1. INTRODUCTION
The past few decades have seen a shift from open surgery to minimally invasive procedures guided by real-time imaging. These techniques often involve directing catheters from a superficial, easily accessible blood vessel to a more remote, distal blood vessel to access essentially every organ in the body endovascularly. Such minimally invasive, image-guided interventions have not only broadened the scope of vascular pathologies that can be treated but also often result in comparatively less post-operative morbidity relative to open surgical techniques. A commonly performed endovascular procedure is embolization, a technique in which an agent is delivered through a catheter to obstruct blood flow within a target vessel. Examples of conditions for which embolization is indicated include eradication of a hepatic tumor by blocking the blood supply or preventing rupture of an intracranial aneurysm. A large variety of occlusive agents have been developed for different clinical scenarios. Here, we summarize the diverse array of engineered materials that are available or being developed for embolization and the clinical scenarios in which they are used.
2. HISTORICAL PERSPECTIVE AND CLINICAL UTILITY OF ENDOVASCULAR EMBOLIZATION
Embolization was first documented in 1904 by Robert Dawbarn who embolized lesions of the head and neck with paraffin and vaselin in various patients via the external carotid arteries [1]. Barney Brooks described vessel occlusion in 1930, proposing that the only sufficient treatment for arteriovenous fistulae was closure of the fistula itself [2]. His concepts were later applied successfully with a thin strip of muscle used to embolize a carotid-cavernous fistula [3]. Charles Dotter from Oregon Health and Science University performed the first percutaneous transluminal angioplasty in 1964, successfully treating atherosclerotic arterial stenosis and pioneering catheter-based interventions [4]. Dr. Dotter and his team also performed the first catheter directed embolization in 1970 when they used an autologous blood clot as an endovascular embolization agent to control an upper gastrointestinal bleed in a patient who was a poor candidate for surgery [5].
Fresh blood clots, as used by Dr. Dotter in the first documented embolization procedure, are prepared by collecting blood in a sterile container and allowing the blood to clot. The clot is cut to obtain emboli of various sizes. Fresh clots are heated in a water bath at 56–66 °C to prolong clot lysis. Aminocaproic acid can also be added to resist fibrinolysis [6]. The clots are injected, undergo fragmentation, migrate distally, and occlude small vessels. Autologous subcutaneous tissue and muscle taken from the patient just before the procedure have also been used as permanent embolization agents in the past [6]. Once harvested from the patient, the muscle is cut into small pieces to form a “meatball” and suspended in isotonic saline.
These agents are not used in current practice given the development of engineered synthetic embolization agents. Though no longer used, autologous blot clots are almost completely biodegradable and the inflammatory response is minimal, which are desirable traits of embolization particles [7].
Embolization is indicated in a large variety of clinical situations. One such scenario is that of hemorrhage, a clinical condition that accounts for more than 80% of trauma deaths in the operating room [8]. Not all bleeding can be controlled surgically. Due to the versatility and precision of endovascular techniques, embolization has become a mainstay for many hemorrhagic conditions. For example, embolization has essentially replaced surgery as the treatment of choice for certain scenarios, such as upper gastrointestinal bleeding [9].
Endovascular procedures are also often used to treat pathologic processes involving blood vessels. For example, embolization using intravascular metallic coils is a common approach to treat intracranial aneurysms with low complication rates [10]. Embolization coiling is now a first line treatment for intracranial aneurysms and has better outcomes than open surgery in appropriately selected patients [11].
Arteriovenous malformations (AVMs) are vascular lesions that are comprised of abnormal connections between arteries and veins without an intervening capillary bed. Peripheral AVMs often contain numerous such connections centered around a nidus [12]. Treatment of these lesions requires elimination of blood flow to this nidus. Embolization using liquid embolic agents that penetrate deeply into the AVM and embolize the nidus is an effective method to treat these AVMs [13]. AVMs that do not feature a nidus, such as those involving renal or pulmonary circulation, can be treated with devices meant for more proximal treatment [12].
Embolization of tumors is a very common procedure performed for patients across a range of malignancies and at every stage of their disease. Depending on the clinical setting, embolization may be used to achieve complete local control of a tumor. For patients with advanced disease, cytoreduction through embolization can provide symptom relief and pain palliation [14].
The clinical scenario determines the type of embolic material necessary to treat the patient. The embolization material could be a metal made out of shape memory alloys, highly calibrated beads that may be soft and malleable or rigid to block vessels based on size, or liquid/gel-like material that is injected into the target vessel and flows like lava. Embolization is complex and requires knowledge of the vascular anatomy, an understanding of the pathology, experience with endovascular catheters and microvascular navigation skills, and experience with the various embolic agents. An unexpected non-target embolization may lead to tissue ischemia, organ failure and, in rare cases, death.
3. CLINICALLY USED EMBOLIC AGENTS
In this section, a brief overview of clinically used embolic agents, including particulates, coils and liquids is presented, with a few clinical examples demonstrated in Figure 1.
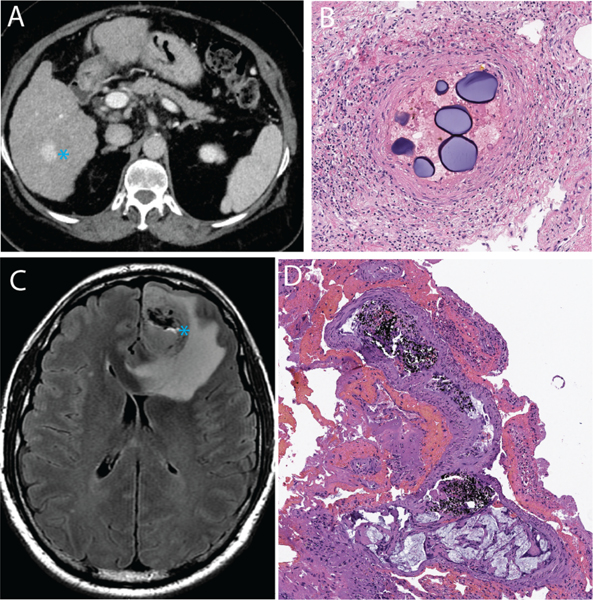
Figure 1.
Clinical examples of embolic agents. A, a patient with cirrhosis and hepatocellular carcinoma (blue asterisk) was treated with microsphere embolization. B, Post-surgical histologic evaluation of the tumor revealed intravascular localization of the microspheres with adjacent inflammatory reaction. C, a patient with glioblastoma multiforme underwent pre-surgical embolization with a liquid embolic agent (Onyx). D, the black-colored liquid embolic agent could be seen within the tumor on the post-surgical resection specimen.
3.1. Particulates
Embolic particulates were the first embolic agents developed and are currently the most commonly used because of their functionality and versatility [7]. These agents have a variety of properties that can be applied to different clinical scenarios, such as being calibrated or irregularly shaped. Particulates can be temporary (biodegradable) or permanent (nonbiodegradable). Particulates can also be natural or synthetic in origin, and they can be loaded with chemotherapy or radioactivity for targeted tumor delivery. As opposed to the proximal embolization provided by coils, embolic particulates are principally designed to embolize tissue at the arteriole and capillary levels. This distal embolization can result in tissue ischemia and necrosis.
3.1.1. Polyvinyl alcohol (PVA) Particulates
Polyvinyl alcohol (PVA), which was initially known for use in household sponges, is a biocompatible polymer that has been used for various medical procedures since the 1950s when Grindlay and Claggett used PVA as a filling material after a pneumonectomy [15]. PVA particles were first introduced as an embolic agent by Tadavarthy et al. in the 1970s [16]. Since then, PVA has been used for a variety of intravascular interventions, including AVM embolization, embolization for lower gastrointestinal bleeding, and embolization of bone metastases for pain palliation [15].
PVA is a linear polymer chain synthesized through hydrolysis of polyvinyl acetate, which removes the acetate groups and creates a soluble polymer [17]. The particles are made from a vacuum dried foam sheet of PVA that is rasped into particles [18]. These particles are then filtered using sieves to separate them based on size, which ranges from 100 μm to 1100 μm [18].
Once injected, PVA particles adhere to the wall of the blood vessel, leading to thrombus formation. Additionally, PVA induces inflammatory processes that lead to angionecrosis of the vessel wall. PVA particles are not biodegradable and are therefore considered a permanent occlusion agent; however, recanalization weeks to months later may occur through angiogenesis within the original thrombus leading to resorption [19].
A drawback of PVA particles is due to the way the particles are prepared, which causes the particles to be irregular in size and shape [7]. This heterogeneity contributes to the tendency of PVA particles to aggregate [20]. The particle aggregation may also occlude the catheter, impeding the injection of particles during the procedure. Several studies regarding the use of PVA particles for embolization procedures have reported high failure and complication rates [21–23].
3.1.2. Gelatin Sponge Particles
Gelatin sponges, such as Gelfoam (Pfizer, New York, USA), is often used preoperatively for hemostasis during surgery [24]. It is prepared from pig skin and made into sheets that are then cut further into particles [24]. The manufacturing process creates a highly porous structure that induces the clotting cascade [25]. Gelatin sponge, like PVA, has been utilized as a transcatheter embolic agent for many different clinical procedures [16]. The biodegradable nature of gelatin foam suggests that the embolic agent is temporary. Gelatin sponge can be used to control gastrointestinal bleeding via embolization of the gastroduodenal artery [26]. Gelatin sponge particles have been shown to provide efficacious treatment of uterine fibroids [27–29]. However, gelatin sponge particles elicit a strong inflammatory response that can lead to proliferation of intimal tissue, causing unintentional permanent occlusion of the target vessel [30], [31].
3.1.3. Calibrated Microspheres
The unpredictable embolization outcomes from irregularly shaped particles has led to the development of calibrated microspheres that allow for tailored embolization. Microspheres of various sizes can be selected for management of different conditions. These can also serve as vehicles for drug delivery with a capability of loading multiple therapeutics [32].
Tris-acryl gelatin microspheres (TAGM) were the first calibrated microsphere embolic agents that were made commercially available (Biosphere Medical’s Embosphere) [33]. These microspheres have been clinically shown to penetrate more distally than PVA particles, providing more significant prevention of blood loss than PVA particles in preoperative embolization of meningiomas [34]. Color can be incorporated into the microspheres to allow for better visualization of the microspheres during injection. TAGM has been used for treatment of benign prostatic hyperplasia (BPH) [35]. The use of TAGM for management of symptoms in patients with uterine fibroids via uterine artery embolization is also well documented [36–39].
To improve upon the drawbacks of PVA particles, PVA microspheres have been developed with regular-shaped spherical bodies that are flexible, allow for complete embolization, and avoid unwanted embolization of normal tissues that are not being targeted [40]. PVA microspheres have been shown to elicit a less aggressive inflammatory response compared to PVA particles and gelatin microspheres in renal models [41]. Hwang et al., demonstrated that in treatment of BPH, prostate artery embolization (PAE) via PVA microspheres showed greater reduction in the volume of the prostate gland compared to nonspherical PVA particles [42]. These microspheres have shown in vivo and in vitro biocompatibility and may serve as effective tools in the future for drug-eluting beads (DEB) and transarterial chemoembolization (TACE) [40].
3.1.4. Chemoembolization with DEBs
Drug eluting beads (DEBs) were developed to deliver therapeutics locally over an extended period of time in a manner that minimizes systemic toxicity. The ideal characteristics of DEBs include visibility on imaging, ability to specify the target, capability of loading multiple modalities of therapeutics, and sustaining effective drug concentrations over time. Transarterial chemoembolization (TACE) is an endovascular intervention that involves the selective catheterization of arteries supplying tumors followed by the delivery of DEBs in a targeted manner. TACE is commonly performed for primary liver tumors such as hepatocellular carcinoma (HCC) and cholangiocarcinoma as well as for hepatic metastases from colorectal cancer [43].
