Abstract
We report the case of a patient who developed a large brain abscess after neurosurgery. Cerebrospinal fluid from the abscess drainage yielded Abiotrophia adiacens-specific PCR products and microorganisms that were identified by conventional microbiological methods and by 16S ribosomal DNA analysis as Abiotrophia adiacens, which was formerly classified as a member of nutritionally variant streptococci.
The genus Abiotrophia comprises fastidious gram-positive bacteria (4), previously referred to as nutritionally variant streptococci (NVS). These organisms grow as satellite colonies around other bacteria or on complex media supplemented with l-cysteine or pyridoxal hydrochloride. NVS are part of the normal oral, intestinal, and genital flora and were first isolated from patients with endocarditis and otitis media (3). Reportedly, NVS cause 5% of the cases of streptococcal endocarditis (6). Isolations have also been made from patients with otitis externa, wound infections, pancreatic abscess, and conjunctivitis (8). We report the isolation of Abiotrophia adiacens from the cerebrospinal fluid (CSF) of a patient who developed a brain abscess after neurosurgery.
Case report.
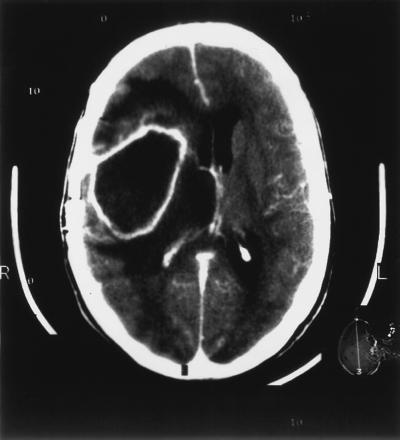
A 46-year-old woman was admitted to the Department of Neurosurgery, University Hospital Mainz, because of persisting headache, nausea, and vomiting for 3 weeks. Her past medical history revealed two neurosurgical operations: excision of an astrocytoma, WHO grade II–III, in 1994 and removal of a tumor recurrence in March 1998, 2 months prior to this admission. On admission, the patient appeared ill and displayed low-grade neck stiffness. She was afebrile, and nothing abnormal was detected at the former site of operation. The patient had a leukocyte count of 17.4 × 109/liter and an elevated C-reactive protein level (6 mg/dl). A computerized tomography (CT) of the brain revealed a large cystic mass with perifocal edema at the former tumor resection site (Fig. 1). On clinical examination, the patient did not display symptoms of sinusitis, dental infections, or other conditions predisposing for the development of a brain abscess. The patient received high-dose steroids and underwent a craniotomy. Purulent material was aspirated following incision of the dura mater. A large brain abscess that could be completely excised was found at the former tumor resection site. The lateral wall of the right ventricle was resected, and a cerebrospinal fluid (CSF) drainage was established. Histopathological examination of the abscess material revealed pus and central necrosis surrounded by a capsule consisting of connective tissue, indicative of a chronic inflammatory reaction. Following removal of the abscess, the patient was treated with ceftriaxone (1 g per day) and gentamicin (240 mg per day) for 10 days. The C-reactive protein level and leukocyte count returned to normal values during therapy. Clinical recovery was achieved, and the patient was discharged after 15 days.
FIG. 1.
Results from a gadolinium-enhanced CT scan demonstrating a cystic mass with perifocal edema in the right frontal temporoparietal hemisphere.
Two swabs were taken from the aspirated pus for microbiological analysis. Microscopic examination of Gram-stained smears revealed polymorphonuclear neutrophils (PMN) but no bacteria. Cultures of this material on blood agar cross-streaked with Staphylococcus aureus, on cysteine–lactose–electrolyte-deficient (CLED) agar, and on Schaedler agar as well as thioglycolate broth remained sterile. A possible explanation for the sterile cultures could be that the swabs were not taken from the active margin of the lesion and did not contain sufficient amounts of bacteria for culture. Possibly fastiduous bacteria did not get enough support for growth during transport. A sample of the purulent abscess aspirate would have been the superior material for microbiological examination.
