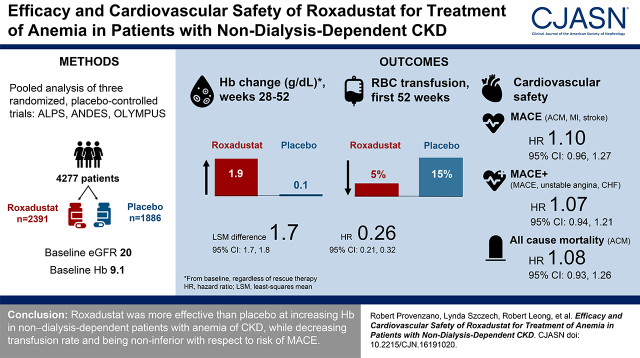
Visual Abstract
Keywords: roxadustat, anemia, chronic kidney disease, efficacy, MACE, safety
Abstract
Background and objectives
We evaluated the efficacy and cardiovascular safety of roxadustat versus placebo by analyzing data pooled from three phase 3 studies of roxadustat in patients with non–dialysis-dependent CKD and CKD-related anemia.
Design, setting, participants, & measurements
In the three phase 3, double-blind studies of roxadustat versus placebo evaluating the treatment of CKD-related anemia in patients not requiring dialysis, the primary efficacy end point was mean change from baseline in hemoglobin averaged over weeks 28–52, regardless of rescue therapy. The primary cardiovascular safety end point was a composite measure of major adverse cardiovascular events (MACE; all-cause mortality, myocardial infarction, stroke). MACE plus (MACE+; MACE plus unstable angina and heart failure requiring hospitalization) and all-cause mortality were key secondary safety end points. These safety end points were adjudicated.
Results
A total of 4277 patients with non–dialysis-dependent CKD were randomized (roxadustat, n=2391; placebo, n=1886). Baseline characteristics were comparable between groups; the mean (SD) hemoglobin was 9.1 (0.7) g/dl and mean eGFR was 20 (12) ml/min per 1.73 m2. Patients treated with roxadustat versus those treated with placebo showed a mean change from baseline in hemoglobin averaged over weeks 28–52, regardless of rescue therapy, of 1.9 versus 0.2 g/dl, a treatment difference of 1.7 (95% confidence interval [95% CI], 1.7 to 1.8). Roxadustat reduced the need for red blood cell transfusion in the first 52 weeks versus placebo (6.1 versus 20.4 per 100 patient-exposure years, respectively; hazard ratio [HR], 0.26; 95% CI, 0.21 to 0.32). There were no increased risks of MACE (HR, 1.10; 95% CI, 0.96 to 1.27), MACE+ (HR, 1.07; 95% CI, 0.94 to 1.21), all-cause mortality (HR, 1.08; 95% CI, 0.93 to 1.26), or individual MACE+ components in patients treated with roxadustat versus those treated with placebo.
Conclusions
Roxadustat was more effective than placebo at increasing hemoglobin in patients with non–dialysis-dependent CKD and anemia, while decreasing transfusion rate and being noninferior to placebo with respect to risk of MACE.
Clinical Trial registry name and registration number:
A Study of FG-4592 for the Treatment of Anemia in Chronic Kidney Disease Patients Not Receiving Dialysis, NCT01750190; Roxadustat in the Treatment of Anemia in Chronic Kidney Disease Patients Not Requiring Dialysis (ALPS), NCT01887600; Safety and Efficacy Study of Roxadustat to Treat Anemia in Patients With Chronic Kidney Disease (CKD), Not on Dialysis, NCT02174627
Introduction
Before erythropoiesis-stimulating agents (ESAs) were approved, patients with kidney failure and severe anemia were often managed with red blood cell (RBC) transfusions, which were associated with higher risk for viral disease, iron overload, and allosensitization (1,2). In 1989, epoetin alfa—the first ESA approved by the Food and Drug Administration for the treatment of CKD-related anemia—transformed the treatment landscape (1). The burden of disease was reduced as hemoglobin levels were increased and the number of patients dependent on transfusion decreased (1,3).
ESAs became the standard of care for CKD-related anemia, without robust clinical research to support their cardiovascular safety (4). This changed when sentinel trials linked ESA therapy to risk of cardiovascular and thromboembolic events (5–7). Given the subsequent decrease in ESA doses used and concomitant increase in RBC transfusions, the cardiovascular safety of a new therapy, as compared with placebo, is a critical clinical question (3).
In the late 1990s/early 2000s, hypoxia-inducible factor was identified as being responsible for increased transcription of the erythropoietin gene in hypoxic conditions, under the regulation of oxygen-sensing prolyl hydroxylases (8–12). Roxadustat is a potent and reversible hypoxia-inducible factor–prolyl hydroxylase inhibitor that mimics the body’s natural response to hypoxia, stimulating a coordinated erythropoietic response, including increased erythropoietin production, decreased hepcidin levels, and increased iron availability (13). Roxadustat is approved to treat anemia of CKD in adults on and not on dialysis in China (14,15) and Japan (16–19). Roxadustat was shown to increase hemoglobin and serum iron levels, resulting in increased RBC production and reduced intravenous (IV) iron use. We report the results of analyses of data pooled from three, similarly designed, phase 3 studies evaluating the efficacy and cardiovascular safety of roxadustat versus placebo in patients with non–dialysis-dependent CKD and CKD-related anemia.
Materials and Methods
Study Design and Participants
This study includes data pooled from three phase 3, randomized, double-blind, placebo-controlled studies of roxadustat in patients with non–dialysis-dependent CKD (ANDES, FGCL-4592-060, NCT01750190 [20]; ALPS, 1517-CL-0608, NCT01887600 [21]; and OLYMPUS, D5740C00001, NCT02174627 [22]). In brief, the trials were designed to evaluate roxadustat efficacy and noninferiority for major adverse cardiovascular events (MACE; all-cause mortality, myocardial infarction, stroke). Sample size determinations were calculated using an upper margin of 1.3 (for details, see Supplemental Methods). Study 608 (ALPS) was not event driven and had a target treatment duration of 52–104 weeks. Studies 060 (ANDES) and 001 (OLYMPUS) were event driven and had a common study end date, when the target number of cardiovascular events was considered to have accrued. In studies 060 and 001, patients who provided consent were followed for cardiovascular events after treatment discontinuation until the end of the study period; public records searches were used to confirm vital status at the end of the study, where allowed, in accordance with local regulations. Events were adjudicated by a central committee blinded to treatment assignment (additional details in Supplemental Methods). Patients with hemoglobin ≤10.0 g/dl and eGFR <60 ml/min per 1.73 m2 were included (Supplemental Table 1). Automated randomization and treatment assignments were prospectively generated and performed by an interactive response system. Study drug dosing is detailed in the Supplemental Methods and Supplemental Table 2. Rescue therapy consisted of RBC transfusion, ESA use, and/or IV iron supplementation. The research protocols were approved by relevant institutional review boards or ethics committees. The study was conducted in accordance with the Declaration of Helsinki, and all participants gave written informed consent.
Efficacy End Points
The primary US (Food and Drug Administration [FDA] submission) efficacy end point in the individual studies was the mean hemoglobin change from baseline averaged over weeks 28–52, regardless of rescue therapy. The primary European Union (EU; European Medicines Agency submission) efficacy end point was the proportion of patients that achieved a hemoglobin response at two consecutive visits during the first 24 weeks of treatment, censored for the use of rescue therapy. Hemoglobin response is defined in Supplemental Table 1. Secondary efficacy end points are listed in the Supplemental Methods. Efficacy was also examined among prespecified subgroups on the basis of iron repletion status (iron replete: ferritin ≥100 ng/ml and transferrin saturation [TSAT] ≥20%). Additional efficacy end points included proportion of patients requiring RBC transfusion and/or rescue therapy.
