Abstract
Background
The cell-based quadrivalent influenza vaccine (QIVc) is now offered as an alternative to egg-based quadrivalent (QIVe) and adjuvanted trivalent (aTIV) influenza vaccines in the UK. While post-licensure studies show non-inferiority of cell-based vaccines, it is not known how its safety profile compares to other types of vaccines in real-world use.
Methods
We conducted a retrospective cohort study using computerised medical records from the Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) sentinel network database. We used a self-controlled case series design and calculated the relative incidence (RI) of adverse events of interest (AEIs) over different risk periods. We then compared the RIs of AEIs within seven days of vaccination overall and between QIVc and QIVe in the 18–64 years age group, and between QIVc and aTIV in the ≥65 years age group.
Findings
The majority of AEIs occurred within seven days of vaccination, and a seasonal effect was observed. Using QIVc as the reference group, QIVe showed similar incidence of AEIs whereas live attenuated influenza vaccine (LAIV) and aTIV had lower incidence of AEIs. In the stratified analyses, QIVe and aTIV were associated with a 16% lower incidence of AEIs in the seven days post-vaccination in both the 18–64 years and ≥65 years age groups.
Interpretation
Routine sentinel network data allow comparisons of safety profiles of equally suitable seasonal influenza vaccines. The higher incidence of AEIs associated with QIVc suggest monitoring of several seasons would allow robust comparisons to be made.
Funding
Public Health England.
Keywords: Influenza; Influenza vaccines; Adverse events of interest; Medical records systems, computerized; Sentinel surveillance
Research in context.
Evidence before this study
To summarise evidence on the safety profile of cell culture-based influenza vaccines, we conducted a search in MEDLINE on 16th October 2020 for journal articles using a combination of search terms including “cell culture-based”, “cell culture-derived”, “influenza vaccine”, “adverse event”, “adverse reaction”, and “safety”. We excluded articles on pandemic influenza vaccines and reviews, and limited the results to articles published in English between 2000 and 2020. We identified 18 relevant studies, of which 16 are reports of clinical trials and two are post-licensure safety surveillance studies. Trials that compared cell culture-based and egg-based trivalent influenza vaccines generally reported comparable safety and reactogenicity profiles across various age groups. Two trials that compared cell culture-based quadrivalent influenza vaccine (QIVc) with cell culture-based trivalent influenza vaccines found slightly more frequent solicited adverse events. Post-licensure safety surveillance studies did not find any concerning patterns of adverse events associated with cell culture-based influenza vaccines.
Added value of this study
This study provides the first comparison of incidence of adverse events of interest associated with QIVc and its egg-based alternatives using real-world evidence. The key finding here is that in age-stratified analyses, the recommended ‘equally suitable’ alternative to QIVc in the 18–64 years (QIVe) and ≥65 years (aTIV) age groups had a 16% lower incidence of AEIs in the seven days post-vaccination. Our post-hoc analysis also showed reactogenicity profiles differed between vaccine types and influenza seasons.
Implications of all the available evidence
Routine sentinel network data can provide real-world evidence for the safety of different types of vaccines. While earlier clinical trials showed comparable safety and reactogenicity profiles between cell culture-based and egg-based vaccines, our study using a much larger sample detected a higher incidence of adverse events of interest in those who received the cell culture-based quadrivalent influenza vaccine. Our findings highlight the importance of ongoing monitoring of adverse events of interest; the availability of longitudinal data would allow for more robust comparisons to be made.
Alt-text: Unlabelled box
1. Introduction
Cell culture-based influenza vaccines may have advantages over traditional egg-based vaccines. Firstly, it requires shorter production time, which allows for quicker scale-up of vaccine production in case of an influenza pandemic [1]. The cell culture-based vaccine also presents no risk to individuals who are allergic to eggs [2]. Most importantly, it circumvents concerns around the haemagglutinin mutations that occur during isolation, adaptation and propagation in eggs, which can alter viral antigenicity and are hypothesised to contribute to lower vaccine effectiveness (VE) [3,4].
Several VE studies have been conducted using real-world data from the United States, but the evidence is mixed. Two large retrospective cohort studies reported that the cell culture-based influenza vaccine (QIVc) was more effective in preventing hospital encounters related to influenza and certain respiratory events [5,6]. One study found QIVc provided better protection against only influenza B than egg-based influenza vaccines [7], while another found the egg-based quadrivalent influenza vaccine (QIVe) provided better protection against only A(H1N1)pdm09 than QIVc [8]. One retrospective cohort study and one test-negative case-control study reported no significant differences in effectiveness between QIVc and QIVe [9,10].
A cell culture-based quadrivalent influenza vaccine (Flucelvax® Tetra, Seqirus) was licensed for use in the United Kingdom (UK) for patients aged nine years and above in 2018, and was first recommended in the national influenza vaccination programme in the 2019/20 influenza season [11]. This QIVc is prepared from influenza virus propagated in Madin-Darby Canine Kidney (MDCK) cells. The Joint Committee on Vaccination and Immunisation (JCVI) considers it ‘equally suitable’ to the QIVe for those aged nine to 64 years in clinically at-risk and other eligible groups, and to the adjuvanted trivalent influenza vaccine (aTIV) in those aged ≥65 years [12].
More studies are needed to better evaluate the differences between QIVc and QIVe, yet there have not been any published studies to date that examine the safety of QIVc relative to other types of vaccines using real-world data. A better understanding of its safety profile will allow us to better weigh the risks and benefits associated with QIVc.
