Abstract
Background
Peripheral artery disease (PAD) patients have high morbidity and mortality rates, demonstrating a need for improved treatment strategies. While underuse and undertreatment have been reported, there is no clear picture of patterns in population-level disease prevalence, prescription of guideline-recommended pharmacotherapy, or frequency of contact with dedicated specialists. We present population-level data on changes in prevalence, care and treatment of PAD from 2009 to 2018 in Germany.
Methods
We analyzed the ambulatory claims data for all statutorily insured patients comprising 70.1 million patients each year and 87% of the German population. Prevalence was assessed by documentation of PAD and stratified by age and sex within the 10-year study timeframe. In addition, current ambulatory care, stratified by vascular specialists (vascular surgeons or angiologists), internists, cardiologists and primary care physicians, were examined.
Findings
Prevalence increased from 1·85% in 2009 to 3·14% in 2018, affecting 2·3 million patients in 2018 and more males (55%) than females (45%). A low level of visits to vascular specialists, with 11·1% receiving care from vascular surgeons and 8·1% from angiologists, was shown. Moreover, analysis of guideline-recommended prescriptions revealed increasing, but still insufficient, prescription frequencies among PAD patients between 2009 and 2016, from 42·6% to 56% for statins and from 40·2% to 48·0% for antiplatelets.
Interpretation
Our results show that the prevalence of PAD in Germany, as assessed by outpatient PAD documentation, is increasing and PAD patients are underutilizing specialized vascular care; moreover, the prescription frequency of guideline-recommended therapies remains low. There is a clear need to improve the referral and treatment algorithms in the high-risk PAD population.
Funding
None.
Keywords: Lower extremity artery disease, Peripheral artery disease, Statin use, Outpatient care, Medication underuse
Research in context.
Evidence before this study
Peripheral artery disease (PAD) is a major manifestation of cardiovascular disease and PAD patients have high morbidity and mortality rates, with a clear need for improved treatment strategies. There is no clear picture of patterns in population-level and nation-wide disease prevalence, prescription of guideline-recommended pharmacotherapy, or frequency of contact with dedicated specialists.
Added value of this study
Population-level data on changes in PAD prevalence, care and treatment for all statutorily insured patients in Germany comprising over 70 million patients each year in a single decade are presented. An increased prevalence of PAD in Germany is revealed in 2009-2018; however, a underutilization of specialized vascular care of PAD patients is disclosed accompanied by a low prescription frequency of guideline-recommended therapies, despite an improvement over the decade investigated.
Implications of all the available evidence
Massive opportunities to improve the referral and treatment algorithms in the high-risk PAD population emerge. The use of secondary prevention in patients with PAD should be widely adopted, as these are key approaches for the immediate improvement of health care in patients with PAD to improve symptoms and reduce mortality in this high-risk population.
Alt-text: Unlabelled box
1. Introduction
Peripheral artery disease (PAD) is caused by atherosclerosis that manifests in the peripheral arteries, and is characterized by a broad spectrum of clinical manifestations with symptoms such as intermittent claudication, rest pain and even tissue loss, depending on the degree of ischaemia. More than 200 million individuals worldwide suffer from PAD [1], and this condition has been reported to affect approximately 10% of people older than 50 years in Western Europe and North America [2,3]. Yet, despite its high prevalence and poor prognosis, PAD attracts relatively little attention in terms of research, prevention and treatment [4,5].
Patients with PAD are at a significantly increased risk for morbidity and mortality, and recent guidelines on cardiovascular disease prevention place PAD patients in the highest risk category [5]. In addition to lower limb tissue loss and the risk of atherothrombosis in the peripheral vasculature, the immediate threat is that these patients have the highest risk for cardiovascular death and events such as myocardial infarction or stroke [6]. Indeed, poly-vascular disease involving the coronary arteries occurs in up to 70% of patients presenting with PAD [7]. Long-term treatment strategies such as lipid-lowering medications, antiplatelet therapy, supervised exercise training and smoking cessation are important; together they lay the foundation for the reduction of cardiovascular mortality and the improvement of limb‐related outcomes, and they are consequently recommended by all major societies, including the European Society of Cardiology (ESC), European Society for Vascular Surgery (ESVS), American Heart Association (AHA) and American College of Cardiology (ACC) [4,8]. Regarding lipid-lowering therapy, recent guidelines emphasize a clear recommendation for strict treatment targets driven by the goal of achieving levels of even below 1·0 mmol/L (40 mg/dL) when multiple vascular events occur [9].
In PAD, the underuse of guideline‐recommended therapy has been described in studies of administrative and procedural databases [10], [11], [12], but up-to date nation-wide and detailed real-world data on physician-care patterns and medically managed patients are lacking. A strong understanding of current treatment patterns in outpatient care is essential for improving treatment, reducing symptoms and ultimately reducing mortality in this high-risk population.
