The nucleoside analog remdesivir exhibits in vitro efficacy against severe acute respiratory syndrome coronavirus 2 and may shorten the time to recovery of patients with coronavirus disease 2019 (COVID-19), which resulted in an emergency authorization by the Food and Drug Administration (FDA) for patients with an eGFR >30 ml/min. This policy excludes a sizeable portion of patients critically ill with COVID-19 as 20%–40% develop AKI or require KRT (1).
Remdesivir is rapidly converted to the predominant metabolite GS-441524 and mainly excreted by the kidneys in this form. Remdesivir has an unbound drug fraction of only 12%, whereas GS-441524 exhibits one of >85%. Therefore, only GS-441524 should be eliminated by KRT (2). Remdesivir and GS-441524 are eliminated by the CytoSorb adsorber (3), but the effect of the Seraph 100 Microbind Affinity Blood Filter, a heparin-coated pathogen adsorber that may remove SARS-CoV-2 nucleocapsid and received an authorization for emergency use in patients with COVID-19 by the FDA (4), has not been investigated so far.
We report pharmacokinetic data of two patients (patient 1: female, 37 years, body mass index 29.4 kg/m2, sequential organ failure assessment score 12; patient 2: male, 68 years, body mass index 42.5 kg/m2, sequential organ failure assessment score 15) treated with remdesivir due to severe COVID-19 pneumonia. Both patients were mechanically ventilated, showed severe vasoplegic shock, and were AKI stage 3. Patient 1 had a residual urine excretion of 77 ml/h, whereas patient 2 was anuric.
Remdesivir was administered with a loading dose of 200 mg and 100 mg once daily for 5 days. After a risk-benefit evaluation, remdesivir therapy was continued despite dialysis initiation.
Both patients received a single therapeutic plasma exchange (TPE) due to septic shock on the day remdesivir therapy was started, with an exchange fluid volume of 2748 and 3310 ml fresh frozen plasma. Additionally, patient 2 was treated with the Seraph 100, which was installed upstream of the dialyzer. Remdesivir and GS-441524 were analyzed by liquid chromatography tandem mass spectrometry.
Prolonged intermittent dialysis (PIRRT) was performed with high-flux dialyzers (surface 1.4 m2; blood/dialysate flow: 220/220 ml per min, patient 1; 130/65 ml per min, patient 2). An overall dialysate turnover of 3 L/h for 52 hours for patient 1 and 3.5 L/h during the first 48 hours of measurement for patient 2 was administered. PIRRT was paused for TPE and stopped 3 hours later in patient 1. The treatment was paused for the last 12 hours of the observation in patient 2.
TPE, 7 hours after first remdesivir administration, eliminated about 70 µg of remdesivir and 1.8 mg of GS-441524 in patient 1. During the next PIRRT session, remdesivir levels decreased from 18 to 15.4 ng/ml and GS-441524 levels rose from 762.2 to 954.5 ng/ml.
