Abstract
Background:
Several studies have demonstrated that brief interactions with natural environments can improve cognitive functioning. However, the neurocognitive processes that are affected by natural surroundings are not yet fully understood. It is argued that the “elements” in natural environment evoke “effortless” involuntary attention and may affect the neural mechanisms underlying inhibition control central to directed attention.
Methods:
The present study used electroencephalography (EEG) to investigate the effects of nature experience on neurocognitive processes involved in directed attention. During EEG recordings, participants (n = 53) were presented nature audio/video as stimuli to evoke nature experience, and flanker task was administered both before and after nature experience. An open eye rest condition was included randomly in either before or after nature experience cognitive task as a control condition.
Results:
The event-related potential analysis demonstrated a significant improvement in the response time after the nature experience. The analysis also demonstrated a significant difference for the inhibitory control process in fronto-parietal N2 (P < .01) and P3 (P < .05) for incongruent trials subsequent to nature experience. The spectral analysis also found an increase in alpha in all five brain regions (all Ps < .01) and fronto-central theta power (P < .01).
Conclusion:
The findings suggest that improved inhibitory control processes could be one of the aspects of enhanced directed attention after nature experience. Increased alpha along with theta indicates a relaxed yet alert state of mind after nature experience.
Keywords: Nature experience, Directed attention, Inhibition, N2, P3
Introduction
The ability to direct attention on tasks at hand is central to the efficiency of attentional mechanism.1, 2 One of the core cognitive subprocesses in the directed attentional mechanism is the ability to suppress distractors that potentially interfere with the primary response or appropriate action. In the literature, this ability to suppress distractors has been broadly referred to as “inhibitory control.”3–5 Studies suggest that the voluntary control of distractors and directing one’s attention on the task in hand are prone to fatigue.6, 7 In an urban setting, often there are many stimulations in the vicinity of the person that require mental “effort” to inhibit the incoming distractions while maintaining focus on a specific task. Thus, over time this cognitive resource tends to get depleted. Studies suggest that the deficits of “inhibitory control” may cause loss of focus, decrease in efficiency, and other attention-related dysfunctions.8, 9 There are several techniques for recovery from attention fatigue that have been investigated, i.e., rest allowances,10 sleep,11 mindfulness, yoga-based therapies,12 and nature experience (NE).13
Recently, there has been a growing interest to investigate the beneficial effects of brief interactions with natural environment on attention restoration. Kaplan and Kaplan14 in their “Attention Restoration Theory” posited that the sensory stimuli present in the elements of natural environment draw “effortless” or involuntary attention. It is argued that “fascinating” landscapes, forests, and green areas such as gardens or parks with “open spaces” generate a feeling of “being away” and “coherence” and are especially found effective as restorative settings.15–20 Experiences at such places termed as “nature experience” are described inherently personal and subjective, yet they have commonalities that are known to be beneficial for overall health.21, 22 NE also finds a mention in the yogic theory on Pratayahara (control of senses), where the “impressions” from nature are suggested to help in creating positive thoughts, cleansing the mind of “external disturbances,” and controlling the senses to gradually build the capability of complete attention.23
Emprical investigations also suggest that NE may help to moderate and diminish states of arousal, and helps in “clearing the head” of distracting thoughts.13, 22–26 It is argued that NE gently engages an individual in a low-stimulation activity, which reduces the internal noise and provides a quiet internal space. While attending to the natural phenomena such as passing clouds and waves of the ocean, it is reported that individuals feel respite from the “sensory bombardments” and stresses of daily life.27, 28 It is further proposed that at emotional level, the natural environment provides a positive emotional experience that may affect the cognitive control process and directed attention.29 At another level, it is posited that the “elements” of nature bring forth a metaphysical experience.30 This experience has been expressed as moments of extreme happiness, a feeling of lightness and freedom, a sense of harmony with the whole world, and moments that felt completely absorbing and that evoked a feeling of spiritual transcendence. Some of the most influential scholars in transcendent experience, e.g., William James, W. T. Stace, Margharita Laski, and Abraham Maslow, have also observed that NE has a close association with transcendent experience. Notably, the transcendental spiritual experiences are also believed to be beneficial in reducing distratctions and enhancing attention.31, 32
The NE helps participants in transitioning from predominantly task-relevant voluntary attention to saliency-dependent involuntary attention.33 The activation of involuntary mechanisms during brief NE is argued to restore the voluntary attentional resources. Further evidence suggests that involuntary attention perhaps differs in the “inhibitory control” required for directed attention.34, 35 It is argued that involuntary control processes employ lower cognitive resources for inhibitory control than those demanded by volunatry control processes.
While the studies have reported that NE is effective in controlling the outward distractions of mind presented by urban environments,26, 36–38 it is yet not evident if NE also helps in controlling the internal distractions of mind. During the resting state, in the absence of any external stimuli, the thoughts, feelings, emotions—the “internal noise” of an individual—may present similar mental activity as, for instance, the visual or auditory senses.39 Studies have reported that the reception of passive audiovisual input without any active stimulus or task processing during the resting state reflects the “baseline activation level.” The activation after NE when contrasted with the baseline activation provided by the resting state may elucidate the incremental benefits of NE for a subsequent cognitive task.
The effect of NE on the neurocognitive processes of directed attention is yet to be substantiated in the literature.
Therefore, the experiments in this research were planned with an attempt to measure the effect of NE on the neurocognitive processes of directed attention using the electroencephalography (EEG). A cognitive task was designed to elicit differences in cognitive processing because of the absence or presence of conflicting and distracting information.40 The task was administered after both nature experience (NE) and a control condition of open eye resting state (OERS). It was theorized that NE might affect the “inhibitory control” required to successfully discriminate the conflicting and distracting information, with greater “inhibitory control” as an indicator of efficient and enhanced attention. Some of the earlier studies have used urban environments as control to demonstrate the differences. In this study, an OERS was introduced in the experimental design as an alternate setting to examine the differences in cognitive recovery of attentional resources because of “internal noise” present during the resting state.39 The use of OERS as control has been reported by studies on other techniques of attention recovery as well, for example, mindfulness and meditation.41, 42 It was assumed that an OERS might not necessarily provide the restoration of attention. The rationale behind this assumption was that OERS arguably is devoid of “fascinating” stimuli, or a feeling of “being away,” and may lack a sense of “coherence”—the elements according to Attention Restoration Theory necessary for the restoration of attentional capacity.
Physiological measures like EEG have the benefit of being objective and have the capacity to peep into the participant’s brain responses during the NE or post-NE cognitive tasks. Brain responses or the event-related potentials (ERP) offer a real-time temporal resolution of neural processes underlying diffrences in task performances.43 A fundamental assumption of ERP research is that the ERP can be separated into different components, each indexing the degree of activity of a particular cognitive function. Most commonly, EEG studies implicate fronto-parietal cortical brain regions with the neurocognitive processes involved in attention. Particularly, a fronto-central negative peak N2 appearing around 180 to 325 ms44, 45 and a centro-parietal positive peak P3 appearing around 350 to 500 ms46, 47 after the stimulus onset have been reported to be associated with directed attention.