We thus aimed to determine the nation-wide age- and sex-stratified prevalence of PAD in Germany from 2009 to 2018 through ambulatory claims data for all statutorily insured patients in Germany. An estimated 87% of the total German population is statutorily insured; the remaining 13% of the population is insured privately [13]. Thus, claims data provide a global picture regarding treatment patterns. We aimed to further analyze outpatient physician care patterns and the patterns of guideline-recommended therapy prescription; we also evaluated these patterns in care and guideline-recommended therapy prescription by age and PAD stage.
2. Methods
2.1. Study population
We analyzed ambulatory claims data of all statutorily insured patients in Germany (with an average of 70·1 million patients per year), which are reported by physicians approved to treat statutory health insured individuals in Germany and are documented according to German Code of Social Law § 295 SGB V, and we analyzed all drug prescription data of the same population according to § 300 SGB V. Claims data contain the outpatient diagnoses, coded according to the German modification of the International Classification of Diseases (ICD-10-GM), of individuals who contact their physician at least once during the study period from 2009 to 2018; these claims data also include the patients’ sociodemographic information, namely, age and sex.
Patients with PAD have an elevated risk of cardiovascular morbidity and mortality even if they are asymptomatic; we thus chose not to limit the inclusion of patients based on clinical endpoints or symptoms and included also asymptomatic PAD patients. For this analysis, we included all ambulatory patients aged ≥ 40 years, as PAD is a rare condition before this age. Patients were considered to have PAD if they had received one of the ICD diagnosis codes consistent with PAD (I70·20, I70·21, I70·22, I70·23, I70·24, I70·25 or I70·29) during the study period; these diagnosis codes correspond to Rutherford PAD stages I-VI, respectively. For each year and patient, the highest code was chosen.
2.2. Assessment of outpatient physician-care and guideline-recommended prescription patterns
Outpatient physician-care patterns were analyzed and stratified according to specialty, namely primary care physicians, internists, cardiologists, vascular surgeon and angiologists. These are based on “lifelong physician codes” (LANR) including primary care physicians (01-03), internists (23-32), cardiologists (28), vascular surgeon (07) and angiologists (24). Potential duplicate counts were not omitted and thus data presents actual outpatient contacts for each specialty.
To analyze the prescription of PAD-related medications, Anatomical Therapeutic Chemical (ATC) codes indicating that a PAD-related medication had been prescribed were extracted from the database. Due to different databases prescription data was available from 2009 to 2016, including C10AA (statins, HMG CoA reductase inhibitors), C10AX (other lipid-modifying agents), B01AA (vitamin K antagonists), B01AC (platelet aggregation inhibitors, excluding heparin), B01AE (direct thrombin inhibitors), and B01AF (direct factor Xa inhibitors).
2.3. Statistical analysis
The absolute and relative prevalence rates of PAD in Germany, stratified by year, were analyzed. We further calculated the crude and age- and sex-standardized annual prevalence rates for PAD; the standardized rates were calculated using the total statutorily insured patient population for each year as the standard population. For patients with a PAD diagnosis, stratification by Rutherford stage was performed. Contact with outpatient doctors was categorized into contact with primary care physicians, internists, cardiologists, vascular surgeons and angiologists. The frequency of the prescription of antiplatelet and statin therapies, stratified by Rutherford stage and year of assessment, was calculated. Descriptive characteristics are expressed using the means ± standard deviations for continuous variables and percentages for categorical variables. Results regarding the significance of trends over time and comparisons were performed with Chi-squared test for trend. All analyses were performed with GraphPad Prism 8·0.
2.4. Ethics approval
The use of claims data for scientific research is regulated by the Code of Social Law (SGB X) in Germany. According to this law, ethics approval and informed consent are not required, as these are routinely collected pseudonymized data.
3. Results
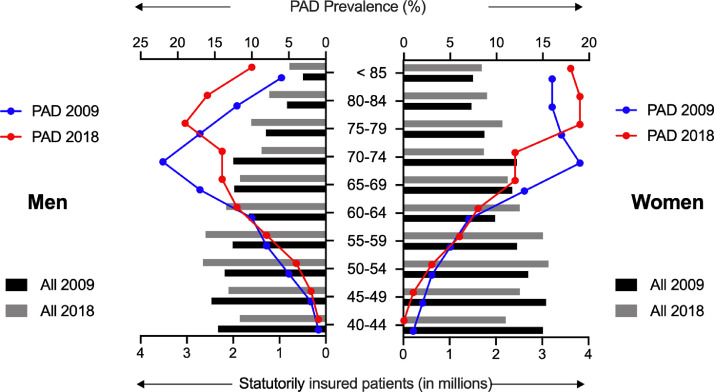
Our study was based on nationwide ambulatory claims data covering approximately 87% of the total German population. We identified, an average of 70·1±1·3 million statutorily insured patients per year, with an average of 1·8±0·4 million PAD patients, yielding a prevalence of 2·5% (Table 1). Notably, a clear increase in the prevalence was observed over time during the 10-year study period (from 1·85% in 2009 to 3·14% in 2018; p<0·0001 for trend), and PAD continuously affected more men (53%) than women (47%, Table 1). This increased prevalence corresponded with an age-related increase in people 70 years in age and older, in both men and women (Fig. 1). Analysis of the patients with PAD by age group further showed a shift in prevalence towards older ages when 2009 and 2018 were compared (Fig. 1).
