Patients on maintenance hemodialysis (HD) are highly susceptible to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (1). Work by us and others shows that patients on maintenance HD mount an antibody (Ab) response following SARS-CoV-2 infection, but the Ab titers decline over time (2,3). The mRNA-1273 SARS-CoV-2 vaccine (Moderna) is directed against the spike protein of SARS-CoV-2 and elicits an Ab response to the receptor binding domain (RBD) of the spike protein, which has neutralizing properties (4).
We collected data on IgG Ab response to the Moderna vaccine in patients on maintenance HD in the outpatient dialysis unit at the James J. Peters Veterans Affairs (VA) Center located in New York City. Of 79 eligible patients, 61 patients received the two-dose Moderna vaccine series at the HD unit and were included in the study. Patients with a positive SARS-CoV-2 RNA on nasal or nasopharyngeal specimen were diagnosed with coronavirus disease 2019 (COVID-19; Cepheid, Abbot, or Roche SARS-CoV-2 RNA assay). SARS-CoV-2 RNA tests were performed on patients who presented with symptoms suggestive of COVID-19 or as part of routine surveillance every 2 weeks on all patients on HD.
SARS-CoV-2 IgG Ab test (Abbot IgG Nucleocapsid assay, anti-N IgG, >1.39 considered positive, 100% sensitivity, 100% specificity; Beckman Coulter IgG Receptor Binding Domain assay, anti-RBD IgG, ≥1.0 considered positive, 97% sensitivity, 100% specificity) was performed on patients 4 weeks prior to the first vaccine dose administration (mean of 29±6 days before vaccine administration) and then serially at 1, 2, 4, and 5 weeks after the first vaccine dose. Comparison of Ab titers was done using the Wilcoxon rank sum for comparison of patients with and without prior COVID-19 and the Wilcoxon signed rank test for comparison of Ab titers across time frames. This project was part of a quality improvement project with approval from the James J. Peters VA Quality Improvement committee and was granted institutional review board exemption.
The mean age of the cohort was 70±11 years, 95% were men, and 85% were Black patients. Twenty of the 61 patients (31%) were diagnosed with COVID-19 prior to their first vaccine dose, and two patients were diagnosed with COVID-19 1 week after the first vaccine dose. Patient characteristics by COVID-19 status at the time of first vaccine dose are presented in Figure 1A.
