Abstract
Background
Resection of colorectal cancer (CRC) metastases provides good survival but is probably underused in real-world practice.
Methods
A prospective Finnish nationwide study enrolled treatable metastatic CRC patients. The intervention was the assessment of resectability upfront and twice during first-line therapy by the multidisciplinary team (MDT) at Helsinki tertiary referral centre. The primary outcome was resection rates and survival.
Findings
In 2012–2018, 1086 patients were included. Median follow-up was 58 months. Multiple metastatic sites were present in 500 (46%) patients at baseline and in 820 (76%) during disease trajectory. In MDT assessments, 447 (41%) were classified as resectable, 310 (29%) upfront and 137 (18%) after conversion therapy. Six-hundred and ninety curative intent resections or local ablative therapies (LAT) were performed in 399 patients (89% of 447 resectable). Multiple metastasectomies for multisite or later developing metastases were performed in 148 (37%) patients. Overall, 414 liver, 112 lung, 57 peritoneal, and 107 other metastasectomies were performed. Median OS was 80·4 months in R0/1-resected (HR 0·15; CI95% 0·12–0·19), 39·1 months in R2-resected/LAT (0·39; 0·29–0·53) patients, and 20·8 months in patients treated with “systemic therapy alone” (reference), with 5-year OS rates of 66%, 40%, and 6%, respectively.
Interpretation
Repeated centralized MDT assessment in real-world metastatic CRC patients generates high resectability (41%) and resection rates (37%) with impressive survival, even when multisite metastases are present or develop later.
Funding
The funders had no role in the study design, analysis, and interpretation of the data or writing of this report.
Keywords: Colorectal cancer, Metastatic, Multidisciplinary, Resection, Resectability, Multisite metastases, Conversion
Research in context.
Evidence before this study
Before designing the RAXO study in 2010, we performed a PubMed search, which was repeated on June 27, 2020, using the terms “metastatic” AND “colorectal cancer” AND “resection/metastasectomy” AND “randomized” (n = 1105/68) OR “prospective” (n = 1701/114) OR “population-based” (n = 229/30) OR “multidisciplinary” (n = 470/39), without date, language, or study type restrictions. We refined the search by looking at “clinical trial”, “meta-analysis”, “randomized controlled trial”, “review”, “systematic review” separately, and on each metastatic site separately; liver, lung, peritoneal, distant lymph nodes, ovarian, and local recurrence. Reports on prospective repeated multisite resectability assessment and resection rates with outcomes in metastatic colorectal cancer (mCRC) were scarce and current literature mainly focuses on single-centre retrospective series focusing on single-site metastases (appendix pp 2–4).
Added value of this study
To our knowledge, RAXO is the first nationwide, real-world prospective intervention study reporting repeated centralized resectability assessment results for any single or multisite metastases, resections and/or local ablations, clinical behavior, and outcomes in mCRC patients. In this setting, all 1086 eligible and consenting patients, regardless of their metastatic site, were centrally evaluated for resectability up to three times during first-line oncological treatment, rendering high conversion and resection rates. Results favoured resection or ablation in all prognostic subgroups. The pattern and dynamics of metastatic sites were recorded during the entire disease trajectory to define potential candidates for resection upfront and during the trajectory. This resulted in high resection rates of new metastatic sites and re-resectability rates mirrored in a high resection/patient ratio. Upfront resectability, conversion and resection rates and outcomes for multisite metastases in a real-world setting nationwide were encouraging and in line with reports from specialized centres for single-site metastases. Addressing metastases during the entire disease trajectory resulted in similar resection rates for upfront as for later appearing metastases (i.e. resections of new or relapsing metastases were actively performed). Resection or ablation of single or multiple organs upfront, after conversion therapy, and of later appearing metastases had higher 5-year overall survival rates than achieved by “systemic therapy alone” (HR 0·18, 95% CI 0·15–0·22).
Implication of all the available evidence
The study emphasizes a practice-changing role for repeated centralized multidisciplinary assessments of resectability also of multisite metastases and the value of adopting an active referral policy by clinical oncologists to perform metastasectomy, not just of liver and lung metastases, but of all resectable sites.
Alt-text: Unlabelled box
1. Introduction
Colorectal cancer is the third most common cancer worldwide with 1.8 million new cases annually and is ranked second in cancer mortality, with 861,000 deaths yearly, mostly due to metastatic colorectal cancer (mCRC). [1] Liver metastases, the most common metastatic site at the time of mCRC diagnosis, are detectable in 68%–75% of patients, while metastases to the lungs are seen in 21%–33%, distant lymph nodes in 16%–26%, peritoneal in 11%–15%, local recurrence in <11%, and rarer metastatic sites like bone and brain in <5%. [2], [3], [4], [5], [6] Single-site mCRC, especially liver metastases (present in 25%–41%), lung metastases (11%–15%), or local recurrence (2%), if oligometastatic, is the best candidate for curative-intent radical surgery and/or local ablative therapy (LAT). [5, [7], [8], [9], [10], [11], [12]]
Clinical outcome studies have shown that 36%–81% of mCRC patients are diagnosed with multisite metastases, [2] and these are frequently considered non-resectable and, thus, left with “systemic therapy alone”. [9], [10], [11], [12] Although the systemic treatments for patients with non-resectable disease have markedly improved during the past decades, with median survival of 30 months in two randomized studies, low 5-year OS rates below 20% are still observed. [13, 14] Reports from specialized centres on single-site metastases have suggested resection rates of 7%–84% and conversion rates with systemic therapy of 0%–61%, with 5-year OS rates of 16%–75%, but substantially lower rates in unselected population-based series (appendix pp 2–4). [15]
Several observations indicate that it is important to assess resectability in a structured manner. Firstly, it is important to assess whether metastasectomy or LAT using various techniques, either upfront or after conversion therapy, are feasible, as long-term outcomes seem favourable after metastasectomy. [5, [9], [10], [11], [12], [16], [17], [18], [19]] Secondly, technical resectability and conversion is difficult to assess, [20] and prone to be underestimated as shown for liver metastases by non-hepatobiliary surgeons and clinical oncologists compared with experienced hepatobiliary surgeons. [21] Whilst present guidelines suggest that patients should be evaluated at a multidisciplinary team (MDT) meeting to ensure proper assessment, [9], [10], [11], [12] repeated or organ-specific assessments at a specialist cancer centre are far from standard practice worldwide for every patient, as was shown in recent trials. [15, 22] Resectability during the entire disease trajectory and data on the clinical behavior of mCRC, mirrored in prognostic and predictive patient characteristics, is often poorly presented in randomized trials, and little is known about the pattern and dynamics of metastatic disease in unselected populations. [2, 3, 7, 8]
Because of the rarity of prospective data concerning, in particular, resectability of multisite metastases, the primary aim was to assess the feasibility and outcomes of repeated centralized MDT intervention to evaluate resectability and conversion leading to resection in a real-world mCRC patient population in Finland.
2. Methods
2.1. Study design and participants
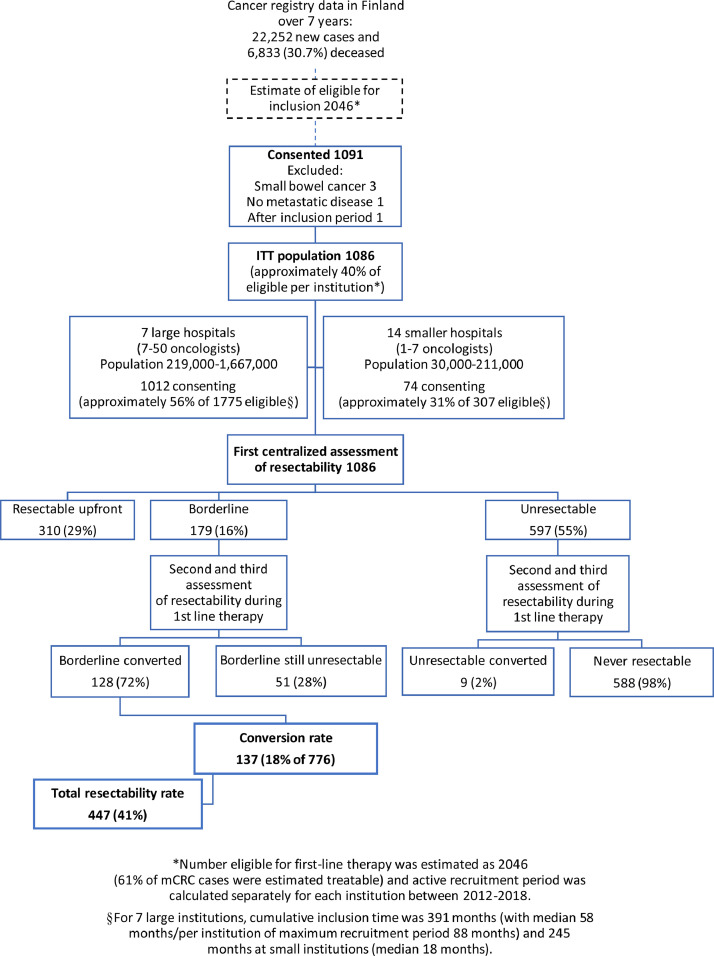
The prospective, investigator-initiated, nationwide RAXO study was conducted in all Finnish hospitals treating mCRC, including five university and 16 regional hospitals (appendix p 5). The primary objective was to evaluate the impact of repeated centralized MDT assessment on technical resectability based on radiology, upfront and after conversion therapy, performed resections and/or LAT, clinical behavior, safety, and outcomes. Inclusion criteria were patients eligible for first-line chemotherapy with any oncological treatment regimen and aged over 18 years, histologically confirmed colorectal adenocarcinoma with distant metastases or locally advanced primary tumor not curatively treatable. Non-colorectal second cancer was not an exclusion criterion. Study design and intervention are presented in Fig. 1. The RAXO study is registered at ClinicalTrials.gov NCT01531621 and EudraCT 2011-003158-24.
