Abstract
The orphan nuclear receptors estrogen-related receptors (ERRs) bind to the estrogen-related receptor response element (ERRE) to regulate transcriptional programs in cellular metabolism and cancer cell growth. In this study, we evaluated the potential for a pyrrole-imidazole polyamide to block ERRα binding to ERREs to inhibit gene expression. We demonstrated that the ERRE-targeted polyamide 1 blocked the binding of ERRα to the consensus ERRE and reduced the transcriptional activity of ERRα in cell culture. We further showed that inhibiting ERRα transcriptional activity with polyamide 1 led to reduced mitochondrial oxygen consumption, a primary biological effect regulated by ERRα. Finally, our data demonstrated that polyamide 1 is an inhibitor for cancer cell growth.
Keywords: ERRα, Pyrrole-imidazole polyamide, Mitochondria
Introduction
The estrogen-related receptors (ERR), also known as nuclear receptor 3B (NR3B), are orphan nuclear receptors that play crucial roles in metabolic homeostasis. The orphan nuclear receptor subfamily comprises three members referred to as NR3B1 (ERRα), NR3B2 (ERRβ), and NR3B3 (ERRγ). The ERRs display constitutive transcriptional activity independent of natural estrogen ligands and do not directly take part in classic estrogen signaling pathways (Zhang et al. 2015). ERRα is abundantly expressed in organs with high oxidative activity and is recognized as a key regulator of adaptive energy metabolism in response to environmental stimuli and energy demands (Villena and Kralli 2008). ERRβ has a vital role in cell fate determination and pluripotency by interacting with OCT4-SOX2 complex and NANOG (van den Berg et al. 2008). ERRγ works to switch bioenergetic responses to hypertrophic stress primarily in the heart (Kwon et al. 2013). Both ERRα and ERRγ orchestrate mitochondrial functions with the coactivator peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α), either by directly activating genes encoding proteins needed to maintain the mitochondrial components, or by indirectly activating major transcription factors governing mitochondrial biogenesis (Kamei et al. 2003; Gleyzer et al. 2005; Gaillard et al. 2007; Takacs et al. 2013). In addition, ERRα directly regulates the transcription of genes encoding proteins involved in the citric acid cycle, mitochondrial oxidative phosphorylation (OXPHOS) and respiratory chain, such as the citrate synthase, succinate dehydrogenase, cytochrome c (CytC), and NADH dehydrogenases (Giguere 2008).
ERR contain two highly conserved zinc finger motifs that preferentially bind to the consensus estrogen-related response element (ERRE) sequence 5′-AAGGTCA-3′ (Fig. 1) (Sladek et al. 1997). This signature motif is also found within the regulatory regions of genes encoding for carnitine/acylcarnitine translocase (SLC25A29), medium chain acyl-CoA dehydrogenase and fatty acid binding protein 3 (Dufour et al. 2007). All three genes are involved in fatty acid β-oxidation (Shekhawat et al. 2005; Gutierrez-Aguilar and Baines 2013) and their expression is reduced when ERRα is absent in adipocytes, intestinal epithelia and hepatocytes (Sladek et al. 1997; Carrier et al. 2004; Alaynick et al. 2007).
Fig. 1.
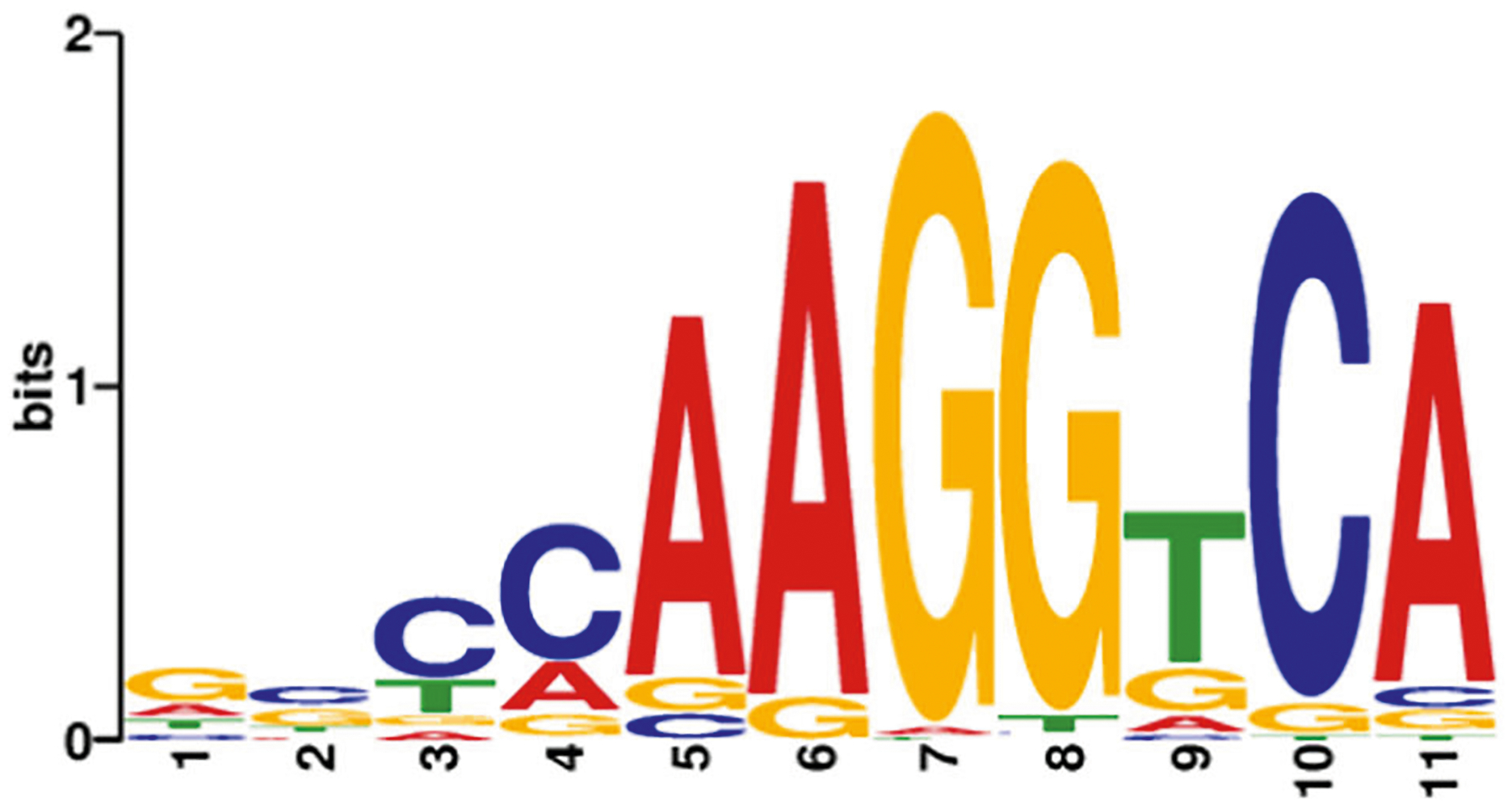
DNA-binding motif sequence for nuclear receptor ERRα. Sequence logos of consensus DNA-binding sites, designated as estrogen-related receptor response element (ERRE). The consensus sequence of ERRα is variable with a number of possible bases at certain positions in the motif, whereas other positions have a fixed base. The height of the letter represents how frequently that nucleotide is observed in that position. AAGGTCA is the most commonly occurring ERRE, for ERRα. The logos were generated from information obtained from the MEME database (meme-suite.org)
Genetic studies in mice also suggest a role for ERRs in lipid metabolism. Mice lacking ERRα were reported to be resistant to high fat diet-induced obesity (Luo et al. 2003). Published ChIP-on-chip data (Charest-Marcotte et al. 2010) show that ERRα bound to the regulatory domain of lipogenic genes and positively regulated their expression. In agreement with its putative function in lipogenesis, ERRα along with its coactivator PGC1-α were found to be essential for adipogenic differentiation induced by glucocorticoid, cAMP and insulin (Ijichi et al. 2007). Furthermore, ERRα was positively regulated by insulin-mediated PI3K/AKT signaling in hepatocytes (Li et al. 2013). Taken together, these results support a role of ERRα in promoting lipogenesis.