The outcomes from this paper indicate that NE significantly improves inhibitory control and in turn, enhances attention in after NE tasks.
Methods
This paper examines the differences in EEG waveforms in a lab experiment conducted with a cognitive (flanker) task before and after NE. NE was manipulated through nature video with real-time natural sounds as stimuli. A control condition of OERS was randomized before or after NE. Both the flanker task and the stimuli were presented on a 52-inch monitor, and a continuous EEG was recorded. The participants were briefed about the experiment and were told about the prerequisites for EEG data collection prior to their consent to participate. The sample size for this study was determined on the basis of power analysis (α = 0.05, β = 0.05).
Participants
A total of 53 healthy participants (37 males) with a mean age of 25 years (age range within 19–33) volunteered to participate in the experiment. All participants were recruited from the resident student groups of the Indian Institute of Technology Delhi. Exclusion criteria included extreme stress, sleep deprivation, a current diagnosis of a physical or psychiatric illness, or regular use of medication. Thirty participants were undergraduates, 18 postgraduates, and five research scholars. All participants were right-handed, and had normal or corrected to normal vision. None of the participants had any prior expertise or background in neuroscience or in studies in NE, and gave their consent voluntarily for participation. They were compensated with food coupons after the experiment.
Cognitive (Flanker) Task
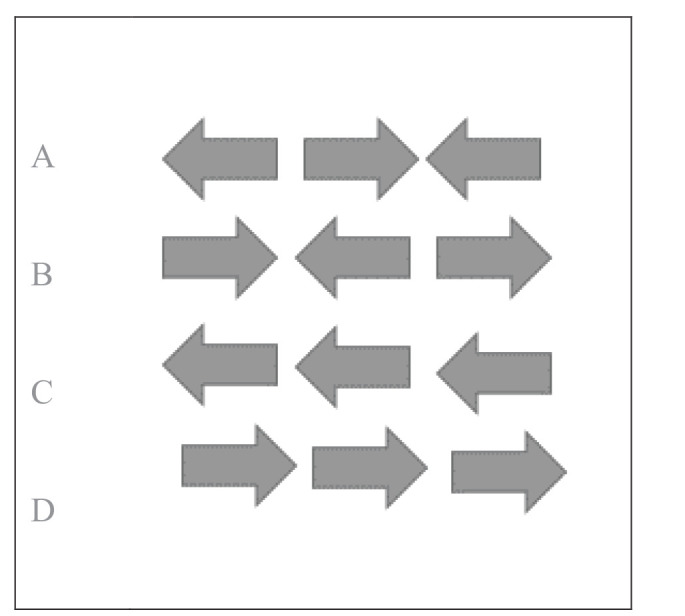
A modified arrow version of the Eriksen flanker task40 was presented using E-Prime version 2.0 software (Psychology Software Tools, Pittsburgh, PA, USA), and Chronos® was used to record the responses. Participants completed the flanker task at the beginning and also after NE. The flanker task composed of two types of trials, congruent and incongruent trials. The congruent trials consisted of the central target being flanked by arrows pointing in the same direction. In contrast, incongruent trials involved the target being flanked by arrows pointing in the opposite direction as shown in Figure 1. A set of instructions preceded the first trial that explained which button press would be used to indicate the direction of the central or target arrow. In addition, participants were instructed to respond as quickly and accurately as possible for each trial. Each trial began with a fixation cross (+) in the center of a white screen for 1000 ms, followed by black arrows centered focally on a white background for 2000 ms. Following the instructions, participants completed 20 practice trials. After the practice, the participants completed two test blocks of 80 trials with open eye rest time in between. Each test block consisted of 64 congruent and 16 incongruent trials. The congruency in the test trials was randomized.
Figure 1. An Example of Flankers Trials Represented at (A) and (B) Refers to Incongruent, and at (C) and (D) Congruent Trials.
NE Stimuli
The nature stimulus presented to the participant was a 15-min video having natural sounds. The video was selected using the method of content validity for the selection process, as described by.48 Open source videos available at BBC, National Geographic, and YouTube were searched with “nature” or “natural environment,” and “natural sounds.” Videos accompanying the audio file containing the original naturalistic sounds were selected. Twenty videos that matched the selection criteria were shortlisted for evaluation. Further screening was done by four subject experts, each from environmental sciences, natural sciences, psychology, and social sciences. Each expert was asked to rate (1 = “least” to 4 = “most”) the video based on “Is the video representative of the natural elements, i.e., sounds and the landscape visuals, that are commonly accessible in the neighborhood areas?” The audio/video that was found “most” representative by at least two experts was selected.
Procedure
Potential participants received detailed information regarding the study and completed a screening questionnaire. Once the inclusion criteria were met, the participants signed a consent form. They were taken to the EEG data acquisition lab, and EasyCap® was placed on their scalps. A 64-channel active electrode system (ActiCHamp, Brain Products GmbH, Germany) based on the 10/20 system was used for raw data acquisition. Figure 2 shows a picture of the experimental settings. The active electrodes placed on the EasyCap® were prepared with SuperVisc conducting gel such that the impedance was maintained below 5 kΩ. All experiments were conducted in the dimly lit experiment room where each participant was seated in a comfortable chair approximately 75 cm away from a 52-inch computer monitor. The recording setup was kept in the observation room with a one-way glass partition in between the two rooms. This setup is particularly useful to avoid making participants conscious of being watched during the experiment.
Figure 2. Picture of Experimental Settings.

The subjects were given a written set of instructions to be followed during the experiment and were also verbally instructed to remain relaxed and avoid any eye or gross body movement during the experiment. A continuous EEG activity was recorded during the flanker task before and after and while the participants were viewing the nature video. The EEG was sampled at 500 Hz, and referenced to default reference FCz and offline re-referenced to mastoids. A checklist of instructions and steps to be followed during data collection both for the investigator and the participant was prepared in advance. This checklist was uniformly applied for each experiment. The experiment lasted for an average of 38 min. The overall flow diagram of the experimental procedure is depicted in Figure 3.
Figure 3. Experimental Flow Diagram.
Control Measures in Experimental Design
The experimental procedure included a practice block in a prebaseline period and randomization of trial type to reduce “task familiarity effects”.49 Controlled lab settings for experimentation were maintained with minimal interference in the experiment room after the start of the experiment to control the “Hawthorne effects” that occur when the subjects know that they are being observed.50
Data Analysis
The EEG data were processed using Brain Vision Analyzer (version 2.0 Brain Products, Munich, Germany). Data were re-referenced to the average of the left and right mastoids (TP9 and TP10).51–53 The artifact correction was performed using the method included in the Brain Vision Analyzer. The details of preprocessing and data extraction for the ERP analysis are presented in Figure 4.
Figure 4. Stepwise EEG Data Processing.