Fig. 1.
Study design, patient flow, and intervention with resectability assessment at tertiary MDT in the RAXO study.
The study was designed to include more than 1000 patients nationwide. Due to differences in patient characteristics and the risk of guarantee-time bias [23, 24] in the outcome groups, no formal power analysis was performed. A sample size estimation was performed (appendix p 6).
This study was conducted in accordance with Declaration of Helsinki and monitored independently (appendix p 7). The protocol (appendix pp 23–45) was approved by the Ethics Committee at Helsinki University Hospital and all patients provided written informed consent.
2.2. Intervention
For the MDT assessment, baseline demographics, fitness for metastasectomy, and cancer characteristics were registered online (appendix p 8) and the imaging examinations relevant to cancer were electronically submitted to Helsinki university hospital. Whole body (chest, abdomen and pelvis) computed tomography (CT) was the standard for centralized assessment. This was supplemented by magnetic resonance imaging in hepatic steatosis if CT was not univocal and 18F-fluoro-deoxyglucose positron emission tomography in select patients to evaluate the extrahepatic spread, as deemed /necessary in online protocol at local or central assessment. Early in the study, the guidelines for CT protocols were standardized (appendix p 10).
The MDT consisted of experienced liver surgeons and abdominal radiologists and other specialists, e.g. medical oncologists, radiation oncologists, gastrointestinal surgeons, thoracic surgeons, cytoreductive surgeons, gynaecologists, thoracic radiologists, and PET/CT specialists, as needed. The MDT assessed resectability of liver, lung, and other metastases based upon available radiology, at baseline and repeated up to twice during first-line systemic therapy. Optional central re-assessment at a later time-point was available and if additional MRI- or PET/CT-scans had been requested. This second opinion on technical resectability and conversion was reported online to the local hospital (appendix p 9). Ultimately, organ-specific MDT re-assessment and resections were performed according to local clinical practice based on full knowledge of the patient's condition and all medical treatments. According to governmental regulation small hospitals did not perform metastasectomies or ablations, and most resections were centralized to six high-volume centres; furthermore, the most demanding surgery from any part of Finland was centralized to Helsinki.
Depending on the estimated resectability of each metastatic site (appendix p 11), patients were upfront categorized into three groups: resectable, borderline, or unresectable. The upfront borderline and unresectable patients were reassessed twice and ultimately classified as converted, borderline still unresectable or never resectable (Fig. 1). Each department used their own standard treatment protocols for systemic therapy (appendix p 10). The reasons for non-resectability and why resectable patients were not resected were recorded.
2.3. Outcomes
The primary outcome of the intervention was the proportion of patients who were resectable at the repeated central assessment and had resection and/or LAT upfront or after systemic conversion therapy, also in comparison with the local assessment upfront. Resectability, radicality as R0, R1 or R2, classification criteria, and guarantee-time bias are presented in (appendix p 11). Data on upfront local organ-specific or CRC-MDT (primary tumor) assessment and specialist surgeon consultations at treatment initiation were retrospectively collected from the patient charts.
Survival is presented for patients with or without procedures i.e. resection and/or LAT, and together with safety outcomes for the curatively “R0/1-resected”, non-radically “R2-resected and/or LAT”, “systemic therapy alone”, or best supportive care (BSC) alone groups, respectively. LAT included thermoablation and stereotactic body radiotherapy for liver and lung metastases. [9]
Overall survival (OS) was calculated from date of diagnosis of mCRC, to the date of death from any cause, and the patients alive were censored at last follow-up. [25] Time from first resection or LAT (OS 1st res) was calculated, to be comparable with surgical patient series. Due to the risk of guarantee-time bias [24] in the outcome groups a landmark analysis at 12 months was performed for the main outcome (explained in detail in appendix p 11). Relapse-free survival (RFS) was calculated from the date of first resection or LAT to mCRC relapse, death, or censored at last follow-up. [25] Progression-free survival (PFS) was calculated from mCRC diagnosis to relapse after resection and/or LAT, progression on treatment or during treatment pause, death from any cause, or censored at time of last follow-up.
The description of clinical behavior consisted of patient and tumor characteristics and the presence of metastases upfront and during disease trajectory, with associations to resections and outcomes. [2, 23]
Nationwide population-based liver resection rates and OS for Helsinki University Hospital during the RAXO inclusion years and the preceding 7 years were collected according to the RAXO subprotocol for the data collection cohort and combined with data from the Finnish cancer registry and Statistics Finland (appendix pp 16, 23–45). Population-based demographics and outcomes according to the RAXO subprotocol for the data collection cohort was collected for Tampere and Turku University Hospitals (appendix p 17, 23–45).
2.4. Statistical methods
OS, RFS and PFS were estimated using the Kaplan-Meier method in the intention-to-treat (ITT) population, supplemented with conditional landmark analysis. [24] Hazard ratios (HR) and corresponding 95% confidence intervals (CI) based on Cox proportional hazard regression were calculated, and proportional hazard assumption was tested by inspection of the HR plots. A post-hoc multivariable analysis was performed adjusted for essential patient characteristics, and minimization factors as T-stage, N-stage, grade, synchronous or metachronous presentation sex, age ≥ 70 years, lung metastases, liver metastases and primary location (appendix pp 12, 15) [17, 23, 26, 27]. The subgroup analyses in the forest plot present crude HRs for each of the factors separately without trying to fit any models. The median follow-up time was calculated with the reverse Kaplan-Meier method. Safety analyses were summarized with descriptive statistics in the safety analysis population, which had received at least one dose of systemic therapy or had one procedure. SPSS Statistics, Version 25.0, Armonk, NY, was used.
2.5. Role of the funding source
This investigator-initiated study was supported by Finska Läkaresällskapet, The Finnish Cancer Foundation, the Competitive State Research Financing of the Expert Responsibility Area of Tampere, Helsinki, Turku, Kuopio, and Satakunta Hospitals, Tampere University Hospital Fund (Tukisäätiö and OOO-project), Helsinki University Hospital, and the infrastructure with database and study nurses partly supported by pharmaceutical companies (Amgen, Eli Lilly, Merck KGaA, Roche Finland, Sanofi and Servier). The funders had no role in the study design, analysis, interpretation of the data, decision to publish, or writing of this report. All authors had full access to the data and had final responsibility for the decision to submit for publication.
3. Results
The RAXO study enrolled 1086 patients between June 29, 2012 and October 7, 2018 at all 21 Finnish oncology departments (Fig. 1). The capture rate during the active screening period was approximately 40% nationwide (40%–73% at larger hospitals and 8%–61% at smaller hospitals; appendix p 5). As of data cut-off, March 27, 2020, 755 (70%) patients were deceased, mostly due to progressive mCRC. Median reverse Kaplan-Meier follow-up estimate was 58·3 months (minimum 18 months, IQR 40–72 months). No patients were lost to follow-up. Baseline characteristics are provided in Table 1 and detailed characteristics in (appendix p 13).
Table 1.