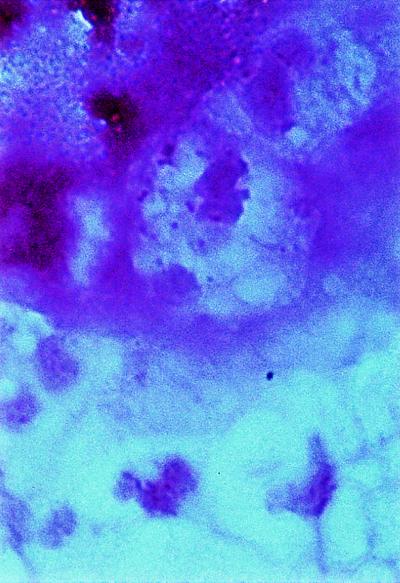
At 36 h after the neurosurgical procedure, CSF drained from the operation site was taken for microbiological analysis. Many PMN and pleomorphic gram-variable coccobacilli were visible on the Gram-stained smear, most of them located within granulocytes (Fig. 2). CSF cultures were performed on blood agar plates (aerobic and anaerobic culture conditions) and on brain heart infusion broth. After 24 h, growth of a few alpha-hemolytic colonies was detected only on blood agar plates cross-streaked with S. aureus. The bacteria could not be cultivated on chocolate agar. The pathogen was identified by sequencing of amplified 16S ribosomal DNA (rDNA) fragments as described previously (9). Sequencing of amplification products obtained directly from the CSF yielded identical results. Similarity searches using the BLAST program (1) were performed with test sequences of various lengths up to 530 bp. For unambiguous species identification, a signature sequence of 50 bases (corresponding to positions 145 through 194 of the Abiotrophia adiacens gene [accession no. dbj/D50450/ABAGIFUA]) was sufficient. All four of the highest-scoring homologous sequences were derived from various Abiotrophia species isolates, with A. adiacens showing the highest alignment score.
FIG. 2.
Direct smear of CSF drained from the operation site showing PMN and intracellular pleomorphic gram-variable coccobacilli.
Results of conventional microbiological tests were in accordance with the 16S rDNA-based identification of our isolate. As described for Abiotrophia species, 0.001% pyridoxal hydrochloride or 0.01% cysteine was required for growth on blood agar plates (8). Neither chocolate agar, thioglycolate broth, brain heart infusion broth, nor anaerobic culture supported growth of the present isolate. Conflicting reports have been published (8) on the usefulness of these culture conditions for growth of NVS. These discrepant results are probably due to inconsistent levels of required nutrients in different formulations of the same medium or variability in the levels of nutrients required by individual NVS strains. Further biochemical characterization was performed by using the API 20 Strep System with modifications as described by Bouvet et al. (2). As shown in Table 1, the isolated strain exhibited the enzyme pattern characteristic of A. adiacens (7, 8).
TABLE 1.
Characterization of the present isolate in comparison with Abiotrophia defectiva and A. adiacens (7)
| Characteristic | Reactiona
|
||
|---|---|---|---|
| A. adiacens | A. defectiva | Present isolatec | |
| Growth on blood agar supplemented with: | |||
| Pyridoxal hydrochloride | + | + | + |
| l-Cysteine | + | + | + |
| Enzyme production | |||
| Pyrrolidonyl-arylamidase | + | + | + |
| Alkaline phosphatase | − | − | − |
| α-Galactosidase | − | + | − |
| β-Galactosidase | − | + | − |
| β-Glucuronidase | +/Vb | − | + |
| β-Glucosidase | −/Vb | − | − |
| Leucine arylamidase | + | + | + |
| Hippurate hydrolysis | − | − | − |
| Acetoin production | −/Vb | − | + |
| Acidification of: | |||
| Trehalose | − | + | − |
| Inulin | +/Vb | − | − |
| Lactose | − | +/Vb | − |
| Raffinose | − | +/Vb | − |
| Starch | − | + | − |
| Glycogen | − | − | − |
+, positive; −, negative; V, variable.
