Abstract
Purpose of Review
This review highlights recent advances on the mechanisms and impact of HDL-small non-coding RNAs (sRNA) on intercellular communication in atherosclerosis.
Recent Findings
Studies demonstrate that HDL-microRNAs (miRNA) are significantly altered in atherosclerotic cardiovascular disease (ASCVD), and are responsive to diet, obesity, and diabetes. Immune cells, pancreatic beta cells, and neurons are shown to export miRNAs to HDL. In turn, HDL can deliver functional miRNAs to recipient hepatocytes and endothelial cells regulating adhesion molecule expression, cytokines, and angiogenesis. With high-throughput sRNA sequencing, we now appreciate the full sRNA signature on circulating HDL, including the transport of rRNA and tRNA-derived fragments. Strikingly, HDL were highly enriched with exogenous microbial sRNAs.
Summary
HDL transport a diverse set of host and non-host sRNAs that are altered in cardiometabolic diseases. Given the bioactivity of these sRNAs, they likely contribute to cellular communication within atherosclerotic lesions, and are potential disease biomarkers and therapeutic targets.
Keywords: HDL, MicroRNA, Atherosclerosis, Macrophages, Obesity, Endothelial cells
Introduction
High-density lipoproteins (HDL) are remarkable to human health in many ways. The number of functions assigned to HDL are rivaled only by the plurality of cargo. HDL’s most famous constituent is cholesterol and while HDL biology has been told through the lens of cholesterol transport, many of HDL’s alternative functions may be conferred by non-cholesterol cargo. In circulation and biofluids, HDL particles continuously load and unload cargo through cellular interactions and exchange cargo with other lipoproteins. The term HDL describes a dynamic range of particle sub-classes (7–12 nm in diameter) that transition between discoidal and quasi-spherical shapes [1]. HDL participate in many biological processes that are beneficial to human health and physiology; however, many of these properties are lost during metabolic and inflammatory diseases [2, 3]. To fully understand HDL function, and disease-associated dysfunction, a greater understanding of HDL’s diverse cargo and their contribution to HDL (dys)function is warranted. HDL has been described colloquially as a circulating ball of loosely associated proteins. Currently, >215 different proteins have been reproducibly detected in HDL pools by mass spectrometry [4–6]. There has been considerable interest in the diversity and biological activities of HDL proteins, and the HDL Proteome Watch maintains a remarkable database of HDL proteomic studies [7]. Approximately 50–60% of HDL mass is proteins, 70% of which is apolipoprotein A-I (APOA1) which provides structure and micellular organization of the many classes of polar lipids. Mass spectrometry has also been used to profile the diverse species of lipids that comprise the shell and core of HDL particles [8]. In addition to lipids and proteins, HDL also transports many types of small non-coding RNAs (sRNA) [9••]. HDL-sRNAs range approximately 10–60 nucleotides (nts) in length and are likely single-stranded [9••]. The most-widely studied sRNA class on HDL is miRNA (18–22 nts in length). In plasma, HDL transports a specific profile of extracellular miRNAs that is consistent across individuals and is distinct from extracellular vesicle (EV) carriers, e.g., exosomes [10, 11•]. The concentration and diversity of miRNAs on HDL are altered in various diseases; therefore, HDL-miRNAs may hold potential as a new class of disease biomarkers. HDL readily accepts miRNAs from multiple cell types and can deliver functional miRNAs to recipient cells (Fig. 1). Cellular HDL-miRNA export is likely a regulated process as each cell type secretes a specific miRNA cassette to accepting HDL, which is not simply a representation of the most abundant miRNAs in each donor cell type [12••]. To date, a common sequence motif or molecular zip code for miRNA selection and export to HDL has not been reported. HDL-miRNAs are also bioactive, i.e., HDL deliver functional miRNAs to recipient cells and alter gene expression. The field of extracellular sRNAs has grown rapidly over the last decade and much of this interest is driven by the potential of sRNA-mediated intercellular communication in disease. Atherosclerosis is a complex, multicellular disease driven by local environmental cues and stimuli. Cell-to-cell communication between local immune cells, smooth muscle cells, and endothelial cells within the sub-endothelial space is well-established; however, recent evidence supports novel roles for extracellular sRNAs in atherosclerosis-associated intercellular communication. Lipoproteins are highly abundant in atherosclerotic lesions and the space-confined nature of the atheroma provides an environment whereby HDL-miRNAs may accumulate to sufficient concentrations to regulate gene expression and contribute to cellular phenotypes.
