Visual Abstract
Keywords: nutrition, dialysis, nutritional status, maintenance hemodialysis
Abstract
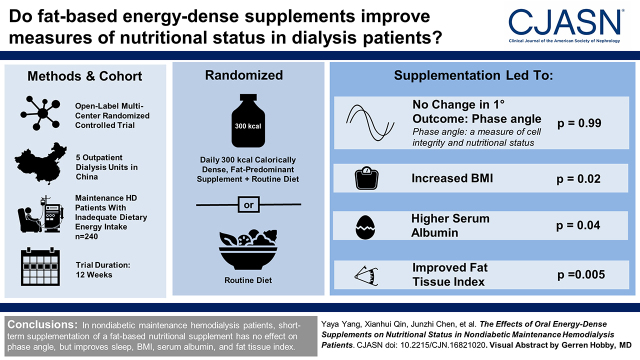
Background and objectives
Fat-based energy-dense nutritional supplements may offer benefits over protein- or carbohydrate-dense supplements for patients receiving dialysis because of the adverse metabolic consequences of the latter. We conducted a randomized controlled trial to assess the effects of the short-term use of a fat-based nutritional supplement on various measures of nutritional status in patients receiving maintenance hemodialysis who have low dietary energy intake.
Design, setting, participants, & measurements
We enrolled nondiabetic patients receiving hemodialysis for >3 months who had inadequate dietary energy intake (<30 kcal/kg per day). The participants were randomly assigned in a 1:1 ratio to receive an oral fat-based energy-dense supplement (300 kcal daily) or routine care for 12 weeks (n=120 per group). The primary outcome was the change in phase angle measured by bioelectrical impedance analysis, a marker of cell integrity and body cell mass, from the baseline to week 12. The secondary outcomes were changes in quality of life. Other outcomes included laboratory nutritional indicators and physical examinations.
Results
The average age of the total population was 47 (SD: 12) years, and 55% were men. The median of dialysis vintage was 43.4 (22.5–76.3) months; 240 participants were randomly assigned to the intervention (n=120) or control group (n=120). In total, 228 (95%) participants completed the trial. The change in phase angle did not differ significantly between the intervention and control groups (estimate, 0.0; 95% confidence interval, −0.1 to 0.1 versus estimate, 0.0; 95% confidence interval, −0.1 to 0.1; estimated difference, 0.0; 95% confidence interval −0.2 to 0.2; P=0.99). None of the 19 domains of quality of life differed between the groups. Adverse events were reported in 23 (19%) participants in the control group and 40 (33%) participants in the intervention group.
Conclusions
In nondiabetic patients on maintenance hemodialysis, short-term administration of fat-based energy-dense nutritional supplement has no clinically significant effect on nutritional status as measured by phase angle.
Podcast
This article contains a podcast at https://https://www.asn-online.org/media/podcast/CJASN/2021_08_03_CJN16821020.mp3
Introduction
Adequate dietary energy intake is necessary to maintain nitrogen balance and body composition in patients on maintenance hemodialysis (1,2). Low dietary energy intake (<30 kcal/kg per day) is associated with a higher risk of mortality (3). However, anorexia, comorbidities, and prescribed dietary restrictions make it challenging for patients on maintenance hemodialysis to achieve adequate energy intake, which contributes to poor nutritional status (2,4,5). The guidelines recommended nutritional interventions in patients on maintenance hemodialysis (2,6). However, evidence regarding the effects of oral nutritional supplements on the nutritional status of such patients is limited and inconsistent (7–12).
In most previous studies, the oral nutritional supplements that were used contained a mixture of nutrients or were amino acid/protein based. However, the strategies used to improve protein intake may also increase the intake of several potentially harmful substances, especially phosphate (4,13). Moreover, the consumption of high-protein supplements may lead to the accumulation of metabolic waste (urea and phosphate) and the acceleration of metabolic acidosis, resulting in the need for additional dialysis (14). A previous randomized controlled trial demonstrated that if energy intake is sufficient, a low-protein diet is nutritionally and metabolically safe for patients with kidney failure (15). In addition, the results of previous prospective cohort studies have suggested that oral fat-based supplements may be effective at preventing energy deficits (16,17). However, no previous studies have determined the effects of oral fat-based energy-dense supplements on the nutritional status of patients on maintenance hemodialysis.
Although there are a number of indicators of nutritional status, including serum albumin, body mass index (BMI), and grip strength, no single parameter can indicate the overall nutritional status of patients on maintenance hemodialysis (2). Recently, bioelectrical impedance analysis has become a commonly used means of estimating body composition and monitoring nutritional status (4,18,19). Resistance (R) and reactance (Xc), obtained from bioelectrical impedance analysis, were used to calculate the phase angle by the following equation: phase angle (°) = arctangent (Xc/R) ×(180/π). Although the biologic meaning of phase angle is not clearly understood, it is thought to be a measure of cell integrity and body cell mass, and it can reflect the nutritional status (20–22). Moreover, phase angle was found to be a predictor of survival in several clinical conditions, such as liver cirrhosis, cancer, and dialysis (22,23).
In this study, we aimed to determine the effects of an oral fat-based energy-dense supplement on various measures of nutritional status of nondiabetic patients on maintenance hemodialysis.
Materials and Methods
Trial Design
We performed an investigator-initiated, multicenter, open-label, randomized controlled trial in which we recruited patients at five outpatient hemodialysis centers in China between March and December 2018. All practices were performed according to the same standard protocol at each dialysis center. The trial was composed of a screening period and a 12-week randomized treatment period.
The study was approved by the Medical Ethics Committee of Nanfang Hospital, Southern Medical University, and written informed consent was obtained from all of the participants. The protocol is registered on the Chinese Clinical Trial Registry (www.chictr.org.cn; ChiCTR1800015068). This trial was conducted in accordance with the principles of the Declaration of Helsinki.
Participants, Randomization, and Intervention
Eligible patients were aged >18 years, had been undergoing maintenance hemodialysis for >3 months, had a normal oral intake, and had a dietary energy intake of <30 kcal/kg per day. Patients were excluded if they received nutritional supplements within the last 6 months or required enteral nutrition; were intolerant of oral nutritional supplementation; or had diabetes, hyperthyroidism, acute infection, liver cirrhosis, active autoimmune disease, multiple organ failure, serious gastrointestinal disease, or an advanced malignant tumor.