DC Bead microspheres (Biocompatibles, UK) are PVA microspheres that can be loaded with doxorubicin [44]. The rate at which these drugs are sequestered and released by the microspheres is dependent on the size of the beads; smaller beads both sequester and release the drug more rapidly because of a larger surface area [45].
Paragon Bead microspheres (Biocompatibles, UK) can be loaded with irinotecan via an ion-exchange process in which the drug, which is positively charged, associates with the negatively charged polymer-bound sulfonate groups within the PVA structure of the beads [46]. These beads have shown evidence supporting clinical evaluation in the treatment of colorectal cancer metastases to the liver [47].
HepaSphere is a superabsorbent polymer (SAP) microsphere (Biosphere Medical, Rockland, MA, USA) that absorbs fluids to a volume up to 64 times the volume in the dry state to occlude vessels by swelling [48]. These microspheres adapt to the vessel and have CE Mark approval for TACE of HCC in combination with doxorubicin [49].
Embozene TANDEM microspheres (CeloNova BioSciences, Inc, San Antonio, TX/Boston Scientific, Marlborough, MA) were developed to embolize tumors more distally with more predictable and controlled high drug loading [50]. Using an ion-exchange-based mechanism, these microspheres can be loaded with doxorubicin or irinotecan and provide controlled drug delivery [50]. These microspheres are involved in studies regarding untreatable HCC. Tanaka et al. demonstrated that in vivo, TANDEM microspheres loaded with irinotecan effectively provided slow release of therapeutics with maximized tumor cytotoxicity and high concentrations of the drug in tumors [51]. The MIRACLE I study included 25 HCC patients who underwent TACE with doxorubicin-loaded TANDEM microspheres [52]. This study demonstrated high rates of tumor control in these patients with few adverse effects [52].
3.1.5. Microspheres Visible on Imaging
Most of embolization microspheres are mixed with intravenous contrast agents for imaging, but the applications in long-term monitoring are limited because the body clears contrast media from blood rapidly [53]. The microspheres currently available are also radiolucent and do not form primary bonds when mixed with contrast agents [54]. The washout of contrast diminishes the visibility of the embolization agents. This has led to the need for microspheres with intrinsic radiopacity, which reduces the need for multiple contrast injections and therefore reduces the potential toxicity of contrast agents.
Intrinsic radiopacity can be introduced to microspheres through polymerization of monomers that are radiopaque or attaching the radiopaque moiety onto the core polymer [55]. Attaching triiodobenzyl acid moieties introduces radiopacity to the embolic agent [56]. Horák et al. developed intrinsically radiopaque beads with a polymeric hydrogel base that are visible by X-ray yet retain their porous structure necessary for effective embolization [56]. Such visibility allows for real time monitoring of the embolization during the procedure without performing an angiogram [54].
LC Bead LUMITM.
LC Bead LUMI™ (Biocompatibles, UK Ltd, a BTG International group company) are intrinsically radiopaque microbeads that have recently been introduced and applied in the management of HCC [55], [57]. Aliberti et al. reported the use of these microbeads, which also serve as DEBs, in a patient cohort for treatment of HCC, demonstrating low levels of toxicity, few adverse events, and the benefit of real time direct visualization of the beads [58].
3.1.6. Biodegradable Microspherical Embolic Agents
Temporary or biodegradable agents are chosen when reintervention may be anticipated in the same artery [32]. TAE applications of these spheres include gastrointestinal bleeding, trauma, and uterine fibroid embolization [59].
Natural.
Various polysaccharides have been used to develop microspheres that are naturally biodegradable. Starch-based microspheres [60], dextran-based microspheres [61], and calcium alginate [62] are examples of microspheres based on natural biomaterials that can be degraded by the body.
Synthetic.
Poly(lactic-co-glycolic acid)(PLGA), a hydrophobic and degradable polymer, is synthesized with different glycolic acid and lactic acid ratios [59]. PLGA’s degradation behavior has applications in surgical sutures because the polymer becomes more hydrophilic as the material degrades, which leads to accelerated degradation [63]. The release pattern and properties of PLGA make it a candidate for drug delivery, especially for short term continuous release [63]. The degradation of the polymer has been well documented elsewhere [64], [65]. Degradable PLGA microspheres are available on the market under the brand name Occlusin™ 500 Artificial Embolization Device (IMBiotechnolgies Ltd., Edmonton, AB, Canada) and have been approved by the FDA [66]. These microspheres are easily suspended in contrast media, but lack multi-modal imageability [59].
3.2. Coils
Coils are the achetype device for proximal vascular embolization. These devices are primarily used to arrest blood flow in medium- to large-sized arteries. This is in distinction to distal embolic devices such as microparticles and liquid embolics, materials designed to flow deeply into target lesions and effect tissue ischemia. Examples of clinical scenarios in which coils are commonly employed are traumatic vascular injuries and arterial aneurysms.
Coils made of metal alloys were the first devices to be used clinically for treatment of hemorrhage and aneurysms [67]. Coils, especially when detachable, afford control and can be adjusted while being deployed [68]. Metal coils reduce the risk of subarachnoid hemorrhage caused by a ruptured intracranial aneurysm, making them the first line choice for embolization of aneurysm sacs. Other devices, such as balloons and stents, are often used in conjunction with coils to maintain the patency of the artery while also increasing the packing rate of the coil [67].
3.2.1. Bioinert Bare Metallic Coils
Guglielmi introduced coils known as Guglielmi detachable coils (GDC) made of platinum alloy for the treatment of aneurysms [69]. These were the first embolization devices approved by the FDA for occlusion of aneurysms [70]. Stainless steel coils with wool strands were first introduced in 1975 for embolization of renal arteries to treat hypernephroma [71]. These coils had limited flexibility and were better suited for large vessel occlusion as may be required during splenic artery embolization for hypersplenism or segmental renal artery embolization for a renal arteriovenous fistula [72]. Platinum-based materials represent a substantial improvement over the initial stainless steel devices because of their flexibility and the inert nature of platinum, making them the ideal choice for permanent placement in the body [67].
Szikora et al. found that GDCs did not result in adequate thrombus organization, suggesting that bioinert bare metallic coils do not elicit an appropriate response from the target tissue [73], [74].
3.2.2. Modified Bioactive Coils
The bare metallic coils discussed previously are bioinert and rely on the patient’s ability to form thrombi. The variability of and dependence on individual physiological processes has led to the development of bioactive coils by modifying the surface of metallic coils.
The first stainless steel coil introduced by Gianturco and his team in 1975 had wool coils for use in large vessels and cotton tails for small vessels [71]. However, these coils were found to elicit strong chronic inflammatory reactions of the vessel wall compared to coils with Ivalon and silk fibers [75]. Fibered platinum coils have been found to significantly improve rates of occlusion compared to bare platinum coils in treatment of aneurysm [76].
The VortX® coil (Stryker, Boston, MA) was one of the first detachable fibered coils introduced for intracranial treatments [77]. This coil was a Guglielmi detachable coil (GDC) with Dacron fibers (DuPont, Wilmington, DE) [77]. Though success in treating aneurysms was documented, the coil was not used extensively due to complications with the catheter and thrombosis [77].
The Matrix detachable coil (Boston Scientific) is a platinum coil covered in PGLA indicated in the treatment of aneurysm [78]. When compared to GDCs in animal models, the Matrix coils exhibited more extensive formation of neointima with fewer instances of recanalization of aneurysm or rupture [78]. However, a study of 25 patients with intracranial aneurysms by Taschner et al. found that Matrix coils provided occlusion, but the stiffness prevented tight packing and led to some recurrence rates [79]. This study concluded that Matrix coils should not be used exclusively [79].
The Cerecyte coil (Micrus Endovascular, San Jose, Calif) is a platinum coil with PGA in the lumen that was developed and studied for reducing recanalization in aneurysms [80]. Compared to bare platinum coils and the Matrix coil, the Cerecyte coil has high occlusion despite low packing attenuation, providing benefit over the bare platinum coils [80].
3.2.3. HydroCoil and HydroSoft
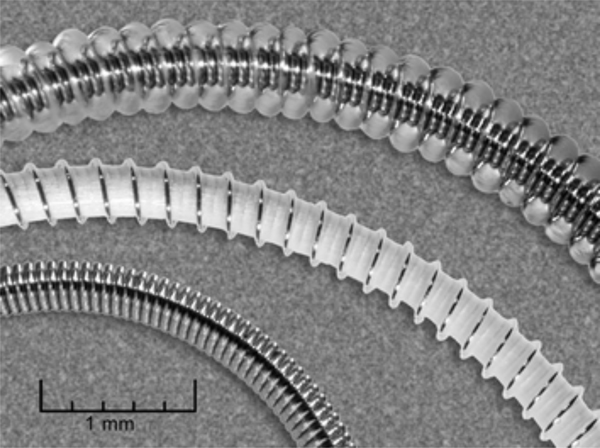
To improve durability of occlusion and reduce recurrence rates, hydrogel-coated coils have been introduced to achieve larger filling volumes compared to those of metallic coils (Figure 2) [81]. HydroCoil, a hydrogel-platinum coil (MicroVention, Aliso Viejo, Calif.), was developed with a hydrogel material that expands upon exposure to blood [82]. Compared to bare platinum coils, the HydroCoils were found to increase packing density of aneurysms when used in addition to the bare coils and also showed significantly increased complete occlusion [82]. However, randomized controlled trials, such as the Hydrocoil Endovascular Aneurysm Occlusion and Packing Study (HELPS) trial, have suggested that hydrogel coils may not provide a significant benefit in treatment of large or recurrent aneurysms compared to bare platinum coils [83], [84].
Figure 2.
From bottom to top: a platinum coil, an unexpanded hydrogel-coated coil, and an expanded hydrogel-coated coil. The increase in volume of the hydrogel-coated coil after expansion is evident compared to its dry state (with white coating). Reproduced with permission from [81].
The limitations of HydroCoil led to the development of HydroSoft coils, a platinum coil with a core made of hydrogel and a stretch-resistant filament [85]. HydroSoft demonstrates less stiffness and better packing attenuation with lower recurrence rates than HydroCoil [85].
3.3. Liquid/Gel Embolic Agents
Interest in liquid and gel embolic agents has increased since these agents do not depend on a patient’s individual ability to coagulate and can penetrate to areas where catheters and coils cannot reach for more peripheral interventions [86]. These agents are typically dissolved in an organic solvent and injected into the target arteries [88]. Once injected, the agent undergoes a phase transition to a solid implant through a process of either polymerization, precipitation, and cross-linking [88].
3.3.1. Ethanol – A Sclerosing Agent
The most commonly used sclerosing agent is ethanol, a potent medication that rapidly causes protein denaturation that eventually leads to endothelial damage and permanent occlusion [86]. The use of absolute alcohol has been documented in treatment of renal carcinoma [89], as well as AVMs in various areas of the body [90–93].
3.3.2. Acrylic Glue
n-butyl cyanoacrylate (n-BCA) has been used for treatment of cerebral AVMs with similar outcomes to embolization using PVA agents [94]. n-BCA can be also be used for preoperative embolization in treatment of meningiomas to reduce blood loss [95], [96]. n-BCA polymerizes rapidly on contact with the ionic medium or surface to form a case of the vessel and is deployed as a glue-like adhesive [94]. However, n-BCA injection is difficult to control and the microcatheter must be withdrawn immediately to prevent the catheter itself from being glued to the vessel wall [96].