Fig. 1.
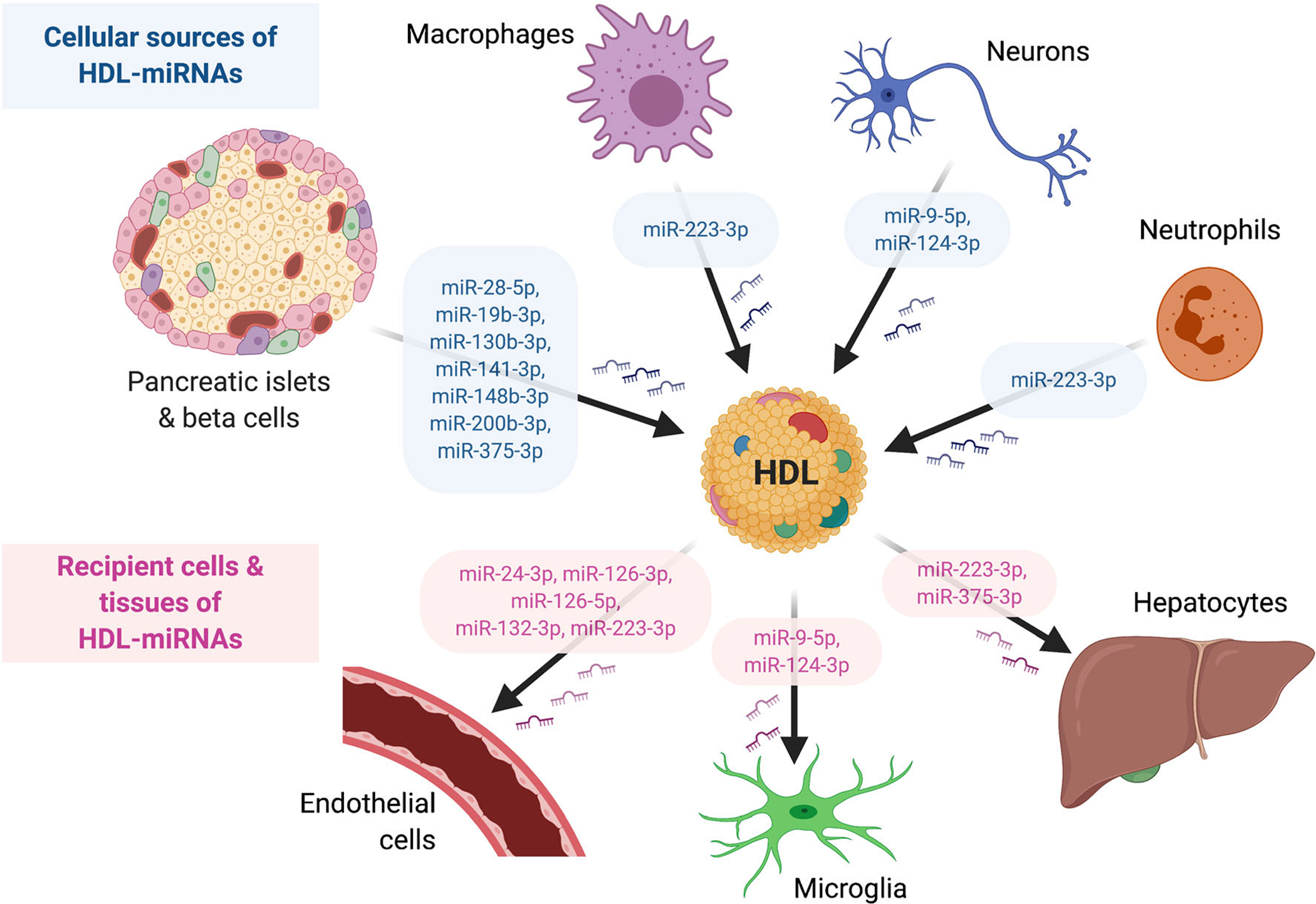
Schematic of HDL-miRNA intercellular communication. Cellular sources include pancreatic islets and beta cells, macrophages, neurons, and neutrophils. Recipient cells include endothelial cells, microglia, and hepatocytes
HDL-miRNAs as Biomarkers of Disease
Due to HDL’s accessibility in plasma, multiple groups have sought to explore HDL-miRNAs as disease biomarkers and determine how HDL-miRNAs change in cardiometabolic diseases [13, 14•]. HDL-miRNAs were first reported to be altered in human subjects with homozygous familial hypercholesterolemia (hoFH), a rare autosomal dominant disorder caused by mutations in the LDL receptor (LDLR) gene [10]. Clinically, FH is characterized by severely elevated LDL-cholesterol levels and increased risk of ASCVD; however, FH also results in dysfunctional HDL [2, 3, 15]. HDL-miR-223-3p, miR-24-3p, and miR-222-3p levels were significantly increased in hoFH compared to control subjects, likely due to increased atherosclerosis-associated inflammation [10]. miR-223-3p is one of the most highly expressed miRNAs in myeloid cells and both macrophages and neutrophils export miR-223-3p to HDL in vitro [10, 16••]. Heterozygous FH (hetFH) is a more common (1:200,000 individuals) genetic disorder, and the severity of hypercholesterolemia and atherosclerotic disease in hetFH subjects is likely determined by the specific genotype of the LDLR gene [17]. Different LDLR gene variants can result in variable levels of LDLR activity and pathophysiology. Recent studies have sought to define the links between LDLR genotypes and cardiovascular phenotypes in hetFH, particularly in the context of functional deficiencies to the LDLR [18–20]. Although FH and LDLR-deficiency primarily affects LDL uptake, specific HDL-miRNAs may serve as valuable predictors of ASCVD risk for individuals harboring distinct LDLR variants in hetFH. This was the basis for a recent study which reported that specific HDL-miRNAs were indeed altered in subjects based on LDLR genotypes causing either severe (LDLR-null) or moderate (LDLR-defective) hetFH phenotypes [21•]. For example, hetFH subjects harboring LDLR-null mutations had increased miR-486-5p, miR-92a-3p, miR-24-3p, and miR-223-3p levels on HDL [21•]. Moreover, HDL-miR-486-5p, and miR-92a-3p levels were independently associated with pulse-wave velocity, a clinical measurement of ASCVD risk [21•]. HDL-miR-486-5p and miR-92a-3p levels were also elevated in hetFH subjects with ASCVD compared to LDLR-defective subjects without ASCVD, thus supporting a potential association with HDL-miR-486-5p and miR-92a-3p levels to disease burden. These findings support earlier studies in which the levels of these two HDL-miRNAs (miR-486-5p and miR-92a-3p) were associated with vulnerable coronary artery disease (CAD) [22]. Furthermore, in a separate study, circulating levels of miR-92a-3p, miR-223-3p, miR-486-5p, miR-122-5p, miR-125a-5p, and miR-146a-5p were significantly increased on HDL in subjects with acute coronary syndrome (ACS) compared to HDL from control subjects [23]. On the contrary, a previous study had reported that HDL-miR-92a-3p, miR-146a-5p, and miR-30c-5p levels were significantly decreased in ACS subjects compared to healthy subjects [24]. The reasons for the discrepancies between these two studies on HDL-miRNA changes in ACS are unknown and will likely require a larger sample size and further analysis of co-variables. HDL-miRNAs have also been quantified over a transcoronary gradient to assess HDL-miRNA flux with coronary lesions [25•]. Most interestingly, HDL-miR-92a-3p, miR-223-3p, and miR-16-5p levels were decreased on HDL through the transcoronary passage in unstable ACS subjects compared to stable CAD subjects, suggesting that unstable coronary lesions may take up more HDL-miRNAs than stable lesions [25•].