Table 1.
Study cohort of statutorily insured patients with PAD over 40 years old in Germany, 2009-2018.
| Year | Statutorily insured patients | Patients with PAD | PAD prevalence (%) | Sex distributions (male gender, %) |
|---|---|---|---|---|
| 2009 | 69·828·504 | 1·290·050 | 1·85 | 52 |
| 2010 | 68·545·893 | 1·330·130 | 1·94 | 52 |
| 2011 | 68·559·492 | 1·420·854 | 2·07 | 53 |
| 2012 | 68·726·180 | 1·532·264 | 2·23 | 53 |
| 2013 | 69·816·686 | 1·652·745 | 2·37 | 53 |
| 2014 | 69·936·324 | 1·790·036 | 2·56 | 53 |
| 2015 | 70·231·946 | 1·958·825 | 2·79 | 53 |
| 2016 | 70·866·212 | 2·151·568 | 3·04 | 53 |
| 2017 | 71·506·853 | 2·228·318 | 3·12 | 53 |
| 2018 | 72·576·432 | 2·279·180 | 3·14 | 54 |
| Mean ± SD | 70·059·452 ± 1·314·726 |
1·763·397 ± 375·135 |
2·51 ± 0,49 | 53 ± 0·6 |
Fig. 1.
Age- and sex-stratified prevalence of PAD in Germany in 2009 vs. 2018.
The total age adjusted patient numbers of statutorily insured patients (in millions) and the PAD prevalence (%) for men and women in 2009 and 2018.
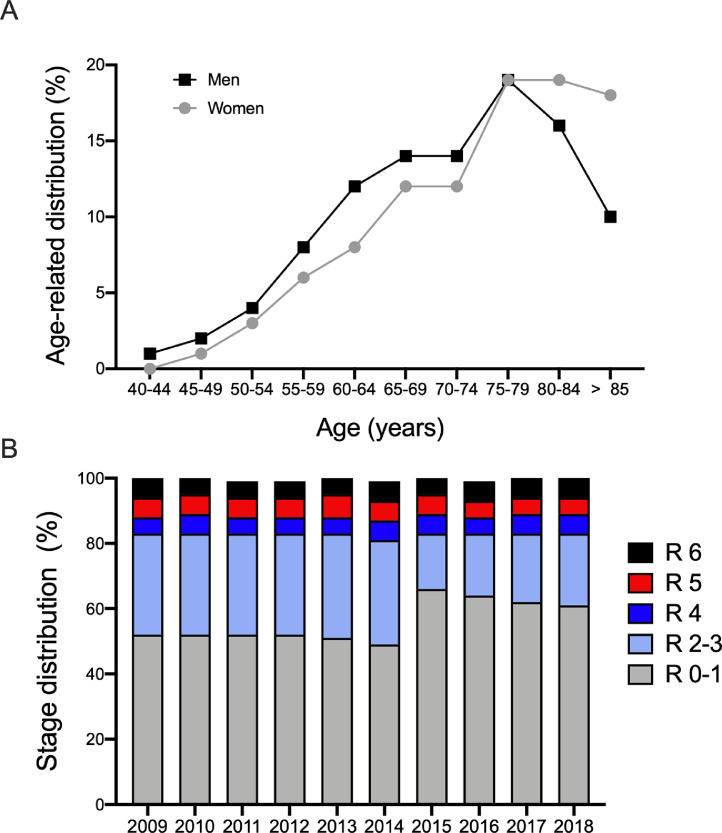
A steep increase in the age-related distribution was also observed in both men and women (maximum of 19% at in the 75- to 79-year-old age group); beyond that age group, there was a decline in age-related distribution in men (16% in the 80- to 85-year-old group) and a plateau for women (19% in the 80- to 85-year-old group; Fig. 2A). We then sought to analyze the distribution of PAD by stage. Throughout the 10-year period investigated, most cases were asymptomatic or mildly symptomatic, with 56·1% classified as Rutherford stages 0-1 (annual prevalence range 49%-66%); 26·7% were classified as having PAD with moderate to severe intermittent claudication (Rutherford stages 2-3; annual prevalence range 17%-32%), and 5·5% to 5·8% were classified as having PAD with critical limb ischemia (CLI) or tissue loss (annual prevalence range 5%-6% for Rutherford stage 4, 5%-7% for Rutherford stage 5, 5%-7% for Rutherford stage 6; Fig. 2B). In the evaluation of temporal trends in the distribution of patients with PAD by stage, we observed a 17% increase in patients with Rutherford stages 0-1 PAD from 2014 to 2015 (49% to 66%, respectively) and a decrease in patients with Rutherford stages 2-3 PAD in these same years (32% in 2014 to 17% in 2015; Fig. 2B). This change might, however, relate to a change in diagnostic coding that took place in 2015. Of note, the proportion of patients with CLI and tissue loss remained equal throughout the study period (Rutherford stage 4: 5·5%; Rutherford stage 5: 5·8% and Rutherford stage 6: 5·7%, p>0.9; Fig. 2B).
