Abstract
Acid-related injury from chronic metabolic acidosis is recognized through growing evidence of its deleterious effects, including kidney and other organ injury. Progressive acid accumulation precedes the signature manifestation of chronic metabolic acidosis, decreased plasma bicarbonate concentration. Acid accumulation that is not enough to manifest as metabolic acidosis, known as eubicarbonatemic acidosis, also appears to cause kidney injury, with exacerbated progression of CKD. Chronic engagement of mechanisms to mitigate the acid challenge from Western-type diets also appears to cause kidney injury. Rather than considering chronic metabolic acidosis as the only acid-related condition requiring intervention to reduce kidney injury, this review supports consideration of acid-related injury as a continuum. This “acid stress” continuum has chronic metabolic acidosis at its most extreme end, and high-acid-producing diets at its less extreme, yet detrimental, end.
Keywords: chronic kidney disease, end stage kidney disease, nutrition, oxidative stress, progression of chronic renal failure
Growing evidence supports kidney injury from a range of acid challenges to acid-base status, including those insufficient to cause metabolic acidosis by reduced plasma bicarbonate concentration ([HCO3 −]). Acid accumulation that is insufficient to cause metabolic acidosis, known as “eubicarbonatemic acidosis” (1,2), “preclinical acidosis” (3), or “subclinical acidosis” (4), is associated with increased risk for kidney stones (5) and exacerbated progression of CKD (6,7). Acid-producing diets in general populations, including those typical of Western societies, are associated with increased risk for kidney injury (8) and incident CKD (8 –10). Indeed, individuals without known kidney disease who are given mineral acid cumulatively excrete less acid than ingested (11,12), consistent with sustained acid accumulation.
These data challenge scientific communities to consider injurious acid accumulation as a continuum of “acid stress” in which metabolic acidosis is its most severe manifestation, but includes high-acid-producing Western diets. Reframing these disorders as a continuum enables recognition of lower, yet clinically meaningful, levels of kidney injury caused by conditions earlier in the acid stress continuum. This reframing also underscores an opportunity for medical and public health professionals to address the apparent contribution of high-acid-producing diets to kidney injury, through practitioner and public health recommendations for lower-acid diets. Although these diets are associated with other organ injury to be discussed briefly, this review will focus primarily on their associated kidney injury.
What Is “Normal” Plasma Bicarbonate Concentration?
The normal range of plasma [HCO3 −] varies widely among clinical laboratories, with low ends as low as ≥18 mmol/L and high ends as high as ≤36 mmol/L (13), lending uncertainty as to a “low” plasma [HCO3 −] that is consistent with metabolic acidosis. In a large US database of apparently healthy individuals who were presumably eating typical Western diets, 91% of plasma [HCO3 −] determinations were between 23 and 30 mmol/L (13), supporting this narrower range for normal. These data also align with expert opinion of current guidelines that define metabolic acidosis in populations eating Western diets as plasma [HCO3 −] <22 mmol/L in the absence of respiratory alkalosis (14). Whether lower ends of this normal range include individuals with injurious acid accumulation needing treatment, and whether it would be higher in populations eating a non-Western, less acid-producing diet, awaits further study.
The Daily Acid Challenge to Acid-Base Status
Our “fixed” acid challenge derives from endogenous metabolism and metabolism of dietary proteins, phospholipids, nucleic acids, and incomplete oxidation of carbohydrates (15), and is approximately 0.7–1.0 mmol/kg body wt per day in healthy adults eating Western diets (16,17). These diets are acid-producing because of the preponderance of acid-producing meats and refined grains compared with base-producing fruits and vegetables (18). Animal-sourced compared with plant-sourced foods have more protein per mass and more sulfur-containing amino acids (e.g., methionine, cysteine) that yield acid when metabolized, than plant-sourced foods. Even fresh animal-sourced foods have various amounts of sodium chloride (NaCl) that increase acid production (19,20), and processed foods—an increasing proportion of Western diets—typically have added NaCl. Most fresh plant-sourced foods are very low in NaCl, and many have potassium and magnesium salts of organic acids (e.g., citrate) that metabolize to yield HCO3 −.
The acid-producing capacity of a diet can be estimated by calculating its potential renal acid load (PRAL) through tabulation of the type and quantity of its foods, and assigning the amount of acid (positive value) or base (negative value) produced when metabolized (15). This calculation excludes an estimate of organic acid excretion, about 40 mmol in average adults, that, combined with PRAL, estimates urine net acid excretion (NAE) (15). Table 1 lists PRAL of various food groups and composite PRALs for indicated diets calculated by the author, applying PRAL for various foods (15) to the types and food amounts in published diets (21,22). The table shows that the average US diet (21) has higher PRAL than the diet recommended by US Department of Agriculture (22), supporting that typical Western diets are highly acid-producing (18). Table 1 shows even higher PRAL for diets of study participants from a low-income, “food desert” community in the United States (23), which was reduced to lower dietary acid for participants given fresh fruits and vegetables. Sample Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and vegan diets, each of which is associated with reduced CKD risk (24 –26), had lower PRAL. Overall, Table 1 shows that typical US diets are acid-producing, more so in food desert communities who appear to be at very high CKD risk (27), and that recommended healthy diets are comparatively low acid. Unfortunately, there are no published guidelines regarding a healthy dietary acid content.
Table 1.
