Abstract
Background
Paediatric outpatient prescription (POP) monitoring is pivotal to identify inadequate prescriptions and optimize drug use. We aimed at describing recent trends in POPs in France.
Methods
All reimbursed dispensations of outpatient prescribed drugs (excluding vaccines) were prospectively collected for the paediatric population (<18 years old) in the French national health database in 2010–2011 and 2018–2019 (mean 117,356,938/year). POP prevalence (proportion of children receiving ≥1 drug prescriptions/year) was calculated by age groups and compared by prevalence rate ratios (PRRs). Given the large sample size, 95% confidence intervals of POP prevalences and PRRs did not differ from estimates.
Findings
Among the 14,510,023 children resident in France in 2018–2019, mean POP prevalence was 857‰ children. Most prescribed therapeutic classes were analgesics (643‰), antibiotics (405‰), nasal corticosteroids (328‰), nonsteroidal anti-inflammatory drugs (NSAIDs) (244‰), antihistamines (246‰) and systemic corticosteroids (210‰). POPs decreased with age from 976‰ for infants to 782‰ for adolescents. Children <6 years old were notably more exposed to inhaled corticosteroids (PRR=3.06), non-penicillin beta-lactam antibacterial agents (PRR=3.05) and systemic corticosteroids (PRR=2.11) than older ones. The POP prevalence was slightly higher (PRR=1.04) during 2018–2019 than 2010–2011, with marked increases for anti-emetics (PRR=1.84), vitamin D (PRR=1.49), proton pump inhibitors (PRR=1.42), systemic contraceptives (PRR=1.24) and nasal corticosteroids (PRR=1.21) and decreases for propulsive/prokinetic agents (PRR=0.09), NSAIDs (PRR=0.73) and systemic antibiotics (PRR=0.88).
Interpretation
POP remained highly prevalent in France throughout the 2010s, especially for children <6 years old, with only a few improvements for selected therapeutic classes. These findings should prompt clinical guidance campaigns and/or regulatory policies.
Funding
Internal funding
Research in Context.
Evidence before this study
The monitoring of paediatric outpatient prescriptions (POPs) at the population level is of paramount importance to identify chronic or emerging areas of inappropriate prescriptions and prepare corrective actions such as clinical guidance or regulatory decisions.
In France, the only available study dates from 2011 and reported concerning findings, with the world's highest prevalence of drug dispensation for paediatric patients before age 2 years (97% of infants had been exposed to ≥1 drug over the year). This over-prescription included drugs with high risk of adverse events such as antibiotics and systemic corticosteroids or drugs without a demonstrated benefit but with safety concerns. Since then, several regulations came into force in France and POPs may have significantly changed.
Added value of this study
This comprehensive study of the French paediatric prescription database — including a mean of 117,356,938 prescriptions/year — showed up-to-date POP prevalences and the last decade trends by age groups. POPs remained highly prevalent in France throughout the 2010s and consistently by sex and different age groups including neonates, with only few improvements in selected therapeutic classes. This study especially highlighted a persistent high POP prevalence for children <6 years old (971‰), for systemic corticosteroids (210‰) and antibiotics (405‰). Some regulatory decisions and safety warnings probably contributed to a decrease in some POP trends (eg. antibiotics, NSAIDs). Other decisions led to a substitution of drugs with additional safety concerns.
Implication of all the available evidence
The French POP level is amongst the highest amongst countries with advanced economies and is rather due to an inadequate positive attitude of physicians and the public toward drug use in children than to epidemiological differences of disease prevalences between countries. These findings should prompt new clinical guidance campaigns and/or regulatory decisions such as drug reimbursement cessation or incentives to optimize practices, also taking into account the risk of drug substitution. Priority targets should probably be corticosteroids and antibiotics, given their adverse side effects and their high level of POPs. Finally, determinants of the very high POP prevalence need to be explored to better target prescribers and populations at risk of high drug prescription. Regular assessments of POP trends are needed to evaluate the impact of corrective actions and detect the emergence of inappropriate POPs.
Alt-text: Unlabelled box
1. Introduction
The paediatric population and especially the youngest children are particularly vulnerable to short- and long-term adverse drug effects because of their physiological and developmental immaturity [1,2]. Paradoxically, paediatric medicine development has been neglected for decades [3], leading to highly frequent use of drugs not or insufficiently evaluated for this specific age group [3,4]. Severe drug adverse events and massive off-label prescribing in the paediatric population [5], [6], [7] paved the way for legislation ensuring access to evidence-based use of drugs in children [8]. These regulations mainly allowed for a better assessment of frequent short-term adverse effects [9], with rare and/or long-term adverse effects of drugs in the paediatric population often remaining unknown. Such a gap in knowledge should prompt avoiding inappropriate and unnecessary prescriptions in the paediatric population. Thus, the monitoring of paediatric outpatient prescriptions (POPs) at the population level is of paramount importance to identify chronic or emerging areas of inappropriate prescriptions and prepare corrective actions such as clinical guidance or regulatory decisions [4,[10], [11], [12]].
POPs should be monitored regularly, but few population-based studies have been performed in the last decade in countries with advanced economies. In 2009, Clavenna et al. reported that the prevalence of children exposed to ≥1 drug over a year ranged from 510‰ in Denmark to 70‰ in Greenland and the median number of different drugs used per child over a year ranged from 0·8 in Norway to 3·2 in the United States [4]. In this last country, Hales et al. showed an overall decrease in drug prescription in children between 1999 and 2014, in particular for antibiotics, anti-histamines and anti-cough medications, but an increasse in the prescription of anti-asthmatic drugs and stimulants for attention-deficit disorders [13]. In New Zealand, Tomlin et al. also showed a decrease in antibiotic prescription between 2010 and 2015, with a slight increase in prescription of non-steroidal anti-inflammatory drugs (NSAIDs) and antihistamines and striking increase in anti-emetic agents [14]. In France, the only available study reported concerning findings [15], with the world's highest prevalence of drug dispensation for paediatric patients before age 2 years: 97% of infants had been exposed to ≥1 drug over the year 2011, with a median of 9 drugs per infant per year. This over-prescription included drugs with high risk of adverse events such as antibiotics [16] and systemic corticosteroids [17] or drugs without a demonstrated benefit but with safety concerns (nasal decongestants [18], cough drugs [19] and propulsive/prokinetic agents [20]). This high prevalence of dispensation is probably related, amongst other explanations, to the positive attitude toward drugs by physicians and public because 90% of consultations end with a prescription in France [21,22].
Since this publication, several regulations came into force in France and included the cessation of the reimbursement of nasal decongestants [23], the removal of cough drugs from the market for the youngest children [24], warnings related to the safety of NSAIDs [25], and recommendations promoting the better use of antibiotics for upper respiratory tract infection [26], with the latter leading to significant change in prescription patterns . In this context, POPs may have significantly changed. Our objective was to investigate recent POPs in France and to compare them with those in 2010–2011, at the national level.
2. Methods
2.1. General methodology
We conducted a national administrative database analysis and followed the RECORD-PE guidelines to report the results [27]. The French National Health Data System (Système national de données de santé [SNDS]) covers 98•8% of the French population and includes reimbursement data for ambulatory care (the health insurance claims database, or Datamart de Consommation Inter-Régime [DCIR]) for all children covered by the Universal Public Health Insurance in France [29], [29], [30]. Every user (or parent user for children) of the French Public Health Insurance is informed of his/her opposition right regarding the use of their data for research purposes. The EPI-PHARE scientific group has regulatory permanent access to the SNDS database (French Decree no. 2016–1871 from December 26, 2016).
In this study, the term “prescriptions” refers to prescribed and dispensed reimbursed drugs in outpatient settings and excludes vaccines and not-reimbursed over-the-counter (OTC) drugs. Vaccines were excluded because free-of-charge (non-reimbursed) vaccinations are performed in dispensaries and account for as much as 5% of vaccinations in children <6 years old, thereby preventing a population-based exhaustive evaluation with the DCIR database. In France, some drugs available as OTCs are also reimbursed when prescribed because they are included on the list of reimbursable pharmaceutical specialities (e.g., paracetamol, NSAIDs). They are then evaluable in the DCIR. However, drugs not included in this list (e.g., homoeopathy, phytotherapy, carbocysteine) or purchased as OTCs without a prescription were not evaluable in the DCIR.