Fig. 2.
Age-related distribution and distribution of PAD patients by stage in 2009-2018.
(A) Age-related PAD distribution divided by gender in 2018. (B) Distribution of PAD patients by stage in 2009-2018. R 0-6 denotes Rutherford stages 0-6.
3.1. Outpatient physician-care patterns
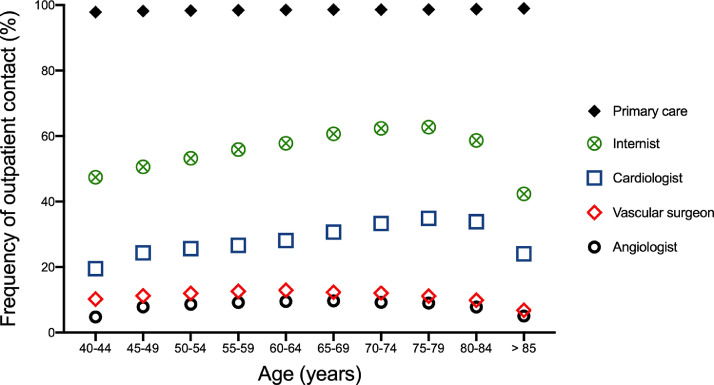
Because quality of care can be dependent on the specialty of the treating physicians [14], we sought to investigate stage- and age-stratified outpatient contact with physicians by specialty. While nearly all PAD patients had contact with a primary care physician (98% in 2009 and 99% in 2018) and more than half had contact with an internist (55% in 2009 and 57% in 2018), less than a third were treated by cardiologists (25% in 2009 and 31% in 2018), and only a minority were treated by vascular specialists: 10% of PAD patients in 2009 and 11% in 2018 were treated by vascular surgeons and only 8% in both 2009 and 2018 were treated by dedicated angiologists (Fig. 3). The analysis of outpatient physician-care revealed age-dependent patterns: The frequency of contact with primary care physicians was highest among those aged 85 years and older (98·9%), while the frequency of contact with internists and cardiologists was highest among those aged 75-79 years (62·7% for internists and 34·9% for cardiologists). Regarding vascular specialists, the frequency of contact with vascular surgeons was highest among those aged 60-64 years (13%) and with angiologists highest among those aged 65-69 years (9·7%, Fig. 3). Sex stratification showed similar trends regarding age-dependent outpatient care utilization patterns (Figure S1).
Fig. 3.
Physician-stratified outpatient care for PAD patients in 2018.
Age-adjusted ambulatory care of patients with PAD, stratified by physician specialty (primary care physicians, internists, cardiologists, vascular surgeons and angiologists) in 2018.
Next, we compared the distribution of patients by PAD stage across the outpatient care specialties. A greater proportion of PAD patients treated by vascular specialists in 2009 were clinically symptomatic (i.e., claudication, Rutherford stages 2-3: 44% were treated by vascular surgeons and 46% were treated by angiologists compared with 36% treated by cardiologists, 35% by internists and 31% by primary care physicians), while cardiologists, primary care physicians and internists treated primarily asymptomatic patients or those with only mild claudication (Rutherford stages 0-1: 51% were treated by cardiologists, 52% by primary care physicians and 50% by internists), with a relatively small proportion of stage 0-1 patients being treated by angiologists (36%) or vascular surgeons (35%, Figure S2A). In contrast, the data presented from 2018 highlight that vascular specialists treated more patients with early stages of the disease (angiologists: Rutherford stages 0-1: 49% and Rutherford stages 2-3: 32%; vascular surgeons: stages Rutherford 0-1: 45% and Rutherford stages 2-3: 32%); nonvascular specialists also treated more patients with early stages of the disease in 2018 (cardiologists: Rutherford stages 0-1: 61% and Rutherford stages 2-3: 24%; primary care physicians: Rutherford stages 0-1: 61% and Rutherford stages 2-3: 22%; internists: Rutherford stages 0-1: 59% and Rutherford stages 2-3: 24%; Figure S2B). The proportion of patients with critical limb ischemia and chronic wounds treated, by physician specialty, remained similar over the study period (4-7% in 2009 and 4-9% in 2018; Figure S2).
3.2. Guideline-recommended prescription patterns
To address the cardiovascular risk associated with atherosclerotic peripheral disease pharmacotherapy is the therapeutic basis. We focused on the prescription frequencies for antiplatelet therapy and statins, both considered first-line therapies for PAD. Prescription data were available from 2009 to 2016.