Baseline demographics in all patients and in the different therapy groups*.
| All patients | R0–1 resection | R2-resection and/or LAT | Systemic therapy alone | Best supportive care | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | <70 | 715 | 66% | 239 | 73% | 51 | 70% | 418 | 63% | 7 | 30% |
| ≥70 | 371 | 34% | 87 | 27% | 22 | 30% | 246 | 37% | 16 | 70% | |
| ECOG | PS 0 | 295 | 27% | 140 | 43% | 19 | 26% | 136 | 21% | 0 | 0% |
| PS 1–3 | 791 | 73% | 186 | 57% | 54 | 74% | 528 | 80% | 23 | 100% | |
| Primary tumor location | Right colon | 310 | 29% | 73 | 22% | 13 | 18% | 214 | 33% | 10 | 44% |
| Left colon | 396 | 37% | 142 | 44% | 34 | 47% | 218 | 33% | 2 | 9% | |
| Rectum | 374 | 35% | 111 | 34% | 26 | 36% | 226 | 34% | 11 | 48% | |
| Primary tumor resection | Operated upfront | 726 | 67% | 274 | 84% | 50 | 69% | 396 | 60% | 6 | 26% |
| Not operated | 360 | 33% | 52 | 16% | 23 | 32% | 268 | 40% | 17 | 74% | |
| Prior adjuvant chemotherapy | No | 748 | 69% | 178 | 55% | 46 | 63% | 503 | 76% | 21 | 91% |
| Adjuvant chemotherapy | 338 | 31% | 148 | 45% | 27 | 37% | 161 | 24% | 2 | 9% | |
| Number of metastatic sites | 1 | 586 | 54% | 278 | 85% | 31 | 43% | 267 | 40% | 10 | 44% |
| 2 to 6 | 500 | 46% | 48 | 15% | 42 | 58% | 397 | 60% | 13 | 57% | |
| Liver only metastases | Yes | 430 | 40% | 236 | 72% | 26 | 36% | 161 | 24% | 7 | 30% |
| No | 656 | 60% | 90 | 28% | 47 | 64% | 503 | 76% | 16 | 70% | |
| Liver involvement | <25% | 265 | 24% | 156 | 48% | 23 | 32% | 82 | 12% | 4 | 17% |
| ≥25% | 547 | 50% | 106 | 33% | 33 | 45% | 392 | 59% | 16 | 70% | |
| No liver metastases | 274 | 25% | 64 | 20% | 17 | 23% | 190 | 29% | 3 | 13% | |
| Presentation | Synchronous | 736 | 68% | 186 | 57% | 45 | 62% | 484 | 73% | 21 | 91% |
| Metachronous | 350 | 32% | 140 | 43% | 28 | 38% | 180 | 27% | 2 | 9% | |
| RAS status¶ | KRAS/NRAS wildtype | 520 | 49% | 165 | 52% | 25 | 35% | 317 | 49% | 13 | 65% |
| KRAS/NRAS mutant | 540 | 51% | 151 | 48% | 46 | 65% | 336 | 52% | 7 | 35% | |
| BRAF status¶ | BRAF wildtype | 539 | 85% | 188 | 94% | 34 | 92% | 310 | 81% | 7 | 58% |
| BRAF mutant | 93 | 15% | 12 | 6% | 3 | 8% | 73 | 19% | 5 | 42% | |
| Mismatch repair status¶ | pMMR | 302 | 95% | 102 | 94% | 19 | 100% | 178 | 96% | 3 | 75% |
| dMMR | 15 | 5% | 7 | 6% | 0 | 0% | 7 | 4% | 1 | 25% | |
| Not tested | 769 | ·· | 217 | ·· | 54 | ·· | 479 | ·· | 19 | ·· | |
Patients were divided into four groups: curative resection (R0–1), R2 resection of metastases or primary, Local Ablative Therapy (LAT) or not all tumor sites resected curatively (R2/LAT); systemic therapy only or best supportive care only (BSC) group.
Proportions of total number of tested for RAS, BRAF, and MMR status
dMMR = deficient mismatch repair, ECOG = Eastern Cooperative Oncology Group; pMMR = proficient mismatch repair; PS = performance status.
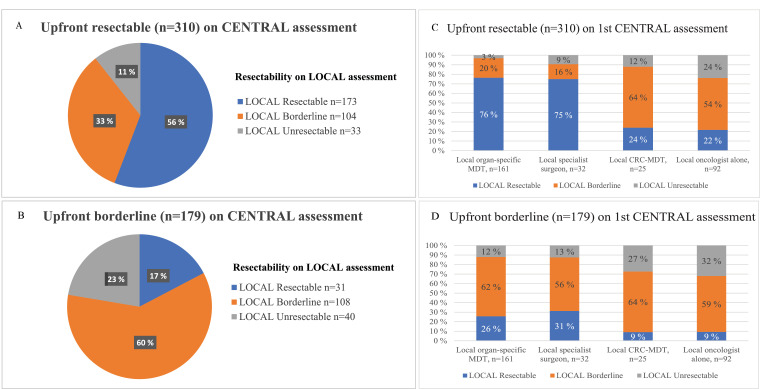
According to the first centralized MDT assessment of technical resectability based on radiology, 310 (29%) of 1086 patients were classified resectable upfront, 179 (16%) were borderline, and 597 (55%) were unresectable (Fig. 1). Local assessment of resectability prior to systemic treatment initiation (at time of referral to first central assessment) was discordant in 137 (44%) of those being upfront resectable by centralized MDT and in 71 (40%) of borderline (Fig. 2A and B). The local decision was based on an MDT and/or consultation of a specialist surgeon beforehand in 218 (70%) of upfront resectable and in 104 (58%) of borderline (Fig. 2C and D). The CRC-MDT (primary tumor) and clinical oncologists underestimated resectability more often than the organ-specific MDT or specialist surgeons did (Fig. 2C and D). The conversion rate was 18% (137 of 776 upfront borderline or unresectable). The concordance of the central vs. the local repeated assessment in the conversion setting cannot be reliably assessed. When summarizing all assessments, 447 (41%) were considered resectable at first or subsequent assessment and 639 never resectable.
Fig. 2.
Upfront resectable (panel A & C) and borderline (panel B & D) in the resectability assessment at central tertiary MDT compared with upfront resectability in the local assessment according by whom the assessment was done before treatment initiation and inclusion to the RAXO-study.
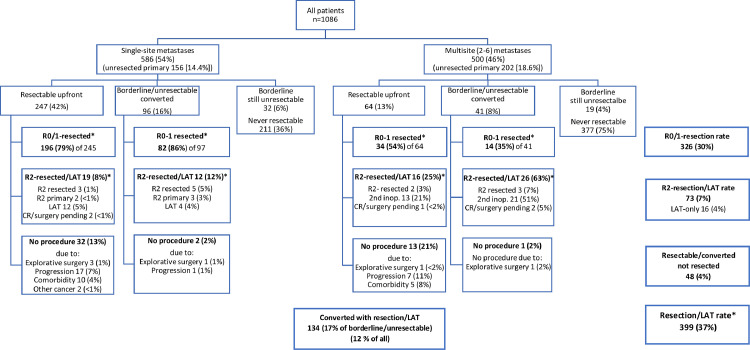
A curative intent procedure with resection and/or LAT (including thermoablation or stereotactic body radiation therapy) was performed in 399 patients (37%). Of these, 66% were resectable upfront and 34% after conversion (Fig. 3). Of the 447 resectable (either upfront or after conversion therapy), 89% had a procedure and 48 (11%) did not, due to disease progression during neoadjuvant therapy in 25, comorbidities contraindicating procedure in 15, or other reasons in 8 patients (Fig. 3). “Systemic therapy alone” was given to 664 patients. At inclusion, all patients were willing and eligible for chemotherapy but 23 (2%) eventually received only BSC (Table 1). Median OS was 30·0 months (95% CI 27·9–32·0) in all patients, 71·5 months (95% CI 62·6–80·4) in the group with procedures versus 20·4 months (19·1–21·7) in the group without procedures (HR 0·18; 95% CI 0·15–0·22; and in post-hoc adjusted analysis 0·21; 95% CI 0·15–0·28 and 0·20; 0·17–0·25, respectively) (appendix p 12), and 5-year OS rates were 27%, 61% and 6%, respectively. Analyses of OS according to baseline patient characteristics favoured resection and/or LAT (HR 0·03–0·45, appendix p 14).
Fig. 3.
Upfront resectability, conversion and resection rates and outcomes, and reasons why a curative intent resection was not undertaken. Data are shown for single-site (n = 586) and multisite (n = 500) metastases with intact or removed primary tumor. Procedures are divided into R0/1-resection, and R2-resection and/or Local Ablative Therapy (LAT).
Resection of the primary tumor and all metastatic sites (R0/1-resected i.e. macroscopically and/or microscopically radical) was performed in 326 (30%) and a non-radical R2-resection and/or LAT in 73 patients (7%). Resectability upfront, conversion and resection rates for single-site metastases were higher than for multisite metastases (Fig. 3). A total of 690 resections and/or LATs in 399 patients were performed, with 414 liver, 112 lung, 57 peritoneal, and 107 other procedures (appendix pp 18–19). Mean number of resections was 1·7 per patient.