The participants were randomly assigned (1:1) to receive an oral energy-dense supplement or routine care using permuted blocks, with block sizes of four, stratified for centers. The allocation of the participants was performed via telephone by a research member using a computer-generated random number sequence. Blinding was done where research members involved in the data collection remained unaware of the group assignments.
The participants in the intervention group consumed 60 ml (300 kcal) of an oral supplement (Fresubin 5 kcal shot; Fresenius Kabi Deutschland, Bad Homburg, Germany) once per day after a meal at home for 12 weeks. The supplements were dispensed every 4 weeks. Adherence was verified by researchers during dialysis sessions, and the total amount of supplement consumed was checked by counting the returned empty cans. Fresubin is highly energy dense, and fat provides 97% of the total energy, of which the monounsaturated fatty acids provide 44% of energy and polyunsaturated fatty acids provide 23% of energy. We did not include patients with diabetes in this trial because the product manual for Fresubin states that excessive fat intake may impair insulin sensitivity and cause dyslipidemia in such individuals. Dietary advice was provided to both groups. For the control group, no nutritional supplementation was provided. The methods were not modified after the trial commenced.
Data Collection and Follow-Up Evaluation
Each participant was interviewed using a questionnaire that was designed specifically for this study. Study procedures consisting of body composition, quality of life, physical examinations, laboratory assays, and dietary intake were collected at baseline and after 12 weeks.
Body composition was determined using a Body Composition Monitor (Fresenius Medical Care, Bad Homburg, Germany) 20–30 minutes after a hemodialysis session. The nonfistula side arm was used for measurements in participants who had arteriovenous fistulas, and the dominant arm was used in participants who had a central venous catheter. The Body Composition Monitor describes body composition using a three-compartment model, providing a lean tissue index (kilograms per meter squared) and fat tissue index (kilograms per meter squared) and indicating overhydration. The hydration status can be calculated from the difference between the normal extracellular water expected and the measured extracellular water (24). The reference range for normal hydration provided by the Body Composition Monitor is ±1.1 L (25), and the 10th to 90th percentiles in an age- and sex-matched healthy population are regarded as the reference ranges for lean tissue index and fat tissue index (19,26). A previous cohort study conducted in patients on maintenance hemodialysis suggested that the ranges 15–20 and 4–15 kg/m2 appeared optimal for lean tissue index and fat tissue index, respectively (19). The R and Xc, measured at 50 kHz, were used to calculate the phase angle: phase angle (°) = arctangent (Xc/R) ×(180°/π) (20). The phase angle differs according to sex, age, and BMI. Therefore, the use of population-specific reference values for the phase angle is recommended (27–29). In healthy individuals, the phase angle is usually between 5° and 7° (20).
Quality of life was assessed using the Kidney Disease Quality of Life Short Form (30).
All anthropometric measurements were made postdialysis. Midarm muscle circumference was calculated as follows: midarm muscle circumference = midarm circumference − (3.1415× triceps skin fold). Grip strength was measured using an electronic hand dynamometer (Camry, Shanghai, China) for the dominant hand or in the nonfistula hand. Each measurement was repeated twice, and the higher value was recorded. The bioimpedance device and dynamometers were routinely calibrated during the trial.
Dietary intake was assessed using three 24-hour diet recalls (one dialysis day and two nondialysis days) using the Automated Multiple-Pass Method. The energy intake of all food and drink items was sourced using a computer-aided dietary software (Zhending, Shanghai, China; software version 2.0), in which nutrient models were on the basis of the Chinese Food Composition Table developed by the Chinese Center for Disease Control and Prevention in 2009.
Blood samples were obtained from each participant before a hemodialysis session to measure their serum albumin, prealbumin, transferrin, hemoglobin, calcium, phosphate, and lipid concentrations.
Study Outcomes
The primary outcome was the change in phase angle from baseline to week 12. Secondary outcomes included 19 domains of quality of life. The other outcomes were laboratory markers of nutritional status (albumin, prealbumin, transferrin, and hemoglobin concentrations), physical examination parameters (BMI, handgrip strength, and midarm muscle circumference), and body composition (lean tissue index and fat tissue index). An increase in fat tissue index of 1 kg/m2 was considered to be clinically significant (31).
Statistical Analyses
The sample size was calculated on the basis of the results from a previous trial (30) that demonstrated a 0.31° increase in phase angle following 12 weeks of oral supplements. On the basis of the previous study, we assumed a treatment group difference in phase angle of 0.3° following 12 weeks of follow-up, with an SD of 0.59, type 1 error rates of 5% and 80% power, a dropout rate of 20%, and a nonadherence rate of 15%; thus, a total sample size of 220 participants was required (R version 3.5.3). We did not have plans for interim analyses and termination of the study before the end of the trial.
The primary analytic approach was according to the intention-to-treat principle, including all participants who had undergone randomization. The missing data were handled with multiple imputations. The secondary analytic approaches included complete case and per protocol analyses. The complete case analyses were performed for participants for whom data were available at baseline and at the 12-week time point. Participants with an adherence of <80% were excluded from the per protocol analysis. Data are presented as means ± SDs, medians (quartiles 1–3), or counts (percentages). The two groups were compared at baseline and 12 weeks using the t test or the Wilcoxon–Mann–Whitney test with respect to continuous data and using the chi-squared test with respect to categorical data. The changes between the treatment groups were compared and tested using the Wilcoxon–Mann–Whitney test. The effects of the supplement on outcomes were also assessed using linear mixed effects models.
A two-tailed P=0.05 was considered statistically significant in all analyses. The secondary and other outcomes were regarded as exploratory. We did not account for multiple testing. All of the analyses were performed using R (http://www.R-project.org; version 3.5.3).
Results
Study Participants and Baseline Characteristics
Of the 1078 patients screened, 240 participants were randomly assigned to the intervention (n=120) or control group (n=120). In total, 112 (93%) participants in the control group and 116 (97%) participants in the intervention group completed the week 12 testing and were included in the complete case analyses (Figure 1).
Figure 1.