Given the diverse role of ERRs in the regulation of mitochondria and lipid metabolism, it is proposed that ERRs may play a role in tumor metabolism (Conzen 2008; Bernatchez et al. 2013; Fradet et al. 2016; Ye et al. 2019). Transformed cells are known to reprogram their energy homeostasis and metabolism with various strategies to meet energetic and anabolic demands (Zheng 2012; Cheng et al. 2018). Recent studies have highlighted the role of dysfunctional lipid metabolism in promoting tumor cell transformation and growth (Liu et al. 2018; Long et al. 2018; Nakagawa et al. 2018). In order to understand the function of ERRα in metabolic regulation and explore the potential therapeutic benefit of targeting ERRα, we designed a pyrrole–imidazole (Py-Im) polyamide inhibitor to disrupt ERRα-DNA binding. Py-Im polyamides are a class of synthetic DNA minor groove-binding ligands with programmable sequence selectivity, and have been shown to modulate gene expression by inhibiting transcription factor-DNA binding (Dervan and Edelson 2003; Olenyuk et al. 2004; Nickols and Dervan 2007; Nickols et al. 2013; Taniguchi et al. 2017; Wei et al. 2018). Here, we report the design and validation of an ERRE-targeting Py-Im polyamide that inhibits ERRα-mediated cellular functions.
Materials and methods
Cell culture
Mouse hepatocytes were isolated from mouse livers as previously described (Zeng et al. 2011). Mouse and human hepatocytes (SNU398 and Huh7 cells purchased from ATCC) were cultured in Dulbecco’s Modified Eagle’s Medium (Mediatech) supplemented with 10% FBS (Atlas Biologicals), 5 μg/ml insulin (Sigma-Aldrich), and 10 ng/ml epidermal growth factor (Invitrogen). The C4–2b human prostate cancer cell line was cultured in RPMI-1640 medium (Mediatech) supplemented with 10% FBS. All cell culture was supplemented with 1% of penicillin–streptomycin and incubated at 37 °C with 85% relative humidity/5% CO2.
Py-Im polyamide synthesis
Py-Im polyamides 1 and 2 were provided by the Dervan Group (California Institute of Technology) and were synthesized on Oxime resin as described (Puckett et al. 2012).
Electromobility shift assay (EMSA)
Oligonucleotide containing the 5′-NNNNAAGGTCAN NNN-3′ consensus sequence corresponding to the ERRE binding site was synthesized by and purchased from Integrated DNA technologies. NE-PER Nuclear and Cytoplasmic Extraction Reagents, Biotin 3′ End DNA Labeling and LightShift Chemiluminescent EMSA kits were purchased from Thermo Fisher Scientific. In brief, two complementary oligonucleotides were labeled separately with biotin according to manufacturer’s instruction and allowed to reanneal. For binding and competition reactions, 20 fmole biotin-labeled ERRE dsDNA was incubated with 10 μg nuclear protein extract from ERRα overexpressing mouse hepatocytes with or without unlabeled ERRE dsDNA, ERRα antibody (ab76228, Abcam), and indicated concentrations of two different polyamides (final concentration: 1 nM of biotin-labeled ERRE dsDNA, 0.5 μg/μl of protein extract and 10 pM to 1 μM polyamides). The mixture was then separated on polyacrylamide gel following incubation and DNA transferred into membrane. Biotin-labeled DNA-protein complexes were detected using X-ray film following the instructions of the EMSA kit.
Luciferase reporter assay
For the luciferase reporter plasmid, the −686 to +55 human genomic sequences relative to the transcription initiation site of CytC, containing ERRE (Schreiber et al. 2004), was amplified and cloned upstream of the luciferase coding sequences of pGL4 (Promega). Subsequently, the pGL4-CytC (−686/+55) reporter plasmid was introduced to mouse hepatocytes using Lipofectamine 2000 (Invitrogen) and selected with puromycin. Prior to polyamide treatment, the mouse hepatocytes stably expressing pGL4-CytC luciferase were seeded in six-well plates (1 × 105 cells per well) and allowed to attach for 24 h. These cells were then treated with the indicated concentration of polyamides for another 24 h. All experiments were repeated at least three times. Cell lysate preparation and luminescence detection was performed according to manufacturer’s instructions (Dual-Luciferase Reporter Assay System, Promega).
RNA interference, RNA isolation and quantitative PCR (qPCR)
siRNAs for ERRα were purchased from Santa Cruz Bio-technology (sc-44707, siERRα−1) and OriGene (SR414015, siERRα−2). Control siRNA was from Santa Cruz Bio-technology (sc-37007). Plasmids and siRNA were delivered using Lipofectamine 2000 according to the manufacturer’s instruction. Overall, 4 μg of DNA or 100 pmol siRNA and 10 μl of Lipofectamine 2000 were added to cells growing at 70–90% confluence in six-well plates in triplicate and incubated for 24 h. Total RNA was isolated using TRIzol reagent (Invitrogen) following manufacturer’s instruction. cDNA was produced by reverse transcription from RNA samples using the Reverse Transcription System (Promega). Quantitative PCR was performed using SYBR green qPCR mix (Thermo) and 7900 HT fast real-time PCR system (Applied Biosystems). Gene-specific primers used for qPCR: mouse CytC forward 5′-CCAGTGCCACACCGTTGAA-3′ and reverse 5′-TCCCCAGATGATGCCTTTGTT-3′. Mouse GAPDH: forward 5′-GCACAGTCAAGGCCGAGAAT-3′ and reverse 5′-GCCTTCTCCATGGTGGTGAA-3′. Human CytC forward 5′-TCAGGCCCCTGGATACTCTT-3′ and reverse 5′-GCTATTAAGTCTGCCCTTTCTTCC-3′. Human GAPDH 5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse 5′-GAAGATGGTGATGGGA TTTC-3′.
Western blot
Cell lysates were prepared in lysis buffer (50 mM Tris-HCl pH 7.4, 1 mM EDTA, 150 mM NaCl, 1% NP40, 5 mM NaF, 0.25% sodium deoxycholate and 2 mM NaVO3) supplemented with phosphatase inhibitors and protease inhibitors (Roche). Protein electrophoresis and Western blotting were performed using Mini Trans-Bolt Cell system (Bio-Rad). Blots were probed with anti-ERRα (ab76228, Abcam) and anti-β-actin (a2228, Sigma-Aldrich) antibodies. The protein blot membranes were visualized with ECL Western Blotting Substrate (Thermo Fisher Scientific) and images were taken with X-ray film or ChemiDoc Imaging System (Bio-Rad).
Seahorse oxygen consumption rate (OCR) assay
Cells treated with polyamides or transfected with siERRα were harvested and replated (2 × 104 and 4 × 104 cells per well for mouse hepatocytes and human hepatocytes Huh7, respectively) on XF24 cell assay plates (Agilent technologies). After being allowed to attach for 16 h, cells were washed twice with Mito Assay Medium and preincubated in a non-CO2 incubator for 1 h before the assay. During the Seahorse OCR assay, four baseline respiration rates were recorded for each condition followed by sequential injections of the four mitochondrial inhibitors, oligomycin (1 μM), FCCP (1 μM), and rotenone/antimycin A (1 μM) to measure OCR.
Cell proliferation assay
Cells were seeded (1 × 104 cells per dish) onto 60 mm dishes with complete medium supplemented with polyamides. Medium and polyamides were replaced daily. To measure cell proliferation rate, the cells were completely trypsinized and counted using a hemocytometer for five consecutive days. Each experiment was repeated at least three times.
Statistical analysis
Data in this study are presented as mean ± standard error of the mean. For multiple group comparisons, multivariance ANOVA was used to determine if there were intergroup differences. This was followed by the post hoc Tukey’s test. For two-group comparison, data were analyzed by the two-tailed Student’s t test. p values < 0.05 and p < 0.01 were considered statistically significant and denoted by * and **, respectively.
Results and discussion
Inhibition of ERRα DNA-binding activity by a Py-Im polyamide
To inhibit transcription activation by ERRα, we sought to inhibit the binding of ERRα to ERREs. We used a Py-Im polyamide that was previously shown to inhibit the binding of the estrogen receptor to the estrogen-response element, which comprises two half-sites that bear resemblance to the ERRE sequence of 5′-AAGGTCA-3′ (Fig. 1) (Nickols et al. 2013). In addition to Py-Im polyamide 1, which targets the ERRE, we also included Py-Im polyamide 2, which targets the sequence 5′-WGWWCW-3′, as a mismatch control (Fig. 2).
Fig. 2.