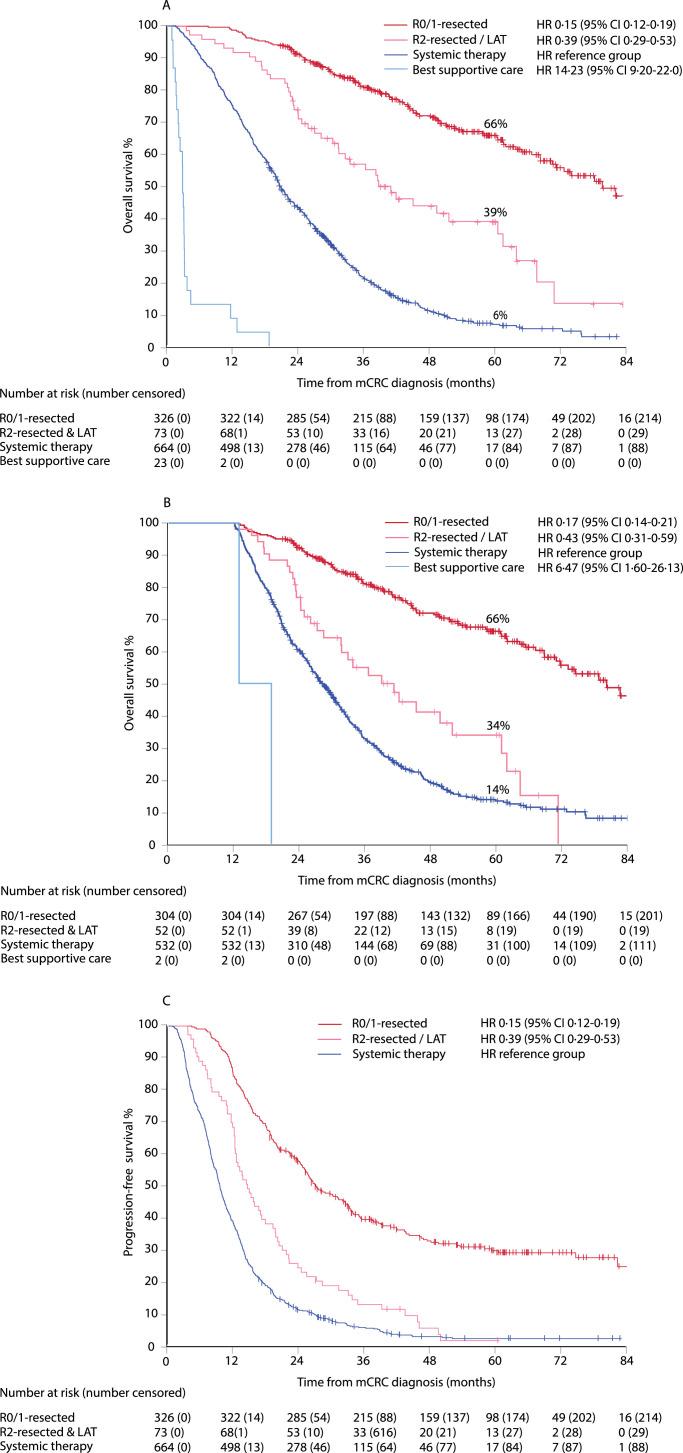
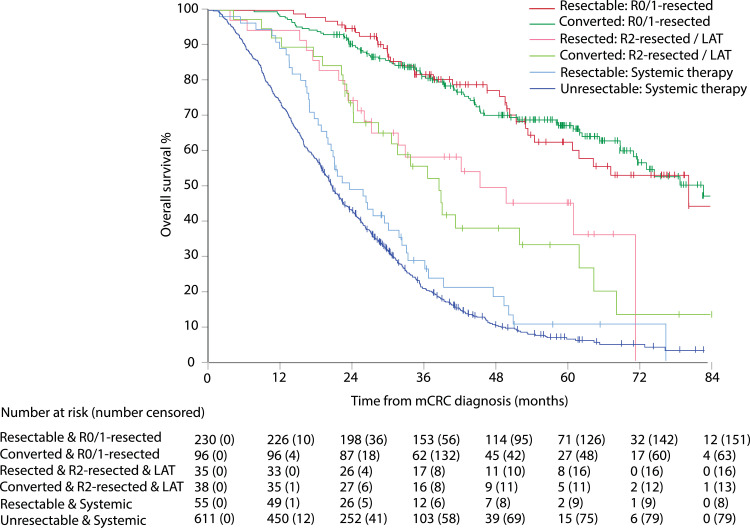
Median OS was 80·4 months (95% CI 69·8–90·9) in the R0/1-resected group, 39·1 months (29·6–48·5) in the R2-resected and/or LAT group, 20·8 months (19·4–22·1) in the “systemic therapy alone” group (reference), and 2·9 months (2·6–3·1) in the BSC group, with HR of 0·15, 0·39, ··, and 14·23, respectively (Fig. 4A), and post-hoc adjusted HR of 0·16, 0·47, 1·00, 46·9, and 0·16, 0·43, 1·00, 15·4, respectively (appendix p 15), with 5-year estimated OS rates of 66%, 39%, 6%, and 0%, respectively. In Fig. 4B, OS by conditional landmark analysis is presented to control for guarantee-time bias i.e. allocation to groups is not performed at mCRC diagnosis, but within 12 months for patients alive in this analysis. Median PFS was 27·0 months (95% CI 23·1–31·0) in the R0/1-resected, 14·5 months (11·7–17·3) in R2-resected and/or LAT, and 9·6 months (9·0–10·2) in the “systemic therapy alone” groups (Fig. 4C) with 5-year PFS rates of 30%, 2%, and 3%, respectively.
Fig. 4.
Overall survival (OS) and 5-year OS rate (panel A), with 12-months landmark (panel B), and progression-free survival (PFS) and 5-year PFS rate (panel C). Data shown from mCRC diagnosis in R0/1-resected, R2-resected or LAT, systemic therapy only, and best supportive care (BSC) groups. Hazard ratio (HR) and 95% confidence interval (CI) with systemic therapy used as reference group.
In resected and/or LAT, mOS was similar in upfront resectable and in those converted to resectable (Fig. 5). In R0/1-resected, mOS was 82·8 months (95% CI 70·7–95·0) in upfront resectable and 80·4 months (95% CI 53·3–107·5) in converted, and in the R2-resected and/or LAT group, 45·4 months (95% CI 22·1–68·8) and 38·6 months (32·1–45·0), respectively. In 48 patients who were technically resectable but received “systemic therapy alone”, mOS was 23·5 months (95% CI 17·9–29·2) compared to 20·6 months (95% CI 19·2–22·0) in the never resectable group receiving “systemic therapy alone”.
Fig. 5.
Median OS from mCRC diagnosis. Data shown for upfront resectable and converted resectable with chemotherapy and/or biologics, according to R0/1-resection, R2-resection and/or local ablative therapy (LAT), upfront or converted resectable treated with “systemic therapy alone” versus borderline still unresectable or never resectable that were treated with “systemic therapy alone”.
Postoperative complications were encountered in 215 (33%) of 660 surgeries with infections being most frequent (appendix p 20). The 30-day mortality rate was 0·5% (2 of 399).
First-line oncologic therapy was mainly doublet or triplet chemotherapy consisting of a fluoropyrimidine (99% of 1060 who received systemic therapy) with oxaliplatin (62%) and/or irinotecan (26%), combined with a biological, i.e. anti-VEGF-inhibitor (58%) or anti-EGFR-inhibitor (29% of RAS wt). Conversion, neoadjuvant, and/or adjuvant therapy was given in 357 (89%) of 399 resected and/or LAT. Lines of therapy and drug exposures are presented in Table 2. The objective response rate to first-line therapy was 60% with a disease control rate of 87% (appendix pp 18–19). Median total time on systemic therapy was 9 months in R0/1-resected, 14 months in R2-resected and/or LAT, and 12 months in the “systemic therapy alone” group. Grade 3–4 toxicity during first-line therapy was seen in 681 of 1060 (64%) and grade 5 in 9 patients (0·8%; appendix p 21).
Table 2.