2.2. Inclusion criteria, cohort constitution and data extracted
The eligible participants were children <18 years old present in the DCIR between January 1, 2010 and December 31, 2011 and/or between January 1, 2018 and December 31, 2019. Each child was historically followed from the beginning of the study period if they belonged to the DCIR or from the date of their inclusion if it occurred during the study period. The follow-up ended with the occurrence of one of the following events: end of the study period, 18-year birthday or death.
Two open cohorts of 2 years each were constituted at a 6-year interval. A 2-year period was chosen for each cohort to allow for modulating annual fluctuations in prescriptions related to the variable intensity of viral epidemics. The 2018–2019 cohort allowed for studying current prescriptions, and comparisons with the 2010–2011 cohort allowed for identifying trends, assuming their monotony.
For each participant, we collected data on sex, all reimbursed dispensed drugs classified according to the Anatomical Therapeutic Chemical (ATC) classification [31], and age at dispensation.
2.3. Statistical analyses
To describe the general characteristics of the study population in 2010–2011 and 2018–2019, we calculated the total distribution of person-years by sex and age groups over these 2 periods. Then, we calculated the median (interquartile range [IQR]) number of drugs prescribed by year, for the overall paediatric population then by age groups adapted from those suggested by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals [1] (≤ 6 weeks of life, 0–23 months, 2–5 years, 6–11 years, 12–17 years). Because the DCIR database is not exhaustively updated each year for children without any drug prescription, we approximated the total number of person-years spent in an age category by averaging the number of children residing in France on January 1 of the investigated year and the following year by using the census figures provided by the National Institute of Statistics and Economical studies [32,33].
For the 2 periods (2010–2011 and 2018–2019), we estimated the POP prevalence (i.e., the mean annual prevalence of ≥1 prescription) by dividing the total number of children with at least one drug prescription over a calendar year by the total number of person-years for this year [10]. For neonates and infants ≤6 weeks old, the POP prevalence was similarly estimated except that the total number of infants born in a year with at least one drug prescription during the first 6 weeks of life was divided by the total number of live births the same year. These POP prevalences were calculated for the overall paediatric population and by age groups, sex and drug anatomical classes (level 1 ATC classification) and therapeutic classes (levels 2 to 4 ATC classification) and, for the most prescribed drugs, by active substances (level 5 ATC classification). In addition, yearly prevalence of prescription of at least 2 drugs of the same therapeutic class were calculated overall and by age groups to have an indicator of repeated prescription over the year mixing repeated acute prescriptions and chronic prescriptions. The therapeutic classes (ATC levels 2 to 4) and ATC level 5 drugs at high POP (i.e., prescribed to >100‰ of the paediatric population per year) [12], were described. Crude prevalence rate ratios (PRRs) were used to evaluate ratios in POP prevalence by the 2 studied periods and sex. We described the main increasing and decreasing trends of POP prevalence by therapeutic classes from 2010 to 2011 to 2018–2019. To prepare key messages for future targeted corrective actions, we compared PRRs between the age groups < 6 versus ≥ 6 years old during 2018–2019. Indeed, many consultations are due to self-limited diseases amongst children < 6 years old and result in potentially avoidable prescriptions [34,35]. In contrast, amongst older children, the consultation rate is lower [34] and adherence to the follow-up and treatment may be less optimal in some chronic diseases [36,37]. Given the very large sample size, 95% confidence intervals (CI) of POP prevalence and PRR were reported only when their values with 2 decimals differed from estimates.
2.4. Role of funding source
Not applicable as there was no external funding for this study.
3. Results
3.1. Overall drug prescriptions
During the 2018–2019 period, the 14,510,023 French paediatric residents received a mean of 117,356,938 prescriptions/year, with a median of 5 [IQR 3–8] different drugs/child/year; 12,431,002 paediatric patients received ≥1 outpatient prescription/year, for a POP prevalence of 857‰/year (Table 1 and Supplementary Table S1). POP prevalences were 976‰, 969‰, 828‰, and 782 ‰ for paediatric patients <2, 2–5, 6–11 and 12–17 years old, respectively (Table 1). The POP prevalence was similar for girls (863‰) and boys (851‰; PPR=1•01), except during adolescence, when it was higher for girls (PRR=1•06) (Table 1).
Table 1.
Distribution by sex and age group (years) of drug prescriptions during 2010–2011 and 2018–2019.
| 2010–2011 |
2018–2019 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All ages | ≤6 weeks | <2 years | 2–5 years | 6–11 years | 12–17 years | All ages | ≤6 weeks | <2 years | 2–5 years | 6–11 years | 12–17 years | |
| n = 14,446,249 | n = 897,151 | n = 1600,769 | n = 3234,444 | n = 4876,185 | n = 4734,851 | n = 14,510,023 | n = 754,398 | n = 1434,707 | n = 3085,950 | n = 4990,884 | n = 4998,483 | |
| ≥1 prescription/year/child | ||||||||||||
| N | 11,923,309 | 384,466 | 1,453,807 | 3,044,437 | 3,893,127 | 3,531,940 | 12,431,002 | 443,522 | 1,399,577 | 2,990,384 | 4,130,945 | 3,910,096 |
| Prevalence (‰) All |
825 | 429 | 908 | 941 | 798 | 746 | 857 | 588 | 976 | 969 | 828 | 782 |
| Boys | 818 | 431 | 910 | 945 | 796 | 723 | 851 | 590 | 978 | 974 | 828 | 762 |
| Girls | 833 | 426 | 907 | 937 | 801 | 770 | 863 | 585 | 968 | 960 | 829 | 807 |
| No of drugs/year/child | ||||||||||||
| Median (IQR) | 6 (3–9) | 3 (2–4) | 8 (5–12) | 8 (5–12) | 5 (3–8) | 5 (3–8) | 5 (3–8) | 2 (1–3) | 6 (4–9) | 7 (4–10) | 4 (3–7) | 4 (2–7) |
| 0, n | 2,522,940 | 512,685 | 146,963 | 190,007 | 983,058 | 1,202,913 | 2,079,021 | 310,876 | 35,130 | 95,566 | 859,939 | 1,088,387 |
| (%) | (17%) | (57%) | (9%) | (6%) | (20%) | (25%) | (14%) | (41%) | (2%) | (3%) | (17%) | (22%) |
| 1–4, n | 4,450,196 | 338,411 | 367,047 | 677,451 | 1,743,626 | 1,649,898 | 5,622,908 | 392,398 | 473,233 | 915,646 | 2,133,087 | 2,019,764 |
| (%) | (31%) | (38%) | (23%) | (21%) | (36%) | (35%) | (39%) | (52%) | (33%) | (30%) | (43%) | (40%) |
| ≥5, n | 7,485,289 | 46,056 | 1,086,760 | 2,366,987 | 2,149,502 | 1,882,041 | 6,889,273 | 51,124 | 926,345 | 2,074,738 | 1997,859 | 1890,332 |
| (%) | (52%) | (5%) | (68%) | (73%) | (44%) | (40%) | (47%) | (7%) | (65%) | (67%) | (40%) | (38%) |
3.2. Main drug prescriptions
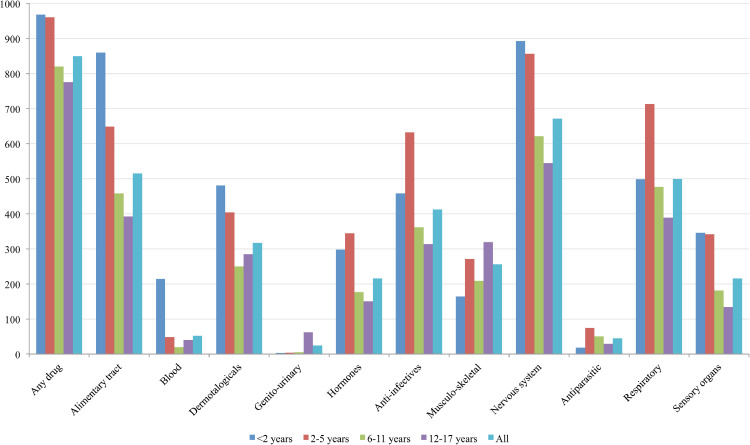
The most commonly prescribed ATC level 1 drugs were for the nervous system (672‰), the alimentary tract and metabolism (516‰), and respiratory system (499‰) as well as anti-infective agents for systemic use (412‰) (Fig. 1). The prevalence rates of prescribing for some ATC level 1 drugs were mainly due to a unique therapeutic class or one specific drug (e.g., prevalence for paracetamol was 641‰; after excluding paracetamol, the prevalence for nervous system drugs decreased from 672‰ to 165‰; Supplementary Figure S1). Children <6 years old had the highest prescription prevalence for most ATC levels, except for sex hormones and musculoskeletal products, which were more often prescribed to adolescents.
