Abstract
Background:
Chronotype is the circadian time preference for sleep–wake timings. However, its impact on cognitive performance is least explored.
Objective:
The present study investigated the effect of chronotype (morning “M” vs. evening “E”) on cognitive measures as a function of time of the day. In addition, the correlation between electroencephalogram (EEG) waves and subjective/objective cognitive measures were investigated.
Method:
Cognitive status of 28 adult male subjects (15 “M” and 13 “E”) was assessed objectively through event-related potential (ERP) by administering visual odd ball paradigm test and subjectively through Montreal Cognitive Assessment questionnaire. In addition, 20 to 30 min of resting EEG was recorded. Recordings were done from 8 to 10 am and from 4 to 6 pm on a single day. Power spectral analysis of EEG for alpha and beta waves at PZ and FZ cortical sites was done after subjecting selected epochs to fast Fourier transformation. Also, latency and amplitude of P300 potential from event-related potential record were measured. Appropriate statistical tests were applied for analysis.
Results:
Higher alpha and beta power was observed in “E” at PZ in the evening. “M” showed increased P300 latency and amplitude during evening session for frequent and rare stimuli and vice versa in “E.”’ Significant negative correlation was seen between latency of rare stimuli and alpha and beta power at FZ site during evening in “E” chronotype only.
Conclusion:
Result indicates better attention and alertness during evening hours in evening chronotypes and vice versa in morning chronotypes. The findings could be implemented to schedule the mental performance/cognitive load according to individual chronotype.
Keywords: Chronotype, Cognition, Electroencephalography, Event-related potential, P300
Introduction
The ubiquitous and endogenous circadian rhythm (about 24 h rhythm) is visible in almost all biological functions. Based on circadian time preference for sleep-wake or rest-activity behavior, physical or mental performance, the human population has been classified into three categories known as chronotypes or circadian typology.1 These are (a) “lark or morning type” showing morning time preferences, (b) “owls or night type,” preferred late night hours, and (c) intermediate type who neither have early morning nor extreme evening behavior, but prefer in between. Inventory, e.g. Horne and Östberg Morningness-Eveningness Questionnaire (MEQ),1 is one of the most accepted psychometric screening tool for ascertaining chronotype of an individual. It is generally reported that pure morning or evening people are rare, i.e., only about 10% to 15%, with majority falling in the intermediate group.2 Age and gender also influence the morning–evening preferences.3 The clock genes expression also differ among different chronotypes.4
Modern era has influenced our lifestyle such that eveningness amongst the population is becoming a choice. Several studies have indicated the impact of eveningness on the cognitive ability and daytime sleepiness when compared with morning people. Studies indicate adverse impact of eveningness on cognitive ability and mental performance.2 A strong association was reported between conscientiousness and morningness, whereas extraversion showed a strong correlation with eveningness.2 Also, a possible relationship has emerged between eveningness and certain type of mental health disorders such as schizophrenia, attention deficit hyperactivity disorder, sleep disorders, mood disorders, depression, etc.5 The morning chronotype university students have been found to perform better in academics than their evening counterparts, irrespective of the time of class or examination time and correlates with poor sleep quality in evening type.6
In this context, the use of electroencephalography (EEG) for objective measurement of neuro-cognitive or neuro-physiological cortical activity in terms of perception, memory, attention, is well known.7 Event-related potentials (ERP; time locked EEG changes to sensory, motor, or cognitive events in response to specific events or stimuli), another robust tool of monitoring cognitive status by measuring P300 wave,8 described according to latency (ms, the time from the onset of the stimulus to the occurrence of the positive peak) and amplitude (µV, the distance from the baseline to the highest point of the positive peak) is widely accepted.9, 10 Besides, circadian variation in EEG activity and P300 are also clearly indicated.10, 11
Although the effect of chronotype and circadian variation in many cognitive tasks related to vigilance, executive functions, attention has been reported,12, 13 the effect of chronotypes, specifically morning and evening types, during their odd hours of performances (morning and evening hours) on a single day, has not been explored much. Therefore, the present study was conducted to address the change in neuro-cognitive status objectively through EEG and cognitive component of P300 by Visual-ERP and subjectively through Montreal Cognitive Assessment (MoCA) Questionnaire14 in morning and evening chronotype young healthy male cohort. Additionally, we investigated the correlation if any, between the EEG profile and cognitive parameters in these individuals.
Methods
This was a case-controlled prospective observational study. In this noninvasive study, total 28 normal adult male subjects (age group: 18 to 25 years) comprising of 15 morning chronotype and 13 evening chronotype, voluntarily participated. The study was initiated after obtaining approval from the Institute Ethics Committee. An informed written consent was obtained from each subject after explaining the details of the study and recording methods. A detailed history of all the subjects was taken to meet the inclusion and exclusion criteria. The subjects with body mass index (BMI) between 19.0 and 24.9 were included and the subjects with hypertension or diabetes mellitus or respiratory diseases or any history of other disease including any neurological or psychological disorders, history of habits of smoking, alcohol, caffeine consumption, drug intake, etc. were excluded.