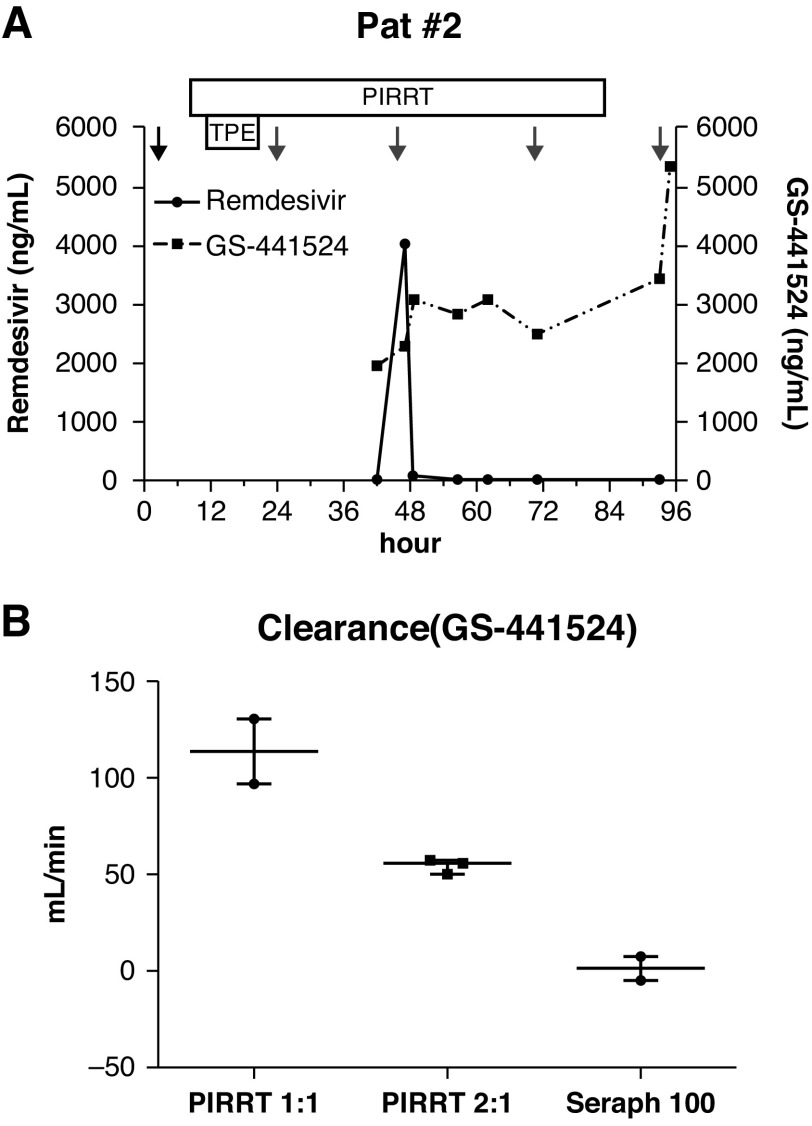
Patient 2 had received remdesivir for 2 days before drug levels were measured. During the next 54 hours, the patient was treated with two PIRRT sessions. Remdesivir and GS-441524 maximum plasma levels were 15.3 and 1937.7 ng/ml on day 1 and 13.9 and 5309.5 ng/ml on day 3 (Figure 1A).
Figure 1.
The course of remdesivir and GS-441524 plasma levels of patient 2 and extracorporeal treatment clearance of GS-441524. (A) The black arrow indicates initial remdesivir dose (200 mg), gray arrows mark additional doses (100 mg). The box marks the duration of prolonged intermittent dialysis (PIRRT). (B) Dialyzer clearance with blood flow/dialysate flow ratios of 1:1 (220 ml and 220 ml) and 2:1 (130 ml and 65 ml) of GS-441524 and the adsorber clearance of the Seraph 100 are shown. The dialyzer and adsorber plasma clearance (CL) was determined by the drug concentration upstream (Cin) and downstream (Cout) of the device, the blood flow (QB), and the patient's hematocrit (Hct) level with the formula: CL = (Cin − Cout)/Cin × QB × (1 − Hct/100). TPE, therapeutic plasma exchange.
GS-441524 dialyzer plasma clearance was 96.7 and 130.3 ml/min in patient 1 and 55.9 ml/min in patient 2 (Figure 1B). There was no significant dialyzer clearance for remdesivir (median −2.2 ml/min, interquartile range, −8.2 to 0 ml/min). Seraph 100 showed no significant clearance for either of the substances (remdesivir: 1.1 ml/min, GS-441524: 1.5 ml/min). Both patients could be discharged from our hospital 30 and 13 days after admission without the need for further PIRRT.