Calculated potential renal acid load of selected relevant diets
| Food | USDA Recommended | Average Intake in the United States | Study Participants | Study Participants Given F+V | Mediterranean Diet | DASH Diet | Vegan Diet |
|---|---|---|---|---|---|---|---|
| Meat/seafood | 13.61 | 22.55 | 27.45 | 25.21 | 6.03 | 22.83 | 0 |
| Vegetables | −24.91 | −12.46 | −5.75 | −13.96 | −22.63 | −20.19 | −6.52 |
| Fruit | −1.81 | −0.91 | 6.23 | −18.68 | −10.04 | −5.91 | −13.72 |
| Grains | 6.34 | 6.43 | 10.64 | 9.57 | 18.26 | 8.15 | 27.6 |
| Dairy | 10.16 | 11.21 | 23.31 | 23.31 | 7.99 | 5.77 | 0 |
| Oils | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0 |
| Total | 3.4 | 26.83 | 61.89 | 25.46 | −0.39 | 10.63 | 7.36 |
All values are shown in millimoles per day. USDA, United States Department of Agriculture; F+V, fruits and vegetables; DASH, dietary approaches to stop hypertension.
Chronic Engagement of Mechanisms to Mitigate Acid Accumulation can Cause Organ Injury
Figure 1 outlines mechanisms that help maintain plasma [HCO3 −] such that even large increases in dietary acid elicit quantitatively small increases in plasma hydrogen ion concentration ([H+]) (expressed clinically as pH) and quantitatively small decreases in plasma [HCO3 −] (approximated clinically by total carbon dioxide [total CO2]), within normal ranges for each (28). Accordingly, plasma [H+]/[HCO3 −] yield inadequate insight for acid accumulation that is not enough to cause metabolic acidosis, yet is enough to cause injury. These data highlight the clinical challenge of using plasma acid-base parameters to detect potentially injurious levels of chronic acid accumulation that are earlier in the continuum of acid stress than metabolic acidosis.
Figure 1.
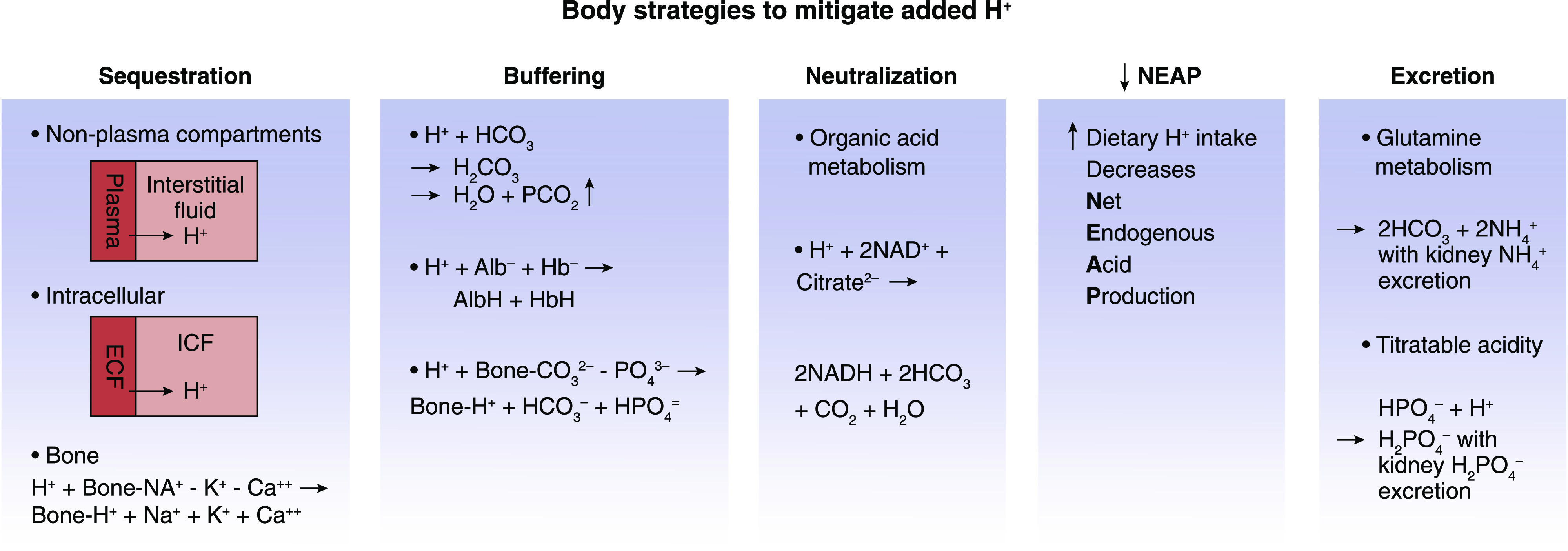
The body has multiple strategies to mitigatethe potential untowardeffects of added acid (H+). Alb−, albumin; AlbH, protonated albumin; Buf−, buffer; Ca++, calcium; CO3 2−, carbonate; CO2, carbon dioxide gas; ECF, extracellular fluid; H+, hydrogen; H2CO3, carbonic acid; Hb−, hemoglobin; HbH, protonated hemoglobin; HCO3 −, bicarbonate; HPO4 =/H2PO4 −, phosphate; ICF, intracellular fluid; K+, potassium; Na+, sodium; NAD+/NADH, nicotinamide adenine dinucleotide; NH4 +, ammonium; PCO2, partial pressure of carbon dioxide; PO4 3−, phosphate.
Acid Sequestration
Most (60%–75%) of H+ added to plasma is sequestered intracellularly (29) and buffered by anionic proteins, phosphate (HPO4 =), and HCO3 −, limiting the proportion of “free” H+ (30). Sequestered H+ from plasma into cells and bone yields the benefit of reduced H+ exposure to tissues that interface with plasma, but increased intracellular acidity yields the detriment of released iron, previously bound to intracellular protein, causing tissue-damaging oxidative stress (31).