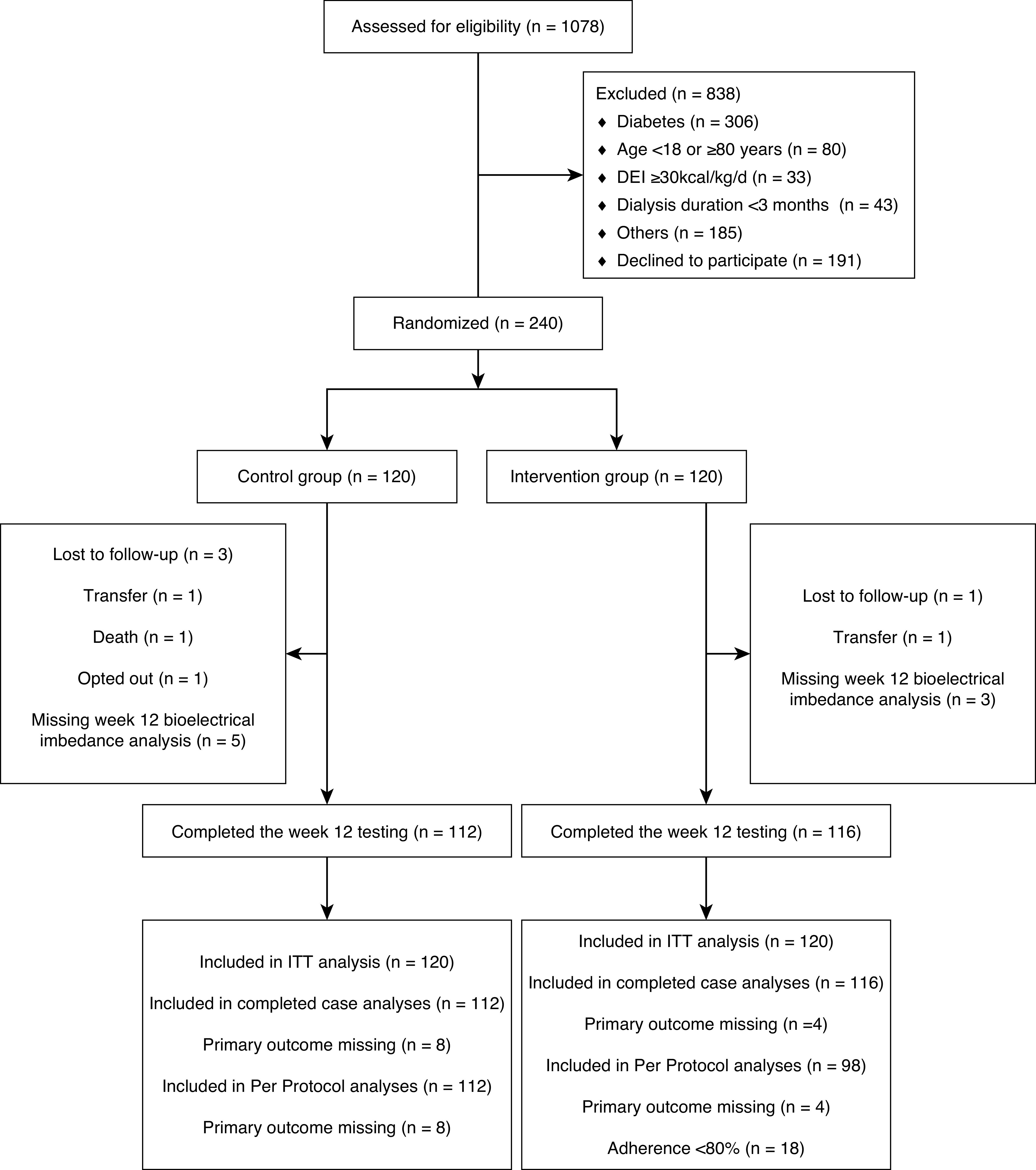
CONSORT trial flow diagram. CONSORT, Consolidated Standards of Reporting Trials; DEI, dietary energy intake; ITT, intention to treat.
The characteristics of the participants in the two groups were well balanced at baseline; there were no differences between the groups, except with respect to BMI (P=0.02) (Table 1).
Table 1.
Baseline characteristics of the study participants
| Variables | Total | Control | Intervention |
|---|---|---|---|
| N | 240 | 120 | 120 |
| Demographics | |||
| Age, yr | 47±12 | 47±13 | 46±11 |
| Men, N (%) | 131 (55) | 67 (56) | 64 (53) |
| Current smoking, N (%) | 39 (16) | 21 (18) | 18 (15) |
| Current alcohol drinking, N (%) | 14 (6) | 9 (8) | 5 (4) |
| Comorbidities | |||
| Hypertension, N (%) | 151 (63) | 78 (65) | 73 (61) |
| Cardiovascular disease, N (%) | 14 (6) | 6 (5) | 8 (7) |
| Dialysis vintage, mo | 43 (23–76) | 43 (20–71) | 47 (24–87) |
| Kt/Va | 1.39±0.35 | 1.34±0.32 | 1.44±0.36 |
| Phase angle, °b | 6.1±1.1 | 6.1±1.0 | 6.1±1.1 |
| Physical examination | |||
| BMI, kg/m2 | 20.5±2.8 | 20.9±3 | 20.0±2.5 |
| Handgrip strength, kg | 29±11 | 29±10 | 29±11 |
| Midarm muscle circumference, cm | 23±3 | 23±3 | 23±3 |
| Laboratory variables | |||
| Albumin, g/dl | 4.0±0.3 | 4.1±0.3 | 4.0±0.3 |
| Prealbumin, mg/dl | 35±12 | 35±12 | 35±12 |
| Transferrin, mg/dl | 172±42 | 175±45 | 169±38 |
| Triglyceride, mg/dl | 160±127 | 153±117 | 167±136 |
| Total cholesterol, mg/dl | 160±38 | 161±41 | 159±34 |
| HDL, mg/dl | 40±11 | 40±10 | 40±11 |
| LDL, mg/dl | 92±29 | 93±31 | 91±27 |
| BUN, mg/dl | 74±19 | 74±18 | 75±19 |
| Hemoglobin, g/dl | 11.1±1.7 | 11.2±1.6 | 11.0±1.7 |
| Phosphate, mg/dl | 6.9±2.1 | 6.7±2.3 | 7.0±1.9 |
| CRP, mg/dl | 0.2 (0.1–0.4) | 0.2 (0.1–0.4) | 0.2 (0.1–0.4) |
| White blood cell, 103/μl | 6.1±1.6 | 6.1±1.7 | 6.1±1.6 |
| Dietary parameters | |||
| Dietary energy intake, kcal/IBW per d | 21.5±4.9 | 20.9±4.9 | 22.1±4.9 |
| Dietary protein intake, g/IBW per d | 0.9±0.3 | 0.8±0.3 | 0.9±0.3 |
Continuous variables are presented as mean (SD) or median (interquartile range); categorical variables are presented as number (percentage). BMI, body mass index; CRP, C-reactive protein; IBW, ideal body weight.