The steps followed for preprocessing were the same as for ERP and spectral analyses. However, there was a difference in the processing pipeline after segmentation 1: prior to the spectral analysis, the data after preprocessing were downsampled to 256 Hz. Fast Fourier transform was performed on epochs of 2 s with 50% overlap to get a maximum frequency resolution of 0.5 Hz. Data were extracted for the following frequency bands: theta (4–7.5 Hz) and alpha (7.5–12.5 Hz). The mean frequency band power for the alpha and theta frequency was calculated for five regions—frontal, fronto-central, central, centro-parietal, and parietal. The statistical analysis for theta, alpha, and beta (before and after NE) was undertaken using a series of paired sample t-tests for all regions over before and after NE.
For the ERP analysis, using a mean amplitude approach,54 based on the grand averaged data, mean activity (signed values) for N2 and P3 was extracted within a 280 to 350 ms and 300 to 650 ms window poststimulus onset, respectively. The global maxima peak in the baseline-corrected average waveform was calculated for all electrodes within the respective time window. The latency of electrode with the highest amplitude of P3 and N2 was used for the statistical analysis.
Before conducting statistical analyses, cognitive outcome measures were assessed for normality. To test the effects of NE, within-subject measure analyses of variance with the condition (before and after NE) and congruency (congruent and incongruent) as a within-subject variable were conducted. Statistical analyses for the N2 and P3 ERP components were performed using the average of five electrode sites across the midline (N2: Fz, FCz, and Cz; P3: Cz, CPz, and Pz).55, 56 The anterior N2 is most robust and frequently examined at frontal and fronto-central midline electrode sites,55, 57 while the posterior P3 component is most prominent and commonly studied at centro-parietal and parietal midline electrode sites.55, 56, 58
The statistical analysis was performed using IBM SPSS version 20.0. Descriptive statistics and box plots were used to identify any outliers, and Mauchly’s test of sphericity was used to check the homogeneity of variance. Where appropriate, the Greenhouse–Geisser correction was applied to counteract the inflation of type 1 error59 and Bonferroni correction for multiple comparisons. All the tests were conducted as two-tailed, and the family-wise alpha level of probability was set at P < .05. Lastly, bivariate correlations were conducted to examine the relationship between response time scores and ERP component amplitudes. Partial eta squared (η2p) values are presented as effect sizes.
Results
A descriptive analysis was conducted, and the outliers were identified based on extreme values more than three interquartile ranges60 computed from Tukey’s hinges in SPSS. Two cases were excluded at this stage. Preliminary analysis measures are presented in Table 1 and show that there was no significant difference with respect to age, gender, or qualification for the response time and accuracy. Further, there was no significant difference in response time for the two test blocks in the pretest (blocks 1 and 2) and posttest (blocks 3 and 4; all Ps > .05), demonstrating that there was no difference in performance because of repeated measurements and that the open eye rest condition had no significant effect on performance. Therefore, for further EEG data analysis, the data from two test blocks in the pretest and posttest were combined and referred to as before and after NE flanker.
Table 1. Mean and Standard Deviation for Age and Gender Groups With Statistical Values for Significant Difference.
| Response Time ( ms) | Statistical Values | ||
| Mean | Standard Deviation | ||
| Age Gender males females |
26.0 years 593.01 ms 604.19 ms |
7.8 years 173.52 144.12 |
p = .872 p = .549 |
Further, the effect of NE on the accuracy and response time for two flanker types was examined using within-subject measure analysis of variance. Because the assumption for sphericity was not met (Mauchly’s W = 0.239, P < .05), the Greenhouse–Geisser adjustments were applied. The results show a significant effect of NE on response time F (1,50) = 14.75, P < .001, ƞ2p = 0.305. Further, the comparisons revealed a significant effect of NE on the response time for congruent trials F (1, 50) = 11.03, P < .005, ƞ2p = 0.251 and incongruent trials F (1, 50) =9.27, P < .01, ƞ2p = 0.219.
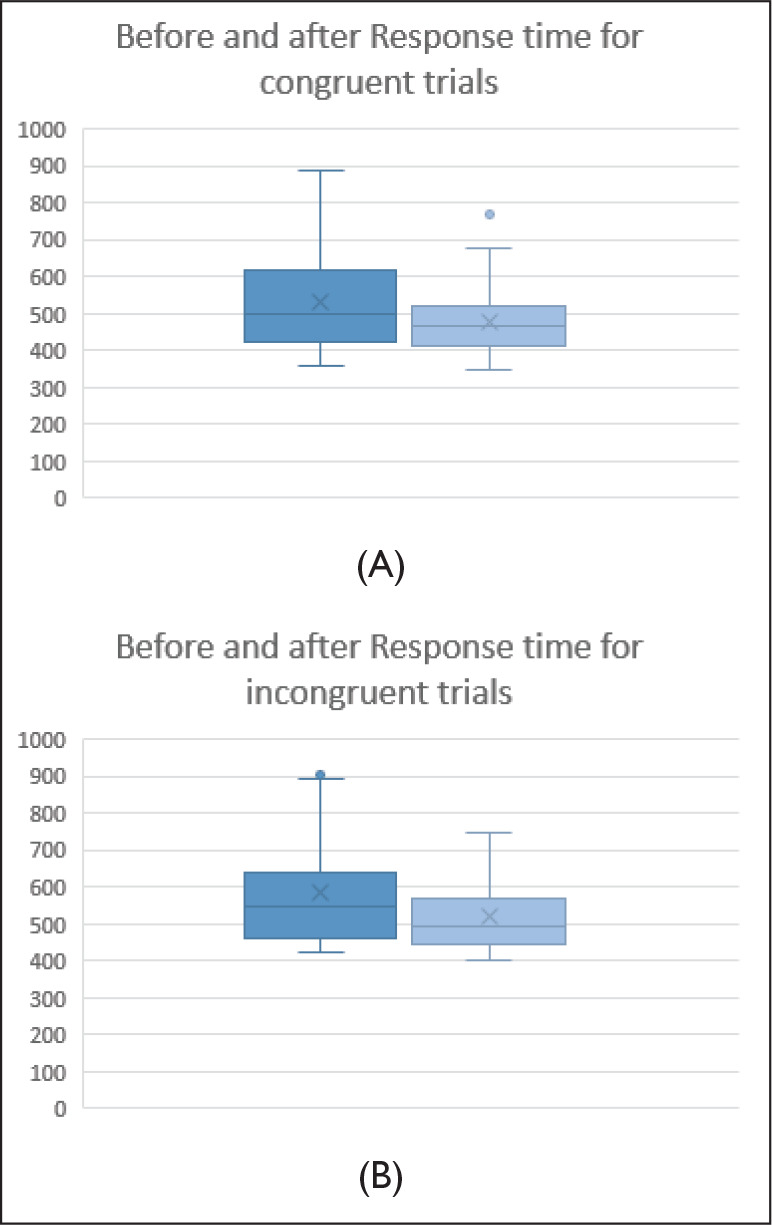
As expected by the experimental paradigm of the flanker task, there was a significant effect of congruency on response time in both before and after NE flanker task: before NE, F (1,50) = 25.225, P < .001, ƞ2p = 0.433, after NE, F (1,50) = 25.86, P < .001, ƞ2p = 0.48, with increased response time for incongruent trials in both before and after NE. Figure 5 depicts the distribution of response time (mean and standard deviation).