The chronotype or circadian typology was assessed using English or Hindi version of MEQ1 as per the preference of the volunteers, which consisted of 19 items pertaining to habitual rising and bedtimes, preferred times of physical and mental performance, and subjective alertness after rising and before going to bed. The individuals having overall MEQ scores from 69 to 86 and 16 to 40 were categorized as the definite morning (M) and evening chronotype (E), respectively.1
Following this, the subjective cognitive status of the participant was assessed using the MoCA Questionnaire14 comprising of a 30-point test administered over approximately 10 min for detecting different cognitive domains, i.e.: attention and concentration, executive functions, memory, language, visuo-constructional skills, conceptual thinking, calculations, and orientation.
Each subject then underwent EEG and ERP recordings two times on the same day: one at 8 to 10 am (morning, session 1) and another at 4 to 6 pm (evening, session 2).15 This was done to help draw parallels and assess diurnal variation between the morning and evening chronotypes with respect to their neuro-cognitive parameters. All the recordings were carried out in a well-ventilated room at thermoneutral ambient temperature (26 ± 2°C).
EEG recording was done with the help of a digital EEG machine B.E.S.S. WF-64 (64 channel-Brain Electro Scan System from Axxonet System Technologies Private Limited, India) following International 10 to 20 system of electrode placement.16 This was done using saline electrode cap with impedance <20kΩ and sampling rate of 1,000 Hz, where CZ was used as reference and AFZ as ground. After 15 min of supine rest, EEG was recorded for 20 to 30 min with the eyes closed under awake and relaxed conditions till alpha waves were observed for greater than 50% time in the occipital region.16 Thereafter, in the analysis mode of the B.E.S.S. software, EEG data were first re-referenced offline and then visually inspected for gross movement and eye-blink artifacts using a rejection criterion of ±75 µV. The high-pass, low-pass, and notch filter frequencies were 1 Hz, 35 Hz, and 50 Hz, respectively. Then, 15 min of artifact-free epochs (i.e., at least 450 epochs of two sec/subject) were subjected to fast Fourier transformation (FFT) to decompose EEG waveform into sine wave components of respective frequencies. These were used to compute absolute power (in uv2) for the alpha (8–13 Hz) and beta (14–30 Hz) waves at FZ and PZ sites with the help of inbuilt power spectral analysis software.7
In addition, cognitive status was assessed objectively through event related potential (ERP) with the help of B.E.S.S. EEG/ERP system (Axxonet System Technologies Private Limited, India). This was done by administering oddball paradigm task for the visual ERP (VERP) recording. In this task, two different visual stimuli, i.e., picture of one circle and one square, were presented in a series on the computer screen such that one of them occurs relatively infrequently. The subject was instructed to respond by pressing a designated key after the presentation of the infrequent or the rare (R) target stimulus and not to the frequently presented or standard stimulus (F). In each recording setup, a total of 240 stimuli were presented, comprising of 80% frequent (F) and 20% rare (R) stimuli,9 while the subject sat in a darkened room on a chair approximately 1 m from a computer screen. Then, after offline re-referencing, ERP waveforms were reduced and analyzed using “B.E.S.S.” software (version 6.9_1). The EEG was converted into epochs, time-locked to the frequent and rare image. A prestimulus interval of 200 ms along with a poststimulus presentation of 1,000 ms was used to extract the epoch. Then, the amplitude (µV) and latency (ms) of averaged P300 of ERP waveform at Cz, as indicators of cognition and perception, was calculated and expressed as mean ± SE at the designated electrode sites.
Data of M and E chronotypes were segregated and entered in MS Excel. All the resting EEG and ERP data were expressed in standard units and statistical analysis was done using StatistiXL Version 1.11 (2018). Within groups comparison of sessions 1 vs. 2 for each chronotype was done using Wilcoxon paired test, whereas between groups (M vs. E) comparison was carried out using Mann–Whitney “U” test for statistical significance (minimum P < 0.05). Pearson’s correlation test was applied to assess correlation between alpha and beta power of EEG at FZ and PZ sites and amplitude and latency of VERP as well as with MoCA scores.
Results
The mean BMI of M type was 23.25 ± 1.65 and that of E type was 22.9 ± 2.9. There was no significant difference in BMI between the groups.
No statistical difference in MoCA scores was observed between M (28.5 ± 1.4) and E type (28.9 ± 0.9) individuals.
