Abstract
Background
Andorra is a small country located in the Pyrenees attracting millions of visitors for tourism, mostly associated with skiing, and nature-related activities. As its neighbouring countries, Spain and France, it has been heavily affected by the COVID-19 pandemic. We estimated SARS-CoV-2 seroprevalence in the entire country by universal serological testing under a lockdown environment.
Methods
A total of 77,543 inhabitants of Andorra were invited to participate in the study. From 4-28 May, 2020, two cross sectional serological surveys were conducted using a rapid serological test (nCOV IgG/IgM) on a finger prick blood sample in 59 drive-through or walk-through checkpoints, all over Andorra. We calculated seroprevalence of antibodies against SARS-CoV-2 and analysed the main sociodemographic factors associated with being seropositive.
Findings
70,494 inhabitants (90.9% of the population) participated in at least one survey. Overall seroprevalence was 11.0%. The most affected age groups were those over 90 years old (15.2%) and 80-89 (13.8%), followed by adults 50-59 (13.6%) and adolescents 10-19 (13.7%). Most seropositive participants, 6,061 (95.1%), were asymptomatic before the surveys. The multivariable analysis showed that the odds of being seropositive was higher among seasonal workers (OR 2.41; 95% CI 1.07-5.45) or in the population living in La Massana region, a popular ski-related area (OR 2.66; 95% CI 2.44-2.89). A higher seroprevalence was observed in those familiar nuclei with greater numbers of cohabitants: 18% in families with 6 household members or more; 13% in medium size families (3/4/5 people) and 12% in small size (1 to 2 people) nuclei.
Interpretation
The prevalence of antibodies against SARS-CoV-2 in the population of Andorra was high during the first wave of the pandemic. Seasonal workers and inhabitants based in La Massana presented a higher seroprevalence. Mass antibody screening allows to identify infection hotspots and should contribute to the design of tailored interventions to prevent SARS-CoV-2 transmission in Andorra.
Funding
Andorran Ministry of Health, Andorran Health Services.
Keywords: COVID-19, Andorra, Screening, SARS-CoV-2, Epidemiology, Diagnostics
Research in context.
Evidence before this study
Few studies have attempted to estimate SARS-CoV-2 seroprevalence in a country by universal serological testing. Previous serological studies were performed using random or convenient population samples, mostly at sub-regional level. SARS-CoV-2 seroprevalence data in European countries during March-May 2020, varied between 1-11%.
Added value of this study
This is the first seroprevalence study universally testing the entire population of a country and one of the largest of its kind worldwide. It contributes to identify country areas of greater transmission and population groups at higher risk of being infected. Seasonal workers, and inhabitants based in ski-related areas presented a higher seroprevalence.
Implications of all the available evidence
This mass serological survey estimated the exposure to SARS-CoV-2 in the Andorran population. Detection of recent exposures allowed for adequate isolation of potentially infectious cases, preventing the spread of the virus at a time of relaxation of containment measures. Serological screenings are fast, feasible and reliable tools for understanding the SARS-CoV-2 exposure in a population. It contributes to identify susceptible population groups with greater risk of infection and guide decision-making policies.
Alt-text: Unlabelled box
1. Introduction
The ongoing pandemic of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected almost every country in the world [1,2]. Despite the rapid spread of the disease, the reported number of cases in many territories is just a proportion of the real number, given the limited diagnostic capacity in many settings, the lack of available and consistent diagnostic protocols during the initial phases of the pandemic or the absence of plans to produce or import diagnostic tests and reagents, among others. A robust screening program for early detection and isolation of SARS-CoV-2 positive individuals is crucial to reduce the transmission of the virus [3]. In addition, the detection of antibodies against SARS-CoV-2 at a population level can provide a minimum estimate of people who have experienced the infection and thus guide decision-making if, for example, there is a good correlation between antibody detection and protection from re-infection [4], [5], [6], [7], [8]. Likewise, as we face new waves of the epidemic [9], seroprevalence studies can contribute to identify high risk population groups who could benefit from tailored preventive strategies.
Andorra is a small country located in the Pyrenean mountains, with about 78,000 inhabitants. It is world famous as a tourist destination attracting over 8 million visitors annually. Located between France and Spain, two of the most affected countries by COVID-19 in Europe, Andorra has also been hardly hit by this pandemic with a total of 9,596 notified cases, and a mortality rate of 1,254 per million population as of 25 January, 2021 [10]. Before the study, the diagnostic capacity in the country was up to 1,100 real-time polymerase chain reaction (rt-PCR) tests per 100,000 persons per week; during the study, it increased to 2,750 per 100,000. On 25 January, 2021, Andorra conducted 8,000 tests per 100,000 inhabitants per week. Given these exceptional circumstances, Andorra offers an ideal and a unique setting to evaluate the feasibility of a serological mass screening program on its entire population. This would provide precise figures on the proportion of the population who have been infected by SARS-CoV-2 and, therefore, contribute to the design of control strategies adapted to the Andorran setting.
Most seroprevalence studies performed in other settings have included specific high-risk groups or randomly-selected participants which exclude certain population groups, such as ill or quarantined people, or institutionalized individuals, among others [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. Thus, their results might not accurately reflect the actual seroprevalence in the population. To date, no studies have been published attempting to estimate SARS-CoV-2 seroprevalence in a country by universal serological testing.
The aim of this study is to estimate the seroprevalence for SARS-CoV-2 in the population of Andorra under a lockdown environment and strict border restrictions after the first two COVID-19 cases were detected. The secondary objectives are a) to estimate the seroconversion patterns over a 15-day period in a confined population, b) to identify high-risk population groups, and c) to estimate the seroprevalence at household level.