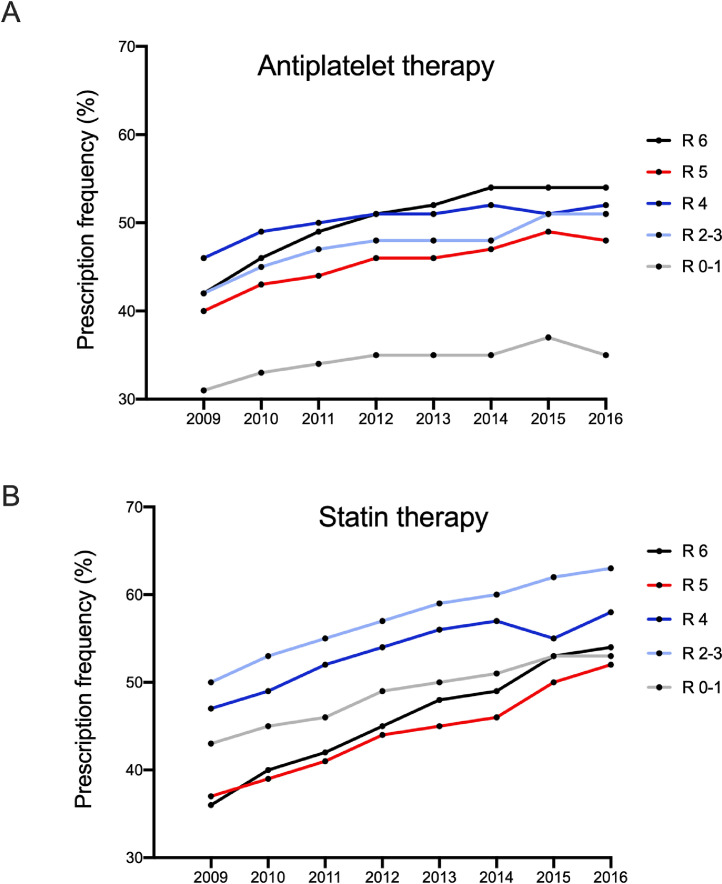
The antiplatelet prescription frequency increased gradually from 40·2% in 2009 (range 31%-46%) to 46·2% in 2012 (range 35%-51%) and 48·0% in 2016 (range 35%-54%; Fig. 4A and Table S1). Notably, the stage-stratified prescription frequency reveals a difference in the frequency of antiplatelet prescription by stage: 34·4% (range 31%-37%) of patients with Rutherford stages 0-1 PAD were prescribed antiplatelets, while 47·5% (range 42%-51%) of patients with Rutherford stages 2-3 PAD were prescribed antiplatelets. Only 50·3% (range 46%-52%) of patients with Rutherford stage 4 PAD were prescribed antiplatelets, and patients with Rutherford stage 5 received antiplatelets even less frequently (mean 45·4%; range 40%-49%), while 50·3% (range 42%-54%; Fig. 4A) of patients with Rutherford stage 6 PAD were prescribed antiplatelets.
Fig. 4.
Guideline-recommended therapy in PAD patients, stratified by Rutherford stage.
(A) Prescription of antiplatelet therapy in 2009-2016 according to Rutherford stage.
(B) Prescription of cholesterol-lowering statin medication in 2009-2016 according to Rutherford stage.
The use of statins showed a similar overall picture, with an increase in the prescription frequency from 42·6% in 2009 (range 36%-50%) to 49·8% in 2012 (range 44%-57%) and 56·0% in 2016 (range 52%-63%; Fig. 4B and Table S1). Analysis of the prescriptions by stage, however, showed a disturbing pattern: while 48·8% (range 43%-53%) of patients with Rutherford stages 0-1 PAD, 57·4% (range 50%-63%) of patients with Rutherford stages 2-3 PAD and 53·5% (range 47%-58%) of patients with Rutherford stage 4 PAD were prescribed statins, only 44·3% (range 37%-52%) and 45·9% (range 36%-54%) of patients with Rutherford stages 5 and 6 were prescribed statins, respectively. Only a small proportion of patients received other lipid-modifying agents (Table S1).
4. Discussion
Cardiovascular disease (CVD) remains one of the leading causes of death and disability in Europe and globally [15]. Based on the enormous burden of the disease, targeting the consequences and sequelae of CVD and especially PAD has been the focus of a major global effort to improve diagnostic, treatment, and preventive strategies [4,8,16]. This study evaluated the PAD prevalence, outpatient care and guideline-recommended prescription patterns in a source population comprising 87% of all German inhabitants in a single decade.
Our results demonstrate a rapidly increasing prevalence of PAD in Germany during the study period. Despite an age-related increase in PAD numbers, outpatient physician-care patterns revealed a low-level of visits to vascular specialists, highlighting an underutilized specialized vascular care. Moreover, an analysis of the prescription of guideline-recommended cholesterol-lowering statin and antiplatelet therapy disclosed low prescription patterns, despite an increase over the study period.
Our findings are in line with those of previous surveys and studies that have shown a consistently increasing prevalence of PAD with increasing age, with a maximum prevalence in septuagenarians [17,18]. Notably, the prevalence and age-related distribution showed a decline for octo- and nonagenarians, which could be based on the increased CV mortality in the elderly PAD population. The data are corroborated by studies demonstrating that most peripheral artery disease cases are in the septuagenarian age-group [1]. In the study period examined, an unexpected time-dependent increase was observed from 2009 to 2018. The reasons for this may be threefold: first, there is a demographic trend towards ageing such that absolute and relative numbers increase. Second, the concurrent projected increase in the prevalence of important risk factors may have led to a larger burden of PAD. And third, an increased awareness due to recent guidelines and publications focusing on PAD as well as sensibilization and screening campaigns might lead to increased screening and reporting especially for early-stage PAD.