Figure 5. Box Plot Diagram Depicting the Distribution of Response Time for (A) Congruent and (B) Incongruent.
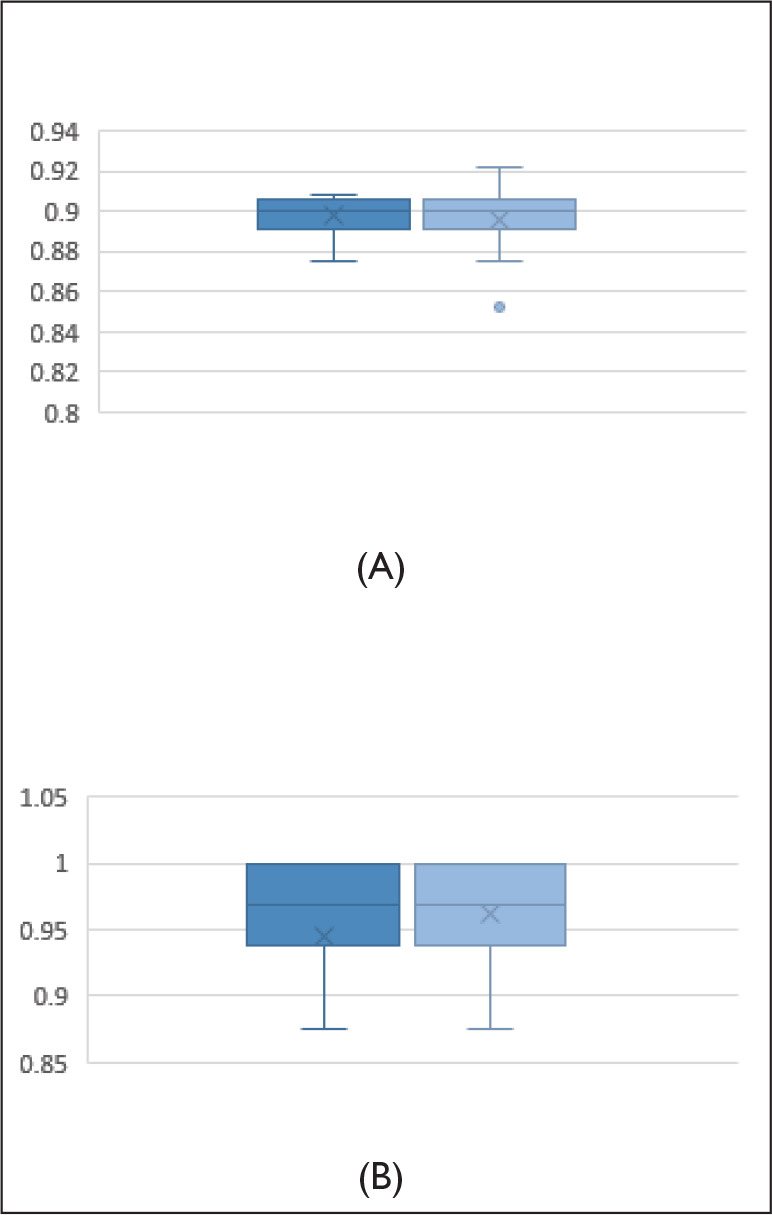
The examination of the difference in accuracy for the congruent and incongruent trials in the before and after NE shows that there was no significant effect of NE on the accuracy of either congruent trials or incongruent trials, Ps > .1. But there was a significant effect of congruency on the accuracy in both before NE, F (1, 50) = 94.54, P < .001, ƞ2p = 0.75, and after NE, F (1, 50) = 47.5, P < .001, ƞ2p = 0.60, with more accuracy for congruent trials than incongruent trials. Figure 6 depicts the distribution of accuracy (mean and standard deviation). The means and standard deviations are given in Table 1. It is worth noting that there was only a marginal increase in the accuracy for incongruent trials in after NE flanker task.
Figure 6. Box Plot Diagram Depicting the Distribution of Accuracy for (A) Congruent and (B) Incongruent.
A paired-samples t-test was conducted to compare the effect of NE on theta and alpha power. One case was further omitted at this stage for the poor quality of raw data. An analysis of theta power in the five regions was examined for its role in inhibitory control in attentional processing. There was a significant difference in the mean theta activity in the frontal [t (49) = 2.23, P < .05], fronto-central [t (49) = 2.57, P < .05], central [t (49) = 3.07, P < .01], and centro-parietal [t (49) = 2.50, P < .05] regions in before and after NE. However, there was no significant difference in the parietal region (P > .05). A summary of means and standard deviations is given in Table 2. Specifically, the results show a significant difference in fronto-central theta, indicating improved inhibitory control to distractors associated with enhanced theta.
Table 2. Mean and Standard Deviation (SD) for Congruent and Incongruent Trials Before and After Nature Experience.
| Response Time ( ms) | Accuracy | |||
| Mean | SD | Mean | SD | |
| Congruent before NE | 544.81 | 147.70 | .90 | .01 |
| Incongruent before NE | 594.15 | 147.61 | .94 | .14 |
| Congruent after NE | 483.75 | 98.39 | .90 | .01 |
| Incongruent after NE | 531.78 | 101.52 | .96 | .04 |
There was a significant difference in the mean alpha activity in all the five brain regions in before and after NE, frontal [t (49) = 3.83, P < .005], fronto-central [t(49) = 3.72, P < .005], central [t (49) = 3.20, P < .005], centro-parietal [t (49) = 3.14, P < .005], and parietal [t (49) = 3.50, P < .001] regions. The summary of mean and standard deviation is given in Table 2. The results signify that the NE has a significant effect on alpha mean activity, indicating enhancement of the directed attention or the decreased arousal associated with an increase in alpha.
To confirm that the resulting ERPs are in line with the typical patterns of electrical activity observed for the flanker task, the grand mean ERP averaged for incongruent trials was plotted. The ERP deflections were found to resemble the pattern usually found with the incongruent trials of the flanker task, representing a robust reflection of the interference because of flankers, which was present in the data. Figure 7 shows the N2 and P3 components in the midline electrodes.
Figure 7. Grand Mean Averaged Waveforms for (A) Frontal, (B) Fronto-Central, (C) Central, (D) Centro-Parietal, and (E) Parietal Midline Electrodes. (A) to (C) Highlight N2 and (C) to (E) Highlight P3.
