Abstract
The field of mosquito mating biology has experienced a considerable expansion in the last decade. Recent work has generated many key insights about specific aspects of mating behaviour and physiology. Here, we synthesize these findings and classify swarming mosquito systems as polygynous. Male mating success is highly variable in swarms and evidence suggests it is likely determined by both scramble competition between males and female choice. Incorporating this new understanding will improve both implementation and long-term stability of reproductive control tools.
Keywords: Mosquito, Sexual Selection, Mating System, Mating Ecology, Reproductive Control
Mosquito Mating Systems
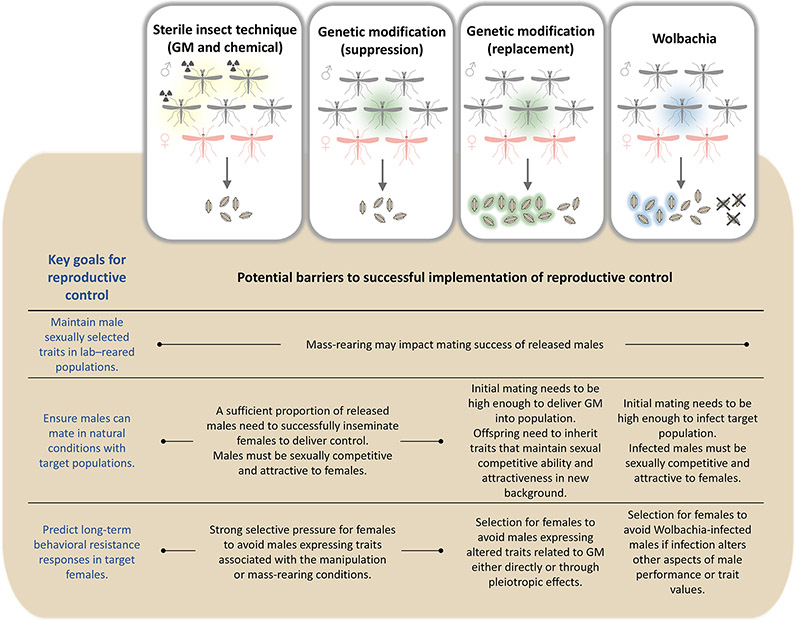
Mosquito control strategies which rely on manipulating or exploiting mating behaviours are rapidly becoming a key tool in the management of mosquito populations [1,2]. Generally, these strategies involve laboratory rearing of mosquitoes that have been chemically sterilized [3,4] or carry either a pathogen or a genetic construct designed to reduce the wild population or curtail its ability to transmit disease agents [2,5-7] (Figure 1). A clear view of mosquito mating systems and how we expect sexual selection to act within in them can be used to both optimize the deployment of control tools and ensure their long-term success. Here, we apply common frameworks for describing animal mating systems to medically important mosquito species and discuss how a deeper understanding of sexual selection can improve control efficacy.
Figure 1. Applications for mosquito control.
The implementation and long-term stability of many control interventions will be enhanced by filling key gaps in our understanding of mosquito mating systems. For example, many strategies rely on the mass-rearing and release of modified males (genetically [1] or chemically [3,7] sterilised, infected with a pathogen [2], or carrying a transgene [5]). Improved understanding of the degree to which male-male competition and female choice determine male mating success can inform the conditions under which these males should be maintained to maximize mating success. There are important gaps in our understanding of the variation and selection of female preferences that need to be filled in order to assess and mitigate the potential for behavioural resistance. Important evolutionary questions also remain about the relationships between traits important for male mating, male viability, and female traits that can influence vectorial capacity.
Mosquitoes display a diverse array of mating behaviours, from discrete intricate courtship dances [8] to mass aerial swarming [9], We focus on Aedes, Anopheles, and Culex mosquitoes which are the primary targets of current reproductive control programs (see Glossary) and for which most data have been collected. Mating in these species is readily observed in swarms ([10], but see [11] for exceptions). Swarm aggregation sizes, locations, and circadian patterns vary widely even among the relatively few species we have adequate data for [4,9,12,13]. For example, Aedes aegypti form small swarms around the human host coinciding with peak female blood feeding activity [12,14], whereas Anopheles freeborni swarm at dusk by the thousands over rice fields [9]. Males locate and orient to females within swarms using the flight tone emissions of females (the phonotactic response [15]). Mating interactions between male and female occurs in mid-air within swarms. These brief pre-copulatory interactions (<1 min) typically end in either a successful synchronized mating flight or decisive rejection by the female (reviewed by [10]).
While there is general agreement about these basic aspects of mating in swarming species, several key gaps in our understanding of mating behaviour remain. Many of these questions revolve around the factors that determine variation in male fitness. These questions are central to both understanding the basic biology of these mating systems and informing reproductive control strategies. Recent work has eroded the long held belief that male mating success within swarms is essentially random [10]. Here we put this evidence into a sexual selection framework and discuss the implications of these for reproductive control strategies.
Reproductive Skew
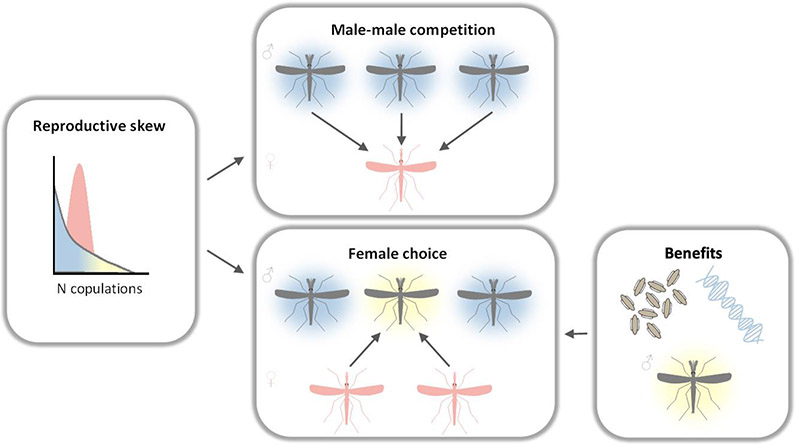
Reproductive skew can be quantified directly by measuring the statistical variance in reproductive success within a sample of males and females [16]. Swarming mosquito mating systems appear to be polygynous with high variation in male reproductive success and low variation in female reproductive success (Figure 2) [17].