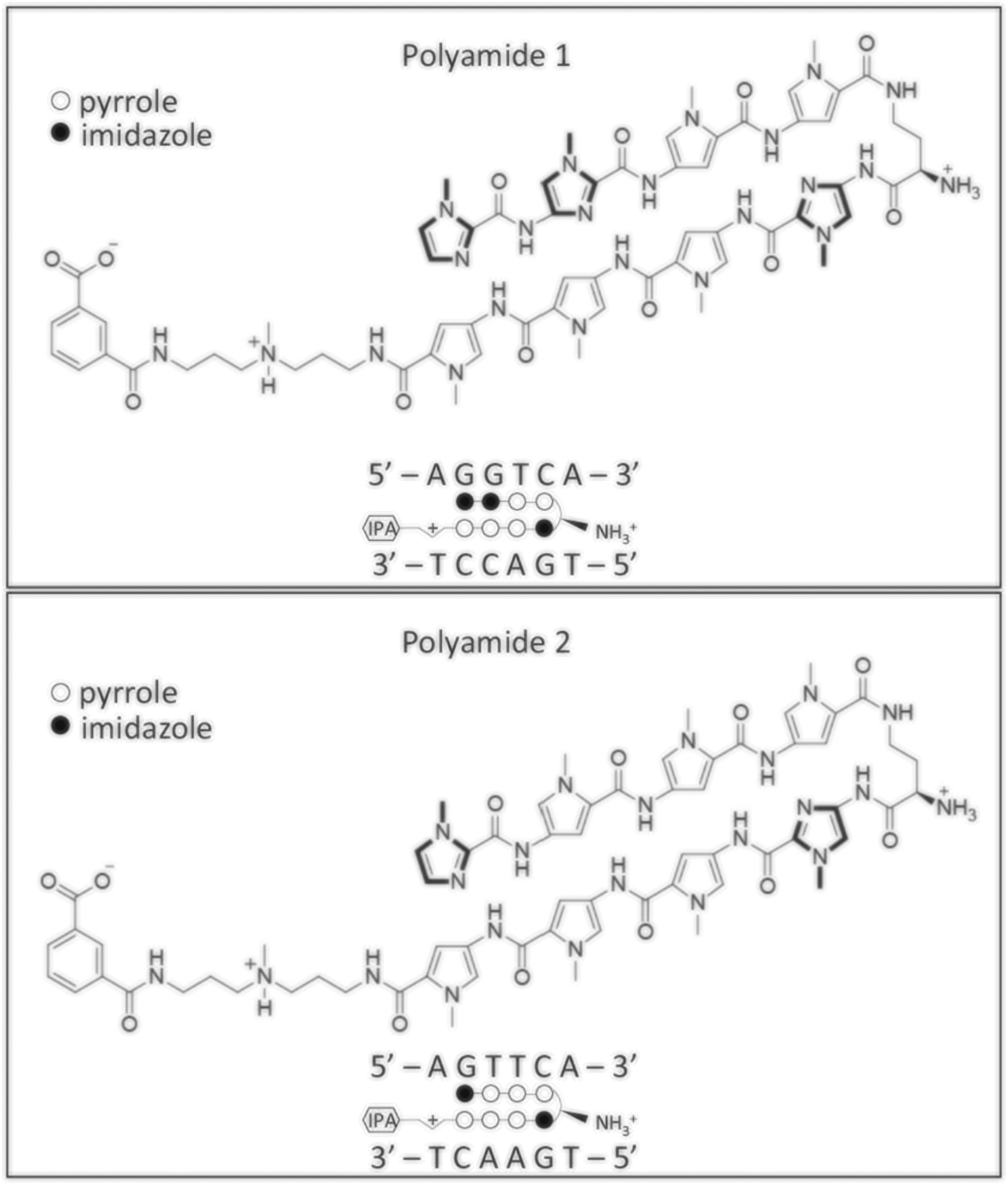
Structure of the two polyamides that target the ERRE site. Polyamide 1 and 2 are consisted of two polyamide strands covalently linked by a γ-aminobutyric acid (γ-turn) linker. Within this structure design, the Py/Im pair target C/G; Im/Py pair target G/C; Py/Py target A/T or T/A. The γ-turn linker has demonstrated selectivity for A, T over G, C. For each panel: top, chemical structure of the polyamides; bottom, the ball-and-stick models with the target sequence of polyamides shown together. Solid circle, imidazole; Open circle, pyrrole. IPA isophthalic acid
To evaluate the ability of the two Py-Im polyamides to inhibit ERRα binding to DNA oligos containing the consensus ERRE, we employed the EMSA assay (Fig. 3). In this assay, the biotin-labeled DNA probe containing ERRE (ERRE dsDNA) was incubated alone (lane 1) or with nuclear extract from cultured hepatocytes that over-expressed ERRα protein (lane 2). As expected, a band migrating at higher molecular weight than the probe alone (lane 1) was observed when nuclear extract was incubated with the free probe (lane 2), indicating the complex of ERRE dsDNA with ERRα (ERRα + ERRE). When unlabeled competitor (cold probe) was added in lane 3, the cold probe displaced the biotin-labeled probe and diminished the observed ERRα + ERRE complex band, suggesting that the upper band observed in lane 2 was indeed complexed with the ERRE dsDNA probe. When the monoclonal antibody for ERRα was added (lane 4), the antibody bound to the complex of ERRα + ERRE and caused further upwards shift of the complex due to increased overall molecular weight, suggesting that the complex indeed contained the ERRα protein. For lanes 5 to 10, increasing dose of polyamide 1 (10 pM to 1 μM in log scale) was added to the lysate and probe mixture. The data showed that incubation with polyamide 1 at 0.1 μM concentration and beyond completely blocked the binding of ERRα to ERRE dsDNA as demonstrated by the diminishing band of ERRα + ERRE in lanes 9 and 10. In contrast, a tenfold higher dose of polyamide 2 (1 μM) was needed before any loss of complex formation was observed (lane 16). This result suggested that polyamide 1 was able to disrupt the ERRα–ERRE complex and establishes a dose range for subsequent evaluations.
Fig. 3.
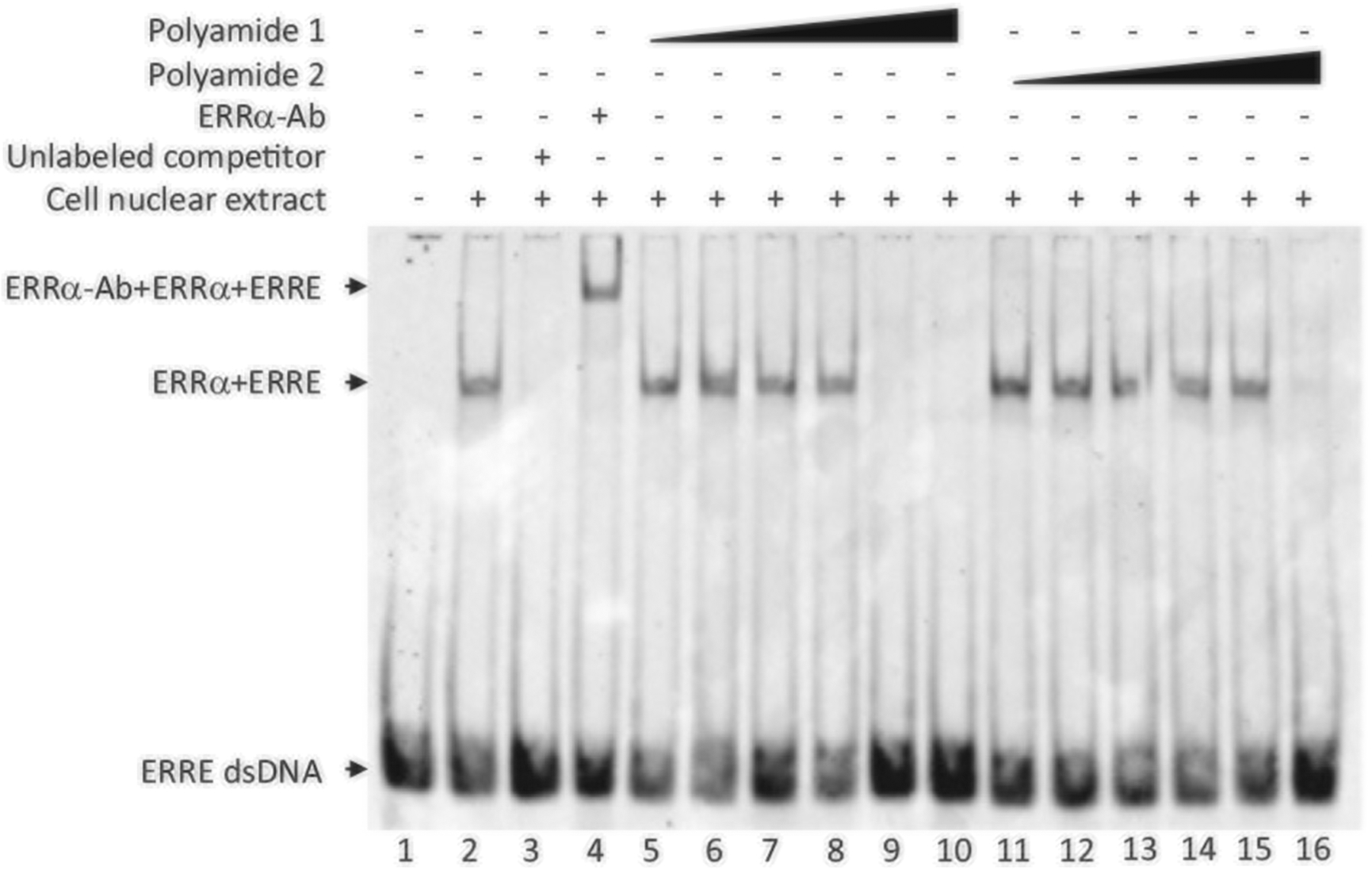
Polyamide 1 reduces ERRα binding to the ERRE motif. Electromobility shift assay performed with the polyamide 1 and polyamide2. From left, lane 1, biotin-labeled ERRE dsDNA only; lane 2, labeled ERRE dsDNA (10 nM) + nuclear extract containing overexpressed ERRα; lane 3, unlabeled competitor added; lane 4, antibody for ERRα (ERRα-Ab) added; lanes 5–10, increasing dose (10 pM to 1 μM in log scale) of polyamide 1; lanes 11–15, increasing dose (10 pM to 1 μM in log scale) of polyamide 2. Arrows point to the complex of ERRα + ERRE or ERRα + ERRE + antibody used for ERRα detection
Polyamide 1 inhibits ERRα transcriptional activity
To evaluate the ability of polyamide 1 to inhibit ERRα-mediated transcriptional activity, we utilized a dual-luciferase reporter assay where the transcription of firefly luciferase was controlled by ERRα. CytC is a component in mitochondrial OXPHOS, and the CytC promoter contains an ERRE under the transcriptional regulation of ERRα (Schreiber et al. 2004). A pGL4-CytC construct containing a promoter of CytC from codon −686 to +55 was introduced to and stably expressed in mouse hepatocytes (Li et al. 2013). In the CytC-driven luciferase expressing hepatocytes, treatment with 1 μM polyamide 1 reduced the ERRα-dependent luciferase expression by 55%. Comparatively, cells treated with 0.1 and 1 μM polyamide 2 showed no significant reductions (Fig. 4).