Currently, the impact of statins on HDL-miRNAs is unknown; however, statin treatments would likely have a great effect of HDL-miRNAs based on reduced LDL concentrations and decreased inflammation in statin-treated patients. miR-30c-5p has been shown to be enriched on small HDL particles and plasma levels of miR-30c-5p were significantly increased with pravastatin treatments (1 year); however, HDL-miR-30c changes in response to pravastatin use have not been reported [26]. In addition to inflammation, HDL-miRNA levels may also be linked to metabolic status. For example, hyperglycemia further increased circulating HDL-miRNAs in ACS subjects, as HDL-miR-223-3p, miR-486-5p, miR-92a-3p, miR-122-5p, miR-125a-5p, and miR-146a-5p levels were significantly increased compared to HDL from ACS subjects with normo-glycemic levels [23]. HDL-miRNAs were also altered in diabetic subjects with nephropathy as HDL-miR-132-3p levels were significantly decreased compared to diabetic subjects without nephropathy [27••]. Loss of HDL-miR-132-3p in diabetic nephropathy subjects may contribute to microvascular injury and loss of angiogenesis, as HDL-miR-132-3p levels were inversely correlated with a marker of microvascular injury (plasma angiopoietin-2 levels) and HDL-miR-132-3p delivery to recipient endothelial cells enhanced angiogenesis [27••]. Although the full impact of hyperglycemia and diabetes on the HDL-miRNA profile is currently unknown, there is a strong possibility that cellular miRNA export to HDL is altered in diabetes. We have recently reported that pancreatic beta cells export miRNAs to HDL and this processes in inversely regulated by glucose-stimulated insulin secretion [12••]. HDL-miRNAs may also contribute to the physiological response to diabetic ischemia, potentially angiogenesis, as the levels of angiogenic miRNAs – miR-223-3p, miR-27b-3p, and miR-92a-3p – were significantly increased on HDL in diabetic mice (injected with reconstituted HDL) in early (6h-post) hindlimb ischemia [28•]. Further investigation is needed to determine if circulating HDL-miRNA changes can be used to monitor beta cell function in pre-diabetes and type 2 diabetes mellitus (T2DM) patients or monitor HDL function related to diabetic complications.
Specific HDL-miRNAs may also be linked to diet. For example, HDL-miRNAs were increased in postprandial states, as HDL-miR-223-3p and miR-92a-3p levels were significantly increased 2h after high-fat meal consumption [29]. HDL-miRNA changes associated with chronic dietary content may be more complicated to resolve as diets enriched with trans-fat failed to alter HDL-miRNA levels after 4 weeks [30, 31]. Recently, HDL-miRNA changes were assessed in a porcine (Sus scrofa) model of high-fat diet and hypercholesterolemia [32••]. Pigs were fed a high-fat/ high-cholesterol diet for 10 days to reach total cholesterol levels – HFD-treated (mean 321 mg/dL) and control-treated (mean 74 mg/ dL) – that were similar to levels observed in humans with hypercholesterolemia. A panel of HDL-miRNAs were quantified using Locked-Nucleic Acid-based multiplex PCR assays and the levels of five HDL-miRNAs were significant differentially altered, including 3 increased (miR-126-5p, miR-126-3p, miR-30b-5p) and 2 decreased (miR-103a-3p, let-7g-5p) [32••]. This study further demonstrated that the diet-associated increase in HDL-miR-126-5p levels likely altered HDL function, as HDL from hypercholesterolemic pigs transferred functional miR-126-5p to recipient endothelial cells and suppressed the expression of the predicted miR-126-5p target gene hypoxia inducible factor 1α (HIF1A) both in vitro and in vivo [32••].
To the delight of those that favor sweets, chocolate consumption may decrease the levels of pro-atherogenic miRNAs on HDL. Theobromine, an active ingredient in chocolate, was shown to decrease fasting HDL-miR-92a-3p levels in the absence of changes to HDL cholesterol acceptance capacity [29]. miR-92a-3p likely promotes atherogenesis through multiple mechanisms, specifically in endothelial cells [33, 34]. Chocolate consumption is associated with decreased risk of ASCVD; however, it is unknown if the biochemical links underlying these associations are mediated by HDL-miRNAs [35]. Vitamin C (1250 mg/day) may also regulate HDL-miRNA levels, as dietary vitamin C significantly decrease HDL-miR-155-5p levels by 49% in non-smokers and 75% in smokers after 8 weeks of consumption [36]. HDL-miRNA changes have also been reported to be linked to obesity and weight loss. For example, HDL-miR-223-3p levels were significantly increased in severely obese subjects compared to healthy control subjects, which may also be linked to increased inflammation observed in obesity [37•]. Extracellular miR-223-3p is tightly linked to inflammation, including adipose tissue inflammation [38]. Diet-induced weight loss (high protein vs normal protein diets) in obese subjects was associated with a significant decrease in HDL-miR-223-3p levels [39]. Most interestingly, the method of weight loss appears to influence HDL-miRNA changes, as HDL-miRNA levels, including miR-223-3p, were significantly increased, not decreased, in obese subjects after surgical weight loss [37•]. Specifically, HDL-miR-223-3p, miR-24-3p, and miR-222-3p levels were significantly increased on HDL from severe obese subjects 12-months post Roux-en-Y gastric bypass surgery [37•]. Differences in HDL-miRNA changes associated with the method of weight loss – dietary (decreased HDL-miR-223-3p) or surgical (increased HDL-miR-223-3p) – may be attributed to the amount of weight loss, HDL functionality, or residual inflammation.
Most HDL-miRNA studies have focused on miRNA changes on circulating HDL in plasma/serum; however, HDL-miRNAs in other biofluids may also hold biomarker value. For example, recent findings suggest that insulin resistance (IR) can result in decreased HDL-miRNA levels in mesenteric lymph, as HDL-miR-223-3p levels in lymph were significantly decreased by 60% in a rat model of IR compared to controls [40••]. Remarkably, dietary niacin for 6 weeks restored HDL-miR-223-3p levels in lymph [40••]. This study also reported an inverse relationship between HDL-cholesterol (HDL-C) and HDL-miRNAs, as IR was associated with increased lymphatic HDL-C and HDL-triglyceride levels [40••]. In addition to lymph, HDL are present in other biofluids, including cerebral spinal fluid; however, it remains to be determined if HDL transports miRNAs in these fluids and if they are altered in disease.
Cellular miRNA Export to HDL
Most miRNAs are transcribed and processed in multiple cell types within the body, and many of them are present in extracellular fluids. HDL transports a limited number of miRNAs; however, many of these miRNAs could be exported from many different cell types making it difficult to know the exact origin. Nonetheless, a few of the top-most abundant miRNAs on HDL have been reported to have restricted or limited expression to specific cell types. For example, miR-122-5p is present on circulating HDL and has been described to be a hepatocyte-specific miRNA with limited expression outside of the liver. miR-375-3p is another top HDL-miRNA and its transcription is largely restricted to pancreatic beta cells [10]. Recently, we reported that human pancreatic islets and beta cells export miR-375-3p to HDL [12••]. Most interestingly, beta cell HDL-miRNA export was inversely regulated by glucose-stimulated insulin secretion [12••]. For example, miR-375-3p export was suppressed in high glucose conditions [12••]. Moreover, tolbutamide, a chemical inhibitor ATP-sensitive potassium channel (KATP) channel that induces insulin secretion from beta cells, inhibited miR-375-3p export to HDL [12••]. Likewise, pancreatic islet HDL-miR-375-3p export was significantly decreased in a mouse (genetic) knockout of Abcc8, a gene that produces a subunit of the KATP channel [12••]. If HDL mediates intercellular communication between pancreatic beta cells and other cell types, this communication may be suppressed in hyperglycemia and T2DM. Another source of HDL-miRNAs is likely immune cells. We have previously demonstrated that mouse J774 macrophages export miR-223-3p to HDL in vitro and that macrophage HDL-miR-223-3p export was inversely regulated to exosome release through the secretory pathway [10]. More recently, we reported that human monocyte-derived macrophages (HMDM) and polymorphonuclear neutrophils (PMN) also export miR-223-3p to HDL [16••]. Strikingly, HDL-miR-223-3p export from immune cells was determined to be mediated by a scavenger receptor class B member 1 (SCARB1)-independent process, as blocking antibodies against SCARB1, which reduce HDL binding to SCARB1 on the cell surface, significantly increasing miRNA export to HDL [16••]. These results suggest that HDL either accepts cellular miRNAs through an unknown HDL-binding transporter or that HDL binds to exported miRNAs without binding to the donor cell surface. Recently, it was also reported that neurons can export miRNAs to HDL, miR-124-3p, and miR-9-5p, and this process was associated with electrical activity [41••]. Based on biochemical analyses of neuron conditioned media, HDL may facilitate the transfer of miR-124-3p from neurons to microglia within a novel intercellular communication network; however, further investigation into this process in the central nervous system is warranted [41••].
