Glomerular hyperfiltration is one of the earliest mechanisms contributing to CKD initiation and progression. Experimental and clinical studies have shown glomerular hyperfiltration is associated with kidney injury in many CKD etiologies, and is a consequence of complex interactions between growth factors, vasoactive substances, and tubuloglomerular feedback (1). Nephroprotective pharmacotherapies and dietary interventions, including dietary protein moderation and use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), generally reduce glomerular hyperfiltration by reducing glomerular pressure. This manifests clinically as an acute and reversible dip in eGFR, with greater dipping patterns associated with long-term kidney function preservation (2).
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a relatively new class of drugs that are cardiovascular protective and reduce kidney disease progression (3). SGLT2 inhibitors induce an acute, reversible reduction in GFR, which is often referred to as the GFR “dip.” This response pattern suggests these agents reduce glomerular hypertension—an effect that is reminiscent of ACE inhibitors/ARBs. The clinical implications of the acute eGFR dip were unknown and led to concerns about the safety of SGLT2 inhibitors because observational reports suggested an increase in the risk of AKI with these therapies (4). Given the clear cardiorenal protective effects of SGLT2 inhibitors, it is important to resolve uncertainty around the safety and long-term consequences of eGFR dipping to avoid clinical inertia with these therapies (5).
Fortunately, new experimental and clinical studies have provided important insights into the mechanisms and clinical relevance of the eGFR dip. SGLT2 inhibitors exert a variety of physiologic effects that are either attributable to glucosuria, such as HbA1c reduction and weight loss, or to simultaneous inhibition of proximal tubular sodium reabsorption (3). This leads to augmented distal nephron sodium delivery, an effect linked with macula densa sodium uptake, which generates adenosine from ATP breakdown (3). Adenosine binds to the adenosine type 1 receptor at the afferent arteriole, leading to vasoconstriction (3,6). As a result, this proximal natriuresis causes vasoconstriction and a reduction in glomerular hyperperfusion and hyperfiltration. In this model, when the adenosine receptor is pharmacologically antagonized, the acute hemodynamic effect of SGLT2 inhibition is abolished (6).
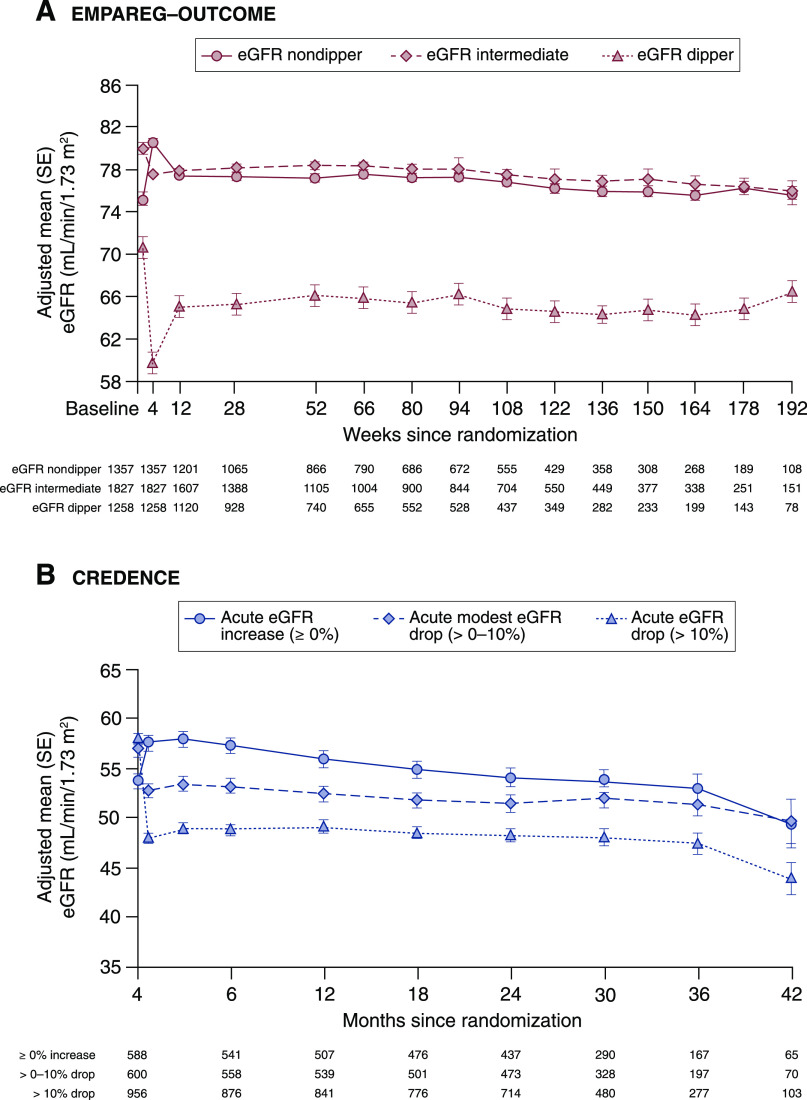
The fact that SGLT2 inhibitors induce an acute dip in eGFR has been known for more than 8 years. Less clear, however, was the relevance of the eGFR dip, and whether it represented potential signals around kidney safety or, conversely, clinical benefit. It is for this reason that three recent analyses—one from Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME), one from Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial (VERTIS-CV), and one from Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE)—are of importance. The first report was a post-hoc analysis from EMPA-REG OUTCOME in participants with type 2 diabetes and cardiovascular disease. The analysis showed that after 4 weeks, 28% of empagliflozin-treated participants versus 13% placebo-treated participants experienced an acute dip in eGFR ≥10% (odds ratio, 2.7; 95% confidence interval, 2.3 to 3.0). Diuretic use at baseline and a higher Kidney Disease: Improving Global Outcomes category were associated with higher likelihood of eGFR dipping. Importantly, long-term eGFR trajectories and safety outcomes, including AKI, were similar regardless of the initial dip in eGFR with empagliflozin treatment (Figure 1) (7). Similar data were reported in an analysis from the VERTIS-CV randomized controlled trial in