Figure 2. Key characteristics of mosquito mating systems.
Mosquitoes are thought to be polygynous, with laboratory studies demonstrating that males can mate multiply. In polygynous systems we expect variance in male reproductive success to be high, with a small number of males achieving most of the matings. Males engage in scramble competition. Evidence of active rejection by females suggests female choice is at play in the swarm. This begs the question: what male traits do they select on, and what do they stand to gain (and risk) by choosing?
There are three lines of evidence to support a classification of polygyny. First, the few quantitative observations of natural swarms report operational sex ratios (OSRs) skewed towards males [4,11,12,14]. At any given time, there are more males than females available to mate and therefore a greater opportunity for variance in male reproductive success. Second, males can inseminate multiple females over their lifetime and even consecutively within a swarming period. Even under controlled laboratory conditions, studies report variance in the number of females that can be inseminated by a single male (Table 1). Finally, females are able to store enough sperm from a single mating to last their lifetime [18] and effectively resist male mating attempts after their first mating [18-22], In Aedes, Culex and Anopheles, female refractory behaviour is induced by receipt of seminal fluid molecules from the male [20,21,23-25]. Work in Ae. aegypti indicates that seminal fluids act quickly (within 2 hr of mating) and females remain refractory for up to five egg-laying cycles [26]. Most Anopheline males additionally produce and transfer a mating plug to females [20,27]. These laboratory observations of refractory behaviour are complemented by field and semi-field experiments which report low rates of multiple mating in females under natural conditions [28-30].
Table 1.
Examples of studies that have measured male insemination capacity.
| Species | Females Inseminated (Mean ± SE) |
Duration (Days) |
Methods (how virgin females were provided) |
Ref. |
|---|---|---|---|---|
| Aedes aegypti | 3.50 ± 0.070a | 1 | 1 at a time | [85] |
| 11.78 ± 1.21a | 7 | 5/ 3 days | [86] | |
| 17.60b | 21 | 8 initial + 5 per week | [87] | |
| 5.26 ± 0.32 | 8 | 5 every 2 days | [52] | |
| 11.50 ± 0.53 | 14.62 ± 0.74* | 5 per day | [88] | |
| Aedes albopictus | 8.63 ± 0.91 | 14 | 10 per day | [89] |
| 19.20 ± 1.69a | 14 | 10 per day | [90] | |
| Anopheles coluzzi | 7.95 ± 0.31 | 3 | 20 on day 1 | [82] |
| 10.13 ± 0.36 | 3 | 20 on day 1 | [82] | |
| Anopheles gambiae | 8.30 ± 1.00 | 1 | 30 on day 1 | [91] |
| Culex tarsalis | 4.00 ± 0.36a | 10 | 10 on day 1 | [92] |
| 5.30 ± 0.42a | 10 | 10 on day 1 | [92] | |
| Sabathes cyans | 3.03 ± 2.16 | 51.79 ± 4.14* | 4 per week | [93] |
While there is agreement that males can inseminate more than one female consecutively, variation in lifetime insemination capacity and the relationship between insemination capacity and total male reproductive success are less clear.
indicates insemination success was measured over the entire male lifetime.
indicates that mean was calculated from published data instead of reported.
Data not available to calculate errors
Male-Male Competition
Given the operational sex ratio (OSR) and reproductive skew, it is likely that males compete for access to the limited number of females available. There are a few reports of males directly interfering with other males [31] and no evidence that males within a swarm defend particular “territories” [32]. Competition between males in mosquito swarms appears consistent with scramble competition, in which males attempt to find and mate with females before other males [33].
While males can theoretically increase their access to females by maximizing their time in mating swarms, swarming is a costly activity [11]. For example, Maïga et al. [34] estimated that 25 min of swarming consumed 50% of a male An. gambiae’s total sugar and glycogen reserves. Observations of Anopheles swarms suggest that the timing of swarming and not just duration may also be important for male mating success with the majority of matings occurring during peak swarming activity [9,35,36]. Within the swarm, male success can be enhanced by detecting and orienting to females faster than competitors. The elaborate sensory organs of male mosquitoes and the high aerial mobility males display during swarming support the role of male scramble competition [15]. There has been little work assessing the role of post-copulatory male competition in overall male reproductive success and this remains an important question.
Mate Choice
While larger, potentially more fecund, females are more likely to be mated [37], male choice is an unlikely explanation for these observations. Time spent searching within the swarm for a preferred female has costs [9,34,38]. Further, with intense scramble competition, males which pass up an encountered female risk failing to mate at all. An alternative explanation for the observed pattern of larger females being mated is that these females are easier to detect, intercept, or inseminate than smaller females [39].
There is also some evidence for assortative mating, in which males and females preferentially mate with partners sharing similar characteristics. In Anopheles and Aedes, studies indicate assortative preferences body size [11,40,41]. However, in these cases a single size class of male or female was investigated [11,41], or assortative mating was only detected for one size class [40]. Other work has provided some evidence for both assortative mating by genetic background and rearing environment in Anopheles [11]. The degree of assortative mating in nature and its relative importance in determining male mating success remains unclear.