HDL-miRNA Delivery to Recipient Tissues
Due to HDL’s small size and mobility in tissues, HDL particles likely interact with many different cell types; however, it is unknown how many of these cell types accept HDL-sRNAs. Moreover, the mechanisms by which HDL delivers sRNAs to recipient cells require further investigation. We have previously reported that HDL’s primary receptor, SCARB1, likely regulates HDL-miRNA delivery to human hepatocytes and others have reported that HDL-miRNA delivery to porcine endothelial cells may also require SCARB1 [10, 32••]. However, global SCARB1-deficiency in mice failed to cause a backup of miRNA levels on circulating HDL, as overall HDL-miRNA levels were not significantly increased in SCARB1 knockout mice compared to control mice [9••, 10]. These results suggest that SCARB1 may not be a major route of miRNA delivery in vivo or that other uptake pathways exist. In circulation, HDL likely have extensive interactions with endothelial cells and HDL have been demonstrated to suppress endothelial cell inflammation and promote NO-mediated vasorelaxation [42]. We have previously reported that HDL’s ability to inhibit endothelial cell adhesion molecule and cytokine expression was mediated, in part, through the delivery of miRNAs to recipient endothelial cells [43]. Specifically, we showed that native HDL (1 mg/mL) transferred functional miR-223-3p to human coronary artery endothelial cells (HCAEC) at 24h [43]. In this study, HDL-miR-223-3p delivery was associated with decreased intercellular adhesion molecule-1 (ICAM1) expression and reduce neutrophil adhesion to HDL-treated cells [43]. Recently, HDL delivery of miR-223-3p to endothelial cells was confirmed by a separate group using 100 μg/mL of HDL with human umbilical vein endothelial cells (HUVEC) at 24h, suggesting that HDL can deliver miR-223-3p at lower concentrations and to multiple types of endothelial cells [44••]. It should be noted that a previous study had failed to observe a significant increase in mature miR-223-3p levels in HUVEC treated with 1 mg/mL HDL at time-points up to 24h [24]. HDL have previously been reported to inhibit the beta-amyloid-induced adhesion of peripheral blood mononuclear cells (PBMC) to human cerebral endothelial cells (hCMEC/D3) [45]. However, HDL-miR-223-3p delivery was also ruled out in this study as a contributing mechanism, as HDL-miR-223 transfer was not observed in hCMEC/D3 during 5h HDL-incubation [45]. The underlying reasons for the different results in these studies are unknown; however, the type of endothelial cells, length of incubation, HDL concentration, and the levels of miR-223-3p on HDL used for the experiment may potentially contribute to the observed discrepancies. In addition to miR-223-3p, HDL also delivered functional miR-24-3p to endothelial cells (HUVEC) [44••]. Dysfunctional HDL, but not normal HDL, suppressed angiogenesis and increased reactive oxygen species in recipient endothelial cells through HDL-miR-24-3p delivery and silencing of the miR-24-3p target gene vinculin (VCL) [44••]. This study also demonstrated HDL-miR-24-3p delivery to endothelial cells by loading HDL with fluorescence-labeled miR-24-3p mimetics for tracing studies to recipient endothelial cells. HDL dysfunction in the setting of hypercholesterolemia may also manifest as increased HDL-miR-126-5p transfer to recipient endothelial cells [32••]. As noted above, HDL-miR-126-5p levels are increased in hypercholesterolemia [32••]. HDL from hypercholesterolemic pigs were demonstrated to transfer miR-126-5p to porcine endothelial cells (in vitro) and coronary endothelium (in vivo), where miR-126-5p was observed to target and inhibit HIF-1α expression, and this process was not observed with HDL from normo-cholesterolemic pigs [32••].
Multiple studies have also demonstrated HDL-miRNA delivery to endothelial cells through HDL-miRNA mimetic loading, including the use exogenous C. elegans miRNA (cel-miR-39), and PCR quantification in recipient cells [24, 27••]. HDL have been reported to deliver functional miR-132-3p to endothelial cells (HUVEC), as a significant increase in miR-132-3p levels in endothelial cells coincided with a significant decrease in mRNA levels of the miR-132-3p target gene p120RasGap (RASA1) [27••]. Moreover, HDL-miR-132-3p delivery increased angiogenic tube formation in endothelial cells [27••]. HDL-miR-132-3p delivery likely promotes angiogenesis and this process may be defective in diabetic nephropathy; and HDL-miR-132-3p levels were decreased in this disease, as mentioned above [27••]. A previous study reported that HDL failed to deliver candidate miRNAs to human vascular smooth muscle cells and PBMCs; however, HDL from human subjects with CAD and ACS increased cellular levels of miR-92a-3p, miR-223-3p, and miR-126-5p in PBMCs after 6h in the absence of a significant increase in primary and precursor miRNA transcripts, supporting the potential of HDL-miRNA delivery in these cells in the setting of atherosclerosis [24].
