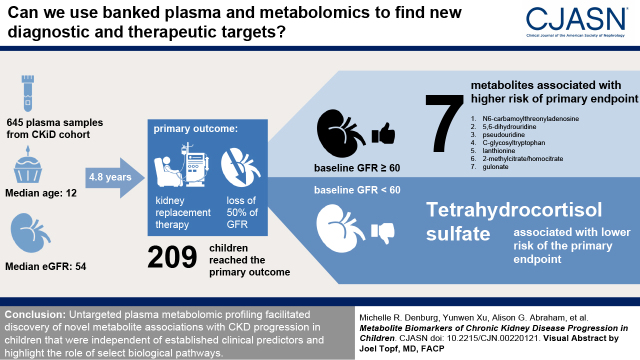
Visual Abstract
Keywords: children, chronic kidney disease, metabolism, pediatric nephrology, progression of chronic renal failure, biomarkers
Abstract
Background and objectives
Metabolomics facilitates the discovery of biomarkers and potential therapeutic targets for CKD progression.
Design, setting, participants, & measurements
We evaluated an untargeted metabolomics quantification of stored plasma samples from 645 Chronic Kidney Disease in Children (CKiD) participants. Metabolites were standardized and logarithmically transformed. Cox proportional hazards regression examined the association between 825 nondrug metabolites and progression to the composite outcome of KRT or 50% reduction of eGFR, adjusting for age, sex, race, body mass index, hypertension, glomerular versus nonglomerular diagnosis, proteinuria, and baseline eGFR. Stratified analyses were performed within subgroups of glomerular/nonglomerular diagnosis and baseline eGFR.
Results
Baseline characteristics were 391 (61%) male; median age 12 years; median eGFR 54 ml/min per 1.73 m2; 448 (69%) nonglomerular diagnosis. Over a median follow-up of 4.8 years, 209 (32%) participants developed the composite outcome. Unique association signals were identified in subgroups of baseline eGFR. Among participants with baseline eGFR ≥60 ml/min per 1.73 m2, two-fold higher levels of seven metabolites were significantly associated with higher hazards of KRT/halving of eGFR events: three involved in purine and pyrimidine metabolism (N6-carbamoylthreonyladenosine, hazard ratio, 16; 95% confidence interval, 4 to 60; 5,6-dihydrouridine, hazard ratio, 17; 95% confidence interval, 5 to 55; pseudouridine, hazard ratio, 39; 95% confidence interval, 8 to 200); two amino acids, C-glycosyltryptophan, hazard ratio, 24; 95% confidence interval 6 to 95 and lanthionine, hazard ratio, 3; 95% confidence interval, 2 to 5; the tricarboxylic acid cycle intermediate 2-methylcitrate/homocitrate, hazard ratio, 4; 95% confidence interval, 2 to 7; and gulonate, hazard ratio, 10; 95% confidence interval, 3 to 29. Among those with baseline eGFR <60 ml/min per 1.73 m2, a higher level of tetrahydrocortisol sulfate was associated with lower risk of progression (hazard ratio, 0.8; 95% confidence interval, 0.7 to 0.9).
Conclusions
Untargeted plasma metabolomic profiling facilitated discovery of novel metabolite associations with CKD progression in children that were independent of established clinical predictors and highlight the role of select biologic pathways.
Introduction
CKD and its progression to kidney failure in childhood threaten growth, development, and life expectancy. Most young adults with a history of childhood-onset kidney failure suffer considerable comorbidities, with as much as a ten- to 100-fold greater risk of cardiovascular disease, infectious complications, and metabolic bone disease, compared with the general population (1–6). Age-specific mortality is 30- to 150-fold higher in children requiring KRT than in the general pediatric population (7–9), and cardiovascular mortality is 1000-fold higher (10).
At present, antihypertensive and antiproteinuric agents represent the mainstay of therapy to delay CKD progression, and the rationale for blockade of the renin-angiotensin-aldosterone system in hypertensive and/or proteinuric CKD is well established (11,12). However, although hypertension and proteinuria are important factors associated with kidney disease progression (13,14), they are not universally prevalent. In contrast to CKD in adults, congenital anomalies of the kidney and urinary tract, including renal hypodysplasia, obstructive uropathy, and reflux nephropathy, collectively compose about 60% of the underlying etiologies (15,16), and are frequently unaccompanied by hypertension or proteinuria. Even when present, such clinical markers of progression do not allow for early identification of those at risk for disease progression, when novel therapeutics and earlier intervention may be effective.
Given the intrinsic metabolic activity of the kidney and the effect of kidney function on circulating metabolites and systemic metabolism, nontargeted metabolomics is an increasingly utilized tool in nephrology research (17). Recent advances in metabolomic technologies allow for high-throughput, high-resolution metabolic phenotyping of small volume human blood samples, with >1000 analytes measured simultaneously. In combination with well-phenotyped cohort studies, these technologies hold promise for the discovery of highly discriminant biomarkers of, and new therapeutic targets for, kidney disease and its complications. Several groups have applied these technologies to study altered metabolic pathways associated with impaired kidney function in adults (18–26). In contrast to adults in whom CKD typically arises secondary to, and is confounded by, comorbid conditions, CKD in children is usually the consequence of congenital anomalies of the kidney and urinary tract or primary glomerular disorders. Therefore, specific biomarkers of CKD progression may be more readily identified in children. The largest prospective cohort study of CKD progression conducted to date, the Chronic Kidney Disease in Children (CKiD) study, has enrolled >1000 children and adolescents across North America since 2005. The objective of this investigation was to leverage the longitudinal clinical phenotyping of this unique cohort and use untargeted metabolomic profiling to perform the first large-scale discovery of novel metabolite biomarkers of CKD progression in children.
Materials and Methods
Study Population and Study Design
CKiD is an ongoing multicenter prospective cohort study of children with CKD. Participants included in the present investigation were enrolled in CKiD between January 2005 and December 2014 in two recruitment waves at 54 participating medical centers in the United States and Canada. Children were enrolled if they were between the ages of 6 months and 16 years and had an eGFR of 30–90 ml/min per 1.73 m2 (based on the original Schwartz equation). They were followed annually for up to 11.5 years. Exclusion criteria included: history of solid organ or bone marrow transplantation, dialysis within 3 years, malignancy or HIV within 12 months, structural cardiac disease, and genetic syndromes involving the central nervous system. The complete details of study design, methods, and population characteristics have been published previously (14,27). Blood samples were collected annually to measure kidney function and other biomarkers to observe the natural history of pediatric CKD. Written informed consent was obtained from all parents or legal guardians, along with assent, when appropriate, from the enrolled children. The CKiD study was approved by the institutional review board of each participating institution and adhered to the Declaration of Helsinki.
Metabolomic Profiling
Metabolite profiling was performed on plasma samples collected at the 6-month visit after CKiD study enrollment. These samples were inventoried and stored at −80°C until processed and were assayed with an untargeted ultra-high performance liquid chromatography tandem mass spectrometry–based metabolomics quantification protocol by Metabolon, Inc. (Durham, North Carolina) (28–30). In total, 35 samples were repeated as blind replicates. Samples were excluded if >50% metabolite values were missing. Metabolites were excluded if ≥80% of the values were missing across all samples. Of the remaining metabolites, named nondrug compounds were retained for the analysis. Missing values were imputed with the minimum value, and metabolite values were normalized to the run-day medians to correct for variations across runs or instruments. After logarithmic (base 2) transformation, we further removed metabolites with high variability or values deemed outliers.
Composite Outcome
CKD progression was defined as initiation of KRT (dialysis or transplantation), or a 50% reduction of eGFR as the composite outcome, consistent with prior CKiD studies (14). The analysis was anchored at the first annual visit, with a median time since enrollment of 1.2 years (interquartile range, 1.0–1.3). This anchoring point was chosen because most participant data were collected at annual visits, and it was the closest annual visit after metabolite measurement. For analyses, the time to this composite outcome was defined as the years from this first annual visit (baseline for the present investigation) to the initiation of KRT, >50% eGFR decline, or the end of study (March 1, 2018), whichever came first. The study baseline was chosen as it was the first full CKiD visit with outcome measures after the metabolite measurement time point.