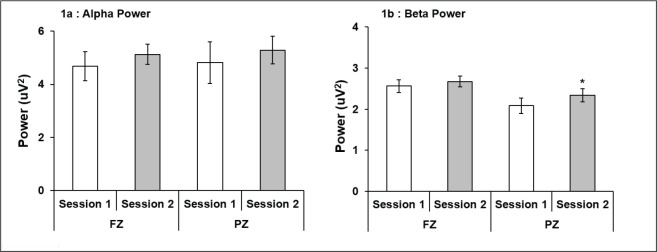
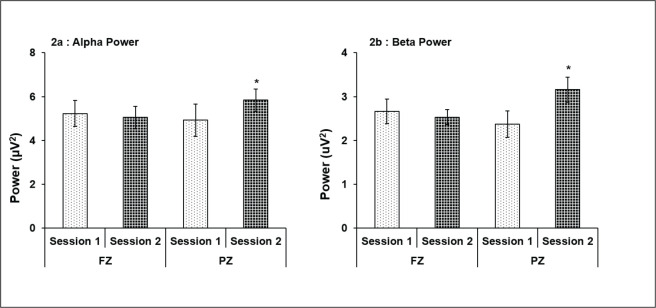
The M group subjects showed higher alpha and beta power at FZ and PZ during session 2 as compared to session 1 with a statistically significant higher beta at PZ only (P = .05; Figures 1a and b). A statistically significant higher alpha (P < .05) and beta power (P < .05) at PZ was also recorded in E type subjects in session 2 (Figures 2a and b). Comparison between M vs. E chronotypes showed significantly high power for both alpha and beta in E-type subjects at PZ site during session 2 as compared to M-type (Table 1).
Figure 1. Alpha and beta power, respectively, of morning chronotype subjects at FZ and PZ in sessions 1 and 2. Higher alpha power at PZ in session 2 was evident, though statistically not significant (a), while beta power of morning chronotype subjects at FZ and PZ in session 1 and 2 showed higher power in both sessions, which was statistically significant at PZ (b). *P < .05 (session 1 vs. 2); all values are expressed as mean ± SE.
Table 1. Alpha and Beta Power (µV2) at FZ and PZ Cortical Sites in Adult Male Subjects of Morning and Evening Chronotype Recorded in Session 1 and Session 2.
| Mean ± SE at FZ site | |||||
| Session | EEG Wave | Chronotype | Mann–Whitney U Test | ||
| Morning | Evening | U | P | ||
| Session 1 | Alpha | 4.68 ± 0.55 | 5.25 ± 0.82 | 85.0 | .65 |
| Beta | 2.63 ± 0.51 | 3.30 ± 0.81 | 89.0 | .98 | |
| Session 2 | Alpha | 5.07 ± 0.39 | 5.04 ± 0.93 | 77.0 | .93 |
| Beta | 2.67 ± 0.13 | 2.56 ± 0.35 | 108.0 | .31 | |
| Mean ± SE at PZ site | |||||
| Morning | Evening | U | P | ||
| Session 1 | Alpha | 4.81 ± 0.79 | 4.93 ± 1.02 | 77.0 | .93 |
| Beta | 2.23 ± 0.44 | 2.97 ± 0.81 | 98.0 | .64 | |
| Session 2 | Alpha | 5.17 ± 0.53 | 5.75 ± 1.00 | 121.0 | .05* |
| Beta | 2.37 ± 0.16 | 3.13 ± 0.72 | 133.5 | .02** | |
Note: Morning vs. evening chronotype *P < .05;**P < .01; all the values are expressed as mean ± SE.
Figure 2. Alpha and beta power of evening chronotype subjects at FZ and PZ in sessions 1 and 2. Statistically significant higher alpha power was recorded in session 2 at PZ (a), whereas session 2 at PZ showed significantly more beta power (b). *P < .05 (session 1 vs. 2); all values are expressed as mean ± SE.
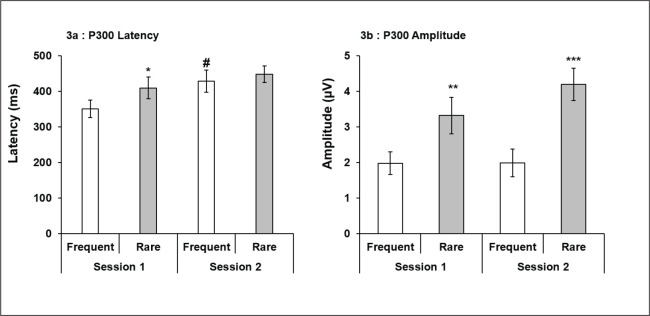
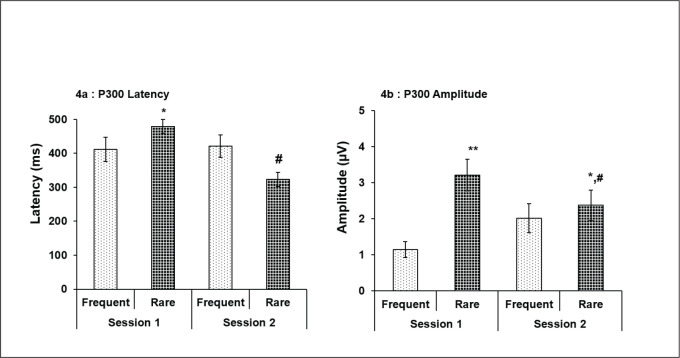
With regard to ERP, a statistically significant higher P300 latency of VERP for R stimuli compared to F stimuli (P < .05) was observed in session 1 only in M chronotype (Figure 3a), whereas P300 latency was significantly more for F than R stimuli in session 2 compared to session 1 (P < .05; Figure 3a). Also, significantly higher amplitude for R stimuli compared to F stimuli was recorded in both sessions 1 (P < .01) and 2 (P < .001) in M chronotype subjects (Figure 3b). However, between sessions comparison was not significant for both the stimuli in morning type. Whereas, in evening chronotype, though significantly higher P300 latency was recorded for R stimuli compared to F stimuli in session 1 (P < .05; Figure 4a), reduced latency was noted for R stimuli as compared to F stimuli in session 2, though not significant (Figure 4a). Besides, evening chronotypes also displayed significantly increased P300 amplitude for R stimuli than F stimuli at both session 1 (P < .01) and session 2 (P <.05; Figure 4b). But between sessions comparison showed a significant decrease in VERP P300 amplitude for R stimuli, only in session 2 (P < .05; Figure 4b).