Fig. 4.
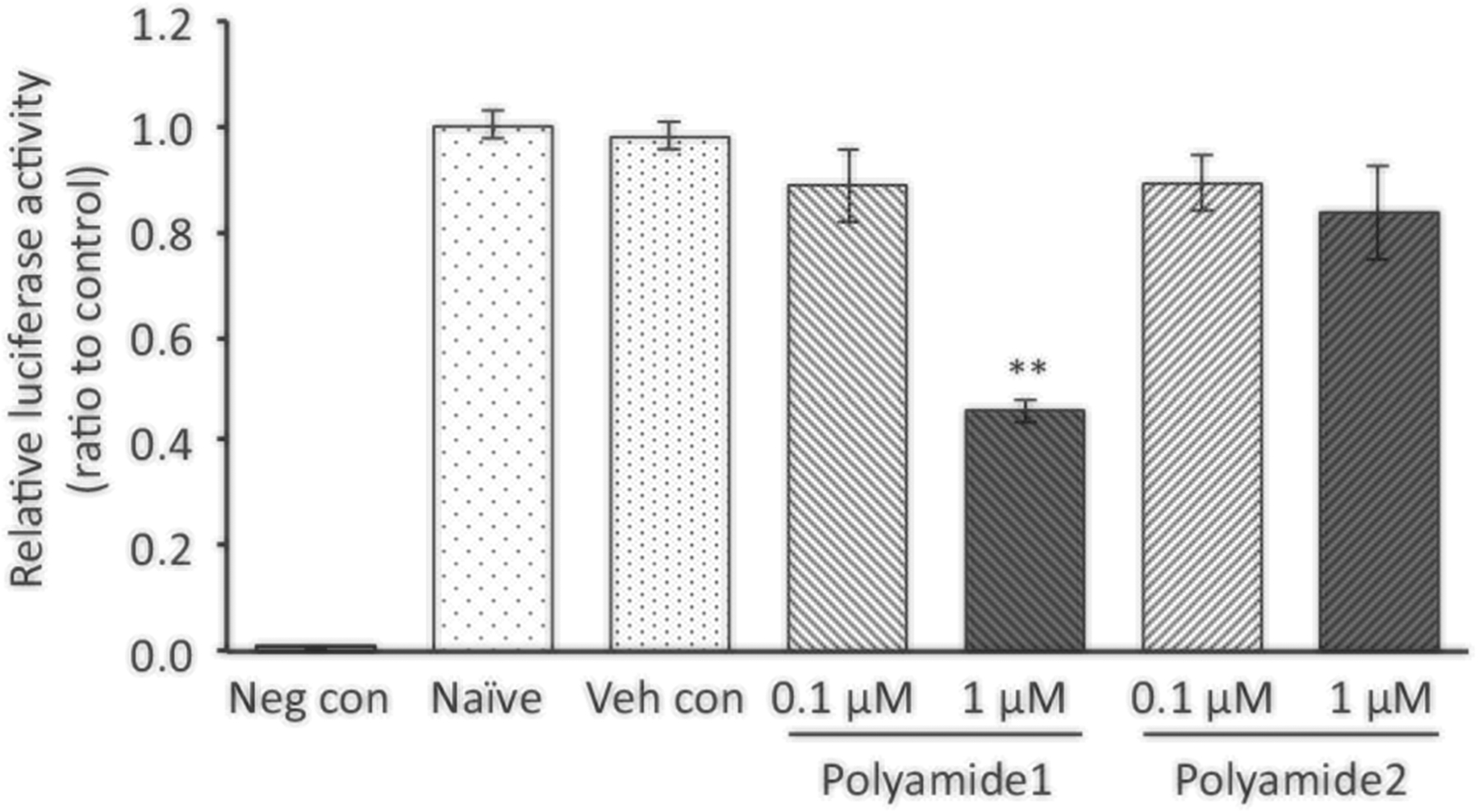
Polyamide 1 suppresses the ERRα-dependent luciferase expression. Mouse hepatocytes stably expression cytochrome c-driven luciferase were treated with polyamide 1 or 2 for 24 h at indicated dose. The cells were then lysed and used to determine the luciferase activity using a luminometer. Polyamide 1 treatment at 1 μM reduced the luciferase activity whereas polyamide 2 did not. Neg con, the mouse hepatocyte without luciferase expression vector; Naive, untreated cells expressing the luciferase reporter; Veh con, cells expressing the luciferase reporter treated with vehicle. n = 3. ** statistically significant at p < 0.01 when compared to vehicle control group
Next, we tested the endogenous expression of CytC in response to polyamide 1 treatment in human and mouse hepatocytes. In human hepatocellular carcinoma SNU398 cells and mouse hepatocytes (Li et al. 2013) where high ERRα expression was found, we showed that polyamide 1 significantly reduced the expression of CytC (Fig. 5a). This observation was consistent with the effects of ERRα knockdown using the siRNA approach (Fig. 5b). Taken together, these results demonstrate the ability of polyamide 1 to modulate ERRα-mediated expression of CytC.
Fig. 5.