The OSR and reproductive skew reported from the literature are the ideal conditions for the evolution of female choice (Box 1). In line with this prediction, there are several observations of females effectively rejecting males. These include reports of evasive flight [31,42], timing of swarm participation [35] and more “active” rejection behaviours such as kicking and otherwise displacing male attempts [24,42-45] in Aedes and Culex species (Table 2). Using high-speed videography, Aldersley and Cator [45] found that female Ae. aegypti delivered fewer kicks at a slower rate to males that were eventually accepted suggesting that females may alter mating responses based on their assessment males attempting to form the copula. Charlwood and Jones [46] report similarly low success rates in Anopheles (Table 2) and note that removal of female legs increased copulation rates by making females less resistant. Further, video tracking of wild Anopheles swarms suggests that females interact with multiple males prior to mating [11]. Benelli [42] documented similar female active rejection behaviours in Culex. Overall, while this is an active area of study, current data suggest that females of swarming mosquito species exercise rejection-based choice.
Box 1. Key Concepts in Sexual Selection.
Mate choice is the outcome of an individual’s selective response for a given phenotype of mate. Females most often exercise this choice, which is a major mechanism of sexual selection [72].
Direct benefits models propose that selection favours females with a genetically heritable preference for males that provide her with immediate benefits in the present generation. Such benefits can take the form of parental care, increased survival or resource acquisition, among others (see [73]).
Indirect benefits arise when a female’s preference for a male trait results not in immediate benefits for her, but in the form of increased offspring fitness. Indirect models of sexual selection are contingent on having sufficient detectable variation among males upon which female choice can act. Models of indirect benefits are distinct but not exclusive, and some encapsulate principles of both, making the case for a single process of indirect selection whereby females exhibit preference for males with a high reproductive value [74].
Fisherian Selection also known as “sexy sons” is a type of indirect selection in which alleles for female choosiness for attractive male ornaments are inherited by their offspring. Choosy females have attractive sons which achieve a greater than average reproductive success as they are preferred by females which can ultimately lead to lead to self-reinforced runaway selection in what is known as the Fisher process [75-77]. In the runaway process, even if male traits decrease survival, they will still be selected for if they increase male mating success.
“Good genes” or “viability indicator” [17] models of indirect benefits postulate that females show preferences for male traits that signal a heritable quality which translates to increased offspring reproductive value. The central tenet to these models is a self-reinforcing genetic correlation between female preference alleles and male fitness genotypes. This raises the question of how males advertise their quality status, and additionally how females can reliably identify the highest quality males. Sexually selected traits have been shown to have higher additive variation (VA) than non-sexually selected traits [62], despite the expectation that variation should be eroded as a result of female preference for beneficial alleles in what is known as the “paradox of the lek” [63]. Condition-dependence of traits is one proposed resolution of this paradox [78], among others [79].
Table 2.
Summary of studies in which the outcomes of individual mating attempts were tracked.
Models for the evolution of female choice address the fact that a female can garner direct and indirect benefits from the mate she selects. Direct benefits contribute to female fitness by increasing her survival and/or reproductive success (i.e. parental care, nuptial gifts) whereas indirect benefits improve the reproductive success of her offspring (i.e. increased offspring viability or mating success) (Box 1). While males do not contribute to parental care, they may provide material benefits to females via components of their ejaculate. Recent work has demonstrated that male ejaculate positively influences survival in female Ae. aegypti [47]. In An. gambiae, male transfer of the steroid hormone 20E triggers expression of multiple genes to increase egg development and initiate egg laying [20,48]. However, in both cases it is not known if females actively select males based on variation in ejaculate composition.
Work in Ae. aegypti suggests that females may receive indirect or genetic benefits [17,49,50] from mating with particular males. Females have been found to increase offspring competitive mating success [44] and immune function [51] by mating with males performing certain acoustic signals [15]. Other important fitness traits, such as insemination capacity and flight performance were not found to be associated with acoustic signals or inherited [52]. To date there has been no exploration of genetic benefits outside of this species.
Applying Sexual Selection to Control
There is currently enormous potential to improve our understanding of sexual selection in mosquitoes (see Outstanding Questions). Even with these questions outstanding, the current evidence suggests that swarms can be characterized as polygynous systems with both male-male competition and female choice (potentially based on indicators of male genetic quality), and these features of mosquito mating systems likely play a role in determining male reproductive success. A better understanding of the evolutionary forces which shape mosquito mating systems will enhance our ability to both maximize the impact of reproductive control tools in the short term and anticipate evolutionary response of wild populations to these interventions the longer term.
Outstanding Questions.
How do male sexually selected traits evolve?
What costs and benefits are associated with female mate choice, and can preferences vary and evolve?
How does the evolution and maintenance of traits key to male mating success affect both male and female traits?
Firstly, these data indicate that, in addition to producing an adequate quantity of males, mass-rearing programs need to produce males of adequate quality. Instead of variation in male mating success within swarms being random [10], it appears this variation is determined by both male traits and female preferences for these traits. Continued empirical work is needed to identify which traits are important for determining overall mating success. There is a plethora of metrics used to quantify male reproductive success (insemination capacity, body size etc.) including some which favor laboratory adaptation. However, unlike control programmes for other species, there is not an agreed set of metrics applied across studies [53]. Application of genetic tools to directly measure the number of offspring males produce in natural settings could be used to identify which proxies are best for monitoring and assessment (for example see [54]).