We conducted this study to: (1) calculate the relative incidence (RI) of adverse events of interest (AEIs) following seasonal influenza vaccination across all vaccine types, (2) compare RIs between QIVc and QIVe in at-risk adults aged 18 to 64 years, and (3) compare RIs between QIVc and aTIV in older adults aged ≥65 years.
2. Methods
2.1. Data source
We used the Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) sentinel network database. This is derived from pseudonymised extracts of computerised medical record (CMR) system data. The UK lends itself to this type of study as it has registration-based primary care (one patient registers with a single general practice) and CMRs have been in routine use in the UK for over 20 years. The RCGP RSC was established in 1957, and has been involved in influenza monitoring and assessing influenza vaccine effectiveness since 1967 [13]. The scheme includes practices recruited to be representative of the population [14]. At the time of this study, the RCGP RSC included around 5 million patient records from >500 practices across England; but has more recently expanded considerably in response to the COVID-19 pandemic [15].
We included all patients who had received a seasonal influenza vaccination between 1st September 2019 and 30th April 2020, but excluded those who were aged over 100 years at the time of vaccination and those who received monovalent pandemic influenza vaccines. Patients who attended practices involved in other enhanced surveillance programmes were also excluded. From our experience, this more active surveillance increased awareness and recording at the practices, and captured more adverse events such as local minor reactions for which vaccines may not have sought medical attention otherwise [16], which may distort comparisons between types or brands of vaccine and between years. Patients were followed up retrospectively for a list of adverse events of interest (AEIs) following vaccination as pre-specified by the European Medicines Agency (EMA) [17]. We had used a set of Read codes that matched the EMA's list to identify AEIs in earlier studies as well as three years of observation for a vaccine manufacturer, and this set of codes has been updated to SNOMED clinical terms (the UK's new clinical terminology) for this study. The AEIs identified include common conditions which may not be causally related to the vaccine, any signal is inferred from a change in incidence in the seven days following vaccination.
We extracted the following data: age, sex, self-reported ethnicity using an ontology to maximise data capture [18], socioeconomic status using Index of Multiple Deprivation (IMD) quintiles, vaccination date, vaccine type, vaccine manufacturer, AEI date, AEI type, and dates of registration and deregistration at the practice. IMD is derived from post code of the patient at the individual level at the point of data extraction, the post code is not retained [19].
Finally, we compared the rates of AEIs with the previous season, where available. We did this because in previous research we saw year-on-year changes in AEIs [20].
2.2. Statistical analyses
We used the self-controlled case series (SCCS) design [21,22] for this study. The SCCS method is a case-only method that compares the rate of events during pre-defined exposure risk periods with the rate of events during all remaining time in the observation period (i.e. baseline risk periods) within individuals. The major advantage of this method is that it eliminates potential time-invariant confounding effects of between-person characteristics, as each individual acts as their own control.
We conducted three separate SCCS models to investigate the incidence of AEIs following seasonal influenza vaccination, with the observation period defined as 1st September 2019 to 30th April 2020 in all models. In Model 1, the exposure risk periods were days −7 to −1, days 0 to 6, days 7 to 13, and days 14 to 45 where day 0 is the day of vaccination. Given the seasonal variation in the incidence of some of the events of interest, we incorporated an additional variable with the observation period divided into 8 seasonal periods: days 0 to 29, days 30 to 59, days 60 to 89, days 90 to 119, days 120 to 149, days 150 to 179, days 180 to 209, days 210 to 241 where day 0 is the beginning of the influenza season. We calculated the RI of AEIs following vaccination for the different exposure risk periods and different seasonal periods. In Model 2, we modelled modification effects of vaccine type by including an interaction term. Models 3 and 4 were stratified analyses in which we compared the RIs of AEIs between QIVc and QIVe in those aged 18 to 64 years and between QIVc and aTIV in those aged ≥65 years respectively by adding an interaction term in the models. In Models 2–4 we focused only on the exposure risk period of days 0 to 6, as previous studies showed the risk of AEIs were highest in this period [20].
All statistical analyses were conducted in R version 3.4.4 [23], with the following packages: tidyverse version 1.2.1 [24], SCCS version 1.1 [25], lubridate version 1.7.4 [26], tableone version 0.10.0 [27]. Graphical output was generated using packages ggplot2 version 3.1.1 [28] and ggthemes version 4.1.0 [29].
2.3. Ethical considerations
All potentially identifiable data were pseudonymised as close to source as possible and not made available to researchers; data were not extracted for patients who opted out of data sharing. All data were stored and processed at the RCGP RSC secure data and analytics hub, the University of Surrey. According to the Health Research Authority and Medical Research Council Regulatory Support Centre's online decision tool, this study falls under the category of service evaluation and does not require further ethical review. This study was approved by the RCGP.
2.4. Role of the funding source
The RCGP RSC's principal funder is Public Health England (PHE). PHE had no role in study design; data collection, analysis, or interpretation; writing of the report; or the decision to submit it for publication. No specific funding was allocated for the writing of this annual report paper, which focusses on a topic issue, in this case whether the introduction of cell culture-based manufactured influenza vaccine would be associated with any difference in the pattern of adverse events of interest. The corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
3. Results
3.1. Study participants
A total of 1,108,632 patients in the RCGP RSC network who met the inclusion criteria received a seasonal influenza vaccine in the 2019/20 season. This cohort had a mean age of 56 years and a slight female preponderance (54.5% female); a large majority (72.9%) was of white ethnicity. Detailed demographic characteristics of the cohort are presented in Table 1.
Table 1.