Figure 1.
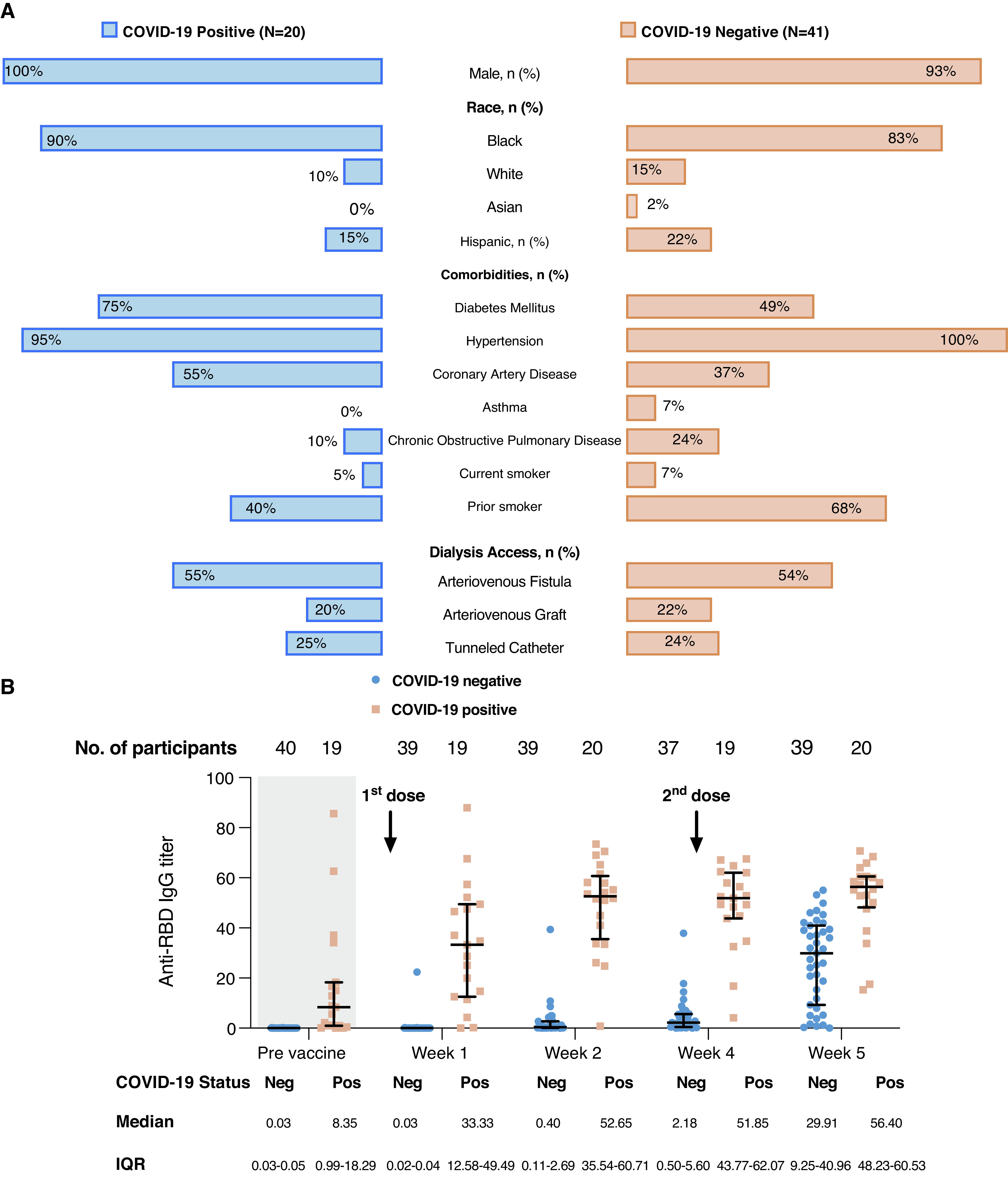
Patient characteristics and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antireceptor binding domain (anti-RBD) IgG antibody titer following SARS-CoV-2 mRNA-1273 vaccine administration. (A) Butterfly plot of patient characteristics in patients on hemodialysis who tested positive (Pos) or negative (Neg) for coronavirus disease 2019 (COVID-19) prior to vaccination. (B) Results of SARS-CoV-2 anti-RBD IgG antibody titer results before and after SARS-CoV -2 mRNA-1273 vaccine administration. “Prevaccine” antibody titers were measured 4 weeks prior to first vaccine dose administration. Week-1 and week-2 antibody titers were measured 1 and 2 weeks after the first vaccine dose administration, respectively. The second vaccine dose was administered 4 weeks after the first vaccine dose, and week-4 antibody titers were done immediately before the second vaccine dose administration. Week-5 antibody titers were done 1 week after the second vaccine dose administration. IQR, interquartile range.
Prevaccine median anti-N IgG Ab titer was 3.98 (interquartile range [IQR], 0.71–5.05) and anti-RBD IgG titer was 8.35 (IQR, 0.99–18.29) in patients with prior COVID-19. Prevaccine median anti-N IgG Ab titer was 0.03 (IQR, 0.02–0.05) and anti-RBD IgG titer was 0.03 (IQR, 0.03–0.05) in patients without prior COVID-19. The change in the anti-N IgG Ab titer was not significant at any time point following the vaccine administration, except in two patients who developed COVID-19 1 week after the first vaccine dose.
In patients with prior COVID-19, the median anti-RBD IgG Ab titers were 33.33, 52.65, 51.85, and 56.40 at 1, 2, 4, and 5 weeks following the first vaccine dose, respectively. This group received the second vaccine at 4 weeks (28±0.6 days) after the first vaccine dose, and the 4-week Ab titer was done immediately before the second vaccine dose administration (Figure 1B). The median Anti-RBD IgG Ab titers rose significantly at 1 week after the first vaccine dose (8.35 versus 33.33; P<0.001). Anti-RBD IgG Ab titers at 5 weeks were significantly higher than at 1 week following the first vaccine dose (33.33 versus 56.4; P<0.001).