Notably, the potential role of female choice in addition to male-male competition suggests it is important to consider both traits which will enhance male competitive performance and attractiveness to females [55]. While manipulation of the environment experienced by males directly before release can be used to enhance their participation in swarms [11,56], increased swarming duration will increase mating opportunities but will not necessarily improve success rates upon encountering females [56,57]. In addition to traits involved in male-male competition, traits important for female choice need to be considered. Besides the few studies in Ae. aegypti discussed above these traits remain largely unknown.
We also need to address how male traits that contribute to reproductive success evolve. In general, most mass-rearing protocols have focused on environmental effects on males prior to release (diet and density in larval conditions, etc.) and have not addressed genetic or evolved contribution to male mating success (see Box 2 for exceptions). Traits under sexual selection are expected to respond to changes in mating environment and intensity of competition. Experimental evolution approaches [69] coupled with next generation sequencing methods could reveal genetic targets of sexual selection and how this is expected to evolve in different control scenarios such as interventions that release large numbers of males (e.g. SIT) or genetic techniques that distort the sex ratio (e.g. female-specific lethal).
Box 2. Genetic sources of decreased performance in release lines.
There are several factors that can lead to decreased performance in release lines:
1. The manipulation (genetic construct, Wolbachia infection) can affect male mating performance either directly or indirectly via pleiotropic effects. For example, Bargielowski et al. found costs associated with a genetic construct in Ae. aegypti [64]. In a separate study, Bargielowski et al. documented decreased flight performance in males carrying a construct that was only expressed in female offspring [65].
2. Loss of genetic diversity can also lead to performance deficits in laboratory reared strains. For example, in Anopheles mosquitoes, inbreeding depression can decrease sperm length, sperm motility, and survival [66,67]. In Ae. aegypti inbreeding depression can impair immature development, survival, and fecundity in the laboratory [68].
3. Adaptation to laboratory conditions can also impact performance in field conditions. The laboratory environment can select for life history and behavioural phenotypes that differ from field populations [69]. Long-term laboratory rearing favours males which maximize early mating opportunities and produce poorer quality sperm compared to wild males [66]. In a recent study, we reported evolved changes in mating behaviour occurred in as little as five generations of experimental selection in Ae. aegypti [61].
Strategies for mitigating these effects have been proposed. Manipulation design and early assessment is used to determine if off-target effects of manipulations will make males unlikely to mate. While it is standard to include viability and small cage insemination success, there has been a call to include a wider range of behaviours in these assessments [80]. It has been suggested that effects of captive mass-rearing could be mitigated with techniques such as hybridization of laboratory lines [81,82] or maintaining large population sizes [83]. These largely address the effects of loss of genetic diversity as opposed to laboratory adaptation. “Clean stream” rearing protocols, in which lines are held under more natural, less high-throughput conditions prior to release, have been established for other mass-rearing operations [84] and could be adapted for mosquitoes if deemed appropriate. In some instances, there is also the option of crossing lines with field material directly prior to release to enhance both diversity and introduce alleles from the natural population. More work is needed to understand the relative contribution of adaptation and inbreeding on mating performance to determine which are appropriate for a given intervention.
Improved understanding of sexual selection is also critical for predicting how wild populations will respond to the selective pressures that will be imposed by these control tools. For example, release strategies could theoretically be undermined by the appearance of female behavioural resistance in target populations [11]. There is potential for behavioural resistance with both population replacement and reduction strategies. (Figure 1). We expect there to be standing variation in female mating preferences. Applying strong selection to this standing variation will likely lead to the development of behavioural resistance in the long term. Female resistance to released males has been identified in mass-rearing programs for other insects such as medfly and screw-worm [58,59]. Evolutionary responses of female mosquito choice behaviours have been documented in the context of interspecific interactions [82,83], but variation and evolution of intraspecific choice is relatively unexplored. However, the development of female resistance has been suggested as a contributing factor to the reduced effectiveness of an intensive SIT program in Brazil [60]. It is critical that we better understand the role of female choice in determining male mating success and develop protocols for detecting this type of resistance.
While there is good evidence for choice in these systems, there is scarce data on the costs and benefits associated with female mate choice, and the degree to which preferences vary and can evolve. In order to anticipate evolutionary responses to male release strategies, a comprehensive assessment of female choice is required, including measurement of costs and benefits associated with choice [84]. Also, while there is compelling evidence that acoustic signals may be involved in female choice behaviours [44,61], the cues by which females assess males remain unclear and could have utility for monitoring release line quality [15].
We also need to improve our understanding of how the evolution and maintenance of traits important for male mating success affect other male and female traits. Evolutionary models predict trade-offs between costly traits when resources are finite [85]. For example, increased expression of male pre-copulatory traits may come at the cost of post-copulatory ones. Additionally, traits that maximise male mating success may have deleterious effects on female fitness (sexual conflict). Sexually selected traits have been shown to have higher additive variation than non-sexually selected traits [62], despite the expectation that variation should be eroded as a result of female preference for beneficial alleles [63] (“paradox of the lek”, Box 1). One way that variation may be maintained is via pleiotropic relationships or trade-offs between sexually selected traits and those under natural selection in either male or female offspring (Box 1). For example, An. gambiae immune gene thioester-containing protein 1 (TEP1) is highly polymorphic, with some alleles conferring resistance to Plasmodium, and others rendering females susceptible to infection [64]. It is thought that variation in this trait is maintained by pleiotropic antagonism as specific TEP1 alleles enhance male fertility by flagging defective sperm cells for removal whilst increasing female susceptibility to Plasmodium infection [65]. This is a salient example of how male sexually selected traits can have pleiotropic effects on traits that influence female vectorial capacity. Putative trade-offs between male mating success and female immunity [63] suggest further work is necessary to characterize genetic associations (e.g. pleiotropy, genetic coupling/linkage disequilibrium) between traits that are important to male mating success and for traits underlying vectorial capacity. All interventions that rely on the release of laboratory-reared males must be aware of the implications of this question to their control strategy.