2. Methods
2.1. Study design and population
We conducted a two-phase screening study on the entire population in Andorra above the age of 2 who voluntarily signed the informed consent. Beyond Andorran citizens, we also included registered cross-border workers. The only exclusion criterion was the refusal to provide the signed informed consent.
The study included two cross-sectional serological surveys using a rapid serological test on a finger prick blood sample. The first survey took place from 4 to 14 May, 2020, and the second started 4 days after the end of the first one, from 18 to 28 May, 2020 (Supplementary, Figure 2). The objectives of the second survey were two-fold: a) to detect seroconversion in participants between the two surveys, and b) to account for indeterminate results or potential false negative results of the test in the first survey.
2.2. Timeline of public health measures around the study period
Andorra was locked down on 13 March, 2020 and a voluntary quarantine was requested to the entire population. The country borders were not closed, but transit in neighbouring countries was heavily restricted and controlled. Only personnel working in the provision of basic services such as groceries stores, pharmacies, special security forces and health workers who provide service at Hospital Nostra Senyora de Meritxell (the main referral hospital facility in the country) were allowed to cross the border. By the beginning of the first survey, there were 750 COVID-19 lab-confirmed COVID-19 cases and 48 deaths (Supplementary Figure 1). When the study was performed, COVID-19 cases and hospitalisations were showing a downward trend. However, restrictions and reduced mobility were still in place due to the epidemic indicators in neighbouring countries. On 18 May, 2020, around 30% of the country's activity was restarted. Sports activities such as walking and running were allowed on alternate days and at specific times of the day. On 1 June, the entire country's economic activities and services resumed with the end of lockdown (Supplementary Table 1).
2.3. Study procedures
Participants were invited to participate through television and social media advertisements. Registration for the test was performed online through the website (http://coronavirus.govern.ad/), designed for providing information about the pandemic as well as monitoring COVID-19 related symptoms in affected patients.
To perform both surveys, 59 drive-through or walk-through checkpoints called ‘StopLabs’ were enabled across the country with 1,500 volunteers participating in its setup. During sample collection, public officials collected patient's data and registered them into an electronic database (Supplementary Figure 3). Finger prick samples were collected by qualified health care workers. “Drive-thru” testing took around 5 minutes per person. The date of the second survey was scheduled at the end of first survey to ensure that a minimum time lapse of 14 days between both surveys. Personal identifiers prevented from participants getting tested more than once per survey. The test result was available within 15 minutes and the results were uploaded into the database. People with reduced mobility and from social health centres were screened through mobile units at their households. Within twenty-four hours, all participants received the results, via a text message. Individuals testing positive for IgM received a message with the recommendation to contact the referring general practitioner who would then assess the need for a rt-PCR test. Clinical decisions derived from testing, such as isolation or quarantine, followed national guidelines (Supplementary Table 2 and Fig. 4). At the end of the two tests, tested people had access to their results, on the web platform using a personal identifier.
2.4. Serological test
We used the Livzon® rapid test, a diagnostic kit for IgM/IgG antibody detection against SARS-Cov-2 based on a lateral flow assay (nCOV 2019 IgG/IgM- Zhuhai Livzon Diagnostics, Inc. - IgM and IgG kits, Colloidal gold). The test was selected based on a list of recommended tests from FIND (Foundation for Innovative Diagnostics) (https://www.finddx.org/covid-19/sarscov2-eval-immuno/). The kit is CFDA/NMPA approved (China's State Food and Drug Administration/ National Medical Products Administration), and it detects IgM and IgG on the same test providing a maximum combined sensitivity and specificity of 90.6% and 99.2%, respectively (according to the manufacturer). When the rapid test was selected to be used in the study, there were no guidelines on how the test was validated by the vendor and that these were published at a later stage, and therefore, a validation study was performed. First evaluation was performed in Nostra Senyora de Meritxell Hospital (Andorra) in 87 COVID-19 patient samples; 48 symptomatic individuals diagnosed of COVID-19 (cases) who had tested positive by SARS-CoV-2 rt-PCR and 48 healthy donors who served as controls (rt-PCR negative). Among cases, serum samples were obtained 10 days or more after symptoms onset. We found a specificity of 100% (CI 95%; 0.95-1) and sensitivity of 92% (CI 95%; 0.84-0.96). A second evaluation of the test was performed, after the study, at the ISGlobal laboratory (Barcelona, Spain) in August 2020 following FIND recommendations. The study tested 119 plasma/serum samples from individuals with a confirmed past/current diagnosis of COVID-19 (symptomatic and asymptomatic), including 109 rt-PCR-confirmed positive samples, and 129 pre-pandemic negative controls. We found that the combined sensitivity (IgM-IgG) ranged from 0.72 – 0.78 depending on the days since symptoms onset (7 or 14 days) 0.71 – 0.81 when positive samples were rt-PCR-confirmed (Supplementary, Table 3A and 3B). Specificity ranged from 0.98–0.99 [23].