HDL-sRNA Intercellular Communication in Atherosclerosis
Atherosclerosis is an inflammatory disease driven by lipids and cell-to-cell communication. The functional relevance of HDL-miRNA intercellular communication to atherosclerosis is unknown and has not been tested in vivo. Multiple in vitro studies suggest that HDL has the potential to accept miRNAs from macrophages and deliver functional miRNAs to recipient endothelial cells; however, this specific cell-to-cell network has not been demonstrated in vitro or in vivo. HDL-miR-223-3p suppression of endothelial cell activation and adhesion molecule expression may antagonize atherogenesis and HDL-miR-223-3p levels may increase in specific examples of ASCVD and enhance HDL’s anti-atherogenic properties; however, this theory has not been proven. The sub-endothelial space and confined lesion environment may be conducive for paracrine HDL-miRNA communication, as HDL particles and their sRNA cargo may accumulate to a sufficient degree to impact gene expression and phenotypes in cells that take up HDL particles or cargo. In terms of functional activity, HDL-sRNAs may act more as a singular cargo instead of individual entities or specific post-transcriptional gene regulation through canonical miRNA-like silencing mechanisms. Extracellular miRNAs have been reported to activate RNA-sensing toll like receptors, and it remains to be determined if HDL-sRNAs could do the same upon holo-particle uptake [46]. Although we cannot rule out that HDL facilitates long distance endocrine-like cell-to-cell communication, as circulating HDL contain a diverse set of sRNAs, it is more plausible that HDL mediates paracrine communication networks in space-confined tissues. A prime example of this would be the atherosclerotic lesion.
HDL-miRNA Quantification
HDL particles are highly abundant in circulation with approximately 1016 HDL particles/mL of plasma [47]. Low-density lipoproteins (LDL), which also transport miRNAs, are also abundant in plasma at 1015 particles/mL [47]. Conversely, the most-widely studied class of extracellular sRNA carriers, EVs are 107–9 vesicles/mL of plasma, substantially less concentrated than lipoproteins in plasma [47]. Investigation of these different sRNA carriers requires some technical considerations. For example, HDL (1.063–1.21 g/mL) and EVs (1.08–1.21 g/mL) share overlapping densities; therefore, cross-contamination in density-gradient ultracentrifugation (DGUC) fractions may occur. Moreover, apolipoprotein B-containing lipoproteins, namely LDL (22–25 nm in diameter) and very-low-density lipoproteins (VLDL, 30–80 nm in diameter), are similar in size to sub-classes of EVs, specifically exosomes (30–100 nm in diameter) and exomeres (approximately 30–40 nm in diameter) [48–50]. Size-exclusion chromatography (SEC) can be used to purify HDL (7–12 nm in diameter) from larger carriers in plasma and biofluids; however, there is some potential for free ribonucleoproteins to co-fractionate with small HDL particles in commonly used SEC resins. This issue can be resolved by using resins that provide greater resolution for proteins at 50–150 kDa. Lipid binding agents can also be used to separate lipid-associated and lipid-free sRNA carriers in SEC fractions to achieve greater-purity of HDL for down-stream sRNA analyses. The most common method for HDL purification is DGUC as this method yields high concentrations of HDL particles; however, as noted above, there is potential for EV contamination in HDL-DGUC samples and a secondary purification method, e.g., SEC, after DGUC is recommended. Recently, phosphotungstic acid/MgCl2 precipitation was used after DGUC to isolate highly-pure HDL samples for miRNA quantification [51•]. In addition to plasma, DGUC can also be used to purify HDL from other biofluids for miRNA quantification. For example, extracellular miRNAs have recently been quantified on HDL isolated from mesenteric lymph using DGUC [40••]. The only caution for starting material should be plasma collected in heparin-containing tubes, as heparin may interfere with downstream methods for miRNA quantification, e.g., PCR. In addition to DGUC and SEC, APOA1 immunoprecipitation and apolipoprotein B (APOB)-depleted plasma using polyethylene glycol have been used for HDL-miRNA quantification [9••, 10, 29, 52]. Each method produces different levels of HDL purity and yields, and combining multiple methods is advised to prevent cross-contamination of other sRNA carriers. HDL-miRNAs have been reported to be remarkably stable through multiple freeze-thaws, resistant to RNase A treatments, and survive weeks of HDL storage at 4°C or −80°C [53]. The required starting concentration of HDL particles for miRNA analyses is unknown, and depending on the miRNA quantification method, >100μg of HDL total protein may be necessary to reliably quantify HDL-miRNAs. Newer methods for lipoprotein isolation, e.g., asymmetric field flow fraction (AF4), may not separate sufficient quantities of HDL or be scalable for use in miRNA studies [54, 55]. There is great interest in the field of extracellular RNA and technological advances will likely lead to the development of new methods, equipment, and approaches for HDL purification and extracellular sRNA quantification.
miRNAs were the first class of sRNAs to be detected on HDL, and were quantified using TaqMan low-density PCR arrays and individual real-time PCR assays [10]. Quantitative PCR and droplet-digital PCR can also be used to quantify HDL-miRNAs, and the benefit of these assays is the ability to quantify copy numbers of candidate miRNAs based on a standard curve [56]. The current state-of-the-art method for HDL-miRNA profiling is high-throughput sRNA-sequencing (sRNA-seq); however, this approach is more costly and requires informatic support for data analysis [9••, 11•, 52]. The benefits of sRNA-seq over PCR-based arrays/assays include (i) lack of required prior knowledge of candidate sRNAs or sequences, (ii) analysis of miRNA isoforms, and (iii) additional analysis of non-miRNA sRNA classes [9••, 12••]. Recently, we released a sRNA-seq analysis pipeline (TIGER) specifically for the analysis of lipoprotein sRNA datasets [9••].
The Complete HDL-sRNA Signature
Although most research on HDL-sRNAs is focused on host miRNA cargo, miRNAs are just one of many classes of non-coding sRNAs present on circulating HDL. The most abundant class of host sRNAs on HDL is ribosomal RNA (rRNA)-derived sRNAs (rDR) [9••]. Parent rRNA transcripts are likely cleaved at specific regions to produce small rDRs. miRNAs are the second most abundant host sRNA class on HDL followed by transfer RNA (tRNA)-derived sRNAs (tDR) [9••]. Other classes of host sRNAs have been detected by sRNA-seq at very low levels, including sRNA fragments cleaved from parent small nuclear RNAs (snDR), small nucleolar RNAs (snoDR), and other miscellaneous transcripts [9••, 57]. The biological functions of miRNAs are well-established in post-transcriptional gene silencing mechanisms and a considerable amount of evidence has been reported on the role of tDRs in suppression of protein synthesis. Nevertheless, the biological functions of the other classes of sRNAs are largely unknown. Furthermore, the value of non-miRNAs as extracellular disease biomarkers in plasma is emerging; however, the prognostic value of these non-miRNAs in the HDL pool has not been investigated. There are some unique features of sRNAs on HDL compared to sRNAs in tissues, e.g., the liver [9••, 10]. For example, HDL-miRNAs are distinct from cellular miRNAs based on post-transcriptional modifications, specifically non-templated nucleotide additions (NTA) on the 3′ terminal end of miRNAs. HDL-miRNAs are enriched with non-templated 3′ end uridylation and cellular miRNAs are enriched with non-templated 3′ end adenylation [9••]. The biological relevance of miRNA NTAs and 3′ end variance is not fully understood but may be related to cellular retention and miRNA export [58, 59]. One of the benefits of using sRNA-seq to quantify HDL-miRNAs is the ability to observe changes to miRNA isoforms and NTAs. In addition to miRNAs, other sRNAs on HDL harbor distinct features. For example, rDRs in the liver are enriched for fragments approximately 44 nts in length and rDRs on HDL are equally distributed across a range of sizes 18–60 nts [9••]. In addition, HDL-tDRs were distinct from liver tDRs based on principal coordinate analyses [9••]. It remains to be determined if these defining features of HDL-sRNAs facilitate their export to HDL, influence biological function of HDL-sRNAs in intercellular communication, or hold value as disease biomarkers.