Finally, most work on mating biology has focused on Ae. aegypti and An. gambiae. This is likely due to their major vector status and because they can be maintained reliably in colony. As new pathogens emerge and as secondary vectors increasingly become the targets of control, we strongly caution against overextrapolation of findings from these two species to others. While frameworks and experimental methods may be transferable it should not be assumed that mating system parameters will be the same even in closely related species.
Concluding remarks
The field is well positioned to address these long-standing gaps in knowledge (see Outstanding Questions). The wealth of genetic resources (assembled genomes, gene editing tools such as CRISPR etc.) available to mosquito biologists [66-68] allows for explicitly testing predictions of evolutionary models. Experimental evolution approaches can be powerful in identifying traits under selection by manipulating mating systems [55,69,70]. Combining selection experiments with next-generation sequencing methods can be effective in identifying male traits under selection, particularly since phenotypic correlations do not always predict genetic ones and significant variation in gene expression means that genetic correlations do not always predict phenotypic ones [71]. Uniquely, because of existing control programs, many of these species have existing infrastructure to measure traits under both controlled laboratory conditions and semi-field or field conditions. Combining cutting-edge techniques with sexual selection theory offers a powerful approach to enhance our understanding of sexual selection in mosquitoes and ultimately ensure the success of novel control strategies that target mosquito reproduction.
Highlights.
The mating systems of medically important mosquitoes are characterized by aerial swarming, within which many complex behaviours unfold.
Evidence suggests that females mate once, whereas males can mate multiply.
This combined with swarms that consist of many more males than females generates intense mating competition between males and allows females to be choosy.
A lack of data on male and female sexually selected traits and evolutionary relationships between them are a key knowledge gap in these systems.
A comprehensive understanding of mosquito mating biology is essential for the development and successful deployment of reproductive control methods.
Acknowledgements
This research was funded by NIH/NIAID grant R01AI095491 awarded to LCH, NIH/NIAID and grant R21AI118593 awarded to LJC. CASW was supported by a Natural Environment Research Council (NERC) Doctoral Training Programme in Science and Solutions for a Changing Planet grant NE/S007415/1.
Glossary:
- Operational sex ratio (OSR)
the ratio of sexually competing males that are ready to mate to sexually competing females that are ready to mate, or alternatively the local ratio of fertilizable females to sexually active males at any given time.
- Polygyny
mating system in which males mate multiply and females mate once. Males are expected to exhibit high variance in mating success and females exhibit low variance in mating success.
- Pre-copulatory success
refers to portion of reproductive success that results in the formation of a copula. In this case it would include swarming, mate location, and successful formation of a copula.
- Post-copulatory success
refers to portion of reproductive success that is determined after the copula is formed. This includes insemination, sperm competition, and fertility.
- Reproductive control
umbrella term for a suite of strategies that rely on the successful mating of laboratory reared males with natural populations to deliver population or disease control
- Reproductive skew
is a measure of the proportion of individuals of each sex that breed in a group.
- Reproductive success
an individual's production of offspring per breeding event or lifetime.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carvalho DO et al. (2015) Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Neglected Tropical Diseases DOI: 10.1371/journal.pntd.0003864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caragata EP et al. (2016) Exploiting Intimate Relationships: Controlling Mosquito-Transmitted Disease with Wolbachia. Trends in Parasitology DOI: 10.1016/j.pt.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 3.Helinski MEH et al. (2009) Radiation biology of mosquitoes. Malaria Journal DOI: 10.1186/1475-2875-8-S2-S6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reisen WK et al. (1977) Observations on the Swarming and Mating of Some Pakistan Mosquitoes in Nature. Annals of the Entomological Society of America DOI: 10.1093/aesa/70.6.988 [DOI] [Google Scholar]
- 5.Burt A (2014) Heritable strategies for controlling insect vectors of disease. Philosophical Transactions of the Royal Society B: Biological Sciences DOI: 10.1098/rstb.2013.0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alphey L (2014) Genetic control of mosquitoes. Annual Review of Entomology DOI: 10.1146/annurev-ento-011613-162002 [DOI] [PubMed] [Google Scholar]
- 7.Reisen WK et al. (1982) Attempted suppression of a semi-isolated Culex tarsalis population by the release of irradiated males: a second experiment using males from a recently colonized strain. Mosquito News [Google Scholar]
- 8.Zsemlye JL et al. (2005) Analysis of a complex vertical copulatory-courtship display in the yellow fever vector Sabethes chloropterus. Medical and Veterinary Entomology DOI: 10.1111/j.1365-2915.2005.00570.x [DOI] [PubMed] [Google Scholar]
- 9.Yuval B and Bouskila A (1993) Temporal dynamics of mating and predation in mosquito swarms. Oecologia DOI: 10.1007/BF00649508 [DOI] [PubMed] [Google Scholar]