Acid Buffering
HCO3/H2CO3 Buffer System.
Adding H+ to body fluids containing HCO3 leads to the following equation: H+ + HCO3 − → H2CO3 → H2O + CO2 ↑ (lungs excrete CO2 gas).
This response removes H+ from plasma as CO2 gas that would yield H+ (reversal of the above equation) were it to accumulate. The price paid is reduced plasma [HCO3 −], which kidneys must regenerate through NAE, while suffering potential detrimental effects from sustained engagement of kidney H+ excretory mechanisms (see Enhanced Urine Acid Excretion).
Non-bicarbonate Buffers.
Increased dietary acid in animals augmented H+ titration of extracellular non-HCO3 buffers (32). Bone calcium carbonate and dibasic HPO4 = chronically buffer increased net endogenous acid production (NEAP), which progressively reduces bone mineral content (12).
Endogenous Acid Neutralization
The pH-sensitive metabolite citrate is the most abundant urine organic base-equivalent, and proximal tubule reabsorption determines its urine excretion (33). Proximal tubule cell secreted H+ into luminal fluid partially titrates citrate3− to H-citrate2−, the latter being preferred substrate for reabsorption by the apical Na+ dicarboxylate cotransporter NaDC-1 (34,35), with NAD+ conversion to NADH. Metabolism of retained citrate yields HCO3 −, so its reabsorption is base gain, but its excretion is base loss (36). pKa of the three citrate valences (6.40, 4.76, and 3.14 for its −3, −2, and −1 valences, respectively) makes it mostly dissociated at typical urine pH, limiting H+ binding, making its urine excretion yield little H+ excretion. Citrate metabolism to HCO3 − yields benefit by replacing HCO3 − titrated by accumulated H+, but yields detriment by reducing body H+ buffering capacity and increased kidney stone risk through reduced urine citrate excretion (37). Individuals with eubicarbonatemic acidosis have reduced urine citrate excretion (7,38) that can be increased by adding dietary fruits and vegetables (38).
Reduced Net Endogenous Acid Production
Individuals with increased NEAP, as with starvation-induced ketoacidosis, reduce NEAP in response to an increment in dietary acid given as ammonium chloride (39). By contrast, they maintain higher NEAP when given sodium bicarbonate or NaCl (39), supporting the idea that individuals with high baseline NEAP can reduce NEAP in response to high dietary acid.
Enhanced Urine Acid Excretion
Importance of Ammonium.
Ammonium (NH4 +) increases in response to augmented dietary acid (18,40), forming from H+ titration of ammonia (NH3 + H+ → NH4 +) after metabolism of the amino acid glutamine that also yields α-ketoglutarate. Urine acid excretion as NH4 +, along with α-ketoglutarate metabolism to yield HCO3 −, constitutes NAE, with regeneration of HCO3 − to replace that titrated by accumulated acid (Figure 1). Dietary acid in animals stimulated kidney NH4 + production, enhancing complement deposition in kidney tissue (41). Complement could be a marker of an inflammatory response to NH4 + that contributes to kidney injury and mediates progressive kidney function decline in response to dietary acid (41). Furthermore, patient studies showed an association between urine NH4 + and the profibrotic marker TGF-β1, supporting a potential role for NH4 + in progressive kidney injury in patients (42,43). The data support that increased urine NH4 + production, and excretion in response to increased dietary acid, yields the benefit of increased NAE but the possible detriment of kidney injury, with progressive kidney function decline.
Increased Kidney Tubule Acidification.
Increasing dietary acid with mineral acid (44) or acid-producing dietary protein (45,46) increased kidney angiotensin II (47,48), aldosterone (49), and endothelin-1 (ET-1) (44,49) in animals. Higher kidney levels contributed short-term benefit of enhanced kidney acidification with increased urine NAE (44 –47), but long-term detriment of increased kidney interstitial fibrosis (50 –52), a component of progressive nephropathy (53). By contrast, dietary base as oral mineral alkali (47 –49,54) or base-producing dietary components (45,46,55) decreased their urine excretion. Furthermore, mineral alkali reduced these levels in patients with eubicarbonatemic acidosis (56). These data support that sustained engagement of H+ excretory mechanisms in response to dietary acid has long-term detrimental kidney consequences that can be ameliorated by reducing dietary acid and/or neutralizing accumulated acid of eubicarbonatemic acidosis.
Influence of Glomerular Filtration Rate on Acid Accumulation in Response to Dietary Acid
Animals with normal GFR given high compared with lower dietary acid that increases urine NAE but with normal plasma [HCO3 −] nevertheless had acid accumulation by microdialysis (32). Acid accumulation that was insufficient to reduce plasma [HCO3 −] below normal, and so not manifest as metabolic acidosis, was greater in animals with reduced than normal GFR (54,57). Likewise, patients with reduced compared with normal eGFR and a high-acid diet had greater acid accumulation despite normal plasma [HCO3 −] (38,58). Higher acid diets directly associated with higher anion gap in individuals with CKD, and the anion gap was higher in those with eGFR 30–59 ml/min per 1.73 m2 than with eGFR >60 ml/min per 1.73 m2, including individuals with plasma [HCO3 −] within normal ranges (59). Furthermore, acid accumulation in individuals with reduced eGFR and eubicarbonatemic acidosis increased as eGFR further declined over time (58), and increased accumulated acid directly associated with increased plasma anion gap despite maintenance of plasma [HCO3 −] with the normal range (9).