Kt shows effective urea clearance and duration of dialysis, and V represents the volume of distribution of urea in the body calculated as Kt/V=−ln(post-BUN/pre-BUN–0.008×t)+(4–3.5×post-BUN/pre-BUN) × UF/postweight, where t is effective dialysis time and UF is ultrafiltration.
In healthy individuals, the phase angle is usually between 5° and 7°.
Treatment Adherence
Throughout the study period, the mean treatment adherence was 88% in the intervention group. The reasons for nonadherence to the treatment included adverse events and dislike of the taste of the supplement in some of the participants.
Dietary Intake
After 12 weeks, the mean energy intakes from food in the control and intervention groups were 23.0±6.5 and 23.2±6.1 kcal/kg per day, respectively. The additional energy intake provided by the oral nutritional supplement was 4.9±1.4 kcal/kg per day. Participants in the intervention group achieved a higher energy intake than those in the control group (28.2±6.3 versus 23.0±6.5 kcal/kg per day; P<0.001).
Efficacy of Oral Energy-Dense Supplement on the Study Outcomes
Using the intention-to-treat set, there was no significant difference in phase angle between baseline and 12 weeks in either the control (estimate, 0.0; 95% confidence interval [95% CI], −0.1 to 0.1) or intervention group (estimate, 0.0; 95% CI, −0.1 to 0.1) and no difference in the change in phase angle from baseline to 12 weeks between groups (estimated difference, 0.0; 95% CI, −0.2 to 0.2; P=0.99). Similar results were obtained using complete case analysis and the per protocol analysis (Table 2, Supplemental Table 1).
Table 2.
Effects of the 12-week energy supplementation on primary outcome and other outcomes
| Variables | Control | Intervention | Group Difference Mean (95% Confidence Interval) | P Value |
|---|---|---|---|---|
| Primary study outcome, phase angle, mean ± SD, ° | ||||
| Baseline | 6.2±1.0 | 6.1±1.1 | 0.0 (–0.3 to 0.2) | — |
| 12 wk | 6.2±1.0 | 6.1±1.1 | 0.0 (–0.3 to 0.2) | — |
| Change (complete case analysis)a | 0.0±0.5 | 0.0±0.6 | 0.0 (–0.1 to 0.1) | 0.82 |
| Change (intention-to-treat analysis)b | 0.0 (–0.1, 0.1) | 0.0 (–0.1, 0.1) | 0.0 (–0.2 to 0.2) | 0.99 |
| Physical examination | ||||
| BMI, mean ± SD, kg/m2 | ||||
| Baseline | 20.9±2.9 | 20.0±2.5 | −0.9 (–1.6 to –0.2) | — |
| 12 wk | 20.9±3 | 20.1±2.5 | −0.7 (–1.4 to 0) | — |
| Change (complete case analysis) a | 0.0±0.5 | 0.1±0.6 | 0.2 (0.0 to 0.3) | 0.01 |
| Change (intention-to-treat analysis) b | −0.1 (–0.2, 0.1) | 0.1 (0, 0.2) | 0.2 (0.0 to 0.4) | 0.02 |
| Handgrip strength, mean ± SD, kg | ||||
| Baseline | 29.7±10.4 | 28.9±10.7 | −0.8 (–3.5 to 1.9) | — |
| 12 wk | 29.9±10.0 | 29.9±11.0 | 0.1 (–2.7 to 2.8) | — |
| Change (complete case analysis) a | 0.2±5.3 | 0.8±5.6 | 0.6 (–0.8 to 2.1) | 0.45 |
| Change (intention-to-treat analysis) b | 0.5 (–0.5, 1.6) | 0.9 (–0.1, 2.0) | 0.5 (–0.9 to 1.9) | 0.46 |
| Midarm muscle circumference, mean ± SD, cm | ||||
| Baseline | 23.3±2.6 | 22.9±2.5 | −0.4 (–1.0 to 0.3) | — |
| 12 wk | 23.4±2.5 | 23.0±2.5 | −0.3 (–1.0 to 0.3) | — |
| Change (complete case analysis) a | 0.1±1.0 | 0.2±1.2 | 0.1 (–0.2 to 0.4) | 0.24 |
| Change (intention-to-treat analysis) b | 0.2 (–0.0, 0.4) | 0.2 (–0.1, 0.4) | 0.0 (–0.3 to 0.3) | 0.85 |
| Laboratory variables | ||||
| Albumin, mean ±S D, g/dl | ||||
| Baseline | 4.1±0.3 | 4.0±0.3 | −0.1 (–0.2 to 0) | — |
| 12 wk | 4.0±0.3 | 4.0±0.3 | 0 (–0.1 to 0.1) | — |
| Change (complete case analysis) a | −0.1±0.3 | 0±0.3 | 0.1 (0 to 0.2) | 0.008 |
| Change (intention-to-treat analysis) b | −0.1 (–0.1, –0.0) | 0.0 (–0.0, 0.1) | 0.1 (0 to 0.2) | 0.04 |
| Prealbumin, mean ± SD, mg/dl | ||||
| Baseline | 35.4±10.9 | 35.2±12.3 | −0.3 (–3.3 to 2.7) | — |
| 12 wk | 34.8±11.3 | 34.6±12.2 | −0.2 (–3.2 to 2.9) | — |
| Change (complete case analysis) a | −0.8±8.1 | −0.6±4.8 | 0.2 (–1.5 to 1.9) | 0.47 |
| Change (intention-to-treat analysis) b | −0.1 (–1.5, 1.2) | −0.5 (–1.8, 0.9) | −0.3 (–2.2 to 1.6) | 0.73 |
| Transferrin, mean ± SD, mg/dl | ||||
| Baseline | 174.6±45.1 | 168.9±38 | −5.6 (–16.4 to 5.1) | — |
| 12 wk | 172.4±43.3 | 170.7±42.6 | −1.7 (–12.8 to 9.4) | — |
| Change (complete case analysis) a | −2.6±27.1 | 1.2±27 | 3.8 (–3.3 to 10.8) | 0.80 |
| Change (intention-to-treat analysis) b | −4.8 (–10.2, 0.7) | 1.4 (–4.0, 6.8) | 6.2 (–1.5 to 13.9) | 0.11 |
| Hemoglobin, mean ± SD, g/dl | ||||
| Baseline | 11.2±1.6 | 11.1±1.7 | −0.1 (–0.5 to 0.3) | — |
| 12 wk | 11.2±1.7 | 11.3±1.6 | 0.1 (–0.3 to 0.5) | — |