2.5. Statistical analysis
Seroprevalence of antibodies against SARS-CoV-2 were calculated as proportions. Since the total Andorran population was invited to participate in the study and the resulting 95% confidence intervals would be extremely narrow and potentially misleading given that they do not account for the potential bias that non-participating individuals could cause on our central seroprevalence estimate, confidence intervals are not provided. Given the moderate sensitivity the test in our two-step validation process, the numerator for the overall seroprevalence was the number of individuals who had a positive result of IgG and/or IgM at any of the two surveys. The denominator was the population estimates of the Andorra's Government Department of Statistics, as of 1st April, 2020 [24]. Consequently, to calculate the overall proportion of seronegative individuals we used a numerator which includes those participants with a negative result in both surveys, an inconclusive result and one inconclusive result, or with just one negative result if the individual only participated in one survey. Seroconversion was defined as a transition of the test results (IgM or IgG) from negative to positive from the first to the second survey. Conversely, seroreversion was defined as a transition of the test results for IgG or IgM against SARS-CoV-2 from positive to negative results. Inconclusive results were those that could not be interpreted correctly. The asymptomatic proportion was calculated as the percentage of people with initial infection (IgM+ / IgG-), advanced infection (IgM+ / IgG+) or resolved infection (IgM- / IgG+) who did not report COVID-19 related symptoms from the beginning of the epidemic until the study concluded.
Seasonal workers were defined as those who worked only seasonally in the country during the period from October to May and provide services mostly related to ski resorts activities (97%) [24].
Spatial analysis included the seven parish or territories in which the Principality of Andorra is administratively divided. The descriptive analysis of the main socio-demographic variables is reported with absolute frequencies and percentages for categorical variables and with mean and standard deviation or interquartile range for quantitative variables, as appropriate. We described the variables with frequency values (for categorical variables) and means with standard deviations (for continuous quantitative variables). Univariable and multivariable logistic regression models (MLM) were run to evaluate factors associated with seroprevalence of antibodies against SARS-CoV-2. The analysis was carried out using the statistical software Stata v16.1 (College Station, TX: StataCorp LLC) and R studio version R-3.5.1.
2.6. Ethical issues
The protocol of the study was approved by the relevant Andorran regulatory agencies and the local Research Ethics Committee. Participation in the study was entirely voluntary. The Institutional Review Board of the Andorra Health Services (SAAS) approved the study (register number 0720). This study was supported and approved by the Andorran government.
Role of the funding source: Government of the Principality of Andorra and Andorra Health Services contributed to the acquisition of medical equipment, supplies and the essential tools. Funders were not involved in the analysis or decision to submit this manuscript for publication.
3. Results
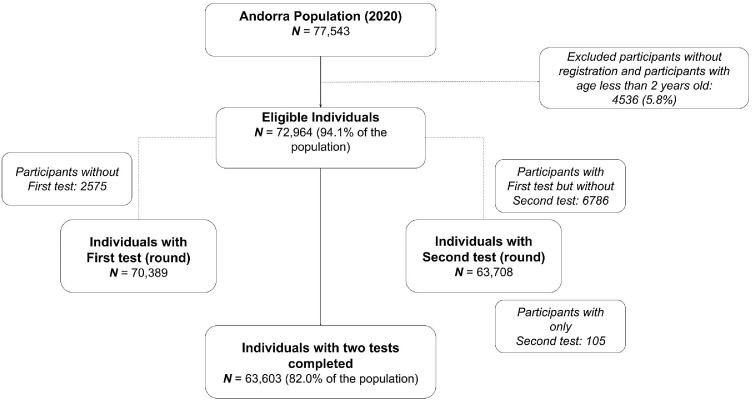
Of the estimated population of Andorra in 2020 (n=77,543), 72,964 inhabitants registered to participate and were older than 23 months of age. Of those, 70,389 (96.4%) participated in the first survey and 63,708 (87.3%) in the second one. A total of 63,603 (87.2%) completed both surveys (Figure 1). Among the 70,494 participants (90.1% of the total population) who participated in at least one of the surveys, the mean age was 40.4 (SD: 20.0) years old, 32,204 (45.7%) were male, and the region that contributed with more participants was Andorra la Vella with 21,102 (29,9%) participants. The most frequent age group among study participants was the 40-49 years old (19.5% of all participants) (Table 1). There was a higher frequency of seasonal workers in regions related to skiing activities: 66.6% of seasonal workers resided in the region of Canillo/Encamp, and 16.5% in the regions of La Massana/Ordino (Supplementary, Figure 5).
Figure 1.
Study participants flowchart.
Table 1.
Baseline demographic characteristics
| Number of participants (n=63,992) | |
|---|---|
| Sex | |
| Female | 31,471 (49.4%) |
| Male | 32,204 (50.6%) |
| Age, years (SD) | |
| 2-9 | 4,312 (7.0%) |
| 10-19 | 6,669 (10.8%) |
| 20-29 | 7,703 (12.5%) |
| 30-39 | 9,798 (15.9%) |
| 40-49 | 12,049 (19.5%) |
| 50-59 | 10,506 (17.0%) |
| 60-69 | 6,164 (10.0%) |
| 70-79 | 3,033 (4.9%) |
| 80-89 | 1,241 (2.0%) |
| ≥ 90 | 265 (0.4%) |
| Occupational Status | |
| Student | 12,290 (17.7%) |
| Seasonal worker | 2,885 (4.0%) |
| Health care worker | 2,167 (3.1%) |
| Others | 52,993 (75.2%) |
| Self-reported Symptoms | |
| Before first survey | 1,746 (2.6%) |
| At time of survey | 226 (0.3%) |
| Parish | |
| Andorra la Vella | 21,102 (29.0%) |
| Canillo | 4,729 (6.5%) |
| Encamp | 11,222 (15.4%) |
| Escaldes-Engordany | 13,347 (18.3%) |
| La Massana | 9,200 (12.6%) |
| Ordino | 4,330 (5.9%) |
| Sant Julia de Lòria | 8,863 (12.2%) |
*Percentages may vary due to missing cases in different groups.
