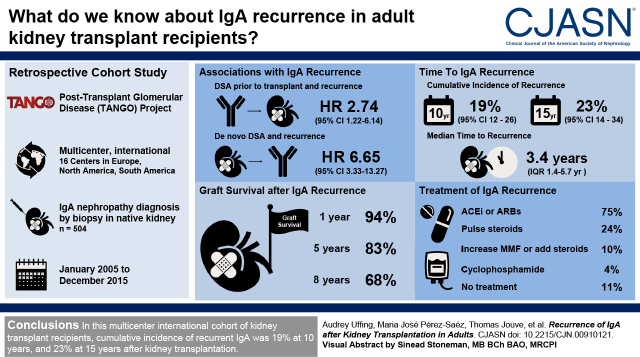
Visual Abstract
Keywords: kidney transplantation, IgA nephropathy, IgA deposition, glomerular disease, recurrence, graft survival
Abstract
Background and objectives
In patients with kidney failure due to IgA nephropathy, IgA deposits can recur in a subsequent kidney transplant. The incidence, effect, and risk factors of IgA nephropathy recurrence is unclear, because most studies have been single center and sample sizes are relatively small.
Design, setting, participants, & measurements
We performed a multicenter, international, retrospective study to determine the incidence, risk factors, and treatment response of recurrent IgA nephropathy after kidney transplantation. Data were collected from all consecutive patients with biopsy-proven IgA nephropathy transplanted between 2005 and 2015, across 16 “The Post-Transplant Glomerular Disease” study centers in Europe, North America, and South America.
Results
Out of 504 transplant recipients with IgA nephropathy, recurrent IgA deposits were identified by kidney biopsy in 82 patients; cumulative incidence of recurrence was 23% at 15 years (95% confidence interval, 14 to 34). Multivariable Cox regression revealed a higher risk for recurrence of IgA deposits in patients with a pre-emptive kidney transplant (hazard ratio, 3.45; 95% confidence interval, 1.31 to 9.17) and in patients with preformed donor-specific antibodies (hazard ratio, 2.59; 95% confidence interval, 1.09 to 6.19). After kidney transplantation, development of de novo donor-specific antibodies was associated with subsequent higher risk of recurrence of IgA nephropathy (hazard ratio, 6.65; 95% confidence interval, 3.33 to 13.27). Immunosuppressive regimen was not associated with recurrent IgA nephropathy in multivariable analysis, including steroid use. Graft loss was higher in patients with recurrence of IgA nephropathy compared with patients without (hazard ratio, 3.69; 95% confidence interval, 2.04 to 6.66), resulting in 32% (95% confidence interval, 50 to 82) graft loss at 8 years after diagnosis of recurrence.
Conclusions
In our international cohort, cumulative risk of IgA nephropathy recurrence increased after transplant and was associated with a 3.7-fold greater risk of graft loss.
Introduction
In patients who received a kidney transplantation for kidney failure due to IgA nephropathy, IgA deposits can recur in the transplanted kidney. The clinical course of recurrent IgA nephropathy is variable because it can be diagnosed in patients on a protocol biopsy who are asymptomatic, in patients with mild hematuria or proteinuria, or in patients with rapidly deteriorating kidney function. As a result, reported rates of recurrence vary significantly between 9% and 61%, mainly due to diverse biopsy protocols and differences in follow-up (1).
Recent studies have shown that recurrence of IgA nephropathy usually manifests a couple of years after transplantation, and longer follow-up studies showed lower survival rates after 5–10 years (2). Reported graft loss due to recurrent IgA nephropathy varies from 2% to 14% in studies with medium follow-up (3), but increase up to 29% in patients with symptomatic recurrent disease in long follow-up studies (2). A number of risk factors for IgA nephropathy recurrence have been described, including younger age at transplant, transplant without an induction agent, higher HLA-mismatch, and early steroid withdrawal immunosuppressive regimens (4–13). Because most performed studies are single center with relatively small sample sizes, outcomes are difficult to generalize and risk factors often cannot be validated in subsequent studies (Supplemental Table 1).
As part of The Post-Transplant Glomerular Disease (TANGO) project, we analyzed detailed retrospective clinical data from patients with biopsy-proven IgA nephropathy in 16 centers located in three continents. In this study, we report IgA nephropathy recurrence rates, risk factors for recurrence, treatment strategies, and outcomes.
Materials and Methods
Study Design, Objectives, and Risk Factors
We performed a multicenter, international, retrospective study in patients from 16 TANGO kidney transplant centers in Europe, North America, and South America (14). The primary objective was to determine the incidence of recurrent IgA nephropathy after kidney transplantation in patients with a biopsy-proven native diagnosis of IgA nephropathy. Secondary objectives included identification of risk factors for IgA nephropathy recurrence, clinical outcomes of patients with and without IgA nephropathy recurrence, and treatment strategies of IgA nephropathy recurrence (see Supplemental Methods for further details).
Patient Selection and Data Collection
In participating centers, all adult (aged >16 years) kidney transplant recipients between January 2005 and December 2015, with a biopsy-proven native diagnosis of IgA nephropathy were included. Detailed patient information was extracted from medical records. Patients were censored at the time of graft loss, patient death, loss to follow-up, or in January 2020.
In the primary analysis on incidence and risk factors for IgA nephropathy recurrence, one participating center in Brazil was excluded because biopsies on native kidneys were not routinely performed. However, we included patients with recurrent IgA nephropathy and pretransplant clinical course suspect for IgA nephropathy (e.g., active urine sediment, proteinuria, no other explanation of symptoms) from this center (n=26) in the analysis to treatment and outcomes of recurrent IgA deposits.