Cardiovascular Safety End Points
The primary cardiovascular safety end point was the time to first MACE, a composite measure of all-cause mortality, myocardial infarction, and stroke. The key secondary safety composite end point, MACE plus (MACE+), comprised MACE plus unstable angina and heart failure requiring hospitalization. Key secondary safety end points included time to first MACE+ and time to all-cause mortality. End point definitions are given in the Supplemental Methods. Cardiovascular safety events were adjudicated by a central independent event review committee blinded to study treatment, as described in the Supplemental Methods.
There is a high risk of cardiovascular mortality in patients who initiate dialysis, especially during the initial months of therapy (23). Although progression to dialysis did not require study discontinuation, we also evaluated the subgroup of patients with baseline eGFR ≥10 ml/min per 1.73 m2, excluding the patients with non–dialysis-dependent CKD who were most likely to initiate dialysis during the studies.
General Safety/Tolerability
Safety was assessed by monitoring treatment-emergent adverse events (AEs) and treatment-emergent serious AEs (SAEs) during treatment and for 28 days after study drug discontinuation (on treatment+28) in the safety population (all randomized patients who received one or more dose of study drug). Seizures were assessed using the “convulsions” standardized Medical Dictionary for Regulatory Activities query.
Statistical Analyses
The pooled analysis for the primary US efficacy end point was performed on the intention-to-treat (ITT) population (all randomized/enrolled patients) using multiple imputation analysis of covariance. All other efficacy end points were evaluated using the full analysis set (all randomized/enrolled subjects who received one or more dose of study drug and had one or more postdose hemoglobin assessment). There is no adjustment for multiplicity in pooled analyses for efficacy end points; all efficacy P-values were therefore considered nominal and are not reported herein. The evaluation window for the primary cardiovascular safety end point encompassed all events that occurred after randomization, regardless of whether the patient was on or off study treatment. This is referred to as the ITT population throughout the manuscript to differentiate from the ITT analysis (period following long-term follow-up) for safety. Safety analysis was performed on the safety population (all randomized patients who received one or more dose of the study drug). Statistical analysis procedures for secondary end points are described in the Supplemental Methods. All statistical analyses were performed using Statistical Analysis System version 9.3 or higher.
The phase 3 program to evaluate the safety of roxadustat in patients with non−dialysis-dependent CKD was designed to demonstrate the noninferiority of roxadustat compared with placebo in the incidence of MACE using an on-treatment analysis. For this pooled analysis, a reference noninferiority margin of <1.3 was used, consistent with previous ESA trials (5–7,24) and FDA guidance on antidiabetic and oncologic drugs (25,26). Given the potential for an informative censoring due to differential dropout (placebo greater than roxadustat) and with the agreement of the FDA, the ITT analysis was designated the appropriate analysis method (i.e., on-study analysis, which includes long-term follow-up for MACE events). The pooled population hazard ratio (HR) was estimated using the meta-analysis method to combine the logarithm of the HRs from the individual studies weighted by the inverse of the variances (27). The HR from each study was estimated using a Cox regression modeling treatment effect (roxadustat versus placebo), stratified by baseline hemoglobin values (≤8.0 versus >8.0 g/dl); history of cardiovascular, cerebrovascular, or thromboembolic diseases (yes versus no); baseline eGFR <30 versus ≥30 ml/min per 1.73 m2; and geographic region (OLYMPUS and ANDES, United States versus others; ALPS, Western Europe versus others). Further details regarding the statistical analysis, including sensitivity analyses, are provided in the Supplemental Methods.
In all studies, patients discontinued the study drug at a greater rate in the placebo arm compared with the roxadustat arm, especially among those with lower baseline eGFRs. It has been observed that the lowest eGFRs carry disproportionately higher risks for MACE and other cardiovascular events (28,29). Therefore, treatment discontinuation resulted in informative censoring, or a “depletion of the susceptibles,” in the placebo group relative to the roxadustat group. To minimize the potential bias introduced by informative censoring in the placebo arm, cardiovascular safety and fatal AEs were analyzed using an ITT analysis with long-term follow-up, as described in the Supplemental Methods.
Results
Patient Disposition and Demographics
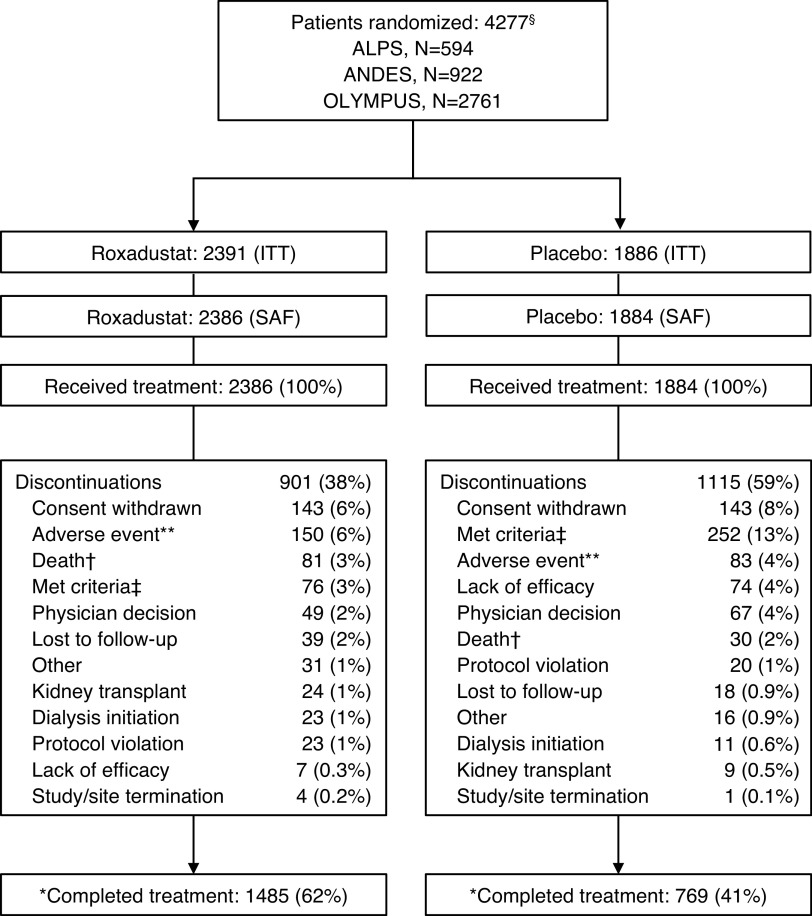
In total, 4277 patients were randomized to roxadustat (n=2391) or placebo (n=1886) (ITT population); 4270 received one or more dose of the study drug and were included in the safety analysis set (Figure 1). Baseline demographic and clinical characteristics were generally comparable between the treatment groups (Table 1; Supplemental Table 3 for individual studies). Patient age averaged 62 years (roxadustat) versus 63 years (placebo); mean baseline hemoglobin was 9.1 g/dl in both groups; and mean baseline eGFR was 19.7 and 20.0 ml/min per 1.73 m2 in the roxadustat and placebo groups, respectively.
Figure 1.