The seroprevalence in the first survey, conducted under a strict population lockdown, was 9.7% while the seroprevalence of the second survey, conducted under a partial population confinement, was 8.5%. The overall seroprevalence (considering seropositivity in either survey) was 11.0% (Table 2). A total of 55,571 (76%) participants were negative in both surveys, 99361 (13%) participants had an inconclusive result after two surveys. Between the first and the second cross-sectional survey, a total of 2,066 people (2.8%) seroconverted and 2,612 people (3.6%) seroreverted.
Table 2.
Seroprevalence of SARS-Cov2 by general characteristics
| Seroprevalence first survey | Seroprevalence second survey | Overall seroprevalence | |
|---|---|---|---|
| Overall | 6,816 (9.7%) | 5,433 (8.5%) | 8,032 (11.0%) |
| Sex | |||
| Female | 2,945 (9.7%) | 2,414 (8.6%) | 3,490 (11.4%) |
| Male | 2,931 (9.4%) | 2,348 (8.3%) | 3,521 (11.3%) |
| Age, years (%) | |||
| 2-9 | 325 (7.9%) | 239 (7.3%) | 447 (10.9%) |
| 10-19 | 626 (9.6%) | 601 (9.7%) | 889 (13.7%) |
| 20-29 | 577 (7.9%) | 423 (6.7%) | 675 (9.2%) |
| 30-39 | 751 (7.9%) | 572 (6.7%) | 899 (9.5%) |
| 40-49 | 1,003 (8.6%) | 894 (8.1%) | 1,287 (11.0%) |
| 50-59 | 1,213 (11.9%) | 989 (10.3%) | 1,396 (13.6%) |
| 60-69 | 672 (11.3%) | 536 (9.5%) | 767 (12.8%) |
| 70-79 | 336 (11.6%) | 267 (9.6%) | 381 (13.1%) |
| 80-89 | 138 (11.8%) | 133 (12.1%) | 162 (13.8%) |
| ≥ 90 | 43 (16.8%) | 33 (14.6%) | 39 (15.2%) |
| Occupational status | |||
| Student | 1,104 (9.1%) | 959 (8.5%) | 1,507 (12.4%) |
| Seasonal worker | 358 (13.3%) | 188 (11.2%) | 288 (10.7%) |
| Health care worker | 227 (10.6%) | 176 (9.9%) | 222 (10.4%) |
| Other jobs | 4,988 (9.5%) | 3,993 (8.3%) | 5,888 (11.2%) |
| Self-reported Symptoms | |||
| Symptoms at test moment | 34 (15%) | 53 (7.8%) | |
| Symptoms before test | 313 (17.9%) | 48 (27.4%) | |
| No symptoms | 6,042 (9.3%) | 4,982 (8.4%) | |
| Parish | |||
| Andorra la Vella | 1,502 (7.4%) | 1,228 (6.7%) | 1,699 (8.1%) |
| Canillo | 429 (9.7%) | 303 (8.2%) | 457 (9.7%) |
| Encamp | 919 (8.5%) | 657 (6.7%) | 970 (8.6%) |
| Escaldes -Engordany | 1,331 (10.2%) | 955 (8.1%) | 1,527 (11.4%) |
| La Massana | 1,163 (13.2%) | 1,145 (14.1%) | 1,636 (17.8%) |
| Ordino | 402 (9.6%) | 360 (9.4%) | 482 (11.1%) |
| Sant Julià de Lòria | 1,038 (12.1%) | 774 (9.6%) | 1,255 (14.2%) |
*Percentages may vary due to missing cases in different groups.
** Overall seroprevalence analysis we only used participants who were part of at least one survey
Among participants that tested positive, the mean age was 43.2 years in both surveys (SD: 20.3 and SD: 20.5 in first and second survey respectively). Most seropositive participants were asymptomatic at the time of the surveys, only 313/6374 (4.9%) reported symptoms before or at the time of the first survey and 48/5079 (0.9%) in the second one. Among participants with at least one positive antibody isotype, the most frequently observed pattern was IgM+/IgG- (3.6%) which, according to our algorithm, was considered to be due to a recent acute infection (Supplementary Table 2).
A total of 4,364/73,265 (6.0%) participants had been tested by rt-PCR prior to the survey. Among those who had a previous positive rt-PCR, 305/378 (78.0%) were seropositive in the first survey. After the second survey and following the national algorithm (Supplementary Figure 2), a total of 1,518 rt-PCR tests were performed, of which 23 were positive.
3.1. Seroprevalence in different population groups (univariable analysis)
Seroprevalences in different population groups are reported in table 2. No differences were observed by sex (11.4% of seroprevalence in females and 11.3% in males). The age groups with higher seroprevalence were those over 90 years old (15.2%) and 80-89 (13.8%), followed by adults 50-59 (13.6%) and adolescents aged 10-19 (13.7%).
Among health care workers (n=2,167 (3.1%) of all participants), seroprevalence was 10.4%. Only 68 (3.1% of 2,193 health care workers) of all health care workers reported having COVID-19 compatible symptoms before the first survey. Seasonal workers presented higher seroprevalence in the first survey than the general population 13.3% vs 9.7%.