Statistical Analysis
Data are shown as frequencies (percentages) for categorical variables, and medians (interquartile range) or mean±SD for continuous variables. Statistical analysis of Table 1 was done by complete case analysis. Continuous variables were analyzed by t test; binary and categorical variables by chi-squared or Fisher’s exact test, depending on group size.
Table 1.
Baseline characteristics of participants in The Post-Transplant Glomerular Disease project with kidney failure due to IgA nephropathy and their kidney donors
| Baseline Characteristic | Overall Cohort (n=504) |
No Recurrence (n=422) |
Recurrence (n=82) |
| Follow-up, yrs | 8.7 (5.5–11.2) | 8.8 (5.5–11.3) | 8.2 (5.7–10.6) |
| Age at transplantation, yrs | 46 (37–55) | 46 (38–56) | 41 (32–54) |
| Age at diagnosis, yrs | 33 (26–44) | 34 (26–44) | 31 (25–41) |
| Male sex | 362 (72) | 302 (72) | 60 (73) |
| Race/Ethnicity | |||
| White | 354 (70) | 294 (70) | 60 (73) |
| Black | 13 (3) | 12 (3) | 1 (1) |
| Hispanic | 16 (3) | 14 (3) | 2 (2) |
| Asian | 33 (7) | 28 (7) | 5 (6) |
| Mixed | 5 (1) | 5 (1) | 0 (0) |
| Other/unknown | 83 (16) | 69 (16) | 14 (17) |
| BMI at transplantation, kg/m2 | 25.8±4.6 | 25.9±4.6 | 25.1±4.4 |
| Diseases associated with IgA nephropathy | |||
| Symptoms of Henoch-Schönlein purpura | 27 (5) | 23 (5) | 4 (5) |
| Autoimmune disease | 22 (4) | 18 (4) | 4 (5) |
| Liver disease | 7 (1) | 7 (2) | 0 (0) |
| Time from diagnosis to KF, mos | 66 (15–135) | 72 (17–143) | 48 (12–96) |
| Time on dialysis, mos | 17 (4–44) | 19 (5–44) | 11 (0–43) |
| Type of dialysis | |||
| Hemodialysis | 283 (56) | 239 (57) | 44 (54) |
| Peritoneal dialysis | 94 (19) | 87 (21) | 7 (9) |
| Both | 35 (7) | 29 (7) | 6 (7) |
| None (pre-emptive transplant) | 92 (18) | 67 (16) | 25 (30) |
| First degree family member with kidney disease | 11 (2) | 11 (3) | 0 (0) |
| Number of prior transplants | |||
| None | 434 (86) | 362 (86) | 72 (88) |
| 1 | 63 (13) | 55 (13) | 8 (10) |
| 2 | 7 (1) | 5 (1) | 2 (2) |
| PRA >50% | 35 (8) | 30 (8) | 5 (7) |
| DSA at time of transplant | 17 (4) | 11 (3) | 6 (8) |
| Deceased donor | 279 (56) | 238 (57) | 41 (51) |
| Extended criteria donor (KDPI>85%) | 48 (18) | 42 (19) | 6 (16) |
| Cold ischemia time, hours | 16±7 | 16±7 | 16±6 |
| Living donor | 222 (44) | 182 (43) | 40 (49) |
| Living related donor | 131 (60) | 107 (60) | 24 (62) |
| Donor age, years | 49 (39–58) | 50 (40–58) | 49 (35–57) |
| HLA-A/B/DR mismatch | 3.1±1.7 | 3.1±1.7 | 3.3±1.7 |
| Induction therapy | |||
| None | 74 (15) | 61 (15) | 13 (16) |
| Basiliximab | 203 (41) | 171 (41) | 32 (39) |
| Antithymocyte globulin | 176 (35) | 143 (34) | 33 (40) |
| Daclizumab | 39 (8) | 38 (9) | 1 (1) |
| Other | 8 (2) | 5 (1) | 3 (4) |
| Baseline immunosuppressive regimen | |||
| Tacrolimus + MMF + steroids | 355 (71) | 296 (70) | 59 (73) |
| Cyclosporine + MMF + steroids | 89 (18) | 77 (18) | 12 (15) |
| Tacrolimus + MMF | 19 (4) | 15 (4) | 4 (5) |
| Other | 40 (8) | 34 (8) | 6 (7) |
| Steroid free/ early steroid withdrawal | 76 (15) | 59 (14) | 17 (21) |
Values represent frequency (percentage), mean±SD or median (interquartile range). BMI, body mass index; KF kidney failure; PRA, panel reactive antibody; DSA, donor-specific antibody; KDPI, Kidney Donor Profile Index; MMF, mycophenolate mofetil.
Cumulative incidence, Kaplan–Meier curves and 95% confidence intervals (95% CIs) were graphed by Prism 7.02 software (GraphPad software, Inc). Log-rank test or Log-rank test for trend was used to compare two groups or three or more groups, respectively. Missing data are shown in Supplemental Table 2. STATA’s multiple imputation by chained equations procedure was used to impute missing data (Supplemental Methods).
Univariable and multivariable Cox proportional hazards regression was performed with imputed data (Table 2). Categorical variables were entered as binary variables. Schoenfeld residuals were evaluated to assess the proportional-hazard assumption, and deviance residuals were used to examine model accuracy and outliers (Supplemental Methods). In Cox regression to graft failure, IgA nephropathy recurrence was treated as a time-varying covariate. In Cox regression analysis of Table 3, adverse events after kidney transplantation (acute rejection, cytomegalovirus [CMV], cancer, BK viremia, and de novo donor-specific antibodies [dnDSA]) were treated as time-varying covariates to assess the association between the occurrence of adverse events and subsequent development of recurrent IgA nephropathy. A two-sided P value of <0.05 was deemed significant in all tests. Statistical analyses were performed using Prism 7.02 software (GraphPad software, Inc) and STATA (v. 15.1, StataCorp LLC). Sensitivity analyses are described in the Supplemental Methods.