CONSORT flow diagram.§In study 001 (OLYMPUS), 2781 patients were randomized but 20 were excluded from statistical analysis because of system technical issues and major good clinical practice violations. In study 608 (ALPS), 597 patients were randomized but three were excluded from statistical analysis because of good clinical practice violations. **Includes adverse event and progressive disease. †Patients who died while on study drug or after discontinuation of study drug were categorized as completing the study in study 001. ‡Development of study-specific discontinuation criteria: either the patient’s decision (the patient is, at any time, free to discontinue treatment, without prejudice to further treatment) or investigator’s decision (including but not limited to these examples: incorrectly randomized patient in whom the inclusion/exclusion criteria violation would put the patient at undue risk, adverse event for which the investigator thinks continued treatment may put the patient at undue risk, severe nonadherence to study protocol, pregnancy, patients who have received two courses of erythropoietin analogue rescue therapy and for whom there is a need for a third course of rescue with erythropoietin analogue, for patients initiating dialysis during the study and for whom there is a need for rescue with erythropoietin analogue). *Vital status was ascertained at the end of the study among 91% of the roxadustat arm and 91% of the placebo arm. CONSORT, Consolidated Standards of Reporting Trials; ITT, intention to treat; SAF, safety analysis set.
Table 1.
Baseline characteristics of participants in three phase 3 studies of roxadustat with non–dialysis-dependent CKD (ITT)
| Characteristics | Roxadustat (n=2391) | Placebo (n=1886) |
| Age (yr), mean (SD)a | 62 (14) | 63 (14) |
| Male sex, n (%) | 974 (41) | 832 (44) |
| Weight (kg), mean (SD) | 71 (18) | 71 (19) |
| Hemoglobin (g/dl), mean (SD) | 9.1 (0.7) | 9.1 (0.7) |
| <8.0, n (%) | 204 (9) | 164 (9) |
| ≥8.0, n (%) | 2187 (92) | 1722 (91) |
| eGFR (ml/min per 1.73 m2), mean (SD) | 20 (12) | 20 (12) |
| <10.0, n (%) | 481 (20) | 359 (19) |
| 10.0 to <15.0, n (%) | 526 (22) | 452 (24) |
| 15.0 to <30.0, n (%) | 954 (40) | 724 (38) |
| ≥30.0, n (%) | 430 (18) | 351 (19) |
| hs-CRP (mg/L), mean (SD) b | 7.4 (17.4) | 7.2 (16.7) |
| Median (min–max) | 2.2 (0.1–338) | 2.2 (0.1–187) |
| Greater than upper limit of normal, n (%) | 526 (22) | 357 (19) |
| LDL cholesterol (mg/dl), mean (SD) | 99 (44) | 95 (42) |
| Iron repletion status, n (%) | ||
| Ferritin ≥100 ng/ml and TSAT ≥20% | 1433 (60) | 1127 (60) |
| Diabetes mellitus, n (%) | 1337 (56) | 1096 (58) |
ITT, intention-to-treat population; hs-CRP, high-sensitivity C-reactive protein; min, minimum; max, maximum; TSAT, transferrin saturation.
Age was calculated in years from birth date to date of informed consent or first-dose date.
Upper limit of normal for hs-CRP was defined as 4.6 mg/l.
A larger percentage of patients treated with roxadustat versus those treated with placebo completed the treatment (62% versus 41%). The most common reasons for study discontinuation were withdrawal of consent, development of study-specific discontinuation criteria, AEs (including death), and physician decision (Figure 1). Vital status was ascertained at the end of the study among 91% of the roxadustat arm and 91% of the placebo arm. In the roxadustat group, total patient-exposure years were 3870.7 (mean patient exposure, 1.6 years) versus the placebo group, in which total patient-exposure years were 2323.2 (mean patient exposure, 1.2 years) (Supplemental Table 4).
Primary Efficacy End Point
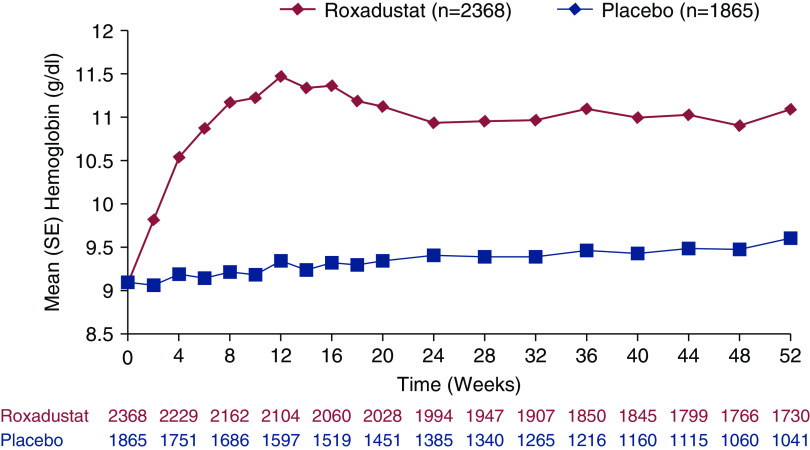
For the primary US efficacy end point, roxadustat was superior to placebo (Figure 2, Table 2). The least-squares mean (SEM) change from baseline in hemoglobin averaged over weeks 28–52, regardless of rescue therapy, was higher in the roxadustat versus placebo group in the pooled ITT population (+1.9 [0.02] versus +0.2 [0.03] g/dl, respectively; least-squares mean treatment difference, 1.7; 95% confidence interval [95% CI], 1.7 to 1.8) and individual phase 3 studies (Table 2).
Figure 2.
Hemoglobin levels in the overall non–dialysis-dependent patient population over time (weeks 0–52) (full analysis set).
Table 2.
Hemoglobin changes from baseline averaged over weeks 28–52, regardless of rescue therapy, by study and for pooled data in patients with non–dialysis-dependent CKD (ITT population)
| Hemoglobin Variables (g/dl) | Study 608 (ALPS) | Study 060 (ANDES) | Study 001 (OLYMPUS) | Pooled Data | ||||
| Roxadustat (n=391) |
Placebo (n=203) |
Roxadustat (n=616) |
Placebo (n=306) |
Roxadustat (n=1384) |
Placebo (n=1377) |
Roxadustat (n=2391) |
Placebo (n=1886) |
|
| Mean (SD) Hb at baselinea | 9.1 (0.8) | 9.1 (0.7) | 9.1 (0.8) | 9.1 (0.7) | 9.1 (0.7) | 9.1 (0.7) | 9.1 (0.7) | 9.1 (0.7) |
| Mean (SD) Hb in weeks 28–52 | 11.2 (0.8) | 9.6 (1.0) | 11.1 (0.7) | 9.3 (1.1) | 10.8 (0.9) | 9.5 (1.2) | 11.0 (0.8) | 9.2 (1.1) |
| Mean (SD) Hb change from baseline | 2.0 (1.0) | 0.4 (1.0) | 2.0 (1.0) | 0.2 (0.9) | 1.7 (1.0) | 0.4 (1.2) | 1.9 (0.9) | 0.1 (1.0) |
| ANCOVA with multiple imputations b | ||||||||
| Least-squares mean (SEM); 95% CI | 2.0 (0.09); 1.8 to 2.2 | 0.3 (0.11); 0.1 to 0.5 | 2.0 (0.04); 2.0 to 2.1 | 0.2 (0.05); 0.1 to 0.3 | 1.8 (0.03); 1.7 to 1.8 | 0.4 (0.03); 0.3 to 0.5 | 1.9 (0.02); 1.9 to 2.0 | 0.2 (0.03); 0.2 to 0.3 |
| Least-squares mean difference (SEM); 95% CI | 1.7 (0.09); 1.5 to 1.9 | 1.9 (0.06); 1.7 to 2.0 | 1.4 (0.04); 1.3 to 1.4 | 1.7 (0.04); 1.7 to 1.8 | ||||
| P value | <0.001 | <0.001 | <0.001 | |||||
ITT, intention to treat; Hb, hemoglobin; ANCOVA, analysis of covariance; 95% CI, 95% confidence interval.