Fig. 1.
Prevalence of paediatric outpatient prescriptions in France in 2018–2019 (expressed as the frequency of children receiving ≥1 prescription(s) per 1000 children-years) by age groups and anatomical classes.
For 14 therapeutic classes, the POP prevalence was >100‰: analgesics (643‰), antibiotics (405‰), nasal corticosteroids (328‰), vitamin D (304‰), antihistamines (246‰), NSAIDs (244‰), systemic corticosteroids (210‰), cough suppressants (172‰), antiseptics (155‰), drugs for functional gastrointestinal disorders (142‰), antidiarrheal agents (119‰), anti-emetic agents (113‰), topical anaesthetics (113‰) and short-acting β2-agonists (111‰) (Table 2 with the corresponding ATC level 5 agents in Supplementary Table S2). The most repeatedly prescribed therapeutic classes over a year also corresponded to the 7 therapeutic classes with the highest POP prevalence: 371‰ for children receiving at least 2 prescriptions/year for analgesics, 180‰ for antibiotics, 125‰ for nasal corticosteroids, 102‰ for vitamin D, 80‰ for antihistamines, 70‰ for NSAIDs, and 64‰ for systemic corticosteroids (Table 3).
Table 2.
Prevalence of most commonly prescribed therapeutic classes in outpatient pediatrics by age groups (expressed as the frequency of children receiving ≥1 prescription per 1000 children-years) and prevalence rate ratios (PRRs) between 2018–2019 and 2010–2011.
| ATC level 1 | ATC level 2, 3 or 4 drugs | < 2 years |
2–5 years |
6–11 years |
12–17 years |
All |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010–2011 | 2018–2019 | 2010–2011 | 2018–2019 | 2010–2011 | 2018–2019 | 2010–2011 | 2018–2019 | 2010–2011 | 2018–2019 | PRR | ||
| A | Caries prophylactic agents | 424 | 177 | 96 | 46 | 41 | 40 | 74 | 79 | 106 | 68 | 0.64 |
| A | Drugs for GORD | 96 | 123 | 23 | 21 | 17 | 20 | 38 | 53 | 34 | 42 | 1.24 |
| A | Proton pump inhibitors | 36 | 61 | 14 | 15 | 11 | 14 | 31 | 45 | 21 | 30 | 1.42 |
| A | Drugs for FGD | 133 | 23 | 174 | 131 | 187 | 172 | 157 | 154 | 168 | 142 | 0.85 |
| A | Propulsives | 192 | 9 | 194 | 16 | 111 | 9 | 83 | 13 | 130 | 12 | 0.09 |
| A | Antiemetics/antinausea agents | 55 | 108 | 95 | 175 | 60 | 111 | 43 | 77 | 61 | 113 | 1.84 |
| A | Drugs for constipation | 43 | 46 | 67 | 73 | 41 | 45 | 26 | 28 | 42 | 45 | 1.08 |
| A | Antidiarrhea agents (others) | 234 | 251 | 211 | 219 | 83 | 93 | 43 | 46 | 115 | 119 | 1.04 |
| A | Vitamin D | 521 | 739 | 338 | 428 | 138 | 245 | 74 | 160 | 204 | 304 | 1.49 |
| B | Vitamin K/hemostatics | 203 | 147 | 1 | 1 | 0 | 0 | 0 | 0 | 23 | 15 | 0.65 |
| B | Iron preparations | 43 | 38 | 35 | 22 | 13 | 10 | 18 | 19 | 23 | 18 | 0.81 |
| D | Antifungals (topic) | 150 | 140 | 85 | 71 | 50 | 47 | 64 | 65 | 74 | 67 | 0.92 |
| D | Emollients and protectives | 222 | 86 | 113 | 65 | 47 | 38 | 29 | 25 | 76 | 44 | 0.58 |
| D | Antibiotics (topic) | 55 | 58 | 85 | 85 | 54 | 53 | 42 | 43 | 57 | 57 | 0.99 |
| D | Corticosteroids | 124 | 135 | 134 | 138 | 87 | 92 | 68 | 70 | 95 | 99 | 1.03 |
| D | Antiseptics/disinfectants | 285 | 279 | 229 | 234 | 118 | 116 | 108 | 109 | 158 | 155 | 0.98 |
| D | Anti-acne agents (topic) | 1 | 0 | 1 | 1 | 8 | 5 | 102 | 78 | 37 | 29 | 0.79 |
| D | Anti-acne agents (systemic) | 0 | 0 | 0 | 0 | 1 | 1 | 31 | 31 | 11 | 11 | 1.04 |
| G | Contraceptives (systemic) | 2 | 2 | 1 | 1 | 0 | 0 | 44 | 52 | 15 | 19 | 1.24 |
| H | Corticosteroids (systemic) | 283 | 298 | 366 | 343 | 177 | 169 | 129 | 143 | 215 | 210 | 0.97 |
| J | Antibacterial agents (systemic) | 444 | 434 | 691 | 627 | 413 | 357 | 350 | 307 | 458 | 405 | 0.88 |
| J | Tetracyclines | 0 | 0 | 0 | 0 | 1 | 1 | 54 | 45 | 18 | 16 | 0.88 |
| J | β-lactam antibiotics, penicillin | 312 | 384 | 490 | 541 | 272 | 290 | 193 | 206 | 299 | 324 | 1.08 |
| J | Other β-lactam antibiotics | 268 | 125 | 395 | 183 | 157 | 69 | 86 | 39 | 199 | 88 | 0.44 |
| J | Macrolides, streptogramins | 65 | 40 | 151 | 91 | 85 | 50 | 77 | 51 | 95 | 58 | 0.61 |
| J | Direct-acting antiviral drugs | 6 | 7 | 14 | 14 | 7 | 8 | 9 | 10 | 9 | 10 | 1.08 |
| M | NSAIDs | 277 | 164 | 434 | 271 | 296 | 201 | 333 | 295 | 337 | 244 | 0.73 |
| M | Products for muscular pain | 1 | 0 | 4 | 1 | 30 | 16 | 93 | 74 | 41 | 31 | 0.75 |
| N | Anesthetics (topic) | 371 | 420 | 182 | 130 | 87 | 90 | 28 | 38 | 121 | 113 | 0.94 |
| N | Opioids | 16 | 1 | 40 | 9 | 19 | 9 | 46 | 47 | 32 | 21 | 0.66 |
| N | Analgesics and antipyretics | 778 | 863 | 807 | 843 | 558 | 589 | 463 | 510 | 607 | 643 | 1.06 |
| N | Anxiolytic agents | 6 | 4 | 18 | 12 | 12 | 10 | 24 | 23 | 16 | 14 | 0.87 |
| P | Antinematodal agents | 12 | 13 | 66 | 69 | 46 | 48 | 20 | 24 | 38 | 40 | 1.06 |
| R | Nasal corticosteroids | 377 | 386 | 449 | 510 | 220 | 298 | 169 | 227 | 272 | 328 | 1.21 |
| R | Other nasal preparations | 260 | 0 | 260 | 0 | 80 | 0 | 32 | 0 | 125 | 0 | 0.00 |
| R | β−2-agonists (inhaled) | 138 | 182 | 140 | 167 | 81 | 91 | 64 | 75 | 95 | 111 | 1.17 |
| R | Adrenergics+corticosteroids (inhaled) | 4 | 3 | 19 | 18 | 34 | 30 | 32 | 31 | 27 | 25 | 0.95 |
| R | Corticosteroids (inhaled) | 114 | 131 | 125 | 140 | 53 | 59 | 26 | 31 | 67 | 74 | 1.10 |
| R | Leukotriene receptor antagonist | 9 | 9 | 24 | 23 | 25 | 19 | 19 | 14 | 21 | 17 | 0.80 |
| R | Cough suppressants | 72 | 10 | 289 | 246 | 195 | 187 | 166 | 157 | 193 | 172 | 0.89 |
| R | Antihistamines (systemic) | 175 | 104 | 460 | 386 | 293 | 249 | 210 | 197 | 290 | 246 | 0.85 |
| S oph | Anti-infectives | 257 | 280 | 185 | 191 | 52 | 56 | 36 | 31 | 99 | 98 | 0.99 |
| S oph | Anti-inflammatory + anti-infective agents | 15 | 10 | 26 | 21 | 20 | 18 | 22 | 17 | 22 | 17 | 0.81 |
| S oph | Decongestants/antiallergics | 6 | 5 | 22 | 20 | 40 | 41 | 40 | 43 | 32 | 34 | 1.04 |
| S oto | Anti-infective agents | 36 | 40 | 56 | 65 | 24 | 32 | 13 | 19 | 29 | 35 | 1.23 |
| S oto | Corticosteroids + anti-infective agents | 53 | 41 | 82 | 72 | 47 | 48 | 30 | 35 | 50 | 48 | 0.96 |
A: alimentary tract and metabolism, B: blood and blood forming organs, D: dermatologicals, G: genito-urinary system and sex hormones, H: systemic hormonal preparations, J: anti-infective agents for systemic use, M: musculoskeletal products, N: nervous system, R: respiratory system, S oph: sensory organs, ophthalmological, S oto: sensory organs, otological; NSAID: non-steroidal anti-inflammatory drugs; FGD: functional gastrointestinal disorders; GORD: gastroesophageal reflux disease.