Figure 3. P300 latency and amplitude of morning chronotype subjects for frequent and rare stimuli in session 1 and 2. Session 1 showed significant higher latency for rare stimuli. Also, latency increased significantly for frequent stimuli and markedly for rare stimuli in session 2 (a). Significantly higher amplitude for rare stimuli was observed in both sessions 1 and 2 which was also markedly increased between sessions 1 and 2 (b). *P < .05, **P < .01, and ***P < .001 (frequent vs. rare); #P < .05 (session 1 vs. 2); all values are expressed as mean ± SE.
Figure 4. P300 latency and amplitude of evening type subjects for frequent and rare stimuli in sessions 1 and 2. Significantly increased latency during session 1 and reduced latency in session 2 for rare stimuli was recorded. Also, significant reduction in latency for rare stimuli was seen in session 2 (a). Amplitude for rare stimuli showed significant increase during both sessions 1 and 2. Also, amplitude in session 2 was markedly low for rare stimuli (b). *P < .05 and **P < .01 (frequent vs. rare); #P < .05 (session 1 vs. 2); all values are expressed as mean ± SE.
The difference in P300 VERP latency and amplitude as a function of chronotype and sessions (1 vs. 2, i.e., morning vs. evening hours) was observed, while a comparison between M and E chronotypes revealed higher P300 latency for both F and R stimuli in evening type during session 1; it was statistically significant for F stimuli only (Table 2). On the contrary, the same evening chronotype group exhibited shorter P300 latency for both F and R stimuli than morning chronotype in session 2/evening hours (Table 2); however, this reduction was statistically significant for R event only in evening type (Table 2). Also, the P300 amplitude for both the F and R stimuli was found to be shorter in evening chronotype compared to morning chronotype in session 1 (morning hours) and higher for F stimuli in session 2 (evening hours), though it was statistically not significant (Table 2). The P300 amplitude for R stimuli was significantly reduced in evening type compared to morning type in session 2 (Table 2).
Table 2. P300 Latency (ms) and Amplitude (µV) in Adult Male Subjects of Morning and Evening Chronotype Recorded in Session 1 and Session 2.
| VERP Latency (ms) | |||||
| Session | Stimuli | Chronotype | Mann–Whitney U Test | ||
| Morning | Evening | U | P | ||
| Session 1 | Frequent | 351.06 ± 24.54 | 410.81 ± 35.83 | 126.5 | .05* |
| Rare | 410.18 ± 30.28 | 478.72 ± 20.27 | 120.12 | .12 | |
| Session 2 | Frequent | 429.37 ± 33.17 | 420.45 ± 33.17 | 97.0 | .68 |
| Rare | 448.43 ± 23.72 | 323.18 ± 20.29 | 148.0 | .002** | |
| VERP Amplitude (µV) | |||||
| Morning | Evening | U | P | ||
| Session 1 | Frequent | 1.98 ± 0.31 | 1.14 ± 0.22 | 127.5 | .05* |
| Rare | 3.32 ± 0.50 | 3.21 ± 0.44 | 92.5 | .82 | |
| Session 2 | Frequent | 1.99 ± 0.38 | 2.01 ± 0.39 | 90.0 | .94 |
| Rare | 4.19 ± 0.65 | 2.37 ± 0.42 | 128.5 | .04* | |
Note: Morning vs. evening chronotype *P < .05; all the values are expressed as mean ± SE.
A significant negative correlation was observed between latency of R stimuli with alpha (r = –0.85; P = .03) and beta (r = –0.89; P < .01) power at FZ site in session 2 in E type subjects only. No other significant correlation was found at any other point of analysis.