Systemic, conversion, and neo/-adjuvant therapy*.
| All systemic |
R0–1 resection |
R2-resected/LAT |
”Systemic therapy alone” |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1060 | 100 | 324 | 100 | 72 | 100 | 664 | 100 | ||
| Resectability | Resectable upfront | 305 | 29% | 228 | 70% | 34 | 47% | 43 | 7% |
| Borderline/unresectable converted | 137 | 13% | 96 | 30% | 38 | 53% | 3 | 1% | |
| Borderline still unsresectable | 51 | 5% | 0 | 0% | 0 | 0% | 51 | 8% | |
| Unresectable | 567 | 53% | 0 | 0% | 0 | 0% | 567 | 85% | |
| 1st line drug combination | Comb. CT & anti-EGFR | 139 | 13% | 59 | 18% | 10 | 14% | 70 | 11% |
| Comb. CT & anti-VEGF | 550 | 52% | 147 | 45% | 39 | 54% | 364 | 55% | |
| Comb. CT (no biological) | 224 | 21% | 90 | 28% | 19 | 26% | 115 | 17% | |
| Single CT +/- anti-VEGF/-EGFR | 147 | 14% | 28 | 9% | 4 | 6% | 115 | 17% | |
| Lines of therapy | 1 line of therapy | 414 | 39% | 155 | 48% | 21 | 29% | 238 | 36% |
| 2 lines of therapy | 271 | 26% | 78 | 24% | 25 | 35% | 168 | 25% | |
| 3+ lines of therapy | 375 | 35% | 91 | 28% | 26 | 36% | 258 | 39% | |
| Exposure to drugs during trajectory | Fluoropyrimidine | 1051 | 99% | 320 | 99% | 72 | 100% | 659 | 99% |
| Oxaliplatin | 840 | 79% | 258 | 80% | 62 | 86% | 520 | 78% | |
| Irinotecan | 765 | 72% | 218 | 67% | 52 | 72% | 495 | 75% | |
| Bevacizumab | 758 | 72% | 206 | 64% | 53 | 74% | 499 | 75% | |
| Anti-EGFR | 341 | 32% | 115 | 35% | 19 | 26% | 207 | 31% | |
| Aflibercept | 75 | 7% | 19 | 6% | 5 | 7% | 51 | 8% | |
| Regorafenib | 162 | 15% | 29 | 9% | 16 | 22% | 117 | 18% | |
| Trifluridin/tipiracil | 74 | 7% | 20 | 6% | 6 | 8% | 48 | 7% | |
| Time on systemic therapy during trajectory | Median (IQR) time on therapy (months) | 11·1 | (6; 19) | 8·5 | (6; 17) | 14·1 | (8; 24) | 12·4 | (6; 20) |
IQR = interquartile range.
Excluded are 23 patients with best supportive care only and 3 patients with metastasectomy without systemic therapy.
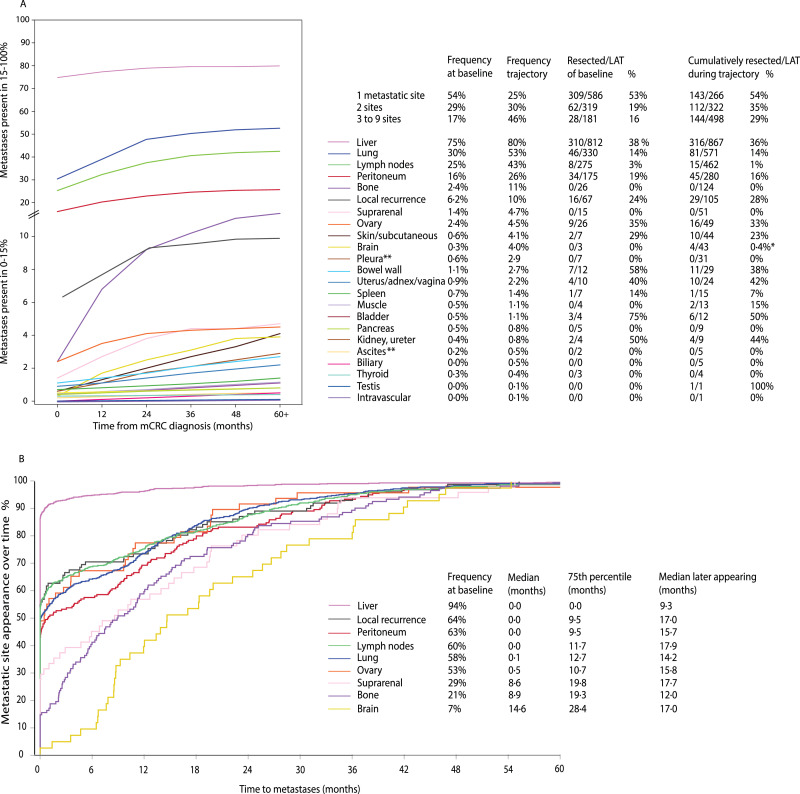
Clinical behavior of metastases and metastatic sites in mCRC is presented in Fig. 6. Presentation of metastases was synchronous in 738 of 1086 (68%). The mean number of metastatic sites was 1·7 at baseline and 2·6 during the entire disease trajectory. Multisite metastases were seen in 46% at baseline and in 75% during disease trajectory. Of the ever-appearing metastases 53%–94% were already present upfront for liver, local, peritoneum, distant lymph nodes, lung, and ovary, whereas suprarenal, bone, and brain metastases clearly appeared later, with 7%–29% present upfront and with median time to appearance of 8·8–14·6 months (Fig. 6B). For metastatic sites not present at baseline, median time to appearance was 9·3–17·9 months. Median time to first new metastatic site was 10·8 months (95% CI 9·6–11·7), to second 16·9 months (14·5–19·3), to third 26·7 months (20·0–30·7), and to fourth 29·1 months (16·2–39·1).
Fig. 6.
Metastatic sites. Frequency as curve and numbers of the 23 most common metastatic sites at baseline and during disease trajectory (presented to 60+ months) in panel A (color- and number-coded in order from most to least frequent). Appearance of metastases at 9 specific sites during the first 60 months of follow-up when 99% of eventual metastases had appeared (proportion of metastases appearing) in panel B (color and number-coded as in A panel).
In the RAXO subprotocol (data collection cohort including all patients with mCRC and liver metastases in Finland) the liver resection rates were 14% during the RAXO inclusion years (2012–2018) compared with 9% in the preceding 7 years (2005–2011) (appendix p 16). Five-year OS rate from 1st liver resection for patients operated at Helsinki University Hospital was 60% (HR reference) for 2005–2011 (n = 260), 56% (HR 1.05; 95% CI 0·80–1·37) for non-RAXO patients (n = 328) and 56% (HR 1.05; 0·73–1·51) for RAXO-patients (n = 142) for 2012–2018.
Population-based characteristics and outcomes for Tampere (n = 866) and Turku (n = 716) University Hospitals are presented in (appendix p 17). Median OS for all patients (including BSC) was 16 and 16 months in Tampere and Turku. For resected patients in Tampere and Turku mOS was 65 and 79 months and 5-year OS-rates 53% and 67% (HR 0·30; 0·24), respectively, in “systemic therapy only” 22 and 22 months (HR reference) and for BSC 3 and 6 months (HR 6·17; 1·78).
Resection and/or LAT were performed in 38% (310/812) of patients with liver metastases present at baseline and in 36% (316/867) of patients with liver metastases anytime during trajectory (Fig. 6A), with mOS 73·3 (95% CI 64·8–81·8) months in resected and/or LAT. Lung metastases were resected and/or LAT in 14% of those present at baseline and in 14% of those during trajectory, respectively, with mOS 86·0 (63·5–108·5) months, distant lymph nodes in 3% and 1%, respectively, with mOS 61·1 months (20·4–101·8), peritoneal in 19% and 16%, respectively, with mOS 45·4 months (30·3–60·5), and other metastases such as local recurrence, gynaecologic, urologic, or skin/subcutaneous in 29%–75% and 28%–50%, respectively, with mOS 53·5 months (32·4–74·5).
In patients having one metastatic site at baseline, 53% were resected with a mOS of 75 months, if two sites, 21% were resected with a mOS of 86 months, and if three or more sites, 15% were resected with a mOS of 39 months (Table 3). OS and RFS from 1st resection and/or LAT are presented in Table 3. Detailed surgical procedures and oncological treatments are presented in (appendix pp 18–19).
Table 3.
Baseline metastatic sites and R0/1/2-resection and/or local ablative therapy (LAT) rates*.