Table 2.
Associations of clinical characteristics with recurrence of IgA nephropathy
| Variable | Missing Values (%) | Total Number of Events | Unadjusted Analysis Hazard Ratio (95% Confidence Interval) |
Multivariable Analysis Hazard Ratio (95% Confidence Interval) |
| Geographic location of center | 0 (0) | |||
| Brazil | 6 | ref | ref | |
| Europe | 48 | 0.78 (0.33 to 1.84) | 0.93 (0.37 to 2.36) | |
| United States | 28 | 0.96 (0.39 to 2.32) | 0.91 (0.33 to 2.49) | |
| Age at diagnosis IgA nephropathy, per 10 yrs | 37 (7) | 77 | 0.94 (0.79 to 1.12) | 1.14 (0.78 to 1.67) |
| White race | 79a (16) | 68 | 1.25 (0.61 to 2.54) | 1.59 (0.70 to 3.62) |
| BMI, per kg/m2 | 0 (0) | 82 | 0.96 (0.91 to 1.01) | 0.96 (0.91 to 1.01) |
| Time on dialysis, per mo (log)b | 10 (2) | 82 | 0.84 (0.73 to 0.97)* | 1.13 (0.84 to 1.53) |
| Age at transplantation, per 10 yrs | 1 (0) | 81 | 0.81 (0.68 to 0.96)* | 0.79 (0.53 to 1.18) |
| Pre-emptive transplant | 0 (0) | 82 | 2.27 (1.41 to 3.57)* | 3.45 (1.31 to 9.17)* |
| Living donor | 3 (1) | 81 | 1.34 (0.87 to 2.08) | 1.03 (0.57 to 1.87) |
| Age donor, per 10 yrs | 55 (11) | 72 | 0.93 (0.79 to 1.10) | 0.99 (0.82 to 1.21) |
| HLA mismatch >3 | 68 (13) | |||
| 0 | 7 | ref | ref | |
| 1–3 | 35 | 1.06 (0.47 to 2.39) | 1.10 (0.45 to 2.65) | |
| 4–6 | 29 | 1.03 (0.46 to 2.33) | 1.08 (0.43 to 2.69) | |
| Presence of DSA at transplantation | 43 (9) | 73 | 3.11 (1.38 to 7.00)* | 2.59 (1.09 to 6.19)* |
| Induction | 8 (2) | |||
| None | 13 | ref | ref | |
| Basiliximab/caclizumab | 33 | 0.83 (0.44 to 1.58) | 0.97 (0.48 to 1.96) | |
| ATG/alemtuzumab | 34 | 1.21 (0.63 to 2.29) | 1.12 (0.54 to 2.31) | |
| IgA associated autoimmune diseasesc | 0 (0) | 82 | 0.86 (0.40 to 1.88) | 0.73 (0.32 to 1.64) |
| Immunosuppression with tacrolimus + MMF + steroids | 1 (0) | 81 | 1.28 (0.78 to 2.09) | 1.20 (0.68 to 2.13) |
| Time to KF, per mo (log)b | 32 (6) | 80 | 0.88 (0.77 to 1.00)* | 0.90 (0.75 to 1.08) |
| Steroid free/early steroid withdrawal | 3 (1) | 82 | 0.78 (0.37 to 1.64) | 1.53 (0.80 to 2.90) |
ref, reference; BMI, body mass index; DSA, donor-specific antibody; MMF, mycophenolate mofetil; KF, kidney failure.
Most patients with missing race come from France, where race/ethnicity is not allowed to be reported.
Variables “time on dialysis” and “time to kidney failure” were natural log-transformed; hazard ratios are to be interpreted per natural log unit increase.
IgA associated autoimmune diseases: Henoch-Schönlein purpura, infective bowel disease, rheumatoid arthritis, psoriasis, systemic lupus erythematosus, ankylosing spondylitis, diabetes mellitus type 1, Hashimoto’s thyroiditis. P value is *statistically significant.
Table 3.
Associations of post-transplantation time-dependent adverse events with development of IgA nephropathy recurrence
| Adverse Event, Treated as Time-Varying Covariate | Overall Cohort (n=455) | No Recurrence (n=378) | Recurrence (n=77) | Number of Recurrences after Adverse Event | Hazard Ratio (95% Confidence Interval) |
| Acute rejection | 67 (15) | 49 (13) | 18 (23) | 17 | 1.77 (0.97 to 3.21)a |
| Cellular-mediated | 53 (12) | 40 (11) | 13 (17) | ||
| Antibody-mediated | 13 (3) | 8 (2) | 5 (6) | ||
| CMV | 74 (16) | 64 (17) | 10 (13) | 6 | 0.50 (0.22 to 1.15) |
| Cancer | 101 (22) | 83 (22) | 18 (23) | 11 | 1.53 (0.77 to 3.05) |
| BK viremia | 36 (8) | 27 (7) | 9 (11) | 6 | 1.51 (0.66 to 3.50) |
| De novo DSA | 51 (11) | 35 (9) | 16 (21) | 12 | 6.65 (3.33 to 13.27)a |
In one European center, not all post-transplant complications were registered, therefore analysis of Table 3 was limited to 455 patients from 14 centers. Hazard ratios to the effect of adverse events on development of IgA nephropathy recurrence were calculated by Cox regression, treating the adverse event (acute rejection, CMV, cancer, BK viremia, and de novo DSA, respectively) as time-varying covariate. CMV, cytomegalovirus; DSA, donor-specific antibodies.