- 10.Yuval B (2006) Mating systems of blood-feeding flies. Annual Review of Entomology DOI: 10.1146/annurev.ento.51.110104.151058 [DOI] [PubMed] [Google Scholar]
- 11.Diabate A and Tripet F (2015) Targeting male mosquito mating behaviour for malaria control. Parasites and Vectors. DOI: 10.1186/s13071-015-0961-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartberg WK (1971) Observations on the mating behaviour of Aedes aegypti in nature. Bulletin of the World Health Organization [PMC free article] [PubMed] [Google Scholar]
- 13.Reisen WK et al. (1985) Swarming and Mating Behavior of Laboratory and Field Strains of Culex tarsalis (Diptera: Culicidae). Annals of the Entomological Society of America DOI: 10.1093/aesa/78.5.667 [DOI] [Google Scholar]
- 14.Cator LJ et al. (2011) Behavioral observations and sound recordings of free-flight mating swarms of Ae. aegypti (Diptera: Culicidae) in Thailand. Journal of Medical Entomology DOI: 10.1603/ME11019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrés M et al. (2020) Buzzkill: targeting the mosquito auditory system. Current Opinion in Insect Science DOI: 10.1016/j.cois.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 16.Schuster S and Wade M (2003) Mating Systems and Strategies, Princeton University Press. [Google Scholar]
- 17.Andersson M (1994) Sexual selection, Princeton University Press [Google Scholar]
- 18.Clements AN (1999) The Biology of Mosquitoes: Sensory Reception and Behaviour [Google Scholar]
- 19.Young ADM and Downe AER (1987) Male accessory gland substances and the control of sexual receptivity in female Culex tarsalis. Physiological Entomology DOI: 10.1111/j.1365-3032.1987.tb00746.x [DOI] [Google Scholar]
- 20.Gabrieli P et al. (2014) Sexual transfer of the steroid hormone 20E induces the postmating switch in Anopheles gambiae. Proceedings of the National Academy of Sciences of the United States of America DOI: 10.1073/pnas.1410488111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell SN et al. (2015) Evolution of sexual traits influencing vectorial capacity in anopheline mosquitoes. Science DOI: 10.1126/science.1259435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw WR et al. (2014) Mating activates the heme peroxidase HPX15 in the sperm storage organ to ensure fertility in Anopheles gambiae. Proceedings of the National Academy of Sciences of the United States of America DOI: 10.1073/pnas.1401715111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Craig GB (1967) Mosquitoes: Female monogamy induced by male accessory gland substance. Science DOI: 10.1126/science.156.3781.1499 [DOI] [PubMed] [Google Scholar]
- 24.Helinski MEH et al. (2012) Duration and dose-dependency of female sexual receptivity responses to seminal fluid proteins in Aedes albopictus and Ae. aegypti mosquitoes. Journal of Insect Physiology DOI: 10.1016/j.jinsphys.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.League GP et al. (2019) Male accessory gland molecules inhibit harmonic convergence in the mosquito Aedes aegypti. Current Biology DOI: 10.1016/j.cub.2019.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degner EC and Harrington LC (2016) Polyandry depends on postmating time interval in the dengue vector Aedes aegypti. American Journal of Tropical Medicine and Hygiene DOI: 10.4269/ajtmh.15-0893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giglioli MEC and Mason GF (1966) The mating plug in anopheline mosquitoes. Proceedings of the Royal Entomological Society of London. Series A, General Entomology DOI: 10.1111/j.1365-3032.1966.tb00355.x [DOI] [Google Scholar]
- 28.Tripet F et al. (2003) Frequency of multiple inseminations in field-collected Anopheles gambiae females revealed by DNA analysis of transferred sperm. American Journal of Tropical Medicine and Hygiene DOI: 10.4269/ajtmh.2003.68.1.0680001 [DOI] [PubMed] [Google Scholar]
- 29.Richardson JB et al. (2015) Evidence of limited polyandry in a natural population of Aedes aegypti. American Journal of Tropical Medicine and Hygiene DOI: 10.4269/ajtmh.14-0718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helinski MEH et al. (2012) Evidence of polyandry for Aedes aegypti in semifield enclosures. American Journal of Tropical Medicine and Hygiene DOI: 10.4269/ajtmh.2012.11-0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones JC and Pilitt DR (1973) Observations on the sexual behavior of free flying Aedes aegypti mosquitoes. BIOL.BULL. DOI: 10.2307/1540302 [DOI] [Google Scholar]
- 32.Diabaté A et al. (2011) Spatial distribution and male mating success of Anopheles gambiae swarms. BMC Evolutionary Biology DOI: 10.1186/1471-2148-11-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alcock J and Thornhill R (2001) The Evolution of Insect Mating Systems. [Google Scholar]
- 34.Maïga H et al. (2012) Variation in energy reserves and role of body size in the mating system of Anopheles gambiae. Journal of Vector Ecology DOI: 10.1111/j.1948-7134.2012.00230.x [DOI] [PubMed] [Google Scholar]
- 35.Charlwood JD et al. (2002) Male size does not affect mating success (of Anopheles gambiae in São Tomé). Medical and Veterinary Entomology DOI: 10.1046/j.0269-283x.2002.00342.x [DOI] [PubMed] [Google Scholar]
- 36.Diabate A et al. (2003) Natural swarming behaviour of the molecular M form of Anopheles gambiae. Transactions of the Royal Society of Tropical Medicine and Hygiene DOI: 10.1016/S0035-9203(03)80110-4 [DOI] [PubMed] [Google Scholar]
- 37.Sawadogo SP et al. (2013) Effects of age and size on Anopheles gambiae s.s. male mosquito mating success. Journal of Medical Entomology DOI: 10.1603/ME12041 [DOI] [PubMed] [Google Scholar]
- 38.Dao A et al. (2010) Reproduction-Longevity Trade-Off in Anopheles gambiae (Diptera: Culicidae). Journal of Medical Entomology DOI: 10.1603/ME10052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dechaume-Moncharmont FX et al. (2016) Opportunity costs resulting from scramble competition within the choosy sex severely impair mate choosiness. Animal Behaviour DOI: 10.1016/j.anbehav.2016.02.019 [DOI] [Google Scholar]