| Change (complete case analysis) a | 0±1.9 | 0.2±1.9 | 0.2 (–0.3 to 0.7) | 0.51 |
| Change (intention-to-treat analysis) b | 0.0 (–0.3, 0.4) | 0.3 (–0.1, 0.6) | 0.2 (–0.3 to 0.7) | 0.36 |
| Bioimpedance measurements c | ||||
| Lean tissue index, mean ± SD, kg/m2 | ||||
| Baseline | 14.1±2.5 | 13.9±2.7 | −0.2 (–0.9 to 0.5) | — |
| 12 wk | 14.1±2.5 | 13.5±2.6 | −0.6 (–1.2 to 0.1) | — |
| Change (complete case analysis) a | −0.1±1.1 | −0.4±1.1 | −0.3 (–0.6 to 0) | 0.08 |
| Change (intention-to-treat analysis) b | −0.1 (–0.4, 0.1) | −0.4 (–0.7, –0.1) | −0.3 (–0.6 to 0.1) | 0.15 |
| Fat tissue index, mean ± SD, kg/m2 | ||||
| Baseline | 7±3.5 | 6.5±3.1 | −0.5 (–1.3 to 0.4) | — |
| 12 wk | 7.2±3.3 | 7.1±3.3 | −0.1 (–0.9 to 0.8) | — |
| Change (complete case analysis) a | 0.1±1.1 | 0.6±1.1 | 0.5 (0.2 to 0.8) | <0.001 |
| Change (intention-to-treat analysis) b | 0.1 (–0.2, 0.4) | 0.7 (0.4, 0.9) | 0.5 (0.2 to 0.9) | 0.005 |
| Overhydration,d mean ± SD, L | ||||
| Baseline | −0.9±1.3 | −1.0±1.3 | 0 (–0.4 to 0.3) | — |
| 12 wk | −1.1±1.2 | −1.2±1.2 | −0.1 (–0.4 to 0.2) | — |
| Change (complete case analysis) a | −0.1±0.8 | −0.2±1.1 | −0.1 (–0.3 to 0.2) | 0.51 |
| Change (intention-to-treat analysis) b | −0.1 (–0.3, 0.1) | −0.2 (–0.4, 0.0) | −0.0 (–0.4 to 0.3) | 0.73 |
The 10th to 90th percentiles in an age- and sex-matched healthy population are regarded as the reference ranges for lean tissue index and fat tissue index. The intention-to-treat analyses included 240 participants; the complete case analyses included 228 participants. BMI, body mass index.
Change was defined as the value at week 12 minus the value at baseline; difference in the change between groups was compared and tested using the Wilcoxon–Mann–Whitney test.
From linear mixed models.
The Body Composition Monitor describes body composition using a three-compartment model, providing a lean tissue index (kilograms per meter squared) and a fat tissue index (kilograms per meter squared) and indicating overhydration.
The hydration status can be calculated from the difference between the normal extracellular water expected and the measured extracellular water. Normal hydration: ±1.1 L; overhydration: >1.1 L; underhydration: <−1.1 L.
There were also no differences in secondary outcomes of 19 domains of quality of life (Table 3).
Table 3.
The quality of life at baseline and after intervention by the Kidney Disease Quality of Life Short Form
| Variables | Control | Intervention | Group Difference Mean (95% Confidence Interval)b | P Valueb | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 wk | Changea | Baseline | 12 wk | Changea | |||
| Specific domain | ||||||||
| Symptoms | 79±13 | 77±16 | −2±13 | 77±16 | 77±16 | 0±15 | 2 (–2 to 6) | 0.32 |
| Effects of kidney disease | 58±21 | 57±22 | −1±18 | 55±21 | 57±22 | 2±19 | 1 (–3 to 6) | 0.58 |
| Burden of kidney disease | 24±22 | 25±24 | 1±20 | 24±23 | 27±26 | 3±18 | 1 (–4 to 6) | 0.59 |
| Work status | 53±28 | 48±32 | −4±35 | 49±28 | 49±31 | 1±36 | 6 (–3 to 15) | 0.18 |
| Cognitive function | 70±23 | 68±23 | −2±22 | 72±19 | 73±19 | 1±21 | 2 (–4 to 8) | 0.51 |
| Quality of social interaction | 70±21 | 67±21 | −3±21 | 70±20 | 68±20 | −2±21 | 0 (–6 to 5) | 0.94 |
| Sexual function | 64±29 | 60±30 | −6±17 | 67±29 | 67±28 | 1±18 | 1 (–9 to 11) | 0.82 |
| Sleep | 59±22 | 57±22 | −2±16 | 61±18 | 63±18 | 2±18 | 4 (–1 to 9) | 0.08 |
| Social support | 75±22 | 69±26 | −6±25 | 76±24 | 75±25 | −1±27 | 5 (–1 to 11) | 0.13 |
| Dialysis staff encouragement | 84±21 | 89±17 | 4±25 | 81±20 | 84±18 | 3±18 | −1 (–7 to 4) | 0.63 |
| Patient satisfaction | 61±24 | 60±25 | −1±27 | 61±23 | 62±24 | 1±26 | 2 (–5 to 8) | 0.66 |
| Generic domain | ||||||||
| Physical functioning | 77±19 | 75±18 | −1±16 | 75±20 | 76±19 | 0±16 | 0 (–4 to 5) | 0.83 |
| Role—physical | 39±38 | 36±39 | −2±40 | 39±37 | 43±40 | 4±40 | 5 (–5 to 15) | 0.36 |
| Pain | 65±26 | 67±25 | 3±24 | 67±24 | 66±24 | −2±25 | −4 (–10 to 2) | 0.21 |
| General health | 39±19 | 38±20 | −2±17 | 38±20 | 39±21 | 1±17 | 2 (–3 to 6) | 0.45 |
| Emotional well-being | 69±18 | 68±18 | −1±15 | 72±16 | 69±17 | −2±14 | −2 (–6 to 2) | 0.38 |
| Role—emotional | 52±41 | 46±41 | −6±45 | 51±41 | 52±43 | 1±45 | 7 (–5 to 18) | 0.25 |
| Social function | 59±25 | 58±23 | −1±22 | 65±24 | 60±26 | −6±26 | −4 (–11 to 2) | 0.18 |
| Energy/fatigue | 55±23 | 53±22 | −2±19 | 58±19 | 55±21 | −3±17 | −2 (–7 to 3) | 0.42 |
Data are presented as means ± SDs.