Covariate Ascertainment
Covariate information included demographic characteristics (e.g., age, sex, race), underlying diagnosis (glomerular versus nonglomerular etiology), urine protein-creatinine ratio (UPCR), body mass index (BMI) Z-score, hypertension, and eGFR. BMI Z-score was calculated as weight in kilograms divided by height in meters squared, standardized for age and sex. Hypertension was defined as systolic or diastolic blood pressure ≥95th percentile for age, sex, and height, or any self-reported high blood pressure medication use. eGFR was calculated from serum creatinine, cystatin C, and blood urea nitrogen concentrations according to the equation developed in the CKiD study (31). Covariate data were obtained from the first annual CKiD visit (baseline for the current study), or, when missing, at the 6-month visit or CKiD enrollment visit (n=37, n=20, and n=22 for UPCR, BMI Z-score, and hypertension, respectively).
Statistical Analysis
Participant characteristics were compared by underlying etiology (glomerular versus nonglomerular) using two-sided tests (chi squared, Kruskal–Wallis, or t tests, as appropriate). To determine plasma metabolites associated with CKD progression, separate Cox proportional hazards models were used to regress the time to the composite outcome on each log base 2 metabolite level. Models were adjusted for the following potential baseline confounders: age, sex, race, BMI Z-score, hypertension, glomerular versus nonglomerular diagnosis, baseline eGFR, and log base 2 transformed UPCR at baseline. To account for multiple statistical testing across all metabolites, we used a strict significance testing threshold and compared the observed P value to a conservative Bonferroni-corrected alpha of 6.06 × 10−5 (0.05/825, where 825 was the number of tested metabolites). Analyses were performed in the overall sample and stratified by the baseline eGFR (≥60 ml/min per 1.73 m2 versus <60 ml/min per 1.73 m2) and by glomerular versus nonglomerular etiology, to identify both unique and universal metabolomic signatures. In a sensitivity analysis, we excluded participants who had <0.75 years of follow-up time from our study baseline (n=37), 31 of whom initiated KRT in that period, and repeated all analyses described above. These observations could be highly influential as a result of very short times to the event, because individuals who proceed very rapidly to the event are unlikely to have had their progression influenced by an exposure of interest. As an alternative approach to multiple testing, we also examined metabolites that met a less stringent significance threshold using the false discovery rate (FDR) method (Benjamini and Hochberg method) (32).
Study Variability and Robustness of Inferences.
To examine the robustness of the study results, we used bootstrap resampling with replacement, drawing 200 samples. The primary analysis was run in each sample, and we estimated the median hazard ratio (HR) and empirical 95% confidence interval (95% CI). In addition, we evaluated the stability of the study inferences by comparing the P values from the repeated regression models to the Bonferroni-corrected alpha thresholds, summarizing the results across the 200 samples as the proportion of P values smaller than the threshold.
Further, a permutation analysis was conducted to obtain empirically based P values. We randomly reassigned metabolite concentrations across participants (to assure a null relationship between the metabolite levels and progression), creating 33,000 null datasets that allowed us to have sufficient discrimination around the Bonferroni significance threshold. Cox regression was run on each dataset to find the empirical distribution of the Wald chi-squared test statistic. Then we compared the observed test statistic from the original analysis to the empirical null distribution and determined the empirical P value as the percentile where the observed test statistic fell. The empirical P value was evaluated for significance against the Bonferroni-corrected threshold (0.05/825=6.06 × 10-5) to determine statistical significance. Analyses were performed using Stata/SES 15.1 (College Station, TX) and SAS 9.4 for Windows (SAS Institute Inc, NC).
Results
Baseline Cohort Characteristics
There were 673 CKiD children with sufficient plasma samples at the 6-month follow-up visit for metabolomics profiling. Of these, outcome and covariate data could not be obtained in 28 participants (18 participants had no data on kidney disease progression, six lacked calibrated serum creatinine laboratory test results, and four had missing UPCR). This left 645 participants for inclusion in our analyses. Baseline demographic and clinical characteristics of the participants are shown in Table 1. Participants with glomerular disease were more likely to be older, Black, female, have greater values for BMI and UPCR, higher baseline eGFR, and a higher percentage of participants with anemia and hypertension, but had lower median years of follow-up. A comparison to the full CKiD population can be seen in Supplemental Table 1.
Table 1.
Baseline characteristics of the Chronic Kidney Disease in Children participants included
| Characteristics | Overall (n=645) |
Glomerular Diagnosis (n=197) |
Nonglomerular Diagnosis (n=448) |
| Age, yrs | 12 (9–16) | 15 (12–17) | 11 (8–15) |
| Female sex | 254 (39) | 93 (47) | 161 (36) |
| Black race | 98 (15) | 49 (25) | 49 (11) |
| BMI Z-score | 0.37 (−0.37–1.25) | 0.86 (−0.10–1.68) | 0.22 (−0.48–0.94) |
| eGFR, ml/min per 1.73 m2 | 54 (38–67) | 63 (44–81) | 52 (37–63) |
| Serum creatinine, mg/dl | 1.1 (0.8–1.6) | 1.1 (0.8–1.6) | 1.2 (0.8–1.6) |
| Cystatin C, mg/l | 1.35 (1.07–1.95) | 1.19 (0.86–1.68) | 1.46 (1.15–2.03) |
| UPCR, mg/mg | 0.32 (0.12–1.00) | 0.46 (0.17–1.67) | 0.26 (0.11–0.81) |
| Anemia | 217 (35) | 83 (44) | 134 (31) |
| Hypertension | 325 (50) | 114 (58) | 211 (47) |
| Time with CKD, yrs | 9.1 (4.7–13.3) | 4.4 (2.4–7.7) | 10.4 (7.1–14.2) |
Values for categorical variables are presented as count (%); values for continuous variables are presented as median (interquartile range). Sample size differs due to missing data: Cystatin C level (n=618), anemia (n=627), and time with CKD (n=635). BMI, body mass index; UPCR, urine protein-creatinine ratio.
Progression to Composite Outcome
Over a median follow-up time of 4.8 years (interquartile range, 3.0–7.1), 209 (32%) children progressed to an event of the composite outcome (142 KRT, 68%, and 67 halving of eGFR, 32%). There were no deaths during the follow-up period. About 47% (n=186) of 399 children with baseline eGFR <60 ml/min per 1.73 m2 progressed to the composite event (with median follow-up of 4.8 years), whereas 9% (n=23) of the 246 participants with higher eGFR ≥60 ml/min per 1.73 m2 progressed to the composite outcome (with median follow-up of 4.8 years). About 35% (n=69) of the 197 participants with glomerular CKD diagnosis progressed to the composite event over a median follow-up of 3.6 years, whereas 31% (n=140) of the 448 participants with nonglomerular CKD diagnosis progressed to the composite event over a median follow-up of 5.3 years. Supplemental Figures 1 and 2 show the Kaplan–Meier survival curves for diagnosis and eGFR subgroups.
Metabolites related to CKD Progression
There were 1518 metabolites identified, of which 45 metabolites were excluded due to metabolite-wise missing ≥80%, 16 due to high variability (≥0.01 on log-scale), and 21 with extreme values. From the remaining 1436 metabolites, 825 known, nondrug metabolites were retained for the analysis within eight superpathways and 93 subpathways. The full list of metabolites and their standard deviations are listed in Supplemental Table 2. In blind replicate samples, 546 (66%) of the 825 metabolites had a correlation ≥0.8, and 512 (62%) had a coefficient of variation <20% (Supplemental Table 3).