| Frequency | Resection/LAT | mOS from mCRC |
95% CI | 5-yr rate | mOS 1st resection | 95% CI | 5-yr rate | mRFS 1stR0/1-resection | 95% CI | 1-yr rate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All metastatic sites | 1086 | 100% | 399 | 37% | 71.5 | 62·6 | 80·4 | 61% | 67·0 | 60·0 | 75·0 | 56% | 18·8 | 13·8 | 23·8 | 61% |
| One metastatic site | 586 | 100% | 309 | 53% | 74·6 | 66·0 | 83·3 | 64% | 73·0 | 64·1 | 81·8 | 60% | 18·8 | 13·1 | 24·5 | 60% |
| Liver-limited | 430 | 100% | 262 | 61% | 74·6 | 64·9 | 84·4 | 72% | 73·2 | 62·4 | 83·9 | 60% | 21·2 | 14·6 | 27·7 | 61% |
| Lung-limited | 66 | 100% | 27 | 41% | 71·8 | ·· | ·· | 74% | 70·9 | ·· | ·· | 74% | 13·0 | 6·7 | 19·3 | 56% |
| Peritoneal-limited | 45 | 100% | 11 | 24% | 61·1 | 24·9 | 97·3 | 53% | 61·1 | 23·1 | 99·0 | 53% | 14·1 | 5·4 | 22·9 | 56% |
| Other single metastasis | 45 | 100% | 9 | 20% | NE | ·· | ·· | 62% | NE | ·· | ·· | 62% | 22·3 | ·· | ·· | 63% |
| Two metastatic sites | 319 | 100% | 66 | 21% | 86·0 | 18·5 | 22·9 | 64% | 65·4 | 43·6 | 87·2 | 50% | 18·9 | 8·4 | 29·3 | 67% |
| Liver and lung | 119 | 100% | 23 | 19% | 68·9 | 53·1 | 84·8 | 80% | 65·4 | 51·8 | 79·1 | 56% | 26·8 | 17·6 | 36·1 | 78% |
| Liver and any (not lung) | 118 | 100% | 20 | 17% | 52·0 | ·· | ·· | 48% | 49·3 | ·· | ·· | 46% | 13·3 | 0·0 | 26·9 | 54% |
| Lung and any (not liver) | 31 | 100% | 2 | 6% | NE | ·· | ·· | 100% | NE | ·· | ·· | 100% | 26·4 | 16·7 | 30·1 | 80% |
| Any (not liver and not lung) | 51 | 100% | 17 | 33% | 101·3 | 20·3 | 182·3 | 58% | 97·4 | 29·3 | 165·5 | 54% | 17·6 | 11·5 | 23·7 | 69% |
| 3–6 metastatic sites | 181 | 100% | 28 | 15% | 38·6 | 31·3 | 45·8 | 32% | 31·8 | 23·9 | 39·6 | 17% | 12·7 | 2·7 | 22·8 | 58% |
| Liver, lung and any | 98 | 100% | 8 | 8% | 42·3 | 6·3 | 78·2 | 50% | 37·3 | 0·3 | 74·3 | 27% | 3·0 | ·· | ·· | 50% |
| Liver and any (not lung) | 47 | 100% | 5 | 11% | 23·1 | 13·2 | 33·1 | 20% | 22·7 | 13·5 | 31·8 | 20% | 0·7 | ·· | ·· | 50% |
| Any other (not liver) | 36 | 100% | 15 | 42% | 37·4 | 21·3 | 53·5 | 23% | 28·4 | 22·2 | 34·8 | ·· | 12·7 | 4·5 | 20·9 | 63% |
According to number and combination of metastatic sites. Median overall survival (mOS) in months and estimated 5-year OS-rate for resected patients calculated from diagnosis of metastatic disease (mOS mCRC) and from 1st resection (mOS 1st resection), and recurrence-free survival (mRFS 1st resection) and 1-year RFS-rate calculated from first resection.
mOS = median overall survival from metastatic colorectal cancer diagnosis, mOS 1st resection = median overall survival from first resection, mRFS 1st R0/1-resection = median relapse-free survival from first resection with R0/1 and/or local ablative therapy (LAT), NE = no estimate.
Recurrence in resected organ or new metastatic sites were seen in 216 (66%) of 326 R0/1-resected, at median 18·8 (95% CI 13·8–23·8) months from 1st resection. Re-resections were performed in 121 (37%) patients with relapse in resected organ or new metastases.
4. Discussion
To the best of our knowledge, RAXO is the only prospective study to report results from centralized repeated MDT intervention on metastasis resectability (41%) upfront and after conversion therapy in a nationwide real-world patient population with mCRC, and to evaluate how often this practice leads to a metastasis resection and/or LAT (37%) of any single- or multisite metastases. These encouraging resection and/or LAT rates are in line with those from selected single-site series (11%–54%) and higher than in unselected single-site series (1%–30%) or in population-based series, also ours from Tampere and Turku university hospitals (12%–16%) (appendix pp 2–4, 17). [5, [16], [17], [18]] Our apparently high rates are probably due to high action rates on resectability, including LAT (4%) for non-optimally fit and elderly patients. Resections and/or LAT were performed not just for upfront resectable, but especially for conversion opportunities (18%), that are usually reported in 28%–61% for single-site and 5%–16% for any single- or multisite metastases (appendix pp 2–4). [22]
MDT practice is essential in treatment planning and guidelines recommend that all mCRC patients should be evaluated upfront by an MDT, but no clear guidance is given on repeated assessments at organ-specific MDTs, such as liver, thoracic, peritoneal etc., for multisite metastases. [9], [10], [11], [12] MDT practices vary between hospitals and may be reflected in the wide variability of reported resection rates, [21, 22] clearly seen in the pre-RAXO era (2005–2011) in the population-based RAXO subprotocol for the data collection cohort separated for the five university districts (appendix p 16). Resectability is also highly dependent on the experience and the skills of the MDT members and the surgeons performing the metastasectomies. [15, 28] In our study, underestimation of resectability locally was two to three times more common for CRC-MDT (general colorectal surgeon) or oncologist assessed, than in organ-specific MDT or specialist surgeon assessed cases. These findings are also in line with findings that specialist hepatobiliary surgeons refer patients for liver resection more often than general surgeons and oncologists. [21] Disagreement on resectability is common, [20, 29] and this was also observed in our study as upfront resectability or borderline status was underestimated in 12%–25% and overestimated in 9%–25% by organ-specific MDT or specialist surgeons compared with central assessment. Our encouraging results are likely a result of all metastatic patients, not liver- and lung-only, being assessed repeatedly by an experienced MDT at a high-volume academic centre, being able to optimize many of the challenges mentioned above.
Our high 5-year OS-rates of 66% and long mOS of 80 months among any-site R0/1-resected compare well with 16%–75%/13–72 months for single-site metastases in the literature (appendix pp 2–4), and in the population-based RAXO subprotocol for the data collection cohort with 53%–67%/65–79 months for Tampere and Turku University Hospitals (appendix p 17). All our resections were intended to be curative, but 15% became non-radical due to an R2-resection of the metastases or primary, or progression between planned sequential metastasectomy. Our 5-year OS rate among R2-resected (39%) with caveat of inclusion of LAT patients also compares well with 15%–30% in reviews of debulking surgery. [30, 31] Results of a clarifying randomized study investigating debulking surgery are awaited. [30] There was no difference in survival for upfront resectable versus converted, contrary to early findings, [32] and in line with a recent finding. [33]
Quadrupled or doubled survival compared with “systemic therapy alone” was achieved with maximized curative intent resections and effective use of systemic therapy. Mean number of resections per patient or re-resections is rarely reported, (appendix pp 2–4) [15, 26] but seems higher (1.7 procedures/patient) in our study implicating high multisite resection and re-resection rates (37%). In the literature, a re-resection rate of up to 40% has been noted, with apparently improved survival in re-resected patients. [34] Duration of all lines of systemic therapy for mCRC has not previously been reported as far as we are aware. In our study, just 9 months of systemic therapy in total, including conversion, neoadjuvant, adjuvant and all lines of disease control therapy in R0/1-resected, 14 months in R2-resected and/or LAT and 12 months in “systemic therapy alone” were given. This is short compared with 6–8 months already for 1st line according to the minireview (appendix pp 2–4). Neoadjuvant/conversion therapy was given in 74% of resected and adjuvant in 77%; in line with a regional Finnish study, [5] and more often than in previously published studies with 5%–38% and 20%–68% (appendix pp 2–4). Disease control therapy, i.e. palliative chemotherapy, was given to 82% during trajectory, with only first-line, second line, and third or later line of therapy given in 39%, 26% and 35%, respectively. In the population-based data collection cohort for Tampere and Turku University Hospitals systemic therapy was given in 55%–66% in line with previously published, and mOS for “systemic therapy only” was 22 months compared with 21 months in the RAXO prospective study and 15 months in previously published studies (appendix p 17).
The most common resectable metastatic sites, liver, local, peritoneum, lung, and ovary, were present in well over half of the patients at mCRC diagnosis, in line with previous findings, [3] and of these single or multiple metastatic sites, our resection rates were in the high range for respective single-site rates according to the minireview (appendix pp 2–4, 17). [5] Of metastases at these sites developing during the entire disease trajectory, up to one-third were resected, i.e. nearly as often as baseline metastases, with little published previously. [34]
Re-resections, not just of the resected organs, but of all new metastatic sites, could probably be one key to long OS in our study, in line with Japanese findings, [34] as RFS was modest and in line with previous findings (13–27 months for 1–2 metastatic sites in our study versus 8–33 months for single-site in the literature; appendix pp 2–4).