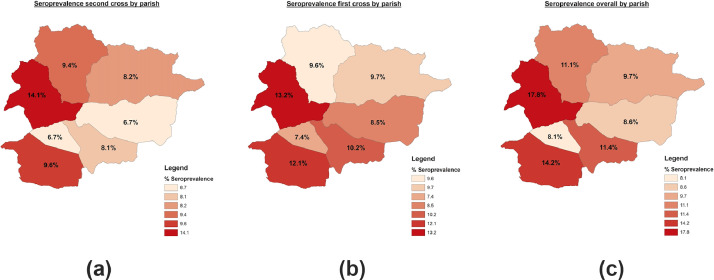
Figure 2 shows the spatial distribution of the seroprevalence in Andorra, per parish. The overall highest seroprevalence was observed in La Massana (17.8%), Sant Julià de Lòria (14.2%) and Escaldes-Engordany (11.4%).
Figure 2.
Seroprevalence against SARS-CoV-2 in Andorra by parish (May 2020). Panel A: first survey; Panel B: second survey; Panel C: overall.
3.2. Multivariable analysis of factors affecting seroprevalence
The odds of being seropositive were higher in elderly participants age above 90 years (OR: 1.61, 95% CI: 1.31-2.31), than in 80-89 years (OR: 1.34, 95% CI: 1.11-1.63) and 50-59 years (OR: 1.32, 95% CI: 1.18-1.48). Participants who presented any COVID-19 related symptoms, especially in the first survey, presented higher odds (OR: 1.80, 95% CI: 1.60-2.04)) of being seropositive than asymptomatic participants. The occupations more strongly associated with a seropositive result were seasonal workers (OR 2.41; 95% CI: 1.07- 5.45). The parishes with higher odds were La Massana (OR 2.66; 95% CI: 2.44-2.89) and Sant Julià de Lòria (OR 1.82; 95% CI: (1.66-2.00) followed by Ordino (OR: 1.47, 95% CI: 1.30-1.67), on the multivariable analysis (Table 3).
Table 3.
Univariable and multivariable analysis
| Variable | n | Seroprevalence | Univariate Odds Ratio | Multivariate Odds Ratio (95% CI) | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 27858 | 3490 (12.5%) | 1.00 | 1.00 | |
| Male | 28301 | 3521 (12.4%) | 0.99 | 1.01 (0.95; 1.07) | |
| Age (years) | |||||
| 2-9 | 3899 | 447 (11.5%) | 1.00 | 1.00 | |
| 10-19 | 6196 | 889 (14.3%) | 1.29 | 1.22 (1.07; 1.40) | |
| 20-29 | 6305 | 675 (10.7%) | 0.93 | 0.92 (0.78; 1.08) | |
| 30-39 | 8587 | 899 (10.5%) | 0.90 | 0.95 (0.80; 1.14) | |
| 40-49 | 10985 | 1287 (11.7%) | 1.02 | 1.12 (0.95; 1.33) | |
| 50-59 | 9560 | 1396 (14.6%) | 1.32 | 1.44 (1.22; 1.71) | |
| 60-69 | 5655 | 767 (13.6%) | 1.21 | 1.42 (1.18; 1.70) | |
| 70-79 | 2776 | 381 (13.7%) | 1.23 | 1.42 (1.17; 1.74) | |
| 80-89 | 1093 | 162 (14.8%) | 1.34 | 1.65 (1.30; 2.11) | |
| ≥ 90 | 226 | 39 (17.3%) | 1.61 | 1.56 (0.97; 2.50) | |
| Symptoms | |||||
| Symptoms first survey | no | 58781 | 7261 (32.6%) | 1.00 | 1.00 |
| yes | 1667 | 338 (20.3%) | 1.80 | 2.00 (1.72; 2.33) | |
| Symptoms second survey | no | 59253 | 7463 (12.59%) | 1.00 | 1.00 |
| yes | 836 | 140 (16.7%) | 1.40 | 1.06 (0.84; 1.33) | |
| Occupational status | |||||
| Student | no | 50918 | 6318 (12.4%) | 1.00 | 1.00 |
| yes | 11296 | 1507 (13.3%) | 1.09 | 1.44 (0.64; 3.25) | |
| Seasonal worker | no | 61864 | 7715 (12.4%) | 1.00 | 1.00 |
| yes | 1681 | 288 (17.1%) | 1.45 | 2.41 (1.07; 5.45) | |
| Health care worker | no | 60985 | 7670(12.5%) | 1.00 | 1.00 |
| yes | 1771 | 222 (12.5%) | 1.0 | 1.33 (0.60; 2.95) | |
| Parish | |||||
| Andorra la Vella | 18277 | 1699 (9.3%) | 1.00 | 1.00 | |
| Canillo | 3708 | 457 (12.3%) | 1.37 | 1.16 (1.02; 1.33) | |
| Encamp | 9801 | 970 (9.9%) | 1.07 | 1.09 (0.98; 1.21) | |
| Escaldes-Engordany | 11804 | 1527 (12.9%) | 1.45 | 1.44 (1.32; 1.57) | |
| La Massana | 8108 | 1636 (20.2%) | 2.47 | 2.66 (2.44; 2.89) | |
| Ordino | 3807 | 481 (12.6%) | 1.41 | 1.47 (1.30; 1.67) | |
| Sant Julià de Lòria | 8021 | 1255 (15.6%) | 1.81 | 1.82 (1.66; 2.00) |
3.3. Household level seroprevalence
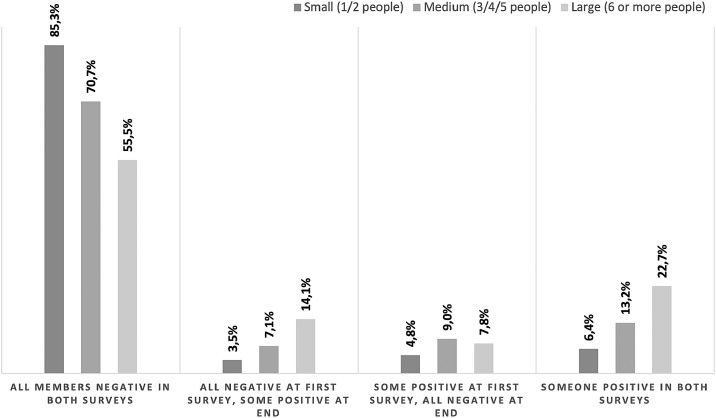
The median household size was 3 people (95% CI: 2.0–3.0). Higher mean seroprevalence was observed in households with a greater number of cohabitants, being highest in those with 6 or more people (18%), followed by those with medium size (4 to 5 people) (13%) and small size (1 to 2 people) (12%). The proportion of seroconverters increased in larger households, from 3.5% in small households, to 7.1% in medium and 14.1% in large households (Figure 3).