- 40.Callahan AG et al. (2018) Small females prefer small males: Size assortative mating in Aedes aegypti mosquitoes. Parasites and Vectors DOI: 10.1186/s13071-018-3028-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aldersley A et al. (2019) Too “sexy” for the field? Paired measures of laboratory and semi-field performance highlight variability in the apparent mating fitness of Aedes aegypti transgenic strains. Parasites and Vectors DOI: 10.1186/s13071-019-3617-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benelli G (2018) Mating behavior of the West Nile virus vector Culex pipiens – role of behavioral asymmetries. Acta Tropica DOI: 10.1016/j.actatropica.2017.12.024 [DOI] [PubMed] [Google Scholar]
- 43.Gwadz RW and Craig GB (1968) Sexual receptivity in female Aedes aegypti. Mosquito News 28, 586–593 [Google Scholar]
- 44.Cator LJ and Harrington LC (2011) The harmonic convergence of fathers predicts the mating success of sons in Aedes aegypti. Animal Behaviour DOI: 10.1016/j.anbehav.2011.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aldersley A and Cator LJ (2019) Female resistance and harmonic convergence influence male mating success in Aedes aegypti. Scientific Reports DOI: 10.1038/s41598-019-38599-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charlwood J and Jones M (2008) Mating behaviour in the mosquito, Anopheles gambiae s.l. I. Close range and contact behaviour. Physiological Entomology 4, 111–120 DOI: 10.1111/j.1365-3032.1979.tb00185.x [DOI] [Google Scholar]
- 47.Villarreal SM et al. (2018) Male contributions during mating increase female survival in the disease vector mosquito Aedes aegypti. Journal of Insect Physiology DOI: 10.1016/j.jinsphys.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baldini F et al. (2013) The Interaction between a Sexually Transferred Steroid Hormone and a Female Protein Regulates Oogenesis in the Malaria Mosquito Anopheles gambiae. PLoS Biology DOI: 10.1371/journal.pbio.1001695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kokko H et al. (2003) The evolution of mate choice and mating biases. Proceedings of the Royal Society B: Biological Sciences. DOI: 10.1098/rspb.2002.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neff BD and Pitcher TE (2005) Genetic quality and sexual selection: An integrated framework for good genes and compatible genes. Molecular Ecology DOI: 10.1111/j.1365-294X.2004.02395.x [DOI] [PubMed] [Google Scholar]
- 51.Reitmayer C et al. (2020) Sex, Age, and Parental Harmonic Convergence Behavior Affect the Immune Performance of Aedes aegypti Offspring. bioRxiv DOI: 10.1101/2020.09.25.312991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.League GP et al. (2020) Sexual selection theory meets disease vector control: Testing harmonic convergence as a "good genes" signal in Aedes aegypti mosquitoes bioRxiv DOI: 10.1101/2020.10.29.360651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cáceres C et al. (2007) Quality management systems for fruit fly (Diptera: Tephritidae) sterile insect technique. Florida Entomologist DOI: 10.1653/0015-4040(2007)90[1:QMSFFF]2.0.CO;2 [DOI] [Google Scholar]
- 54.Jasper M et al. (2019) A genomic approach to inferring kinship reveals limited intergenerational dispersal in the yellow fever mosquito. Molecular Ecology Resources DOI: 10.1111/1755-0998.13043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qureshi A et al. (2019) Male competition and the evolution of mating and life-history traits in experimental populations of Aedes aegypti. Proceedings of the Royal Society B: Biological Sciences DOI: 10.1098/rspb.2019.0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lang BJ et al. (2018) The Effect of Larval Diet on Adult Survival, Swarming Activity and Copulation Success in Male Aedes aegypti (Diptera: Culicidae). Journal of Medical Entomology DOI: 10.1093/jme/tjx187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cator LJ and Zanti Z (2016) Size, sounds and sex: interactions between body size and harmonic convergence signals determine mating success in Aedes aegypti. Parasites and Vectors DOI: 10.1186/s13071-016-1914-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McInnis DO et al. (1996) Behavioral resistance to the sterile insect technique by Mediterranean fruit fly (Diptera: Tephritidae) in Hawaii. Annals of the Entomological Society of America DOI: 10.1093/aesa/89.5.739 [DOI] [Google Scholar]
- 59.Wyss JH (2001) Overview of the sterile insect technique in screw-worm fly eradication. Proceedings of the screw-worm fly emergency preparedness conference, Canberra, Australia [Google Scholar]
- 60.Powell JR (2018) Genetic variation in insect vectors: Death of typology? Insects DOI: 10.3390/insects9040139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reitmayer C et al. (2020) Sex, Age, and Parental Harmonic Convergence Behavior Affect the Immune Performance of Aedes aegypti Offspring. bioRxiv DOI: 10.1101/2020.09.25.312991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pomiankowski A and Moller AP (1995) A resolution of the lek paradox. Proceedings of the Royal Society B: Biological Sciences DOI: 10.1098/rspb.1995.0054 [DOI] [Google Scholar]
- 63.Borgia G (1979) Sexual Selection and the Evolution of Mating Systems. In Sexual Selection and Reproductive Competition in Insects [Google Scholar]