3.3.3. Precipitating Agents
The first alternative to acrylic glues for embolization of AVM, such as n-BCA mentioned previously, was proposed by Taki [98]. His team developed a copolymer made of ethylene vinyl alcohol (EVOH) and metrizamide dissolved in dimethyl sulfoxide (DMSO), which successfully treated cerebral AVMs [98]. The DMSO rapidly diffused upon contact with blood to form an elastic EVOH sponge that provided occlusion [98]. This agent is radiolucent and therefore must have a contrast agent, such as tantalum powders, added for visibility on imaging [99]. The ev3 ONYX system (Medtronic, formerly Covidien, Dublin, Ireland) is an FDA-approved EVOH gelling solution that is well recognized for its use in treatment of cerebral AVMs [99]. This agent contains a PVA copolymer dissolved in DMSO, which is a powerful solvent [99]. Rabinov et al. found that in embolization of cranial dural AVFs, patients treated with ONYX had better occlusion and long term outcomes compared to patients treated with n-BCA [100]. Although ONYX can achieve complete occlusion, the liquid agent can migrate intracranially or into the parent artery [101]. Seron et al. prepared an agent using copolymers of tertiobutylacrylamide (tBA) and 2-hydroxypropyl methacrylate (HPMA) to improve upon the ONYX [99]. In vivo studies showed that this compound provided complete occlusion [99]. ONYX’s main limitation is the difficulty in obtaining forward flow and achieving penetration of the fistula [97]. After receiving over 100 reported patient cases of catheter breakage, the FDA issued a warning in June of 2012 regarding the risks of microcatheter entrapment within a viscous cast of Onyx [102], [104]. Onyx is therefore only approved for treatment of AVM in patients who will undergo surgical removal and not as a long-term implant [102].
PHIL (Precipitating Hydrophobic Injectable Liquid) (PHIL) (MicroVention, Tustin, California) is a new liquid embolic non-adhesive agent comprised of a copolymer dissolved in DMSO [97]. The agent also has triiodophenol, an iodine component, bound to the copolymer, which provides intrinsic radiopacity [104]. This agent overcomes some of the drawbacks of n-BCA and Onyx. Studies by Leyon et al. and Lamin et al. demonstrated that forward flow was achieved without difficulty and venous penetration was successful in the treatment of AVMs and AVFs [97], [105]. PHIL is easier to prepare than Onyx since it is intrinsically radiopaque and does not require the addition of tantalum and also results in improved visualization of the occluding cast [105].
4. EMBOLIC AGENTS IN DEVELOPMENT
The ideal embolic agent is an off-the-shelf bioengineered material within a syringe that can be injected via any catheter without any prior preparation such as vortexing and visualized as it exits the catheter tip without any fear of non-target distal embolization. These embolic agents could be temporary or permanent, biocompatible, non-toxic, and easy to make. Such a one-size-fits-all embolic agent would eliminate the need to have large coil and bead inventories of various sizes, shapes, and lengths in the procedure rooms, reducing the financial burden to the hospital and to the patient. Some of the embolic agents that are in development addressing the limitations of the embolic agents we use today are reviewed below.
4.1. Particulates
4.1.1. Biodegradable Agents
Biodegradable embolic materials are of clinical relevance when a temporary embolization is desired. For example, in patients with bleeding following trauma, temporary interruption of the arterial perfusion to the affected organ can help stabilize the patient, with subsequent reconstitution of the blood supply preserving long-term organ function. This is commonly performed following pelvic trauma or for patients with post-partum hemorrhage due to uterine bleeding. As with other endovascular embolic agents, there exists a broad range of desired biomaterial characteristics for biodegradable embolic agents due to the myriad clinical scenarios in which they may be applied.
Resorbable Embolization Microsphere (REM).
Verret et al. studied a resorbable embolization microsphere (REM) (ResMic; Occlugel SAS, Jouy-en-Josas, France) made from polyethylene glycol (PEG) hydrogel crosslinked with PLGA-PEG-PLGA that would occlude the target vessel but be eliminated before any chronic inflammation took place [106]. PEGs are polymers made of ethylene glycol that are excreted in urine and therefore typically regarded as not degradable [59]. PLGA-PEG-PLGA crosslinks are degraded into PLGA and PEG as byproducts. PLGA and PEG have previously been used in the development of synthetic platelets for nanoparticle hemostasis techniques [107]. Verret et al. injected REM into the uterine artery of an animal model and found complete disappearance and recanalization and no residual inflammation of the tissue [108]. Louguet et al. combined the PLGA-PEG-PLGA crosslinker with poly(ethylene glycol) methacylate (PEGMA) to develop another degradable microsphere with minimal inflammatory response [108]. Animal models of these microspheres showed rapid degradation, low toxicity of degradation products, and minimal inflammation [108].
Chitin- and Chitosan-Derived Microspheres and Nanoparticles.
Chitin and chitosan, which is a derivative of chitin, are biocompatible and biodegradable materials that can serve as carriers of therapeutics for chemoembolization [109]. They can be harvested from the shells of crustaceans and are degraded by lysozymes that are present in the human body [110]. Chitosan nanoparticles have potential applications in many clinical circumstances, particularly drug delivery to certain tissues [110]. For example, such nanoparticles loaded with immunosuppressive rapamycin have been studied in animal models to treat allografts in patients with corneal transplantation [111]. Chitosan nanoparticles loaded with acyclovir for drug delivery to the skin have been found to provide improved permeation of the drug [112].
Chitosan microspheres prepared by a Water-in-Oil (W/O) emulsification technique are shown to have a uniform size and spherical shape. The final microsphere sizes and morphology can be tuned by stirring speed, W/O phase proportion, emulsification time, and cross-linking time [109]. Protein adsorption decreases as the particle size decreases and clotting weight increases with increasing particle size. Surface properties such as electric charge, hydrophilicity and hydrophobicity are keys to thrombosis at the molecular level, as well as adhesion between the microsphere and blood plasma proteins [109].
4.1.2. Microspheres Visible on Imaging
Nano-on-micro.
Nanoparticles are another vehicle to introduce radiopacity into microspheres. Chitosan microspheres loaded with superparamagnetic iron oxide nanoparticles have been synthesized with the potential for use in anti-cancer embolotherapy [113]. Barium alginate microspheres loaded with barium sulfate have been synthesized as another form of microspheres with radiopacity [114]. Tantalum nanoparticles have also been used to synthesize radiopaque microspheres. These nanoparticles are loaded onto calcium alginate microspheres through electrospraying and have been shown to be assessed successfully by CT and digital radiography four weeks after embolization [115]. PLGA microspheres loaded with doxorubicin and magnetic iron-cobalt nanoparticles for MRI imageability demonstrated successful in vivo steering and tracking [116].
Multimodal Visibility.
Embolization particles with multimodal visibility have been synthesized to contain a core with iodine that is visible by x-ray and a coating with ultra-small paramagnetic iron oxide (USPIO) that is visible by MRI [117], [118]. In animal models, these particles have shown successful embolization without the need to add radiopaque agents and are visible in various different imaging modalities, providing visibility during and after embolization [117]. Such agents are favorable because they might reduce the amount of contrast agent needed. In another study, Embozene Microspheres were modified with different densities of radiopaque barium sulfate and iodine as well as magnetic iron oxide to provide visibility on radiography, MRI, and CT in animal models [119].
4.1.3. Drug eluting microspheres
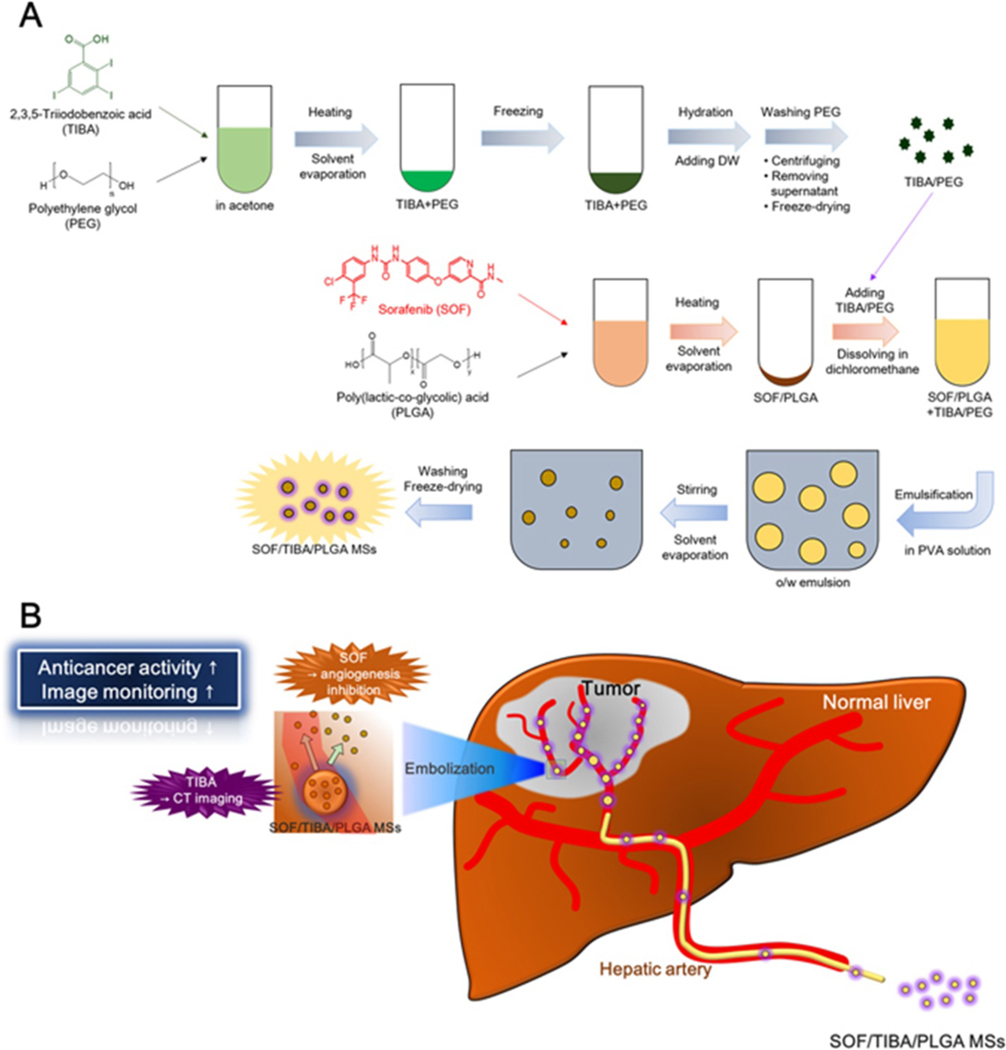
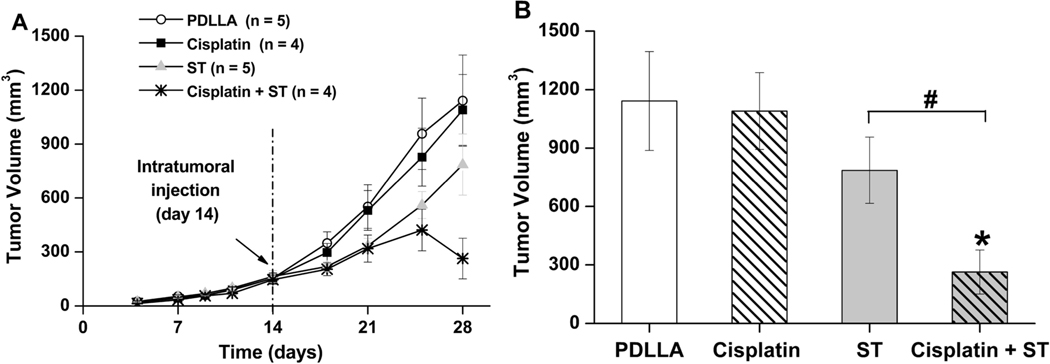
Drug eluting microspheres, especially bioresorbable carriers, have received tremendous attention nowadays due to their multi-functionality in both embolization and ablation for tumor treatment. Zhang et al. prepared two types of alginate-chitosan microspheres for treatment of hepatic chemoembolization via emulsification-gelation loaded with Norcantharidin (NCTD/LSD-ACMs) [120]. In vitro studies showed favorable sustained release of the drug and significantly decreased tumor growth rates, suggesting that NCTD/LSD-ACMs can effectively embolize liver tumors [120]. Biodegradable DEBs made from poly(D-L-lactic acid) and loaded with cisplatin or sorafenib are degraded by absorption of water, hydrolysis, erosion of the polymer, and bulk degradation [121]. These microspheres have shown high therapeutic efficacy in vitro [121], [122]. Choi et al. developed PLGA microspheres loaded with sorafenib for HCC embolization (Figure 3)[123]. The PLGA matrix degraded into lactic acid and glycolic acid in biological fluids [123]. Cisplatin- and sorafenib- loaded biodegradable poly(D,L-lactic acid) (PDLLA) microspheres were synthesized through an emulsion/solvent evaporation method [121]. The drug was dissolved in organic solvent and mixed with PDLLA solution to produce the eventual drug-loaded microspheres with diameters ranging from 200–400 μm. In nude mice, cisplatin-loaded microspheres had no effect on tumor growth compared to plain PDLLA microsphere. Though sorafenib-loaded PDLLA spheres slowed tumor growth, combined cisplatin and sorafenib led to tumor shrinkage at day 28, showing the synergetic cytostatic and antiangiogenic effects on a solid tumor (Figure 4). Additional benefit arises from the radiopacity of cisplatin (PDLLA sphere is translucent) to fluoroscopy and CT imaging for local targeting and monitoring of microspheres in the vessels [122].