Demographic characteristics of all recipients of seasonal influenza vaccines in the 2019/20 season in the RCGP RSC network (n = 1,108,632).
| Mean±SD / n (%) | |
|---|---|
| Age (years) | 56.04±26.79 |
| Sex | |
| Female | 604,048 (54.5%) |
| Male | 504,584 (45.5%) |
| Ethnicity | |
| White | 808,086 (72.9%) |
| Asian | 53,538 (4.8%) |
| Black | 21,395 (1.9%) |
| Mixed | 9,750 (0.9%) |
| Other | 6,665 (0.6%) |
| Missing | 209,198 (18.9%) |
| Index of Multiple Deprivation (IMD) Quintile | |
| 1 – most deprived | 148,646 (13.4%) |
| 2 | 182,643 (16.5%) |
| 3 | 217,221 (19.6%) |
| 4 | 247,451 (22.3%) |
| 5 – least deprived | 285,674 (25.8%) |
| Missing | 26,997 (2.4%) |
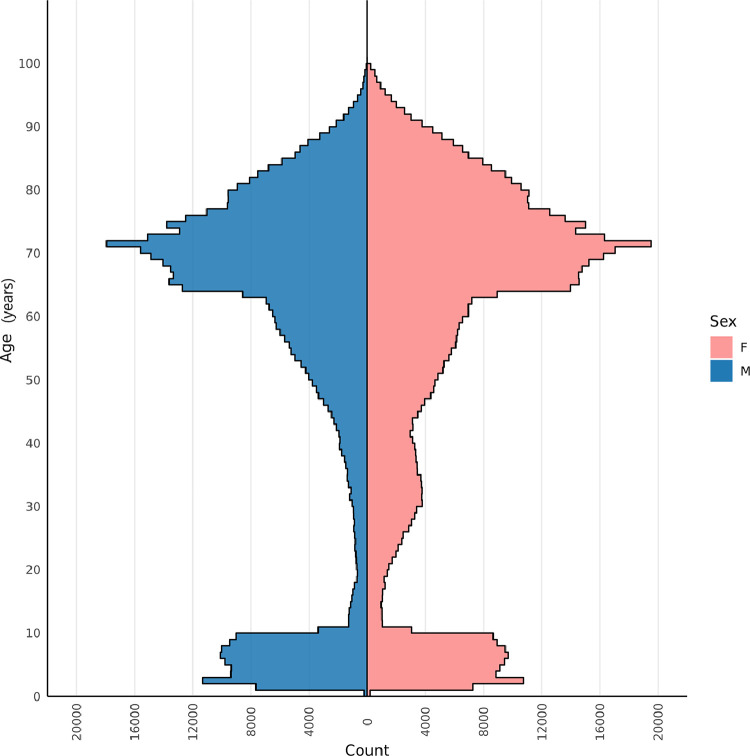
The age-sex profile (Fig. 1) shows a peak in the older age group, with the sharp increase beginning from 64 years, and reaching the peak at 71 years. Children between the ages of one and ten years are the next biggest group of vaccines. In addition, there is a small increase in numbers of women aged between 20 and 40 years, probably reflecting the recommendation for vaccination in pregnant women.
Fig. 1.
Age-sex profile for all seasonal influenza vaccine recipients in the RCGP RSC network in the 2019/20 season.
3.2. Types of vaccines
We were able to identify 98.6% of the vaccines administered. There were six categories of seasonal influenza vaccines in our dataset, comprising the adjuvanted trivalent influenza vaccine (aTIV), quadrivalent live attenuated influenza vaccine (LAIV), QIV (unspecified whether cell- or egg-based), QIVc, QIVe, trivalent influenza vaccine (TIV) and high-dose trivalent influenza vaccine (TIV-HD) (Table 2). We excluded the latter two categories from the analysis due to low numbers. We further excluded the QIV (unspecified) category and conducted complete case analysis in the stratified models.
Table 2.
Total number of different types of vaccines by age group.
| 0–1yr | 2–17yr | 18–64yr | ≥65yr | |
|---|---|---|---|---|
| aTIV | 0 | 267 | 10,073 | 616,648 |
| LAIV | 56 | 123,094 | 175 | 5 |
| QIV | 1 | 108 | 10,345 | 2,769 |
| QIVc | 5 | 305 | 26,687 | 29,384 |
| QIVe | 765 | 7,052 | 260,574 | 4,051 |
| TIV | 0 | 20 | 228 | 356 |
| TIV-HD | 0 | 0 | 2 | 4 |
Note. yr: years of age; aTIV: adjuvanted trivalent influenza vaccine; LAIV: live attenuated influenza vaccine; QIV: quadrivalent influenza vaccine; QIVc: cell culture-based quadrivalent influenza vaccine; QIVe: egg-based quadrivalent influenza vaccine; TIV: trivalent influenza vaccine; TIV-HD: high-dose trivalent influenza vaccine.
3.3. Incidence of AEIs
We observed AEIs in every EMA category, with the most commonly reported being arthropathy, cough and rash (Table 3). Of all AEIs, the ones that were most frequently reported within seven days of vaccination were wheezing, nasal congestion and cough. More severe conditions such as Guillain-Barré syndrome (n = 1) and anaphylaxis (n = 6) were rare within seven days of vaccination.
Table 3.