The Peripheral Arterial Disease Awareness Risk and Treatment: New Resources for Survival (PARTNERS) program previously showed a distinct discrepancy between the high prevalence of PAD and the low awareness of the PAD diagnosis in the primary care setting in the US [19]. The low awareness on the part of primary physicians might additionally explain the alarmingly low proportions of PAD patients we observed who were treated by vascular specialists, as referrals of patients from the primary care sector to medical specialists are usual in the German outpatient healthcare system. Furthermore, a lack of specialists in certain areas and scheduling issues in overcrowded outpatient centers might also lead to relatively low percentages. Specialist care, however, is clearly needed and has been shown to improve outcomes, especially when targeting atherosclerotic CVD [20]. Further improvements in PAD awareness due to updated guidelines and recent large trials focusing on adverse limb events may additionally lead to enhanced diagnosis and medical documentation of PAD by treating specialized physicians [4,21,22].
A striking finding of the present study is the clear under-prescription of antiplatelet therapies and statins (both of which are guideline-recommended therapies), which indicates that the treatment of the German PAD population is currently inadequate. Indeed, our data provide a disturbing picture of the current treatment situation in a high-income country with a sophisticated healthcare system. While an improvement in treatment patterns can be seen from 2009 to 2016, there is still an unequivocal need to improve the prescription of guideline-recommended therapy. Our numbers are in line with those reported in previous studies from the UK and US, which described medication prescription patterns in which at least one-third of PAD patients did not receive antiplatelet or statin treatment [11,23,24]. Of note, in the current study we could only rely on claims data and did not have laboratory measurements of lipids and lipoproteins available; therefore, we could not determine the proportion of patients with a priori low LDL-C levels, which could, in theory, exist even without statin therapy. However, published registry and clinical data in patients with coronary artery disease and PAD highlight that these goals are rarely achieved, which provides clear opportunities for treatment improvements [25,26]. Based on these findings, some researchers have suggested that, to achieve strict treatment targets, even novel medications such as PCSK-9 (proprotein convertase subtilisin/kexin type 9) inhibitors should be prescribed to the majority of the CVD population [27].
Another key finding of our study is that the treatment patterns did not correlate with clinical stage; for example, the frequency of statins prescription was lower than expected in patients with minor and major tissue loss. Especially for statins, an abundance of robust data supports prescription to patients with advanced stages of PAD to prevent limb loss and reduce mortality [28], [29], [30]. As suggested by the recent guidelines, PAD patients should receive statins as early as possible to achieve dedicated LDL-C values and aspirin should be administered in patients with claudication and higher staged PAD. Potential future analysis of prescriptions of novel medications targeting hypercholesterolemia or direct oral anticoagulants would be of importance, as both have shown promising results in PAD patients and could change prescription practices based on the increased awareness generated by the improved limb and overall mortality outcomes observed [21,22].
Our results must be interpreted from both the clinical and public health viewpoints. Estimating the population-level prevalence of PAD by age and sex may help inform health policy, as resource utilization and the economic burden related to management may be influenced by the demographics and populations involved. Despite the major progress in reducing cardiovascular morbidity and amputations for PAD, their total impact has increased in the past years. Risk factors such as diabetes have increased sharply, even for younger people [31]. Average annual expenditures per individual for patients with PAD are projected to be $11·553 in the US and €12·549 in Europe with CLI patients producing 65% of in-hospital and 56% of outpatient costs [32], [33], [34]. These costs will further escalate if this epidemic is not halted and reversed and thus, early and guideline-recommended treatment is of huge importance.
Some limitations of our study should also be acknowledged. The claims data we used were not at the individual patient-level and thus did not include actual perfusion measurements or could be supported by clinically driven outcome data. Moreover, the estimation of outpatient PAD contact with physicians in different specialties could be misleading because one patient could be seen by two different doctors, which may result in an apparent duplication of treatment. Additionally, as we relied on outpatient claims data, we could not exclude the possibility of hospital-derived prescriptions that could have potentially led to different medication patterns. Furthermore, given the use of non-patient-level data and the design of our study, detailed cardiovascular risk factors like smoking or elevated blood pressure, intolerance to or even contraindications for certain medications, and the type of statin used were not documented. Additionally, the prevalence of polypharmacy, which may have led to the discontinuation of medications, was not evaluated for the elderly PAD population presented here. Finally, medications were analyzed solely on the prescription patterns with no treatment guarantee or a detailed picture of a change in treatment. Furthermore, due to the use of different databases, prescription patterns could not be stratified by the physician specialty. Nonetheless, we believe that our study presents a real-world and holistic picture including 70 million of the statutorily insured German population, as only a minority (13%) is insured privately and thus their data would have had a minimal impact on our findings.