patients with type 2 diabetes and cardiovascular disease, presented at the American Society of Nephrology in 2020. In this analysis, patients treated with ertugliflozin with the largest tertile for the initial eGFR dip at 6 weeks exhibited the lowest subsequent eGFR slope over time, suggesting a kidney protective effect (8). A third analysis from the CREDENCE trial characterized the acute dip in eGFR and its long-term consequences in patients with type 2 diabetes and CKD (9). In this study, an acute dip in eGFR ≥10% was observed in 45% of patients treated with canagliflozin versus 21% of patients treated with placebo (odds ratio, 3.0; 95% confidence interval, 2.7 to 3.3). Long-term eGFR trajectories and kidney safety profiles were similar, regardless of the magnitude of the initial dip in eGFR after canagliflozin initiation. In the CREDENCE trial, an initial eGFR dip >30% was a rare event and occurred in only 0.5% of canagliflozin-treated participants. In this small subgroup, the risk for kidney-related adverse events was slightly increased compared with nondippers. In this rare circumstance, the SGLT2 inhibitor should be temporarily held so eGFR can return to baseline. Collectively, these data confirm SGLT2 inhibitors preserve kidney function, regardless of the initial dip in eGFR.
Figure 1.
eGFR change over time during empagliflozin treatment in the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPAREG-Outcome) trial and canagliflozin treatment in the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial by category of percentage change in eGFR from baseline to week 4 in EMPAREG-Outcome and from baseline to week 3 in CREDENCE. In both the EMPAREG-Outcome (A) and CREDENCE trial (B), the long-term eGFR trajectories remained stable in all dipping categories. In the CREDENCE trial, long-term annual eGFR decline was slightly but statistically significantly less in patients with an acute eGFR dip (2.0 ml/min per 1.73 m2 per year) compared with patients with an acute eGFR increase (2.1 ml/min per 1.73 m2; P=0.04).
These new data are reassuring and confirm the dip in eGFR is not associated with progressive long-term kidney function loss or AKI. Meta-analyses have also demonstrated SGLT2 inhibitors do not cause AKI, and in fact may even reduce the likelihood of it occurring (10). With the concern about AKI risk no longer significant on the basis of contemporary trials, clinicians still grapple with how to monitor patients after SGLT2 inhibitor initiation, and what to do in response to the eGFR dip when it occurs. On the basis of the new data from EMPA-REG OUTCOME, VERTIS-CV, and CREDENCE, and consistent safety data in real-world evidence studies (11), it seems reasonable to conclude that in the majority of patients, there is no need to have a routine monitoring strategy to check kidney function or electrolytes, unless there is a clinical concern about volume depletion in specific individuals, such as in patients with BP <120/70 mm Hg, sign/symptoms of volume depletion (e.g., orthostatic symptoms), in patients taking high-dose diuretics, and perhaps in the elderly. This is predicated on the concepts that: (1) AKI risk is not increased; (2) eGFR dipping is not associated with kidney injury; (3) even in the event of dipping, this should not affect management or continuation of therapy; (4) SGLT2 inhibitors do not cause electrolyte abnormalities, so there is no need to check potassium after initiation (i.e., as with ACE inhibitors, ARBs). Therefore, patients can safely have the next set of blood work at subsequent scheduled appointment to avoid additional cost and concerns from clinicians or patients around the eGFR dip. This streamlined approach is also appropriate to remove unnecessary clinical barriers to initiation of therapy, including in primary care.