Baseline hemoglobin defined as the mean of up to four most recent laboratory values before the first dose of the study drug.
Treatment comparison using the multiple imputation strategy by combining the results of an ANCOVA model with baseline Hb and eGFR as covariates, and study; treatment; study-by-treatment interaction; region (United States, Europe, other); and history of cardiovascular, cerebrovascular, or thromboembolic disease (yes versus no) as fixed effects.
Secondary Efficacy End Points
An analysis of efficacy was also performed on the full analysis set for weeks 28–36, and this revealed a treatment difference for hemoglobin (SEM) of 1.8 (0.04) g/dl (95% CI, 1.7 to 1.8). For the primary EU efficacy end point, a larger percentage of patients with non–dialysis-dependent CKD met the definition of a hemoglobin response during the first 24 weeks of treatment compared with placebo (Table 3). The cumulative percentage of patients who satisfied the definition of a hemoglobin response increased to 92% at 52 weeks (data not shown). The outcomes from the three individual phase 3 studies were consistent with those of the pooled analysis (Table 3). Patients who were iron replete (baseline ferritin ≥100 ng/ml and TSAT ≥20%) and iron nonreplete (baseline ferritin <100 ng/ml or TSAT <20%) showed a similar least-squares mean (SEM) change from baseline in hemoglobin averaged over weeks 28–52 (respectively, treatment difference of 1.8 [0.04] versus 1.6 [0.05] g/dl).
Table 3.
Hemoglobin response analysis during the first 24 weeks of treatment, censoring for rescue therapy by study and for pooled data in patients with non–dialysis-dependent CKD (full analysis set)
| Hemoglobin Response | Study 608 | Study 060 | Study 001 | Pooled Data | |||||
| Roxadustat (n=389) |
Placebo (n=203) |
Roxadustat (n=608) |
Placebo (n=305) |
Roxadustat (n=1371) |
Placebo (n=1357) |
Roxadustat (n=2368) |
Placebo (n=1865) |
||
| Patients achieving a Hb response, n (%) a | 308 (79) | 20 (10) | 523 (86) | 20 (7) | 1055 (77) | 115 (9) | 1899 (80) | 163 (9) | |
| 95% CIb | 75 to 83 | 6 to 15 | 83 to 89 | 4 to 10 | 75 to 79 | 7 to 10 | 79 to 82 | 8 to 10 | |
| Treatment group difference (95% CI) | 69 (64 to 75) | 80 (76 to 83) | 69 (66 to 71) | 72 (69 to 74) | |||||
| OR (95% CI) c | 34.7 (20.5 to 58.9)d | 77.6 (44.7 to 134.5) | 36.5 (28.5 to 46.7) | 40.5 (33.0 to 49.7) | |||||
| P value | <0.001 | <0.001 | <0.001 | ||||||
Hb values under the influence of a rescue therapy were censored up to 6 weeks. Hb, hemoglobin; 95% CI, 95% confidence interval; OR, odds ratio; CMH, Cochran–Mantel–Haenszel.
Hb response defined as Hb ≥11.0 g/dl, and Hb increase from baseline ≥1.0 g/dl in patients with baseline Hb >8.0 g/dl, or Hb increase ≥2.0 g/dl in patients with baseline Hb ≤8.0 g/dl at two consecutive visits ≥5 days apart during the first 24 weeks of treatment without rescue therapy.
On the basis of the exact method of Clopper–Pearson.
CMH method adjusting for study, region (United States, Europe, other); baseline Hb (<8.0 versus ≥8.0 g/dl); baseline eGFR (<30 versus ≥30 ml/min per 1.73 m2); and history of cardiovascular, cerebrovascular, or thromboembolic diseases (yes versus no).
CMH test was performed.
The least-squares mean (SEM) change from baseline in hemoglobin averaged over weeks 28–36 and censored for rescue therapy was +1.9 (0.02) g/dl in the roxadustat versus +0.1 (0.03) g/dl in the placebo group (treatment difference, 1.8; 95% CI, 1.7 to 1.8). The percentage of patients with hemoglobin ≥10.0 g/dl averaged over weeks 28–36 was 73% (roxadustat) versus 19% (placebo) (odds ratio, 12.3; 95% CI, 10.5 to 14.3). In the subgroup of patients with baseline high-sensitivity C-reactive protein greater than the upper limit of normal, the least-squares mean change from baseline in hemoglobin averaged over weeks 28–52 was 2.0 g/dl in the roxadustat (n=523) and 0.3 g/dl in the placebo group (n=355); a between-group difference of 1.7 (95% CI, 1.5 to 1.8). The least-squares mean change from baseline in LDL cholesterol averaged over weeks 12–28 was −17.3 mg/dl (roxadustat) versus +2.6 mg/dl (placebo); a between-group difference of −19.8 (95% CI, −22.2 to −17.5).
The percentage of patients who received any rescue therapy during the first 52 weeks of treatment was 9% in the roxadustat versus 31% in the placebo group, with an exposure-adjusted incidence rate of 10.4 versus 41.0 per 100 patient-exposure years, respectively. The HR for the time to first use of rescue therapy was 0.19 (95% CI, 0.16 to 0.23) in favor of roxadustat. The percentage of patients that received an RBC transfusion during the first 52 weeks of treatment was 5% (roxadustat) versus 15% (placebo) (HR, 0.26; 95% CI, 0.21 to 0.32), with an incidence rate of 6.1 versus 20.4 per 100 patient-exposure years.
Cardiovascular Safety End Points
The HR for time to first MACE, comparing roxadustat with placebo, in the pooled non–dialysis-dependent CKD population was 1.10 (95% CI, 0.96 to 1.27) (Figures 3 and 4, Supplemental Figure 1). Two sensitivity analyses to minimize the bias imposed by informative censoring were performed to exclude those with an eGFR of <10 ml/min per 1.73 m2 at baseline and up to 1 year of treatment. The HRs for time to first MACE in these two analyses were 1.00 (95% CI, 0.85 to 1.17; Supplemental Figure 2) and 1.00 (95% CI, 0.81 to 1.23; Supplemental Figure 3), respectively. An additional sensitivity analysis using post hoc stratification factors is presented in the Supplemental Methods (Supplemental Figure 4).
Figure 3.