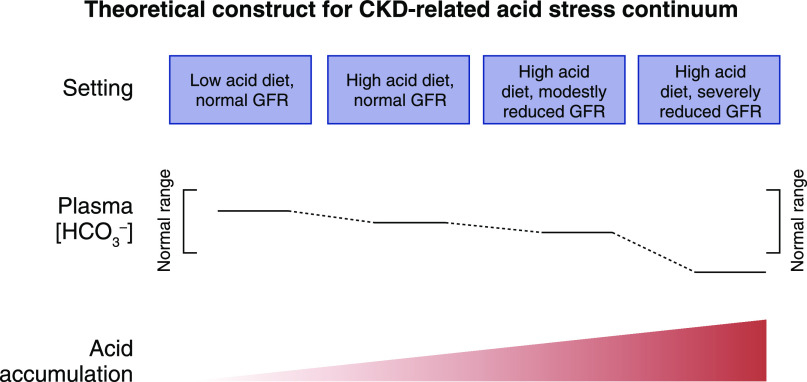
Whether diet-induced acid accumulation manifests as metabolic acidosis depends, in part, on the level of remaining eGFR and the level of dietary acid. Indeed, individuals with CKD and reduced eGFR had greater increases in plasma [H+] and greater decreases in plasma [HCO3 −], even developing metabolic acidosis, at dietary acid levels that did not cause metabolic acidosis in those with higher eGFR (60). The data support that individuals with normal eGFR more completely excrete the acid of Western diets, but do so less with declining eGFR. With high dietary acid, progressive eGFR decline leads to acid accumulation, and eventually to metabolic acidosis, as in Figure 2.
Figure 2.
In the setting of a high acid-producing diet, progressive decreases in glomerular filtration rate are associated with progressively increasing acid accumulation that initially decreases plasma bicarbonate concentration (HCO3−]) within normal ranges but eventually decreases it below normal to manifest as metabolic acidosis. The top horizontal line of the boxes characterizes progressively decreasing levels GFR combined with either low or high dietary acid intake. The middle horizontal portion figuratively compares plasma bicarbonate concentration ([HCO3 −]) either within the brackets of the normal range or below it. The bottom horizontal figuratively compares H+ retention among the four stages. Adapted from ref. 86, with permission.
Acid Accumulation and Organ Injury
Table 2 lists untoward systemic and organ injury of high dietary acid. High-acid diets associate with increased mortality (61,62), insulin resistance (63,64), increased risk for type 2 diabetes (65,66) and hypertension (67,68), and increased glucocorticoid activity (20). These diets also associate with decreased bone health (69,70) and increased catabolism that decreases muscle mass (71,72). Table 3 lists untoward effects associated with reduced GFR/eGFR and eubicarbonatemic acidosis. These effects include vascular endothelial inflammation (73), increased oxidative stress (31), and tissue inflammation and fibrosis (74). Also, patients with CKD and progressively decreasing eGFR developed signs of disturbed Ca/HPO4 = metabolism before the onset of metabolic acidosis manifest by reduced plasma [HCO3 −] (75). Furthermore, patients with eubicarbonatemic acidosis had an increased catabolic state, with augmented protein catabolism (76).
Table 2.
Systemic/organ injury associated with increased dietary acid in individuals/animals without reduced GFR and, presumably, initial normal acid-base status
| Systemic/overall effects |
| Increased mortality (61,62) |
| Insulin resistance (63,64) |
| Type 2 diabetes (65,66) |
| Hypertension (67,68) |
| Increased glucocorticoid activity (20) |
| Decreased bone health (69,70) |
| Muscle: increased catabolic state with decreased muscle mass (71,72) |
| Kidney |
| Increased CKD risk (8 –10) |
| Increased chronic tubulointerstitial injury (52) and increased urine markers of tubulointerstitial injury (57) in animals with normal GFR |
| Increased risk for kidney stones (5,37) |
Table 3.
Systemic/organ injury associated with individuals/animals with reduced GFR and eubicarbonatemic acidosis
| Systemic/overall effects |
| Vascular endothelial cell inflammation (73) |
| Oxidative stress (31) |
| Tissue inflammation and fibrosis (74) |
| Disturbed CKD calcium/phosphate metabolism before metabolic acidosis onset (75) |
| Increased catabolic state with protein catabolism (76) |
| Kidney |
| Decreased urine citrate with increased kidney stone risk (2) |
| Increased kidney production of angiotensin II, aldosterone, and endothelin-1, with increased risk for kidney interstitial inflammation (44 –49,77) |
| Complement activation with increased risk for kidney injury (41,43) |
| Increased risk for progression of established CKD to ESKD (6,7,80) |
Although patients with CKD-related metabolic acidosis have kidney injury (77) and CKD progression (23,77,78,79), acid-induced kidney injury with CKD progression is not limited to those with reduced GFR and reduced plasma [HCO3 −] (Table 2). Chronic high-acid diets in animals with normal GFR and normal plasma [HCO3 −] caused long-term tubulointerstitial injury (52). Switching to a high-acid diet increased a urine marker of tubulointerstitial injury in animals with normal GFR and normal plasma [HCO3 −] (57). High-acid diets in the general population increase the plasma anion gap (59), consistent with acid accumulation, and this is associated with increased CKD risk (9) and increased risk for progression of prevalent CKD to ESKD (80). Also, chronic oral NaHCO3 in patients with CKD, normal GFR, and normal plasma [HCO3 −] reduced urine excretion of endothelin and aldosterone (56), substances associated with CKD progression (50,51). These data support the physiologic construct in Figure 2, in which harmful acid accumulation occurs with transition from a low- to high-acid diet, constituting the earliest stage of the acid stress continuum.