Figure 3.
Schematics of (A) Synthesis and (B) Therapeutics of SOF/TIBA/PLGA microspheres. Reproduced with permission from [123].
Figure 4.
(A) Tumor growth profile (Renca tumor mice model) until day 28 (14 days after injection of microspheres) for PDLLA, cisplatin, sorafenib, and cisplatin+sorafenib treated groups. (B) Tumor volumes on day 28 for all four groups. Reproduced with permission from [121].
4.2. Coils
4.2.1. Modified Bioactive Coils
SDF-1α-coated Coils.
Gao et al. studied platinum coils with silk fibroin and coated in stromal cell-derived factor-1α (SDF-1α) for the occlusion of aneurysms [124]. The study hypothesized that the SDF-1α would recruit endothelial progenitor cells (EPCs) to the damaged tissue and accelerate vascular repair [124]. Animal models demonstrated well organized tissue after implantation of the coil, showing promise as a potential intervention for treatment of aneurysms [124].
Axium MicroFX Coils.
The ACCESS study included a cohort of patients with aneurysms and studied outcomes of the use of Axium MicroFX PLGA coils [125]. The Axium MicroFX PLGA coil contains microfilament technology, which may act hemodynamically in ways that other PLGA coils do not [125]. This study involved a small prospective cohort and further testing with larger patient cohorts comparing the coil to other coils is needed.
Other Modified Coils.
Bare metallic coils coated in materials to aid in thrombus formation and embolic treatment have been developed and studied [126]. Ahuja et al. studied GDCs coated in different thrombogenic materials in animal models and found that compared to bioinert GDCs, GDCs coated in PEU-PEO polyurethane led to faster occlusion of the target vessel [127]. Coils coated in collagen were found in animal models to result in aneurysm treatment with complete obliteration and no recanalization [128]. Kallmes et al. demonstrated that GDCs modified with fibroblasts containing basic fibroblast growth factor led to in vivo proliferation of fibroblasts and formation of collagen [129].
4.2.2. Shape Memory Polymer (SMP) Coils
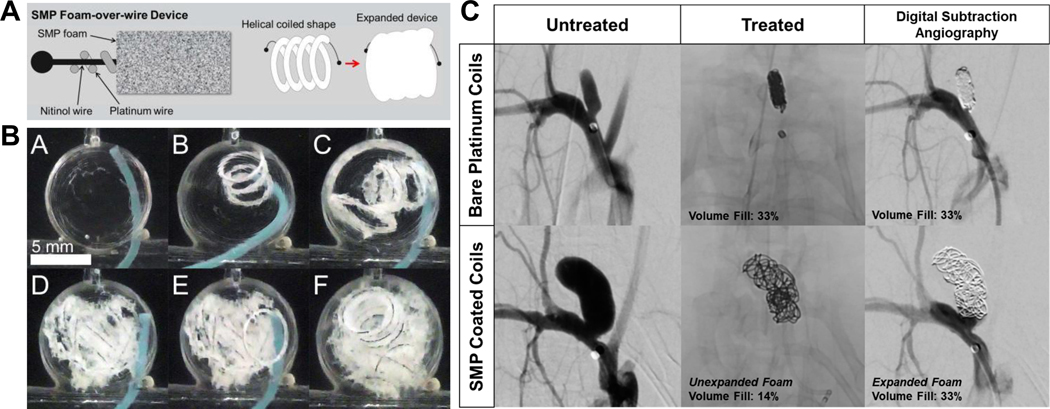
The biomedical applications of shape memory polymers (SMPs) have been reviewed and explored with regards to scaffolds, stents, and tissue engineering frameworks [130]. Heat, light, and water can be used as external stimulation to trigger the shape memory effect. Therefore, a large, bulky device made from these smart and active SMPs can be delivered in its temporarily compressed form through a minimally invasive approach and then be expanded into their permanent form for treatment [130]. While shape memory materials have lingered around for many year, their use in the field of endovascular embolization is still limited. In one example, coils coated in SMP can expand to fill more volume while also enhancing the healing process [131]. SMPs have various properties that make them candidates for embolization, including short shape recovery time upon release [132]. Compared to bare metallic coils in vivo, SMP-coated coils demonstrated more complete aneurysm healing and better occlusion (Figure 5) [131], [133], [134].
Figure 5.
(A) Schematics of fabrication of an SPM foam-over-wire device; (B) In vitro delivery for the device; and (C) Shape memory foam-coated coil for rabbit elastase aneurysm treatment. The volume fill of expanded SMP-coated coils is the same as that of bare platinum coils. Figure components adapted and reproduced with permission. [133], [134].
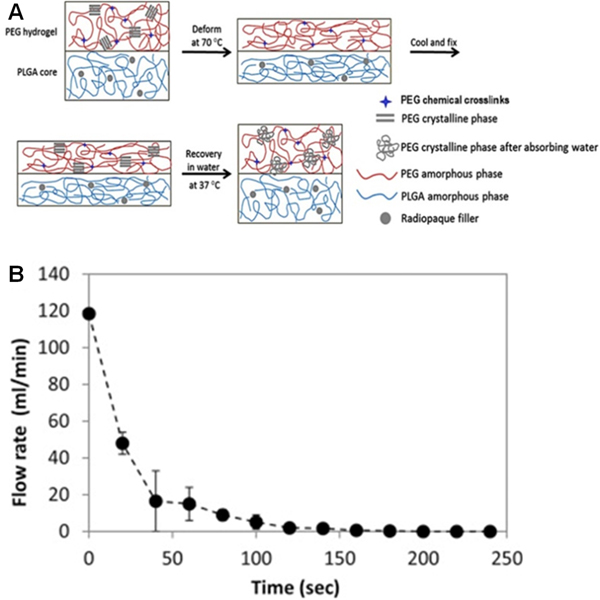
A bioabsorbable water-induced shape memory plug has recently been developed for temporary vascular occlusion [135]. The coil consisted of a PLGA core, which was blended with radiopaque agents (i.e., barium sulfate, tantalum, or bismuth oxychloride) and a poly(ethylene glycol) diacrylate (PEGDA) hydrogel surface coating for enhanced vessel occlusion. The addition of radiopaque agents did not have a significant effect on Young’s modulus but led to a significant decrease (up to 40%) in tensile strength, perhaps due to the loss of adhesion at the particle-matrix interface in the composite material. It was shown that high loadings of bismuth oxychloride reduced the gel ductility, which is an essential property for successful shape programming.
The water- and temperature- induced shape memory behavior of this composite plug was achieved by the transformation between the crystalline and amorphous states of both PLGA and PEGDA polymers, shown in Figure 6A [135]. Its occlusive behavior was tested in various arteries in a rabbit model, including carotid, hepatic, superior mesenteric, celiac axis branches, and renal. The time for complete occlusion ranges between 38 seconds and 2 minutes (Figure 6B). One out of thirteen tested devices was observed with distal migration, whereas no ruptures of any of these vessels were observed. Significant enlargement of the vessels was clear at the embolization sites as a result of the swollen PEGDA hydrogel. The composite plug was observed to degrade in vivo, as its mass was reduced by 70% in 10 weeks, primarily due to hydrolysis. Moreover, PEGDA hydrogel served as a protective coating to encapsulate degraded PLGA to prevent its fragments from migrating downstream.
Figure 6.
(A) Schematic illustration of the water-responsive shape memory mechanism of an embolic device. (B) Time-dependent flow response during in vitro embolization. Reproduced with permission from [135].
4.3. Shape Memory Polymer Foams
Shape memory polymers (SMPs) have also been a focus when designing embolization materials that have tunable properties [136]. These foams induce the coagulation cascade and are able to occlude vessels rapidly in in vivo models [137]. SMP foams have shown effective occlusion of aneurysms in vivo with minimal inflammatory response [138. Monroe et al. synthesized SMP foams with antimicrobial properties through the incorporation of cinnamic acid, an antimicrobial phenolic acid, which has applications in procedures that require additional efforts to reduce risk of infection [136]. SMP foams have structural and mechanical characteristics that make them a favorable alternative for treating intracranial aneurysms, such as lower densities and high porosities, and can be loaded with tungsten to become radiopaque [139].
4.4. Liquid/Gel Embolic Agents
Liquid embolics are a subject of great interest in bioengineering, especially in peripheral interventions. They can penetrate into peripheral, nidus, or distal regimes that coil or catheters cannot reach. Liquid embolics block blood flow by forming casts mechanically, a process that does not depends on the patient’s coagulation system [86].
Some clinically relevant characteristics of injectable liquid delivery systems include viscosity, setting mechanism (e.g. polymerization, precipitation, or ionic crosslinking), and settling time. Systematic investigation of additional key material features, such as associated side effects (e.g. toxicity and adhesion), bio-functionally (biocompatibility, biodegradability, biomechanics and bioactivity), and radiopacity, is also essential for the development of clinically tailored embolic agents in the field of imaging-guided minimally invasive procedures [88].
4.4.1. Temperature-Induced Phase Change Systems
Temperature sensitive polymer solutions can change from a sol-injectable state at room temperature to a gel state inside the body at body temperature [140]. Poly(N-isopropylacrylamide) (PNIPAAm) exhibits a reversible phase transition in response to a lower critical solution temperature (LCST) of 32°C [141]. The inner core of PNIPAAm micelles can be loaded with a drug that is then dispersed in response to the LCST [139]. Qian et al. studied the applications of Poly(N-isopropylacrylamide-co-butyl methylacrylate) gels with iohexol dispersions for TACE of liver cancers [143]. These gels demonstrated effective embolization of renal arteries in rabbits and sustained release of doxorubicin [143]. Wang et al. found that chitosan/β-glycerophosphate (C/GP) successfully occluded renal arteries in rabbits, suggesting its potential as an embolization agent [144]. C/GP is a solution that could transition into a gel at 37°C due to secondary interactions between the two components [145]. The system does not require organic solvents, is well regarded as a nontoxic and biocompatible polymer, and is low cost as the materials can be obtained easily. Zhao et al. investigated temperature sensitive Poly(N-isopropylacrylamide) (PNIPAM)-Iohexol nanogels for embolization [146]. The hydrophobic interactions above phase transition temperature (36.5°C) leads to the formation of a 3D hierarchical gel network for in situ vessel casting. Compared to Lipiodol, a peripheral embolic agent, the nanogel provided better peripheral embolization in VX2 liver tumor in rabbit.