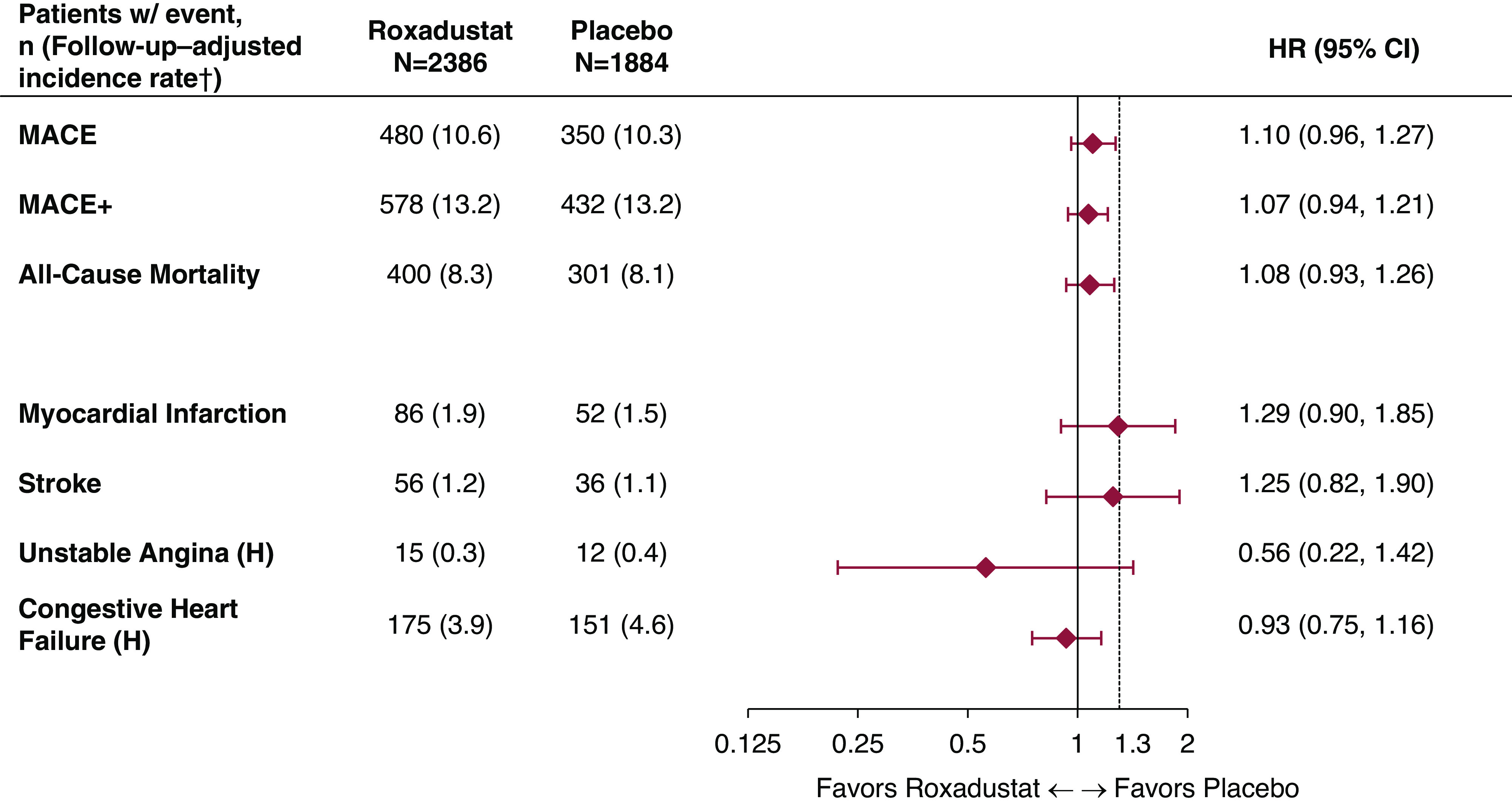
Forest plots for the cardiovascular safety analyses in patients who were not dependent on dialysis (safety analysis set, ITT analysis). ITT analysis refers to analyses comprising both on-treatment and long-term follow-up events. †Follow-up–adjusted incidence rate per 100 patient years: 100×number of patients with events/patient years. Patient years for each patient: (first event occurrence or censor date–first dose date+1)/365.25. 95% CI, 95% confidence interval; H, hospitalization; HR, hazard ratio; MACE, major adverse cardiovascular event defined as all-cause mortality, myocardial infarction, or stroke; MACE+, MACE plus unstable angina and congestive heart failure requiring hospitalization.
Figure 4.
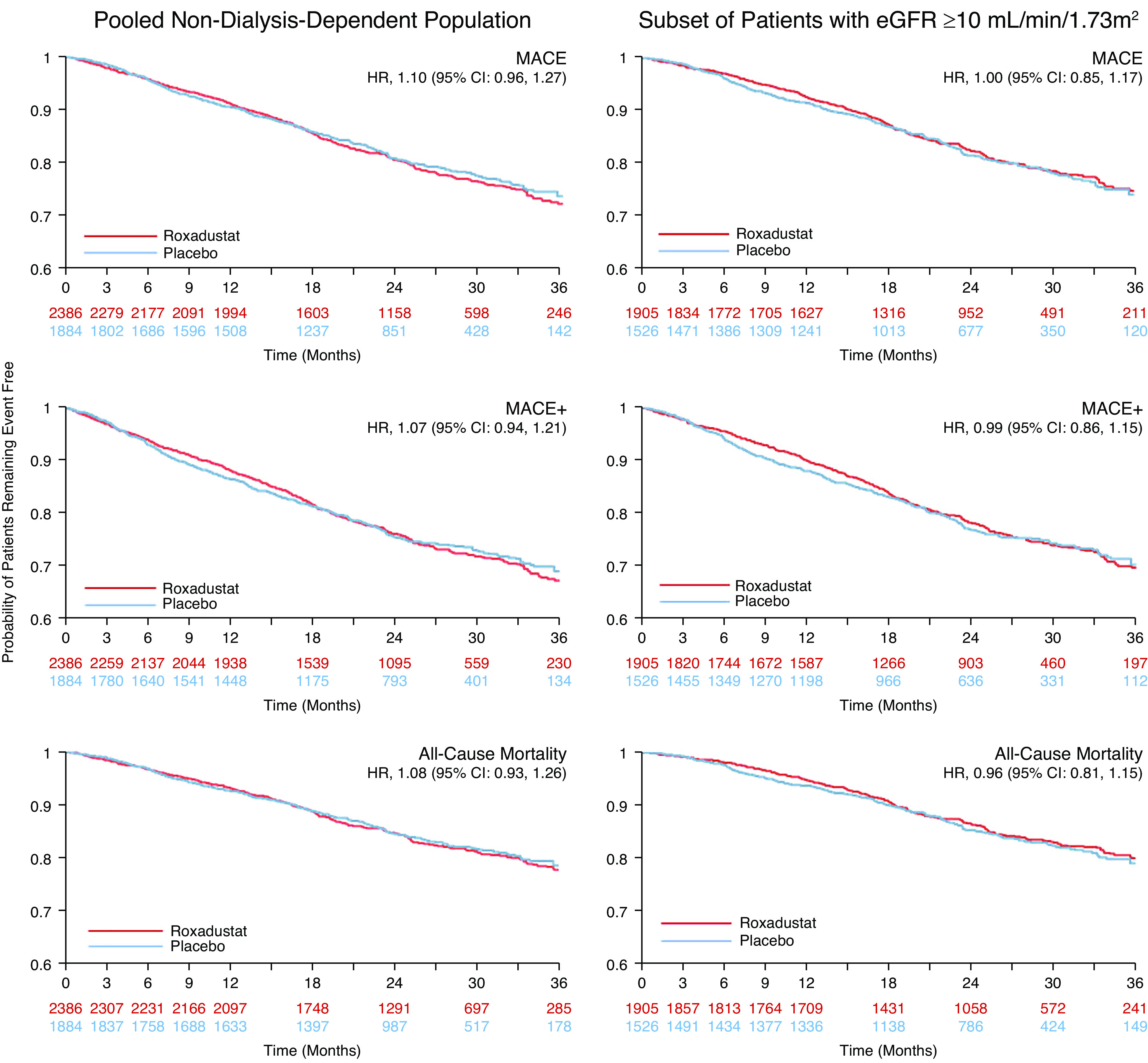
Kaplan–Meier curves for MACE, MACE+, and all-cause mortality in patients who were not dependent on dialysis (safety analysis set, ITT analysis). MACE was defined as all-cause mortality, myocardial infarction, or death.