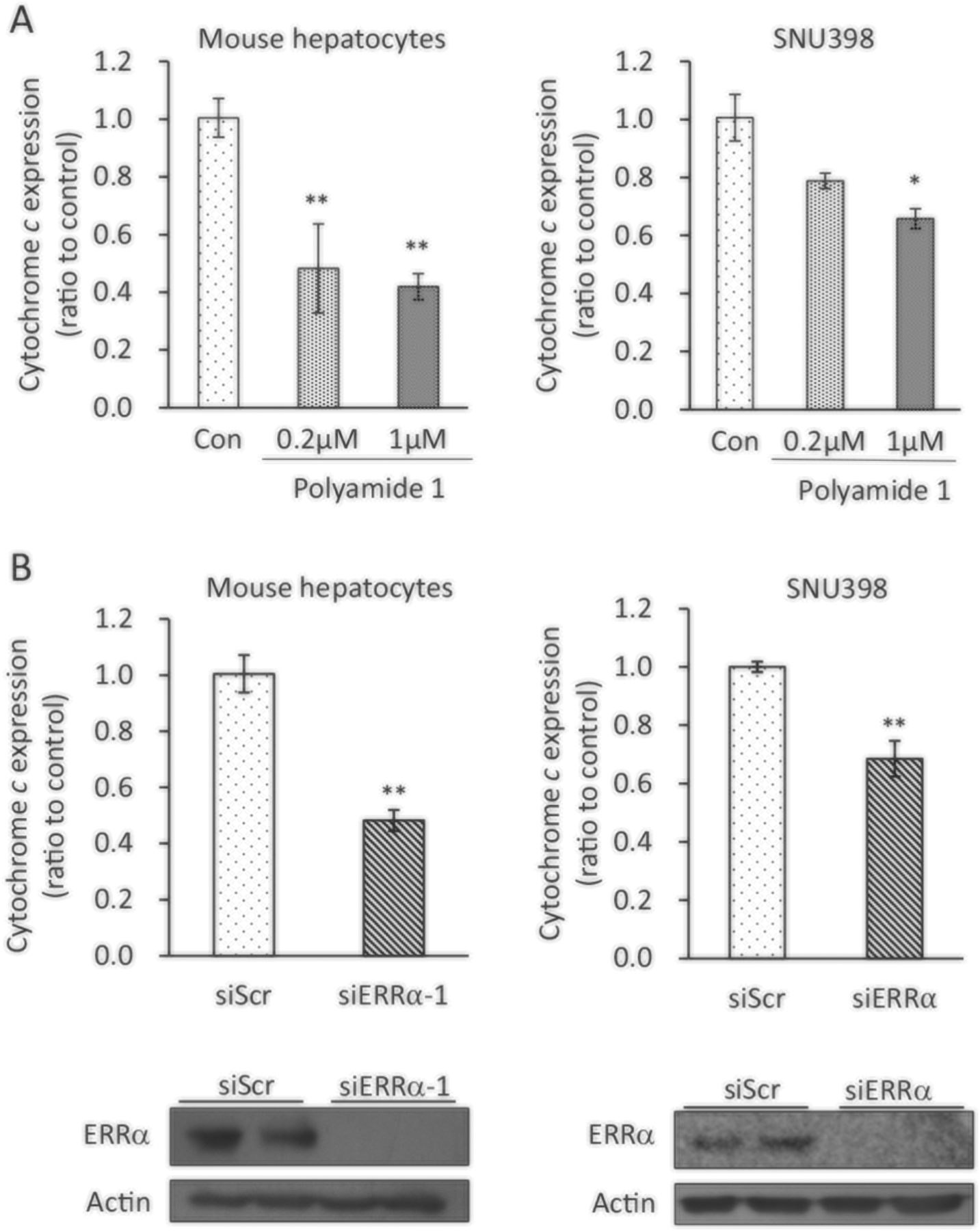
Polyamide 1 suppresses the ERRα downstream gene expression. a Polyamide 1 treatment reduced the ERRα downstream target cytochrome c (CytC) gene expression. Mouse and human (SNU398) hepatocytes were treated with polyamide 1 for 24 h at 0.2 and 1 μM. Cells were then lysed and mRNA prepared for qPCR analysis of CytC expression. b siERRα introduction reduced expression of CytC. siR NAs designed for ERRα was introduced to mouse and human hepatocytes. After 24 h, cells were lysed, mRNA was prepared and used for qPCR analysis to measure relative CytC expression. Top, mRNA expression of CytC; bottom, immunoblotting analysis shows protein expression of ERRα was inhibited with the introduction of siERRα. n = 3; *p < 0.05; **p < 0.01 difference between experimental and control groups. SNU398, a human hepatocyte cells line. Actin was detected as loading control for immunoblotting analysis
ERRα-targeting reduces OCR
In mammalian cells, ATP is generated through mitochondrial OXPHOS. ERRα were initially characterized as a transcriptional activator that promoted mitochondria biogenesis and respiration in collaboration with PGC1-α (Luo et al. 2017). Thus, inhibiting ERRα function in cells is expected to decrease mitochondrial respiration, hence oxygen consumption of the cells. We used the Seahorse XF24 analyzer to measure OCR in cultured cells in order to assess polyamide 1 as an ERRα inhibitor in real time. Mitochondrial respiration was monitored as basal, ATP production-linked, maximal, and proton leak-linked OCR with the use of four mitochondrial inhibitors. Oligomycin blocked ATP synthase, thus allowing measurement of ATP production-linked OCR (Plitzko et al. 2017). The mitochondrial OXPHOS uncoupler, FCCP, permeabilized the inner mitochondrial membrane for protons and allowed maximum electron flux through the electron transport chain (Dranka et al. 2011). Thus, the OCR measured after FCCP treatment represented the maximal mitochondrial respiratory capacity of the cells. Antimycin A and rotenone together inhibited complexes I and III, respectively (Chen et al. 2003). Any OCR measured after treatment with rotenone and antimycin A are nonmitochondrial based oxygen consumption.
To assess the effect of polyamide 1 on ERRα-regulated mitochondrial respiration, cells were pretreated with polyamide 1 or siRNA against ERRα for 24 h followed by 16 h incubation before the OCR assay run (Fig. 6a). In mouse hepatocytes, the introduction of siERRα1 and 2 that target different sequences of ERRα gene led to 70 and 60% reduction of basal and maximal respiration, respectively (Fig. 6b). In the dose-dependency experiment, polyamide 1 treatment at 0.2 μM led to 52 and 54% reduction in basal and maximal respirations, respectively; treatment of poly-amide 1 at 1 μM led to 70 and 72% reduction in basal and maximal respiration, respectively (Fig. 6c). This effect of polyamide 1 on the mitochondrial respiration was comparable with that induced by siRNA treatment targeting ERRα. On the other hand, the same concentration of the mismatched polyamide control (polyamide 2, 1 μM) only caused 9 and 10% reduction on the basal and maximal respiration, respectively (Fig. 7a). Polyamide 2 at 1 μM also has little impact on the protein levels of ERRα (Fig. 7b) or endogenous expression of CytC expression (Fig. 7c). Furthermore, the effect of polyamide 1 is more potent than that of XCT-790, an inverse agonist for ERRα (Willy et al. 2004; Eskiocak et al. 2014), on reducing the mitochondrial OCR.
Fig. 6.