Most interestingly, most sRNAs on HDL likely come from exogenous microbial sources, and the aforementioned host sRNAs only compose a minor fraction of sRNAs on circulating HDL [9••]. Based on genome and database alignments within the TIGER analysis pipeline, non-host microbial sRNAs (msRNA) are likely derived from bacteria, fungi, protists, archaeplastida, embryophytes, and archaea [9••]. A large percentage of HDL-msRNAs were identified to be bacterial rDRs and tDRs, likely from species in the microbiome and environment [9••]. Based on analysis at the phylum level, HDL were highly enriched with msRNA from Proteobacteria, Actinobacteria, and Firmicutes(9••). One key observation from the analysis of HDL-msRNAs is the low variability at the genome level with high variability at the sequence level across all samples and individuals [9••]. In other words, HDL-msRNAs were likely contributed by the same species (or phylum), but the individual msRNA sequences were different for each HDL sample and individual. This highlights one of the main barriers to investigating non-miRNAs and non-host msRNAs as biomarkers or functional molecules [9••, 57]. For example, mature miRNAs harbor relatively precise cleavage from precursor miRNAs and are remarkably consistent between subjects and samples. This allows for miRNAs to be readily quantified with standard PCR assays and antisense strategies across samples in an experiment. Conversely, non-miRNA sRNAs and non-host msRNAs are cleaved from parent transcripts by less precise mechanisms which leads to high variability on both the 5′ and 3′ terminal ends, different sequence lengths, and the requirement for custom PCR probes for quantification.
Conclusions
HDL transport many different classes of sRNAs, including fragments of parent rRNAs from Proteobacteria. The most well-studied class of sRNAs is miRNAs. HDL-miRNAs were altered in ASCVD, including FH, CAD, and ACS. HDL-miRNAs were also differentially altered in obesity, weight loss, and specialized diets. HDL has been shown to accept miRNAs from various cell types, including pancreatic beta cells, neurons, and multiple immune cells (Fig. 1). HDL also delivered functional miRNAs to recipient endothelial cells, where HDL-miRNAs regulated miRNA target expression and cellular processes, including adhesion molecule expression and angiogenesis (Fig. 1). Although HDL-miRNA intercellular communication in atherosclerosis has not been proven in vivo, HDL have been shown to accept miRNAs from macrophages and deliver miRNAs to endothelial cells in vitro, supporting the possibility for cell-to-cell communication within the atheroma. Collectively, HDL-sRNAs are bioactive signals that are part of a promising new class of biomarkers for ASCVD and other cardiometabolic diseases.
Acknowledgments
This work is supported by the W.M. Keck Foundation Medical Research Award and National Institutes of Health Grant P01HL116263. Figure was generated using BioRender.com.
Footnotes
This article is part of the Topical Collection on Vascular Biology
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest The authors declare no competing interests.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med. 2011;17(10):594–603. [DOI] [PubMed] [Google Scholar]
- 2.Ganjali S, Momtazi AA, Banach M, Kovanen PT, Stein EA, Sahebkar A. HDL abnormalities in familial hypercholesterolemia: focus on biological functions. Prog Lipid Res. 2017;67:16–26. [DOI] [PubMed] [Google Scholar]
- 3.Tao H, Huang J, Yancey PG, Yermalitsky V, Blakemore JL, Zhang Y, et al. Scavenging of reactive dicarbonyls with 2-hydroxybenzylamine reduces atherosclerosis in hypercholesterolemicLdlr(−/−) mice. Nat Commun. 2020;11(1):4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlsson H, Leanderson P, Tagesson C, Lindahl M. Lipoproteomics II: mapping of proteins in high-density lipoprotein using two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2005;5(5):1431–45. [DOI] [PubMed] [Google Scholar]
- 5.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117(3):746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon SM, Deng J, Lu LJ, Davidson WS. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J Proteome Res. 2010;9(10):5239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah AS, Davidson WS. updated2020. Available from: homepages.uc.edu/~davidswm/HDLproteome.html.Accessed22 Oct 2020.
- 8.Christinat N, Masoodi M. Comprehensive lipoprotein characterization using lipidomics analysis of human plasma. J Proteome Res. 2017;16(8):2947–53. [DOI] [PubMed] [Google Scholar]
- 9.••.Allen RM, Zhao S, Ramirez Solano MA, Zhu W, Michell DL, Wang Y, et al. Bioinformatic analysis of endogenous and exogenous small RNAs on lipoproteins. J Extracell Vesicles. 2018;7(1): 1506198. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that HDL are highly-enriched with microbial sRNAs and was the release of the TIGER pipeline for HDL-sRNA sequencing analysis.
- 10.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4): 423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.•.Desgagne V, Guerin R, Guay SP, Boyer M, Hutchins E, Picard S, et al. Human high-density lipoprotein microtranscriptome is unique and suggests an extended role in lipid metabolism. Epigenomics. 2019;11(8):917–34. [DOI] [PubMed] [Google Scholar]; This study used high-throughput sRNA sequencing to demonstrate that HDL transports a distinct sRNA profile compared to whole plasma.
- 12.••.Sedgeman LR, Beysen C, Ramirez Solano MA, Michell DL, Sheng Q, Zhao S, et al. Beta cell secretion of miR-375 to HDL is inversely associated with insulin secretion. Sci Rep. 2019;9(1):3803. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported that pancreatic beta cell miR-375-3p export to HDL is suppressed by glucose-stimulated insulin secretion.