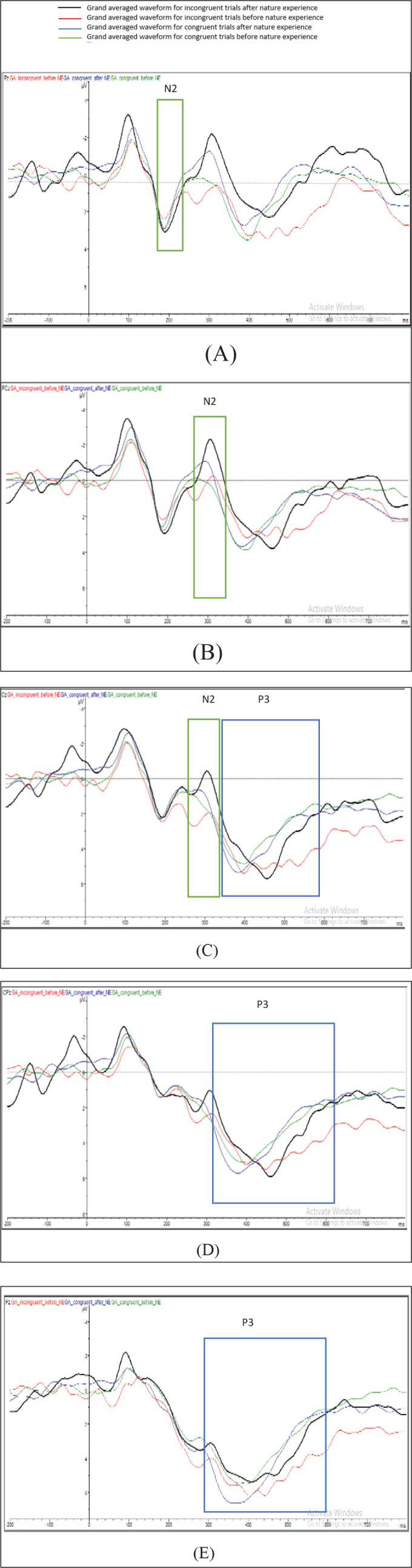
A within-subject measure analysis of variance with two within-subject factors: flanker trial type (congruent/incongruent) and condition (before/after) was conducted. The assumption for sphericity was met (Mauchly’s test P = .63). To evaluate the expected difference between inhibitory controls in before and after NE conditions, the mean amplitude analysis for the N2 component was conducted at averaged fronto-central electrodes Fz, FCz, and Cz within the time window 280 to 350 ms. Grand averaged waveforms highlighting N2 for congruent and incongruent trials in both before and after NE are presented in Figures 7A–C. The EEG signal was maximal at Fz, and this electrode was therefore used to derive peak latencies. An initial analysis of variance assessed a significant effect of NE on N2 mean activity for incongruent trials F (3,93) = 4.62, P < .05, ƞ2p = 0.113, such that more negative N2 values were observed during the incongruent task trials F (1,49) = 6.23, P < .05, ƞ2p = 0.167 in the after NE condition. However, there was no significant effect of NE for congruent trials, P > .1. But, there was a significant effect of NE on postcongruency F (1,49) =7.76, P < .01, ƞ2p = 0.200. The analysis for this condition was of interest because the inhibitory control to the flanker for incongruent trials would be expected to reflect in the N2 waveform after NE, and an improvement would indicate more efficient inhibitory control.
A P3 analysis was conducted for averaged central and parietal electrodes Cz, CPz, and Pz. Grand averaged waveforms highlighting P3 for congruent and incongruent trials in both before and after NE are presented in Figures 7C–E. There was a significant effect of NE on P3 mean activity F (3,108) = 2.69, P < .05, ƞ2p = 0.069. The planned contrast revealed that there was a significant effect of NE on incongruent trials, F (1, 49) =3.45, P < .05, ƞ2p = 0.088, which was not significant for congruent trials P = .49.
For the latency analysis, Fz with maximum mean amplitude for N2 and CPz with maximum amplitude for P3 were used to derive peak latency. There was a significant effect of NE on P3 peak latency F (3,99) =10.55, P < .001, ƞ2p = 0.242 and N2 peak latency F = 3.33, P < .05, ƞ2p = 0.085, with shorter latencies in the after NE condition.
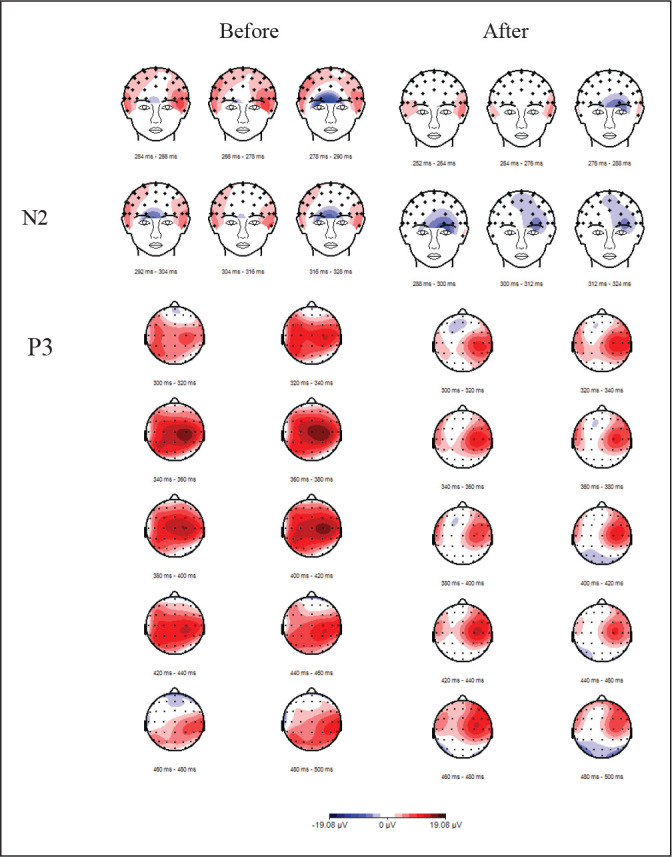
Further, the response time correlated with the average P3 mean amplitudes r = 0.345 to 0.468, all P < .05 for both congruent and incongruent flanker trials. Figure 8 depicts the topographical presentation of frontal N2 and centro-parietal P3 in before and after NE.
Figure 8. Topographical Representation of the Difference Wave Between Grand Averaged Waveform for the Incongruent Trials After and Before Nature Experience: (A) Frontal N2; (B) Parietal P3.
Conclusion and Discussion
This study provides preliminary evidence for the effects of NE on the neurocognitive mechanisms of directed attention. The findings from this study suggest that improved inhibitory control could be one of the aspects of enhanced directed attention associated with NE. The outcomes of this study are also in line with a recent research that suggests that the visual perception of natural environments calls for less attentional and cognitive processing, when compared with urban stimuli.61
This study reports an improved response time after a brief NE for both congruent and incongruent flanker tasks with a large effect size (ƞ2p > 0.20). However, there was no significant improvement in the response times after a brief open eye rest condition. These findings suggest that participants were able to focus and successfully inhibit the distractions from irrelevant information in a subsequent cognitive task to make an appropriate response. Previous studies using similar attention tasks such as Stroop task, backward digit span,13, 62, 63 and attention network test64 have also demonstrated an improvement in the response times.