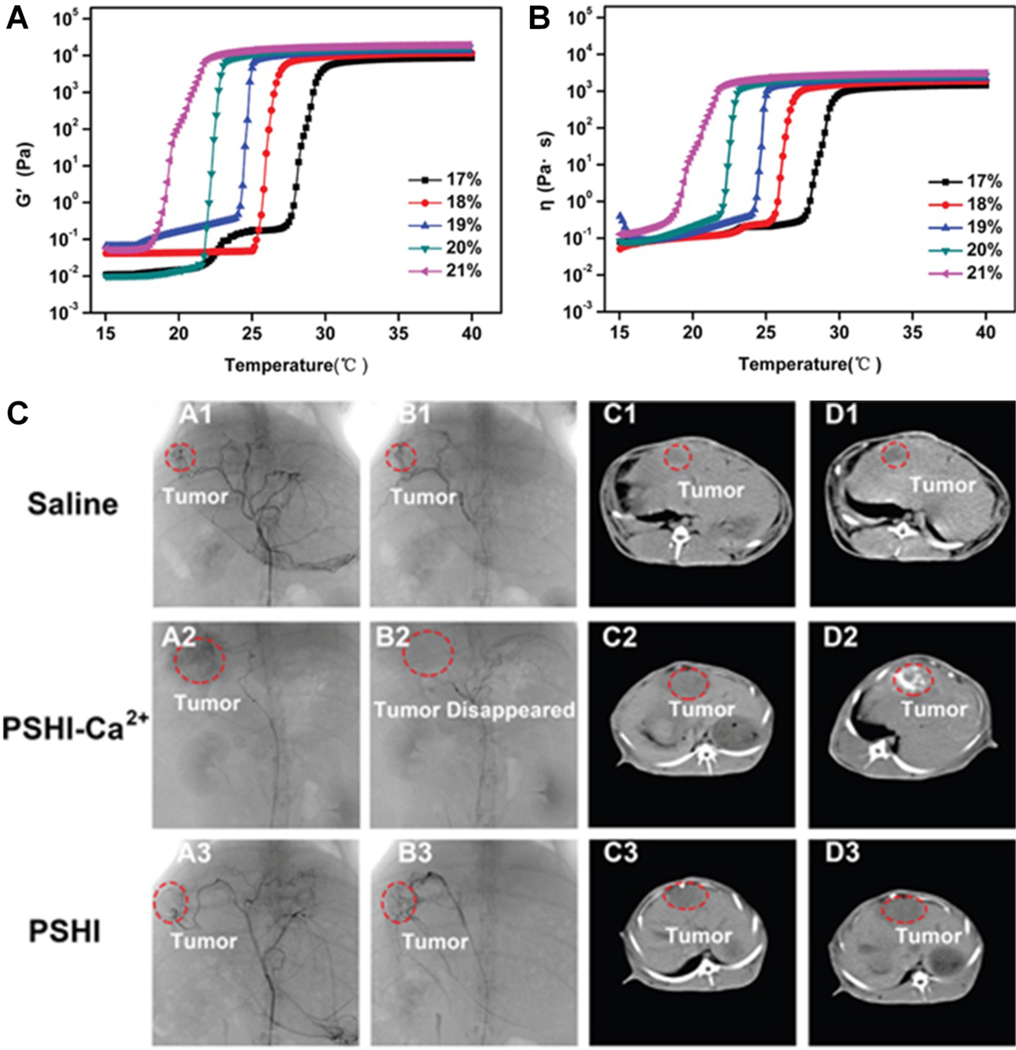
In another example, a poloxamer 407 (a triblock copolymer of polyethylene oxidea–polypropylene oxideb–polyethylene oxidea) containing thermosensitive liquid embolic hydrogel has been proposed for liver cancer therapy [147]. This composite hydrogel composed of poloxamer 407, sodium alginate, hydroxymethyl cellulose, and iodixanol (PSHI) exhibited two phases with increasing temperature: a flowing sol and a shrunken gel. Its storage modulus and viscosity are dependent on the concentration of poloxamer 407 (Figure 7A and B). The PSHI was prepared together with Ca2+, which improves strength at higher temperatures (PSHI-Ca2+), and demonstrated successful occlusion of renal tumors in animal models [147]. Specifically, complete occlusion of VX2 liver cancer model was also demonstrated with PSHI-Ca2+ as the tumor disappeared after embolization. However, for both saline and PSHI embolized groups, tumors still existed in both cases after embolization (Figure 7C) [147].
Figure 7.
Temperature dependent (A) storage modulus (G’) and (B) viscosity (η) of PSHI with P407 at various concentrations. (C) DSA and CT images of VX2 liver tumors in rabbits before (A1-A3, C1-C3) and after embolization (B1-B3, D1-D3) with saline, PSHI-Ca2+ and PSHI. Reproduced with permission from [147].
4.4.2. pH-Triggered Embolic Agents
pH-sensitive agents undergo phase transition due to the change of pH between the in-vitro and physiological environment. These hydrogels swell and collapse in response to pH, which has been used to control the release of drugs [148]. The ionization of different functional groups on the hydrogels based on medium can be exploited and tailored for drug delivery to different organs with varying pH’s, especially the gastrointestinal tract [149]. Nguyen et al. developed PCLA-PUSSM, a copolymer comprised of poly(ε-caprolactone-co-lactide) (PCLA), PEG, and poly(urethane sulfide sulfamethazine) (PUSSM), for TACE of HCC [150]. This biodegradable material was loaded with doxorubicin and exhibited a phase transition in response to decreasing pH [150]. This hydrogel successfully embolized a hepatic tumor of a VX2 rabbit tumor model while maintaining sustained release of the doxorubicin [150]. Park et al. described the development of pH-responsive nanocomposites loaded with sorafenib for treatment of HCC [151]. In a rat model, the embolization stopped the blood supply of the tumor, creating an acidic environment through hypoxia and induced lactic acidosis [151]. The low pH triggered the release of the drug and successfully inhibited in vivo tumor growth [151].
4.4.3. Physically and Chemically Crosslinked N-isopropylacrylamide(NIPAAm)-based System
Hydrogels that undergo both physical and chemical gelation have been developed and investigated with the copolymer base as N-isopropylacrylamide (NIPAAm) for biomedical applications, including embolization of aneurysms. Lee et al. developed a polymer system consisting of poly(NIPAAm-co-HEMA-acrylate) and poly(NIPAAm-co-Cysteamine)/PEGDA (poly(ethylene glycol) [152]. The physical gelation is triggered by increased temperature while the chemical gelation is triggered by crosslinking reactions between acrylates and thiols [152]. The temperature sensitive phase changing occurs mainly by chain entanglement, not covalent cross-linking, which leads to an internal fluidity and the reversible properties of the gel [153]. This is attributable to creep flow under exposure from large external forces, such as high blood pressure, which is undesirable when full occlusion of a vessel is necessary [153]. In evaluation as an agent for embolization of intracranial aneurysms, the NIPAAm-based polymer system was successfully delivered to the target site through catheters both in vivo and in vitro [154].
4.4.4. Ionically Cross-linked System
Calcium alginate gel (ALGEL) (Neural Intervention Technologies, Ann Arbor, MI) is an embolic agent with primary applications in neuroendovascular interventions such as treatment of aneurysms [18]. Alginic acid, a natural polysaccharide that can be derived from brown algae, is a copolymer that can crosslink ionically with a cation, such as calcium chloride [155]. When the cation binds to the alginic acid, a stable alginate polymer gel is formed. Preliminary investigations in swine models have shown that calcium alginate is biocompatible and effectively occludes vessels while providing an effective template for growth of tissue in the treatment of aneurysms [156]. ALGEL offers many advantages, including rapid formation of the occlusive gel, obstruction without depending on thrombosis formation, and no catheter occlusion since the components are injected separately [18].
4.4.5. Lyotropic Liquid Crystalline Phase
Phytantriol.
Phytantriol is a common ingredient found in cosmetics and skin and hair care products [154]. Han et al. investigated the application of phytantriol as an embolic agent and drug carrier for TACE in HCC treatment [158]. The viscous cubic liquid crystalline phase acts as an obstructing plug and is made injectable by preparation with ethanol [158]. The agent converts to the bicontinuous cubic phase upon contact with body fluids [158]. The agent was able to convert into a viscous cubic liquid crystalline phase and successfully embolize the hepatic artery in animal models [158]. Qin et al. developed a phytantriol-based agent that demonstrates potential application as a carrier for delivery of hydroxycamptothecine, a chemotherapeutic [157].
Glycerol Monooleate.
Du et al. developed a potential liquid embolic agent comprised of glyceryl monooleate (GMO) [159]. The agent exhibits low viscosity, which allows for delivery through a microcatheter, but forms a viscous gel cast upon contact with physiological fluids [159]. In vivo and in vitro studies demonstrated compatibility and successful embolization of rabbit renal arteries [159].
4.4.6. Shear-Thinning Biomaterial (STB)
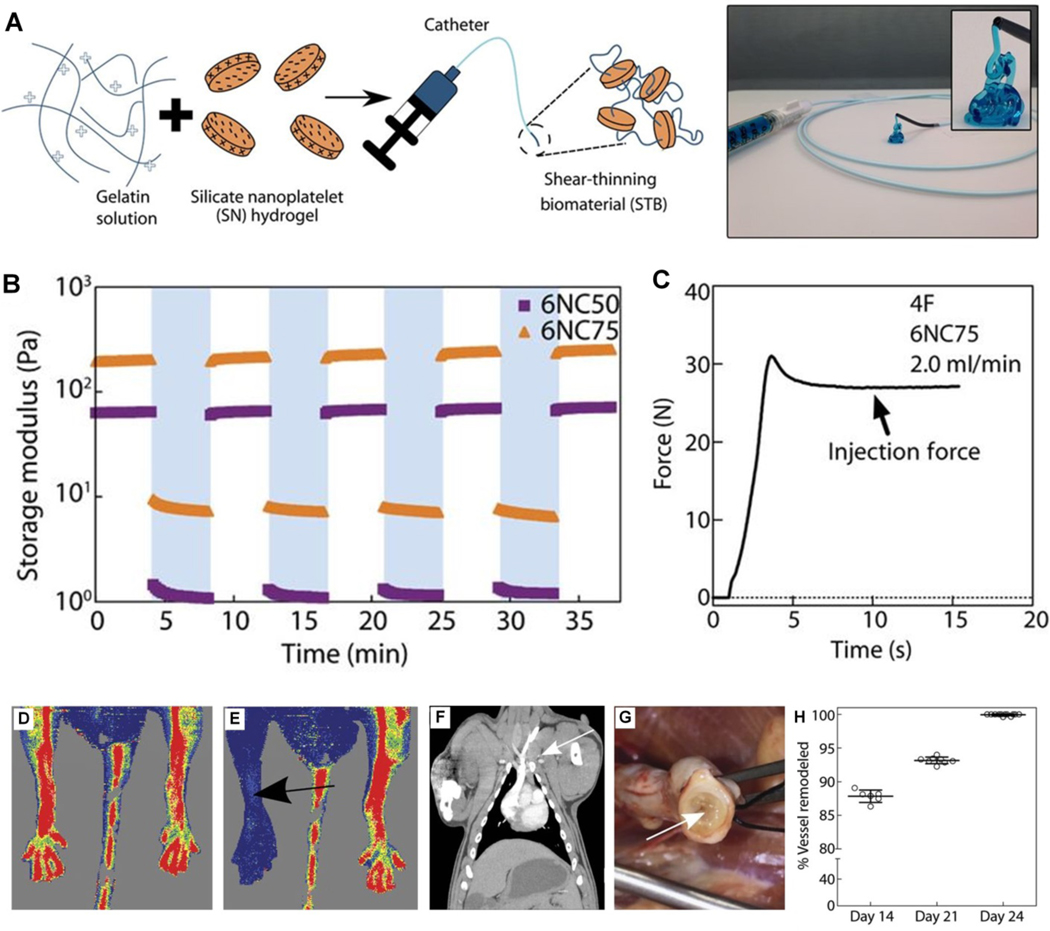
Shear-thinning biomaterial (STB) are hydrogels that are a focus in tissue engineering for a variety of treatments, such as post-myocardial infarction (MI) cardiac remodeling [160]. Recently, a STB containing gelatin and silicate nanoplatelets has been proposed for vascular embolization, giving it instant hemostatic capability [161]. This shear-thinning nanocomposite does not rely on the patient’s ability to form a thrombus the way coils do and therefore may be suitable for patients with coagulopathy or on anticoagulation therapy [161]. It has shown full occlusion in vivo with no fragmentation or non-target embolization [161]. STBs have been studied in vitro for control of hemorrhage [162], as well as embolic treatment of aortic dissection [163], warranting further investigation as a gel-based agent for endovascular embolization (Figure 8).