Figure 3.
Seroprevalence against SARS CoV-2 in Andorra by household size (May 2020).
4. Discussion
This is the first seroprevalence study universally testing the entire population of a country and the largest of its kind worldwide. With 70,494 voluntary participants, the study covered 91% of the population of Andorra [24]. The overall seroprevalence was 11.0% in May 2020, therefore a considerable portion of the population appeared to have been exposed to the virus during the initial peak. The detection of both IgM and IgG allowed us to identify acute (IgM+/IgG-) or subacute (IgM+/IgG+) infections and led to public health recommendations: isolation of the positively tested people and quarantine of their contacts to prevent the spread of virus, at a time when the country was at an early stage of relaxation of lockdown measures. A peculiarity of Andorra is the high presence of ski-related tourists and seasonal workers during the first wave of the pandemic. We observed a high seroprevalence among seasonal workers (> 95% working in ski-related activities) and areas located near to the main ski resorts, such as La Massana and Canillo.
We observed an overall seroprevalence of 11.0%, higher than other studies conducted at around the same time in neighbouring countries [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]. This might be due to the population influx from areas with high seroprevalence in Spain or France, who travel frequently to Andorra. In a study conducted in the Spanish population [11], the overall seroprevalence during the first fortnight of May was 5% with marked regional differences; there was a higher seroprevalence in the central area of the country (11.3%), where Madrid is located and in the province of Barcelona (7.0%), the latter closely communicated with Andorra.
The decrease in seroprevalence between the two surveys was an unexpected finding. This could partially be due to the decrease of IgM levels from the first to second survey in some patients. Given that the majority of patients were asymptomatic and IgM peak declined early after infection, we could have undetectable levels in the second survey with rapid tests [25], [26], [27]. Another potential reason could have been the exit of temporary workers, who had the highest seroprevalence, after the first cross-sectional survey (temporary worker participation rate decline by almost 40% among surveys). In addition, participants with positive test in the first survey were less likely to participate in the second survey (87.3% vs 90.6% participation rate in those with negative result in first survey, respectively), potentially because they already had recent serology results. The potential utilization of damaged test batches could not be verified.
In those cases, a more sensitive and quantitative test would be needed, but it was not within the scope of our study. We found no differences in seroprevalence between males and females, in line with other studies [11,12,17]. Remarkably, the seroprevalence in the 10-19 years age group was one of the highest, and the age range between 2-9 presented a seroprevalence similar to the other age groups. These findings were different to other studies where seroprevalence in children was lower than in older age-groups. A potential explanation for these results could be the fact that one of the first outbreak foci detected in Andorra occurred within schools, therefore children were one of the first population groups to be affected. The fact that children and adolescents share common spaces and participate in leisure activities in groups could have facilitated this spread in the family nucleus and over the country, albeit in an asymptomatic way. Likewise, another group with high prevalence was people over 80 years, as expected and reported by other studies [11]. The high mortality rate in this age group requires more attention and reinforced strategies to protect and treat this vulnerable high-risk group [28].
Most participants with detectable SARS-CoV-2 antibodies were asymptomatic. Even in groups with high seroprevalence such as seasonal workers or students, the proportion of symptomatic cases was also very low. As expected, we found a higher prevalence in participants reporting having symptoms related with COVID-19 prior to the first survey, probably because the first survey was closer to the peak of cases.
Seasonal workers presented higher seroprevalence than the general population, even higher than health workers where we could have expected the highest seroprevalence. Although most seasonal workers work in outdoor spaces, they tend to interact with many people every day as well as to share common spaces, with many of them living in shared houses or dormitories which might have facilitated the spread of the virus. Although some studies show high seroprevalence among health care workers [29], we observed a seroprevalence similar than that of the general population and even lower than other groups such as students. Health care workers used personal protective equipment in the workplace early, and were closely followed-up, with rapid establishment of quarantines if a close interaction with a contact was ascertained. We found that larger households presented higher seroprevalence than smaller familiar nuclei. Prior studies have noted significant transmission of SARS-CoV-2 in households, highlighting the importance of quarantining index patients at home to prevent SARS-Co-2 transmission within households [30,31].
