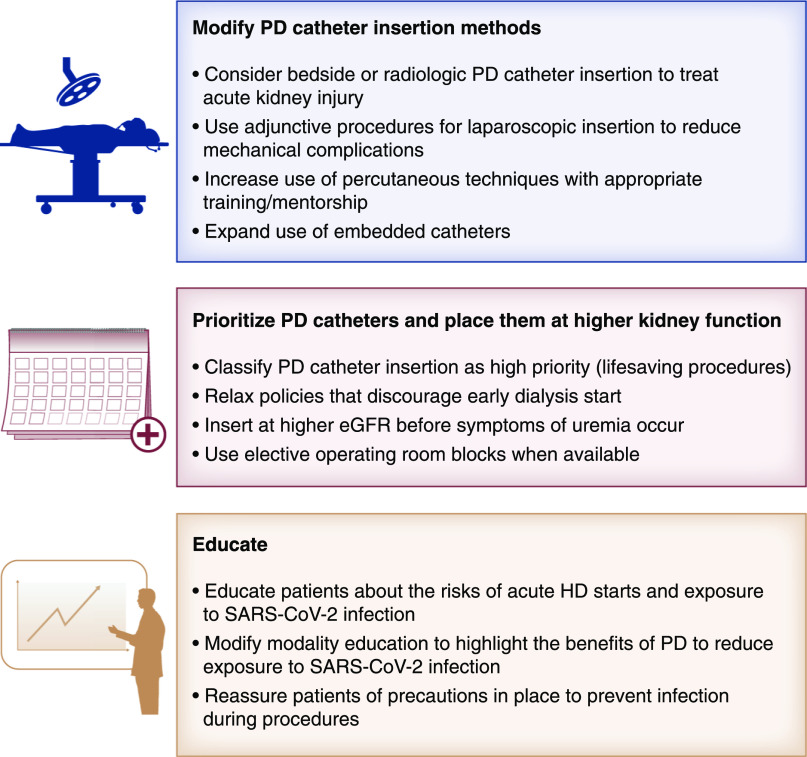
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in individuals receiving dialysis can have devastating consequences with a mortality ranging from 21% to 27% (1 –3). In Ontario, Canada, the risk of infection for patients on in-center hemodialysis was 2.5 times higher than home dialysis (1). In Europe, only 125 patients receiving peritoneal dialysis (PD) contracted SARS-CoV-2 infection compared with 3160 receiving in-center hemodialysis, although the coronavirus disease 2019–attributable mortality was similar at 20% (2). Patients on PD may be at less risk because they have less comorbidity, but also, they have a greater ability to self-isolate. Although hemodialysis programs have instituted strict practices to prevent transmission, outbreaks within hemodialysis units have been reported among staff and patients (4). PD offers the advantage of being a simpler form of home therapy to institute with less training time than home hemodialysis. For these reasons, it seems prudent to promote PD during the coronavirus disease 2019 pandemic. One of the key steps to expanding PD is successful PD catheter insertion, which requires advanced planning, prioritization of operating room time, expansion of percutaneous methods, and new operator training. Government policy and patient education should reframe PD access as a potentially lifesaving procedure (Figure 1).
Figure 1.
Strategies to facilitate peritoneal dialysis (PD) catheter insertion and facilitate PD during the coronavirus disease 2019 pandemic. HD, hemodialysis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The most common method of creating elective PD access in North America is laparoscopic catheter placement. Laparoscopy enables PD access in patients with prior major abdominal surgeries, previous peritonitis, and pronounced central obesity. Laparoscopic PD catheter implantation permits adjunctive procedures (e.g., rectus sheath tunneling, omentopexy, and adhesiolysis) to prevent common mechanical complications, such as leaks and flow restrictions. Disappointingly, the North American PD Catheter Registry reveals that these advanced proactive techniques are only being used less than half the time (5). Laparoscopy requires general anesthesia, representing a contraindication in patients with significant comorbidities, especially acute pulmonary disease. During intubation and extubation, only anesthesia personnel should be in the operating room and attired in appropriate personal protective equipment (N95 masks and face shields or goggles) because it is an aerosol-generating procedure. Even though preoperative SARS-CoV-2 testing may be negative, consideration should still be given to using precautionary measures. To date, there has been no substantial evidence against the use of laparoscopy during the pandemic. Aerosolization of the virus from the abdominal cavity can be minimized by effective evacuation of smoke from electrosurgical devices and using reduced abdominal insufflation pressures. Fortunately, laparoscopic PD catheter insertion seldom requires energy devices or high pneumoperitoneum pressures.