In the overall cohort, 470 metabolites met the FDR threshold. Of them, 270 met the Bonferroni-corrected threshold in partially adjusted models (excluding baseline eGFR and UPCR), all of which were attenuated in the fully adjusted analysis (Figure 1). Only adjusting for eGFR (and not UPCR) did not change results, suggesting the evaluated metabolites were not strong correlates of proteinuria. We observed no statistically significant metabolite signals consistently across models in the overall cohort, or in subgroups stratified by glomerular versus nonglomerular CKD, but detected unique signals in the subgroups stratified by baseline eGFR (Figure 2 and Table 2), although interactions were not formally tested. In the higher kidney function strata (baseline eGFR ≥60 ml/min per 1.73 m2), there were seven metabolites that were significantly associated (by the Bonferroni threshold) with time to either 50% reduction in eGFR or KRT in fully adjusted models. Three nucleotide metabolites involved in purine (N6-carbamoylthreonyladenosine) and pyrimidine (5,6-dihydrouridine and pseudouridine) metabolism were related to substantially higher hazards of composite outcome events. A two-fold higher pseudouridine level was associated with a nearly 40-fold higher hazard of CKD progression (HR, 39.35; 95% CI, 7.73 to 200.39), adjusted for age, sex, race, and clinical risk factors. Higher levels of two amino acids were associated with CKD progression. The adjusted hazards of halving of eGFR or KRT per doubling in C-glycosyltryptophan and lanthionine were 24.11 (95% CI, 6.15 to 94.56) and 2.99 (95% CI, 1.80 to 4.98), respectively. A two-fold higher level of the tricarboxylic acid cycle intermediate, 2-methylcitrate/homocitrate, was associated with a four-fold higher risk of CKD progression (HR, 4.04; 95% CI, 2.24 to 7.29). Gulonate, in the ascorbate and aldarate subpathway, was also associated with greater risk of progression to the composite outcome (HR, 9.76; 95% CI, 3.28 to 29.03). Among participants with baseline eGFR <60 ml/min per 1.73 m2, there was one statistically significant metabolite association identified. A two-fold higher level of the corticosteroid, tetrahydrocortisol sulfate, was associated with lower risk of CKD progression (HR, 0.75; 95% CI, 0.65 to 0.86).
Figure 1.
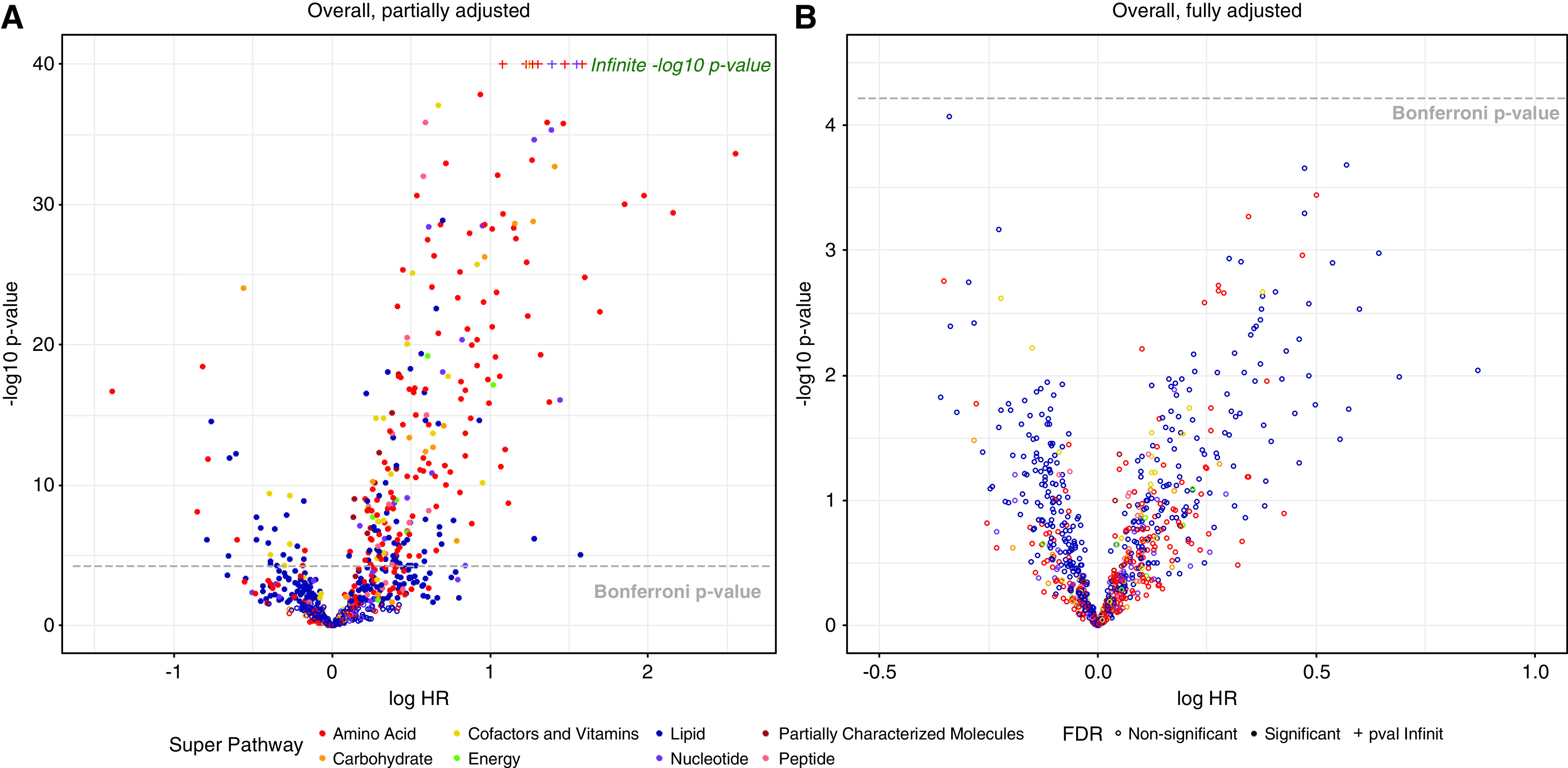
Volcano plot of 825 known, nondrug metabolites for all 645 participants. Fully adjusted included baseline covariates of age, sex, race, BMI Z-score, hypertension, CKD diagnosis (glomerular versus nonglomerular), log base 2 transformed UPCR and eGFR; partially adjusted included all baseline covariates excluding log base 2 transformed UPCR and eGFR. BMI, body mass; FDR, false discovery rate; UPCR, urine protein-creatinine ratio.
Figure 2.
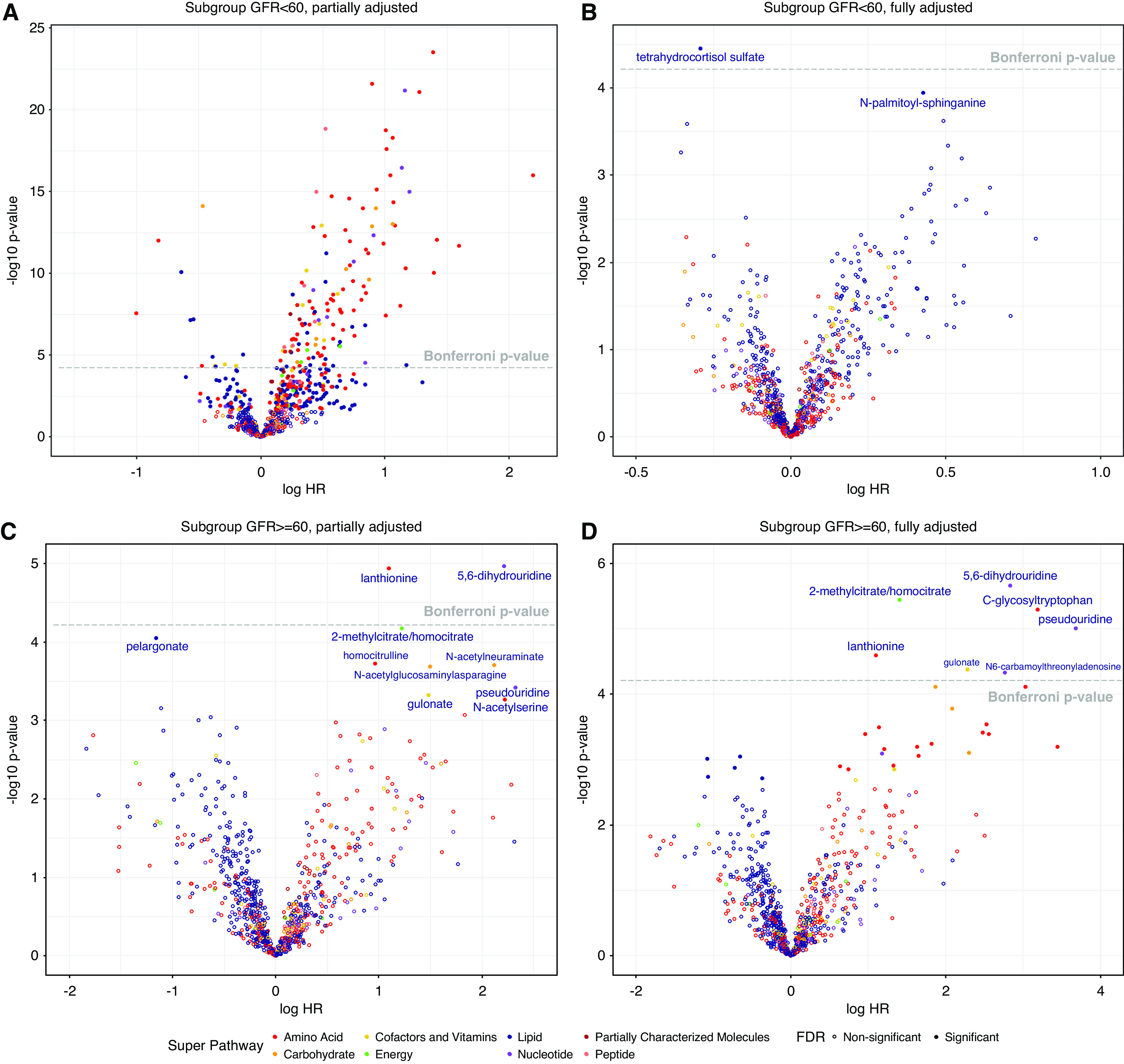
Volcano plot of 825 known, nondrug metabolites for participants by baseline eGFR. Fully adjusted included baseline covariates of age, sex, race, BMI Z-score, hypertension, CKD diagnosis (glomerular versus nonglomerular), log base 2 transformed UPCR and eGFR; partially adjusted included all baseline covariates excluding log base 2 transformed UPCR and eGFR.