Hazard ratio is corrected for pretransplant DSA and pre-emptive kidney transplant.
Results
Cohort Demographics
A total of 577 patients fulfilled the inclusion criteria and were included in a dedicated online database. In total, 47 patients were subsequently excluded because of patient death or loss to follow-up within 1 year after transplant, or primary nonfunction of the kidney transplant (Supplemental Figure 1). Patients from one Brazilian center who had post-transplant IgA deposits without a native biopsy-proven diagnosis of IgA nephropathy were excluded from the primary analysis, but included in secondary analyses (n=26). Finally, 504 patients with a biopsy-proven native diagnosis of IgA nephropathy were included for primary analysis. Patient and donor characteristics are shown in Table 1.
Recurrence of IgA Deposits in the Transplanted Kidney
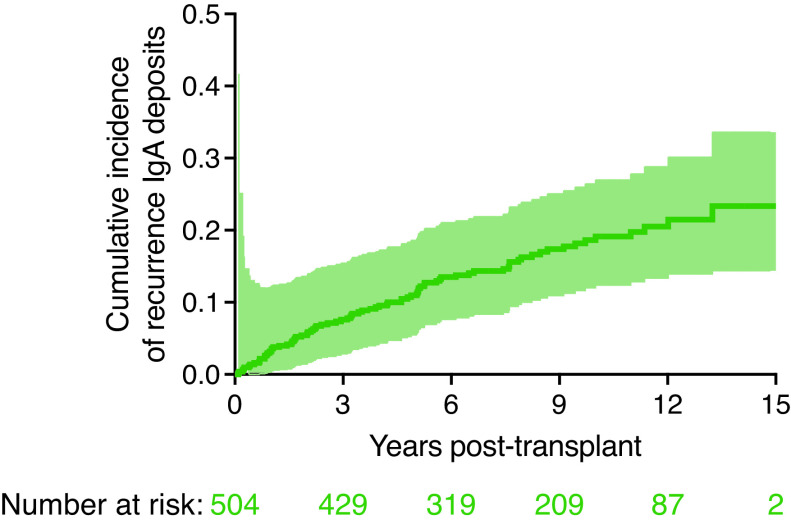
Over a median follow-up period of 8.7 (interquartile range, 5.5–11.2) years, biopsy-proven recurrence of IgA nephropathy occurred in 82 patients. The incidence of IgA nephropathy recurrence increased gradually after transplant (Figure 1), with a cumulative incidence of 19% at 10 years (95% CI, 12 to 26), and 23% at 15 years (95% CI, 14 to 34) after kidney transplantation. Among patients who experienced IgA nephropathy recurrence, median time to recurrence was 3.4 years (interquartile range, 1.4–5.7 years). Four patients had IgA deposits found on protocol biopsy (5%), whereas 78 recurrences were detected on a clinically indicated biopsy (95%).
Figure 1.
Recurrent IgA postkidney transplantation. Cumulative incidence curve of IgA recurrence in kidney transplant recipients with biopsy-proven IgA nephropathy. Cumulative incidence of recurrence of IgA deposits was 19% at 10 years and 23% at 15 years. Among patients with IgA nephropathy recurrence, median time to recurrence was 3.4 years. Shaded area around the curve represents the 95% confidence interval.
In patients with IgA nephropathy recurrence compared with patients without, median age at time of transplant was lower (41 and 46, respectively; P=0.02), median time from IgA nephropathy diagnosis to kidney failure was lower (48 and 72 months, respectively; P=0.04), DSA at time of transplant was observed more frequently (8% versus 3%, respectively; P=0.03), and more patients with recurrent IgA nephropathy had received a pre-emptive transplant (30% versus 16%, respectively; P=0.002). Other variables did not differ between groups (Table 1).
A sensitivity analysis was performed to the recurrence rate of IgA nephropathy in patients who underwent a post-transplant kidney biopsy. Out of 455 patients (as detailed in Supplemental Methods), a post-transplant kidney biopsy was performed in 209 patients (46%). The cumulative incidence of IgA nephropathy recurrence in these patients was 42% (95% CI, 34 to 50) at 10 years after kidney transplantation.
Risk Factors for IgA Nephropathy Recurrence
In univariable Cox regression, IgA nephropathy recurrence was associated with time on dialysis, age at transplantation, pre-emptive transplant, presence of preformed DSA, and time to kidney failure (Table 2). In multivariable analysis, pre-emptive transplant (hazard ratio [HR], 2.56; 95% CI, 1.59 to 4.17; P<0.001) and presence of DSA before transplant (HR, 2.74; 95% CI, 1.22 to 6.14; P=0.01) remained associated with recurrent IgA nephropathy. Because pre-emptive transplantation was associated with IgA nephropathy recurrence, we analyzed subgroups for better interpretation of data. In 92 patients with a pre-emptive transplantation, there was no difference in recurrence between deceased or living donation (HR, 2.33; 95% CI, 0.61 to 8.81), or between related and unrelated living donation (HR, 0.93; 95% CI, 0.37 to 2.31), analyzed by Cox regression adjusted for time to kidney failure and DSA. In 412 patients treated with dialysis pretransplant, time on dialysis was not associated with recurrence (HR, 1.05 per month; 95% CI, 0.82 to 1.36; P=0.69). Interestingly, peritoneal dialysis was associated with lower recurrence rates (HR, 0.42; 95% CI, 0.19 to 0.94; P=0.03) compared with hemodialysis. The geographical location (Europe, United States, or Brazil) of the patient was not associated with recurrence (Table 2). Furthermore, the presence of systemic autoimmune diseases associated with IgA nephropathy did not affect recurrence of IgA nephropathy, neither did early steroid withdrawal or a younger age at kidney transplantation.
