fenton J.J., Weyrich M.S., Durbin S., Liu Y., Bang H., Melnikow J. Prostate-specific antigen-based screening for prostate cancer: evidence report and systematic review for the US preventive services task force. JAMA. 2018;319(18):1914–1931. doi: 10.1001/jama.2018.3712. [DOI] [PubMed] [Google Scholar]
- 31.Brzoska P., Abdul-Rida C. Participation in cancer screening among female migrants and non-migrants in Germany: a cross-sectional study on the role of demographic and socioeconomic factors. Med Baltim. 2016;95(30):e4242. doi: 10.1097/MD.0000000000004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaucher S., Khil L., Kajüter H. Breast cancer incidence and mammography screening among resettlers in Germany. BMC Public Health. 2020;20(1):417. doi: 10.1186/s12889-020-08534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brzoska P., Aksakal T., Yilmaz-Aslan Y. Utilization of cervical cancer screening among migrants and non-migrants in Germany: results from a large-scale population survey. BMC Public Health. 2020;20(1):5. doi: 10.1186/s12889-019-8006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronellenfitsch U., Kyobutungi C., Ott J.J. Stomach cancer mortality in two large cohorts of migrants from the Former Soviet Union to Israel and Germany: are there implications for prevention? Eur J Gastroenterol Hepatol. 2009;21(4):409–416. doi: 10.1097/MEG.0b013e3283155220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.