In conclusion, SGLT2 inhibitors reduced the risk of kidney failure and cardiovascular outcomes in two independent large kidney outcome trials in patients with CKD, including individuals with and without type 2 diabetes. Clinical practice guidelines are evolving and recommend the use of SGLT2 inhibitors for kidney protection. The dip in eGFR observed soon after SGLT2 inhibitor initiation likely reflects their protective mechanism of action and should not lead to safety concerns and/or barriers for their widespread implementation.
Disclosures
D.Z.I. Cherney reports employment with Toronto General Hospital; reports receiving consulting fees or speaking honorarium, or both, from Abbvie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim-Eli Lilly, Bristol Myers Squibb, Janssen, Johnson and Johnson, MAZE, Merck & Co. Inc., Mitsubishis-Tanabe, Novo Nordisk, Otsuka, Prometic, and Sanofi; reports receiving research funding from AstraZeneca, Boehringer Ingelheim-Lilly, Janssen, Merck, Novo Nordisk, and Sanofi; and has served as a scientific advisor or member of AstraZeneca, Boehringer Ingelheim, Janssen, Merck, Novo Nordisk, and Sanofi. H.J.L. Heerspink reports employment with University Medical Center Groningen; ongoing consultancy agreements with AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Pharma, Dimerix Fresenius, Gilead, Janssen, Merck Mundi Pharma, Mitsubishi Tanabe, NovoNordisk, and Travere Pharmaceuticals; reports receiving research support from AbbVie, AstraZeneca, Boehringer Ingelheim, and Janssen (grant funding directed to employer); and speakers bureau for AstraZeneca.
Funding
None.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or CJASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Tonneijck L, Muskiet MH, Smits MM, van Bommel EJ, Heerspink HJ, van Raalte DH, Joles JA: Glomerular hyperfiltration in diabetes: Mechanisms, clinical significance, and treatment. J Am Soc Nephrol 28: 1023–1039, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, Parving HH, Brenner BM, Shahinfar S, Lambers Heerspink HJ: An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 80: 282–287, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Heerspink HJL, Perkins BA, Fitchett DH, Husain M, Cherney DZI: Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: Cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134: 752–772, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Perlman A, Heyman SN, Matok I, Stokar J, Muszkat M, Szalat A: Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: Analysis of the FDA adverse event report system database. Nutr Metab Cardiovasc Dis 27: 1108–1113, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Sridhar VS, Tuttle KR, Cherney DZI: We can finally stop worrying about SGLT2 inhibitors and acute kidney injury. Am J Kidney Dis 76: 454–456, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Kidokoro K, Cherney DZI, Bozovic A, Nagasu H, Satoh M, Kanda E, Sasaki T, Kashihara N: Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation 140: 303–315, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Kraus BJ, Weir MR, Bakris GL, Mattheus M, Cherney DZI, Sattar N, Heerspink HJL, Ritter I, von Eynatten M, Zinman B, Inzucchi SE, Wanner C, Koitka-Weber A: Characterization and implications of the initial estimated glomerular filtration rate ‘dip’ upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int 99: 750–762, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Cherney D, Charbonnel B, Cosentino F, Pratley RE, Dagogo-Jack S, Shih WJ, Mcguire DK, Frederich R, Maldonado M, Liu J, Pong A, Liu CC, Cannon CP: Effect of ertugliflozin on initial eGFR decline and chronic slope: Analyses from the VERTIS CV trial. J Am Soc Nephrol S1: B5, 2020 [Google Scholar]
- 9.Oshima M, Jardine MJ, Agarwal R, Bakris G, Cannon CP, Charytan DM, de Zeeuw D, Edwards R, Greene T, Levin A, Lim SK, Mahaffey KW, Neal B, Pollock C, Rosenthal N, Wheeler DC, Zhang H, Zinman B, Perkovic V, Heerspink HJL: Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int 99: 999–1009, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Zhao M, Sun S, Huang Z, Wang T, Tang H: Network meta-analysis of novel glucose-lowering drugs on risk of acute kidney injury. Clin J Am Soc Nephrol 16: 70–78, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iskander C, Cherney DZ, Clemens KK, Dixon SN, Harel Z, Jeyakumar N, McArthur E, Muanda FT, Parikh CR, Paterson JM, Tangri N, Udell JA, Wald R, Garg AX: Use of sodium-glucose cotransporter-2 inhibitors and risk of acute kidney injury in older adults with diabetes: A population-based cohort study. CMAJ 192: E351–E360, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]