Table 2.
Metabolite associations with time to composite outcome event (either initiation of kidney replacement therapy or a 50% reduction in eGFR from baseline)
| Metabolite | Pathway | Subpathway | Main Analysis | 200 Bootstrap Samples | Exact Test | ||
| Adjusted Hazard Ratio (95% Confidence Interval) | Median Adjusted Hazard Ratio (95% Confidence Interval) | % P value Crossing Bonferroni, % | Permutation P Value (95%) | ||||
| Overall | No results met Bonferroni threshold | ||||||
| eGFR, ml/min per 1.73 m2 ≥60 | |||||||
| Lanthioninea | Amino acid | Methionine, cysteine, SAM, and taurine metabolism | 2.99 (1.80 to 4.98) | 3.32 (1.78 to 9.58) | 50.5% | <3.0×10−5 | |
| C-glycosyltryptophana | Amino acid | Tryptophan metabolism | 24.11 (6.15 to 94.56) | 42.10 (5.37 to 2751.77) | 74.5% | <3.0×10−5 | |
| Gulonatea | Cofactors/vitamins | Ascorbate and aldarate metabolism | 9.76 (3.28 to 29.03) | 11.47 (1.97 to 89.57) | 50.0% | 9.1×10−5 | |
| 2-methylcitrate/ homocitratea | Energy | TCA cycle | 4.04 (2.24 to 7.29) | 4.71 (2.01 to 23.22) | 54.0% | 1.2×10−4 | |
| N6-carbamoylthreonyla denosinea | Nucleotide | Purine metabolism | 15.78 (4.18 to 59.51) | 24.05 (5.99 to 148.12) | 59.0% | 9.1×10−5 | |
| 5,6-dihydrouridinea | Nucleotide | Pyrimidine metabolism | 16.94 (5.26 to 54.63) | 19.89 (5.81 to 105.64) | 76.5% | 6.1×10−5 | |
| Pseudouridinea | Nucleotide | Pyrimidine metabolism | 39.35 (7.73 to 200.39) | 61.56 (7.61 to 1719.86) | 65.0% | 3.0×10−5 | |
| eGFR, ml/min per 1.73 m2 <60 | |||||||
| Tetrahydrocortisol sulfatea | Lipid | Corticosteroids | 0.75 (0.65 to 0.86) | 0.80 (0.71 to 0.92) | 24.0% | 1.2×10−4 | |
Values are given as relative hazard ratio (95% CI) to kidney replacement therapy or 50% decline in eGFR. In total, 209 (32%) children progressed to an event of the composite outcome overall: 186 of 399 children with baseline eGFR <60 ml/min per 1.73 m2 and 23 of the 246 with baseline eGFR ≥60 ml/min per 1.73 m2. Adjusted for baseline covariates, including age, sex, race, BMI Z score, hypertension, CKD diagnosis (glomerular versus nonglomerular), log base 2 transformed UPCR and eGFR. % P value crossing Bonferroni is the proportion of P values that crossed the Bonferroni threshold of 6.06 × 10−5 among the 200 samples. Permutation P value is the percentile of observed Wald chi-square statistic from the Cox regression on the basis of 33,000 permutations. Minimum P value discrimination is 3.03×10−5 and values less than this were indicated by <3.03×10−5. SAM, S-adenosylmethionine; TCA, tricarboxylic acid; 95% CI, 95% confidence interval; BMI, body mass index; UPCR, urine protein-creatinine ratio.
aSignificant metabolite using a Bonferroni P value of 6.06 × 10−5 0.05/825, where 825 was the number of tested metabolites).
Robustness of Metabolite Findings
Table 2 shows the median of the 200 adjusted HRs from bootstrap samples with empirical 95% CI for the eight metabolite hits that crossed the Bonferroni threshold in the main analysis. For most, the estimated HR of the original sample was similar to the median HR from bootstrap resamples, whereas some metabolites with large original effect sizes had even larger median estimates and wider confidence intervals as well, particularly C-glycosyltryptophan (original HR, 24.11, bootstrap median HR, 42.10; 95% CI, 5.37 to 2751.77) and pseudouridine (original HR, 39.35, bootstrap median HR, 61.56; 95% CI, 7.61 to 1719.86). The proportions of P values from 200 bootstrap resamples that crossed the Bonferroni threshold of 6.06 × 10−5, which corresponds to the robustness of the inference, varied across metabolites. The highest proportion was 77% for 5,6-dihydrouridine; 153 of the fully adjusted Cox regression results out of 200 total resamples met the Bonferroni significance threshold, indicating that most of the time, if we were to repeat the analysis 200 times, we would see a consistently significant association for 5,6-dihydrouridine. In the higher eGFR strata, C-glycosyltryptophan had a probability of being a significant association in 75% of resamples, followed by pseudouridine at 65%, whereas the proportion for tetrahydrocortisol sulfate was only 24.0% in the lower eGFR strata.
In permutation analysis, there were four metabolites that met or exceeded a Bonferroni threshold in the higher eGFR stratum. These metabolites included lanthionine, C-glycosyltryptophan, gulonate, 5,6-dihydrouridine, and pseudouridine. In the lower eGFR stratum, tetrahydrocortisol sulfate did not remain significant at the Bonferroni threshold in permutation analysis.
Table 3 shows the sensitivity analysis removing those participants who were followed for <9 months. Significant metabolite associations in the higher eGFR stratum remained the same, whereas no metabolites met the significance threshold in the lower eGFR stratum. Among this sensitivity analysis sample, if we used the less conservative FDR threshold, there were 24 additional metabolites identified in the higher eGFR stratum (14 amino acids, five lipids, three carbohydrates, one nucleotide, and one cofactor/vitamin), and one lipid metabolite signal in the lower eGFR stratum (Table 3). N-acetylated amino acids represented half of the amino acid metabolites associated with a two- to ten-fold higher hazard of CKD progression. However, the robustness test of these additional hits indicated that probabilities of observing them meeting the Bonferroni threshold were consistently low.
Table 3.