Acid accumulation remained unchanged in participants with declining eGFR who receive chronic oral alkali, but increased in those who do not receive it, showing long-term benefit of chronic oral alkali (5 years) to reduce acid accumulation in eubicarbonatemic acidosis (58), in addition to its shorter-term (30 days) benefit to do so (56). Such treatment in patients fitting these criteria reduced urine indices of kidney injury (6,81) and slowed eGFR decline (6), supporting that eubicarbonatemic acidosis caused kidney injury and exacerbated eGFR decline (Table 3). These data support the progressive course of acid stress in Figure 2 in individuals with reduced eGFR who eat high-acid diets and suffer from kidney toxic acid accumulation before it accrues to reduce plasma [HCO3 −], when current guidelines recommend treatment (14).
Strategies to Detect Acid Stress in Individuals without Metabolic Acidosis
As noted earlier and depicted in Figure 2, steady-state eubicarbonatemic acidosis more likely occurs in individuals with CKD, reduced eGFR, and those who eat high-acid diets. Those without metabolic acidosis appear to be appropriate for investigation to determine their candidacy for interventions to reduce dietary acid and/or treatment with Na+-based alkali. Reducing dietary acid by adding base-producing food components appears to be an effective initial step to decrease identified acid accumulation (38).
Microdialysis can detect acid accumulation insufficient to significantly reduce plasma [HCO3 −] in animals (32,49,54,57,82), and indirect techniques do so in patients with plasma [HCO3 −] within normal ranges (38,56,58,83). Acid accumulation can be estimated in patients with plasma [HCO3 −] within normal ranges by comparing the observed to expected plasma [HCO3 −] increase in response to retained HCO3 − (administered minus excreted) after an oral NaHCO3 bolus (0.5 mmol/kg body wt), assuming 50% body wt HCO3 apparent space of distribution (38,83). This procedure is invasive and time-consuming, making it unsuitable for clinical settings.
Rather than measuring acid accumulation directly, identifying its surrogates appears to be a more attractive option. Compromised urine NAE in the setting of high-acid diets might indicate increase risk for acid accumulation, particularly in patients with reduced eGFR. Low urine NH4 + excretion in patients with CKD and reduced eGFR, possibly a reflection of suboptimal urine NAE, is associated with adverse kidney outcomes (42,84), and so its measurement might identify patients at risk for eubicarbonatemic acidosis. Although currently not routinely available in clinical laboratories, advances in urine NH4 + measurement might identify individuals with low NAE in the setting of reduced eGFR with normal plasma [HCO3 −] that increases risk for acid accumulation. On the other hand, clinicians might identify underlying acid accumulation by assessing compensatory mechanisms that help maintain acid-base status, including urine citrate excretion (85). Low levels of timed urine citrate excretion indicated eubicarbonatemic acidosis in patients with CKD (7,38), their response to treatment with either mineral alkali (7) or base-producing food components (38), and indicated changes in acid accumulation over time (7). Other studies support the utility of the more clinically practical citrate-to-creatinine ratio in a “spot” urine specimen to identify patients with underlying acid accumulation despite having normal plasma [HCO3 −] (85). Further studies will determine clinically useful indicators to identify individuals with eubicarbonatemic acidosis.
Future Research Considerations
Data supporting the contribution of high-acid Western diets to kidney and other organ injury highlight the need for noninvasive methods to detect lower, yet injurious, levels of acid accumulation that are earlier in the acid stress continuum than metabolic acidosis. Ongoing research will help develop noninvasive surrogates to identify patients with CKD and compromised NAE, possibly through reduced urine NH4 +, indicating increased risk for injurious acid accumulation. Research to assess engagement of compensatory mechanisms to protect against acid accumulation, like reduced urine citrate excretion, will help determine if this strategy reliably identifies patients with injurious acid accumulation not evident by plasma acid-base parameters. Such diagnostic tools will facilitate large-scale interventional studies to determine if treatment of earlier stages of the acid stress continuum, by practitioner-recommended reductions of dietary acid and/or neutralization of accumulated acid, is kidney- and other organ-protective. Such studies will also help to determine the population benefit(s) of public health strategies to implement lower-acid diets.
Disclosures
D.E. Wesson reports employment with Baylor Scott and White Health; serving as a paid consultant of Tricida, Inc.; serving on the American Journal of Nephrology Editorial Board, CJASN Editorial Board, Journal of Renal Nutrition Editorial Board, and Kidney International Editorial Board; and serving as a Deputy Editor of JASN.