- 13.Simionescu N, Niculescu LS, Sanda GM, Margina D, Sima AV. Analysis of circulating microRNAs that are specifically increased in hyperlipidemic and/or hyperglycemic sera. Mol Biol Rep. 2014;41(9):5765–73. [DOI] [PubMed] [Google Scholar]
- 14.•.Axmann M, Meier SM, Karner A, Strobl W, Stangl H, Plochberger B. Serum and lipoprotein particle miRNA profile in uremia patients. Genes (Basel). 2018;9(11):533. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study quantified HDL-miRNA changes in uremia and the ability of HDL to deliver miRNAs to recipient cells.
- 15.Watts GF, Barrett PH. High-density lipoprotein metabolism in familial hypercholesterolaemia: significance, mechanisms, therapy. Nutr Metab Cardiovasc Dis. 2002;12(1):36–41. [PubMed] [Google Scholar]
- 16.••.Cuesta Torres LF, Zhu W, Ohrling G, Larsson R, Patel M, Wiese CB, et al. High-density lipoproteins induce miR-223-3p biogenesis and export from myeloid cells: role of scavenger receptor BI-mediated lipid transfer. Atherosclerosis. 2019;286:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that macrophages and neutrophils export miR-223-3p to HDL and that this process is likely independent of SR-BI.
- 17.Goldberg AC, Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, Robinson JG, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5(3):133–40. [DOI] [PubMed] [Google Scholar]
- 18.Ten Kate GJ, Neefjes LA, Dedic A, Nieman K, Langendonk JG, Galema-Boers AJ, et al. The effect of LDLR-negative genotype on CT coronary atherosclerosis in asymptomatic statin treated patients with heterozygous familial hypercholesterolemia. Atherosclerosis. 2013;227(2):334–41. [DOI] [PubMed] [Google Scholar]
- 19.Bourbon M, Alves AC, Alonso R, Mata N, Aguiar P, Padro T, et al. Mutational analysis and genotype-phenotype relation in familial hypercholesterolemia: the SAFEHEART registry. Atherosclerosis. 2017;262:8–13. [DOI] [PubMed] [Google Scholar]
- 20.Pirillo A, Garlaschelli K, Arca M, Averna M, Bertolini S, Calandra S, et al. Spectrum of mutations in Italian patients with familial hypercholesterolemia: new results from the LIPIGEN study. Atheroscler Suppl. 2017;29:17–24. [DOI] [PubMed] [Google Scholar]
- 21.•.Scicali R, Di Pino A, Pavanello C, Ossoli A, Strazzella A, Alberti A, et al. Analysis of HDL-microRNA panel in heterozygous familial hypercholesterolemia subjects with LDL receptor null or defective mutation. Sci Rep. 2019;9(1):20354. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study support that HDL-miRNAs may help to resolve the link between LDLR gene variants (genotype) and cardiovascular phenotypes in FH subjects.
- 22.Niculescu LS, Simionescu N, Sanda GM, Carnuta MG, Stancu CS, Popescu AC, et al. MiR-486 and miR-92a identified in circulating HDL discriminate between stable and vulnerable coronary artery disease patients. PLoS One. 2015;10(10):e0140958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simionescu N, Niculescu LS, Carnuta MG, Sanda GM, Stancu CS, Popescu AC, et al. Hyperglycemia determines increased specific micrornas levels in sera and HDL of acute coronary syndrome patients and stimulates microRNAs production in human macrophages. PLoS One. 2016;11(8):e0161201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner J, Riwanto M, Besler C, Knau A, Fichtlscherer S, Roxe T, et al. Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler Thromb Vasc Biol. 2013;33(6):1392–400. [DOI] [PubMed] [Google Scholar]
- 25.•.Choteau SA, Cuesta Torres LF, Barraclough JY, Elder AMM, Martinez GJ, Chen Fan WY, et al. Transcoronary gradients of HDL-associated microRNAs in unstable coronary artery disease. Int J Cardiol. 2018;253:138–44. [DOI] [PubMed] [Google Scholar]; This study demonstrated that sRNA flux between HDL and atherosclerotic lesions likely occurs through the transcoronary passage and is altered in acute coronary syndrome.
- 26.Sodi R, Eastwood J, Caslake M, Packard CJ, Denby L. Relationship between circulating microRNA-30c with total- and LDL-cholesterol, their circulatory transportation and effect of statins. Clin Chim Acta. 2017;466:13–9. [DOI] [PubMed] [Google Scholar]
- 27.••.Florijn BW, Duijs J, Levels JH, Dallinga-Thie GM, Wang Y, Boing AN, et al. Diabetic nephropathy alters the distribution of circulating angiogenic microRNAs among extracellular vesicles, HDL, and Ago-2. Diabetes. 2019;68(12):2287–300 [DOI] [PubMed] [Google Scholar]; This study demonstrated that HDL delivery of miR-132-3p to recipient endothelial cells likely promotes angiogenesis; however, this function may be lost in diabetic nephropathy.
- 28.•.Hourigan ST, Solly EL, Nankivell VA, Ridiandries A, Weimann BM, Henriquez R, et al. The regulation of miRNAs by reconstituted high-density lipoproteins in diabetes-impaired angiogenesis. Sci Rep. 2018;8(1):13596. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that angiogenic miRNAs were increased on HDL in diabetic mice injected with reconstituted HDL.
- 29.Talbot CPJ, Mensink RP, Smolders L, Bakeroot V, Plat J. Theobromine does not affect fasting and postprandial HDL cholesterol efflux capacity, while it decreases fasting miR-92a levels in humans. Mol Nutr Food Res. 2018;62(13):e1800027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desgagne V, Guay SP, Guerin R, Corbin F, Couture P, Lamarche B, et al. Variations in HDL-carried miR-223 and miR-135a concentrations after consumption of dietary trans fat are associated with changes in blood lipid and inflammatory markers in healthy men - an exploratory study. Epigenetics. 2016;11(6):438–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desgagne V, Guerin R, Guay SP, Corbin F, Couture P, Lamarche B, et al. Changes in high-density lipoprotein-carried miRNA contribution to the plasmatic pool after consumption of dietary trans fat in healthy men. Epigenomics. 2017;9(5):669–88. [DOI] [PubMed] [Google Scholar]
- 32.••.Ben-Aicha S, Escate R, Casani L, Padro T, Pena E, Arderiu G, et al. High-density lipoprotein remodelled in hypercholesterolaemic blood induce epigenetically driven down-regulation of endothelial HIF-1alpha expression in a preclinical animal model. Cardiovasc Res. 2020;116(7):1288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported that HDL functional delivery of miR-126-5p to recipient endothelial cells is likely increased in hypercholesterolemia.