Discussion
The present study has examined the cognitive performance in terms of EEG recording and visual ERP test as well as subjective assessment by MoCA questionnaire, of healthy young morning and evening chronotype male subjects at morning (8–10 am) and evening hours (4–6 pm). Substantial number of studies have demonstrated the presence of estrogen and progesterone receptors in the cognitively-relevant brain regions, e.g., hypothalamus, amygdala, hippocampus, and prefrontal cortex, depicting thereby direct neurological influence of sex hormones on cognitive processes.17, 18 Associations between emotion-dependent cognitive processes and menstrual cycle phases have been shown,19 with the luteal phase associated with poorer cognitive performance.20 In light of the above facts, we did not include female subjects for the study as it would have required repeated recording (that too, twice during each session) during different phases of menstrual cycle.
EEG and Cognition
We measured alpha and beta activity as measures of EEG which along with ERP have been shown to provide significant information with respect to mental performance. It has been reported that negative emotional interference increases the load of cognitive processing in terms of electrophysiological and behavioral reactions.21 However, higher activity of the brain is shown to be associated with high-frequency low-amplitude waves, wherein alpha and beta waves, the most prominent waves in awake individuals, qualify.22 Earlier reports have associated alpha wave activity of EEG with cognitive performance showing positive correlation with memory and attention at all ages.23 Also, alpha-band oscillations have been documented to be one of the most basic cognitive processes playing major function in the coalescence of brain activity. Close link of alpha-band oscillations with fundamental functions of attention, i.e., suppression/inhibition and selection/timing, have been strongly exhibited which enables the conscious orientation to time, space, and context.24 Frontal beta activity, however, has been shown to influence stimulus assessment and decision-making during cognitive tasks.25 In line with these above reports, we analyzed alpha and beta activity to assess and correlate them with the outcome of cognitive assessment done by VERP.
We observed significantly higher level of alpha and beta power during evening session in E type individuals at PZ. Spatial attentional shift of different sensory stimuli has been found to be related with the alpha band in the posterior parietal cortex,26 whereas the alpha and beta bands in the occipital region are documented to be linked to preparatory attention.27 Therefore, increased EEG waves’ activity may be indicative of better attention and alertness of E type in the evening hours as compared to morning chronotype.
In the present study, we used midline channels only, i.e., FZ and PZ, to record and analyze alpha and beta rhythms of the EEG signals as per studies done earlier,28, 29 with CZ being a reference site. These studies have documented for the most pronounced appearance of various components on the midline electrodes FZ, CZ, and PZ after examining for their scalp distributions. These midline channels denoting different brain regions are known to be associated with different functions, i.e., FZ, as near intentional and motivational centers and PZ contributing to the activity of perception and differentiation.30
It has been reported earlier that the posterior alpha power is positively correlated to the subjects’ global cognitive status: lower the alpha power, lower is the cognitive status.31 As the PZ activity is known to contribute to perception and differentiation,30 it could be the basis of better cognitive response of evening chronotype subjects in terms of better performance to oddball paradigm for ERP test during evening hours in our study. The result is also in concordance to the validated circadian variation in EEG waves.32
ERP and Cognition
Both P300 latency and amplitude recorded during visual oddball paradigm test were found to be higher for rare stimuli as compared to a frequent one in both chronotype individuals in our study. P300 is known to be associated with cognitive demands and, therefore, stimulus attended with full attention is remembered better, and depicts larger P300 amplitude.33 As the rare stimuli during oddball paradigm also demand higher level of attention eliciting more complex neural processing and cognitive assessment, they gave rise to larger amplitude of P300. However, the interesting observation was that morning-type subjects showed delayed response in terms of increased latency and amplitude during the evening session for both frequent and rare stimuli, whereas E-type subjects showed similar delayed response during the morning session. This finding points toward the impact of chronotypes on the diurnal variation of cognitive processing in terms of P300.10, 34 Increased latency of P300 is indicative of relatively slow neuronal processing, thereby leading to more synchronized spread of neural activity which was reflected as increased amplitude in somatosensory cortical areas.