Table 3.
Prevalence of most common therapeutic classes prescribed at least twice per year in outpatient pediatrics by age groups (expressed as the frequency of children receiving ≥2 prescriptions per 1000 children-years) and prevalence rate ratios (PRRs) between 2018–2019 and 2010–2011.
| ATC level 1 | ATC level 2, 3 or 4 drugs | < 2 years |
2–5 years |
6–11 years |
12–17 years |
All |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010–2011 | 2018–2019 | 2010–2011 | 2018–2019 | 2010 2011 | 2018–2019 | 2010–2011 | 2018–2019 | 2010–2011 | 2018–2019 | PRR | ||
| A | Caries prophylactic agents | 247 | 76 | 28 | 8 | 4 | 4 | 11 | 11 | 39 | 15 | 0.37 |
| A | Drugs for GORD | 38 | 53 | 7 | 6 | 4 | 4 | 8 | 11 | 9 | 12 | 1.23 |
| A | Proton pump inhibitors | 17 | 30 | 5 | 4 | 3 | 3 | 6 | 9 | 6 | 8 | 1.34 |
| A | Drugs for FGD | 32 | 2 | 35 | 23 | 40 | 35 | 35 | 34 | 36 | 29 | 0.79 |
| A | Propulsives | 47 | 1 | 31 | 1 | 12 | 0 | 11 | 1 | 20 | 1 | 0.04 |
| A | Antiemetics/antinausea agents | 7 | 17 | 11 | 28 | 5 | 13 | 4 | 9 | 6 | 15 | 2.47 |
| A | Drugs for constipation | 10 | 11 | 19 | 21 | 8 | 10 | 5 | 5 | 10 | 11 | 1.14 |
| A | Antidiarrhea agents (others) | 64 | 67 | 38 | 40 | 7 | 9 | 3 | 4 | 19 | 19 | 1.01 |
| A | Vitamin D | 297 | 439 | 95 | 145 | 18 | 49 | 9 | 30 | 63 | 102 | 1.60 |
| B | Vitamin K/hemostatics | 74 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0.05 |
| B | Iron preparations | 8 | 9 | 4 | 3 | 1 | 1 | 3 | 4 | 3 | 3 | 0.98 |
| D | Antifungals (topic) | 34 | 29 | 14 | 11 | 6 | 6 | 10 | 11 | 12 | 11 | 0.89 |
| D | Emollients and protectives | 72 | 21 | 28 | 14 | 8 | 7 | 5 | 4 | 18 | 9 | 0.48 |
| D | Antibiotics (topic) | 7 | 7 | 10 | 10 | 5 | 5 | 4 | 5 | 6 | 6 | 1.02 |
| D | Corticosteroids | 29 | 32 | 25 | 27 | 13 | 14 | 11 | 12 | 17 | 18 | 1.07 |
| D | Antiseptics/disinfectants | 36 | 35 | 40 | 40 | 17 | 17 | 20 | 21 | 25 | 25 | 1.00 |
| D | Anti-acne agents (topic) | 0 | 0 | 0 | 0 | 1 | 1 | 29 | 21 | 10 | 7 | 0.76 |
| D | Anti-acne agents (systemic) | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 12 | 4 | 4 | 1.06 |
| G | Contraceptives (systemic) | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 30 | 8 | 10 | 1.28 |
| H | Corticosteroids (systemic) | 109 | 112 | 143 | 131 | 45 | 43 | 27 | 30 | 68 | 64 | 0.94 |
| J | Antibacterial agents (systemic) | 262 | 235 | 436 | 351 | 172 | 130 | 136 | 107 | 234 | 180 | 0.77 |
| J | Tetracyclines | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 17 | 7 | 6 | 0.86 |
| J | β-lactam antibiotics, penicillin | 132 | 181 | 216 | 256 | 78 | 85 | 44 | 48 | 103 | 118 | 1.14 |
| J | Other β-lactam antibiotics | 110 | 36 | 157 | 52 | 36 | 12 | 14 | 5 | 64 | 21 | 0.32 |
| J | Macrolides, streptogramins | 12 | 7 | 36 | 19 | 15 | 8 | 12 | 7 | 18 | 10 | 0.53 |
| J | Direct-acting antiviral drugs | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.92 |
| M | NSAIDs | 113 | 50 | 177 | 80 | 90 | 47 | 118 | 94 | 121 | 70 | 0.58 |
| M | Products for muscular pain | 0 | 0 | 0 | 0 | 2 | 1 | 13 | 9 | 5 | 3 | 0.7 |
| N | Anesthetics (topic) | 238 | 279 | 49 | 28 | 14 | 13 | 4 | 7 | 43 | 40 | 0.93 |
| N | Opioids | 1 | 0 | 3 | 0 | 2 | 1 | 6 | 6 | 4 | 2 | 0.71 |
| N | Analgesics and antipyretics | 580 | 660 | 564 | 606 | 263 | 289 | 197 | 224 | 344 | 371 | 1.08 |
| N | Anxiolytic agents | 1 | 0 | 2 | 1 | 2 | 2 | 5 | 6 | 3 | 3 | 1.02 |
| P | Antinematodal agents | 1 | 1 | 9 | 10 | 6 | 6 | 3 | 3 | 5 | 5 | 1.10 |
| R | Nasal corticosteroids | 179 | 180 | 205 | 249 | 63 | 98 | 42 | 61 | 101 | 125 | 1.24 |
| R | Other nasal preparations | 100 | 0 | 86 | 0 | 12 | 0 | 3 | 0 | 35 | 0 | 0.00 |
| R | β−2-agonists (inhaled) | 49 | 69 | 51 | 69 | 29 | 36 | 21 | 27 | 34 | 43 | 1.29 |
| R | Adrenergics+corticosteroids (inhaled) | 1 | 1 | 9 | 8 | 16 | 15 | 13 | 14 | 12 | 12 | 1.00 |
| R | Corticosteroids (inhaled) | 44 | 55 | 47 | 59 | 16 | 18 | 6 | 6 | 23 | 26 | 1.17 |
| R | Leukotriene receptor antagonist | 3 | 4 | 9 | 10 | 12 | 10 | 9 | 7 | 9 | 8 | 0.87 |
| R | Cough suppressants | 16 | 1 | 96 | 79 | 46 | 44 | 35 | 31 | 50 | 43 | 0.85 |
| R | Antihistamines (systemic) | 51 | 20 | 196 | 133 | 105 | 81 | 67 | 62 | 107 | 80 | 0.75 |
| S oph | Anti-infectives | 81 | 89 | 40 | 42 | 6 | 7 | 4 | 3 | 21 | 21 | 1.01 |
| S oph | Anti-inflammatory + anti-infective agents | 2 | 2 | 4 | 3 | 2 | 2 | 3 | 2 | 3 | 2 | 0.83 |
| S oph | Decongestants/antiallergics | 1 | 0 | 3 | 3 | 7 | 7 | 6 | 6 | 5 | 5 | 1.04 |
| S oto | Anti-infective agents | 8 | 9 | 10 | 12 | 3 | 4 | 1 | 2 | 5 | 5 | 1.16 |
| S oto | Corticosteroids + anti-infective agents | 12 | 8 | 14 | 12 | 5 | 6 | 3 | 4 | 7 | 7 | 0.92 |
A: alimentary tract and metabolism, B: blood and blood forming organs, D: dermatologicals, G: genito-urinary system and sex hormones, H: systemic hormonal preparations, J: anti-infective agents for systemic use, M: musculoskeletal products, N: nervous system, R: respiratory system, S oph: sensory organs, ophthalmological, S oto: sensory organs, otological; NSAID: non-steroidal anti-inflammatory drugs; FGD: functional gastrointestinal disorders; GORD: gastroesophageal reflux disease.