Figure 8.
(A) Schematic illustration of STB fabrication. STB is extruded from the catheter tip. (B) Storage moduli (G′) of 6% (w/v) STBs after repeated cycles of low and high strain. (C) A representative injection force curve to deliver STB through a catheter. Laser Doppler microperfusion imaging showing (D) hindlimb perfusion before STB injection and (E) no hindlimb perfusion after STB injection in rat model. (F) Coronal CT study confirmed no pulmonary embolism occurred in porcine model during 24 days after STB embolization. (G) Gross evaluation at 24 days shows STB (arrow) occluding the vein. (H) Percentage of vessel remodeling (replacement of STB with connective tissue) over time, (n = 6). Reproduced with permission from [161].
4.4.6. Characterization of Injectability and Potential Embolic Strength of Liquid/Gel agents
To predict embolic strength and performance of liquid/gel embolic agents, rheology is widely used to assess their physical and mechanical properties [164]. Relevant rheological parameters include viscosity, storage moduli, and loss moduli. Viscosity represents the material’s ability to resist deformation in response to applied stress. It is also a measure of the response of hydrogel to change from shear strain during injection. Storage and loss moduli represent elastic and viscous properties, respectively, of the material.
Additionally, an understanding of injection force is also of great importance since this parameter directly correlates with a physician’s ability to deliver embolic agents in a practical setting. Hagen–Poiseuille’s law (Eq. 1) can be used to correlate the injection force required to pass Newtonian fluids through a catheter/needle using a syringe. The fluid/gel has a viscosity of η, where Q is volumetric flow rate, Rs is the inner diameter of the syringe, L and Rn represent the length and inner diameter of the catheter. The term represents the injection force that requires to push the fluid through the syringe while the second term, f, represents the sliding fiction between plunger and inner barrel of the syringe [165–167]. This relationship is valid for laminar flow in a tube with Reynolds number <<2300. The diameter variations influence flow resistance more significantly than surface tension variations from different catheter materials [168]. A typical force-time or force-displacement plot consists of plunger-stopper breakloose force (the force to initiate plunger movement) and injection force (also known as dynamic glide force which is the force to sustain the movement of barrel) [169].The break-loose force is important to characterize as in practice, this is the force the physician needs to overcome to initiate liquid dispensing.
| Eq. 1 |
Since the majority of liquids are non-Newtonian, the power-law coefficient in viscosity needs to be included to account for fluid properties (e.g. shear thinning) [166]. The change of rheological properties and injection force after contrast agent mixing should also be thoroughly investigated.
5. CURRENT CHALLENGES AND FUTURE DIRECTIONS
The development of embolic materials continues to progress at an accelerating pace, driven by innovations in biomaterial design as well as an expanding clinical role for minimally invasive, image-guided interventions. A major limitation to current approaches, however, is the lack of standardized metrics with which to evaluate the relative merits of one device design over another. This lack of standardization has led to a tremendous diversity in preclinical embolic agents without clarity regarding the optimal approaches for clinical translation. Such clarity is best provided by a fundamental appreciation for the biological ramifications of each embolic technology. Unfortunately, this level of understanding is often lacking and furthermore complicated by the fact that the biological implications likely vary dramatically from not just one disease type to another but in all probability from one patient to another. Nevertheless, benchtop breakthroughs hold tremendous promise for clinical advancements by tailoring devices for the desired efficacy based upon embolization “first principles”: a) proximal versus distal embolization; b) temporary versus permanent occlusion; c) ischemia/necrosis versus viability of the perfused target area.
6. CONCLUSIONS
The materials available for endovascular embolization have evolved from autologous blood clots and muscle tissues to microspheres and gels comprised of complex polymers that can be modified for a variety of functions, including the loading of therapeutics for targeted drug delivery. While the use of a specific embolic agent may depend on the clinical scenario, one particular condition can be treated with more than one embolic agent. When considering which embolic agent to use, important considerations include: 1) is the target vessel large and/or proximal or small and/or distal and 2) does the case require permanent or temporary embolization? [170].
In the case of tumor embolization, the current focus has been primarily on loading the adjuvant agents (i.e. chemo drugs) into the embolic materials for local delivery with reduced toxicity. Additional efforts should be made to include further biofunctions, such as incorporating peptides or genes to intervene with DNA production in cancer cells. This approach will induce apoptosis of cancer cells and suppress the cancer cell generation both locally and systemically. In addition, radioactive materials have also been ensued for brachytherapy to interrupt the mitotic process and eliminate tumor tissue directly. Immunotherapy can also be used to adjust the internal environment and secrete antibodies or peptides to block the hyperplasia pathway for cancer recurrence. All embolic agents should aim to achieve multi-biofunctionality, maximize clinical outcomes, and minimize side effects. Therefore, a transition from laboratory bench to clinical testing is critical.
As a continuously growing area of bioengineering, embolic agents encompassing a diverse array of shapes, sizes, and advanced materials are available for a vast number of medical conditions. The clinical applications of these technologies will continue to expand as more tools are developed to advance the field of endovascular interventions.
Contributor Information
Courtney Y. Wang, The University of Texas Health Science Center at Houston, McGovern Medical School, 6431 Fannin St., Hourson, TX 77030, USA
Dr. Jingjie Hu, Division of Vascular and Interventional Radiology, Minimally Invasive Therapeutics Laboratory, Mayo Clinic, 13400 East Shea Blvd., Scottsdale, AZ 85259, USA
Dr. Rahul A. Sheth, Department of Interventional Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX 77054, USA
Dr. Rahmi Oklu, Division of Vascular and Interventional Radiology, Minimally Invasive Therapeutics Laboratory, Mayo Clinic, 13400 East Shea Blvd., Scottsdale, AZ 85259, USA.
References
- [1].Dawbarn RHM. The starvation operation for malignancy in the external carotid area. JAMA. 1904;43:792. [Google Scholar]
- [2].Brooks B 1930The treatment of traumatic arteriovenous fistula Southern Medical Journal 23100–106 [Google Scholar]
- [3].Vitek JJ, Smith MJ 2009The myth of the brooks method of embolization: A brief history of the endovascular treatment of carotid-cavernous sinus fistula J. Neurointerv. Surg. 1108–111 [DOI] [PubMed] [Google Scholar]
- [4].Dotter CT, Judkins MP 1964Transluminal treatment of arteriosclerotic obstruction: Description of a new technic and a preliminary report of its application Circulation 30654–670 [DOI] [PubMed] [Google Scholar]
- [5].Lubarsky M, Ray CE, Funaki B 2009Embolization agents - which one should be used when? part 1: Large-vessel embolization Semin. Interv. Radiol. 26352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kunstlinger F, Brunelle F, Chaumont P, Doyon D 1981Vascular occlusive agents AJR Am. J. Roentgenol. 136151–156 [DOI] [PubMed] [Google Scholar]
- [7].Sheth RA, Sabir S, Krishnamurthy S, et al. 2017Endovascular embolization by transcatheter delivery of particles: Past, present, and future J. Funct. Biomater. 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hoyt DB, Bulger EM, Knudson MM, et al. 1994Death in the operating room: An analysis of a multi-center experience J. Trauma 37426–432 [PubMed] [Google Scholar]
- [9].Eriksson LG, Ljungdahl M, Sundborn M, Nyman R 2008Transcatheter arterial embolization versus surgery in the treatment of upper gastrointestinal bleeding after therapeutic endoscopy failure J. Vasc. Interv. Radiol. 191413–1418 [DOI] [PubMed] [Google Scholar]
- [10].Brilstra EH, Rinkel GJ, van der Graaf Y, van Rooij WJ, Algra A 1999Treatment of intracranial aneurysms by embolization with coils: A systematic review Stroke 30470–476 [DOI] [PubMed] [Google Scholar]
- [11].Molyneux AJ, Kerr RS, Yu LM, et al. 2005International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion Lancet 366809–817 [DOI] [PubMed] [Google Scholar]
- [12].Nassiri N, Cirillo-Penn NC, Thomas J 2015Evaluation and management of congenital peripheral arteriovenous malformations J. Vasc. Surg. 621667–1676 [DOI] [PubMed] [Google Scholar]
- [13].Khurana A, Hangge PT, Albadawi H, et al. 2018The use of transarterial approaches in peripheral arteriovenous malformations (AVMs) J. Clin. Med. 7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schell SR, Camp ER, Caridi JG, Hawkins IF Jr 2002Hepatic artery embolization for control of symptoms, octreotide requirements, and tumor progression in metastatic carcinoid tumors J. Gastrointest. Surg. 6664–670 [DOI] [PubMed] [Google Scholar]
- [15].Siskin GP, Englander M, Stainken BF, Ahn J, Dowling K, Dolen EG 2000Embolic agents used for uterine fibroid embolization AJR Am. J. Roentgenol. 175767–773 [DOI] [PubMed] [Google Scholar]
- [16].Tadavarthy SM, Knight L, Ovitt TW, Snyder C, Amplatz K 1974Therapeutic transcatheter arterial embolization Radiology 11213–16 [DOI] [PubMed] [Google Scholar]
- [17].Baker MI, Walsh SP, Schwartz Z, Boyan BD 2012A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications J. Biomed. Mater. Res. B Appl. Biomater. 100B1451–1457 [DOI] [PubMed] [Google Scholar]
- [18].Vaidya S, Tozer KR, Chen J 2008An overview of embolic agents Semin. Interv. Radiol. 25204–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Davidson GS, Terbrugge KG 1995Histologic long-term follow-up after embolization with polyvinyl alcohol particles AJNR Am. J. Neuroradiol. 16843–846 [PMC free article] [PubMed] [Google Scholar]