The HR for time to first MACE+, comparing roxadustat with placebo, was 1.07 (95% CI, 0.94 to 1.21; Figure 3). For all-cause mortality, the HR was 1.08 (95% CI, 0.93 to 1.26; Figure 3). In the non–dialysis-dependent subgroup with an eGFR of ≥10 ml/min per 1.73 m2, HRs for time to first MACE+ and all-cause mortality were 0.99 (95% CI, 0.86 to 1.15) and 0.96 (95% CI, 0.81 to 1.15), respectively (Supplemental Figure 2). Results for MACE, MACE+, and individual MACE+ components are reported in Figure 3. Analyses of MACE were consistent across the subgroups (Supplemental Figure 5).
General Safety and Tolerability
Comparable percentages of patients in the roxadustat and placebo groups experienced at least one treatment-emergent AE during the on-treatment+28 days period: 2132 of 2386 patients (89%) and 1608 of 1884 patients (85%), respectively. The follow-up–adjusted incidence rate per 100 patient follow-up years was 222.6 in the roxadustat versus 211.5 in the placebo group. The most common treatment-emergent AEs occurring in ≥5% of patients in either group were kidney failure, hypertension, peripheral edema, hyperkalemia, diarrhea, and urinary tract infection (Table 4).
Table 4.
Treatment-emergent adverse events occurring in ≥5% of patients with non–dialysis-dependent CKD in either treatment group (on treatment+28)
| Preferred Terma | Roxadustat (n=2386) | Placebo (n=1884) | ||||
| n (%) | PYb | FAIRc | n (%) | PYb | FAIRc | |
| Kidney failured | 473 (20) | 3639.8 | 13.0 | 282 (15) | 2322.9 | 12.1 |
| Hypertension | 329 (14) | 3636.1 | 9.0 | 153 (8) | 2325.2 | 6.6 |
| Edema peripheral | 279 (12) | 3692.4 | 7.6 | 143 (8) | 2334.9 | 6.1 |
| Hyperkalemia | 261 (11) | 3738.3 | 7.0 | 133 (7) | 2341.1 | 5.7 |
| Diarrhea | 248 (10) | 3749.4 | 6.6 | 129 (7) | 2335.6 | 5.5 |
| Urinary tract infection | 248 (10) | 3782.5 | 6.6 | 120 (6) | 2349.2 | 5.1 |
| Nausea | 243 (10) | 3720.6 | 6.5 | 119 (6) | 2344.3 | 5.1 |
| Viral upper respiratory tract infection | 228 (10) | 3694.2 | 6.2 | 137 (7) | 2296.3 | 6.0 |
| Pneumonia | 212 (9) | 3869.7 | 5.5 | 118 (6) | 2396.3 | 4.9 |
| Constipation | 209 (9) | 3769.0 | 5.5 | 102 (5) | 2369.8 | 4.3 |
| Upper respiratory tract infection | 187 (8) | 3762.5 | 5.0 | 110 (6) | 2339.8 | 4.7 |
| Headache | 178 (8) | 3787.2 | 4.7 | 103 (6) | 2355.2 | 4.4 |
| Cough | 170 (7) | 3815.0 | 4.5 | 90 (5) | 2369.3 | 3.8 |
| Vomiting | 148 (6) | 3859.4 | 3.8 | 76 (4) | 2396.5 | 3.2 |
| Dizziness | 146 (6) | 3827.9 | 3.8 | 110 (6) | 2356.9 | 4.7 |
| Hypoglycemia | 146 (6) | 3889.6 | 3.8 | 77 (4) | 2396.3 | 3.2 |
| Back pain | 138 (6) | 3851.4 | 3.6 | 71 (4) | 2370.0 | 3.0 |
| Pruritus | 138 (6) | 3869.6 | 3.6 | 86 (5) | 2376.2 | 3.6 |
| Insomnia | 131 (6) | 3872.1 | 3.4 | 44 (2) | 2421.3 | 1.8 |
| Dyspnea | 124 (5) | 3919.8 | 3.2 | 90 (5) | 2392.6 | 3.8 |
| Arthralgia | 121 (5) | 3874.4 | 3.1 | 73 (4) | 2387.0 | 3.1 |
| AKI | 121 (5) | 3934.6 | 3.1 | 53 (3) | 2431.8 | 2.2 |
| Anemia | 51 (2) | 4003.1 | 1.3 | 101 (5) | 2399.3 | 4.2 |
Patients with more than one event in a category were counted once for that category. On treatment+28, a treatment-emergent adverse event occurred (or a preexisting condition worsened) during the treatment period and within 28 days of the last dose of study drug; PY, patient years; FAIR, follow-up–adjusted incidence rate.
Medical Dictionary for Regulatory Activities version 20.0.
PY for each patient=(first event occurrence or censor date−first-dose date+1)/ 365.25.
FAIR per 100 PY=100×number of patients with events/PY.
Reported as “end stage renal disease.”
A summary of treatment-emergent SAEs occurring in ≥1% of patients in either treatment group during the on-treatment+28 days period is provided in Supplemental Table 5. The follow-up–adjusted incidence rates for treatment-emergent SAEs in total were comparable in the roxadustat versus placebo groups (45.9 versus 43.9 per 100 patient follow-up years, respectively).
The rates of treatment-emergent AEs and SAEs in the neoplasm system organ class were similar between treatment groups (2.5 events per 100 patient-exposure years for both arms; 1.1 and 1.3 events per 100 patient-exposure years for roxadustat and placebo arms, respectively). In the analysis including long-term follow-up, fatal AEs were experienced by 361 of 2386 patients (15%) treated with roxadustat and 247 of 1884 patients (13%) treated with placebo, with follow-up–adjusted incidence rates of 8.3 and 8.1 per 100 patient follow-up years, respectively. When including deaths found by a public records search that may not have a corresponding reported AE, deaths were noted in 400 (17%) patients treated with roxadustat and 301 (16%) patients treated with placebo, with follow-up–adjusted incidence rates of 8.3 and 8.1 per 100 patient follow-up years, respectively. Vascular access thrombosis events were adjudicated at a rate of 1.5 and 0.9 events per 100 patient years for patients treated with roxadustat and those treated with placebo, respectively (Supplemental Table 6). Deep vein thrombosis events were adjudicated for 0.7 and 0.2 events per 100 patient years, pulmonary embolism events for 0.3 and 0.1 events per 100 patient years, and hypertensive emergency events for 1.5 and 1.3 events per 100 patient years for patients treated with roxadustat and those treated with placebo, respectively. Treatment-emergent seizures were reported for roxadustat versus placebo at a rate of 0.6 and 0.2 per 100 patient years, respectively.
Discussion
This study pooled data from three phase 3 studies examining the efficacy and safety of roxadustat relative to placebo in patients with non–dialysis-dependent CKD. Treatment with roxadustat versus placebo met both the US and EU primary efficacy end points in all three individual studies and the pooled analysis. Roxadustat was superior to placebo for increasing hemoglobin, and a larger percentage of patients treated with roxadustat achieved a hemoglobin response, even those with elevated baseline high-sensitivity C-reactive protein, a biomarker for inflammation. Roxadustat also increased hemoglobin versus placebo among patients who were either iron replete or not iron replete at baseline.
Roxadustat markedly reduced the need for RBC transfusion compared with placebo, with an absolute and relative risk reduction of 14.3 events per 100 patient-exposure years and 74%, respectively, during the first 52 weeks of treatment. Roxadustat’s ability to reduce RBC transfusion risk in patients with CKD-related anemia not on dialysis reduces transfusion-related risk for allosensitization, potentially improving a patient’s chance of receiving a kidney transplant (6,30).
Importantly, roxadustat demonstrated noninferior cardiovascular safety to placebo in patients with non–dialysis-dependent CKD and anemia. The HRs for MACE, MACE+, and all-cause mortality met the reference noninferiority risk margin. A decomposition of MACE and MACE+ end points into the first occurrence of its components showed broadly similar rates between the treatment groups, with all-cause mortality being the main driver of first-event counts (74% in MACE and 51% in MACE+). Analyses of MACE showed consistent results across the various subgroups studied (Supplemental Figure 5).