One of the strengths of this study is the universality of testing, which includes aroudn 91% of the inhabitants in Andorra. The study was conducted within a short period thanks to voluntary citizen participation. This strategy can serve as a model for other countries aiming at conducting mass serological screening. Another key strength of the study was the execution of two cross sectional surveys. We adopted this approach to minimize human error, to increase test sensitivity, to account for seroconversion in people that were infected in the first survey and to minimize inconclusive results in either survey. Finally, this study allowed to detect and isolate individuals with asymptomatic recent infections. Given that asymptomatic and pre- symptomatic carriers could transmit the virus, their early isolation and the establishment of quarantine among their contacts, likely led to a reduction of transmission [32]. The only way detect these asymptomatic cases is through mass screening programs which are fundamental for population-wide containment strategies [33]. After the surveys and although the country's activities had been re-opened, only sporadic cases (N=5) were detected in June and no cases were detected in July. Thus, results from the present study allowed a safe re-opening of economic and social activities given that asymptomatic patients, who could have potentially changed the epidemic dynamics, had been identified and isolated. Although rt-PCR is the gold standard to detect acute infections and therefore and should be the basis for a tracking-tracing-isolation strategy, at the time the study was conducted, Andorra had a limited capacity to perform this type of test, and therefore, not all patients with a positive IgM result could be confirmed by RT-PCR and were considered as acute or subacute cases.
The study also had several limitations. First, despite the high participation in the survey, there is a potential source of selection bias in our seroprevalence estimates introduced by non-participants (non-participation bias). Reasons for not being part of the study are unknown given that participation was completely voluntary. Although work permits were given to participate in the surveys, some participants have acknowledged they did not participate because they could not abandon their work. Some quarantined close contacts also showed concerns for participation, although they were encouraged to participate, given that the design of the survey using a “drive thru” system allowed for safe participation. COVID-19 cases isolated at home were less likely to participate and this could have resulted in certain underestimation of seroprevalence. Second, the quality of data collection can be variable, because they were collected by volunteers, and during a short time per participant. In order to prioritize testing speed, it was decided to conduct a short survey, thus the variety of risk factors analysed is limited. Third, symptoms were collected as self-reported symptoms. It could potentially provide inaccurate information about what the symptoms were, and if they were correctly attributed to COVID-19 as well as their severity. Fourth, we were unable to correctly identify around 3,000 inhabitants by census number, thus we were unable to include them in the household level analysis. Fifth, the exclusion of children < 2 years of age precludes the generalization of the conclusions of this study to that age group. Sixth, the way we estimated overall seroprevalence (positive antibodies in any of the survey) maximizes the sensitivity [32]. Given that the seroprevalence of infection in the Andorran population was high and the specificity of the test was very high (Specificity 100%), we believe the proportion of false positive results, although certainly present, was small and would not affect the conclusions of the study [34]. Likewise, the seroprevalence study was performed at the first peak of the 2020 COVID-19 pandemic, thus, the epidemiological situation of the country might have changed, and any future policy decisions should be made based on updated seroprevalence data. Lastly, the geopolitical particularities of Andorra could make it difficult to extrapolate the results of the study to other countries.
In conclusion, this mass serological survey identified the immunological exposure of SARS-CoV-2 in the Andorran population. Detection of new cases of infections allowed for adequate isolation and tracing, preventing the spread of the virus at a time of relaxation of containment measures. The analysis contributed to identify high-risk groups and infection hotspots, such as temporary workers, and ski related areas respectively. These findings should be addressed in future policies of this highly touristic setting.
Contributors
All authors were involved in the study conceptualization. CRC, DV, JL, VA, MP and JMP were involved in study design. CRC, DV, JL, VA, MP, GF and MGC were involved in data acquisition. CRC, DV, JL, SS, CD and ALGB were involved in data analysis or interpretation. CRC, DV, SS, CD and ALGB drafted and revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Data sharing agreement
Anonymized data used for this analysis is available and made public under the title of this publication at http://diposit.ub.edu/dspace/handle/2445/56611.
Declaration of interests
The authors declare no conflicts of interest.
Acknowledgments
We thank the Government of the Principality of Andorra for its support and its financial contribution for the acquisition of medical equipment, supplies and the essential tools, the Health Department for its guidance, and the Foundation for the Innovation and Research Actuatech for performing the study and management of the entire data, Nil De Celis, Carlos Cebrecos. We thank FIND and the ISGlobal personnel for their support in the evaluation of the RDT, Ruth Aguilar, Alfons Jiménez, Diana Barrios, Laura Puyol and Rebeca Santano; and Francisco Carmona-Torre and Gabriel Reina at UNAV for positive COVID-19 samples. We thank Joan Oliva for his help with language correction.
We are grateful to the honourables municipalities of Canillo, Encamp, Ordino, La Massana, Andorra la Vella, Sant Julià de Lòria and Escaldes-Engordany; the Andorran Red Cross and civil protection for their assistance in the operational execution of the screening and management of volunteers. We thank all the volunteers for their participation and the entire population, making it possible to screen 90% of the country.
Footnotes
TRANSPARENCY DECLARATION The authors declare no conflicts of interest.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100119.
Contributor Information
Cristina Royo-Cebrecos, Email: croyo@saas.ad.
Alberto L. García-Basteiro, Email: alberto.garcia-basteiro@isglobal.org.