There was no change in the accuracy reported for congruent trials, and only a marginal improvement in incongruent trials for after NE was observed. It may be reasoned that participants may have found the flanker task only moderately difficult, as also concurred by high accuracy (>0.90) for both congruent and incongruent trials. It is argued that when most of the participants have scores at the upper limit of the test, some of the actual variation in the data is not reflected in the scores obtained from that test, which are known as “ceiling effects”.65
The outcomes from the ERP analysis show a significant change in the neural response to the flanker trials after NE. There were significant differences in the mean amplitude of N2 for congruent and incongruent trials, and for incongruent trials after NE. A substantial effect (ƞ2p = 0.113) on the improved inhibition of distractors in a subsequent flanker task was found with greater negativities of N2 in the frontal and fronto-central regions. Studies suggest that a greater negative N2 corresponds to the avoidance of inappropriate responses, possibly reflecting the inhibition of automatically primed responses.45 The presence of conflict was shown to modulate the N2 potential such that the amplitude increased in incongruent trials relative to congruent ones. This effect has been related to control processes arising in the fronto-central brain regions. Greater N2 negativity in fronto-central topography has been associated with cognitive control,45, 54 particularly related to the suppression of irrelevant stimuli.44, 54, 66, 68 Thus, the greater negative N2 mean amplitude found for incongruent flanker trials indicates efficient inhibitory control for the cognitive conflict presented by the flanker arrows in the incongruent trials.
Further, the P3 amplitude was observed to be significantly lower after NE in the centro-parietal regions. Because P3 is reported to be an index of resource allocation,56 a lower P3 amplitude observed in this research may indicate lower cognitive resources employed for the flanker task after the NE. However, often, the decrease in P3 is also associated with “attentional lapse” and hence decrease in accuracy. In this study, the accuracy was not compromised; therefore, lower P3 may be interpreted as efficient resource allocation. A lower P3 mean amplitude may also indicate the efficient application of “task rules” for an appropriate response. Particularly, localized right parietal activity could suggest “task-relevant” resource allocation. These findings are also in line with previous studies showing that brief meditation practice leads to more effective brain resource allocation66, 68 and improved efficiency.69 In light of the behavioral performance, and N2 and P3 mean amplitude outcomes, it may be inferred that enhancement in directed attention reflected in an improved response time is perhaps because of improved and efficient inhibitory control for incongruent trials after NE.
The spectral analysis shows that there were significant differences across the frontal and fronto-central regions in theta (P < .05) and across the frontal and parietal regions for alpha (P < .005). The alpha and theta—the dominant frequencies during NE—perhaps continued during the cognitively demanding flanker task after the NE. Studies suggest that enhanced theta indicates the improvement in the capacity to control one’s locus of attention.47 Increased theta after NE may also mean an improvement in focus and attention.
Further, in light of existing studies that have investigated the significance of alpha,70–72 an increase in alpha as reported in our study can be interpreted as a state of relaxation and increased directed attention capacity. Together with higher theta, higher alpha during NE indicates a mental state that is relaxed yet alert. Interestingly, the results from our study are in line with the findings from meditative and mindfulness studies that also suggest an emergence of theta and alpha waves in the attention-related frontal and parietal regions.71, 73, 74 Additionally, the presence of lower frequency theta and alpha is generally known for the deactivation of cortical processing. In the context of this study, it may be construed as lower distractions and subsequently reduced task-irrelevant processing. Consequently, the presence of lower distractions may be conjectured as the lesser mental effort required for inhibitory control.
The above-derived conclusions also find support from the neural efficiency hypothesis, which proposes that the effective performance on a cognitive task may not depend on how hard the brain works, but rather on how “economically” the resources are put to use to get an appropriate response.75 Thus, the observations from the experiment as described in this study not only suggest that NE enhances attention, but also provide evidence for more efficient fronto-parietal correlates of neurocognitive processes involved in directed attention. This result could be of direct relevance to present-day needs for improving one’s ability to focus and direct attention at the task in hand. NE offers a cost-effective, alternate intervention for attention-related dysfunctions.
Limitations and Implications for Future Research
This study was designed in controlled lab conditions, and NE was simulated through audiovisual media. It may be suggested here that future studies could include NE through a real-time interaction with nature such as forests, parks, gardens, or urban green spaces in the experiment design to explore the outcomes as listed in this study. Further, the outcomes of this study are based on brief NE; the inclusion of the effect of time and duration of NE could provide evidence for effectivity of NE as an alternate intervention.
The experiment described in our study has used a repeated measure design. This method has been widely used in EEG-based experiments as they provide two main advantages. First, they are suggested to control for the factors that cause variability between subjects, and the subjects act as their own control. Second, fewer subjects need to be recruited, trained, and compensated to complete the entire experiment for the desired effect size. A rest condition was used as control, and significance was analyzed both before and after the NE. However, a comparative condition such as an urban environment could be used as an alternate setting for future studies.
Nevertheless, given the limitations of the study as described above, one merit of the current design is that it gives a good reflection of the likely effects of NE on inhibition control and attention enhancement.
Acknowledgments
This experiment was conducted in the UX Lab of Indian Institute of Technology Delhi. The authors acknowledge the help of the fellow research scholars, Abhijeet Kujur and Sunny Bairsal, in data collection. The authors also thank the student groups of Indian Institute of Technology Delhi for agreeing to participate in the experiment.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authorship Statement
PS and JK were responsible for the conceptualization of the research and deciding on the methodology to be followed. PS carried out the investigations and analysis of the data. The manuscript was prepared by PS and reviewed by JK. The overall mentorship and supervision was provided by JK.
Author Contribution
PS: Conceptualization, EEG data collection, formal analysis, investigation, methodology, project administration, software, validation, writing—original draft, writing—review and editing.
JK: Conceptualization, project administration, resources, supervision, writing—review and editing.