Among 70 patients with a prior kidney transplantation, 23 patients had lost a prior graft due to recurrent IgA nephropathy (n=19) or a combination of recurrent IgA nephropathy and rejection (n=4). In these patients, the incidence of recurrent IgA nephropathy in the new transplanted kidney was similar to the full cohort (n=4, 17%).
Graft Failure
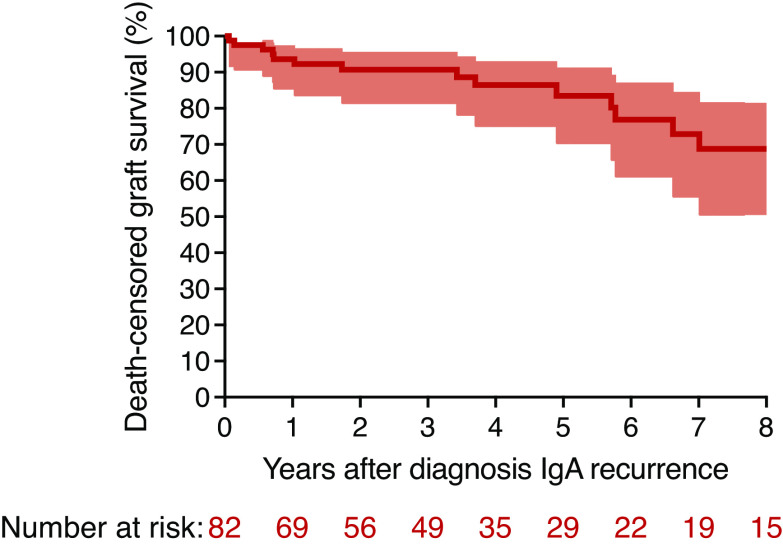
Graft loss occurred in 16 patients (20%) with recurrent IgA nephropathy, compared with 49 (12%) in patients without recurrence. The 10-year death-censored graft survival was 76% in patients with and 89% in patients without recurrence. However, because many patients with IgA nephropathy recurrence experienced recurrence several years after transplant, the analysis comparing recurrence groups is likely to be biased due to immortal time bias. We therefore performed subsequent analyses with IgA nephropathy recurrence as a time-varying covariate. A multivariable Cox regression to graft failure, treating IgA nephropathy recurrence as a time-varying variable and adjusted for HLA mismatch, pretransplant DSA, donor type, donor age, and pre-emptive transplant, revealed an HR 3.69 (95% CI, 2.04 to 6.66; P<0.001) in patients with recurrent IgA nephropathy compared with patients without. Kaplan–Meier analysis to graft failure after diagnosis of IgA nephropathy recurrence revealed graft survival of 94% at 1 year, 83% at 5 years, and 68% at 8 years after diagnosis (Figure 2).
Figure 2.
Graft survival in patients with recurrent IgA nephropathy post-transplantation. Kaplan–Meier graft survival curve of patients with IgA nephropathy recurrence after diagnosis. Area around the curve represents 95% confidence intervals.
In patients with IgA nephropathy recurrence, graft failure was attributed to recurrent IgA nephropathy alone (n=7; 44%), a combination of chronic rejection and recurrent IgA nephropathy (n=2), graft rejection (n=2), and unknown cause (n=5). In patients without recurrence, graft failure was attributed to (chronic) rejection (n=26), BK nephropathy (n=5), de novo glomerular disease (n=1), infection (n=2), calcineurin inhibitor nephropathy (n=2), or unknown or other (n=13) etiology.
In total, 53 patients died with a functioning graft during follow-up: 46 (11%) in patients without versus seven patients (9%) with IgA nephropathy recurrence (P=0.66).
Events Postkidney Transplantation
As native IgA nephropathy is associated with dysregulation of the immune system (15), we investigated the incidence of IgA nephropathy recurrence after development of immune-related events post-transplantation. In unadjusted analysis, the occurrence of CMV, BK viremia, or cancer was not associated with subsequent IgA nephropathy recurrence, although the number of IgA nephropathy recurrences after CMV, BK, or cancer was low and adjusted analysis was therefore not possible (Table 3). In multivariable analysis, adjusted for pre-emptive transplantation and preformed DSA, occurrence of acute rejection was not associated with subsequent IgA nephropathy recurrence; however, IgA nephropathy recurrence rates were higher in patients after development of dnDSA (HR, 6.65; 95% CI, 3.33 to 13.27; P<0.001).
Because IgA deposits could be a coincidental finding as a result of increased surveillance and number of biopsies performed in patients with dnDSA, we performed two sensitivity analyses. Assessing only patients who had undergone a kidney biopsy, occurrence of dnDSA was associated with subsequent IgA nephropathy recurrence (HR, 3.41; 95% CI, 1.74 to 6.70; P<0.001). Post-transplant DSA testing was performed at least once in 270 patients (59%), and in this analysis, development of dnDSA was associated with subsequent IgA recurrence (HR, 6.05; 95% CI, 2.91 to 12.61; P<0.001).