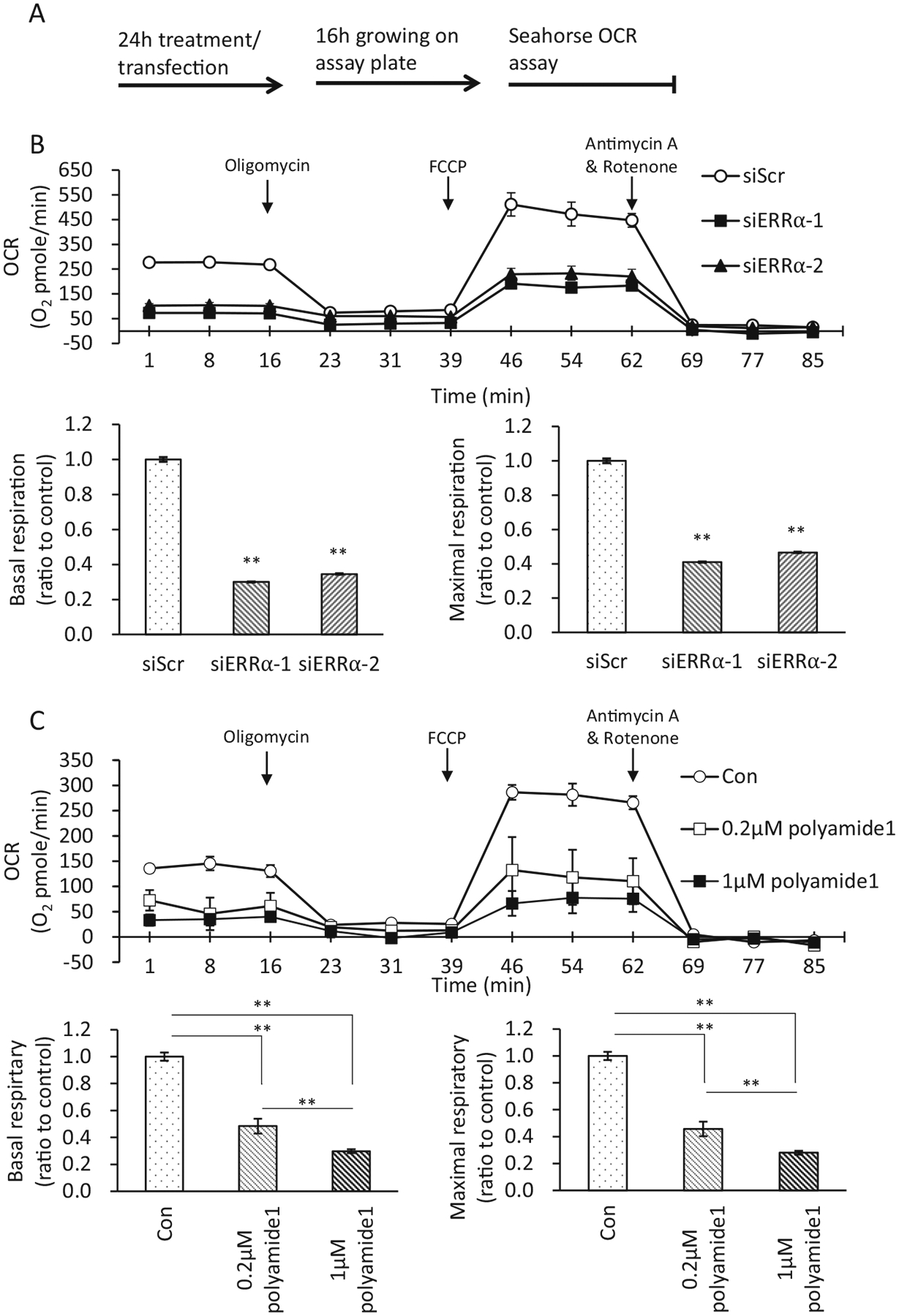
Polyamide 1 dose-dependently suppresses the ERRα-regulated mitochondrial respiration similar to siERRα. a Treatment protocol. Polyamide or siRNA was introduced to mouse hepatocytes and human hepatocytes Huh7 to inhibit ERRα activity. After 24 h of the treatment, cells were harvested and plated into Seahorse oxygen consumption rate (OCR) assay plates and allowed for growth for 16 h. The cellular OCR date was then recorded in real-time for a duration of ~2 h. b siERRα robustly inhibited OCR in mouse hepatocytes. Two independent siRNAs for ERRα were used to inhibit the expression of ERRα. Both siERRα−1 and siERRα−2 led to decreased OCR in mouse hepatocytes. siScr, scrambled siRNA as control. c Mouse hepatocytes were treated with indicated dose of polyamide 1 for 24 h before assaying for OCR. Polyamide 1 treatment dose-dependently inhibited OCR in mouse hepatocytes. Con, vehicle-treated cells. b, c Top, real-time plots for OCR levels, each data point was replication of four readings for each sample with three samples each group for each experiment; bottom left, the basal respiration was calculated by averaging the baseline OCR minus the nonmitochondrial OCR; bottom right, the maximal respiratory capacity was calculated by averaging the maximal OCR minus the nonmitochondrial OCR. Oligomycin, an ATPase inhibitor; FCCP, a mitochondrial uncoupler; antimycin A and rotenone, inhibitors of complexes I and III respectively of the electron transport chain. Arrows indicate time points when these chemicals were added. n = 3. **p < 0.01 difference between experimental and control groups
Fig. 7.
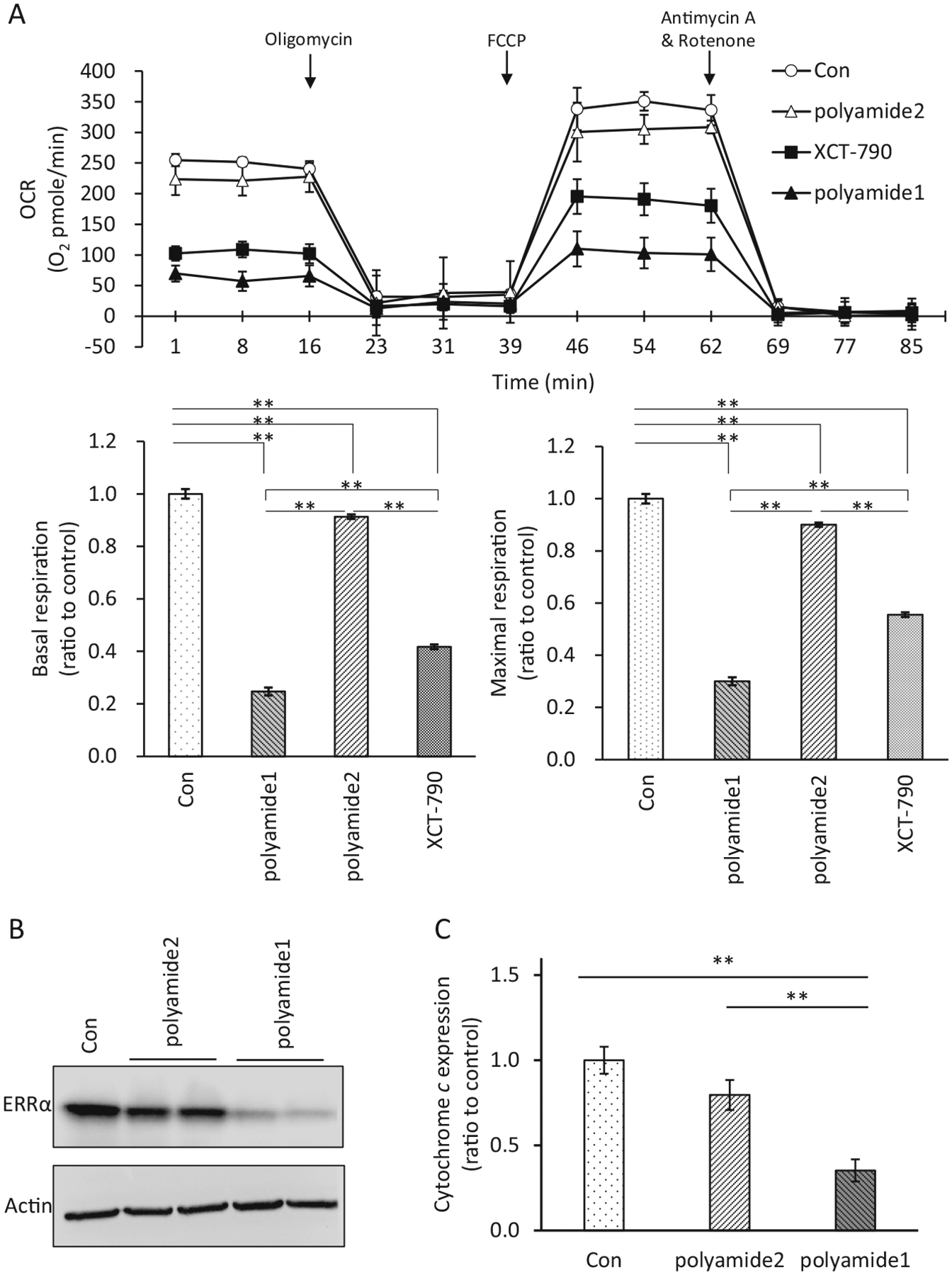
Specificity for Polyamide 1 at reducing mitochondrial respiration. a The effect of polyamide 1 (1 μM) was compared with that of the mismatch polyamide 2 (1 μM) and an ERRα inverse agonist, XCT-790 (2 μM). Con, vehicle-treated cells. Arrows indicated time points when these chemicals were added. n = 3. Top, real-time plots for OCR levels, each data point was replication of four readings for each sample with three samples each group for each experiment. Bottom left, the basal respiration was calculated by averaging the baseline OCR minus the nonmitochondrial OCR; bottom right, the maximal respiratory capacity was calculated by averaging the maximal OCR minus the nonmitochondrial OCR. b Comparison of ERRα levels in Huh7 cells treated with polyamide 1 vs. polyamide 2 and controls. **p < 0.01 difference between experimental and control groups. c Comparison of cytochrome c (CytC) gene expression in Huh7 cells treated with polyamide 1 vs. polyamide 2 and controls
Polyamide 1 inhibits cell proliferation
Recent clinical oncology studies show that elevated ERRα expression is significantly associated with unfavorable clinical outcome and increases the risk of recurrence in human cancers including prostate and liver cancer, and ERRα may serve as a potent predictive biomarker of cancer therapy (Fujimura et al. 2007; Xia et al. 2019). We determined cell growth rate in mouse and two cancer cell lines by counting cell numbers for five consecutive days with sustained polyamide treatment (Fig. 8). In both mouse and human hepatocytes, polyamide 1 treatment led to a dose dependent reduction in cell number as compared to vehicle and polyamide 2 control (Fig. 8 top and middle). The observed inhibition was sustained throughout the entire duration of the experiment. In addition to hepatocytes, incubation with polyamide 1 in the high ERRα expression human prostate cancer lines C4–2b (Cheung et al. 2005) resulted in similar growth repression after 5 days of treatment (Fig. 8 bottom). These findings were consistent with the observation that XCT-790 treatment in HepG2 hepato-carcinoma cells and its multidrug resistant subline led to apoptosis (Wu et al. 2009).