5. Conclusions
Our findings show that there was an increase in the prevalence of PAD, as assessed by PAD documentation, from 2009-2018 in Germany, but only a minority of patients received specialized vascular care. Moreover, we found that the frequencies of the prescription of guideline-recommended therapies are inappropriate, suggesting the need for improved outpatient vascular care. Our analysis also suggests that the use of secondary prevention in patients with PAD is not widely adopted in Germany, an outcome consistent with global patterns [12,35,36]. Although scientific research is currently focused on novel modalities of CVD prevention, our findings highlight an urgent need to refocus on known and established therapies. In addition to drug prescription, supervised exercise therapy and the adoption of healthy dietary patterns can influence the progression of vascular disease and impact traditional and novel risk factors, such as lipid levels, inflammation, blood pressure and vascular function [37,38]. Therefore, these may be key approaches for the immediate improvement of health care in patients with PAD to reduce symptoms and reduce mortality in this high-risk population.
Author Contributions
CR, MSt, AAM, TR conception and design of the work; CR, TR, RH and MSc acquisition and analysis of the data; JL, OP and KK interpretation of the data; CR, MSt, JL, AAM, OP and KK drafting the work. RH, MSc and TR revising it critically for important intellectual content; CR, MSt, JL, AAM, OP, KK RH, MSc and TR approval of the final version to be published; CR, MSt, JL, AAM, OP, KK RH, MSc and TR agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CR, OP and TR verified underlying data.
Conflicts of Interest
All authors declare no conflict of interest.
Acknowledgments
Acknowledgements
None.
Funding
None.
Data sharing statement
Due to German law, data is available upon request from the Central Research Institute for Ambulatory Healthcare in Germany (Zi).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100113.
Appendix. Supplementary materials
References
- 1.Song P, Rudan D, Zhu Y. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7(8):e1020–e1e30. doi: 10.1016/S2214-109X(19)30255-4. [DOI] [PubMed] [Google Scholar]
- 2.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 3.Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. 2017;14(3):156–170. doi: 10.1038/nrcardio.2016.179. [DOI] [PubMed] [Google Scholar]
- 4.Aboyans V, Ricco JB, Bartelink MEL. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS) Eur Heart J. 2017;39(9):763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 5.Piepoli MF, Hoes AW, Agewall S. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ankle Brachial Index C, Fowkes FG, Murray GD. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatt DL, Steg PG, Ohman EM. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180–189. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 8.Gerhard-Herman MD, Gornik HL, Barrett C. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;69(11):e71–e126. doi: 10.1016/j.jacc.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Mach F, Baigent C, Catapano AL. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 10.Saxon JT, Safley DM, Mena-Hurtado C. Adherence to guideline-recommended therapy-including supervised exercise therapy referral-across peripheral artery disease specialty clinics: insights from the international PORTRAIT registry. J Am Heart Assoc. 2020;9(3) doi: 10.1161/JAHA.119.012541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger JS, Ladapo JA. Underuse of prevention and lifestyle counseling in patients with peripheral artery disease. J Am Coll Cardiol. 2017;69(18):2293–2300. doi: 10.1016/j.jacc.2017.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colantonio LD, Hubbard D, Monda KL. Atherosclerotic risk and statin use among patients with peripheral artery disease. J Am Coll Cardiol. 2020;76(3):251–264. doi: 10.1016/j.jacc.2020.05.048. [DOI] [PubMed] [Google Scholar]
- 13.Holstiege J, Akmatov MK, Stork S, Steffen A, Batzing J. Higher prevalence of heart failure in rural regions: a population-based study covering 87% of German inhabitants. Clin Res Cardiol. 2019;108(10):1102–1106. doi: 10.1007/s00392-019-01444-8. [DOI] [PubMed] [Google Scholar]
- 14.Donohoe MT. Comparing generalist and specialty care: discrepancies, deficiencies, and excesses. Arch Intern Med. 1998;158(15):1596–1608. doi: 10.1001/archinte.158.15.1596. [DOI] [PubMed] [Google Scholar]
- 15.Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232–3245. doi: 10.1093/eurheartj/ehw334. [DOI] [PubMed] [Google Scholar]
- 16.Arnett DK, Blumenthal RS, Albert MA. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 18.Kalbaugh CA, Kucharska-Newton A, Wruck L. Peripheral artery disease prevalence and incidence estimated from both outpatient and inpatient settings among medicare fee-for-service beneficiaries in the atherosclerosis risk in communities (ARIC) study. J Am Heart Assoc. 2017;6(5) doi: 10.1161/JAHA.116.003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch AT, Criqui MH, Treat-Jacobson D. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 20.Nash IS, Nash DB, Fuster V. Do cardiologists do it better? J Am Coll Cardiol. 1997;29(3):475–478. doi: 10.1016/s0735-1097(96)00528-1. [DOI] [PubMed] [Google Scholar]