Ethical Statement
All ethical approvals were sought as per the guidelines suggested by the Ethics Committee of the Indian Institute of Technology Delhi.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Nigg J.On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull 2000; 126(2): 220–246. [DOI] [PubMed] [Google Scholar]
- 2.Diamond A. Executive functions. Annu Rev Psychololgy 2013; 64: 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiego J, Testa R, Bellgrove MAet al. A hierarchical model of inhibitory control. Front Psychol 2018; 9: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aron A, Robbins T, Poldrack R.Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8(4), 170–177. [DOI] [PubMed] [Google Scholar]
- 5.Melara RD, Singh S, Hien DAet al. Neural and behavioral correlates of attentional inhibition training and perceptual discrimination training in a visual flanker task. Front Hum Neurosci 2018; 12: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahneman D.Attention and Effort. Prentice-Hall Inc, 1973, 246 p. [Google Scholar]
- 7.Kaplan S Berman MG.. Directed attention as a common resource for executive functioning and self-regulation. Perspect Psychol Sci 2010; 5(1): 43–57. [DOI] [PubMed] [Google Scholar]
- 8.Itti L, Rees G, Tsotsos JK, eds. Neurobiology of Attention. Elsevier Academic Press, 2005. [Google Scholar]
- 9.Hartig T, Böök A, Garvill Jet al. Environmental influences on psychological restoration. Scand J Psychol 1996; 37(4): 378–393. [DOI] [PubMed] [Google Scholar]
- 10.Mital A, Bishu RR, Manjunath SG.Review and evaluation of techniques for determining fatigue allowances. Int J Ind Ergon 1991; 8(2): 165–178. [Google Scholar]
- 11.Wallis A, Jackson M, Ball Met al. Sleep, cognitive and mood symptoms in myalgic encephalomyelitis/ chronic fatigue syndrome: Examining the role of the gut-brain axis. In: Cooper C, Quick J, eds. The Handbook of Stress and Health: A Guide to Research and Practice. John Wiley & Sons, 2017, p. 501–522. [Google Scholar]
- 12.Querstret D, Cropley M, Fife-Schaw C.Internet-based instructor-led mindfulness for work-related rumination, fatigue, and sleep: Assessing facets of mindfulness as mechanisms of change. A randomized waitlist control trial. J Occup Heal Psychol 2017; 22(2): 153–169. [DOI] [PubMed] [Google Scholar]
- 13.Beute F de Kort YAW.. Natural resistance: Exposure to nature and self-regulation, mood, and physiology after ego-depletion. J Environ Psychol 2014; 40: 167–178. [Google Scholar]
- 14.Kaplan S.The restorative benefits of nature: Toward an integrative framework. J Environ Psychol 1995; 15(3): 169–182. https://doi.org/10.1016/0272-4944(95)90001-2 [Google Scholar]
- 15.Bratman GN, Daily GC, Levy BJet al. The benefits of nature experience: Improved affect and cognition. Landsc Urban Plan [Internet] 2015; 138: 41–50. http://dx.doi.org/10.1016/j.landurbplan.2015.02.005 [Google Scholar]
- 16.Staats H, Kieviet A, Hartig T.Where to recover from attentional fatigue: An expectancy-value analysis of environmental preference. J Environ Psychol 2003; 23(2): 147–157. [Google Scholar]
- 17.Gidlow CJ, Jones MV, Hurst Get al. Where to put your best foot forward: Psycho-physiological responses to walking in natural and urban environments. J Environ Psychol 2016; 45: 22–29. [Google Scholar]
- 18.Stigsdotter UK, Corazon SS, Sidenius Uet al. Health and place it is not all bad for the grey city: A crossover study on physiological and psychological restoration in a forest and an urban environment ★. Health Place [Internet] 2017; 46: 145–154. http://dx.doi.org/10.1016/j.healthplace.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Ohly H, White MP, Wheeler BWet al. Attention restoration theory: A systematic review of the attention restoration potential of exposure to natural environments. Part B: Critical reviews. J Toxicol Environ Health 2016; 19. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan S.The restorative benefits of nature: Toward an integrative framework. J Environ Psychol 1995; 15(3): 169–182. [Google Scholar]
- 21.Hartig T, Mitchell R, de Vries Set al. Nature and health. Annu Rev Public Health [Internet] 2014; 35(1): 207–228. http://www.annualreviews.org/doi/10.1146/annurev-publhealth-032013-182443. [DOI] [PubMed] [Google Scholar]
- 22.White MP, Pahl S, Ashbullby Ket al. Feelings of restoration from recent nature visits. J Environ Psychol 2013; 35: 40–51. [Google Scholar]
- 23.Patañjali, Bharati SV.. Yoga sutras of Patanjali. Motilal Banarsidass Publ, 2001. [Google Scholar]
- 24.Roe J, Aspinall P, Mavros Pet al. Engaging the brain : The impact of natural versus urban scenes using novel EEG methods in an experimental setting. Environ Sci 2013; 1(2): 93–104. [Google Scholar]
- 25.I-C 25. Tang, Y-P Tsai, Y-J Linet al. Using functional magnetic resonance imaging (fMRI) to analyze brain region activity when viewing landscapes. Landsc Urban Plan 2017; 162: 137–144. [Google Scholar]
- 26.Chen Z, He Y, Yu Y.Enhanced functional connectivity properties of human brains during in-situ nature experience. Peer J 2016; 4: e2210. https://peerj.com/articles/2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frawley D.. Mantra yoga and the primal sound: Secrets of seed (bija) mantras. Lotus Press, 2010. [Google Scholar]
- 28.Tripathi KM.Concept and scope of pratyāhāra in management of mental health. Found Indian Psychol 2011; 2: 84–97. [Google Scholar]
- 29.Zinchenko A, Kanske P, Obermeier Cet al. Emotion and goal-directed behavior: ERP evidence on cognitive and emotional conflict. Soc Cogn Affective Neuroscience 2015; 10(11): 1577–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams K Harvey D.. Transcendent experience in forest environments. J Environ Psychol 2001; 21(3): 249–260. [Google Scholar]
- 31.Kaplan R Kaplan S.. The Experience of Nature. A Psychological Perspective, 1989. [Google Scholar]
- 32.Gray L.. Ecopsychology: Restoring the Earth, Healing the Mind. Roszak T, Gomes ME, Kanner AD, eds. Sierra Club Books, 1995, 171–172 p. [Google Scholar]
- 33.Jonides J Irwin DE.. Capturing attention. Cognition 1981; 10(1–3): 145–150. [DOI] [PubMed] [Google Scholar]
- 34.Prinzmetal W, Zvinyatskovskiy A, Gutierrez Pet al. Voluntary and involuntary attention have different consequences : The effect of perceptual difficulty. Q J Exp Psychol 2009; 62(2): 352–369. [DOI] [PubMed] [Google Scholar]
- 35.Posner M Sheese B Odludaş Y Tang.. Analyzing and shaping neural networks of attention. Neural Networks 2006; 17059879: 1422–1429. [DOI] [PubMed] [Google Scholar]
- 36.Berman MG, Jonides J, Kaplan S.The Cognitive benefits of interacting with nature. Psychol Sci 2018; 19(12): 1207–1212. [DOI] [PubMed] [Google Scholar]
- 37.Keniger LE, Gaston KJ, Irvine KNet al. What are the benefits of interacting with nature? Int J Environ Res Public Health 2013; 10(3): 913–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van den Berg AE, Joye Y, Koole SL.. Why viewing nature is more fascinating and restorative than viewing buildings: A closer look at perceived complexity. Urban For Urban Green [Internet] 2016; 20: 397–401. http://dx.doi.org/10.1016/j.ufug.2016.10.011. [Google Scholar]