Fig. 8.
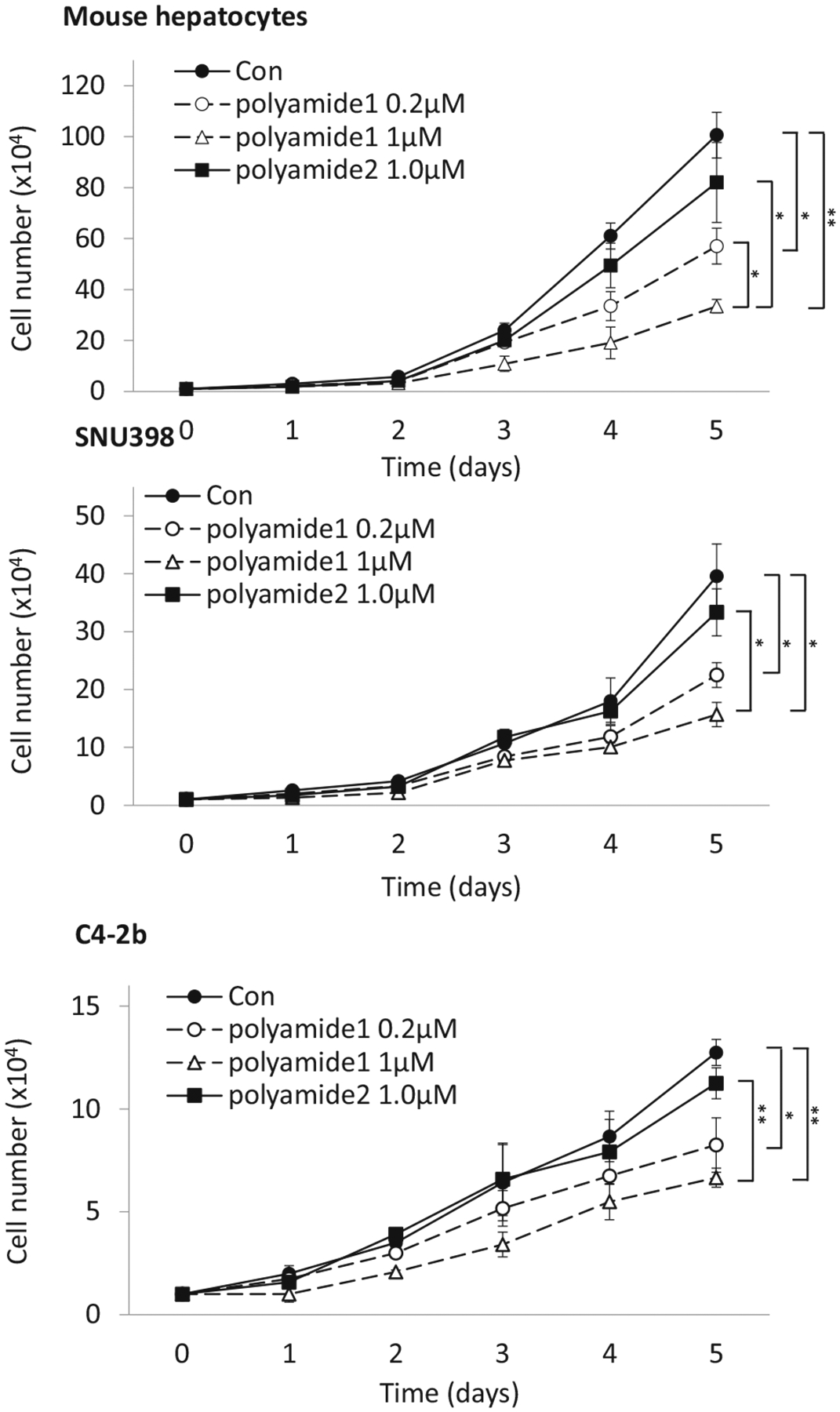
ERRα inhibition by polyamide 1 attenuates cancer cell growth. Mouse hepatocytes, human hepatocellular carcinoma (SNU398) and human prostate cancer cells (C4–2b) were seeded in cultured dishes and treated with the indicated concentrations of polyamide 1 and 2. Cell numbers were counted daily for five consecutive days. Fresh medium containing polyamide was replenished daily. n = 3. *p < 0.05; **p < 0.01 difference between polyamide and control groups
Conclusions
ERRα is a pivotal transcriptional factor that coordinates mitochondrial and lipid homeostasis by mediating the expression of genes vital for maintaining mitochondrial function and lipid metabolism (Vega and Kelly 1997; Eichner and Giguere 2011). Here, we evaluated a Py-Im polyamide as a potential inhibitor of ERRα. Our data indicated that polyamide 1 blocked the ERRα binding to the consensus ERRE sequence that is necessary for transcriptional activation (Sladek et al. 1997). We demonstrated that polyamide 1 treatment is comparable with siRNA knockdown at downregulating ERRα-mediated transcription, and at inhibiting the primary biological effects governed by ERRα, i.e., mitochondrial function. Finally, we showed that polyamide 1 inhibits cell growth/survival. Whether this effect was dependent on its function on mitochondria and lipid metabolism remains to be further explored.
Acknowledgements
BLS was supported by R01CA154986. This work is partially supported by a technology development grant from the University of Southern California.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguere V, Evans RM (2007) ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab 6:13–24 [DOI] [PubMed] [Google Scholar]
- Bernatchez G, Giroux V, Lassalle T, Carpentier AC, Rivard N, Carrier JC (2013) ERRalpha metabolic nuclear receptor controls growth of colon cancer cells. Carcinogenesis 34:2253–2261 [DOI] [PubMed] [Google Scholar]
- Carrier JC, Deblois G, Champigny C, Levy E, Giguere V (2004) Estrogen-related receptor alpha (ERRalpha) is a transcriptional regulator of apolipoprotein A-IV and controls lipid handling in the intestine. J Biol Chem 279:52052–52058 [DOI] [PubMed] [Google Scholar]
- Charest-Marcotte A, Dufour CR, Wilson BJ, Tremblay AM, Eichner LJ, Arlow DH, Mootha VK, Giguere V (2010) The homeobox protein Prox1 is a negative modulator of ERR{alpha}/PGC-1 {alpha} bioenergetic functions. Genes Dev 24:537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ (2003) Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278:36027–36031 [DOI] [PubMed] [Google Scholar]
- Cheng C, Geng F, Cheng X, Guo D (2018) Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun 38:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CP, Yu S, Wong KB, Chan LW, Lai FM, Wang X, Suetsugi M, Chen S, Chan FL (2005) Expression and functional study of estrogen receptor-related receptors in human prostatic cells and tissues. J Clin Endocrinol Metab 90:1830–1844 [DOI] [PubMed] [Google Scholar]
- Conzen SD (2008) Minireview: nuclear receptors and breast cancer. Mol Endocrinol 22:2215–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervan PB, Edelson BS (2003) Recognition of the DNA minor groove by pyrrole-imidazole polyamides. Curr Opin Struct Biol 13:284–299 [DOI] [PubMed] [Google Scholar]
- Dranka BP, Benavides GA, Diers AR, Giordano S, Zelickson BR, Reily C, Zou L, Chatham JC, Hill BG, Zhang J, Landar A, Darley-Usmar VM (2011) Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic Biol Med 51:1621–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguere V (2007) Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab 5:345–356 [DOI] [PubMed] [Google Scholar]
- Eichner LJ, Giguere V (2011) Estrogen related receptors (ERRs): a new dawn in transcriptional control of mitochondrial gene networks. Mitochondrion 11:544–552 [DOI] [PubMed] [Google Scholar]
- Eskiocak B, Ali A, White MA (2014) The estrogen-related receptor alpha inverse agonist XCT 790 is a nanomolar mitochondrial uncoupler. Biochemistry 53:4839–4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradet A, Bouchet M, Delliaux C, Gervais M, Kan C, Benetollo C, Pantano F, Vargas G, Bouazza L, Croset M, Bala Y, Leroy X, Rosol TJ, Rieusset J, Bellahcene A, Castronovo V, Aubin JE, Clezardin P, Duterque-Coquillaud M, Bonnelye E (2016) Estrogen related receptor alpha in castration-resistant prostate cancer cells promotes tumor progression in bone. Oncotarget 7:77071–77086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T, Takahashi S, Urano T, Kumagai J, Ogushi T, Horie-Inoue K, Ouchi Y, Kitamura T, Muramatsu M, Inoue S (2007) Increased expression of estrogen-related receptor alpha (ERRalpha) is a negative prognostic predictor in human prostate cancer. Int J Cancer 120:2325–2330 [DOI] [PubMed] [Google Scholar]
- Gaillard S, Dwyer MA, McDonnell DP (2007) Definition of the molecular basis for estrogen receptor-related receptor-alpha-cofactor interactions. Mol Endocrinol 21:62–76 [DOI] [PubMed] [Google Scholar]
- Giguere V (2008) Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev 29:677–696 [DOI] [PubMed] [Google Scholar]
- Gleyzer N, Vercauteren K, Scarpulla RC (2005) Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol 25:1354–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Aguilar M, Baines CP (2013) Physiological and pathological roles of mitochondrial SLC25 carriers. Biochem J 454:371–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijichi N, Ikeda K, Horie-Inoue K, Yagi K, Okazaki Y, Inoue S (2007) Estrogen-related receptor alpha modulates the expression of adipogenesis-related genes during adipocyte differentiation. Biochem Biophys Res Commun 358:813–818 [DOI] [PubMed] [Google Scholar]
- Kamei Y, Ohizumi H, Fujitani Y, Nemoto T, Tanaka T, Takahashi N, Kawada T, Miyoshi M, Ezaki O, Kakizuka A (2003) PPAR-gamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc Natl Acad Sci USA 100:12378–12383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon DH, Eom GH, Kee HJ, Nam YS, Cho YK, Kim DK, Koo JY, Kim HS, Nam KI, Kim KK, Lee IK, Park SB, Choi HS, Kook H (2013) Estrogen-related receptor gamma induces cardiac hyper-trophy by activating GATA4. J Mol Cell Cardiol 65:88–97 [DOI] [PubMed] [Google Scholar]