3.3. Age sub-groups
As compared with older children, those <6 years old more frequently received POPs for systemic corticosteroids (PRR=2·11), non-penicillin beta-lactam antibacterial agents (PRR=3·05), inhaled corticosteroids (PRR=3·06), topical anaesthetics (PRR= 3·46) and ophthalmological anti-infectives (PRR=5·06; Supplementary Figure S2). Children > 6 and <6 years old had similar POP prevalences for proton pump inhibitors (PPIs; PRR=0·98 [95% CI 0·97; 0·99]), NSAIDs (PRR=0·96) and cough suppressants (PRR=0·99). Systemic contraceptives (104‰ for adolescent girls) and anti-acne drugs (78‰ topical and 31‰ systemic) were mainly prescribed to adolescents (Table 2).
In neonates and infants ≤6 weeks old, the therapeutic classes (ATC levels 2–4) with high POP prevalence (Table 4) were vitamin D (404‰), antiseptics (279‰), vitamin K (260‰) and analgesics (215‰).
Table 4.
Prevalence of most common therapeutic classes prescribed to outpatient infants ≤ 6 weeks old (expressed as the frequency of infants receiving ≥1 prescription per 1000 infants) and prevalence rate ratios (PRRs) between 2018–2019 and 2010–2011.
| ATC | ATC level 3 or 4 label | 2010–2011 | 2018–2019 | PRR [95% CI] |
|---|---|---|---|---|
| A01AA | Caries prophylactic agents | 68 | 4 | 0.06 [0.03;0.09] |
| A01AB | Anti-infective/antiseptic agents (topic) | 11 | 8 | 0.74 [0.71;0.77] |
| A02B | Drugs for GORD | 19 | 59 | 3.16 [3.14;3.18] |
| A02BC | Proton pump inhibitors | 4 | 21 | 4.82 [4.78;4.86] |
| A02BX | Other drugs for GORD | 15 | 48 | 3.22 [3.2;3.24] |
| A03A | Drugs for FGD | 41 | 18 | 0.45 [0.43;0.47] |
| A03F | Propulsives | 19 | 1 | 0.05 [0.00;0.12] |
| A07AA | Intestinal antibiotics | 6 | 22 | 3.91 [3.88;3.94] |
| A11CC | Vitamin D | 249 | 404 | 1.62 [1.61;1.63] |
| B02BA | Vitamin K | 248 | 260 | 1.05 [1.04;1.06] |
| B03A | Iron preparations | 7 | 16 | 2.31 [2.28;2.34] |
| B05X | Solution additive | 4 | 13 | 3.01 [2.97;3.05] |
| D01A | Antifungals (topic) | 13 | 30 | 2.37 [2.35;2.39] |
| D02A | Emollients and protectives | 66 | 20 | 0.30 [0.28;0.32] |
| D08A | Antiseptics/disinfectants | 276 | 279 | 1.01 [1.00;1.02] |
| J02AA | Amphotericin B | 10 | 28 | 2.70 [2.67;2.73] |
| N01B | Anesthetics (topic) | 27 | 82 | 3.07 [3.05;3.09] |
| N02B | Other analgesics and antipyretics | 110 | 215 | 1.95 [1.94;1.96] |
| R01A | Decongestants (topic) | 19 | 20 | 1.07 [1.05;1.09] |
| R01AD | Nasal corticosteroids | 12 | 18 | 1.59 [1.56;1.62] |
| S01A | Anti-infective agents (ophthalmological) | 55 | 78 | 1.43 [1.42;1.44] |
| S01AA | Antibiotics (ophthalmological) | 33 | 54 | 1.65 [1.63;1.67] |
| S01AX | Other anti-infective agents (ophthalmological) | 34 | 40 | 1.19 [1.17;1.21] |
FGD: functional gastrointestinal disorders; GORD: gastroesophageal reflux disease; PRR: prevalence rate ratio; 95% CI; 95% confidence interval.
3.4. Drug prescription trends
The POP prevalence increased from 825‰ to 857‰ from 2010 to 2011 to 2018–2019 (PRR=1·04; Table 1). The main increasing trends of POP prevalence involved alimentary tract drugs with anti-emetics, vitamin D, and PPIs (PRR=1·84, 1·49 and 1·42, respectively), respiratory drugs with nasal corticosteroids, short-acting β2-agonists and inhaled corticosteroids (PRR=1·21, 1·17, and 1·10, respectively), and systemic contraceptives (PRR=1·24; Table 2). The main decreasing trends were for alimentary tract drugs with the propulsive/prokinetics agents (PRR=0·09), respiratory drugs with some nasal preparations, systemic antihistamines, leukotriene receptor antagonists, and cough suppressants (PRR= 0·00, 0·75, 0·80 and 0·85, respectively), antalgics with opioids (PRR=0·66), anti-inflammatory drugs with NSAIDs and topical products for muscle pain (PRR=0·73, and 0·75, respectively), and antibiotics (PRR=0·88). The POP prevalence significantly decreased for the main broad-spectrum antibiotics such as amoxicillin clavulanate (PRR=0·70), josamycin (PRR=0·61), cefpodoxime (PRR=0·42) and clarithromycin (PRR=0·35) but increased for amoxicillin (PRR=1·28; Supplementary Table S2).
POP prevalence in neonates and infants ≤6 weeks old increased from 429‰ to 588‰ from 2010 to 2011 to 2018–2019 (PRR=1·37) (Table 1). Amongst therapeutic classes with high POP prevalence in this age group, the prevalence significantly increased for vitamin D and analgesics (PRR=1·62 [95%CI: 1·61;1·63] and 1·95 [1·94;1·96]) (Table 4). Amongst the other therapeutic classes, the highest increasing trends of POP prevalence concerned PPIs (PRR=4·82 [95%CI: 4·78;4·86]), other drugs for gastrooesophageal reflux disease (alginic acid, PRR=3·22 [95%CI: 3·20;3·24]), antifungal drugs such as nystatin (PRR=3·91 [95%CI: 3·88;3·94]), amphotericin B (PRR=2·70 [2·67;2·73]) and topic econazole (PRR=3·69 [95%CI: 3·66;3·72]), topic anaesthetics (PRR=3·07 [95%CI: 3·05;3·09]), iron preparations (PRR=2·31 [95%CI: 2·28;2·34]), ophthalmological antibiotics (PRR=1·65 [95%CI: 1·63;1·67]), and nasal corticosteroids (PRR=1·59 [95%CI: 1·56;1·62]) (Tables 4 and Supplementary 3).
4. Discussion
4.1. Main results and interpretation
In this first comprehensive analysis of the national paediatric prescription database in France, one of the largest in the world, the current overall POPs (prevalence of 857‰) was the highest as compared with other countries or regions with similar economies, such as New Zealand (731‰) [14], British Columbia (Canada) (550‰) [38], Denmark (508‰) [39], and Italy (491‰) [40]. The high POPs was observed consistently by sex and different age groups including neonates, the most vulnerable group, but also amongst most therapeutic classes. These high levels of POP are not explained by a different epidemiology of diseases in France versus neighbouring countries [41] but more probably by French specificities in prescribing and reimbursing drugs for the paediatric population [21,22,42,43]. In France, prescriptions of drugs also available as OTCs represent a significant proportion of the total reimbursement for the ambulatory paediatric population [22,44]. Indeed, the French health insurance widely reimburses prophylactic drugs (e.g., vitamin D) or antalgics/antipyretics (e.g. paracetamol), and also numerous “old” drugs [45] with questionable benefit-risk ratio [19,46,47][67]. However, some prescription-only therapeutic classes, such as systemic antibiotics, corticosteroids or PPIs, have high levels of prescribing, which suggests substantial overprescribing.