We found significant reduction in latency as well as amplitude for the rare stimuli during evening session of E-type subjects. This reduced latency and amplitude in the evening session may be indicative of a faster neuronal processing spreading in an asynchronous manner, thereby reducing the averaged amplitude. This again suggests a better cognitive processing ability during evening time for E chronotype subjects. In this context, it may also be mentioned here that latency for rare stimuli in E-type subjects during evening hours was significantly and negatively correlated with both alpha and beta power at FZ. This is in concordance to the better spatial attentional shift to the rare visual stimuli given during oddball paradigm test.26, 27 It is pertinent to mention here that clear circadian rhythm has been reported in cognitive abilities (measuring attentional capacities, executive functioning, and memory) in individuals with strong circadian preferences, i.e., morning, evening, or neither types. Evening-type individuals exhibit poor psychomotor vigilance task or executive functions in morning hours compared to morning type in a young age group of males and females.13 Therefore, inter-individual difference in circadian preference which is reflected as their peak periods of circadian arousal is bound to affect the cognitive performance at the time of the day at which testing occurs, this interaction being known as synchrony effect.35 An earlier study had also reported similar diurnal change of cognitive function in M and E chronotype individuals; however, they used auditory oddball paradigm for eliciting P30036 in contrast to visual oddball paradigm administered by us. Besides, all the subjects in that study were moderately M and moderately E type, while subjects included in our study were definitely M and E type with MEQ scores of ≤70 and ≥30, respectively.1
Therefore, clinicians and researchers should take this fact into consideration while interpreting a person’s cognitive performance with respect to the time of day and their chronotypes.12 A very recent study has also documented for compromised cognitive and physical ability at the earlier hours of day in the evening-type personnel.13 Our results provide evidence in support of these findings clearly. The observed reduced P300 latency during session 1 (morning session), both for frequent and rare stimuli, pointing toward a better cognitive ability for the morning chronotype people as compared to evening chronotypes, may also be explained by the concept of early rising, i.e., at Brahma-muhurtha, the last quarter of night, described in ancient Indian tradition. Early morning riser (Brahma–muhurtha) students have been believed to have best concentration in the morning hours.37 Therefore, this period has been associated with hormonal changes conducive to spiritual blossoming of mind. The scientific basis of this claim has been proposed to be because of lowest body temperature and concomitant minimum level of melatonin in the early hours of morning which coincides with increasing level of cortisol, thereby influencing the process of attention and improved ability to recall.38
Chronotyping could also be helpful for the clinicians to track the progression and/or pathophysiology of certain diseases. One such disease, amyotrophic lateral sclerosis (ALS), a neuro-muscular/neuro-cognitive disorder, displays significant relation to the rhythm disturbances in the patients as manifested by circadian sleep–wake rhythm disorders in them.39 Approximately 35% of patients with ALS experience cognitive and behavioral impairment, while majority of patients suffer from neuro-psychological impairment by end-stage disease.40 Other pathological conditions affecting the retina, i.e., age-related macular degeneration (AMD), show similar circadian misalignment like the one seen in Alzheimer's disease, a neuro-degenerative disease. This is proposed to be caused because of defect in the melanopsin-containing retinal ganglion cells, responsible for mediating circadian entrainment of daily light-dark cycle.41 Along with this, cognitive impairment has been a persistent outcome in advanced AMD as well as in glaucoma patients.42, 43 However, the cause for the observed cognitive impairment in these diseases has not yet been answered. The chronotype (morning or evening) determines the persons’ amount of light exposure and are linked to the cognitive performance, as shown in our present study also. Thus, looking at the chronotypes of these patients could open a new avenue for the therapeutic treatment of them.
Overall Cognition Status
No significant deficit in the overall cognitive processing (objective assessment done by ERP analysis) of E chronotype as compared to M type was observed in our study. At this point, it is worth mentioning that studies documenting psychophysiological alterations in evening-type people have been quite recent and that too, most of these studies have been conducted on subjects with the presence of certain symptoms or in-patient population on the basis of their circadian preference, i.e., morning and evening types.2, 5, 44 We ensured that the subjects recruited for the present study did not have presence/manifestation of any cognitive, functional, or psychological deficits. However, our study consisted of only male volunteers. Therefore, we are unable to comment on the gender difference in the cognitive profile with different chronotypes.
As far as subjective cognitive status is concerned, both groups scored >28 in 30 points MoCA questionnaire. This signifies no cognitive impairment in terms of subjective assessment in both groups of subjects (M and E type) in our study, as an average score of less than 26 is taken as mild cognitive impairment.14
As most reported studies on cognitive aspects have been carried out in patient groups with altered chronotypes and rhythmicity, the extent of the effect of circadian rhythmicity on healthy adults is lesser known. Besides, the objective assessment of cognition by means of EEG and ERP recordings has been very rarely attempted before on different chronotypes, with most studies assessing cognition by subjective tools, e.g., questionnaire or performance measurement during various tasks. Therefore, the study clearly provides evidence of diurnal variations in the cognitive processing of normal adults with morning and evening chronotypes.
Conclusion
In nutshell, we conclude that this study has investigated the impact of chronotype and the diurnal variation (at two different times of the day: morning and evening hours) on the neuro-cognitive functions (EEG and P300) of young age, healthy adult males. The study corroborates that morning chronotype’s cognitive ability is better manifested in the morning hours and evening chronotype’s in the evening hours. The current study is of immense importance in predicting the development of neuro-cognitive changes based on an individual chronotype/chrono-preference. This study may help individuals to take circadian benefits and schedule their activities/cognitive load as per their chronotype and the time of the day for the best cognitive, academic, or overall achievements. Such studies are important in view of the fact that circadian preference may be an important predictor of success in jobs and workplace which requires mental alertness or energy, especially at specific times of the day. However, the measurement of reaction time, correct/incorrect trials and their correlation to the P300 latency and amplitude could also add more ramifications to the findings which would be taken up in future. Besides, our study was conducted on a small cohort of male subjects, which is a limitation for conclusions made using extreme chronotypes. Further studies are recommended to explore gender differences on chronotypes in large gender-balanced samples and different age groups.