- 21.Bonaca MP, Nault P, Giugliano RP. Circulation. 2017. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk) [DOI] [PubMed] [Google Scholar]
- 22.Anand SS, Bosch J, Eikelboom JW. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10117):219–229. doi: 10.1016/S0140-6736(17)32409-1. [DOI] [PubMed] [Google Scholar]
- 23.Cea-Soriano L, Fowkes FGR, Johansson S, Allum AM, Garcia Rodriguez LA. Time trends in peripheral artery disease incidence, prevalence and secondary preventive therapy: a cohort study in The Health Improvement Network in the UK. BMJ Open. 2018;8(1) doi: 10.1136/bmjopen-2017-018184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundaram V, Bloom C, Zakeri R. Temporal trends in the incidence, treatment patterns, and outcomes of coronary artery disease and peripheral artery disease in the UK, 2006-2015. Eur Heart J. 2020;41(17):1636–1649. doi: 10.1093/eurheartj/ehz880. [DOI] [PubMed] [Google Scholar]
- 25.Dykun I, Wiefhoff D, Totzeck M. Disconcordance between ESC prevention guidelines and observed lipid profiles in patients with known coronary artery disease. Int J Cardiol Heart Vasc. 2019;22:73–77. doi: 10.1016/j.ijcha.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumbhani DJ, Steg PG, Cannon CP. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35(41):2864–2872. doi: 10.1093/eurheartj/ehu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dykun I, Mahabadi AA, Rassaf T. A clinical perspective on the 2019 ESC/EAS guidelines for the management of dyslipidaemias: PCSK-9 inhibitors for all? Eur Heart J. 2020;41(24):2331. doi: 10.1093/eurheartj/ehaa005. [DOI] [PubMed] [Google Scholar]
- 28.Westin GG, Armstrong EJ, Bang H. Association between statin medications and mortality, major adverse cardiovascular event, and amputation-free survival in patients with critical limb ischemia. J Am Coll Cardiol. 2014;63(7):682–690. doi: 10.1016/j.jacc.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stavroulakis K, Borowski M, Torsello G, Bisdas T, collaborators C Association between statin therapy and amputation-free survival in patients with critical limb ischemia in the CRITISCH registry. J Vasc Surg. 2017;66(5):1534–1542. doi: 10.1016/j.jvs.2017.05.115. [DOI] [PubMed] [Google Scholar]
- 30.Arya S, Khakharia A, Binney ZO. Association of statin dose with amputation and survival in patients with peripheral artery disease. Circulation. 2018;137(14):1435–1446. doi: 10.1161/CIRCULATIONAHA.117.032361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper R, Cutler J, Desvigne-Nickens P. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the national conference on cardiovascular disease prevention. Circulation. 2000;102(25):3137–3147. doi: 10.1161/01.cir.102.25.3137. [DOI] [PubMed] [Google Scholar]
- 32.Scully RE, Arnaoutakis DJ, DeBord Smith A, Semel M, Nguyen LL. Estimated annual health care expenditures in individuals with peripheral arterial disease. J Vasc Surg. 2018;67(2):558–567. doi: 10.1016/j.jvs.2017.06.102. [DOI] [PubMed] [Google Scholar]
- 33.Hasvold P, Nordanstig J, Kragsterman B. Long-term cardiovascular outcome, use of resources, and healthcare costs in patients with peripheral artery disease: results from a nationwide Swedish study. Eur Heart J Qual Care Clin Outcomes. 2018;4(1):10–17. doi: 10.1093/ehjqcco/qcx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinecke H, Unrath M, Freisinger E. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J. 2015;36(15):932–938. doi: 10.1093/eurheartj/ehv006. [DOI] [PubMed] [Google Scholar]
- 35.Subherwal S, Patel MR, Kober L. Missed opportunities: despite improvement in use of cardioprotective medications among patients with lower-extremity peripheral artery disease, underuse remains. Circulation. 2012;126(11):1345–1354. doi: 10.1161/CIRCULATIONAHA.112.108787. [DOI] [PubMed] [Google Scholar]
- 36.Cacoub PP, Zeymer U, Limbourg T. Effects of adherence to guidelines for the control of major cardiovascular risk factors on outcomes in the REduction of Atherothrombosis for Continued Health (REACH) Registry Europe. Heart. 2011;97(8):660–667. doi: 10.1136/hrt.2010.213710. [DOI] [PubMed] [Google Scholar]
- 37.Rammos C, Hendgen-Cotta UB, Sobierajski J, Bernard A, Kelm M, Rassaf T. Dietary nitrate reverses vascular dysfunction in older adults with moderately increased cardiovascular risk. J Am Coll Cardiol. 2014;63(15):1584–1585. doi: 10.1016/j.jacc.2013.08.691. [DOI] [PubMed] [Google Scholar]
- 38.Rassaf T, Rammos C, Hendgen-Cotta UB. Vasculoprotective effects of dietary cocoa flavanols in patients on hemodialysis: a double-blind, randomized, placebo-controlled trial. Clin J Am Soc Nephrol. 2016;11(1):108–118. doi: 10.2215/CJN.05560515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.