In our study, French children were 5- and 20-fold more likely than American and Norwegian children, respectively, to receive POPs for systemic corticosteroids [48,49]. Amongst French children <6 years old, this ratio was increased to 33-fold as compared with Norwegian peers [49]. Systemic corticosteroids are responsible for well-known serious adverse effects [50] such as increased risk of infections [17]. Furthermore, systemic corticosteroid POPs decreased by only 3% during this 10-year period in France. Nasal corticosteroids, which carry some of the risks of systemic ones, were also widely prescribed in France as compared with several other countries [14,39,40,49]. The POP prevalence for nasal corticosteroids was 273‰, whereas that of its main indication, allergic rhinitis, ranges from 38‰ to 80‰ in the paediatric population in western Europe [51]. The POP prevalence for nasal corticosteroids showed an increasing trend during the study period, with an increase of 21% in the overall paediatric population and 59% amongst neonates, although nasal corticosteroids are not labelled for this vulnerable population [47,52]. These increasing trends coincided with the reimbursement cessation of some nasal decongestants in 2011 [23]. In contrast, children and adolescents were 3-fold less likely to receive a POP for inhaled corticosteroids than were preschoolers and infants. This low level of asthma drugs, especially in adolescents (with a POP prevalence for short-acting β2-agonists and inhaled corticosteroids of 75‰ and 62‰, respectively) contrasts with the 12.7% asthma prevalence amongst this age group [53] and international guidelines encouraging the maintenance of controller drugs [54]. Asthma seems undertreated amongst French adolescents as in other countries [53,55].
The POP prevalence for PPIs was also very high in France, especially amongst infants (61‰), as compared with New Zealand (38‰) [14] or Denmark (5‰) [56]. During the study period, the POP prevalence was increased by 42% for the overall paediatric population and by 382% for neonates and infants ≤6 weeks old. During the same period, recent alerts pointed to the association of PPIs with bone fractures, community-acquired pneumonia and bacterial diarrhoea [57]. Furthermore, the PPI efficiency for reflux symptoms is not demonstrated amongst infants [58]. This prompted the re-affirmation of clinical guidelines for the judicious and limited use of PPIs in paediatric patients [59,60]. Similarly, POPs for metopimazine increased by 84% over the 10 years despite the potential neurological and cardiologic side effects of this anti-emetic [46]. These trends are likely due to drug replacement after cessation of the licensing of major propulsives/prokinetics [20]. Such drug replacement was not intended by drug regulatory agencies [20], no prescription being required for gastrooesophageal reflux [59,60] or emesis [46].
Some improvements regarding POP over-prescription were observed over the 10-year period, for example in the field of antibiotics, NSAIDs and cough suppressants. Some of these improvements perhaps followed the publication of clinical guidance and/or regulatory decisions. Indeed, Trinh et al. [26] highlighted a significant impact of French guidelines promoting a better use of antibiotics for upper respiratory tract infections, which led to a decrease by 33% in antibiotics prescription rates per 1000 paediatric inhabitants/year. Our results allowed to show that although slightly fewer children received antibiotics prescriptions per year (decrease by 12%; Table 2), these prescriptions were especially less often repeated in the same year (decrease by 23%; Table 3). Also, for the overall paediatric population, the structure of prescribed antibiotics has evolved toward a decrease of broad-spectrum antibiotics in favour of amoxicillin. However, French children were still 5.3-fold more likely than Dutch children to receive antibiotics.[68] Antibiotics are still mainly prescribed for viral infections [61] and strongly contribute to the increase in antibiotic resistance [26,61].
Although some improvements in POPs were identified in our study in higher prescription of prophylactic agents such as vitamin D (+49% over 10 years in the overall paediatric population) [62], this high level of POPs in the outpatient paediatric settings is worrisome. Reforms in public health such as drug reimbursement cessation [20,23], incentives for preventive practices [63], or guidelines [26] showed beneficial changes in POP trends [24,26], but may also have negative consequences [64]. The replacement of drugs with safety concerns by others with similar concerns supports the existence of obstacles impeding changes in prescribing behaviours [65]. Therefore, it is crucial to anticipate and prevent the increase in prescribing replacement drugs when this type of regulation comes into force.
4.2. Strengths and limitations
This is the first analysis of POPs in France including in-depth analyses by therapeutic classes and ATC level 5 drugs as well as 10-year trends. This time interval allowed for observing the potential impact of some clinical guidelines or regulatory decisions that occurred during this period. The SNDS database is optimal for drug prescription monitoring owing to its exhaustive population coverage including for example 14,510,023 children in the 2018–2019 period.
Our study has limitations. First, the national pharmaceutical claims database does not collect information on the indications for drug prescriptions, which precludes any analysis of the appropriateness of drug prescriptions. Second, as in many other studies, dispensed prescriptions were used as a proxy for drug prescription [14,15]. Third, prescribing and reimbursement of drugs also available as OTCs appears to represent an important share of the total POP [22], contrary to most countries with advanced economies [22,29,30]. For instance in our study, amongst the 14 most-prescribed paediatric outpatient drugs, 5 were drugs also available as OTCs (helicidin, metopimazine, phloroglucinol, racecadotril, tixocortol). Fourth, we studied dispensed POPs but not those administered, knowing the adherence rate ranges from 5% to 50% in other countries with advanced economies [66]. Fifth, our statistical approach did not allow for exploring POPs corresponding to less frequent diseases (such as attention deficit hyperactivity disorder, childhood depression, type I diabetes). Sixth, if the since the date of birth was not available, we used POPs before ≤6 weeks of life to approach POPs for neonates (who should be defined as at < 28 days of life) [1]. Seventh, the choice of the 6-year old threshold for comparing POP between age groups is relatively arbitrary but allows for providing preliminary key messages for two populations that differ by their consultation rate [34].
4.3. Implications
POPs remained highly prevalent in France throughout the 2010s, especially in children <6 years old, with only few improvements in selected therapeutic classes. These findings should prompt clinical guidance campaigns and/or regulatory decisions such as drug reimbursement cessation [23] or incentives to optimize practices [63]. Priority targets should probably be corticosteroids, PPIs, anti-emetic drugs and antibiotics, given their adverse side effects and their high level of POPs. These interventions must also take into account the risk of drug replacement observed in our study. Finally, determinants of the very high POP prevalence need to be explored to better target prescribers and populations at risk of high drug prescription. Regular assessments of POP trends are needed to evaluate the impact of corrective actions and detect the emergence of inappropriate POPs.
Funding
Internal funding
Authors' contributions
Conceptualization/design: Marion Taine, Martin Chalumeau, Rosemary Dray-Spira, Alain Weill, Mahmoud Zureik; Data collection: Lucile Offredo, Marion Taine; Statistical analysis: Lucile Offredo, Marion Taine; Drafting the initial manuscript: Marion Taine; Review or editing the manuscript: Martin Chalumeau, Lucile Offredo, Rosemary Dray-Spira, Alain Weill, Mahmoud Zureik; Supervision: Martin Chalumeau, Rosemary Dray-Spira, Alain Weill, Mahmoud Zureik
Data statement
The procedures carried out with the French data privacy authority (CNIL, Commission nationale de l'informatique et des libertés) do not provide for the transmission of the database. Consultation by the editorial board or interested researchers may nevertheless be considered, subject to prior determination of the terms and conditions of such consultation and in respect for compliance with the applicable regulations. All requests for access must be submitted to the Health data hub. Further information to do this request is available on these websites:
Declaration of Interests
M Chalumeau has received honoraria for expert consultation from Merck Serono outside the submitted work. All remaining authors declare no competing interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100129.
Appendix. Supplementary materials
References
- 1.European Medicines Agency. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use: e11 clinical investigation of medicinal products in the Paediatric population. 2001. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e11r1-guideline-clinical-investigation-medicinal-products-pediatric-population-revision-1_en.pdf. Access July 6,2020.