- Li Y, He L, Zeng N, Sahu D, Cadenas E, Shearn C, Li W, Stiles BL (2013) Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) signaling regulates mitochondrial biogenesis and respiration via estrogen-related receptor alpha (ERRalpha). J Biol Chem 288:25007–25024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Sun P, Dong B, Sehouli J (2018) Key regulator of cellular metabolism, estrogen-related receptor alpha, a new therapeutic target in endocrine-related gynecological tumor. Cancer Manag Res 10:6887–6895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J, Zhang CJ, Zhu N, Du K, Yin YF, Tan X, Liao DF, Qin L (2018) Lipid metabolism and carcinogenesis, cancer development. Am J Cancer Res 8:778–791 [PMC free article] [PubMed] [Google Scholar]
- Luo C, Balsa E, Thomas A, Hatting M, Jedrychowski M, Gygi SP, Widlund HR, Puigserver P (2017) ERRalpha maintains mitochondrial oxidative metabolism and constitutes an actionable target in PGC1alpha-elevated melanomas. Mol Cancer Res 15:1366–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Sladek R, Carrier J, Bader JA, Richard D, Giguere V (2003) Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor alpha. Mol Cell Biol 23:7947–7956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Hayata Y, Kawamura S, Yamada T, Fujiwara N, Koike K (2018) Lipid metabolic reprogramming in hepatocellular carcinoma. Cancers 10:pii: E447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols NG, Dervan PB (2007) Suppression of androgen receptor-mediated gene expression by a sequence-specific DNA-binding polyamide. Proc Natl Acad Sci USA 104:10418–10423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols NG, Szablowski JO, Hargrove AE, Li BC, Raskatov JA, Dervan PB (2013) Activity of a Py-Im polyamide targeted to the estrogen response element. Mol Cancer Ther 12:675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olenyuk BZ, Zhang GJ, Klco JM, Nickols NG, Kaelin WG Jr, Dervan PB (2004) Inhibition of vascular endothelial growth factor with a sequence-specific hypoxia response element antagonist. Proc Natl Acad Sci USA 101:16768–16773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitzko B, Kaweesa EN, Loesgen S (2017) The natural product mensacarcin induces mitochondrial toxicity and apoptosis in melanoma cells. J Biol Chem 292:21102–21116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett JW, Green JT, Dervan PB (2012) Microwave assisted synthesis of Py-Im polyamides. Org Lett 14:2774–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber SN, Emter R, Hock MB, Knutti D, Cardenas J, Podvinec M, Oakeley EJ, Kralli A (2004) The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proc Natl Acad Sci USA 101:6472–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhawat PS, Matern D, Strauss AW (2005) Fetal fatty acid oxidation disorders, their effect on maternal health and neonatal outcome: impact of expanded newborn screening on their diagnosis and management. Pediatr Res 57:78R–86R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek R, Bader JA, Giguere V (1997) The orphan nuclear receptor estrogen-related receptor alpha is a transcriptional regulator of the human medium-chain acyl coenzyme A dehydrogenase gene. Mol Cell Biol 17:5400–5409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs M, Petoukhov MV, Atkinson RA, Roblin P, Ogi FX, Demeler B, Potier N, Chebaro Y, Dejaegere A, Svergun DI, Moras D, Billas IM (2013) The asymmetric binding of PGC-1alpha to the ERRalpha and ERRgamma nuclear receptor homodimers involves a similar recognition mechanism. PLoS One 8:e67810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi J, Pandian GN, Hidaka T, Hashiya K, Bando T, Kim KK, Sugiyama H (2017) A synthetic DNA-binding inhibitor of SOX2 guides human induced pluripotent stem cells to differentiate into mesoderm. Nucleic Acids Res 45:9219–9228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg DL, Zhang W, Yates A, Engelen E, Takacs K, Bezstarosti K, Demmers J, Chambers I, Poot RA (2008) Estrogen-related receptor beta interacts with Oct4 to positively regulate Nanog gene expression. Mol Cell Biol 28:5986–5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega RB, Kelly DP (1997) A role for estrogen-related receptor alpha in the control of mitochondrial fatty acid beta-oxidation during brown adipocyte differentiation. J Biol Chem 272:31693–31699 [DOI] [PubMed] [Google Scholar]
- Villena JA, Kralli A (2008) ERRalpha: a metabolic function for the oldest orphan. Trends Endocrinol Metab 19:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Pandian GN, Yu Z, Zou T, Li Y, Darokar J, Hashiya K, Bando T, Sugiyama H (2018) Synthetic DNA-binding inhibitor of HES1 alters the notch signaling pathway and induces neuronal differentiation. ACS Omega 3:3608–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willy PJ, Murray IR, Qian J, Busch BB, Stevens WC Jr, Martin R, Mohan R, Zhou S, Ordentlich P, Wei P, Sapp DW, Horlick RA, Heyman RA, Schulman IG (2004) Regulation of PPARgamma coactivator 1alpha (PGC-1alpha) signaling by an estrogen-related receptor alpha (ERRalpha) ligand. Proc Natl Acad Sci USA 101:8912–8917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Wang J, Wang Y, Kwok TT, Kong SK, Wong C (2009) Estrogen-related receptor alpha (ERRalpha) inverse agonist XCT-790 induces cell death in chemotherapeutic resistant cancer cells. Chem Biol Interact 181:236–242 [DOI] [PubMed] [Google Scholar]
- Xia H, Dufour CR, Giguere V (2019) ERRalpha as a bridge between transcription and function: role in liver metabolism and disease. Front Endocrinol 10:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Guo J, Zhang H, Meng Q, Ma Y, Lin R, Yi X, Lu H, Bai X, Cheng J (2019) The enhanced expression of estrogen-related receptor alpha in human bladder cancer tissues and the effects of estrogen-related receptor alpha knockdown on bladder cancer cells. J Cell Biochem 120:13841–13852 [DOI] [PubMed] [Google Scholar]
- Zeng N, Li Y, He L, Xu X, Galicia V, Deng C, Stiles BL (2011) Adaptive basal phosphorylation of eIF2alpha is responsible for resistance to cellular stress-induced cell death in Pten-null hepatocytes. Mol Cancer Res 9:1708–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Luo XY, Wu DH, Xu Y (2015) ROR nuclear receptors: structures, related diseases, and drug discovery. Acta Pharm Sin 36:71–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J (2012) Energy metabolism of cancer: glycolysis versus oxidative phosphorylation (Review). Oncol Lett 4:1151–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]