It is important to note, however, that the treatment discontinuation rate for patients in the placebo arm was greater than that in the roxadustat arm. This could indicate that nontreatment of anemia may cause difficulties for patients with non–dialysis-dependent CKD, and is noteworthy from a methodologic perspective. Although there were 27% more patients treated with roxadustat versus those treated with placebo who had non–dialysis-dependent CKD in the pooled dataset due to the randomization ratio, patients in the roxadustat group had 67% more treatment exposure time because of a greater discontinuation rate in the placebo arm, most likely due to the lack of efficacy for anemia correction (Supplemental Figure 6, Supplemental Table 4). The marked between-group difference in exposure widened over time and was particularly prominent among patients with the lowest eGFR who were at the highest risk of morbidity and mortality (28,29). Among patients with baseline eGFR <10 ml/min per 1.73 m2, only approximately 40% had >6 months exposure to placebo, compared with approximately 85% for roxadustat (Supplemental Figure 6). Although this small subgroup of patients experienced a higher point estimate for MACE than the total cohort, it is most affected by the differential dropout and potential for informative censoring, which complicates interpretation of these results.
Recently, Chertow et al. (31) published the results of the phase 3 program for vadadustat in patients with non– dialysis-dependent CKD. In the pooled analysis of the trials comparing vadadustat with darbepoetin alfa, powered for a noninferiority margin of 1.25, the HR for MACE was 1.17 (95% CI, 1.01 to 1.36). Comparison of these results with those presented for roxadustat needs to be interpreted in the context of the different comparators (placebo versus darbepoetin alfa), and how the comparator should affect the choice of noninferiority margin (in other words, the desire to rule out an increased risk against placebo as compared with a comparator with a boxed warning for cardiovascular risk).
Currently available treatments for anemia in patients with non–dialysis-dependent CKD include ESAs, oral/IV iron, and RBC transfusion. Since the completion of sentinel ESA studies such as Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) (7), Cardiovascular Risk Reduction in Early Anemia Treatment with Epoetin Beta (CREATE) (32), and Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) (6), the proportion of patients with non–dialysis-dependent CKD treated for anemia has declined. In 2003, approximately 33% of US patients who initiated dialysis were reported to have received ESA beforehand (33). In 2014, the percentage decreased to 14% (34), most likely related to the ESA label changes made as a result of the cardiovascular safety signal identified in these studies, advising physicians to use the lowest doses sufficient to reduce the need for RBC transfusions (35). The use of placebo as a comparator may more accurately reflect the current standard of care where ESA treatment is less likely to be prescribed, and is arguably one of the gold standards for assessing the safety of any drug. The trials were designed to compare roxadustat against placebo on the basis of epidemiologic data showing that few patients with CKD not requiring dialysis receive either ESAs or IV iron (36). These data cannot, therefore, address the question of how treatment with roxadustat would compare with treatment with an ESA in patients with non–dialysis-dependent CKD.
Although the pooled analyses presented here show results consistent with those of the constituent studies, we acknowledge certain limitations. First, differential treatment exposure between the roxadustat and placebo groups, with greater differences among patients with lower baseline eGFR, introduced the potential for informative censoring for safety outcomes observed on treatment. This complicates interpretation of the safety data and may have biased against roxadustat because higher-risk patients had a greater duration of treatment exposure. Therefore, the cardiovascular safety analysis included the long-term follow-up. Second, the high study drug discontinuation rates in both treatment groups may complicate interpretation of our cardiovascular safety findings. However, all patients who discontinued the study drug before the end of the study were monitored for MACE until the end of the study, unless consent was withdrawn.
This study of data pooled from three phase 3 clinical studies of roxadustat versus placebo in patients with non–dialysis-dependent CKD included patients with more advanced disease, more severe anemia, and less iron repletion at baseline, compared with historical studies in patients with non–dialysis-dependent CKD and anemia. Roxadustat was superior to placebo for increasing hemoglobin, decreased the risk of RBC transfusions, and was noninferior to placebo for the risk of MACE.
Disclosures
L. Frison, J. Houghton, M.T. Houser, and D. J. Little report being employees of AstraZeneca and hold stock and/or stock options in AstraZeneca. T.T. Lee, R. Leong, K.G. Saikali, L. Szczech, and M. Zhong report being employees of FibroGen and hold stock and/or stock options in FibroGen. D.J. Little reports volunteering as a nephrologist at Walter Reed National Military Medical Center. R. Provenzano reports holding stock in AstraZeneca, DaVita, and FibroGen; serving as a consultant for AstraZeneca, DaVita, FibroGen, and Rockwell International; serving on a speakers bureau for, receiving honoraria from, and serving as a scientific advisor or member of AstraZeneca and FibroGen; and having other interests in/relationships with Nephroceuticals and Vasc-Alert. L. Szczech reports serving on the American Society of Nephrology Publications Committee (2017–2019).
Funding
These studies were sponsored by FibroGen, AstraZeneca, and Astellas.
Supplementary Material
Acknowledgments
We would like to acknowledge the participants who volunteered, the clinical staff that who care of them, the study staff who captured and cleaned the data, and the scientists who developed and manufactured roxadustat.
The sponsors had a role in study design, data collection, data analysis, data interpretation, and writing of the manuscript. All authors had full access to all of the study data and had responsibility for the decision to submit the manuscript for publication.
Assistance with manuscript preparation was provided by Dawn Thiselton of FibroGen, Inc. and Linda Goldstein of The Write Source, LLC.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Cardiovascular Safety of Roxadustat in CKD Anemia: A Fig Leaf Named Noninferiority,” on pages 1155–1157.
Data Sharing Statement
Research data will not be shared.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.16191020/-/DCSupplemental.
Supplemental Figure 1. Analysis of MACE by individual study in patients with non–dialysis-dependent CKD (safety analysis set, ITT analysis).
Supplemental Figure 2. Analysis of MACE, MACE+, and all-cause mortality in patients with non–dialysis-dependent CKD and eGFR ≥10 mL/min/1.73 m2 (safety analysis set, ITT analysis).
Supplemental Figure 3. Analysis of MACE, MACE+, and all-cause mortality in patients with non–dialysis-dependent CKD (ITT analysis with follow-up limited to no more than 1 year).
Supplemental Figure 4. Sensitivity analysis in non–dialysis-dependent patients using post hoc stratification factors (safety analysis set, ITT analysis).
Supplemental Figure 5. Subgroup analysis of MACE in patients with non–dialysis-dependent CKD (safety analysis set, ITT analysis).
Supplemental Figure 6. Percentage of patients on treatment over time by baseline eGFR category (safety analysis set).
Supplemental Table 1. Summary of phase 3 clinical trials pooled in patients with non–dialysis-dependent CKD.
Supplemental Table 2. Roxadustat dose adjustment algorithm.
Supplemental Table 3. Baseline characteristics of participants in individual phase 3 studies of roxadustat with non–dialysis-dependent chronic kidney disease.
Supplemental Table 4. Number of patients with non–dialysis-dependent CKD, and exposure by study and overall (safety analysis set).
Supplemental Table 5. Serious treatment-emergent adverse events occurring in ≥1% of patients with non–dialysis-dependent CKD in either treatment group (on-treatment+28).
Supplemental Table 6. Additional adjudicated events (safety analysis set, ITT analysis).