Metabolite associations with time to the composite event (initiation of KRT or a 50% reduction in eGFR from baseline) excluding participants followed for <9 months
| Metabolite | Superpathway | Subpathway | Main Analysis | 200 Bootstrap Samples | ||||
| Partially Adjusted Hazard Ratio (95% Confidence Interval) | Fully Adjusted Hazard Ratio (95% Confidence Interval) | Median Adjusted Hazard Ratio (95% Confidence Interval) | P value across Bonferroni, % | P value across False Discovery Rate, % | 33,000 Permutations P value (95%) | |||
| eGFR, ml/min per 1.73 m2 ≥60 | ||||||||
| Hydroxyasparagine | Amino acid | Alanine and aspartate metabolism | 4.11 (1.36 to 12.41) | 12.87 (3.13 to 53.00) | 18.36 (3.90 to 202.35) | 36.0% | 79.5% | 4.9×10−4 |
| N-acetylalanine | Amino acid | Alanine and aspartate metabolism | 9.81 (1.89 to 50.89) | 31.26 (4.35 to 224.51) | 42.52 (6.75 to 1274.11) | 25.0% | 72.5% | 8.5×10−4 |
| N-acetylserine | Amino acid | Glycine, serine, and threonine metabolism | 9.18 (2.62 to 32.19) | 20.70 (4.61 to 92.95) | 35.87 (4.95 to 972.63) | 56.5% | 88.5% | 9.1×10−5 |
| N-acetylthreonine | Amino acid | Glycine, serine, and threonine metabolism | 5.21 (1.73 to 15.67) | 12.48 (3.19 to 48.79) | 15.49 (2.83 to 98.49) | 38.0% | 76.5% | 4.2×10−4 |
| 1-ribosyl-imidazoleacetate | Amino acid | Histidine metabolism | 3.67 (1.62 to 8.33) | 5.19 (1.97 to 13.65) | 6.62 (1.40 to 32.14) | 31.5% | 71.0% | 1.7×10−3 |
| N-acetyl-1-methylhistidine | Amino acid | Histidine metabolism | 2.05 (1.27 to 3.29) | 3.11 (1.68 to 5.77) | 3.25 (1.62 to 8.41) | 35.5% | 82.0% | 5.1×10−4 |
| N-acetylhistidine | Amino acid | Histidine metabolism | 2.97 (1.38 to 6.40) | 5.07 (2.00 to 12.85) | 6.36 (2.44 to 25.03) | 28.5% | 78.5% | 6.7×10−4 |
| 2,3-dihydroxy-5-methylthio-4- pentenoate | Amino acid | Methionine, cysteine, SAM, and taurine metabolism | 4.59 (1.63 to 12.92) | 11.83 (3.03 to 46.20) | 17.81 (2.97 to 265.07) | 40.0% | 78.5% | 7.0×10−4 |
| Lanthioninea | Amino acid | Methionine, cysteine, SAM, and taurine metabolism | 2.97 (1.83 to 4.84) | 2.99 (1.80 to 4.98) | 3.32 (1.78 to 9.58) | 50.5% | 87.5% | <3.0×10−5 |
| C-glycosyltryptophana | Amino acid | Tryptophan metabolism | 6.23 (2.13 to 18.28) | 24.11 (6.15 to 94.56) | 42.10 (5.37 to 2751.77) | 74.5% | 93.5% | <3.0×10−5 |
| Vanillylmandelate | Amino acid | Tyrosine metabolism | 4.23 (1.65 to 10.89) | 6.11 (2.19 to 17.08) | 8.17 (1.62 to 125.21) | 35.0% | 72.5% | 8.8×10−4 |
| Homocitrulline | Amino acid | Urea cycle; arginine, and proline metabolism | 2.60 (1.58 to 4.30) | 2.61 (1.54 to 4.45) | 2.89 (1.86 to 5.53) | 24.5% | 86.5% | 4.2×10−4 |
| N2,N5-diacetylornithine | Amino acid | Urea cycle; arginine, and proline metabolism | 2.50 (1.41 to 4.42) | 3.32 (1.66 to 6.65) | 3.53 (1.84 to 10.49) | 22.5% | 79.0% | 7.9×10−4 |
| N-acetylglucosaminylasparagine | Carbohydrate | Aminosugar metabolism | 4.45 (2.02 to 9.77) | 6.46 (2.56 to 16.26) | 9.30 (3.13 to 59.15) | 53.0% | 89.5% | 3.3×10−4 |
| N-acetylneuraminate | Carbohydrate | Aminosugar metabolism | 8.27 (2.72 to 25.14) | 7.97 (2.71 to 23.45) | 13.07 (3.10 to 361.41) | 47.5% | 86.5% | 1.5×10−4 |
| Arabitol/xylitol | Carbohydrate | Pentose metabolism | 4.97 (1.69 to 14.58) | 9.96 (2.61 to 38.02) | 13.33 (3.71 to 108.85) | 24.0% | 75.5% | 7.3×10−4 |
| Gulonatea | Cofactors and vitamins | Ascorbate and aldarate metabolism | 4.40 (1.92 to 10.08) | 9.76 (3.28 to 29.03) | 11.47 (1.97 to 89.57) | 50.0% | 83.5% | 9.1×10−5 |
| 2-methylcitrate/homocitratea | Energy | TCA cycle | 3.37 (1.86 to 6.13) | 4.04 (2.24 to 7.29) | 4.71 (2.01 to 23.22) | 54.0% | 76.0% | 1.2×10−4 |
| 9-hydroxystearate | Lipid | Fatty acid, monohydroxy | 0.58 (0.42 to 0.81) | 0.52 (0.35 to 0.76) | 0.49 (0.22 to 0.77) | 30.0% | 65.0% | 1.4×10−3 |
| Pelargonate | Lipid | Medium chain fatty acid | 0.31 (0.18 to 0.56) | 0.34 (0.18 to 0.64) | 0.30 (0.11 to 0.73) | 34.5% | 66.0% | 2.4×10−3 |
| N6-carbamoylthreonyladenosinea | Nucleotide | Purine metabolism, adenine containing | 4.29 (1.61 to 11.39) | 15.78 (4.18 to 59.51) | 24.05 (5.99 to 148.12) | 59.0% | 94.0% | 9.1×10−5 |
| Cytidine | Nucleotide | Pyrimidine metabolism, cytidine containing | 2.88 (1.51 to 5.49) | 3.24 (1.63 to 6.43) | 3.46 (1.58 to 8.08) | 26.5% | 73.5% | 1.4×10−3 |
| 5,6-dihydrouridinea | Nucleotide | Pyrimidine metabolism, uracil containing | 9.12 (3.41 to 24.39) | 16.94 (5.26 to 54.63) | 19.89 (5.81 to 105.64) | 76.5% | 96.5% | 6.1×10−5 |
| Pseudouridinea | Nucleotide | Pyrimidine metabolism, uracil containing | 10.21 (2.84 to 36.74) | 39.35 (7.73 to 200.39) | 61.56 (7.61 to 1719.86) | 65.0% | 91.0% | 3.0×10−5 |
| N-acetyl-isoputreanine | Amino acid | Polyamine metabolism | 3.10 (1.37 to 6.98) | 3.75 (1.68 to 8.35) | 4.81 (1.99 to 20.49) | 22.5% | 76.0% | 1.1×10−3 |
| Indoleacetate | Amino acid | Tryptophan metabolism | 2.25 (1.36 to 3.70) | 2.10 (1.33 to 3.30) | 2.29 (0.88 to 9.12) | 27.0% | 57.5% | 2.1×10−3 |
| 4-methoxyphenol sulfate | Amino acid | Tyrosine metabolism | 1.87 (1.27 to 2.75) | 1.87 (1.28 to 2.74) | 1.97 (1.21 to 3.25) | 17.5% | 69.0% | 1.1×10−3 |
| Quinolinate | Cofactors and vitamins | Nicotinate and nicotinamide metabolism | 2.86 (1.33 to 6.18) | 3.79 (1.67 to 8.56) | 4.76 (1.72 to 14.73) | 24.0% | 67.5% | 1.5×10−3 |
| Palmitoleoyl-arachidonoyl- glycerol | Lipid | Diacylglycerol | 0.69 (0.55 to 0.86) | 0.69 (0.55 to 0.87) | 0.67 (0.49 to 0.86) | 16.5% | 62.5% | 1.8×10−3 |
| Caprate | Lipid | Medium chain fatty acid | 0.33 (0.17 to 0.63) | 0.34 (0.17 to 0.67) | 0.31 (0.14 to 0.61) | 15.0% | 67.0% | 3.2×10−3 |
| Stearidonate | Lipid | Polyunsaturated fatty acid (n3 and n6) |
0.53 (0.37 to 0.77) | 0.48 (0.31 to 0.75) | 0.46 (0.30 to 0.66) | 13.5% | 70.0% | 1.8×10−3 |
| eGFR, ml/min per 1.73 m2 <60 | ||||||||
| Tetrahydrocortisol sulfatea | Lipid | Corticosteroids | 1.02 (0.89 to 1.16) | 0.75 (0.65 to 0.86) | 0.80 (0.71 to 0.92) | 24.0% | 69.0% | 1.2×10−4 |
| N-palmitoyl-sphinganine | Lipid | Dihydroceramides | 1.46 (1.17 to 1.83) | 1.53 (1.23 to 1.90) | 1.38 (1.14 to 1.67) | 20.5% | 59.5% | 1.8×10−4 |
Values are given as relative hazard ratio (95% CI) to kidney replacement therapy or 50% decline in eGFR. Fully adjusted for baseline covariates, including age, sex, race, BMI Z-score, hypertension, CKD diagnosis (glomerular versus nonglomerular), log base 2 transformed urine UPCR and eGFR; partially adjusted for all variables except log base 2 transformed UPCR and eGFR. SAM, S-adenosylmethionine; TCA, tricarboxylic acid; 95% CI, 95% confidence interval; BMI, body mass index; UPCR, urine protein-creatinine ratio; FDR, false discovery rate.