- [20].Tanveer-Ul-Haq, Idris M, Salam B, Akhtar W, Jamil Y 2015Comparison of microcoils and polyvinyl alcohol particles in selective microcatheter angioembolization of non variceal acute gastrointestinal hemorrhage Pak J Med Sci 31751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Carli DFM, Sluzewski M, Beute GN, van Rooij WJ 2010Complications of particle embolization of meningiomas: Frequency, risk factors, and outcome AJNR Am. J. Neuroradiol. 31152–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Duvnjak S, Ravn P, Green A, Andersen PE 2016Clinical long-term outcome and reinterventional rate after uterine fibroid embolization with nonspherical versus spherical polyvinyl alcohol particles Cardiovasc. Intervent. Radiol. 39204–209 [DOI] [PubMed] [Google Scholar]
- [23].Spies JB, Allison S, Flick P, et al. 2005Spherical polyvinyl alcohol versus tris-acyl gelatin microspheres for uterine artery embolization for leiomyomas: Results of a limited randomized comparative study J. Vasc. Interv. Radiol. 161431–1437 [DOI] [PubMed] [Google Scholar]
- [24].Katsumori T and Kasahara T 2006. The size of gelatin sponge particles: Differences with preparation method Cardiovasc. Intervent. Radiol. 29 1077–1083 [DOI] [PubMed] [Google Scholar]
- [25].Abada HT and Golzarian J 2007. Gelatine sponge particles: Handling characteristics for endovascular use Tech. Vasc. and Interv. Radiol. 10 257–260 [DOI] [PubMed] [Google Scholar]
- [26].Kuyumcu G, Latich I, Hardman RL, Fine GC, Oklu R, Quencer KB 2018Gastroduodenal embolization: Indications, technical pearls, and outcomes J. Clin. Med. 7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Katsumori T, Kasahara T and Akazawa K 2006. Long-term outcomes for uterine artery embolization using gelatin sponge particles alone for symptomatic fibroids AJR Am. J. Roentgenol. 186 848–854 [DOI] [PubMed] [Google Scholar]
- [28].Katsumori T, Miura H, Arima H, et al. 2017Tris-acyl gelatin microspheres versus gelatin sponge particles in uterine artery embolization for leiomyoma Acta Radiol. 58834–841 [DOI] [PubMed] [Google Scholar]
- [29].Sone M, Arai Y, Shimizu T, et al. 2010Phase I/II multiinstitutional study of uterine artery embolization with gelatin sponge for symptomatic uterine leiomyomata: Japan interventional radiology in oncology study group study J. Vasc. Interv. Radiol. 211665–1671 [DOI] [PubMed] [Google Scholar]
- [30].Miyayama S, Yamakado K, Anai H, et al. 2014Guidelines on the use of gelatin sponge particles in embolotherapy Jpn J. Radiol. 32242–250 [DOI] [PubMed] [Google Scholar]
- [31].Oh JS, Lee HG, Chun HJ, Choi BG, Choi YJ 2015Evaluation of arterial impairment after experimental gelatin sponge embolization in a rabbit renal model Korean J. Radiol. 16133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Alvarez MM, Aizenberg J, Analoui M, et al. 2017Emerging trends in micro- and nanoscale technologies in medicine: From basic discoveries to translation ACS Nano 115195–5214 [DOI] [PubMed] [Google Scholar]
- [33].Lewis AL, Adams C, Busby W, et al. 2006Comparative in vitro evaluation of microspherical embolisation agents J. Mater. Sci. Mater. Med. 171193–1204 [DOI] [PubMed] [Google Scholar]
- [34].Bendszus M, Klein R, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L 2000Efficacy of trisacryl gelatin microspheres versus polyvinyl alcohol particles in the preoperative embolization of meniogiomas AJNR Am. J. Neuroradiol. 21255–261 [PMC free article] [PubMed] [Google Scholar]
- [35].Torres D, Costa NV, Pisco J, Pinheiro LC, Oliveira AG, Bilhim T 2019Prostatic artery embolization for benign prostatic hyperplasia: Prospective randomized trial of 100–300 μm versus 300–500 μm versus 100- to 300-μm + 300- to 500-μm embospheres J. Vasc. Interv. Radiol. 30638–644 [DOI] [PubMed] [Google Scholar]
- [36].Pelage JP, Le Dref O, Beregi JP, et al. 2003Limited uterine artery embolization with trisacryl gelatin microspheres for uterine fibroids J. Vasc. Interv. Radiol. 1415–20 [DOI] [PubMed] [Google Scholar]
- [37].Spies JB, Benenati JF, Worthington-Kirsch RL, Pelage JP 2001Initial experience with use of tris-acryl gelatin microspheres for uterine artery embolization for leiomyomata J. Vasc. Interv. Radiol. 121059–1063 [DOI] [PubMed] [Google Scholar]
- [38].Spies JB, Cornell C, Worthington-Kirsch R, Lipman JC, Benenati JF 2007Long-term outcome from uterine fibroid embolization with tris-acryl gelatin microspheres: Results of a multicenter study J. Vasc. Interv. Radiol. 18203–207 [DOI] [PubMed] [Google Scholar]
- [39].Shlansky-Goldberg RD, Rosen MA, Mondschein JI, Stavropoulos SW, Trerotola SO, Diaz-Cartelle J 2014Comparison of polyvinyl alcohol microspheres and tris-acryl gelatin microspheres for uterine fibroid embolization: Results of a single-center randomized study J. Vasc. Interv. Radiol. 25823–832 [DOI] [PubMed] [Google Scholar]
- [40].Sun X, Dai H, Guo P, Sha X 2019Biocompatibility of a new kind of polyvinyl alcohol embolic microspheres: In vitro and in vivo evaluation Mol. Biotechnol. 61610–621 [DOI] [PubMed] [Google Scholar]
- [41].Siskin GP, Dowling K, Virmani R, Jones R, Todd D 2003Pathologic evaluation of a spherical polyvinyl alcohol embolic agent in a porcine renal model J. Vasc. Interv. Radiol. 1489–98 [DOI] [PubMed] [Google Scholar]
- [42].Hwang JH, Park SW, Chang IS, et al. 2017Comparison of nonspherical polyvinylalcohol particles and microspheres for prostatic arterial embolization in patients with benign prostatic hyperplasia Biomed. Res. Int. 20178732351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Deipolyi AR, Oklu R, Al-Ansari S, Zhu AX, Goyal L, Ganguli S Safety and efficacy of 70–150 μm and 100–300 μm drug-eluting bead transarterial chemoembolization for hepatocellular carcinoma J. Vasc. Interv. Radiol. 26516–522 [DOI] [PubMed] [Google Scholar]
- [44].Lewis AL, Gonzalez MV, Lloyd AW, Hall B, et al. 2006DC bead: In vitro characterization of a drug-delivery device for transarterial chemoembolization J. Vasc. Interv. Radiol. 17335–342 [DOI] [PubMed] [Google Scholar]
- [45].Lewis AL, Gonzalez MV, Leppard SW, et al. 2007Doxorubicin eluting beads - 1: Effects of drug loading on bead characteristics and drug distribution J. Mater. Sci. Mater. Med. 181691–1699 [DOI] [PubMed] [Google Scholar]
- [46].Tang Y, Taylor RR, Gonzalez MV, Lewis AL, Stratford PW 2006Evaluation of irinotecan drug-eluting beads: A new drug-device combination product for the chemoembolization of hepatic metastases J. Control. Release 116e55–e56 [DOI] [PubMed] [Google Scholar]
- [47].Taylor RR, Tang Y, Gonzalez MV, Stratford PW, Lewis AL 2007Irinotecan drug eluting beads for use in chemoembolization: In vitro and in vivo evaluation of drug release properties Eur. J. Pharm. Sci. 307–14 [DOI] [PubMed] [Google Scholar]
- [48].de Luis E, Bilbao JI, de Ciercoles JAGJ, Martinez-Cuesta A, de Martino Rodriguez A, Lozano MD 2008In vivo evaluation of a new embolic spherical particle (HepaSphere) in a kidney animal model Cardiovasc. Intervent. Radiol. 31367–376 [DOI] [PubMed] [Google Scholar]
- [49].Liapi E, Geschwind JFH 2011Transcatheter arterial chemoembolization for liver cancer: Is it time to distinguish conventional from drug-eluting chemoembolization? Cardiovasc. Intervent. Radiol. 3437–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Delicque J, Guiu B, Boulin M, Schwanz H, Piron L, Cassinotto C 2018Liver chemoembolization of hepatocellular carcinoma using TANDEM® microspheres Future Oncol. 142761–2772 [DOI] [PubMed] [Google Scholar]
- [51].Tanaka T, Nishiofuku H, Hukuoka Y, et al. 2014Pharmacokinetics and antitumor efficacy of chemoembolization using 40 μm irinotecan-loaded microspheres in a rabbit liver tumor model J. Vasc. Interv. Radiol. 251037–1044e2 [DOI] [PubMed] [Google Scholar]
- [52].Richter G, Radeleff B, Stroszczynski C, et al. 2018Safety and feasibility of chemoembolization with doxorubicin-loaded small calibrated microspheres in patients with hepatocellular carcinoma: Results of the MIRACLE I propsective multicenter study Cardiovasc. Intervent. Radiol. 41587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liu Y, Liu J, Ai K, Yuan Q, Lu L 2014Recent advances in ytterbium-based contrast agents for in vivo x-ray computed tomography imaging: Promises and prospects Contrast Media Mol. Imaging 926–36 [DOI] [PubMed] [Google Scholar]
- [54].Hu J, Albadawi H, Chong BW, et al. 2019Advances in biomaterials and technologies for vascular embolization Adv mater 1901071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Duran R, Sharma K, Dreher MR, et al. 2016A novel inherently radiopaque bead for transarterial embolization to treat liver cancer - a pre-clinical study Theranostics 628–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Horák D, Metalová M, Śvec F, et al. 1987Hydrogels in endovascular embolization III radiopaque spherical particles, their preparation and properties Biomaterials 8142–145 [DOI] [PubMed] [Google Scholar]
- [57].Levy EB, Krishnasamy VP, Lewis AL, et al. 2016First human experience with directly image-able iodinated embolization microbeads Cardiovasc. Intervent. Radiol. 391177–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Aliberti C, Carandina R, Sarti D, et al. 2017Transarterial chemoembolization with DC bead LUMITM radiopaque beads for primary liver cancer treatment: Preliminary experience Future Oncol. 132243–2252 [DOI] [PubMed] [Google Scholar]
- [59].Doucet J, Kiri L, O’Connell K, et al. 2018Advances in degradable embolic microspheres: A state of the art review J. Funct. Biomater. 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Iezzi R, Pompili M, Rinninella E, et al. 2019TACE with degradable starch microspheres (DSM-TACE) as second-line treatment in HCC patients dismissing or ineligible for sorafenib Eur. Radiol. 29285–1292 [DOI] [PubMed] [Google Scholar]
- [61].Cheung RY, Ying Y, Rauth AM, Marcon N, Yu Wu X 2005Biodegradable dextran-based microspheres for delivery of anticancer drug mitomycin C Biomaterials 265375–5385 [DOI] [PubMed] [Google Scholar]
- [62].Forster RE, Thürmer F, Wallrapp C, et al. 2010Characterisation of physico-mechanical properties and degradation potential of calcium alginate beads for use in embolisation J. Mater. Sci. Mater. Med. 212243–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gupta B, Revagade N, Hilborn J 2007Poly(lactic acid) fiber: An overview Prog. Polym. Sci. 32455–482 [Google Scholar]
- [64].Xu Y, Kim CS, Paylor DM, Koo D 2016Polymer degradation and drug delivery in PLGA-based drug-polymer applications: A review of experiments and theories J. Biomed. Mater. Res. B Appl. Biomater. 1051692–1716 [DOI] [PubMed] [Google Scholar]
- [65].Houchin ML, Topp EM 2008Chemical degradation of peptides and proteins in PLGA: A review of reactions and mechanisms J. Pharm. Sci. 972395–2404 [DOI] [PubMed] [Google Scholar]
- [66].Owen RJ, Nation PN, Polakowski R, Biliske JA, Tiege PB, Griffith IJ 2012A preclinical study of the safety and efficacy of OcclusinTM 500 artificial embolization device in sheep Cardiovasc. Intervent. Radiol. 35636–644 [DOI] [PubMed] [Google Scholar]