Change was defined as the value at week 12 minus the value at baseline.
From a linear mixed model.
BMI increased in the intervention group from the baseline to week 12 (20.0±2.5 versus 20.1±2.5 kg/m2), and the intervention group showed a slightly larger increase in BMI than the control group (estimated difference, 0.2; 95% CI, 0.0 to 0.4; P=0.02). There were no significant differences in the changes in handgrip strength or midarm muscle circumference from the baseline to week 12 between the groups (Table 2).
The serum albumin concentration decreased from baseline in the control group (4.1±0.3 versus 4.0±0.3 g/dl), whereas there was no significant change in the intervention group (4.0±0.3 versus 4.0±0.3 g/dl). A greater reduction was observed in the control group than the intervention group for serum albumin concentration (−0.1 [−0.1, −0.0] versus 0.0 [−0.0, 0.1]; estimated difference, 0.1; 95% CI, 0 to 0.2; P=0.04). However, no significant differences were found in the changes in the prealbumin, transferrin, and hemoglobin between the groups (Table 2).
There were no significant differences in the changes in lean tissue index or hydration status between the groups. However, the intervention group showed a larger increase in fat tissue index than the control group (estimated difference, 0.5; 95% CI, 0.2 to 0.9; P=0.005) (Table 2).
There were no significant differences in the changes in serum triglyceride, total cholesterol, HDL cholesterol, LDL cholesterol, phosphate, and inflammation markers between the two groups. However, the intervention group showed a slightly greater decrease in BUN than the control group (−6.6±17.1 versus −1.1±19 mg/dl; P=0.05) (Supplemental Table 2).
Adverse Effects
Twenty-three (19%) participants in the control group were reported to experience adverse events during the treatment period, and 40 (33%) in the intervention group were reported to experience adverse events during the treatment period. The most frequent adverse events in the intervention group were gastrointestinal problems, such as nausea, vomiting, abdominal pain, bloating, and diarrhea (Table 4).
Table 4.
Adverse events observed during the study period
| Adverse Event | Control, n=120 | Intervention, n=120 |
|---|---|---|
| No. of patients (%) | 23 (19) | 40 (33) |
| Total no. of events | 25 | 47 |
| Death, n (%) | 1 (1) | 0 (0) |
| Hospitalizations | 15 (13) | 8 (7) |
| Cardiovascular disease | 4 (3) | 3 (3) |
| Pulmonary infection | 3 (3) | 0 (0) |
| Vascular access care | 3 (10) | 0 (0) |
| Pancreatitis | 0 (0) | 2 (2) |
| Other | 5 (4) | 3 (3) |
| Upper respiratory tract infection | 5 (4) | 4 (3) |
| Gastrointestinal adverse events | 2 (2) | 30 (25) |
| Nausea and vomiting | 0 (0) | 8 (7) |
| Abdominal pain | 0 (0) | 5 (4) |
| Bloating | 1 (1) | 8 (7) |
| Diarrhea | 0 (0) | 6 (5) |
| Constipation | 1 (1) | 3 (3) |
| Gastroenteritis | 1 (1) | 0 (0) |
| Hypoglycemic reactions | 0 (0) | 1 (1) |
| Others | 1 (1) | 4 (3) |
Some patients had more than one event.
Discussion
In this randomized controlled trial, 12 weeks of consumption of an oral fat-based energy-dense supplement did not cause a significant improvement in phase angle in nondiabetic patients on maintenance hemodialysis. Compared with the control group, this nutritional intervention may improve BMI, serum albumin, and fat tissue indices. However, the magnitudes of these changes were small, and therefore, they may not be clinically significant. Furthermore, the results must be interpreted with caution because of the likelihood of type 1 statistical errors from multiple comparisons. Therefore, the results of this study should be regarded as hypothesis generating.
In patients on maintenance hemodialysis, fat-based energy-dense supplements may be more beneficial than other types of supplements. Fat is the most energy-dense food component, and fat-based supplements are free of protein, phosphate, and potassium. Therefore, such a supplement can provide additional energy without substantial additional fluid intake and without affecting electrolyte status, making it particularly suitable for patients who are receiving dialysis. In addition, previous observational studies have suggested that higher fat intake is associated with better survival. The Prospective Urban Rural Epidemiology study, a large epidemiologic cohort study in which dietary intake was assessed using validated food frequency questionnaires, showed that high carbohydrate intake is associated with a higher risk of total mortality, whereas the higher total fat intake and higher intake of individual types of fat are associated with lower mortality (16). Similarly, another prospective cohort study conducted in patients with CKD in whom dietary intake was recorded on a food frequency questionnaire showed that an increase in the consumption of sugars is associated with higher cardiovascular mortality, whereas higher fat intake is associated with lower all-cause mortality (17). However, because these were observational studies, the identified associations might have been, in part, the results of residual confounding. There has been a lack of randomized controlled trials that have compared fat-based nutritional supplements with nonfat-dense supplements.
Most previous studies used oral supplements that contained a mixture of nutrients, and these yielded inconsistent results. Geovana Martin-Alemañy et al. (32) compared the effects of an oral nutritional supplement (yielding 434 kcal and containing 19.2 g protein and 22.8 g lipids) and an oral nutritional supplement plus exercise on the nutritional status of patients on maintenance hemodialysis over 12 weeks and found significant increases in markers of nutritional status (phase angle, BMI, and albumin) in both groups (32). A self-controlled study of 85 malnourished patients to whom an oral nutritional supplement (yielding 475 kcal and containing 16.6 g protein, 22.7 g fat, and 52.8 g carbohydrate) was administered during hemodialysis for 6 months found significant increases in serum albumin and prealbumin (7). Another randomized controlled trial conducted by Fouque et al. (12) in which patients in the intervention group received 500 kcal energy, 18.75 g protein, and 15 mg phosphate daily for 3 months found no significant differences in the changes in BMI, albumin, or prealbumin between the groups. Similarly, we demonstrated limited effects of the short-term administration of an oral nutritional supplement on nutritional status. These negative findings may be explained in a number of ways. First, although the participants were enrolled on the basis of their inadequate dietary energy intake (<30 kcal/kg per day), the patient population was relatively well nourished. Second, the phase angle is not likely to change substantially during a 12-week period. Third, the total energy intake (28.2±6.3 kcal/kg per day) of the intervention group was lower than that recommended in the Kidney Disease Outcomes Quality Initiative guidelines (2).