aSignificant metabolite using a Bonferroni P value of 6.06 × 10–5 (0.05/825, where 825 was the number of tested metabolites). P value across Bonferroni (%) is the proportion of P values that crossed the Bonferroni threshold of 6.06 × 10–5 among the 200 samples. P value across FDR (%) is the proportion of P values that crossed the FDR threshold (Benjamin and Hochberg method) among the 200 samples. Permutation P value is the percentile of observed Wald chi-square statistic from the Cox regression on the basis of 33,000 permutations, and the threshold of significance should be adjusted for multiple comparisons as 0.05/825=6×10−5.
Discussion
This study applied untargeted metabolomic profiling for the discovery of metabolite biomarkers associated with CKD progression in children. Unique signals were identified in analyses stratified by baseline kidney function. Among those with relatively preserved eGFR (≥60 ml/min per 1.73 m2), higher levels of seven metabolites were associated with greater risk of progression to the composite outcome, independent of established predictors, including hypertension, proteinuria, and baseline eGFR. Among those with eGFR <60 ml/min per 1.73 m2, only one metabolite was associated with lower hazards of progression using a conservative, Bonferroni correction–based significance threshold. We attribute the different findings in the kidney function strata to the greater variance in risk of progression within the higher eGFR stratum and greater likelihood of identifying biomarkers of progression that are independent of hypertension, proteinuria, and kidney function in earlier-stage CKD.
Some of the metabolite signals identified in our study corroborate prior findings in adult cohorts. Several studies in adult population-based and diabetic cohort studies have demonstrated associations of two C-glycosylated derivatives of tryptophan and uridine (C-glycosyltryptophan and pseudouridine) with incident CKD, eGFR slope, and progression to KRT (24,33–35). These two metabolites and another modified metabolite found to be associated with significantly higher hazards for CKD progression in our study (N6-carbamoylthreonyladenosine) were previously shown to be associated with more rapid eGFR decline and shorter time to KRT in the Joslin Kidney Study prospective cohort study of type 1 diabetes, independent of clinically important factors including hypertension, hemoglobin A1c, albuminuria, and eGFR (33). These metabolites were also among the most strongly, negatively correlated with measured GFR (24).
One of the novel metabolite signals observed in our study was lanthionine. This nonproteinogenic amino acid, a side product of hydrogen sulfide synthesis in the transsulfuration pathway, has been recently identified as a potential uremic toxin. Blood lanthionine has been found to be elevated by approximately two orders of magnitude in hemodialysis patients and has been implicated in the dysregulation of sulfur amino acid metabolism seen in uremia, including low hydrogen sulfide levels and hyperhomocysteinemia (36–38). Gulonate, in the ascorbate/aldarate pathway, was another novel metabolite identified. Gulonate is produced from the reduction of glucuronate by aldehyde reductase in the kidney cortex during inositol catabolism (39). In a recent untargeted metabolomics profiling of blood samples from participants in two community-based adult cohorts (the Bogalusa Heart Study and Multi-Ethnic Study of Atherosclerosis), gulonate was identified as one of 12 novel metabolites associated with kidney function that also belonged to subpathways not previously associated with kidney function (40).
The identified association of the tricarboxylic acid cycle metabolite, 2-methylcitrate/homocitrate, with CKD progression is of considerable interest given its established involvement in inherited disorders of propionate metabolism, such as propionic acidemia, methlymalonic aciduria, and cobalamin disorders. Given the role of dietary modifications and vitamin supplementation in the management of these disorders, this identified metabolite is of relevance and has potential therapeutic implications. Propionyl CoA, an intermediate in branched and odd chain fatty acid catabolism, is converted to methylmalonyl CoA via a biotin-dependent carboxylase, which in the presence of vitamin B12, is then converted to succinyl CoA. In the setting of disordered propionate metabolism, propionyl CoA and methlycitrate, which is generated from the condensation of propionyl CoA with oxaloacetate, accumulate in blood (41,42). Recent studies suggest the accumulation of methylcitrate is neurotoxic (43,44). Methylcitrate has been demonstrated to be elevated in patients who have had a kidney transplant, CKD, or hemodialysis (45,46). Whether elevated methylcitrate represents a disturbance in propionic acid oxidation or a more general disorder of mitochondrial function in CKD is unclear.
The one metabolite identified in the subcohort with baseline eGFR <60 ml/min per 1.73 m2, tetrahydrocortisol sulfate, was associated with lower risk of progression to the composite outcome. Tetrahydrocortisol is one of the main inactive metabolites of cortisol. Concentrations of cortisol metabolites, including tetrahydrocortisol, 5α-tetrahydrocortisol, and tetrahydrocortisone, were demonstrated to be more than five-fold higher in adults on hemodialysis compared with healthy controls, with greater tetrahydrocortisol/tetrahydrocortisone and cortisol/cortisone ratios implicating reduced 11β-hydroxysteroid dehydrogenase type 2 activity in the kidney (47). The protective association of tetrahydrocortisol noted in our study and the role of altered cortisol metabolism in CKD progression warrant further exploration.
Our study had several limitations. Although CKiD is the largest prospective cohort study of CKD progression in children, and the sample size included in this study is comparable to similar studies in adult cohorts with nearly one third of the cohort overall progressing to the composite outcome, the number of outcome events in the higher eGFR stratum was quite modest (only 23). This relatively low event rate motivated the bootstrap resampling that we performed to assess the robustness of our results. We also used a strict Bonferroni-correction statistical significance threshold. Although this conservative approach enhances confidence in our findings, it may be overly stringent given the nonindependence of metabolites in their respective and inter-related pathways. The less conservative FDR also does not account for this nonindependence of metabolites. Given the number of outcome events in the higher eGFR strata, we were also unable to further stratify this group into glomerular versus nonglomerular CKD. In contrast to adults, CKD in children is rare, and opportunities for replication in a similar pediatric cohort are lacking currently. Several of the metabolites identified in our study did replicate prior findings in adult cohorts. Additionally, novel findings in our study demonstrate the highly distinct etiologies of pediatric versus adult CKD, which plausibly explains the fact that these metabolites have not been previously identified in adult cohorts. Finally, although the associations we observed were independent of well-established clinical risk factors, including baseline eGFR, there may be residual confounding by kidney function for certain filtration markers, rendering them more precise indicators of kidney function than biomarkers mechanistically related to disease progression per se. Future studies are needed to determine whether the identified metabolites are mechanistic biomarkers or bystanders of metabolic dysfunction induced by CKD progression.
In summary, untargeted plasma metabolomic profiling identified several novel metabolite biomarkers associated with CKD progression in children, independent of established predictors, including hypertension, proteinuria, and eGFR. Most of these were found among those children with relatively preserved eGFR (≥60 ml/min per 1.73 m2). These included metabolites such as C-glycosyltryptophan, pseudouridine, and N6-carbamoylthreonyladenosine, previously shown to be associated with CKD progression in adult cohorts, and novel metabolite associations with biologic plausibility and mechanistic implications, such as lanthionine and 2-methylcitrate, that warrant further investigation.