Total number of reported adverse events of interest across the 2019/20 season and within 7 days of vaccination.
| Within influenza season |
Within 7 days of vaccination |
|||||
|---|---|---|---|---|---|---|
| Total events | Unique individuals | Total events | Unique individuals | 7-day cumulative incidence (events per 100,000 doses) | Proportion of AEIs within 7 days of vaccination | |
| Fever / pyrexia | ||||||
| Fever (unspecified) | 10,436 | 8,763 | 325 | 315 | 29.32 | 0.0311 |
| Mild fever (<=38.5 °C) | 16,491 | 15,011 | 470 | 465 | 42.39 | 0.0285 |
| Moderate fever (38.6–39.5 °C) | 2,258 | 2,199 | 81 | 81 | 7.31 | 0.0359 |
| High fever (>39.5 °C) | 312 | 309 | 11 | 11 | 0.99 | 0.0353 |
| Gastrointestinal | ||||||
| Decreased appetite | 3,367 | 2,859 | 135 | 133 | 12.18 | 0.0401 |
| Diarrhoea | 21,196 | 14,250 | 677 | 629 | 61.07 | 0.0319 |
| Nausea | 5,801 | 4,070 | 193 | 181 | 17.41 | 0.0333 |
| Vomiting | 8,591 | 6,405 | 248 | 238 | 22.37 | 0.0289 |
| General non-specific symptoms | ||||||
| Drowsiness | 849 | 684 | 22 | 22 | 1.98 | 0.0259 |
| Fatigue | 16,664 | 11,998 | 942 | 909 | 84.97 | 0.0565 |
| Headache | 22,654 | 14,906 | 976 | 937 | 88.04 | 0.0431 |
| Irritability | 261 | 216 | 8 | 8 | 0.72 | 0.0307 |
| Malaise | 8,700 | 6,479 | 322 | 305 | 29.04 | 0.0370 |
| Local symptoms (i.e. local erythema) | 362 | 148 | 8 | 8 | 0.72 | 0.0221 |
| Musculoskeletal | ||||||
| Arthropathy | 162,996 | 88,236 | 8,614 | 8,316 | 776.99 | 0.0528 |
| Muscle aches / myalgia | 13,548 | 5,095 | 560 | 538 | 50.51 | 0.0413 |
| Neurological | ||||||
| Bell's palsy | 601 | 375 | 20 | 18 | 1.80 | 0.0333 |
| Guillain-Barré syndrome (GBS) | 61 | 35 | 1 | 1 | 0.09 | 0.0164 |
| Peripheral tremor | 2,822 | 1,950 | 153 | 148 | 13.80 | 0.0542 |
| Seizure / febrile convulsions | 7,396 | 3,862 | 348 | 337 | 31.39 | 0.0471 |
| Rash | 34,771 | 24,854 | 1,558 | 1,517 | 140.53 | 0.0448 |
| Respiratory / miscellaneous | ||||||
| Conjunctivitis | 8,482 | 7,200 | 339 | 336 | 30.58 | 0.0400 |
| Coryza | 1,348 | 1,260 | 73 | 73 | 6.58 | 0.0542 |
| Cough | 90,246 | 63,019 | 5,185 | 5,080 | 467.69 | 0.0575 |
| Epistaxis | 5,932 | 4,231 | 209 | 203 | 18.85 | 0.0352 |
| Hoarseness | 2,329 | 1,704 | 120 | 119 | 10.82 | 0.0515 |
| Nasal congestion | 2,067 | 1,741 | 120 | 120 | 10.82 | 0.0581 |
| Oropharyngeal pain | 15,281 | 12,137 | 474 | 460 | 42.76 | 0.0310 |
| Rhinorrhoea | 1,131 | 973 | 55 | 55 | 4.96 | 0.0486 |
| Wheezing | 10,542 | 8,629 | 1,152 | 114 | 103.91 | 0.1093 |
| Sensitivity / anaphylaxis | ||||||
| Anaphylactic reactions | 281 | 196 | 7 | 6 | 0.63 | 0.0249 |
| Facial oedema | 82 | 77 | 3 | 3 | 0.27 | 0.0366 |
| Hypersensitivity reactions | 7,662 | 6,585 | 416 | 411 | 37.52 | 0.0543 |
Note. The “total events” columns are the frequencies of events of interest within the indicated periods, including repeated consultations with the same individual. The “unique individuals” columns are counts of individuals who presented with these events of interest within the indicated periods, regardless of whether there were repeated consultations.
The four pre-specified risk periods were associated with different RIs of AEIs, with the majority of AEIs occurring in the seven days post-vaccination (Table 4). The seven days post-vaccination showed a doubling of risk of AEIs (RI=2.05, 95% CI 2.02–2.08) whereas the risk periods of days 7 to 13 and days 14 to 45 were associated with only a marginally increased RI. The seven days leading up to vaccination was associated with a 6% lower RI of AEIs (RI=0.94, 95% CI 0.92–0.96), reflecting the “healthy vaccine” effect that has previously been reported [20].
Table 4.
Model 1: Relative incidence of adverse events of interest in various risk periods and seasonal periods.
| RI | 95% CI | p | |
|---|---|---|---|
| Exposure risk period (with date of vaccination as day 0) | |||
| Days −7 to −1 | 0.94 | 0.92–0.96 | <0.001 |
| Days 0 to 6 | 2.05 | 2.02–2.08 | <0.001 |
| Days 7 to 13 | 1.04 | 1.02–1.06 | <0.001 |
| Days 14 to 45 | 1.03 | 1.01–1.04 | <0.001 |
| Time from start of the influenza season (reference: Days 0 to 29) | |||
| Days 30 to 59 | 1.00 | 0.99–1.02 | 0.694 |
| Days 60 to 89 | 1.05 | 1.03–1.06 | <0.001 |
| Days 90 to 119 | 0.90 | 0.89–0.92 | <0.001 |
| Days 120 to 149 | 1.12 | 1.11–1.13 | <0.001 |
| Days 150 to 179 | 1.11 | 1.09–1.12 | <0.001 |
| Days 180 to 209 | 0.88 | 0.86–0.89 | <0.001 |
| Days 210 to 241 | 0.57 | 0.56–0.58 | <0.001 |
In addition, we found a seasonal pattern in AEIs (Table 4). Using the first 30 days of the season as reference, the third, fifth and sixth seasonal periods showed an increase in AEIs (RI=1.05, 95% CI 1.03–1.06; RI=1.12, 95% CI 1.11–1.13; and RI=1.11, 95% CI 1.09–1.12 respectively), whereas the fourth, seventh and eighth seasonal periods showed a decrease in AEIs (RI=0.90, 95% CI 0.89–0.92; RI=0.88, 95% CI 0.86–0.89; and RI=0.57, 95% CI 0.56–0.58 respectively). The periods that showed decreases in consultations for AEIs roughly correspond to when the UK went into national lockdown due to the COVID-19 pandemic (announced on 23rd March 2020 and continued beyond 30th April 2020).