Ensuring adherence is important for the efficacy of nutritional interventions. In this trial, the overall adherence in the intervention group was 88%, which is similar to previously reported levels (7,11). Gastrointestinal symptoms, such as diarrhea, nausea, and vomiting, were reported by 30 patients in the intervention group in this study. These adverse effects were considered to be the result of intolerance of the supplements, but the mechanisms involved in the development of these gastrointestinal disorders require further investigation. In these cases, a reduction in the daily dose or the provision of advice regarding the incorporation of the supplements into the diet may reduce the severity of such problems, but further studies are required to test such strategies.
The strengths of this study were that it was a multicenter, randomized controlled trial with a larger sample size than those of previous studies. Furthermore, the trial had a dietary energy intake level–based inclusion criterion, and therefore, the intervention was performed only in patients with insufficient dietary energy intake. In addition, it was the first trial to evaluate the effects of a fat-based energy supplement on the nutritional status of patients on maintenance hemodialysis.
The study also had some limitations. First, it was not a placebo-controlled trial. Second, the study was carried out in the south of China, and the mean BMI of the sample was 20 kg/m2. Therefore, it must be determined whether the findings can be generalized to other populations. Third, patients with diabetes were excluded from the trial. Fourth, the study was relatively short. Finally, the negative findings may be explained by the fact that the sample was relatively well nourished (the mean value of phase angle was 6.1° at baseline). Overall, this trial can be regarded as hypothesis generating. Further trials involving longer-term supplementation in a range of populations and, in particular, in patients with poor nutritional status are required.
In conclusion, we found that the short-term administration of oral fat-based energy-dense supplements had limited benefits for the nutritional status of nondiabetic patients on maintenance hemodialysis. Further longer-term clinical trials with large sample sizes should be performed to validate these findings and to determine the effects of oral energy supplementation on clinical outcomes.
Disclosures
All authors have nothing to disclose.
Funding
M. Liang reports High-level Matching Funds of Nanfang Hospital grant 2014070, Key Clinical Research Program of Southern Medical University grant LC2019ZD005, National Key Technology Support Program of China grant 2015BAI12B07, National Science and Technology Major Project of China grant 2020ZX09201017, and Science and Technology Planning Project of Guangzhou grant 2014Y2-00098. X. Qin reports National Natural Science Foundation of China grant 81730019, Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University grant 2017J009.
Supplementary Material
Acknowledgments
We thank the participants, investigators, and staff for their contribution to this study. We are grateful for Beijing Fresenius Kabi Pharmaceutical Co., Ltd for providing some oral supplements for this work.
Data Sharing Statement
The individual deidentified participant data, analytic methods, study materials, and study protocol that support the findings of this study will be available from the corresponding authors on request after the request is submitted and formally reviewed and approved by the Medical Ethics Committee of Nanfang Hospital, Southern Medical University. The data will be available for 3 years after publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Better Nutrition Care for Patients on Hemodialysis: One Step at a Time,” on pages 1143–1145
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.16821020/-/DCSupplemental.
Supplemental Table 1. The per protocol analysis of the effects of oral energy supplements on nutritional status.
Supplemental Table 2. Effects of the 12-week energy supplementation on other laboratory markers.
References
- 1.Slomowitz LA, Monteon FJ, Grosvenor M, Laidlaw SA, Kopple JD: Effect of energy intake on nutritional status in maintenance hemodialysis patients. Kidney Int 35: 704–711, 1989 [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation: KDOQI clinical practice guidelines for nutrition in chronic renal failure. Available at: http://kidneyfoundation.cachefly.net/professionals/KDOQI/guidelines_nutrition/nut_a17.html. Accessed August 10, 2017. [Google Scholar]
- 3.Yang Y, Qin X, Li Y, Lei Z, Li Y, Yang S, Li Y, Kong Y, Lu Y, Zhao Y, Wan Q, Wang Q, Huang S, Liu Y, Liu A, Liu F, Hou F, Liang M: The association between dietary energy intake and the risk of mortality in maintenance haemodialysis patients: A multi-centre prospective cohort study. Br J Nutr 123: 437–445, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Ikizler TA, Cano NJ, Franch H, Fouque D, Himmelfarb J, Kalantar-Zadeh K, Kuhlmann MK, Stenvinkel P, TerWee P, Teta D, Wang AY-M, Wanner C; International Society of Renal Nutrition and Metabolism: Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int 84: 1096–1107, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Treviño-Becerra A, Wanner C: A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease [published erratum appears in Kidney Int 74: 393, 2008]. Kidney Int 73: 391–398, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Cano N, Fiaccadori E, Tesinsky P, Toigo G, Druml W, Kuhlmann M, Mann H, Hörl WH; DGEM (German Society for Nutritional Medicine); ESPEN (European Society for Parenteral and Enteral Nutrition): ESPEN guidelines on enteral nutrition: Adult renal failure. Clin Nutr 25: 295–310, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Caglar K, Fedje L, Dimmitt R, Hakim RM, Shyr Y, Ikizler TA: Therapeutic effects of oral nutritional supplementation during hemodialysis. Kidney Int 62: 1054–1059, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Sharma M, Rao M, Jacob S, Jacob CK: A controlled trial of intermittent enteral nutrient supplementation in maintenance hemodialysis patients. J Ren Nutr 12: 229–237, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Stratton RJ, Bircher G, Fouque D, Stenvinkel P, de Mutsert R, Engfer M, Elia M: Multinutrient oral supplements and tube feeding in maintenance dialysis: A systematic review and meta-analysis. Am J Kidney Dis 46: 387–405, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Eustace JA, Coresh J, Kutchey C, Te PL, Gimenez LF, Scheel