Disclosures
A.G. Abraham reports receiving honoraria from the National Institutes of Health (NIH) for serving on a data monitoring board, on the Rare Kidney Stone disease study data monitoring board, and as an Associate Editor of American Journal of Epidemiology; and reports being funded through the National Institute on Aging, National Institute of Allergy and Infectious Diseases, and National Heart, Lung, and Blood Institute (NHLBI). B.A. Warady reports consultancy agreements with Akebia, Amgen, Baxter, Bayer, GlaxoSmithKline, Reata, and Relypsa; reports receiving research funding from Baxter Healthcare; reports receiving honoraria from Akebia, GlaxoSmithKline, Reata, Relypsa, and University of Missouri; and reports serving as a scientific advisor or member of National Kidney Foundation (NKF), North American Pediatric Renal Trials and Collaborative Studies, and Nephrologists Transforming Dialysis Safety (NTDS) Board of Directors. C. Rebholz reports serving as an Editorial Board member for Diabetes Care and as an Editorial Fellow for JASN. H.I. Feldman reports consultancy agreements with DLA Piper, InMed Inc., Kyowa Hakko Kirin Co, Ltd. (ongoing), and NKF (ongoing); reports receiving honoraria from Rogosin Institute (invited speaker); reports serving as scientific advisor or member of the American Journal of Kidney Disease, Editor-in-Chief Steering Committee, Chronic Renal Insufficiency Cohort Study, Editor-in-Chief; and the NKF (member of Advisory Board). J. Coresh reports consultancy agreements with Healthy.io, Kaleido, and Ultragenyx; ownership interest in Health.io; receiving research funding from National Institutes of Health and the NKF (which receives industry support); and reports serving as a scientific advisor or member of Healthy.io and NKF. M. Grams reports receiving honoraria from academic institutions for giving grand rounds and ASN for Young Investigator Award; reports serving as a scientific advisor or member of American Journal of Kidney Diseases, CJASN, JASN Editorial Fellowship Committee, NKF Scientific Advisory Board, Kidney Disease: Improving Global Outcomes (KDIGO) Executive Committee, and United States Renal Data System Scientific Advisory Board; reports other interests/relationships with the NKF, which receives funding from Abbvie, Relypsa, and Thrasos; and reports receiving travel support from Dialysis Clinic, Inc (DCI) to speak at the annual meeting and KDIGO for participation in scientific meetings and the executive committee. M.R. Denburg reports receiving research funding from Mallinckrodt and serving on the NKF Delaware Valley Medical Advisory Board; reports grants from Mallinckrodt Pharmaceuticals, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Center for Complementary and Integrative Health (NCCIH), Patient-Centered Outcomes Research Institute (PCORI), The Children's Hospital of Philadelphia, outside the submitted work; and their spouse reports consultancy agreements with Trisalus Life Sciences, ownership interest in Precision Guided Interventions and In-Bore, and serving on the Trisalus Life Sciences Scientific Advisory Board. P.L. Kimmel reports serving as and receiving royalties as Co-Editor of Chronic Renal Disease Academic Press and Psychosocial Aspects of Chronic Kidney Disease Academic Press. R.S. Vasan is supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine; and reports reports consultancy agreements with NIDDK. All remaining authors have nothing to disclose.
Funding
This work was supported by NIDDK grants U01DK106982, U01DK085689, U01DK103225, and K23DK093556.
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The CKiD study is funded by the NIH: NIDDK, National Institute of Child Health and Human Development (NICHD), and the NHLBI. The views expressed in this article are those of the authors and do necessarily represent the official view of the NIDDK, the NIH, the Department of Health and Human Services, or the government of the United States.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “The Promise of Metabolomics in Decelerating CKD Progression in Children,” on pages 1152–1154.
Contributor Information
Collaborators: Vasan S. Ramachandran, Joseph Massaro, Clary Clish, Jeffrey Schelling, Michelle Denburg, Susan Furth, Bradley Warady, Joseph Bonventre, Sushrut Waikar, Gearoid McMahon, Venkata Sabbisetti, Josef Coresh, Morgan Grams, Casey Rebholz, Alison Abraham, Adriene Tin, Chirag Parikh, Jon Klein, Steven Coca, Bart S Ferket, Girish N. Nadkarni, Eugene Rhee, Paul L. Kimmel, Daniel Gossett, Brad Rovin, Michael G. Shlipak, M Sarnak, Andrew S. Levey, Lesley A. Inker, Meredith Foster, Orlando M. Gutiérrez, Joachim Ix, Ruth Dubin, Jesse Seegmiller, Tom Hostetter, Rajat Deo, Harold I. Feldman, Amanda Anderson, Theodore Mifflin, Dawei Xie, Haochang Shou, Shawn Ballard, Krista Whitehead, Heather Collins, Jason Greenberg, and Peter Ganz
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00220121/-/DCSupplemental.
Supplemental Table 1. Baseline characteristics of the study cohort and the full CKiD cohort.
Supplemental Table 2. Mean and standard deviations for all 825 metabolites.
Supplemental Table 3. Quality control data from 35 blind replicate samples for all 825 metabolites: correlations and coefficients of variation (CVs).
Supplemental Figure 1. Kaplan–Meier curves by CKD diagnosis (nonglomerular versus glomerular).
Supplemental Figure 2. Kaplan–Meier curves by baseline eGFR (≥60 versus <60 mL/min per 1.73 m2).
References
- 1.Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, Wong C, Stehman-Breen C: Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 58: 396–399, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Groothoff J, Gruppen M, de Groot E, Offringa M: Cardiovascular disease as a late complication of end-stage renal disease in children. Perit Dial Int 25: S123–S126, 2005 [PubMed] [Google Scholar]
- 4.Groothoff JW, Offringa M, Grootenhuis M, Jager KJ: Long-term consequences of renal insufficiency in children: Lessons learned from the Dutch LERIC study. Nephrol Dial Transplant 33: 552–560, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Groothoff JW, Offringa M, Van Eck-Smit BL, Gruppen MP, Van De Kar NJ, Wolff ED, Lilien MR, Davin JC, Heymans HS, Dekker FW: Severe bone disease and low bone mineral density after juvenile renal failure. Kidney Int 63: 266–275, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F: Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106: 100–105, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Groothoff JW, Gruppen MP, Offringa M, Hutten J, Lilien MR, Van De Kar NJ, Wolff ED, Davin JC, Heymans HS: Mortality and causes of death of end-stage renal disease in children: A Dutch cohort study. Kidney Int 61: 621–629, 2002 [DOI] [PubMed] [Google Scholar]
- 8.McDonald SP, Craig JC; Australian and New Zealand Paediatric Nephrology Association: Long-term survival of children with end-stage renal disease. N Engl J Med 350: 2654–2662, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Mitsnefes MM, Laskin BL, Dahhou M, Zhang X, Foster BJ: Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990-2010. JAMA 309: 1921–1929, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parekh RS, Carroll CE, Wolfe RA, Port FK: Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr 141: 191–197, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Abraham AG, Betoko A, Fadrowski JJ, Pierce C, Furth SL, Warady BA, Muñoz A: Renin-angiotensin II-aldosterone system blockers and time to renal replacement therapy in children with CKD. Pediatr Nephrol 32: 643–649, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F; ESCAPE Trial Group: Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Furth SL, Pierce C, Hui WF, White CA, Wong CS, Schaefer F, Wühl E, Abraham AG, Warady BA; Chronic Kidney Disease in Children (CKiD); Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients (ESCAPE) Study Investigators: Estimating time to ESRD in children with CKD. Am J Kidney Dis 71: 783–792, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, Mitsnefes M, Kaskel F, Greenbaum LA, Mak RH, Flynn J, Moxey-Mims MM, Furth S: Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: The Chronic