- 33.Wiese CB, Zhong J, Xu ZQ, Zhang Y, Ramirez Solano MA, Zhu W, et al. Dual inhibition of endothelial miR-92a-3p and miR-489-3p reduces renal injury-associated atherosclerosis. Atherosclerosis. 2019;282:121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loyer X, Potteaux S, Vion AC, Guerin CL, Boulkroun S, Rautou PE, et al. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res. 2014;114(3):434–43. [DOI] [PubMed] [Google Scholar]
- 35.Buitrago-Lopez A, Sanderson J, Johnson L, Warnakula S, Wood A, Di Angelantonio E, et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;343:d4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SM, Lim SM, Yoo JA, Woo MJ, Cho KH. Consumption of high-dose vitamin C (1250 mg per day) enhances functional and structural properties of serum lipoprotein to improve anti-oxidant, anti-atherosclerotic, and anti-aging effects via regulation of antiinflammatory microRNA. Food Funct. 2015;6(11):3604–12. [DOI] [PubMed] [Google Scholar]
- 37.•.Ho JH, Ong KL, Cuesta Torres LF, Liu Y, Adam S, Iqbal Z, et al. High density lipoprotein-associated miRNA is increased following Roux-en-Y gastric bypass surgery for severe obesity. J Lipid Res. 2021;62:100043. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study suggest that HDL-miRNAs are increased in severe obesity and are further increased, not decreased, with surgical weight loss (Roux-en-Y gastric bypass).
- 38.Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125(23):2892–903. [DOI] [PubMed] [Google Scholar]
- 39.Tabet F, Cuesta Torres LF, Ong KL, Shrestha S, Choteau SA, Barter PJ, et al. High-density lipoprotein-associated miR-223 is altered after diet-induced weight loss in overweight and obese males. PLoS One. 2016;11(3):e0151061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.••.Mangat R, Borthwick F, Haase T, Jacome M, Nelson R, Kontush A, et al. Intestinal lymphatic HDL miR-223 and ApoA-I are reduced during insulin resistance and restored with niacin. FASEB J. 2018;32(3):1602–12. [DOI] [PubMed] [Google Scholar]; This study reported changes to HDL-miRNAs associated with insulin resistance within mesenteric lymph and the impact of niacin treatment.
- 41.••.Veremeyko T, Kuznetsova IS, Dukhinova M, Yung AWY, Kopeikina E, Barteneva NS, et al. Neuronal extracellular microRNAs miR-124 and miR-9 mediate cell-cell communication between neurons and microglia. J Neurosci Res. 2019;97(2):162–84. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated a potential neuron-to-microglia miRNA intercellular communication network mediated by HDL.
- 42.Kratzer A, Giral H, Landmesser U. High-density lipoproteins as modulators of endothelial cell functions: alterations in patients with coronary artery disease. Cardiovasc Res. 2014;103(3):350–61. [DOI] [PubMed] [Google Scholar]
- 43.Tabet F, Vickers KC, Cuesta Torres LF, Wiese CB, Shoucri BM, Lambert G, et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat Commun. 2014;5: 3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.••.Li HM, Mo ZW, Peng YM, Li Y, Dai WP, Yuan HY, et al. Angiogenic and antiangiogenic mechanisms of high density lipoprotein from healthy subjects and coronary artery diseases patients. Redox Biol. 2020;36:101642. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that dysfunctional HDL deliver miR-24-3p to recipient endothelial cells where it regulated the expression of vinculin, suppressed angiogenesis, and increased reactive oxygen species.
- 45.Robert J, Button EB, Stukas S, Boyce GK, Gibbs E, Cowan CM, et al. High-density lipoproteins suppress Abeta-induced PBMC adhesion to human endothelial cells in bioengineered vessels and in monoculture. Mol Neurodegener. 2017;12(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109(31):E2110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simonsen JB. What are we looking at? Extracellular vesicles, lipoproteins, or both? Circ Res. 2017;121(8):920–2. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Q, Higginbotham JN, Jeppesen DK, Yang YP, Li W, McKinley ET, et al. Transfer of functional cargo in exomeres. Cell Rep. 2019;27(3):940–54 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boon RA, Vickers KC. Intercellular transport of microRNAs. Arterioscler Thromb Vasc Biol. 2013;33(2):186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. [DOI] [PubMed] [Google Scholar]
- 51.•.Ishikawa H, Yamada H, Kondo K, Ota T, Yamazaki M, Ando Y, et al. Establishment of a simpler method for measuring HDL-microRNAs. Ann Clin Biochem. 2019;56(1):49–55. [DOI] [PubMed] [Google Scholar]; This study reported an improved method for isolating HDL-sRNAs for quantitative PCR analysis.
- 52.Michell DL, Allen RM, Landstreet SR, Zhao S, Toth CL, Sheng Q, et al. Isolation of high-density lipoproteins for non-coding small RNA quantification. J Vis Exp. 2016;(117):54488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishikawa H, Yamada H, Taromaru N, Kondo K, Nagura A, Yamazaki M, et al. Stability of serum high-density lipoproteinmicroRNAs for preanalytical conditions. Ann Clin Biochem. 2017;54(1):134–42. [DOI] [PubMed] [Google Scholar]
- 54.Bria CRM, Afshinnia F, Skelly PW, Rajendiran TM, Kayampilly P, Thomas TP, et al. Asymmetrical flow field-flow fractionation for improved characterization of human plasma lipoproteins. Anal Bioanal Chem. 2019;411(3):777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuklenyik Z, Jones JI, Gardner MS, Schieltz DM, Parks BA, Toth CA, et al. Core lipid, surface lipid and apolipoprotein composition analysis of lipoprotein particles as a function of particle size in one workflow integrating asymmetric flow field-flow fractionation and liquid chromatography-tandem mass spectrometry. PLoS One. 2018;13(4):e0194797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Axmann M, Karner A, Meier SM, Stangl H, Plochberger B. Enrichment of native lipoprotein particles with microRNA and subsequent determination of their absolute/relative microRNA content and their cellular transfer rate. J Vis Exp. 2019;(147). [DOI] [PubMed] [Google Scholar]
- 57.Michell DL, Zhao S, Allen RM, Sheng Q, Vickers KC. Pervasive small RNAs in cardiometabolic research: great potential accompanied by biological and technical barriers. Diabetes. 2020;69(5): 813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vickers KC, Sethupathy P, Baran-Gale J, Remaley AT. The complexity of microRNA function and the role of IsomiRs in lipid homeostasis. J Lipid Res. 2013;54(5):1182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koppers-Lalic D, Hackenberg M, Bijnsdorp IV, van Eijndhoven MAJ, Sadek P, Sie D, et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8(6):1649–58. [DOI] [PubMed] [Google Scholar]