PJ, Walser M: Randomized double-blind trial of oral essential amino acids for dialysis-associated hypoalbuminemia. Kidney Int 57: 2527–2538, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Jeong JH, Biruete A, Tomayko EJ, Wu PT, Fitschen P, Chung HR, Ali M, McAuley E, Fernhall B, Phillips SA, Wilund KR: Results from the randomized controlled IHOPE trial suggest no effects of oral protein supplementation and exercise training on physical function in hemodialysis patients. Kidney Int 96: 777–786, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fouque D, McKenzie J, de Mutsert R, Azar R, Teta D, Plauth M, Cano N; Renilon Multicentre Trial Study Group: Use of a renal-specific oral supplement by haemodialysis patients with low protein intake does not increase the need for phosphate binders and may prevent a decline in nutritional status and quality of life. Nephrol Dial Transplant 23: 2902–2910, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Kovesdy CP, Shinaberger CS, Kalantar-Zadeh K: Epidemiology of dietary nutrient intake in ESRD. Semin Dial 23: 353–358, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakao T, Matsumoto H, Okada T, Kanazawa Y, Yoshino M, Nagaoka Y, Takeguchi F: Nutritional management of dialysis patients: Balancing among nutrient intake, dialysis dose, and nutritional status. Am J Kidney Dis 41[Suppl 1]: S133–S136, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Bernhard J, Beaufrère B, Laville M, Fouque D: Adaptive response to a low-protein diet in predialysis chronic renal failure patients. J Am Soc Nephrol 12: 1249–1254, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, Iqbal R, Kumar R, Wentzel-Viljoen E, Rosengren A, Amma LI, Avezum A, Chifamba J, Diaz R, Khatib R, Lear S, Lopez-Jaramillo P, Liu X, Gupta R, Mohammadifard N, Gao N, Oguz A, Ramli AS, Seron P, Sun Y, Szuba A, Tsolekile L, Wielgosz A, Yusuf R, Hussein Yusufali A, Teo KK, Rangarajan S, Dagenais G, Bangdiwala SI, Islam S, Anand SS, Yusuf S; Prospective Urban Rural Epidemiology (PURE) study investigators: Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 390: 2050–2062, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Iff S, Wong G, Webster AC, Flood V, Wang JJ, Mitchell P, Craig JC: Relative energy balance, CKD, and risk of cardiovascular and all-cause mortality. Am J Kidney Dis 63: 437–445, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Chertow GM, Lowrie EG, Wilmore DW, Gonzalez J, Lew NL, Ling J, Leboff MS, Gottlieb MN, Huang W, Zebrowski B: Nutritional assessment with bioelectrical impedance analysis in maintenance hemodialysis patients. J Am Soc Nephrol 6: 75–81, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Marcelli D, Usvyat LA, Kotanko P, Bayh I, Canaud B, Etter M, Gatti E, Grassmann A, Wang Y, Marelli C, Scatizzi L, Stopper A, van der Sande FM, Kooman J; MONitoring Dialysis Outcomes (MONDO) Consortium: Body composition and survival in dialysis patients: Results from an international cohort study. Clin J Am Soc Nephrol 10: 1192–1200, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norman K, Stobäus N, Pirlich M, Bosy-Westphal A: Bioelectrical phase angle and impedance vector analysis—Clinical relevance and applicability of impedance parameters. Clin Nutr 31: 854–861, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Oliveira CM, Kubrusly M, Mota RS, Silva CA, Choukroun G, Oliveira VN: The phase angle and mass body cell as markers of nutritional status in hemodialysis patients. J Ren Nutr 20: 314–320, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Beberashvili I, Azar A, Sinuani I, Shapiro G, Feldman L, Stav K, Sandbank J, Averbukh Z: Bioimpedance phase angle predicts muscle function, quality of life and clinical outcome in maintenance hemodialysis patients. Eur J Clin Nutr 68: 683–689, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Shin J-H, Kim CR, Park KH, Hwang JH, Kim SH: Predicting clinical outcomes using phase angle as assessed by bioelectrical impedance analysis in maintenance hemodialysis patients. Nutrition 41: 7–13, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Chazot C, Wabel P, Chamney P, Moissl U, Wieskotten S, Wizemann V: Importance of normohydration for the long-term survival of haemodialysis patients. Nephrol Dial Transplant 27: 2404–2410, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Wabel P, Moissl U, Chamney P, Jirka T, Machek P, Ponce P, Taborsky P, Tetta C, Velasco N, Vlasak J, Zaluska W, Wizemann V: Towards improved cardiovascular management: The necessity of combining blood pressure and fluid overload. Nephrol Dial Transplant 23: 2965–2971, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Wang Y-W, Lin T-Y, Peng C-H, Huang J-L, Hung S-C: Factors associated with decreased lean tissue index in patients with chronic kidney disease. Nutrients 9: 434, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbosa-Silva MCG, Barros AJD, Wang J, Heymsfield SB, Pierson RN Jr.: Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am J Clin Nutr 82: 49–52, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Bosy-Westphal A, Danielzik S, Dörhöfer RP, Later W, Wiese S, Müller MJ: Phase angle from bioelectrical impedance analysis: Population reference values by age, sex, and body mass index. JPEN J Parenter Enteral Nutr 30: 309–316, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Kuchnia AJ, Teigen LM, Cole AJ, Mulasi U, Gonzalez MC, Heymsfield SB, Vock DM, Earthman CP: Phase angle and impedance ratio: Reference cut-points from the United States National Health and Nutrition Examination Survey 1999-2004 from bioimpedance spectroscopy data. JPEN J Parenter Enteral Nutr 41: 1310–1315, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Hays RD, Kallich J, Mapes D, Coons S, Amin N, Carter WB, Kamberg C: Kidney Disease Quality of Life Short Form (KDQOL-SF™), Version 1.3: A Manual for Use and Scoring, 1997. Available at: http://www.rand.org/content/dam/rand/pubs/papers/2006/P7994.pdf. Accessed August 10, 2017
- 31.Ziolkowski SL, Long J, Baker JF, Chertow GM, Leonard MB: Chronic kidney disease and the adiposity paradox: Valid or confounded? J Ren Nutr 29: 521–528, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin-Alemañy G, Valdez-Ortiz R, Olvera-Soto G, Gomez-Guerrero I, Aguire-Esquivel G, Cantu-Quintanilla G, Lopez-Alvarenga JC, Miranda-Alatriste P, Espinosa-Cuevas A: The effects of resistance exercise and oral nutritional supplementation during hemodialysis on indicators of nutritional status and quality of life. Nephrol Dial Transplant 31: 1712–1720, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.