Ethical Statement
The study has complied with the guidelines for human studies and includes evidence that the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The subjects have given their written informed consent and the study protocol was approved by the Institute Human Ethics Committee.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
ORCID iD: Meenakshi Sinha  https://orcid.org/0000-0003-4913-7369
https://orcid.org/0000-0003-4913-7369
Author Contribution
First author: Manuscript preparation, data collection and analysis.
Second author: Conception of the study, manuscript preparation and editing.
Third author: Manuscript preparation and editing, data collection.
Fourth author: Manuscript preparation, data collection.
Fifth author: Manuscript preparation, data collection
Funding
The study received financial support from Indian Council of Medical Research (ICMR) under short term studentship (STS) program (Ref. No.: STS 2015-03953).
References
- 1.Horne JA and Östberg O.. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol1976; 4(2): 97–110. [PubMed] [Google Scholar]
- 2.Lipnevich AA, Credè M, Hahn E,. et al. How distinctive are morningness and eveningness from the big five factors of personality? A meta-analytic investigation. J Pers Soc Psychol2017; 112(3): 491–509. [DOI] [PubMed] [Google Scholar]
- 3.Fischer D, Lombardi DA, Marucci-Wellman H,. et al. Chronotypes in the USA: Influence of age and sex. PloS One2017; 12(6): e0178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archer SN, Robilliard DL, Skene DJ. et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep2003; 26(4):413–415. [DOI] [PubMed] [Google Scholar]
- 5.Kivelä L, Papadopoulos M, and Antypa N.. Chronotype and psychiatric disorders. Curr Sleep Med Rep2018; 4(2): 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enright T and Refinetti R.. Chronotype, class times, and academic achievement of university students. Chronobiol Int2017; 34(4): 445–450. [DOI] [PubMed] [Google Scholar]
- 7.Niedermeyer E and Lopes-da-Silva FH.. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. In: Donald L Schomer and Fernando H Lopes da Silva, eds. Wolters Kluwer Health, 2011: p. 119–141. [Google Scholar]
- 8.Peterson NN, Schroeder CE, and Arezzo JC.. Neural generators of early cortical somatosensory evoked potentials in the awake monkey. Electroencephalogr Clin Neurophysiol1995; 96(3): 248–260. [DOI] [PubMed] [Google Scholar]
- 9.Sur S and Sinha V.. Event-related potential: An overview. Ind Psychiatry J2009; 18(1): 70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higuchi S, Liu Y, Yuasa T,. et al. Diurnal variation in the P300 component of human cognitive event-related potential. Chronobiol Int2000; 17(5): 669–678. [DOI] [PubMed] [Google Scholar]
- 11.Croce P, Quercia A, Costa S,. et al. Circadian rhythms in fractal features of EEG signals. Front Physiol2018; 9: 1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt C, Collette F, Cajochen C,. et al. A time to think: Circadian rhythms in human cognition. Cogn Neuropsychol2007; 24(7): 755–789. [DOI] [PubMed] [Google Scholar]
- 13.Facer-Childs E, Boiling S, and Balanos G.. The effects of time of day and chronotype on cognitive and physical performance in healthy volunteers. Sports Med Open2018; 4(1): 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasreddine ZS, Phillips NA, Bédirian V,. et al. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc2005; 53(4): 695–699. [DOI] [PubMed] [Google Scholar]
- 15.Roeser K, Obergfell F, Meule A,. et al. Of larks and hearts morningness/eveningness, heart rate variability and cardiovascular stress response at different times of day. Physiol Behav2012; 106(2): 151–157. [DOI] [PubMed] [Google Scholar]
- 16.Klem GH, Lüders HO, Jasper HH,. et al. The ten-twenty electrode system of the International federation. The International federation of clinical neurophysiology. Electroencephalogr Clin Neurophysiol Suppl1999; 52: 3–6. [PubMed] [Google Scholar]
- 17.Hara Y, Waters EM, McEwen BS,. et al. Estrogen Effects on cognitive and synaptic health over the lifecourse. Physiol Rev2015; 95: 785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinton RD. Thompson RF, Foy MR. et al. Progesterone receptors: Form and function in brain. Front Neuroendocrinol2008; 29: 313–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jessica Le, Natalie Thomas, and Caroline Gurvich. Cognition, the menstrual cycle, and premenstrual disorders: A review. Brain Sci2020; 10: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundström-Poromaa I.The menstrual cycle influences emotion but has limited effect on cognitive function. Vitam Horm2018; 107: 349–376. [DOI] [PubMed] [Google Scholar]