In patients without prior COVID-19, the median anti-RBD IgG Ab titers were 0.03, 0.4, 2.18, and 29.91 at 1, 2, 4, and 5 weeks, respectively. This group received the second vaccine dose at 4 weeks (29±3.9 days) after the first vaccine dose, and the 4-week Ab titer was done immediately before the second vaccine dose administration. The anti-RBD IgG Ab titer did not begin to increase until 4 weeks after the first vaccine dose (0.03 versus 2.18; P<0.001). The median anti-RBD IgG Ab titer rose significantly at 5 weeks after the first vaccine dose (0.03 versus 29.91; P<0.001) (Figure 1B).
Twenty patients had prior COVID-19 before their first vaccine dose, and the median time from COVID-19 infection to first vaccine dose administration was 253 days (IQR, 38–284 days). There was no correlation between days from COVID-19 infection and 1-week anti-RBD IgG Ab titer (Pearson correlation of 0.45; P=0.05).
No patient was diagnosed with COVID-19 after 34 days (IQR, 34–35) following the second vaccine dose. Three of 61 patients (5%) failed to mount an anti-RBD IgG Ab response at 1 week following the second vaccine dose, and one of these patients was receiving rituximab and prednisone during the time of vaccine administration. None of these three patients had prior COVID-19.
In this report, 95% patients on HD mounted an anti-RBD IgG Ab response to the Moderna vaccine. The timing of the Ab response was different in patients on HD depending on prior COVID-19 history. Anti-RBD IgG Ab response in patients on HD with prior COVID-19 was robust at 1 week after the first vaccine dose, a finding similar to that of a recently published study of patients not on dialysis (5). However, the robust anti-RBD Ab response in patients on HD without prior COVID-19 did not occur until 5 weeks after the first vaccine dose (1 week after the second dose), highlighting the importance of following the clinical trial vaccine administration protocol in patients on HD. Whether a single Moderna vaccine dose provides effective protection in patients on HD with prior COVID-19 requires further investigation. The study results may not be generalizable to patients not on HD, to patients receiving non-Moderna vaccine, and to women as the majority of the patients in our cohort were men. Seroconversion following vaccination in patients on HD is reassuring, but the mere presence of antibodies may not confer complete immunity against the SARS-CoV-2 virus.
Disclosures
K.N. Campbell reports consultancy agreements with Calliditas, Goldfinch Bio, Mallinckrodt Pharmaceuticals, Travere, and Vertex; receiving research funding from Goldfinch Bio and Mallinckrodt Pharmaceuticals; and serving as a scientific advisor or member of the Medical Advisory Board, National Kidney Foundation of Greater New York, Nephcure Foundation. L. Chan reports consultancy agreements with Vifor Pharma, Inc.; receiving research funding from the National Institutes of Health; and receiving honoraria from Fresenius. A. Shaikh reports serving as a member of the Educational and Communication Committee of the American Society of Diagnostic and Interventional Nephrology. All remaining authors have nothing to disclose.
Funding
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Valeri AM, Robbins-Juarez SY, Stevens JS, Ahn W, Rao MK, Radhakrishnan J, Gharavi AG, Mohan S, Husain SA: Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol 31: 1409–1415, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaikh A, Zeldis E, Campbell KN, Chan L: Prolonged SARS-CoV-2 viral RNA shedding and IgG antibody response to SARS-CoV-2 in patients on hemodialysis. Clin J Am Soc Nephrol 16: 290–292, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labriola L, Scohy A, Seghers F, Perlot Q, De Greef J, Desmet C, Romain C, Morelle J, Yombi JC, Kabamba B, Rodriguez-Villalobos H, Jadoul M: A longitudinal, 3-month serologic assessment of SARS-CoV-2 infections in a Belgian hemodialysis facility. Clin J Am Soc Nephrol 16: 613–614, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group: Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384: 403–416, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, Bermúdez-González MC, Bielak DA, Carreño JM, Chernet RL, Eaker LQ, Ferreri ED, Floda DL, Gleason CR, Hamburger JZ, Jiang K, Kleiner G, Jurczyszak D, Matthews JC, Mendez WA, Nabeel I, Mulder LCF, Raskin AJ, Russo KT, Salimbangon AT, Saksena M, Shin AS, Singh G, Sominsky LA, Stadlbauer D, Wajnberg A, Simon V: Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med 384: 1372–1374, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]