- 2.Kearns G.L., Abdel-Rahman S.M., Alander S.W., Blowey D.L., Leeder J.S., Kauffman R.E. Developmental pharmacology–drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 3.Christensen M.L., Helms R.A., Chesney R.W. Is pediatric labeling really necessary? Pediatrics. 1999;104(3 Pt 2):593–597. [PubMed] [Google Scholar]
- 4.Clavenna A., Bonati M. Drug prescriptions to outpatient children: a review of the literature. Eur J Clin Pharmacol. 2009;65(8):749–755. doi: 10.1007/s00228-009-0679-7. [DOI] [PubMed] [Google Scholar]
- 5.Pandolfini C., Bonati M. A literature review on off-label drug use in children. Eur J Pediatr. 2005;164(9):552–558. doi: 10.1007/s00431-005-1698-8. [DOI] [PubMed] [Google Scholar]
- 6.Kimland E., Odlind V. Off-label drug use in pediatric patients. Clin Pharmacol Ther. 2012;91(5):796–801. doi: 10.1038/clpt.2012.26. [DOI] [PubMed] [Google Scholar]
- 7.Palmaro A., Bissuel R., Renaud N. Off-label prescribing in pediatric outpatients. Pediatrics. 2015;135(1):49–58. doi: 10.1542/peds.2014-0764. [DOI] [PubMed] [Google Scholar]
- 8.Permanand G., Mossialos E., McKee M. The EU's new paediatric medicines legislation: serving children's needs? Arch Dis Child. 2007;92(9):808–811. doi: 10.1136/adc.2006.105692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European parliament resolution of 15 December 2016 on the regulation on paediatric medicines (2016/2902(RSP)). http://www.europarl.europa.eu/doceo/document/TA-8-2016-0511_EN.pdf. Access July 1, 2020.
- 10.World Health Organization. Introduction to drug utilization research. 2003. https://apps.who.int/medicinedocs/en/d/Js4876e/. Access July 1, 2020.
- 11.Sanz E.J., Hernandez M.A., Ratchina S. Prescribers' indications for drugs in childhood: a survey of five European countries (Spain, France, Bulgaria, Slovakia and Russia) Acta Paediatr. 2005;94(12):1784–1790. doi: 10.1111/j.1651-2227.2005.tb01854.x. [DOI] [PubMed] [Google Scholar]
- 12.Sturkenboom M.C., Verhamme K.M., Nicolosi A. Drug use in children: cohort study in three European countries. BMJ. 2008;337:a2245. doi: 10.1136/bmj.a2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hales C.M., Kit B.K., Gu Q., Ogden C.L. Trends in prescription medication use among children and adolescents-United States, 1999-2014. JAMA. 2018;319(19):2009–2020. doi: 10.1001/jama.2018.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomlin A.M., Woods D.J., Lloyd H.S., Tilyard M.W. Trends in outpatient prescription medicine use in New Zealand children 2010-2015: a national population-based study. Paediatr Drugs. 2018;20(5):465–474. doi: 10.1007/s40272-018-0303-3. [DOI] [PubMed] [Google Scholar]
- 15.Benard-Laribiere A., Jove J., Lassalle R., Robinson P., Droz-Perroteau C., Noize P. Drug use in French children: a population-based study. Arch Dis Child. 2015;100(10):960–965. doi: 10.1136/archdischild-2014-307224. [DOI] [PubMed] [Google Scholar]
- 16.Gerber J.S., Ross R.K., Bryan M. Association of broad- vs narrow-spectrum antibiotics with treatment failure, adverse events, and quality of life in children with acute respiratory tract infections. JAMA. 2017;318(23):2325–2336. doi: 10.1001/jama.2017.18715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schacke H., Docke W.D., Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96(1):23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 18.Deckx L., De Sutter A.I., Guo L., Mir N.A., van Driel M.L. Nasal decongestants in monotherapy for the common cold. Cochrane Database Syst Rev. 2016;10 doi: 10.1002/14651858.CD009612.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morice A., Kardos P. Comprehensive evidence-based review on European antitussives. BMJ Open Respir Res. 2016;3(1) doi: 10.1136/bmjresp-2016-000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mt-Isa S., Tomlin S., Sutcliffe A. Prokinetics prescribing in paediatrics: evidence on cisapride, domperidone, and metoclopramide. J Pediatr Gastroenterol Nutr. 2015;60(4):508–514. doi: 10.1097/MPG.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 21.General Commission for Strategy and Foresight. Les médicaments et leurs usages: comment favoriser une consommation adaptée ? 2014. https://www.strategie.gouv.fr/sites/strategie.gouv.fr/files/atoms/files/2014-03-04-Medicaments-Usages2.pdf Access January 1, 2021.
- 22.Rosman S., Le Vaillant M., Schellevis F., Clerc P., Verheij R., Pelletier-Fleury N. Prescribing patterns for upper respiratory tract infections in general practice in France and in the Netherlands. Eur J Public Health. 2008;18(3):312–316. doi: 10.1093/eurpub/ckm118. [DOI] [PubMed] [Google Scholar]
- 23.Pichetti S., Sermet C. Le déremboursement des médicaments en France entre 2002 et 2011: éléments d’évaluation. Questions d’économie de la santé. 2011;(167) http://www.irdes.fr/Publications/2011/Qes167.pdf Juillet-août. Access July 1, 2020. [Google Scholar]
- 24.Agence Nationale de Sécurité du Médicament et des produits de santé . 15 March 2011. Communiqué de presse: contre-indication chez l'enfant de moins de 2 ans des médicaments antitussifs antihistaminiques H1 de 1ère génération et du fenspiride utilisés dans le traitement de la toux.https://ansm.sante.fr/S-informer/Communiques-Communiques-Points-presse/Contre-indication-chez-L-enfant-de-moins-de-deux-ans-des-medicaments-antitussifs-antihistaminiques-H1-de-1ere-generation-et-du-fenspiride-utilises-dans-le-traitement-de-la-toux-Communique Access July 1, 2020. [Google Scholar]
- 25.de Martino M., Chiarugi A., Boner A., Montini G., De' Angelis G.L. Working towards an appropriate use of ibuprofen in children: an evidence-based Appraisal. Drugs. 2017;77(12):1295–1311. doi: 10.1007/s40265-017-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trinh N.T.H., Bruckner T.A., Lemaitre M. Association between national treatment guidelines for upper respiratory tract infections and outpatient pediatric antibiotic use in france: an interrupted time-series analysis. J Pediatr. 2020;216:88–94. doi: 10.1016/j.jpeds.2019.09.017. e4. [DOI] [PubMed] [Google Scholar]
- 28.Langan S.M., Schmidt S.A., Wing K. The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE) BMJ. 2018;363:k3532. doi: 10.1136/bmj.k3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bezin J., Duong M., Lassalle R. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26(8):954–962. doi: 10.1002/pds.4233. [DOI] [PubMed] [Google Scholar]
- 29.Lassalle M., Le Tri T., Bardou M. Use of proton pump inhibitors in adults in France: a nationwide drug utilization study. Eur J Clin Pharmacol. 2020;76(3):449–457. doi: 10.1007/s00228-019-02810-1. [DOI] [PubMed] [Google Scholar]
- 30.Coste J., Karras A., Rudnichi A. Statins for primary prevention of cardiovascular disease and the risk of acute kidney injury. Pharmacoepidemiol Drug Saf. 2019;28(12):1583–1590. doi: 10.1002/pds.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO Collaborating Centre for Drug Statistics Methodology. ATC (Anatomical Therapeutic Chemical Classification)/DDD Index 2020. https://www.whocc.no/atc_ddd_index/.
- 32.Vandenbroucke J.P., Pearce N. Incidence rates in dynamic populations. Int J Epidemiol. 2012;41(5):1472–1479. doi: 10.1093/ije/dys142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Institut National des Statistiques et des Etudes Economiques. Population totale par sexe et âge au 1er janvier 2020, France. Bilan démographique 2019.https://www.insee.fr/fr/statistiques/1912926.