Appendix. Supplementary materials
References
- 1.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Practice BB. Coronavirus disease 2019. World Health Organ. 2020;2019(April):2633. doi: 10.1001/jama.2020.2633. [DOI] [Google Scholar]
- 3.Gudbjartsson DF, Helgason A, Jonsson H. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. April 2020 doi: 10.1056/NEJMoa2006100. NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellewell J, Abbott S, Gimma A. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Heal. 2020;8(4):e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan A, Liu L, Wang C. Association of Public Health Interventions with the Epidemiology of the COVID-19 Outbreak in Wuhan, China. JAMA - J Am Med Assoc. 2020;02115(19):1915–1923. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebrahim SH, Ahmed QA, Gozzer E, Schlagenhauf P, Memish ZA. Covid-19 and community mitigation strategies in a pandemic. BMJ. 2020;368:m1066. doi: 10.1136/bmj.m1066. Published 2020 Mar 17. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Yuan Q, Wang H. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. March 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okba NMA, Müller MA, Li W. Severe acute respiratory syndrome coronavirus 2−specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://www.govern.ad/coronavirus
- 11.Pollán M, Pérez-Gómez B, Pastor-Barriuso R. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet (London, England) July 2020 doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, Sun J, Nie S. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med. 2020 doi: 10.1038/s41591-020-0949-6. [DOI] [PubMed] [Google Scholar]
- 13.Sood N, Simon P, Ebner P. Seroprevalence of SARS-CoV-2-specific antibodies among adults in los Angeles county, California, on April 10-11, 2020. JAMA - J Am Med Assoc. 2020;323(23):2425. doi: 10.1001/jama.2020.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bendavid E, Mulaney B, Sood N. COVID-19 antibody seroprevalence in santa Clara county, California. medRxiv. 2020 doi: 10.1101/2020.04.14.20062463. 2020.04.14.20062463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stringhini S, Wisniak A, Piumatti G. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. www.thelancet.com. 2020:396. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salje H, Kiem CT, Lefrancq N. Estimating the burden of SARS-CoV-2 in France. Science. 2020 doi: 10.1126/science.abc3517. published online May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snoeck CJ, Vaillant M, Abdelrahman T. Prevalence of SARS-CoV-2 infection in the Luxembourgish population: the CON-VINCE study. medRxiv. 2020 doi: 10.1101/2020.05.11.20092916. published online May 18preprint. [DOI] [Google Scholar]
- 18.Gudbjartsson DF, Norddahl GL, Melsted P. Humoral immune response to SARS-CoV-2 in Iceland [published online ahead of print, 2020 Sep 1] N Engl J Med. 2020 doi: 10.1056/NEJMoa2026116. NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallal PC, Hartwig FP, Horta BL. SARS-CoV-2 antibody prevalence in Brazil: results from two successive nationwide serological household surveys [published online ahead of print, 2020 Sep 23] Lancet Glob Health. 2020 doi: 10.1016/S2214-109X(20)30387-9. S2214-109X(20)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murhekar MV, Bhatnagar T, Selvaraju S. Prevalence of SARS-CoV-2 infection in India: findings from the national serosurvey, May-June 2020. Indian J Med Res. 2020;152(1 & 2):48–60. doi: 10.4103/ijmr.IJMR_3290_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anand S, Montez-Rath M, Han J. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study [published online ahead of print, 2020 Sep 25] Lancet. 2020;396(10259):1335–1344. doi: 10.1016/S0140-6736(20)32009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshiyama T, Saito Y, Masuda K. Prevalence of SARS-CoV-2-Specific antibodies, Japan, June 2020. Emerg Infect Dis. 2021;27(2):628–631. doi: 10.3201/eid2702.204088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobaño C, Vidal M, Santano R. Highly sensitive and specific multiplex antibody assays to quantify immunoglobulins M, A, and G against SARS-CoV-2 antigens. J Clin Microbiol. 2021;59(2) doi: 10.1128/JCM.01731-20. e01731-20Published 2021 Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Department of statistics of the government of andorra (https://www.estadistica.ad/serveiestudis/web/index.asp?lang=2).
- 25.Moncunill G, Mayor A, Santano R. SARS-CoV-2 seroprevalence and antibody kinetics among health care workers in a spanish hospital after 3 months of follow-up. J Infect Dis. 2021;223(1):62–71. doi: 10.1093/infdis/jiaa696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang AT, Garcia-Carreras B, Hitchings MDT. A systematic review of antibody mediated immunity to coronaviruses: antibody kinetics, correlates of protection, and association of antibody responses with severity of disease. medRxiv. 2020 doi: 10.1101/2020.04.14.20065771. Preprint. 2020.04.14.20065771Published 2020 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siracusano G, Pastori C, Lopalco L. Humoral immune responses in COVID-19 patients: a window on the state of the art. Front Immunol. 2020;11:1049. doi: 10.3389/fimmu.2020.01049. Published 2020 May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heras E, Garibaldi P, Boix M. COVID-19 mortality risk factors in older people in a long-term care center [published online ahead of print, 2020 Nov 27] Eur Geriatr Med. 2020:1–7. doi: 10.1007/s41999-020-00432-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Basteiro AL, Moncunill G, Tortajada M. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. medRxiv. 2020 doi: 10.1101/2020.04.27.20082289. published online May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Zhang B, Lu J. The characteristics of household transmission of COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa450. published online April 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jing Q-L, Liu M-J, Yuan J, Zhang Z-B, Zhang A-R, Dean NE, et al. Household secondary attack rate of COVID-19 and associated determinants. medRxiv. 2020:2020.04.11.20056010. [DOI] [PMC free article] [PubMed]
- 32.Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles’ heel of current strategies to control Covid-19. N Engl J Med. 2020;382:2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilmes P, Zimmer J, Schulz J, Glod F, Veiber L, Mombaerts L. SARS-CoV-2 transmission risk from asymptomatic carriers: results from a mass screening programme in Luxembourg. Lancet Region. Health. 2021;4:1–9. doi: 10.1016/j.lanepe.2021.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leeflang MM, Rutjes AW, Reitsma JB, Hooft L, Bossuyt PM. Variation of a test's sensitivity and specificity with disease prevalence. CMAJ. 2013;185(11):E537–E544. doi: 10.1503/cmaj.121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.