- 64.Obbard DJ et al. (2008) The evolution of TEP1, an exceptionally polymorphic immunity gene in Anopheles gambiae. BMC Evolutionary Biology DOI: 10.1186/1471-2148-8-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pompon J and Levashina EA (2015) A New Role of the Mosquito Complement-like Cascade in Male Fertility in Anopheles gambiae. PLoS Biology DOI: 10.1371/journal.pbio.1002255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matthews BJ et al. (2018) Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature DOI: 10.1038/s41586-018-0692-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jenkins AM et al. (2015) Long non-coding RNA discovery across the genus anopheles reveals conserved secondary structures within and beyond the Gambiae complex. BMC Genomics DOI: 10.1186/s12864-015-1507-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen XG et al. (2015) Genome sequence of the Asian tiger mosquito, Aedes albopictus, reveals insights into its biology, genetics, and evolution. Proceedings of the National Academy of Sciences of the United States of America DOI: 10.1073/pnas.1516410112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawecki TJ et al. (2012) Experimental evolution. Trends in Ecology and Evolution DOI: 10.1016/j.tree.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 70.Snook RR et al. (2005) Experimental manipulation of sexual selection and the evolution of courtship song in Drosophila pseudoobscura. Behavior Genetics DOI: 10.1007/s10519-005-3217-0 [DOI] [PubMed] [Google Scholar]
- 71.Hunt J et al. (2004) What is genetic quality? Trends in Ecology and Evolution. DOI: 10.1016/j.tree.2004.03.035 [DOI] [PubMed] [Google Scholar]
- 72.Kokko H et al. (2006) Unifying and testing models of sexual selection. Annual Review of Ecology, Evolution, and Systematics DOI: 10.1146/annurev.ecolsys.37.091305.110259 [DOI] [Google Scholar]
- 73.Møller A and Jennions M (2001) How important are direct fitness benefits of sexual selection? Naturwissenschaften DOI: 10.1007/s001140100255 [DOI] [PubMed] [Google Scholar]
- 74.Kokko H et al. (2002) The sexual selection continuum. Proceedings of the Royal Society B: Biological Sciences DOI: 10.1098/rspb.2002.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fisher R (1930) Chapter 6: Sexual reproduction and sexual selection. The Genetical Theory of natural Selection. [Google Scholar]
- 76.Lande R (1981) Models of speciation by sexual selection on polygenic traits. Proceedings of the National Academy of Sciences of the United States of America DOI: 10.1073/pnas.78.6.3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirkpatrick M (1982) Sexual Selection and the Evolution of Female Choice. Evolution DOI: 10.2307/2407961 [DOI] [PubMed] [Google Scholar]
- 78.Rowe L and Houle D (1996) The lek paradox and the capture of genetic variance by condition dependent traits. Proceedings of the Royal Society B: Biological Sciences DOI: 10.1098/rspb.1996.0207 [DOI] [Google Scholar]
- 79.Radwan J (2008) Maintenance of genetic variation in sexual ornaments: A review of the mechanisms. Genetica DOI: 10.1007/s10709-007-9203-0 [DOI] [PubMed] [Google Scholar]
- 80.Su MP et al. (2020) Assessing the acoustic behaviour of Anopheles gambiae s.l. dsxF mutants: Implications for Vector Control. Parasites and Vectors DOI: 10.1186/s13071-020-04382-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wise De Valdez MR et al. (2011) Genetic elimination of dengue vector mosquitoes. Proceedings of the National Academy of Sciences of the United States of America DOI: 10.1073/pnas.1019295108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ekechukwu NE et al. (2015) Heterosis increases fertility, fecundity, and survival of laboratory produced F1 hybrid males of the malaria mosquito Anopheles coluzzii. G3: Genes, Genomes, Genetics DOI: 10.1534/g3.115.021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ross PA et al. (2019) A comprehensive assessment of inbreeding and laboratory adaptation in Aedes aegypti mosquitoes. Evolutionary Applications DOI: 10.1111/eva.12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Calkins CO and Parker AG (2005) Sterile insect quality. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management [Google Scholar]
- 85.Gwadz RW and Craig GB (1970) Female polygamy due to inadequate semen transfer in Aedes aegypti. Mosquito News [Google Scholar]
- 86.Jones JC (1973) A study on the fecundity of male Aedes aegypti. Journal of Insect Physiology DOI: 10.1016/0022-1910(73)90118-2 [DOI] [PubMed] [Google Scholar]
- 87.Foster WA and Lea AO (1975) Renewable fecundity of male Aedes aegypti following replenishment of seminal vesicles and accessory glands. Journal of Insect Physiology DOI: 10.1016/0022-1910(75)90120-1 [DOI] [PubMed] [Google Scholar]
- 88.Bargielowski I et al. (2011) Cost of mating and insemination capacity of a genetically modified mosquito Aedes aegypti OX513A compared to its wild type counterpart. PLoS ONE DOI: 10.1371/journal.pone.0026086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boyer S et al. (2011) Sexual performance of male mosquito Aedes albopictus. Medical and Veterinary Entomology DOI: 10.1111/j.1365-2915.2011.00962.x [DOI] [PubMed] [Google Scholar]
- 90.Oliva CF et al. (2012) The Sterile Insect Technique for Controlling Populations of Aedes albopictus (Diptera: Culicidae) on Reunion Island: Mating Vigour of Sterilized Males. PLoS ONE DOI: 10.1371/journal.pone.0049414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Okanda FM et al. (2002) Behavioural determinants of gene flow in malaria vector populations: Anopheles gambiae males select large females as mates. Malaria Journal DOI: 10.1186/1475-2875-1-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zalom FG et al. (1981) Determining the mating ability of irradiated male Culex tarsalis (Diptera: Culicidae). Journal of Medical Entomology DOI: 10.1093/jmedent/18.2.167 [DOI] [PubMed] [Google Scholar]
- 93.South SH et al. (2009) Male mating costs in a polygynous mosquito with ornaments expressed in both sexes. Proceedings of the Royal Society B: Biological Sciences DOI: 10.1098/rspb.2009.099 [DOI] [PMC free article] [PubMed] [Google Scholar]