- 34.Bruijnzeels M.A., Foets M., van der Wouden J.C., van den Heuvel W.J., Prins A. Everyday symptoms in childhood: occurrence and general practitioner consultation rates. Br J Gen Pract. 1998;48(426):880–884. [PMC free article] [PubMed] [Google Scholar]
- 35.Hay A.D., Heron J., Ness A., team As. The prevalence of symptoms and consultations in pre-school children in the Avon Longitudinal Study of Parents and Children (ALSPAC): a prospective cohort study. Fam Pract. 2005;22(4):367–374. doi: 10.1093/fampra/cmi035. [DOI] [PubMed] [Google Scholar]
- 36.Desai M., Oppenheimer J.J. Medication adherence in the asthmatic child and adolescent. Curr Allergy Asthma Rep. 2011;11(6):454–464. doi: 10.1007/s11882-011-0227-2. [DOI] [PubMed] [Google Scholar]
- 37.Fiese B.H., Everhart R.S. Medical adherence and childhood chronic illness: family daily management skills and emotional climate as emerging contributors. Curr Opin Pediatr. 2006;18(5):551–557. doi: 10.1097/01.mop.0000245357.68207.9b. [DOI] [PubMed] [Google Scholar]
- 38.Zhang T., Smith M.A., Camp P.G., Shajari S., MacLeod S.M., Carleton B.C. Prescription drug dispensing profiles for one million children: a population-based analysis. Eur J Clin Pharmacol. 2013;69(3):581–588. doi: 10.1007/s00228-012-1343-1. [DOI] [PubMed] [Google Scholar]
- 39.The Danish Health Data Authority. Statistics on the total sales of medicines in Denmark 1996-2019. https://www.medstat.dk/en.Access July 1, 2020.
- 40.Agenzia Italiana del Farmaco L'uso dei farmaci in Italia. Rapporto OsMed. 2018 https://www.aifa.gov.it/fr/rapporti-osmed Access July 1, 2020. [Google Scholar]
- 41.Disease G.B.D., Injury I., Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The Commonwealth Fund. International health care system profiles. https://www.commonwealthfund.org/international-health-policy-center/countries. Access January 1, 2021.
- 43.Mangione-Smith R., McGlynn E.A., Elliott M.N., Krogstad P., Brook R.H. The relationship between perceived parental expectations and pediatrician antimicrobial prescribing behavior. Pediatrics. 1999;103(4 Pt 1):711–718. doi: 10.1542/peds.103.4.711. [DOI] [PubMed] [Google Scholar]
- 44.Mossialos E., Oliver A. An overview of pharmaceutical policy in four countries: france, Germany, the Netherlands and the United Kingdom. Int J Health Plann Manage. 2005;20(4):291–306. doi: 10.1002/hpm.816. [DOI] [PubMed] [Google Scholar]
- 45.Blanchard C., Pouchain D., Vanderkam P., Perault-Pochat M.C., Boussageon R., Vaillant-Roussel H. Efficacy of phloroglucinol for treatment of abdominal pain: a systematic review of literature and meta-analysis of randomised controlled trials versus placebo. Eur J Clin Pharmacol. 2018;74(5):541–548. doi: 10.1007/s00228-018-2416-6. [DOI] [PubMed] [Google Scholar]
- 46.Haute Autorité de Santé. Commission de Transparence sur la metopimazine. Avis du 4 avril 2018. https://www.has-sante.fr/upload/docs/evamed/CT-15607_VOGALENE_PIC_RI_Avis2_CT15607.pdf. Access July 1, 2020.
- 47.Gupta R., Fonacier L.S. Adverse effects of nonsystemic steroids (Inhaled, Intranasal, and Cutaneous): a review of the literature and suggested monitoring tool. Curr Allergy Asthma Rep. 2016;16(6):44. doi: 10.1007/s11882-016-0620-y. [DOI] [PubMed] [Google Scholar]
- 48.Zhong W., Maradit-Kremers H., St Sauver J.L. Age and sex patterns of drug prescribing in a defined American population. Mayo Clin Proc. 2013;88(7):697–707. doi: 10.1016/j.mayocp.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The Norwegian Institute of Public Health (NIPH). Norwegian prescription database. create a report. http://www.norpd.no. Access July 1, 2020.
- 50.Meyers R.S., Thackray J., Matson K.L. Key potentially inappropriate drugs in pediatrics: the KIDs list. J Pediatr Pharmacol Ther. 2020;25(3):175–191. doi: 10.5863/1551-6776-25.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ait-Khaled N., Pearce N., Anderson H.R. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: the international study of asthma and allergies in childhood (ISAAC) phase three. Allergy. 2009;64(1):123–148. doi: 10.1111/j.1398-9995.2008.01884.x. [DOI] [PubMed] [Google Scholar]
- 52.Joshi R.R., Maresh A. Iatrogenic Cushing's syndrome and adrenal insufficiency in infants on intranasal dexamethasone drops for nasal obstruction - Case series and literature review. Int J Pediatr Otorhinolaryngol. 2018;105:123–126. doi: 10.1016/j.ijporl.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Delmas M.C., Guignon N., Leynaert B. Prevalence of asthma among children in France. Arch Pediatr. 2009;16(9):1261–1269. doi: 10.1016/j.arcped.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Global Initiative for Asthma. Global strategy for asthma management and prevention, 2019. www.ginasthma.org Access January 1, 2021.
- 55.Kaplan A., Price D. Treatment adherence in adolescents with asthma. J Asthma Allergy. 2020;13:39–49. doi: 10.2147/JAA.S233268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aznar-Lou I., Reilev M., Lodrup A.B., Rubio-Valera M., Haastrup P.F., Pottegard A. Use of proton pump inhibitors among Danish children: a 16-year register-based nationwide study. Basic Clin Pharmacol Toxicol. 2019;124(6):704–710. doi: 10.1111/bcpt.13191. [DOI] [PubMed] [Google Scholar]
- 57.Safe M., Chan W.H., Leach S.T., Sutton L., Lui K., Krishnan U. Widespread use of gastric acid inhibitors in infants: are they needed? Are they safe? World J Gastrointest Pharmacol Ther. 2016;7(4):531–539. doi: 10.4292/wjgpt.v7.i4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Angelidou A., Bell K., Gupta M., Tropea Leeman K., Hansen A. Implementation of a guideline to decrease use of acid-suppressing medications in the NICU. Pediatrics. 2017;140(6) doi: 10.1542/peds.2017-1715. [DOI] [PubMed] [Google Scholar]
- 59.Mouterde O., Chouraqui J.P., Ruemmele F. Let's stop proton pump inhibitor prescriptions for suspected GERD in non-validated indications! Arch Pediatr. 2014;21(7):686–689. doi: 10.1016/j.arcped.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 60.Rosen R., Vandenplas Y., Singendonk M. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for pediatric gastroenterology, hepatology, and nutrition and the european society for pediatric gastroenterology, hepatology, and nutrition. J Pediatr Gastroenterol Nutr. 2018;66(3):516–554. doi: 10.1097/MPG.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trinh N.T.H., Cohen R., Lemaitre M. Community antibiotic prescribing for children in France from 2015 to 2017: a cross-sectional national study. J Antimicrob Chemother. 2020;75(8):2344–2352. doi: 10.1093/jac/dkaa162. [DOI] [PubMed] [Google Scholar]
- 62.Wagner C.L., Greer F.R., American Academy of Pediatrics Section on B. American Academy of Pediatrics Committee on N Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 63.Pomey M.P., Denis J.L., Bernier M., Vergnaud S., Préval J., Saint-Lary O. Innovation in physician remuneration in France. What lessons for Canada? Health Reform Observer. 2019 doi: 10.13162/hro-ors.v7i2.3578. [DOI] [Google Scholar]
- 64.Dusetzina S.B., Higashi A.S., Dorsey E.R. Impact of FDA drug risk communications on health care utilization and health behaviors: a systematic review. Med Care. 2012;50(6):466–478. doi: 10.1097/MLR.0b013e318245a160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cabana M.D., Rand C.S., Powe N.R. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 66.Winnick S., Lucas D.O., Hartman A.L., Toll D. How do you improve compliance? Pediatrics. 2005;115(6):e718–e724. doi: 10.1542/peds.2004-1133. [DOI] [PubMed] [Google Scholar]
- 67.Rachelefsky G, Farrar JR. Are you comfortable with over-the-counter intranasal steroids for children? A call to action. J Allergy Clin Immunol Pract. 2014;2(3):271–274. doi: 10.1016/j.jaip.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 68.Zorginstituut Nederland. GIPdatabank 2019. https://gipdatabank.nl/.Access March 22, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.