During the pandemic, acute PD has been used to treat AKI with percutaneous catheter placement at the bedside by a surgeon or nephrologist or percutaneous insertion by a radiologist using ultrasonographic and fluoroscopic guidance (6). The advantage of this approach is that the procedure can be performed under local anesthesia. Contraindications for percutaneous placement include previous multiple or major abdominal surgeries, marked central obesity, significant abdominal wall hernias, and the inability of the patient to lay flat or control anxiety. Percutaneous techniques, even with image guidance, do not allow adhesiolysis or omentopexy, resulting in a higher incidence of catheter flow dysfunction, necessitating laparoscopic revision at a time when operating room resources are already strained. Despite these limitations, expanding percutaneous approaches, which may be more common outside North America, should occur where possible.
Pericatheter leakage is a possible complication with all methods of insertion. The risk of leaks can be minimized by paramedian catheter insertion through the body of the rectus sheath and muscle, musculofascial tunneling of the catheter in a long tangential passage through the rectus sheath, positioning the deep Dacron cuff in the rectus muscle, and placement of a fascial purse-string suture around the catheter (7). Both laparoscopic and percutaneous methods introduce port and sheath conduits for catheter insertion by radial tissue expansion as opposed to cutting a pathway through the abdominal wall with open dissection approaches. The resiliency of the tissues with radial expansion results in the collapse of the tissue track against the catheter tubing, helping to further reduce the risk of a leak. In addition to these technical details, the risk of leaks is influenced by how soon dialysis is initiated, the volume of instilled fluid, and physical activities that increase intraperitoneal pressure. For percutaneous insertions to treat AKI, PD was started <24 hours after catheter insertion, which increases the risk of exit site leaks (6). Programs modifying their methods of PD access should periodically audit complications and compare them with best practice benchmarks. Guidelines suggest clinical goals of hemorrhage requiring transfusion or surgical intervention of <1%, visceral injury (bowel, bladder, or solid organ) of <1%, and risk of both exit site/tunnel infection or peritonitis of <5% within 30 days of catheter insertion (7). Pericatheter leakage rates for urgent and planned starts will differ and should be reported separately.
Health care policy should prioritize PD catheter placement. The Centers for Medicare & Medicaid Services clarified that PD access is a lifesaving procedure. The Ontario Renal Network and the British Renal Association recommended PD catheter insertions not be considered elective or routine procedures. These policies allowed surgical PD catheter insertions to continue, even when other important surgeries were postponed. PD catheter insertions at higher kidney function should also be considered. Prior to the pandemic, the mean eGFR at insertion for patients predialysis was 8.8 ml/min, which is relatively low and could increase the risk of urgent hemodialysis starts. In Canada, policies that discourage earlier dialysis starts at higher kidney function should be relaxed (8).
Expanding the role of embedded catheters is another strategy to consider during the pandemic. Catheter embedding consists of burying the catheter's external limb under the skin at the time of implantation (9). The external limb is exteriorized months later in the clinic or at bedside when dialysis is needed. If there are periods when surgical centers resume more normal operating room capacity, catheter embedment facilitates earlier insertion. Because embedded catheters have extended healing time, patients can proceed to full-volume PD without the necessity of a break-in period or concerns for pericatheter leakage. Catheter embedding is contraindicated in patients having previous major abdominopelvic surgery in which the need for adhesiolysis is likely because adhesions will reform and obstruct a nonflushed catheter. PD access providers who have a history of poor outcomes with conventional catheter placements should not be embedding catheters without first gaining proficiency with the former.
Finally, for this PD strategy to succeed, patient education is paramount. Modality education is associated with a two- to three-fold increase in PD use (10), and about 50% of patients elect PD when offered it (10). Because of the pandemic, more patients are likely to choose PD to reduce their risk of exposure to the SARS-CoV-2 virus. PD programs should update their modality education to emphasize this new benefit. Patients should also be educated to have PD catheter insertions before they are symptomatic to reduce the risk of an urgent hemodialysis start. Patients should be reassured that precautions are in place to prevent infection during procedures.
These recommended strategies may also benefit patients after the pandemic. Diversifying insertion methods provides a nonsurgical option for high-risk patients and off-loads operating room resources that could be limited in the future due to another pandemic, a natural disaster, or financial constraints. Moving procedures outside of hospitals can also reduce exposure to infections in general. Broadening the timing of insertion to range from embedded catheters to urgent PD starts will also provide more opportunities for patients to receive PD.