Clinical Signs, Treatment, and Outcomes in Patients with Recurrent IgA Nephropathy
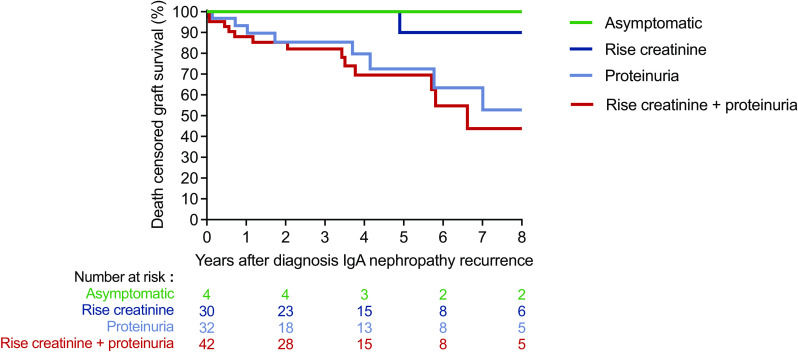
A total of 108 patients were included for analysis of clinical signs and treatment of IgA nephropathy recurrence: 82 from the general cohort and 26 patients with recurrent IgA nephropathy without native biopsy from one center in Brazil. At time of recurrence, 32 (30%) patients had proteinuria with or without hematuria, 30 (28%) patients only had a rise in creatinine, whereas 42 (39%) patients experienced a rise in creatinine with proteinuria or hematuria. Four patients had no significant kidney manifestations (IgA deposits found on protocol biopsy) and did not experience graft loss during follow-up. Patients with proteinuria at time of recurrence were more likely to develop graft failure after diagnosis compared with patients with only a rise in creatinine (P=0.002, Figure 3).
Figure 3.
Clinical signs at time of IgA nephropathy recurrence and graft survival. Death-censored Kaplan–Meier analysis to graft survival in patients with IgA nephropathy recurrence, stratified by the presence of proteinuria and/or rise in creatinine.
In 95 out of 105 kidney biopsy reports, a score was given to the amount of mesangial proliferation (no, mild, moderate, severe). No difference in graft survival between groups was found (Supplemental Figure 2). Additionally, the strength of IgA staining (1+ to 4+) did not correlate with graft outcome (data not shown).
After diagnosis of recurrent IgA nephropathy, most patients with available information on treatment regimen, received angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) (n=78, 75%). Other treatments included pulse steroids (n=25; 24%), increased dose of mycophenolate mofetil or adding steroids to the immunosuppressive regimen (n=10, 10%), cyclophosphamide (n=4, 4%), and/or treatment for concurrent rejection (n=8, 8%). A total of 11 patients (11%) received no treatment for IgA recurrence. Patients who received ACEi/ARB did not have better graft outcomes after diagnosis of IgA nephropathy recurrence (P=0.86) (Supplemental Figure 3).
Discussion
In this multicenter international cohort of kidney transplant recipients, because of IgA nephropathy, cumulative incidence of recurrent IgA nephropathy was 19% at 10 years and 23% at 15 years after kidney transplantation. If only patients who had undergone a post-transplant kidney biopsy are analyzed, the recurrence rate was 42% after 10 years. In multivariable analysis to risk factors, a pre-emptive transplant and presence of DSA at time of transplantation were associated with recurrence of IgA deposits. Postkidney transplantation, development of dnDSA was also associated with subsequent IgA nephropathy recurrence. Recurrent IgA nephropathy was mostly found on clinically indicated biopsy (95%), and was independently associated with a 3.7-fold higher risk of graft loss. Clinical presentation at time of recurrence was of importance for graft survival because patients with proteinuria had worse outcomes compared with patients without. Management of recurrent IgA deposits was mainly focused on starting or increasing ACEi or ARB.
The incidence of recurrent IgA nephropathy found in our cohort is in accordance with previous literature, because it lies within most confidence intervals of other studies (Supplemental Table 1). The incidence is, however, on the lower side, which might be explained by the fact that most centers in this study did not perform protocol biopsies postkidney transplantation. Patients with Henoch-Schönlein purpura in our cohort had equal recurrence rates compared with patients with solely IgA nephropathy, which is also in accordance with prior studies (16).
We found a strong association between a pre-emptive transplant and recurrence of IgA deposits. To our knowledge, a pre-emptive transplant has not been linked to IgA nephropathy recurrence before, possibly because this variable was usually not included in prior studies. Because most pre-emptive transplants were from living donors (85%) and few previous studies had found an association between living (related) donation and IgA nephropathy recurrence (6,17,18), it is important to exclude any bias regarding donor type. In our cohort, the association between pre-emptive transplantation and IgA nephropathy recurrence was not affected by type of donation (deceased, living related, living unrelated), both in the general cohort and in patients with a pre-emptive transplant. The reason why pre-emptive transplanted patients had an associated higher risk of recurrence is not clear. It could be hypothesized that, similar to lupus nephritis, active and/or aggressive IgA nephropathy disease may “burn-out” on dialysis (19), although there was no effect of length of dialysis on recurrence.
The association between pretransplant DSA and recurrence of IgA nephropathy should be interpreted with caution. The outcomes of patients with DSA could be confounded by the increased surveillance that these patients might have had. However, we also found a post-transplant association between dnDSA and subsequent IgA nephropathy recurrence, which makes a possible relation between IgA nephropathy recurrence and DSA more likely. Although our results cannot be used to state any definitive conclusion due to the low number of patients with preformed or dnDSA who experienced IgA nephropathy recurrence, they do provide a rationale for subsequent studies testing the relationship between DSA and IgA nephropathy recurrence. Both IgA nephropathy recurrence and dnDSA could reflect a primary lack of sufficient immunosuppression. Nonetheless, we found no evidence for an effect of baseline immunosuppression and induction therapy on IgA nephropathy recurrence. Of note, immunosuppressant use was homogeneous with 89% of patients receiving calcineurin inhibitors, mycophenolate mofetil, and steroids. Few prior studies have reported an association between early steroid withdrawal and IgA nephropathy recurrence, although these studies have important limitations, such as large differences in groups at baseline (including immunosuppression), no or only limited multivariable analysis, and/or possibility of bias due to biopsy practices or patient selection (10,13,20,21). In agreement with other studies (7,22,23), we did not find an association between early steroid withdrawal and IgA nephropathy recurrence in multivariable analysis.