To our knowledge, these are the first reported clinical data regarding remdesivir and GS-441524 levels measured in critically ill patients during PIRRT and Seraph 100 treatment. The high protein binding of remdesivir explains the neglectable measured dialyzer clearance. Remdesivir area under the curve (AUC) was comparable to healthy volunteers with normal kidney function (2.59 versus 3.6 h*µg/ml) (2), because remdesivir is metabolized rapidly to GS-441524.
In contrast, GS-441524 (molecular mass 291.3 D, protein binding <20%) can be eliminated by PIRRT, as evidenced by the dialyzer clearances we calculated. Ongoing drug conversion and redistribution after TPE might have led to a metabolite increase during PIRRT in patient 1, whereas low dialysate flow rates, less residual kidney function, and a pause in PIRRT are possible factors for the accumulation of GS-441524 in patient 2. We measured plasma levels 35-fold higher than in healthy volunteers and 26-fold higher than in AKI without dialysis dependency (5). Additionally, GS-441524 plasma AUC and t1/2 were higher than in patients without AKI (AUC, 68.6 versus 2.23 h*µg/mL, t1/2: 75.3 versus 25.3 h). We observed a transient transaminase elevation (maximum aspartate aminotransferase 67 and 69 U/L), but aspartate aminotransferase levels normalized during remdesivir therapy. This may reflect that remdesivir, which did not accumulate, is most likely accountable for transaminase elevation. Other possible adverse events could not be detected.
Our data suggest GS-441524, but not remdesivir, accumulates in dialysis-dependent AKI, but normal dosed daily KRT may eliminate the metabolite to a significant amount. The Seraph 100 has no effect on remdesivir or GS-441524 levels. These findings need further studies for the evaluation of other KRT settings.
Disclosures
J.J. Schmidt reports receiving research funding from ExThera Medical. J.T. Kielstein reports consultancy agreements with Vifor Pharma; reports having an ownership interest in ChemoCentryx; reports receiving research funding from ExThera Medical and Fresenius Medical Care; reports receiving honoraria from Berlin-Chemie, Boehringer, DiaMed, Fresenius Medical Care, Novartis, and Terumo Blood and Cell Technologies; serving as Section Editor of Nephrology Dialysis Transplantation; and speakers bureau for Astellas, Berlin Chemie, Menarini, and Vifor Pharma. M.M. Hoeper has received support for lectures and/or consultations from Acceleron, Actelision, Bayer, GlaxoSmithKline, Janssen, Merck & Co., Inc., and Pfizer, all outside this work. All remaining authors have nothing to disclose.
Funding
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Adamsick ML, Gandhi RG, Bidell MR, Elshaboury RH, Bhattacharyya RP, Kim AY, Nigwekar S, Rhee EP, Sise ME: Remdesivir in patients with acute or chronic kidney disease and COVID-19. J Am Soc Nephrol 31: 1384–1386, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jorgensen SCJ, Kebriaei R, Dresser LD: Remdesivir: Review of pharmacology, pre-clinical data, and emerging clinical experience for COVID-19. Pharmacotherapy 40: 659–671, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biever P, Staudacher DL, Sommer MJ, Triebel H, Neukamm MA, Bode C, Supady A, Lother A: Hemoadsorption eliminates remdesivir from the circulation: Implications for the treatment of COVID-19. Pharmacol Res Perspect 9: e00743, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kielstein JT, Borchina D-N, Fuhner T, Hwang S, Mattoon D, Ball AJ: Hemofiltration with the Seraph® 100 Microbind® Affinity filter decreases SARS-CoV-2 nucleocapsid protein in critically ill COVID-19 patients. Critical Care 25: 1–4, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tempestilli M, Caputi P, Avataneo V, Notari S, Forini O, Scorzolini L, Marchioni L, Ascoli Bartoli T, Castilletti C, Lalle E, Capobianchi MR, Nicastri E, D’Avolio A, Ippolito G, Agrati C; COVID 19 INMI Study Group: Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19. J Antimicrob Chemother 75: 2977–2980, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]