Median time to appearance of first, second, and third new metastatic sites were 11, 17, and 27 months, in line with previous timelines, and time to recurrence after resection of 19 months, which confirms that relatively long follow-up is needed in patients fit for re-resection. [3, 8, 35, 36] Metastatic sites like bone, brain, and suprarenal clearly presented later and are rarely curatively resectable and linked to shorter survival, seen in our study and in the literature. [8] The longer survival for resected versus non-resected in all prognostic groups, in line with previous findings (appendix pp 2–4), and also in adjusted analysis, is clinically meaningful and all patients should be regularly assessed for resectability, regardless of prognostic factors and metastatic sites. The adjusted and unadjusted models whether adjusted for essential patient characteristics or stage, grade, presentation, tumor location etc., were significant, contrary to recent findings for adjusted models for lung resection or combined liver and lung resection. [17, 26, 27]
The gap between rates of technical resectability versus performed resections and/or LAT and reasons for this gap are important to consider. A poor prognosis in resectable patients who were not resected was noted in our study, in line with older series (appendix p 2), but contrary to the PULMICC- and SEER database findings for lung metastases. [17, 26, 27] Progression during neoadjuvant therapy is a relative contraindication for resection, [37] and was the reason why more than half of patients with resectable disease were not resected. Marked comorbidity (second most common reason), including frailty and poor performance status, are known negative prognostic markers, but in our study, the apparently longer OS seen in patients with comorbidities, poor performance status and higher age (HRs 0·18–0·24) speak against a restrictive attitude to these patient groups. Second non-CRC malignancy is an exclusion criterion in most previous studies but was present in 13% in this study and was a contraindication for metastasectomy only if metastatic.
Our online second opinion practice is applicable to diverse healthcare settings as patients are not referred for treatment at the tertiary referral centre performing the MDT, but treated according to local practice, with caution that metastasectomies should be centralized to hospitals with sufficient organ-specific expertise. [38]
The non-randomized study design limits causality assessments and the patient groups cannot be formally compared. Nevertheless, the long-term observational nature with a high number of patients, with zero loss to follow-up, has allowed us to describe the clinical behavior, pattern of metastases, treatments, and outcomes in detail. Nationwide we achieved approximately 40% enrolment of eligible mCRC patients in line with high-recruiting academic centres, and clearly higher than the general <5% enrolment rate reported in trials. [39] Thus, our prospective intervention study was not population-based and may be prone to selection bias, but on the other hand, it was nationwide and all hospitals, irrespective of size and expertise, contributed, increasing generalizability of results. The goal of the study was to assess resectability of metastases and thus inclusion is probably skewed towards inclusion of resectable and borderline, e.g. patients with liver and lung metastases, rather than never resectable patients, increasing the resectability rate. We do not have full information on the impact of second opinion on final resection and conversion decisions taken locally, only of baseline assessments before inclusion showing discrepancy in nearly half. According to our ongoing population-based data collection there are no major differences in demographics or outcomes between population-based data for two university hospitals and the prospective RAXO-study data. We are collecting data on population-based metastasectomies and can so far show an increase in liver resection rates from 9% during the preceding 7 years to 14% nationwide during the RAXO-era, without difference in survival for more than half of the Finnish patients, which were resected at Helsinki University Hospital. Reasons therefore are multiplex but presumably to some extent attributable to the intervention and the improved care practice. Central assessment without full knowledge of the patients’ condition may be criticized but provided a good estimate for technical resectability. This may carry a risk for overestimating resectability, which can be reflected in slightly higher R2-resection rates in multisite metastases compared with single-site metastases. This, on the other hand, probably led to higher resectability and/or LAT in patients who were elderly, not fully fit or with adverse prognostic features such as multisite metastases and BRAF mutations, groups who still appeared to benefit from resection.
The next step is to make the data fully population-based. Postoperative morbidity and systemic treatments are being analyzed in detail. Long-term quality-of-life and cost-utility evaluations have started. Prognostic and predictive biomarker studies using tissue samples and consecutively collected blood samples are ongoing.
5. Conclusions
To conclude, nationwide, repeated resectability assessment by centralized MDT was offered online to all Finnish hospitals. The local oncologist organized the systemic therapy and oversaw repeated referrals to organ-specific MDTs and resections at high-volume centres. This resulted in high any-site and multisite resectability, conversion, and resection rates, with impressive survival, not just for upfront resectable and converted but also for recurrences after resection and later appearing metastases. We hope this study encourages others to adopt similar practices.
Congresses
Presented in part at the American Society of Clinical Oncology (ASCO) congress, Chicago, May 31-June 4, 2019; (ESMO) World Congress on Gastrointestinal Cancer, Barcelona, July 3–6, 2019; and the European Society of Medical Oncology (ESMO) Congress, Barcelona, September 27-October 1, 2019. Presentation was cancelled due to COVID-19 at the European Surgical Association (ESA) congress, Köln, Germany, May 22–23, 2020.
Authors’ Contributions
PO, HI, LMS, PH, TS, AÅ, RR, EH, RK, AML, and KL comprised the steering committee and participated in all phases of the study, including the design or conduct of the study, analyses, and interpretation of the data and preparation of the manuscript. All authors recruited patients or gathered data for the study. PO did the statistical analyses and TP checked data and verified the main results of the statistical analyses. All authors interpreted the data and were involved in the review and writing of the manuscript and the decision to submit for publication.
Declaration of Interests
All authors report institutional research funding from Eli Lilly, Merck KGaA, Roche Finland, Sanofi and unrestricted grants from Amgen and Servier, during the conduct of the study. PO, HI, LMS, PH, TS, AÅ, RR, EH, RK, AML, KL and TML report grants, personal fees or non-financial support from Abbvie, Amgen, Astra-Zeneca, Bayer, Celgene, Eli Lilly, Eisai, Erytech Pharma, Incyte, Fresenius, Jansen-Cilag, Merck, MSD, Nordic Drugs, Nutricia, Pierre-Fabre, Roche, Sanofi, Servier, Sobi or Varian.
Data sharing
The data collected for this study can be made available to others in de-identified form after all primary and secondary endpoints have been published, in the presence of a data transfer agreement, and if the purpose of use complies with Finnish legislation. Requests for data sharing can be made to the corresponding author, including a proposal that must be approved by the steering committee.
Acknowledgments
We thank the patients and their families, the investigators, study personnel, and the hospitals that have participated in this study. This investigator-initiated study was supported by Finska Läkaresällskapet, Cancer Foundation Finland, The Competitive State Research Financing of the Expert Responsibility Area of Tampere, Helsinki, Turku and Satakunta Hospitals, Tampere University Hospital Fund (Tukisäätiö and OOO-project), Research Fund of Helsinki University Hospital, and the infrastructure with database and study nurses partly by pharmaceutical companies (Amgen, Eli Lilly, Merck KGaA, Roche Finland, Sanofi and Servier). The funders had no role in the study design and conduct of the study, collection, analysis, interpretation of the data or in writing of this report. We thank the study units in Helsinki, Tampere and Oulu for monitoring the study and the OOO-project at Tampere University Hospital and Auria biobank at Turku University Hospital for data acquisition. We thank professor Halfdan Sorbye, professor Per Pfeiffer and professor Bengt Glimelius for sharing data on file for the population-based Scandinavian SPCRC study. The authors acknowledge professor Bengt Glimelius for sharing his expertise regarding study design, database content, data analysis and writing of the manuscript, professor Heikki Joensuu for critical comments on the manuscript, and the medical writers at Meducom BV, Wilko Coers, PhD and Sandy Field, PhD, who were compensated for their support by the first author.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100049.
Contributor Information
Pia Osterlund, Email: pia.osterlund@pshp.fi, pia.osterlund@helsinki.fi.
Tapio Salminen, Email: tapio.salminen@pshp.fi.
Leena-Maija Soveri, Email: leena-maija.soveri@keusote.fi.
Raija Kallio, Email: raija.kallio@ppshp.fi.
Ilmo Kellokumpu, Email: ilmo.kellokumpu@ksshp.fi.
Annamarja Lamminmäki, Email: annamarja.lamminmaki@kuh.fi.
Päivi Halonen, Email: paivi.halonen@hus.fi.
Raija Ristamäki, Email: raija.ristamaki@tyks.fi.
Eila Lantto, Email: eila.lantto@phhyky.fi.
Aki Uutela, Email: aki.uutela@hus.fi.
Emerik Osterlund, Email: emerik.osterlund@gmail.com.
Ali Ovissi, Email: ali.ovissi@hus.fi.
Arno Nordin, Email: arno.nordin@hus.fi.
Eetu Heervä, Email: eetu.heerva@tyks.fi.
Kaisa Lehtomäki, Email: kaisa.lehtomaki@pshp.fi.
Jari Räsänen, Email: jari.rasanen@hus.fi.
Maija Murashev, Email: maija.murashev@satasairaala.fi.
Laura Aroviita, Email: laura.aroviita@khshp.fi.
Antti Jekunen, Email: antti.jekunen@vshp.fi.
Reneé Lindvall-Andersson, Email: renee.lindvall-andersson@ahs.ax.
Paul Nyandoto, Email: paul.nyandoto@phhyky.fi.
Juha Kononen, Email: juha.kononen@docrates.com.
Anna Lepistö, Email: anna.lepisto@hus.fi.