There are no universally accepted guidelines for the treatment of recurrent IgA nephropathy (24). ACEi/ARB treatment was often used after IgA nephropathy recurrence and was not associated with graft outcomes, but the control group of patients not receiving such therapies was small. Additional therapies, such as pulse steroids or intravenous cyclophosphamide, lack strong evidence in literature. Nonetheless, in >25% of patients, one of these treatments was used.
The main limitations of this study comprise the retrospective design, noncentralized pathology, and the possibility of patients with undiagnosed recurrent IgA nephropathy due to the absence of protocol biopsy. A retrospective study inherently has the potential of bias regarding selection, imputation of missing data, and adjustment of confounders. Because of the large number of centers, we were only able to correct for continent, not for center-to-center variability and different practices. Pathology was not centralized and, therefore, differences across centers could be present, although detailed history and biopsy reports were obtained to minimize variation. A clinical limitation of the study entails the variety in which IgA nephropathy recurrence can manifest, which can be very similar to other pathology, such as transplant glomerulopathy, and therefore can be missed. Lastly, because most patients in our cohort had kidney manifestations at presentation, our data can only be extracted to IgA nephropathy recurrence with clinical signs, not to IgA nephropathy recurrences found on protocol biopsy.
In conclusion, our study shows patients with recurrent IgA deposits detected on clinically indicated biopsy are at higher risk of graft failure. Additionally, our findings reveal an association between recurrence of IgA nephropathy and a pre-emptive transplantation, preformed DSA, and dnDSA after kidney transplantation. In contrast to previous studies, steroid withdrawal did not correlate with higher incidence of IgA nephropathy recurrence. Future studies are needed to test ideal management and treatment strategies to improve the outcomes of affected individuals.
Disclosures
A. Buxeda reports having a Rio Hortega contract CM19/00004, Instituto de Salud Carlos III ; Hospital del Mar. E. David Neto reports receiving research funding from AstraZeneca. A.C. Bauer reports serving on speakers bureau for AstraZeneca and reports other interests/relationships with Associação Brasileira de Transplantes de Orgãos, Sociedade Brasileira de Nefrologia, and Sociedade Brasileira de Diabetes. E. Akalin reports having consultancy agreements with, receiving honoraria from, and serving as a scientific advisor or member of CareDx and Immucor and reports receiving research funding from Angion, Astellas, CareDx, and the National Institutes of Health. G. Comai reports receiving honoraria from Astellas and Novartis. H. Seeger reports receiving honoraria from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Menarini, Mundipharma, and Vifor and reports other interests/relationships with Innovative Medicines Initiative Transbioline (European Union). H. Silva reports consultancy agreements with Novartis and Pfizer; receiving research funding from Novartis, Pfizer, and Sanofi; receiving honoraria from and serving on speakers bureau for Novartis and Pfizer; serving as a scientific advisor or member of Transplantation. J.D. Schold reports consultancy agreements with Guidry and East, Novartis, Sanofi Corporation, and Transplant Management Group; reports receiving honoraria from Novartis and Sanofi Inc; and reports serving as a Data Safety Monitoring Board Member for Bristol Myers Squibb. J. Pascual reports receiving honoraria from Chiesi (sporadic as speaker) and Novartis (sporadic as speaker). L.V. Riella reports receiving research funding from Bristol-Meyers Squibb, CareDx, Natera, and Visterra and reports receiving honoraria from, and serving as a scientific advisor or member of, CareDx. N. Agrawal reports employment with and having an ownership interest in CareDx Inc. and reports serving as a scientific advisor or member of Biosurfaces Inc. and FreeFlow Medical Devices Inc. R.C. Manfro reports serving as a scientific advisor or member of Brazilian Society of Nephrology and Brazilian Society of Transplantation. R. Oberbauer reports receiving research funding from Chiesi, Fresenius, Novartis, Roche, and Sandoz; receiving honoraria from Chiesi, Neovii, Sandoz, and Teva; patents and inventions with Amgen (sold patent, no royalties); serving as a scientific advisor or member of Amgen, Astellas, Chiesi, and Novartis; receiving speaker honoraria from Amgen, Astellas, Chiesi, Fresenius, Novartis, and Sandoz; and other interests/relationships with Austrian Society of Nephrology, European Society of Nephrology, and European Society of Organ Transplantation. S. Berger reports having consultancy agreements with Novartis; reports receiving research funding from Chiesi and Novartis; reports receiving honoraria from Novartis; and reports serving on the Advisory Board for Novartis and the Supervisory Board for Dutch Transplant Foundation. S. Farouk reports serving on the Editorial Boards of American Journal of Kidney Diseases, Clinical Transplantation, and Journal of Nephrology. T. Jouve reports receiving research funding from Chiesi Pharmaceuticals. X.S. Cheng reports receiving honoraria from ClarityCo and Medscape Education and reports receiving research funding from American Heart Association and National Institutes of Health. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
This work was conducted with support from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102), and financial contributions from Harvard University and its affiliated academic health care centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic health care centers, or the National Institutes of Health. This study was supported in part by the Harold and Ellen Danser Endowed/Distinguished Chair in Transplantation at Massachusetts General Hospital (Boston, MA, USA).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00910121/-/DCSupplemental.
Supplemental Table 1. Published nonregistry studies on incidence of recurrence of IgA nephropathy since 2000 including >50 subjects.
Supplemental Table 2. Missing data per variable in patients without and with recurrent IgA nephropathy.
Supplemental Figure 1. Flow chart of the study population .
Supplemental Figure 2. Kaplan–Meier analysis to death-censored graft survival in patients with IgA nephropathy recurrence after time of diagnosis, stratified by degree of mesangial expansion on kidney biopsy.