Funding
This work was supported in part by the National Institutes of Health grant R21DK113440 (D.E. Wesson, Principal Investigator).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Alpern RJ: Trade-offs in the adaptation to acidosis. Kidney Int 47: 1205–1215, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Alpern RJ, Sakhaee K: The clinical spectrum of chronic metabolic acidosis: Homeostatic mechanisms produce significant morbidity. Am J Kidney Dis 29: 291–302, 1997 [DOI] [PubMed] [Google Scholar]
- 3.DuBose TD Jr.: Urine ammonium and preclinical acidosis in CKD. J Am Soc Nephrol 28: 2258–2260, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raphael KL: Metabolic acidosis and subclinical metabolic acidosis in CKD. J Am Soc Nephrol 29: 376–382, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haghighatdoost F, Sadeghian R, Clark CCT, Abbasi B: Higher dietary acid load is associated with an increased risk of calcium oxalate kidney stones [published online ahead of print September 24, 2020]. J Ren Nutr 10.1053/j.jrn.2020.08012 [DOI] [PubMed] [Google Scholar]
- 6.Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE: Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int 78: 303–309, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Goraya N, Simoni J, Sager LN, Mamun A, Madias NE, Wesson DE: Urine citrate excretion identifies changes in acid retention as eGFR declines in patients with chronic kidney disease. Am J Physiol Renal Physiol 317: F502–F511, 2019. 31215805 [DOI] [PMC free article] [PubMed]
- 8.Banerjee T, Tucker K, Griswold M, Wyatt SB, Harman J, Young B, Taylor H, Powe NR: Dietary potential renal acid load and risk of albuminuria and reduced kidney function in the Jackson Heart study. J Ren Nutr 28: 251–258, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Banerjee T, Crews DC, Wesson DE, Tilea A, Saran R, Rios Burrows N, Williams DE, Powe NR; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team: Dietary acid load and chronic kidney disease among adults in the United States. BMC Nephrol 15: 137–148, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rebholz CM, Coresh J, Grams ME, Steffen LM, Anderson CAM, Appel LJ, Crews DC: Dietary acid load and incident chronic kidney disease: Results from the ARIC study. Am J Nephrol 42: 427–435, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemann J Jr., Litzow JR, Lennon EJ: The effects of chronic acid loads in normal man: Further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest 45: 1608–1614, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemann J Jr., Bushinsky DA, Hamm LL: Bone buffering of acid and base in humans. Am J Physiol Renal Physiol 285: F811–F832, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Kraut JA, Lew V, Madias NE: Re-evaluation of total CO2 concentration in apparently healthy younger adults. Am J Nephrol 48: 15–20, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapter 3. Management of progression and complications of CKD. Kid Int Suppl 3: 73–90, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remer T, Manz F: Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc 95: 791–797, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Relman AS, Lennon EJ, Lemann J Jr.: Endogenous production of fixed acid and the measurement of the net balance of acid in normal subjects. J Clin Invest 40: 1621–1630, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scialla JJ, Anderson CA: Dietary acid load: A novel nutritional target in chronic kidney disease? Adv Chronic Kidney Dis 20: 141–149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remer T: Influence of nutrition on acid-base balance--Metabolic aspects. Eur J Nutr 40: 214–220, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Frassetto LA, Morris RC Jr., Sebastian A: Dietary sodium chloride intake independently predicts the degree of hyperchloremic metabolic acidosis in healthy humans consuming a net acid-producing diet. Am J Physiol Renal Physiol 293: F521–F525, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Buehlmeier J, Remer T, Frings-Meuthen P, Maser-Gluth C, Heer M: Glucocorticoid activity and metabolism with NaCl-induced low-grade metabolic acidosis and oral alkalization: Results of two randomized controlled trials. Endocrine 52: 139–147, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Rhodes D, Clemens J, Goldman J, LaComb R, Moshfegh A: What we eat in America. NHANES 2009–2010, Tables 1–36, Food Surveys Research Group, 2015. Available at: https://www.ars.usda.gov/.../tables_1-40_2009-2010.pdf [Google Scholar]
- 22.US Department of Health and Human Services and US Department of Agriculture : 2015–2020 Dietary Guidelines for Americans. 8th Ed., 2015. Available at: https://health.gov/our-work/food-and-nutrition/2015-2020-dietary-guidelines/. Accessed July 28, 2020
- 23.Goraya N, Simoni J, Jo C-H, Wesson DE: Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int 86: 1031–1038, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Khatri M, Moon YP, Scarmeas N, Gu Y, Gardener H, Cheung K, Wright CB, Sacco RL, Nickolas TL, Elkind MS: The association between a Mediterranean-style diet and kidney function in the Northern Manhattan Study cohort. Clin J Am Soc Nephrol 9: 1868–1875, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee T, Crews DC, Tuot DS, Pavkov ME, Burrows NR, Stack AG, Saran R, Bragg-Gresham J, Powe NR; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team: Poor accordance to a DASH dietary pattern is associated with higher risk of ESRD among adults with moderate chronic kidney disease and hypertension. Kidney Int 95: 1433–1442, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chauveau P, Koppe L, Combe C, Lasseur C, Trolonge S, Aparicio M: Vegetarian diets and chronic kidney disease. Nephrol Dial Transplant 34: 199–207, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Wesson DE, Kitzman H, Montgomery A, Mamun A, Parnell W, Vilayvanh B, Tecson KM, Allison P: A population health dietary intervention for African American adults with chronic kidney disease: The Fruit and Veggies for Kidney Health randomized study. Contemp Clin Trials Commun 17: 100540, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtz I, Maher T, Hulter HN, Schambelan M, Sebastian A: Effect of diet on plasma acid-base composition in normal humans. Kidney Int 24: 670–680, 1983 [DOI] [PubMed] [Google Scholar]