Incidence of AEIs by vaccine type
We added an interaction term to compare the incidence of AEIs associated with the different vaccine types across the entire cohort. The results showed that QIVc and QIVe were associated with a similar incidence of AEIs, whereas LAIV and aTIV were associated with 43% and 26% lower RIs than QIVc respectively (Table 5).
Table 5.
Model 2: Relative incidence of adverse events of interest and interaction term for vaccine type.
| RI | 95% CI | p | |
|---|---|---|---|
| Exposure risk period (with date of vaccination as day 0) | |||
| Days 0 to 6 | 2.42 | 2.30–2.55 | <0.001 |
| Time from start of influenza season (reference: Days 0 to 29) | |||
| Days 30 to 59 | 1.00 | 0.99–1.02 | 0.557 |
| Days 60 to 89 | 1.05 | 1.04–1.07 | <0.001 |
| Days 90 to 119 | 0.91 | 0.90–0.92 | <0.001 |
| Days 120 to 149 | 1.12 | 1.11–1.14 | <0.001 |
| Days 150 to 179 | 1.11 | 1.09–1.12 | <0.001 |
| Days 180 to 209 | 0.88 | 0.86–0.89 | <0.001 |
| Days 210 to 241 | 0.57 | 0.56–0.58 | <0.001 |
| Vaccination type (reference: QIVc) | |||
| QIVe | 0.96 | 0.90–1.02 | 0.148 |
| LAIV | 0.57 | 0.53–0.61 | <0.001 |
| aTIV | 0.74 | 0.70–0.78 | <0.001 |
Note. QIVc: cell culture-based quadrivalent influenza vaccine; egg-based quadrivalent influenza vaccine; LAIV: live attenuated influenza vaccine; aTIV: adjuvanted trivalent influenza vaccine.
3.4. Stratified analyses
We compared the incidence of AEIs associated with QIVc and QIVe in adults aged 18 to 64 years by incorporating an interaction term to the model. The results showed that relative to QIVc, QIVe was associated with 16% lower RI of AEIs (RI=0.84, 95% CI 0.78–0.91) (Table 5).
Similarly, we conducted a stratified analysis in older adults aged ≥65 years to compare the incidence of AEIs associated with QIVc and aTIV. The results showed that relative to QIVc, aTIV was associated with 16% lower RI of AEIs (RI=0.84, 95% CI 0.78–0.91) (Table 6).
Table 7.
Model 4: Relative incidence of adverse events of interest and interaction term for vaccine type in older adults aged ≥65 years.
| RI | 95% CI | p | |
|---|---|---|---|
| Exposure risk period (with date of vaccination as day 0) | |||
| Days 0 to 6 | 2.13 | 1.97–2.30 | <0.001 |
| Time from start of influenza season (reference: Days 0 to 29) | |||
| Days 30 to 59 | 1.00 | 0.98–1.01 | 0.556 |
| Days 60 to 89 | 1.04 | 1.02–1.06 | <0.001 |
| Days 90 to 119 | 0.89 | 0.88–0.91 | <0.001 |
| Days 120 to 149 | 1.16 | 1.14–1.18 | <0.001 |
| Days 150 to 179 | 1.11 | 1.09–1.13 | <0.001 |
| Days 180 to 209 | 0.86 | 0.84–0.87 | <0.001 |
| Days 210 to 241 | 0.59 | 0.58–0.60 | <0.001 |
| Vaccination type (reference: QIVc) | |||
| aTIV | 0.84 | 0.78–0.91 | <0.001 |
Table 6.
Model 3: Relative incidence of adverse events of interest and interaction term for vaccine type in adults aged 18 to 64 years.
| RI | 95% CI | p | |
|---|---|---|---|
| Exposure risk period (with date of vaccination as day 0) | |||
| Days 0 to 6 | 2.84 | 2.63–3.06 | <0.001 |
| Time from start of influenza season (reference: Days 0 to 29) | |||
| Days 30 to 59 | 0.97 | 0.94–0.99 | 0.006 |
| Days 60 to 89 | 0.98 | 0.95–1.00 | 0.069 |
| Days 90 to 119 | 0.83 | 0.81–0.85 | <0.001 |
| Days 120 to 149 | 1.07 | 1.04–1.10 | <0.001 |
| Days 150 to 179 | 1.05 | 1.02–1.07 | <0.001 |
| Days 180 to 209 | 0.88 | 0.86–0.90 | <0.001 |
| Days 210 to 241 | 0.59 | 0.57–0.60 | <0.001 |
| Vaccination type (reference: QIVc) | |||
| QIVe | 0.84 | 0.78–0.91 | <0.001 |
Note. QIVc: cell culture-based quadrivalent influenza vaccine; QIVe: egg-based quadrivalent influenza vaccine.