- 39.Northoff G, Duncan NW, Hayes DJ.Progress in neurobiology the brain and its resting state activity: Experimental and methodological implications. Prog Neurobiol [Internet] 2010; 92(4): 593–600. http://dx.doi.org/10.1016/j.pneurobio.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Eriksen BA, Eriksen CW.Effects of noise letters upon the identification of a target letter in a nonsearch task.pdf. Percept Psychophys 1974; 16: 143–149. [Google Scholar]
- 41.Wong WP, Camfield DA, Woods W, Sarris J, Pipingas A.Spectral power and functional connectivity changes during mindfulness meditation with eyes open: A magnetoencephalography (MEG) study in long-term meditators. Int J Psychophysiol 2015; 98(1): 95–111. [DOI] [PubMed] [Google Scholar]
- 42.Aftanas LI, Golocheikine SA.Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neurosci Lett 2001; 310(1), 57–60. [DOI] [PubMed] [Google Scholar]
- 43.Luck SJ.Ten simple rules for designing ERP experiments. In: Todd C.. Handy ed., Event-related potentials: A methods handbook, MIT Press, 2005. [Google Scholar]
- 44.Xie L, Ren M, Cao Bet al. Distinct brain responses to different inhibitions : Evidence from a modified Flanker Task. Sci Rep 2017; 7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kopp B., Rist F., Mattler UWE.N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology 1996; 3(33): 282–294. [DOI] [PubMed] [Google Scholar]
- 46.Norris CJ, Creem D, Hendler Ret al. Brief mindfulness meditation improves attention in novices: Evidence from ERPs and moderation by neuroticism. Front Hum Neurosci 2018; 12: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cahn BR, Polich J.Meditation states and traits : EEG, ERP, and neuroimaging studies. Psychol Bull 2006; 132(2): 180–211. [DOI] [PubMed] [Google Scholar]
- 48.Lawshe CH.A quantitative approach to content validity. Personnel Psychology 1975; 28(4): 563–575. [Google Scholar]
- 49.Goldberg T.E., Harvey P.D., Wesnes K. A., Snyder P. J., Schneider L. S.Practice effects due to serial cognitive assessment: Implications for preclinical Alzheimer’s disease randomized controlled trials. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 2015; 1(1): 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barker Bausell R.The Design and Conduct of Meaningful Experiments Involving Human Participants: 25 Scientific Principles. Oxford University Press, 2015. [Google Scholar]
- 51.Tucker D, Liott M, Potts Get al. Spatiotemporal analysis of brain electrical fields. Hum Brain Mapp 1994; 1: 134–152. [Google Scholar]
- 52.Bertrand O, Perrin F, Pernier J.A theoretical justification of the average reference in topographic evoked potential studies. Electroencephalogr Clin Neurophysiol 1985; 62: 462–464. [DOI] [PubMed] [Google Scholar]
- 53.Sänger J, Bechtold L, Schoofs Det al. The influence of acute stress on attention mechanisms and its electrophysiological correlates Front Behav Neurosci 2014; 8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luck SJ.. An Introduction to the Event-Related Potential Technique. The MIT Press, 2014, 356 p. [Google Scholar]
- 55.Folstein J Van Petten C.. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 2008; 45: 152–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polich J.Updating P300 : An integrative theory of P3a and P3b. Clin Neurophysiol 2007; 118(10): 2128–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gehring W, Gratton G, Coles Met al. Probability effects on stimulus evaluation and response processes. J Exp Psychol Hum Percept Perform 1992; 18: 198–216. [DOI] [PubMed] [Google Scholar]
- 58.Donchin E.Surprise!…Surprise? Psychophysiology 1981; 13: 493–511. [DOI] [PubMed] [Google Scholar]
- 59.Jennings JR, Wood CC.The e-adjustment procedure for repeated-measures analyses of variance. Psychophysiology 1976; 13(3): 277–278. [DOI] [PubMed] [Google Scholar]
- 60.Hoaglin DC, Iglewicz B.Fine-tuning some resistant rules for outlier labeling. J Am Stat Assoc 1987; 82(400): 1147–1149. [Google Scholar]
- 61.Grassini S, Revonsuo A, Castellotti Set al. Processing of natural scenery is associated with lower attentional and cognitive load compared with urban ones. J Environ Psychol 2019; 62: 1–11. [Google Scholar]
- 62.Bailey A.The cognitive benefits of outdoor exercise versus indoor exercise. World Liesure J 2018; 60(4): 293–305. [Google Scholar]
- 63.Bratman GN, Hamilton JP, Daily GC.The impacts of nature experience on human cognitive function and mental health. Ann N Y Acad Sci 2012; 1249, 118–136. [DOI] [PubMed] [Google Scholar]
- 64.Berman MG, Jonides J, Kaplan S.The cognitive benefits of interacting with nature. Psychol Sci 2008; 19(12): 1207–1212. [DOI] [PubMed] [Google Scholar]
- 65.Vogt WP.. Dictionary of statistics & methodology: A nontechnical guide for the social cciences. 3rd ed. SAGE, 2005, p. 40. [Google Scholar]
- 66.Moore A, Gruber T, Derose Jet al. Regular , brief mindfulness meditation practice improves electrophysiological markers of attentional control. Hum Neurosci 2012; 6: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanger KL, Dorjee D.Mindfulness training with adolescents enhances metacognition and the inhibition of irrelevant stimuli : Evidence from event-related brain potentials. Trends Neurosci Educ 2016; 5: 1–11. [Google Scholar]
- 68.Slagter H, Lutz A, Greischar Let al. Mental training affects distribution of limited brain resources. PLoS Biol 2007; 6(5): e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kozasa E, Sato J, Lacerda Set al. Meditation training increases brain efficiency in an attention task. Neuroimage 2012; 59(1): 9. [DOI] [PubMed] [Google Scholar]
- 70.Shaw J.Intention as a component of the alpha-rhythm response to mental activity. Int J Psychophysiol 1996; 24: 7–23. [DOI] [PubMed] [Google Scholar]
- 71.Aftanas LI, Golocheikine SA.Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention : High-resolution EEG investigation of meditation. Neurosci Lett 2001; 310: 57–60. [DOI] [PubMed] [Google Scholar]
- 72.Lomas T, Ivtzan I, HY Fu C.A systematic review of neurophysiology of mindfulness on EEG. Neurosci Biobehav Rev 2015; 57: 401–410. [DOI] [PubMed] [Google Scholar]
- 73.Lagopoulos J, Xu J, Rasmussen Iet al. Increased theta and alpha EEG activity meditation, during nondirective. J Altern Complement Med 2009; 15(11): 1187–1192. [DOI] [PubMed] [Google Scholar]
- 74.Baijal S Srinivasan N.. Theta activity and meditative states: Spectral changes during concentrative meditation. Cogn Process 2010; 11(1): 31–38. [DOI] [PubMed] [Google Scholar]
- 75.Shcherbakova OV, Gorbunov IA, Golovanova IVet al. The neural efficiency hypothesis : Further evidence from the EEG-study of conceptual thinking. Int J Psychophysiol 2014; 94(2): 218. [Google Scholar]
- 76.Chiang YC, Li D, Jane HA.Wild or tended nature? The effects of landscape location and vegetation density on physiological and psychological responses. Landsc Urban Plan 2017; 167: 72–83. [Google Scholar]