Kidney Disease in Children (CKiD) cohort. Am J Kidney Dis 65: 878–888, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ardissino G, Daccò V, Testa S, Bonaudo R, Claris-Appiani A, Taioli E, Marra G, Edefonti A, Sereni F; ItalKid Project: Epidemiology of chronic renal failure in children: Data from the ItalKid project. Pediatrics 111: e382–e387, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Wong CJ, Moxey-Mims M, Jerry-Fluker J, Warady BA, Furth SL: CKiD (CKD in children) prospective cohort study: A review of current findings. Am J Kidney Dis 60: 1002–1011, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalim S, Rhee EP: An overview of renal metabolomics. Kidney Int 91: 61–69, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grams ME, Tin A, Rebholz CM, Shafi T, Köttgen A, Perrone RD, Sarnak MJ, Inker LA, Levey AS, Coresh J: Metabolomic alterations associated with cause of CKD. Clin J Am Soc Nephrol 12: 1787–1794, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu JR, Coresh J, Inker LA, Levey AS, Zheng Z, Rebholz CM, Tin A, Appel LJ, Chen J, Sarnak MJ, Grams ME: Serum metabolites are associated with all-cause mortality in chronic kidney disease. Kidney Int 94: 381–389, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo S, Coresh J, Tin A, Rebholz CM, Appel LJ, Chen J, Vasan RS, Anderson AH, Feldman HI, Kimmel PL, Waikar SS, Köttgen A, Evans AM, Levey AS, Inker LA, Sarnak MJ, Grams ME; Chronic Kidney Disease Biomarkers Consortium Investigators: Serum metabolomic alterations associated with proteinuria in CKD. Clin J Am Soc Nephrol 14: 342–353, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tin A, Nadkarni G, Evans AM, Winkler CA, Bottinger E, Rebholz CM, Sarnak MJ, Inker LA, Levey AS, Lipkowitz MS, Appel LJ, Arking DE, Coresh J, Grams ME: Serum 6-bromotryptophan levels identified as a risk factor for CKD progression. J Am Soc Nephrol 29: 1939–1947, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalim S, Clish CB, Deferio JJ, Ortiz G, Moffet AS, Gerszten RE, Thadhani R, Rhee EP: Cross-sectional examination of metabolites and metabolic phenotypes in uremia. BMC Nephrol 16: 98, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee EP, Clish CB, Wenger J, Roy J, Elmariah S, Pierce KA, Bullock K, Anderson AH, Gerszten RE, Feldman HI: Metabolomics of chronic kidney disease progression: A case-control analysis in the chronic renal insufficiency cohort study. Am J Nephrol 43: 366–374, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekula P, Goek ON, Quaye L, Barrios C, Levey AS, Römisch-Margl W, Menni C, Yet I, Gieger C, Inker LA, Adamski J, Gronwald W, Illig T, Dettmer K, Krumsiek J, Oefner PJ, Valdes AM, Meisinger C, Coresh J, Spector TD, Mohney RP, Suhre K, Kastenmüller G, Köttgen A: A metabolome-wide association study of kidney function and disease in the general population. J Am Soc Nephrol 27: 1175–1188, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu B, Zheng Y, Nettleton JA, Alexander D, Coresh J, Boerwinkle E: Serum metabolomic profiling and incident CKD among African Americans. Clin J Am Soc Nephrol 9: 1410–1417, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah VO, Townsend RR, Feldman HI, Pappan KL, Kensicki E, Vander Jagt DL: Plasma metabolomic profiles in different stages of CKD. Clin J Am Soc Nephrol 8: 363–370, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans A, Bridgewater B, Liu Q, Mitchell MW, Robinson RJ, Dai H, Stewart SJ, DeHaven CD, Miller LAD: High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics 4: 2, 2014 [Google Scholar]
- 29.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E: Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 81: 6656–6667, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Dehaven CD, Evans AM, Dai H, Lawton KA: Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform 2: 9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamini Y, Hochberg Y: Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300, 1995 [Google Scholar]
- 33.Niewczas MA, Mathew AV, Croall S, Byun J, Major M, Sabisetti VS, Smiles A, Bonventre JV, Pennathur S, Krolewski AS: Circulating modified metabolites and a risk of ESRD in patients with type 1 diabetes and chronic kidney disease. Diabetes Care 40: 383–390, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niewczas MA, Sirich TL, Mathew AV, Skupien J, Mohney RP, Warram JH, Smiles A, Huang X, Walker W, Byun J, Karoly ED, Kensicki EM, Berry GT, Bonventre JV, Pennathur S, Meyer TW, Krolewski AS: Uremic solutes and risk of end-stage renal disease in type 2 diabetes: Metabolomic study. Kidney Int 85: 1214–1224, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solini A, Manca ML, Penno G, Pugliese G, Cobb JE, Ferrannini E: Prediction of declining renal function and albuminuria in patients with type 2 diabetes by metabolomics. J Clin Endocrinol Metab 101: 696–704, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Perna AF, Di Nunzio A, Amoresano A, Pane F, Fontanarosa C, Pucci P, Vigorito C, Cirillo G, Zacchia M, Trepiccione F, Ingrosso D: Divergent behavior of hydrogen sulfide pools and of the sulfur metabolite lanthionine, a novel uremic toxin, in dialysis patients. Biochimie 126: 97–107, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Perna AF, Zacchia M, Trepiccione F, Ingrosso D: The sulfur metabolite lanthionine: Evidence for a role as a novel uremic toxin. Toxins (Basel) 9: 26, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vigorito C, Anishchenko E, Mele L, Capolongo G, Trepiccione F, Zacchia M, Lombari P, Capasso R, Ingrosso D, Perna AF: Uremic toxin lanthionine interferes with the transsulfuration pathway, angiogenetic signaling and increases intracellular calcium. Int J Mol Sci 20: 2269, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barski OA, Papusha VZ, Ivanova MM, Rudman DM, Finegold MJ: Developmental expression and function of aldehyde reductase in proximal tubules of the kidney. Am J Physiol Renal Physiol 289: F200–F207, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Nierenberg JL, He J, Li C, Gu X, Shi M, Razavi AC, Mi X, Li S, Bazzano LA, Anderson AH, He H, Chen W, Kinchen JM, Rebholz CM, Coresh J, Levey AS, Inker LA, Shlipak M, Kelly TN: Novel associations between blood metabolites and kidney function among Bogalusa Heart Study and Multi-Ethnic Study of Atherosclerosis participants. Metabolomics 15: 149, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wongkittichote P, Ah Mew N, Chapman KA: Propionyl-CoA carboxylase: A review. Mol Genet Metab 122: 145–152, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al Dhahouri N, Langhans CD, Al Hammadi Z, Okun JG, Hoffmann GF, Al-Jasmi F, Al-Dirbashi OY: Quantification of methylcitrate in dried urine spots by liquid chromatography tandem mass spectrometry for the diagnosis of propionic and methylmalonic acidemias. Clin Chim Acta 487: 41–45, 2018 [DOI] [PubMed] [Google Scholar]
- 43.Cudré-Cung HP, Zavadakova P, do Vale-Pereira S, Remacle N, Henry H, Ivanisevic J, Tavel D, Braissant O, Ballhausen D: Ammonium accumulation is a primary effect of 2-methylcitrate exposure in an in vitro model for brain damage in methylmalonic aciduria. Mol Genet Metab 119: 57–67, 2016 [DOI] [PubMed] [Google Scholar]
- 44.Tangney CC, Aggarwal NT, Li H, Wilson RS, Decarli C, Evans DA, Morris MC: Vitamin B12, cognition, and brain MRI measures: A cross-sectional examination. Neurology 77: 1276–1282, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Busch M, Franke S, Müller A, Wolf M, Gerth J, Ott U, Niwa T, Stein G: Potential cardiovascular risk factors in chronic kidney disease: AGEs, total homocysteine and metabolites, and the C-reactive protein. Kidney Int 66: 338–347, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Henning BF, Riezler R, Tepel M, Langer K, Raidt H, Graefe U, Zidek W: Evidence of altered homocysteine metabolism in chronic renal failure. Nephron 83: 314–322, 1999 [DOI] [PubMed] [Google Scholar]
- 47.N’Gankam V, Uehlinger D, Dick B, Frey BM, Frey FJ: Increased cortisol metabolites and reduced activity of 11beta-hydroxysteroid dehydrogenase in patients on hemodialysis. Kidney Int 61: 1859–1866, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.