4. Discussion
4.1. Key findings
In this study, we examined the incidence of AEIs following seasonal influenza vaccination, and compared that of QIVc versus QIVe in clinically at-risk adults aged 18 to 64 years, and that of QIVc and aTIV in older adults aged ≥65 years. In line with data from earlier influenza seasons [20], we observed AEIs mainly occur within seven days of vaccination. The seasonal pattern observed was different to that of previous seasons; the RIs of AEIs was significantly lower in the last two seasonal periods, which was expected with the national lockdown in place at the time. This sharp decline in the number of consultations for respiratory conditions during that period can also be seen in our COVID-19 Observatory weekly return (orchid.phc.ox.ac.uk/index.php/cov-19/), and a recent analysis of primary care consultations in older adults similarly reported a reduction in total consultations [30].
Overall, the incidence of AEIs in the seven days post-vaccination did not appear to differ among patients who received QIVc or QIVe, but aTIV and LAIV were associated with lower RIs of AEIs. This general pattern was observed in the 2018/19 season too, with QIVe associated with higher incidence of AEIs relative to aTIV and LAIV [31]. This may partly be due to differences in reactogenicity in the different age groups, so we also conducted stratified analyses between two equally suitable vaccines within an age group.
In the stratified analyses, we found that QIVc was associated with higher RIs of AEIs within seven days of vaccination when compared to QIVe in adults aged 18 to 64 years and to aTIV in older adults aged ≥65 years. This is an unanticipated finding, as phase III trials showed the safety profile of QIVc was similar to two comparator TIVc, with only slightly higher incidence of solicited local AEIs [32,33], and post-licensure studies have repeatedly shown that the safety of QIVc is similar to cell-based TIV (TIVc), which in turn is similar to egg-based TIV [34].
We also explored whether the different types of vaccines were associated with different categories of AEIs. We found that the incidence of AEIs in most EMA categories were comparable between the four vaccines. The only category in which the incidence of AEIs associated with QIVc was evidently higher than the other vaccines was musculoskeletal symptoms (Figure S1 in the Supplementary); specifically, arthropathy accounted for a large part of this higher incidence.
It is possible that the differences in incidence of AEIs partly reflect health disparities between demographic groups. In the stratified analyses, there appears to be some differences in the age, sex, ethnicity and IMD quintile distributions between the vaccine groups (see Table S1 in Supplementary). Within the 18 to 64 years age group, a slightly greater proportion of QIVc recipients were female, from an ethnic minority and in the least deprived quintile compared to the QIVe group. For the ≥65 years age group, QIVc recipients were older, a greater proportion of them were female, from an ethnic minority as well as in the least deprived quintile compared to the aTIV group. Earlier studies that examined sex differences in the safety of seasonal influenza vaccines have reported higher rates of AEIs in females [35]. Greater pain sensitivity in females, hypersensitivity reactions, route of administration and hormonal factors were suggested to contribute to such differences [36].
4.2. Strengths and limitations
To our knowledge, this is the first study to evaluate the relative safety of QIVc versus other types of vaccines using real-world data. The use of data from a large nationally representative sentinel network like the RCGP RSC provides us with adequate sample sizes to compare the safety of ‘equally suitable’ vaccines in stratified analyses. These models yielded important insights that have not previously been reported in clinical trials or other post-licensure studies. Our cohort is large and nationally representative in terms of demographics and clinical outcomes, so we believe the findings could be generalisable to other countries with a similar demographic composition.
Given 2019/20 is the first influenza season in which the QIVc was used, only a small proportion of patients received the QIVc (8.06% in the 18 to 64 years group, and 4.51% in the ≥65 years group). While our data are as good as they get in primary care, we acknowledge that incomplete data is an important limitation, for instance where vaccinations took place outside the general practice and the practice had not been notified [37], or where information on vaccine brand or batch was not recorded. We make enormous efforts to capture complete data, including asking individual sentinel network practices to inform us in advance of the brand and batch of vaccines they have ordered at the start of each influenza season. Moreover, as the study is observational in nature, there is some degree of demographic difference between the vaccine groups. In particular, sex, ethnic and socioeconomic disparities in health are well documented, and we are unable to rule out potential confounding effects of these factors. Last but not least, reduced vaccinations as well as consultations due to the COVID-19 pandemic may have had an effect on our data, which could reduce the generalizability of these findings to other influenza seasons.
4.3. Future research
This study provides preliminary evidence that QIVc may be associated with a higher incidence of AEIs. Further evaluation of its safety using data from different countries and future influenza seasons would be beneficial. It would also be informative to explore the specific events of interest associated with the different types of vaccines as well as their severity. With the likely co-circulation of influenza and COVID-19 in the 2020/21 season, the safety of seasonal influenza vaccines in patients who have recovered from COVID-19, particularly those who are experiencing post-acute COVID-19 (‘long COVID’) [38], remain to be investigated. In addition, when a COVID-19 vaccine becomes available, the safety of concomitant administration of seasonal influenza vaccines with the COVID-19 vaccine would also need to be assessed.
5. Conclusion
Safety profiles of recommended seasonal influenza vaccines, particularly those of equally suitable vaccines within an age group, can be compared using routine sentinel network data. We report that QIVc is associated with a higher incidence of AEIs compared to QIVe and aTIV in the 18 to 64 years and ≥65 years age groups respectively. Despite the advantages that cell culture-based influenza vaccines may have, the higher risk of AEIs observed in patients who received QIVc suggest further monitoring for several influenza seasons would allow for robust comparisons to be made that will inform future seasonal influenza vaccination recommendations.