- 29.Schwartz WB, Jenson RL, Relman AS: The disposition of acid administered to sodium-depleted subjects: The renal response and the role of the whole body buffers. J Clin Invest 33: 587–597, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swan RC, Pitts RF: Neutralization of infused acid by nephrectomized dogs. J Clin Invest 34: 205–212, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bandyopadhyay U, Das D, Banerjee RK: Reactive oxygen species: Oxidative damage and pathogenesis. Curr Sci 77: 658–666, 1999 [Google Scholar]
- 32.Wesson DE: Dietary acid increases blood and renal cortical acid content in rats. Am J Physiol 274: F97–F103, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Brennan TS, Klahr S, Hamm LL: Citrate transport in rabbit nephron. Am J Physiol 251: F683–F689, 1986 [DOI] [PubMed] [Google Scholar]
- 34.Wright SH, Kippen I, Wright EM: Effect of pH on the transport of Krebs cycle intermediates in renal brush border membranes. Biochim Biophys Acta 684: 287–290, 1982 [DOI] [PubMed] [Google Scholar]
- 35.Brennan S, Hering-Smith K, Hamm LL: Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am J Physiol 255: F301–F306, 1988 [DOI] [PubMed] [Google Scholar]
- 36.Hamm LL, Simon EE: Roles and mechanisms of urinary buffer excretion. Am J Physiol 253: F595–F605, 1987 [DOI] [PubMed] [Google Scholar]
- 37.Nicar MJ, Skurla C, Sakhaee K, Pak CYC: Low urinary citrate excretion in nephrolithiasis. Urology 21: 8–14, 1983 [DOI] [PubMed] [Google Scholar]
- 38.Goraya N, Simoni J, Pruszynski J, Madias NE, Wesson DE: Urine citrate excretion as a marker of acid retention in patients without overt metabolic acidosis. Kidney Int 95: 1190–1196, 2019 [DOI] [PubMed] [Google Scholar]
- 39.Hood VL: pH regulation of endogenous acid production in subjects with chronic ketoacidosis. Am J Physiol 249: F220–F226, 1985 [DOI] [PubMed] [Google Scholar]
- 40.Breslau NA, Brinkley L, Hill KD, Pak CY: Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab 66: 140–146, 1988 [DOI] [PubMed] [Google Scholar]
- 41.Nath KA, Hostetter MK, Hostetter TH: Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest 76: 667–675, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raphael KL, Carroll DJ, Murray J, Greene T, Beddhu S: Urine ammonium predicts clinical outcomes in hypertensive kidney disease. J Am Soc Nephrol 28: 2483–2490, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raphael KL, Gilligan S, Hostetter TH, Greene T, Beddhu S: Association between urine ammonium and urine TGF-β1 in CKD. Clin J Am Soc Nephrol 13: 223–230, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wesson DE: Endogenous endothelins mediate increased distal tubule acidification induced by dietary acid in rats. J Clin Invest 99: 2203–2211, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khanna A, Simoni J, Hacker C, Duran M-J, Wesson DE: Increased endothelin activity mediates augmented distal nephron acidification induced by dietary protein. J Am Soc Nephrol 15: 2266–2275, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Khanna A, Simoni J, Wesson DE: Endothelin-induced increased aldosterone activity mediates augmented distal nephron acidification as a result of dietary protein. J Am Soc Nephrol 16: 1929–1935, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Wesson DE, Jo C-H, Simoni J: Angiotensin II receptors mediate increased distal nephron acidification caused by acid retention. Kidney Int 82: 1184–1194, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Wesson DE, Jo C-H, Simoni J: Angiotensin II-mediated GFR decline in subtotal nephrectomy is due to acid retention associated with reduced GFR. Nephrol Dial Transplant 30: 762–770, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Wesson DE, Simoni J: Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int 78: 1128–1135, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Ruíz-Ortega M, Gómez-Garre D, Alcázar R, Palacios I, Bustos C, González S, Plaza JJ, González E, Egido J: Involvement of angiotensin II and endothelin in matrix protein production and renal sclerosis. J Hypertens Suppl 12: S51–S58, 1994 [PubMed] [Google Scholar]
- 51.Greene EL, Kren S, Hostetter TH: Role of aldosterone in the remnant kidney model in the rat. J Clin Invest 98: 1063–1068, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wesson DE, Nathan T, Rose T, Simoni J, Tran RM: Dietary protein induces endothelin-mediated kidney injury through enhanced intrinsic acid production. Kidney Int 71: 210–217, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Remuzzi G, Bertani T: Pathophysiology of progressive nephropathies. N Engl J Med 339: 1448–1456, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Wesson DE, Simoni J: Increased tissue acid mediates progressive decline in glomerular filtration rate of animals with reduced nephron mass. Kidney Int 75: 929–935, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Wesson DE, Simoni J, Prabhakar S: Endothelin-induced increased nitric oxide mediates augmented distal nephron acidification as a result of dietary protein. J Am Soc Nephrol 17: 406–413, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Wesson DE, Simoni J, Broglio K, Sheather S: Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol 300: F830–F837, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Wesson DE, Pruszynski J, Cai W, Simoni J: Acid retention with reduced glomerular filtration rate increases urine biomarkers of kidney and bone injury. Kidney Int 91: 914–927, 2017 [DOI] [PubMed] [Google Scholar]
- 58.Goraya N, Simoni J, Sager LN, Pruszynski J, Wesson DE: Acid retention in chronic kidney disease is inversely related to GFR. Am J Physiol Renal Physiol 314: F985–F991, 2018 [DOI] [PubMed] [Google Scholar]