Tuija Poussa, Email: tpoussa@netti.fi.
Timo Muhonen, Email: timo.muhonen@iki.fi.
Annika Ålgars, Email: annika.algars@tyks.fi.
Helena Isoniemi, Email: helena.isoniemi@hus.fi.
Appendix. Supplementary materials
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sorbye H., Köhne C.-.H., Sargent D.J., Glimelius B. Patient characteristics and stratification in medical treatment studies for metastatic colorectal cancer: a proposal for standardization of patient characteristic reporting and stratification. Ann Oncol. 2007;18(10):1666–1672. doi: 10.1093/annonc/mdm267. [DOI] [PubMed] [Google Scholar]
- 3.Holch J.W., Demmer M., Lamersdorf C. Pattern and dynamics of distant metastases in metastatic colorectal cancer. Visceral Med. 2017;33(1):70–75. doi: 10.1159/000454687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Gijn W., Marijnen C.A., Nagtegaal I.D. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12(6):575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 5.Vayrynen V., Wirta E.V., Seppala T. Incidence and management of patients with colorectal cancer and synchronous and metachronous colorectal metastases: a population-based study. BJS Open. 2020;4(4):685–692. doi: 10.1002/bjs5.50299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J., Li S., Liu Y., Zhang C., Li H., Lai B. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: a population-based analysis. Cancer Med. 2020;9(1):361–373. doi: 10.1002/cam4.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Augestad K.M., Bakaki P.M., Rose J. Metastatic spread pattern after curative colorectal cancer surgery. A retrospective, longitudinal analysis. Cancer Epidemiol. 2015;39(5):734–744. doi: 10.1016/j.canep.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Stewart C.L., Warner S., Ito K. Cytoreduction for colorectal metastases: liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr Probl Surg. 2018;55(9):330–379. doi: 10.1067/j.cpsurg.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Cutsem E., Cervantes A., Adam R. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 10.Benson A.B., Venook A. NCCN guidelines for rectal cancer. Natl Compr Cancer Netw Guidel. 2020 doi: 10.6004/jnccn.2020.0032. (Accessed 05.09.2020) [DOI] [PubMed] [Google Scholar]
- 11.Benson A., Venook A. NCCN guidelines for colon cancer. Natl Compr Cancer Netw Guidel. 2020 (Accessed 05.09.2020) [Google Scholar]
- 12.Yoshino T., Arnold D., Taniguchi H. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29(1):44–70. doi: 10.1093/annonc/mdx738. [DOI] [PubMed] [Google Scholar]
- 13.Heinemann V., von Weikersthal L.F., Decker T. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 14.Venook A.P., Niedzwiecki D., Lenz H.J. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317(23):2392–2401. doi: 10.1001/jama.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenton H.M., Taylor J.C., Lodge J.P.A. Variation in the use of resection for colorectal cancer liver metastases. Ann Surg. 2019;270(5):892–898. doi: 10.1097/SLA.0000000000003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Geest L.G., Lam-Boer J., Koopman M., Verhoef C., Elferink M.A., de Wilt J.H. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. 2015;32(5):457–465. doi: 10.1007/s10585-015-9719-0. [DOI] [PubMed] [Google Scholar]
- 17.Siebenhüner A.R., Güller U., Warschkow R. Population-based SEER analysis of survival in colorectal cancer patients with or without resection of lung and liver metastases. BMC Cancer. 2020;20(1):246. doi: 10.1186/s12885-020-6710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamers P.A.H., Elferink M.A.G., Stellato R.K. Informing metastatic colorectal cancer patients by quantifying multiple scenarios for survival time based on real-life data. Int J Cancer. 2021;148(2):296–306. doi: 10.1002/ijc.33200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed F., Kallioinen M., Braun M., Fenwick S., Shackcloth M., Davies R.J. Management of colorectal cancer metastases to the liver, lung or peritoneum suitable for curative intent: summary of NICE guidance. Br J Surg. 2020;107(8):943–945. doi: 10.1002/bjs.11609. [DOI] [PubMed] [Google Scholar]
- 20.Folprecht G., Gruenberger T., Bechstein W.O. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11(1):38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 21.Aubin J.M., Bressan A.K., Grondin S.C. Assessing resectability of colorectal liver metastases: how do different subspecialties interpret the same data? Can J Surg. 2018;61(4):251–256. doi: 10.1503/cjs.014616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modest D.P., Denecke T., Pratschke J. Surgical treatment options following chemotherapy plus cetuximab or bevacizumab in metastatic colorectal cancer-central evaluation of FIRE-3. Eur J Cancer. 2018;88:77–86. doi: 10.1016/j.ejca.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Goey K.K.H., Sørbye H., Glimelius B. Consensus statement on essential patient characteristics in systemic treatment trials for metastatic colorectal cancer: supported by the ARCAD Group. Eur J Cancer. 2018;100:35–45. doi: 10.1016/j.ejca.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Giobbie-Hurder A., Gelber R.D., Regan M.M. Challenges of guarantee-time bias. J Clin Oncol. 2013;31(23):2963–2969. doi: 10.1200/JCO.2013.49.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birgisson H., Wallin U., Holmberg L., Glimelius B. Survival endpoints in colorectal cancer and the effect of second primary other cancer on disease free survival. BMC Cancer. 2011;11:438. doi: 10.1186/1471-2407-11-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treasure T., Farewell V., Macbeth F. Pulmonary metastasectomy versus continued active monitoring in colorectal cancer (PulMiCC): a multicentre randomised clinical trial. Trials. 2019;20(1):718. doi: 10.1186/s13063-019-3837-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milosevic M., Edwards J., Tsang D. Pulmonary metastasectomy in colorectal cancer: updated analysis of 93 randomized patients - control survival is much better than previously assumed. Colorectal Dis. 2020;22(10):1314–1324. doi: 10.1111/codi.15113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones R.P., Vauthey J.N., Adam R. Effect of specialist decision-making on treatment strategies for colorectal liver metastases. Br J Surg. 2012;99(9):1263–1269. doi: 10.1002/bjs.8835. [DOI] [PubMed] [Google Scholar]
- 29.Huiskens J., Bolhuis K., Engelbrecht M.R. Outcomes of resectability assessment of the Dutch colorectal cancer group liver metastases expert panel. J Am Coll Surg. 2019;229(6) doi: 10.1016/j.jamcollsurg.2019.08.1445. 523-32.e2. [DOI] [PubMed] [Google Scholar]
- 30.Gootjes E.C., Bakkerus L., Ten Tije A.J. The value of tumour debulking for patients with extensive multi-organ metastatic colorectal cancer. Eur J Cancer. 2018;103:160–164. doi: 10.1016/j.ejca.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Adam R., Kitano Y., Abdelrafee A., Allard M.A., Baba H. Debulking surgery for colorectal liver metastases: foolish or chance? Surg Oncol. 2020;33:266–269. doi: 10.1016/j.suronc.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Adam R., Delvart V., Pascal G. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644–657. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawamura J., Yazawa T., Sumida K. Clinical efficacy of liver resection after downsizing systemic chemotherapy for initially unresectable liver metastases. W J Surg Oncol. 2016;14:56. doi: 10.1186/s12957-016-0807-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto T., Hasegawa S., Hida K., Kawada K., Sakai Y., Sugihara K. Role of repeat resection in patients with metastatic colorectal cancer: a multicenter retrospective study. Dis Colon Rectum. 2019;62(5):561–567. doi: 10.1097/DCR.0000000000001311. [DOI] [PubMed] [Google Scholar]
- 35.Jones R.P., Jackson R., Dunne D.F. Systematic review and meta-analysis of follow-up after hepatectomy for colorectal liver metastases. Br J Surg. 2012;99(4):477–486. doi: 10.1002/bjs.8667. [DOI] [PubMed] [Google Scholar]
- 36.Brudvik K.W., Bains S.J., Seeberg L.T. Aggressive treatment of patients with metastatic colorectal cancer increases survival: a scandinavian single-center experience. HPB Surg. 2013;2013 doi: 10.1155/2013/727095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vigano L., Capussotti L., Barroso E. Progression while receiving preoperative chemotherapy should not be an absolute contraindication to liver resection for colorectal metastases. Ann Surg Oncol. 2012;19(9):2786–2796. doi: 10.1245/s10434-012-2382-7. [DOI] [PubMed] [Google Scholar]
- 38.Weledji E.P. Centralization of liver cancer surgery and impact on multidisciplinary teams working on stage IV colorectal cancer. Oncol Rev. 2017;11(2) doi: 10.4081/oncol.2017.331. 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorbye H., Pfeiffer P., Cavalli-Bjorkman N. Clinical trial enrollment, patient characteristics, and survival differences in prospectively registered metastatic colorectal cancer patients. Cancer. 2009;115(20):4679–4687. doi: 10.1002/cncr.24527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.