Contributors
SdeL conceived the study. SdeL, OA, MJ contributed to study design. JS performed data extraction. RSMT performed data analysis. RSMT and SdeL contributed to manuscript preparation. All authors contributed to elements of the study, manuscript revisions, and approved the final version of the manuscript.
Data sharing statement
The RCGP RSC dataset can be accessed by researchers, approval is on a project-by-project basis (www.rcgp.org.uk/rsc). Ethical approval by an NHS Research Ethics Committee is needed before any data release/other appropriate approval. Researchers wishing to directly analyse patient-level pseudonymised data will be required to complete information governance training and work on the data from the secure servers at the University of Oxford. Patient-level data cannot be taken out of the secure network.
Declaration of Competing Interest
SdeL is the Director of the RCGP RSC, its principal funder is Public Health England, who had no direct involvement with this publication. SdeL has received funding through his University for studies from AstraZeneca, Daiichi Sankyo, Eli Lilly, Sanofi, GSK, MSD, Seqirus and Takeda; and has been a member of advisory boards for Seqirus and Sanofi. The GSK funding included monitoring adverse events of interest for their quadrivalent influenza vaccine for three seasons 2015-2018. FDRH has received personal fees from Boehringer Ingelheim, Novartis and Pfizer. All other authors have no competing interests to declare.
Acknowledgments
The principal funder of the RCGP RSC is Public Health England. The authors would like to thank patients and member practices of RCGP RSC, who allowed their pseudonymised clinical medical records to be used for this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100029.
Contributor Information
Simon de Lusignan, Email: simon.delusignan@phc.ox.ac.uk.
Ruby S.M. Tsang, Email: ruby.tsang@phc.ox.ac.uk.
Gayatri Amirthalingam, Email: ayatri.amirthalingam@phe.gov.uk.
Oluwafunmi Akinyemi, Email: oluwafunmi.akinyemi@phc.ox.ac.uk.
Julian Sherlock, Email: julian.sherlock@phc.ox.ac.uk.
Manasa Tripathy, Email: manasa.tripathy@phc.ox.ac.uk.
Alexandra Deeks, Email: alexandra.deeks@phc.ox.ac.uk.
Filipa Ferreira, Email: filipa.ferreira@phc.ox.ac.uk.
Gary Howsam, Email: gary.howsam@nhs.net.
F.D.Richard Hobbs, Email: richard.hobbs@phc.ox.ac.uk.
Mark Joy, Email: mark.joy@phc.ox.ac.uk.
Appendix. Supplementary materials
References
- 1.Centers for Disease Control and Prevention. Cell-based flu vaccines. 2020.www.cdc.gov/flu/prevent/cell-based.htm (accessed 2020/12/03).
- 2.Bühler S., Ramharter M. Flucelvax Tetra: a surface antigen, inactivated, influenza vaccine prepared in cell cultures. ESMO Open. 2019;4(1) doi: 10.1136/esmoopen-2018-000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr I.G., Donis R.O., Katz J.M. Cell culture-derived influenza vaccines in the severe 2017–2018 epidemic season: a step towards improved influenza vaccine effectiveness. NPJ Vaccines. 2018;3(1):44. doi: 10.1038/s41541-018-0079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajaram S., Wojcik R., Moore C. The impact of candidate influenza virus and egg-based manufacture on vaccine effectiveness: literature review and expert consensus. Vaccine. 2020;38(38):6047–6056. doi: 10.1016/j.vaccine.2020.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Izurieta H.S., Chillarige Y., Kelman J. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017–2018. J Infect Dis. 2019;220(8):1255–1264. doi: 10.1093/infdis/jiy716. [DOI] [PubMed] [Google Scholar]
- 6.Divino V., Krishnarajah G., Pelton S.I. A real-world study evaluating the relative vaccine effectiveness of a cell-based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017–18 influenza season. Vaccine. 2020;38(40):6334–6343. doi: 10.1016/j.vaccine.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Klein N.P., Fireman B., Goddard K. Vaccine effectiveness of cell-culture relative to egg-based inactivated influenza vaccine during the 2017-18 influenza season. PLoS One. 2020;15(2) doi: 10.1371/journal.pone.0229279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeMarcus L., Shoubaki L., Federinko S. Comparing influenza vaccine effectiveness between cell-derived and egg-derived vaccines, 2017–2018 influenza season. Vaccine. 2019;37(30):4015–4021. doi: 10.1016/j.vaccine.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Izurieta H.S., Chillarige Y., Kelman J. Relative effectiveness of influenza vaccines among the United States elderly, 2018–2019. J Infect Dis. 2020;222(2):278–287. doi: 10.1093/infdis/jiaa080. [DOI] [PubMed] [Google Scholar]
- 10.Bruxvoort K.J., Luo Y., Ackerson B. Comparison of vaccine effectiveness against influenza hospitalization of cell-based and egg-based influenza vaccines, 2017–2018. Vaccine. 2019;37(39):5807–5811. doi: 10.1016/j.vaccine.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 11.The national flu immunisation programme 2019/20. 2019.assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/788903/Annual_national_flu_programme_2019_to_2020_.pdf (accessed 2020/12/03).