Ruoff et al. (8) reported these reactions to be variable.
Partial 16S rDNA analysis indicated that the present isolate was A. adiacens.
Central nervous system infections due to Abiotrophia or NVS have never been reported to our knowledge. One reason could be that these organisms are easily overlooked because of their nutritional requirements. Perhaps NVS can cause cerebral infections only under special conditions. In the present case, it remains unclear whether the infection was acquired from an endogenous or exogenous source. The patient did not display clinical signs of an additional focus of infection. The histological findings of a chronic inflammation suggest that this abscess had developed over a long period. It is possible that the bacteria had entered the brain during the neurosurgical procedure performed 2 months previously. Alternatively, a transient bacteremia could have resulted in colonization of the tumor resection site. A CT scan done after the craniotomy in March had revealed intracranial hemorrhage. As in two other cases (5, 10) of brain abscesses due to a commensal bacterium (Mycoplasma hominis), hematoma formation might have been an important prerequisite for the development of this central nervous system infection with Abiotrophia adiacens.
Acknowledgments
We thank the members of our routine laboratory for skillful work, Bernhard Jahn for helpful discussions, and P. Stoeter for kindly providing the CT scans.
This work was supported by grant 836-386261/190 of the “Stiftung Rheinland-Pfalz für Innovation” awarded to M. Husmann and S. Bhakdi.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipmann D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Bouvet A, Villeroy F, Cheng F, Lamesch C, Williamson R, Gutmann L. Characterization of nutritionally variant streptococci by biochemical tests and penicillin-binding proteins. J Clin Microbiol. 1985;22:1030–1034. doi: 10.1128/jcm.22.6.1030-1034.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frenkel A, Hirsch W. Spontaneous development of L forms of streptococci requiring secretions of other bacteria or sulphydryl compounds for normal growths. Nature. 1961;191:728–730. doi: 10.1038/191728a0. [DOI] [PubMed] [Google Scholar]
- 4.Kawamura Y, Hou X G, Sultana F, Liu S, Yamamoto H, Ezaki T. Transfer of Streptococcus adjacens and Streptococcus defectivus to Abiotrophia gen. nov. as Abiotrophia adiacens comb. nov. and Abiotrophia defectiva comb. nov., respectively. Int J Syst Bacteriol. 1995;45:798–803. doi: 10.1099/00207713-45-4-798. [DOI] [PubMed] [Google Scholar]
- 5.Payan D G, Seigal N, Madoff S. Infection of a brain abscess by Mycoplasma hominis. J Clin Microbiol. 1981;14:571–573. doi: 10.1128/jcm.14.5.571-573.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts R B, Krieger A G, Schiller N L, Gross K C. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis. 1979;1:955–966. doi: 10.1093/clinids/1.6.955. [DOI] [PubMed] [Google Scholar]
- 7.Roggenkamp A, Abele-Horn M, Trebesius K, Tretter U, Autenrieth I B, Heesemann J. Abiotrophia elegans sp. nov, a possible pathogen in patients with culture-negative endocarditis. J Clin Microbiol. 1998;36:100–104. doi: 10.1128/jcm.36.1.100-104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruoff K L. Nutritionally variant streptococci. Clin Microbiol Rev. 1991;4:184–190. doi: 10.1128/cmr.4.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saweljew P, Kunkel J, Feddersen A, Baumert M, Baehr J, Ludwig W, Bhakdi S, Husmann M. Case of fatal systemic infection with an Aureobacterium sp.: identification of isolate by 16S rRNA gene analysis. J Clin Microbiol. 1996;34:1540–1541. doi: 10.1128/jcm.34.6.1540-1541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng X, Olson D A, Tully J G, Watson H L, Cassell G H, Gustafson D R, Svien K A, Smith T F. Isolation of Mycoplasma hominis from a brain abscess. J Clin Microbiol. 1997;35:992–994. doi: 10.1128/jcm.35.4.992-994.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]