Supplemental Figure 3. Kaplan–Meier analysis to graft survival in patients with recurrent IgA after time of diagnosis, treated with or without ACEi/ARB.
References
- 1.Marinaki S, Lionaki S, Boletis JN: Glomerular disease recurrence in the renal allograft: A hurdle but not a barrier for successful kidney transplantation. Transplant Proc 45: 3–9, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Wyld ML, Chadban SJ: Recurrent IgA nephropathy after kidney transplantation. Transplantation 100: 1827–1832, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Floege J: Recurrent IgA nephropathy after renal transplantation. Semin Nephrol 24: 287–291, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Ponticelli C, Traversi L, Feliciani A, Cesana BM, Banfi G, Tarantino A: Kidney transplantation in patients with IgA mesangial glomerulonephritis. Kidney Int 60: 1948–1954, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Berthoux F, El Deeb S, Mariat C, Diconne E, Laurent B, Thibaudin L: Antithymocyte globulin (ATG) induction therapy and disease recurrence in renal transplant recipients with primary IgA nephropathy. Transplantation 85: 1505–1507, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Han SS, Huh W, Park SK, Ahn C, Han JS, Kim S, Kim YS: Impact of recurrent disease and chronic allograft nephropathy on the long-term allograft outcome in patients with IgA nephropathy. Transpl Int 23: 169–175, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Ortiz F, Gelpi R, Koskinen P, Manonelles A, Räisänen-Sokolowski A, Carrera M, Honkanen E, Grinyó JM, Cruzado JM: IgA nephropathy recurs early in the graft when assessed by protocol biopsy. Nephrol Dial Transplant 27: 2553–2558, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Moroni G, Longhi S, Quaglini S, Gallelli B, Banfi G, Montagnino G, Messa P: The long-term outcome of renal transplantation of IgA nephropathy and the impact of recurrence on graft survival. Nephrol Dial Transplant 28: 1305–1314, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Ishida H, Shimizu T, Tanabe K: Evaluation of tonsillectomy before kidney transplantation in patients with IgA nephropathy. Transpl Immunol 30: 12–17, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Von Visger JR, Gunay Y, Andreoni KA, Bhatt UY, Nori US, Pesavento TE, Elkhammas EA, Winters HA, Nadasdy T, Singh N: The risk of recurrent IgA nephropathy in a steroid-free protocol and other modifying immunosuppression. Clin Transplant 28: 845–854, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Nijim S, Vujjini V, Alasfar S, Luo X, Orandi B, Delp C, Desai NM, Montgomery RA, Lonze BE, Alachkar N: Recurrent IgA nephropathy after kidney transplantation. Transplant Proc 48: 2689–2694, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Avasare RS, Rosenstiel PE, Zaky ZS, Tsapepas DS, Appel GB, Markowitz GS, Bomback AS, Canetta PA: Predicting post-transplant recurrence of IgA nephropathy: The importance of crescents. Am J Nephrol 45: 99–106, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Vico MC, Messina M, Fop F, Barreca A, Segoloni GP, Biancone L: Recurrent IgA nephropathy after renal transplantation and steroid withdrawal. Clin Transplant 32: e13207, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Uffing A, Pérez-Sáez MJ, La Manna G, Comai G, Fischman C, Farouk S, Manfro RC, Bauer AC, Lichtenfels B, Mansur JB, Tedesco-Silva H, Kirsztajn GM, Manonelles A, Bestard O, Riella MC, Hokazono SR, Arias-Cabrales C, David-Neto E, Ventura CG, Akalin E, Mohammed O, Khankin EV, Safa K, Malvezzi P, O’Shaughnessy MM, Cheng XS, Cravedi P, Riella LV: A large, international study on post-transplant glomerular diseases: The TANGO project. BMC Nephrol 19: 229, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA: The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanaan N, Mourad G, Thervet E, Peeters P, Hourmant M, Vanrenterghem Y, De Meyer M, Mourad M, Maréchal C, Goffin E, Pirson Y: Recurrence and graft loss after kidney transplantation for Henoch-Schonlein purpura nephritis: A multicenter analysis. Clin J Am Soc Nephrol 6: 1768–1772, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Wang AY, Lai FM, Yu AW, Lam PK, Chow KM, Choi PC, Lui SF, Li PK: Recurrent IgA nephropathy in renal transplant allografts. Am J Kidney Dis 38: 588–596, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Freese P, Svalander C, Nordén G, Nyberg G: Clinical risk factors for recurrence of IgA nephropathy. Clin Transplant 13: 313–317, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Lionaki S, Skalioti C, Boletis JN: Kidney transplantation in patients with systemic lupus erythematosus. World J Transplant 4: 176–182, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clayton P, McDonald S, Chadban S: Steroids and recurrent IgA nephropathy after kidney transplantation. Am J Transplant 11: 1645–1649, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Leeaphorn N, Garg N, Khankin EV, Cardarelli F, Pavlakis M: Recurrence of IgA nephropathy after kidney transplantation in steroid continuation versus early steroid-withdrawal regimens: A retrospective analysis of the UNOS/OPTN database. Transpl Int 31: 175–186, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulay AV, van Walraven C, Knoll GA: Impact of immunosuppressive medication on the risk of renal allograft failure due to recurrent glomerulonephritis. Am J Transplant 9: 804–811, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Barbour S, Djurdjev O, Gill JS, Dong JJ, Gill J: A propensity score matched analysis shows no adverse effect of early steroid withdrawal in non-diabetic kidney transplant recipients with and without glomerulonephritis. Kidney Int 96: 460–469, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group : KDIGO clinical practice guideline for the care of kidney transplant recipients. Available at: https://kdigo.org/guidelines. Accessed December 14, 2020 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.