- 59.Banerjee T, Crews DC, Wesson DE, McCulloch C, Johansen K, Sayhad S, Burrows NR, Saran R, Gillespie B, Bragg-Gresham J, Powe NR: Elevated serum anion gap in adults with moderate chronic kidney disease increases risk for progression to end-stage renal disease. Am J Physiol Renal Physiol 316: F1244–F1253, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adeva MM, Souto G: Diet-induced metabolic acidosis. Clin Nutr 30: 416–421, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Xu H, Åkesson A, Orsini N, Håkansson N, Wolk A, Carrero JJ: Modest U-shaped association between dietary acid load and risk of all-cause and cardiovascular mortality in adults. J Nutr 146: 1580–1585, 2016 [DOI] [PubMed] [Google Scholar]
- 62.Akter S, Nanri A, Mizoue T, Noda M, Sawada N, Sasazuki S, Tsugane S; Japan Public Health Center–based Prospective Study Group: Dietary acid load and mortality among Japanese men and women: The Japan public health center-based prospective study. Am J Clin Nutr 106: 146–154, 2017 [DOI] [PubMed] [Google Scholar]
- 63.Williams RS, Heilbronn LK, Chen DL, Coster ACF, Greenfield JR, Samocha-Bonet D: Dietary acid load, metabolic acidosis and insulin resistance - Lessons from cross-sectional and overfeeding studies in humans. Clin Nutr 35: 1084–1090, 2016 [DOI] [PubMed] [Google Scholar]
- 64.Williams RS, Kozan P, Samocha-Bonet D: The role of dietary acid load and mild metabolic acidosis in insulin resistance in humans. Biochimie 124: 171–177, 2016 [DOI] [PubMed] [Google Scholar]
- 65.Fagherazzi G, Vilier A, Bonnet F, Lajous M, Balkau B, Boutron-Rualt M-C, Clavel-Chapelon F: Dietary acid load and risk of type 2 diabetes: The E3N-EPIC cohort study. Diabetologia 57: 313–320, 2014 [DOI] [PubMed] [Google Scholar]
- 66.Akter S, Kurotani K, Kashino I, Goto A, Mizoue T, Noda M, Sawada N, Tsugane S; Japan Public Health Center–based Prospective Study Group: High dietary acid load score is associated with increased risk of type 2 diabetes in Japanese men: The Japan public health center-based prospective study. J Nutr 146: 1076–1083, 2016 [DOI] [PubMed] [Google Scholar]
- 67.Murakami K, Sasaki S, Takahashi Y, Uenishi K; Japan Dietetic Students’ Study for Nutrition and Biomarkers Group: Association between dietary acid-base load and cardiometabolic risk factors in young Japanese women. Br J Nutr 100: 642–651, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Zhang L, Curhan GC, Forman JP: Diet-dependent net acid load and risk of incident hypertension in United States women. Hypertension 54: 751–755, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alexy U, Remer T, Manz F, Neu CM, Schoenau E: Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am J Clin Nutr 82: 1107–1114, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Remer T, Manz F, Alexy U, Schoenau E, Wudy SA, Shi L: Long-term high urinary potential renal acid load and low nitrogen excretion predict reduced diaphyseal bone mass and bone size in children. J Clin Endocrinol Metab 96: 2861–2868, 2011 [DOI] [PubMed] [Google Scholar]
- 71.Chan R, Leung J, Woo J: Association between estimated net endogenous acid production and subsequent decline in muscle mass over four years in ambulatory older Chinese people in Hong Kong: A prospective cohort study. J Gerontol A Biol Sci Med Sci 70: 905–911, 2015 [DOI] [PubMed] [Google Scholar]
- 72.Welch AA, MacGregor AJ, Skinner J, Spector TD, Moayyeri A, Cassidy A: A higher alkaline dietary load is associated with greater indexes of skeletal muscle mass in women. Osteoporos Int 24: 1899–1908, 2013 [DOI] [PubMed] [Google Scholar]
- 73.Dong L, Li Z, Leffler NR, Asch AS, Chi J-T, Yang LV: Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLoS One 8: e61991, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murphy AM, Wong AL, Bezuhly M: Modulation of angiotensin II signaling in the prevention of fibrosis. Fibrogenesis Tissue Repair 8: 7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Frassetto L, Morris RC Jr., Sebastian A: Potassium bicarbonate reduces urinary nitrogen excretion in postmenopausal women. J Clin Endocrinol Metab 82: 254–259, 1997 [DOI] [PubMed] [Google Scholar]
- 77.Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, Wesson DE: Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int 77: 617–623, 2010 [DOI] [PubMed] [Google Scholar]
- 78.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Di Iorio BR, Bellasi A, Raphael KL, Santoro D, Aucella F, Garofano L, Ceccarelli M, Di Lullo L, Capolongo G, Di Iorio M, Guastaferro P, Capasso G; UBI Study Group: Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: The UBI study. J Nephrol 32: 989–1001, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Banerjee T, Crews DC, Wesson DE, Tilea AM, Saran R, Ríos-Burrows N, Williams DE, Powe NR; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team: High dietary acid load predicts ESRD among adults with CKD. J Am Soc Nephrol 26: 1693–1700, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goraya N, Simoni J, Jo C-H, Wesson DE: Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int 81: 86–93, 2012 [DOI] [PubMed] [Google Scholar]
- 82.Siragy HM, Ibrahim MM, Jaffa AA, Mayfield R, Margolius HS: Rat renal interstitial bradykinin, prostaglandin E2, and cyclic guanosine 3′,5′-monophosphate. Effects of altered sodium intake. Hypertension 23: 1068–1070, 1994 [DOI] [PubMed] [Google Scholar]
- 83.Wesson DE: Assessing acid retention in humans. Am J Physiol Renal Physiol 301: F1140–F1142, 2011 [DOI] [PubMed] [Google Scholar]
- 84.Vallet M, Metzger M, Haymann J-P, Flamant M, Gauci C, Thervet E, Boffa JJ, Vrtovsnik F, Froissart M, Stengel B, Houillier P; NephroTest Cohort Study group: Urinary ammonia and long-term outcomes in chronic kidney disease. Kidney Int 88: 137–145, 2015 [DOI] [PubMed] [Google Scholar]
- 85.Gianella FG, Prado VE, Poindexter JR, Adams-Huet B, Li X, Miller RT, Sakhaee K, Maalouf NM, Moe OW: Spot urinary citrate-to-creatinine ratio is a marker for acid-base status in chronic kidney disease. Kidney Int 99: 208–217, 2021 [DOI] [PubMed] [Google Scholar]
- 86.Goraya N, Wesson DE: Management of the metabolic acidosis of chronic kidney disease. Adv Chronic Kidney Dis 24: 298–304, 2017 [DOI] [PubMed] [Google Scholar]