References
- 1.Coyne DW, Goldsmith D, Macdougall IC: New options for the anemia of chronic kidney disease. Kidney Int Suppl (2011) 7: 157–163, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes : KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl 2: 279–335, 2012 [Google Scholar]
- 3.Gilbertson DT, Monda KL, Bradbury BD, Collins AJ: RBC transfusions among hemodialysis patients (1999-2010): Influence of hemoglobin concentrations below 10 g/dL. Am J Kidney Dis 62: 919–928, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K: History of erythropoiesis-stimulating agents, the development of biosimilars, and the future of anemia treatment in nephrology. Am J Nephrol 45: 235–247, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R; TREAT Investigators: A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D; CHOIR Investigators: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Haase VH: HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial Int 21: S110–S124, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr: HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science 292: 464–468, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ: Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Wang GLJB, Jiang BH, Rue EA, Semenza GL: Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 92: 5510–5514, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang GLSG, Semenza GL: Purification and characterization of hypoxia-inducible factor 1. J Biol Chem 270: 1230–1237, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Koury MJ, Haase VH: Anaemia in kidney disease: Harnessing hypoxia responses for therapy. Nat Rev Nephrol 11: 394–410, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen N, Hao C, Liu BC, Lin H, Wang C, Xing C, Liang X, Jiang G, Liu Z, Li X, Zuo L, Luo L, Wang J, Zhao MH, Liu Z, Cai GY, Hao L, Leong R, Wang C, Liu C, Neff T, Szczech L, Yu KP: Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med 381: 1011–1022, 2019b [DOI] [PubMed] [Google Scholar]
- 15.Chen N, Hao C, Peng X, Lin H, Yin A, Hao L, Tao Y, Liang X, Liu Z, Xing C, Chen J, Luo L, Zuo L, Liao Y, Liu BC, Leong R, Wang C, Liu C, Neff T, Szczech L, Yu KP: Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med 381: 1001–1010, 2019a [DOI] [PubMed] [Google Scholar]
- 16.Akizawa T, Otsuka T, Reusch M, Ueno M: Intermittent oral dosing of roxadustat in peritoneal dialysis chronic kidney disease patients with anemia: A randomized, phase 3, multicenter, open-label study. Ther Apher Dial 24: 115–125, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akizawa T, Iwasaki M, Yamaguchi Y, Majikawa Y, Reusch M: Phase 3, randomized, double-blind, active-comparator (darbepoetin alfa) study of oral roxadustat in CKD patients with anemia on hemodialysis in Japan. J Am Soc Nephrol 31: 1628–1639, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akizawa T, Ueno M, Shiga T, Reusch M: Oral roxadustat three times weekly in ESA-naïve and ESA-converted patients with anemia of chronic kidney disease on hemodialysis: Results from two phase 3 studies. Ther Apher Dial 24: 628–641, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akizawa T, Yamaguchi Y, Otsuka T, Reusch M: A phase 3, multicenter, randomized, two-arm, open-label study of intermittent oral dosing of roxadustat for the treatment of anemia in Japanese erythropoeisis-stimulating agent-naive chronic kidney disease patients not on dialysis. Nephron 144: 372–382, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coyne DW, Roger SD, Shin SK, Kim SG, Cadena AA, Moustafa MA, Chan TM, Besarab A, Chou W, Bradley C, Eyassu M, Leong R, Lee TT, Saikali KG, Szczech L, Yu KP: Roxadustat for CKD-related anemia in non-dialysis patients. Kidney Int Rep 6: 624–635, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shutov E, Sulowicz W, Esposito C, Tataradze A, Andric B, Reusch M, Valluri U, Dimkovic N: Roxadustat for the treatment of anemia in chronic kidney disease patients not on dialysis: A phase 3, randomized, double-blind, placebo-controlled study (ALPS) [published online ahead of print February 25, 2021]. Nephrol Dial Transplant [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fishbane S, El-Shahawy MA, Pecoits-Filho R, Van BP, Houser MT, Frison L, Little DJ, Guzman NJ, Pergola PE: Roxadustat for treating anemia in patients with CKD not on dialysis: Results from a randomized phase 3 study. J Am Soc Nephrol 32: 737–755, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins AJ, Foley RN, Gilbertson DT, Chen SC: United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int Suppl (2011) 5: 2–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macdougall IC, Provenzano R, Sharma A, Spinowitz BS, Schmidt RJ, Pergola PE, Zabaneh RI, Tong-Starksen S, Mayo MR, Tang H, Polu KR, Duliege AM, Fishbane S; PEARL Study Groups: Peginesatide for anemia in patients with chronic kidney disease not receiving dialysis. N Engl J Med 368: 320–332, 2013 [DOI] [PubMed] [Google Scholar]
- 25.United States Department of Health and Human Services, Food and Drug Administration : Guidance for industry on diabetes mellitus–Evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes; availability. Fed Regist 73: 77724–77725, 2008 [Google Scholar]
- 26.United States Food and Drug Administration : FDA Briefing Document for the Oncologic Drugs Advisory Committee (ODAC), 2011. Available at: https://www.fda.gov/advisory-committees/human-drug-advisory-committees/oncologicdrugs-advisory-committee. Accessed July 16, 2021
- 27.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F: Methods for Meta-Analysis in Medical Research, Blackwell, Chichester, United Kingdom, Wiley, 2000. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1002/sim.1565. Accessed July 16, 2021 [Google Scholar]
- 28.United States Renal Data System : Medicare expenditures for persons with CKD. USRDS Annual Data Report, Bethesda, MD, National Institute of Diabetes and Digestive and Kidney Diseases, 2016, pp 133–144. Available at: https://usrds.org/annual-data-report/previous-adrs/. Accessed July 16, 2021 [Google Scholar]
- 29.Khan YHSA, Sarriff A, Adnan AS, Khan AH, Mallhi TH, Jummaat F: Progression and outcomes of non-dialysis dependent chronic kidney disease patients: A single center longitudinal follow-up study. Nephrology (Carlton) 22: 25–34, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Lawler EV, Bradbury BD, Fonda JR, Gaziano JM, Gagnon DR: Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol 5: 667–672, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chertow GM, Pergola PE, Farag YMK, Agarwal R, Arnold S, Bako G, Block GA, Burke S, Castillo FP, Jardine AG, Khawaja Z, Koury MJ, Lewis EF, Lin T, Luo W, Maroni BJ, Matsushita K, McCullough PA, Parfrey PS, Roy-Chaudhury P, Sarnak MJ, Sharma A, Spinowitz B, Tseng C, Tumlin J, Vargo DL, Walters KA, Winkelmayer WC, Wittes J, Eckardt KU; PRO2TECT Study Group: Vadadustat in patients with anemia and non-dialysis-dependent CKD. N Engl J Med 384: 1589–1600, 2021 [DOI] [PubMed] [Google Scholar]
- 32.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A; CREATE Investigators: Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 33.United States Renal Data System : USRDS Annual Data Report: Epidemiology of kidney disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014. Available at: https://usrds.org/annual-data-report/previous-adrs/. Accessed July 16, 2021 [Google Scholar]
- 34.United States Renal Data System : Incidence, prevalence, patient characteristics, and treatment modalities. USRDS Annual Data Report, Bethesda, MD, National Institute of Diabetes and Digestive and Kidney Diseases, 2018, pp 291–332 [Google Scholar]
- 35.Amgen : Epogen (epoetin alfa): Highlights of prescribing information, Thousand Oaks, CA, Amgen, Inc., 2018 [Google Scholar]
- 36.United States Renal Data System : USRDS Annual Data Report: Epidemiology of kidney disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2020. Available at: https://usrds.org/annual-data-report/previous-adrs/. Accessed July 16, 2021 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.