- 21.Lee C, Kim B, and Yoo SK.. Analysis of the emotional-cognitive processes using a modified multisource interference task and recording of EEG and behavioral responses. Neurophysiol2018; 50: 436–444. [Google Scholar]
- 22.Posada-Quintero HF, Reljin N, Bolkhovsky JB,. et al. Brain activity correlates with cognitive performance deterioration during sleep deprivation. Front Neurosci2019; 13: 1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grandy T, Werkle-Bergner M, Chicherio C,. et al. Individual alpha peak frequency is related to latent factors of general cognitive abilities. Neuroimage2013; 79: 10–18. [DOI] [PubMed] [Google Scholar]
- 24.Wolfgang K.Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci2012; 16(12): 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juri D, Kropotov . Chapter 3: Beta rhythms. In: Quantitative EEG, Event-Related Potentials and Neurotherapy. Academic Press, Elsevier Inc., 2009: p. 59–76. [Google Scholar]
- 26.Ruzzoli M and Soto-Faraco S.. Alpha stimulation of the human parietal cortex attunes tactile perception to external space. Curr Biol2014; 24(3): 329–332. [DOI] [PubMed] [Google Scholar]
- 27.Gomez CM, Marco-Pallares J, and Grau C.. Location of brain rhythms and their modulation by preparatory attention estimated by current density. Brain Res2006; 1107(1): 151–160. [DOI] [PubMed] [Google Scholar]
- 28.Amin HU, Malik AS, Badruddin N,. et al. Effects of stereoscopic 3D display technology on event-related potentials (ERPs). Proceedings of the 7th annual International IEEE EMBS conference on Neural Engineering, 2015 April 22, Montpellier, France. [Google Scholar]
- 29.Qazi E-u-H, Hussain M, Aboalsamh H,. et al. Single trial EEG patterns for the prediction of individual differences in fluid intelligence. Front Hum Neurosci2017; 10: 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teplan M.Fundamentals of EEG measurement. Meas Sc Rev2002; 2: 1–11. [Google Scholar]
- 31.Luckhaus C, Grass-Kapanke B, Blaeser I,. et al. Quantitative EEG in progressing vs. stable mild cognitive impairment (MCI): Results of a 1-year follow-up study. Int J Geriatr Psychiatry 2008 November; 23(11): 1148–1155. [DOI] [PubMed] [Google Scholar]
- 32.Gündel A and Hilbig A.. Circadian acrophases of powers and frequencies in the waking EEG. Int J Neurosci1983; 22(1–2): 125–133. [DOI] [PubMed] [Google Scholar]
- 33.Polich J.Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol 2007 October; 118(10): 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wesensten NJ, Badia P, and Harsh J.. Time of day, repeated testing, and interblock interval effects on P300 amplitude. Physiol Behav1990; 47(4): 653–658. [DOI] [PubMed] [Google Scholar]
- 35.May CP and Hasher L.. Synchrony effects in inhibitory control over thought and action. J Exp Psychol Hum Percept Perform1998; 24(2): 363–379. [DOI] [PubMed] [Google Scholar]
- 36.Huang J, Katsuura T, Shimomuray Y,. et al. Diurnal changes in salivary melatonin concentrations and ERP in response to sound stimuli in morning-type and evening-type subjects. J Hum-Environ Syst2006; 9(1–2): 7–12. [DOI] [PubMed] [Google Scholar]
- 37.Sahu DK, Ratre G, Harjpal LC,. et al. Brahm Muhurta. World J Pharmaceutical Res 2019; 8(2): 294–298. [Google Scholar]
- 38.Kumaran VS, Raghavendra BR, and Manjunath NK.. Influence of early rising on performance in tasks requiring attention and memory. Ind J Physiol Pharmacol2012; 56(4): 337–344. [PubMed] [Google Scholar]
- 39.Huang Z, Liu Q, Peng Y,. et al. Circadian rhythm dysfunction accelerates disease progression in a mouse model with amyotrophic lateral sclerosis. Front Neurol2018; 9: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crockford C, Newton J, Lonergan K,. et al. ALS-specific cognitive and behavior changes associated with advancing disease stage in ALS. Neurology2018; 91(15): e1370–e1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.La Morgia C, Ross-Cisneros FN, Sadun AA,. et al. Retinal ganglion cells and circadian rhythms in Alzheimer's disease, Parkinson's disease, and beyond. Front Neurol2017; 8: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clemons TE, Rankin MW, and McBee WL. Age-related eye disease study research group. Cognitive impairment in the age-related eye disease study: AREDS report no. 16. Arch Ophthalmol2006; 124(4): 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varin M, Kergoat MJ, Belleville S,. et al. Age-related eye disease and cognitive function. The search for mediators. Ophthalmology2020; 127(5): 660–666. [DOI] [PubMed] [Google Scholar]
- 44.Rumble M, Dickson D, McCall WV,. et al. The relationship of person-specific eveningness chronotype, greater seasonality, and less rhythmicity to suicidal behavior: A literature review. J Affective Disorders2018; 227: 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]