- 12.Public Health England . London: Public Health England; 2019. Influenza: the green book, chapter 19. immunisation against infectious disease. [Google Scholar]
- 13.de Lusignan S., Correa A., Smith G.E. RCGP research and surveillance centre: 50 years’ surveillance of influenza, infections, and respiratory conditions. Br J Gen Pract. 2017;67(663):440. doi: 10.3399/bjgp17X692645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Correa A., Hinton W., McGovern A. Royal college of general practitioners research and surveillance centre (RCGP RSC) sentinel network: a cohort profile. BMJ Open. 2016;6(4) doi: 10.1136/bmjopen-2016-011092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Lusignan S., Lopez Bernal J., Zambon M. Emergence of a novel coronavirus (COVID-19): protocol for extending surveillance used by the royal college of general practitioners research and surveillance centre and public health England. JMIR Public Health Surveill. 2020;6(2):e18606. doi: 10.2196/18606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lusignan S., Damaso S., Ferreira F. Brand-specific enhanced safety surveillance of GSK's Fluarix Tetra seasonal influenza vaccine in England: 2017/2018 season. Hum Vaccin Immunother. 2020;16(8):1762–1771. doi: 10.1080/21645515.2019.1705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Medicines Agency (EMA). Interim guidance on enhanced safety surveillance for seasonal influenza vaccines in the EU. 2014.www.ema.europa.eu/en/documents/scientific-guideline/interim-guidance-enhanced-safety-surveillance-seasonal-influenza-vaccines-eu_en.pdf (accessed 2020/10/08).
- 18.Tippu Z., Correa A., Liyanage H. Ethnicity recording in primary care computerised medical record systems: an ontological approach. BMJ Health Care Inform. 2016;23(4):799. doi: 10.14236/jhi.v23i4.920. [DOI] [PubMed] [Google Scholar]
- 19.National Statistics: english indices of deprivation 2019. 2019. www.gov.uk/government/statistics/english-indices-of-deprivation-2019 (accessed 2020/10/08).
- 20.Cross J.W., Joy M., McGee C., Akinyemi O., Gatenby P., de Lusignan S. Adverse events of interest vary by influenza vaccine type and brand: sentinel network study of eight seasons (2010–2018) Vaccine. 2020;38(22):3869–3880. doi: 10.1016/j.vaccine.2020.03.034. [DOI] [PubMed] [Google Scholar]
- 21.Whitaker H.J., Hocine M.N., Farrington C.P. The methodology of self-controlled case series studies. Stat Methods Med Res. 2009;18(1):7–26. doi: 10.1177/0962280208092342. [DOI] [PubMed] [Google Scholar]
- 22.Petersen I., Douglas I., Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354:i4515. doi: 10.1136/bmj.i4515. [DOI] [PubMed] [Google Scholar]
- 23.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: a language and environment for statistical computing. [Google Scholar]
- 24.Wickham H., Averick M., Bryan J. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. [Google Scholar]
- 25.Farrington P., Whitaker H., Weldeselassie Y.G. CRC Press; 2018. Self-controlled case series studies: a modelling guide with r. [Google Scholar]
- 26.Grolemund G., Wickham H. Dates and times made easy with lubridate. J Stat Softw. 2011;40(3):1–25. [Google Scholar]
- 27.Yoshida K., Bartel A. 2020. tableone: create ‘Table 1’ to describe baseline characteristics with or without propensity score weights. [Google Scholar]
- 28.Wickham H. Springer-Verlag; New York: 2016. ggplot2: elegant graphics for data analysis. [Google Scholar]
- 29.Arnold J.B. 2019. ggthemes: extra themes, scales and geoms for ‘ggplot2. [Google Scholar]
- 30.Joy M., McGagh D., Jones N. Reorganisation of primary care for older adults during COVID-19: a cross-sectional database study in the UK. Br J Gen Pract. 2020;70(697):e540. doi: 10.3399/bjgp20X710933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Lusignan S, Tsang R, Akinyemi O. Comparing the incidence of common adverse events of interest following influenza vaccination in the first season adjuvanted trivalent immunisation was introduced: English sentinel network annual report paper 2018/19. JMIR Preprints. 2020:25803. doi: 10.2196/preprints.25803. [DOI] [Google Scholar]
- 32.Bart S., Cannon K., Herrington D. Immunogenicity and safety of a cell culture-based quadrivalent influenza vaccine in adults: a Phase III, double-blind, multicenter, randomized, non-inferiority study. Hum Vaccin Immunother. 2016;12(9):2278–2288. doi: 10.1080/21645515.2016.1182270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi W.S., Noh J.Y., Song J.Y. Immunogenicity and safety of a cell culture-derived inactivated quadrivalent influenza vaccine (NBP607-QIV): a randomized, double-blind, multi-center, phase III clinical trial in adults and elderly subjects. Hum Vaccin Immunother. 2017;13(7):1653–1660. doi: 10.1080/21645515.2017.1297351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Medicines Agency (EMA). Flucelvax Tetra: EPAR - Public assessment report. 2018.www.ema.europa.eu/en/documents/assessment-report/flucelvax-tetra-epar-public-assessment-report_en.pdf (accessed 2020/10/08).
- 35.Tadount F., Doyon-Plourde P., Rafferty E., MacDonald S., Sadarangani M., Quach C. Is there a difference in the immune response, efficacy, effectiveness and safety of seasonal influenza vaccine in males and females? – A systematic review. Vaccine. 2020;38(3):444–459. doi: 10.1016/j.vaccine.2019.10.091. [DOI] [PubMed] [Google Scholar]
- 36.Cook I.F. Sex differences in injection site reactions with human vaccines. Hum Vaccin. 2009;5(7):441–449. doi: 10.4161/hv.8476. [DOI] [PubMed] [Google Scholar]
- 37.de Lusignan S., Hoghton M., Rafi I. Flu vaccination by pharmacists leads to suboptimal medical records. BMJ. 2017;359:j5084. doi: 10.1136/bmj.j5084. [DOI] [PubMed] [Google Scholar]
- 38.Mahase E. Covid-19: what do we know about “long covid”? BMJ. 2020;370:m2815. doi: 10.1136/bmj.m2815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.