Disclosures
J.H. Crabtree reports consultancy agreements with Baxter Healthcare, DaVita, Medtronic, and Merit Medical; receiving honoraria from Baxter Healthcare, DaVita, Fresenius Medical Care, Medtronic, and Merit Medical; serving as a scientific advisor or member of Peritoneal Dialysis International Journal; and speakers bureau for Baxter Healthcare, DaVita, Fresenius Medical Care, and Merit Medical. M.J. Oliver reports receiving honoraria from Amgen, Baxter Healthcare, and Janssen; receives a stipend from the Ontario Renal Network as the Regional Medical Lead for Toronto Central; has participated in advisory boards for Janssen and Amgen; and reports that Baxter Healthcare and Medtronic have provided funding support to create a PD catheter registry for the International Society of Peritoneal Dialysis. M.J. Oliver is the sole owner of Oliver Medical Management Inc., a private corporation that licenses the Dialysis Measurement Analysis and Reporting (DMAR) software system. Oliver Medical Management Inc. is co-owner of a Canadian patent for DMAR systems.
Funding
None.
Acknowledgments
This manuscript was produced on behalf of the American Society of Nephrology COVID-19 Home Dialysis Subcommittee.
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or CJASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Jager KJ, Kramer A, Chesnaye NC, Couchoud C, Sánchez-Álvarez JE, Garneata L, Collart F, Hemmelder MH, Ambühl P, Kerschbaum J, Legeai C, Del Pino Y Pino MD, Mircescu G, Mazzoleni L, Hoekstra T, Winzeler R, Mayer G, Stel VS, Wanner C, Zoccali C, Massy ZA: Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int 98: 1540–1548, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taji L, Thomas D, Oliver MJ, Ip J, Tang Y, Yeung A, Cooper R, House AA, McFarlane P, Blake PG: COVID-19 in patients undergoing long-term dialysis in Ontario. CMAJ 193: E278–E284, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sim JJ, Huang CW, Selevan DC, Chung J, Rutkowski MP, Zhou H: COVID-19 and survival in maintenance dialysis. Kidney Med 3: 132–135, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yau K, Muller MP, Lin M, Siddiqui N, Neskovic S, Shokar G, Fattouh R, Matukas LM, Beaubien-Souligny W, Thomas A, Weinstein JJ, Zaltzman J, Wald R: COVID-19 outbreak in an urban hemodialysis unit. Am J Kidney Dis 76: 690–695.e1, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver MJ, Perl J, McQuillan R, Blake PG, Jain AK, McCormick B, Yang R, Pirkle JL Jr, Fissell RB, Golper TA, Shen JI, Hu SL, Pellegrino B, Liebman SE, Krishna VN, Ravani P, Clarke A, Quinn RR: Quantifying the risk of insertion-related peritoneal dialysis catheter complications following laparoscopic placement: Results from the North American PD Catheter Registry. Perit Dial Int 40: 185–192, 2020 [DOI] [PubMed] [Google Scholar]
- 6.El Shamy O, Patel N, Abdelbaset MH, Chenet L, Tokita J, Lookstein R, Lee DS, Cohen NA, Sharma S, Uribarri J: Acute start peritoneal dialysis during the COVID-19 pandemic: Outcomes and experiences. J Am Soc Nephrol 31: 1680–1682, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crabtree JH, Shrestha BM, Chow KM, Figueiredo AE, Povlsen JV, Wilkie M, Abdel-Aal A, Cullis B, Goh BL, Briggs VR, Brown EA, Dor FJMF: Creating and maintaining optimal peritoneal dialysis access in the adult patient: 2019 update. Perit Dial Int 39: 414–436, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Nesrallah GE, Mustafa RA, Clark WF, Bass A, Barnieh L, Hemmelgarn BR, Klarenbach S, Quinn RR, Hiremath S, Ravani P, Sood MM, Moist LM; Canadian Society of Nephrology: Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. CMAJ 186: 112–117, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown PA, McCormick BB, Knoll G, Su Y, Doucette S, Fergusson D, Lavoie S: Complications and catheter survival with prolonged embedding of peritoneal dialysis catheters. Nephrol Dial Transplant 23: 2299–2303, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Devoe DJ, Wong B, James MT, Ravani P, Oliver MJ, Barnieh L, Roberts DJ, Pauly R, Manns BJ, Kappel J, Quinn RR: Patient education and peritoneal dialysis modality selection: A systematic review and meta-analysis. Am J Kidney Dis 68: 422–433, 2016 [DOI] [PubMed] [Google Scholar]