- [67].Zhu Y, Zhang H, Zhang Y, et al. 2019Endovascular metal devices for the treatment of cerebrovascular diseases Adv Mater 31 [DOI] [PubMed] [Google Scholar]
- [68].Lazzaro MA, Badruddin A, Zaidat OO, Darkhabani Z, Pandya DJ, Lynch JR Endovascular embolization of head and neck tumors Front. Neurol. 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Guglielmi G, Viñuela F, Sepetka I, Macellari V 1991Electrothrombosis of saccular aneurysms via endovascular approach part 1: Electrochemical basis, technique, and experimental results J. Neurosurg. 751–7 [DOI] [PubMed] [Google Scholar]
- [70].Rodriguez JN, Hwang W, Horn J, et al. 2015Design and biocompatibility of endovascular aneurysm filling devices J. Biomed. Mater. Res. A 1031577–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gianturco C, Anderson JH, Wallace S 1975Mechanical devices for arterial occlusion AJR Am. J. Roentgenol. Radium There. Nucl. Med. 124428–435 [DOI] [PubMed] [Google Scholar]
- [72].Wallace S, Gianturco C, Anderson JH, Goldstein HM, Davis LJ, Bree RL 1976Therapeutic vascular occlusion utilizing steel coil technique: Clinical applications AJR Am. J. Roentgenol. 127381–387 [DOI] [PubMed] [Google Scholar]
- [73].Horn J, Hwang W, Jessen SL, et al. 2016Comparison of shape memory polymer foam versus bare metal coil treatments in an in vivo porcine sidewall aneurysm model J. Biomed. Mater. Res. B Appl. Biomater. 1051892–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Szikora I, Seifert P, Hanzely Z, et al. 2006Histopathologic evaluation of aneurysms treated with guglielmi detachable coils or matrix detachable microcoils AJNR Am. J. Neuroradiol. 27283–288 [PMC free article] [PubMed] [Google Scholar]
- [75].Barth KH, Strandberg JD, Kaufman SL, White RI Jr. 1978Chronic vascular reactions to steel coil occlusion devices AJR Am. J. Roentgenol. 131455–458 [DOI] [PubMed] [Google Scholar]
- [76].Liebig T, Henkes H, Fischer S, et al. 2004Fibered electrolytically detachable platinum coils used for the endovascular treatment of intracranial aneurysms Interv. Neuroradiol 105–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Vance A and Welch BG 2014. The utility of bioactive coils in the embolization of aneurysms Neurol. Res. 36 356–362 [DOI] [PubMed] [Google Scholar]
- [78].Murayama Y, Tateshima S, Gonzalez NR, Vinuela F 2003Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms Stroke 342031–2037 [DOI] [PubMed] [Google Scholar]
- [79].Taschner CA, Leclerc X, Rachdi H, Barros AM, Privo JP 2005Matrix detachable coils for the endovascular treatment of intracranial aneurysms Stroke 362176–2180 [DOI] [PubMed] [Google Scholar]
- [80].Bendszus M and Solymosi L 2006. Cerecyte coils in the treatment of intracranial aneurysms: A preliminary clinical study AJNR Am. J. Neuroradiol. 27 2053–2057 [PMC free article] [PubMed] [Google Scholar]
- [81].Cruise GM, Shum JC, Plenk H 2007Hydrogel-coated and platinum coils for intracranial aneurysm embolization compared in three experimental models using computerized angiographic and histologic morphometry J. Mater. Chem. 173965–3973 [Google Scholar]
- [82].Fanning NF, Berentei Z, Brennan PR, Thornton J 2007HydroCoil as an adjuvant to bare platinum coil treatment of 100 cerebral aneurysms Neuroradiology 49139–148 [DOI] [PubMed] [Google Scholar]
- [83].Raymond J, Klink R, Chagnon M, et al. 2017Hydrogel versus bare platinum coils in patients with large or recurrent aneurysms prone to recurrence after endovascular treatment: A randomized controlled trial AJNR Am. J. Neuroradiol. 38432–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].White PM, Lewis SC, Cholkar A, et al. 2011Hydrogel-coated coils versus bare platinum coils for the endovascular treatment of intracranial aneurysms (HELPS): A randomised controlled trial Lancet 2771655–1662 [DOI] [PubMed] [Google Scholar]
- [85].Guo X, Fan Y and Zhang J 2011. HydroSoft coil versus HydroCoil for endovascular aneurysm occlusion study: A single center experience Eur. J. Radiol. 79 e42–e46 [DOI] [PubMed] [Google Scholar]
- [86].Medsinge A, Zajko A, Orons P, Amesur N, Santos E 2014A case-based approach to common embolization agents used in vascular interventional radiology AJR Am. J. Roentgenol. 203699–708 [DOI] [PubMed] [Google Scholar]
- [87].Zhou F, Chen L, An Q, et al. 2016Novel hydrogel material as a potential embolic agent in embolization treatments Sci. Rep. 632145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Jordan O, Doelker E, Rüfenacht DA 2005Biomaterials used in injectable implants (liquid embolics) for percutaneous filling of vascular spaces Cardiovasc. Intervent. Radiol. 28561–569 [DOI] [PubMed] [Google Scholar]
- [89].Maxwell NJ, Amer NS, Rogers E, Kiely D, Sweeney P, Brady A P2007 Renal artery embolisation in the palliative treatment of renal carcinoma Br. J. Radiol. 8096–102 [DOI] [PubMed] [Google Scholar]
- [90].Fan XD, Su LX, Zheng JW, Zheng LZ, Zhang ZY 2009Ethanol embolization of arteriovenous malformations of the mandible AJNR Am. J. Neuroradiol. 301178–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hyun D, Do YS, Park KB, et al. 2013Ethanol embolotherapy of foot arteriovenous malformations J. Vasc. Surg. 581619–1626 [DOI] [PubMed] [Google Scholar]
- [92].Su LX, Jia RB, Wang DM, Lv MM, Fan XD 2015Absolute ethanol embolization of arteriovenous malformations in the periorbital region Cardiovasc. Intervent. Radiol. 38632–641 [DOI] [PubMed] [Google Scholar]
- [93].Zheng LZ, Fan XD, Zheng JW, Su LX 2009Ethanol embolization of auricular arteriovenous malformations: Preliminary results of 17 cases AJNR Am. J. Neuroradiol. 301679–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Rosen RJ and Contractor S 2004. The use of cyanoacrylate adhesives in the management of congenital vascular malformations Semin. Interv. Radiol. 21 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ishihara H, Ishihara S, Niimi J, et al. 2015The safety and efficacy of preoperative embolization of meningioma with N-butyl cyanoacrylate Interv. Neuroradiol. 21624–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ohnishi H, Miyachi S, Murao K, et al. 2016Infiltrated embolization of meningioma with dilute cyanoacrylate glue Neurol. Med. Chir. (Tokyo) 5744–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Leyon JJ, Chavda S, Thomas A, Lamin S 2016Preliminary experience with the liquid embolic material agent PHIL (precipitating hydrophobic injectable liquid) in treating cranial and spinal dural arteriovenous fistulas: Technical note J. Neurointerv. Surg. 8596–602 [DOI] [PubMed] [Google Scholar]
- [98].Taki W, Yonekawa Y, Iwata H, Uno A, Yamashita K, Amemiya H 1990A new liquid material for embolization of arteriovenous malformations AJNR Am. J. Neuroradiol. 11163–168 [PMC free article] [PubMed] [Google Scholar]
- [99].Seron A, Moine L, Laurent A, et al. 2009Am embolic gelling solution based on acrylic copolymers in ethanol for the treatment of arteriovenous malformations Biomaterials 303436–3443 [DOI] [PubMed] [Google Scholar]
- [100].Rabinov JD, Yoo AJ, Ogilvy CS, Carter BS, Hirsch JA 2013ONYX versus n-BCA for embolization of cranial dural arteriovenous fistulas J. Neurointerv. Surg. 5306–310 [DOI] [PubMed] [Google Scholar]
- [101].Murayama Y, Vinuela F, Tateshima S, Vinuela F Jr, Akiba Y 2000Endovascular treatment of experimental aneurysms by use of a combination of embolic agents and protective devices AJNR Am. J. Neuroradiol. 211726–1735 [PMC free article] [PubMed] [Google Scholar]
- [102].Food and Drug Administration. ev3 onyx liquid embolic system: Safety communication - risk of catheter entrapment. 2012 https://waybackarchive-itorg/7993/20170112164957/http://wwwfdagov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm310199htm Updated.
- [103].Qureshi AI, Mian N, Siddiqi H, et al. 2015Occurrence and management strategies for catheter entrapment with onyx liquid embolization J. Vasc. Interv. Radiol. 837–41 [PMC free article] [PubMed] [Google Scholar]
- [104].Vollherbst DF, Sommer CM, Ulfert C, Pfaff J, Bendszus M, Möhlenbruch MA 2017Liquid embolic agents for endovascular embolization: Evaluation of an established (ONYX) and a novel (PHIL) embolic agent in an in vitro AVM model AJNR Am. J. Neuroradiol. 381377–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Lamin S, Chew HS, Chavda S, et al. 2017Embolization of intracranial dural arteriovenous fistulas using PHIL liquid embolic agent in 26 patients: A multicenter study AJNR Am. J. Neuroradiol. 38127–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Verret V, Pelage JP, Wassef M, et al. 2014A novel resorbable embolization microsphere for transient uterine artery occlusion: A comparative study with trisacryl-gelatin microspheres in the sheep model J. Vasc. Interv. Radiol. 251759–1766 [DOI] [PubMed] [Google Scholar]
- [107].Hangge P, Stone J, Albadawi H, Zhang YS, Khademhosseini A, Oklu R 2017Hemostasis and nanotechnology Cardiovasc. Diagn. There. 7S267–S275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Louguet S, Verret V, Bédouet L, et al. 2014Poly(ethylene glycol) methacrylate hydrolyzable microspheres for transient vascular embolization Acta Biomater. 101194–1205 [DOI] [PubMed] [Google Scholar]
- [109].Ahmed TA and Aljaeid BM 2016. Preparation, characterization, and potential applications of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery Drug Des. Dev. and Ther. 10 483–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sezer AD, Cevher E 2012Topical drug delivery using chitosan nano- and microparticles Expert Opin. Drug Deliv. 91129–1146 [DOI] [PubMed] [Google Scholar]
- [111].Yuan XB, Yuan YB, Jiang W, et al. 2008Preparation of rapamycin-loaded chitosan/PLA nanoparticles for immunosuppression in corneal transplantation Int. J. Pharm. 349241–248 [DOI] [PubMed] [Google Scholar]
- [112].Hasanovic A, Zehl M, Reznicek G, Valenta C 2010Chitosan-tripolyphosphate nanoparticles as a possible skin drug delivery system for aciclovir with enhanced stability J. Pharm. Pharmacol. 611609–1616 [DOI] [PubMed] [Google Scholar]
- [113].Chung EY, Kim HM, Lee GH, et al. 2012Design of deformable chitosan microspheres loaded with superparamagnetic iron oxide nanoparticles for embolotherapy detectable by magnetic resonance imaging Carbohydr. Polym. 901725–1731 [DOI] [PubMed] [Google Scholar]
- [114].Wang Q, Qian K, Liu S, et al. 2015X-ray visible and uniform alginate microspheres loaded with in situ synthesized BaSO4 nanoparticles for in vivo transcatheter arterial embolization Biomacromolecules 161240–1246 [DOI] [PubMed] [Google Scholar]
- [115].Zeng J, Li L, Zhang H, et al. 2018Radiopaque and uniform alginate microspheres loaded with tantalum nanoparticles for real-time imaging during transcatheter arterial embolization Theranostics 84591–4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Pouponneau P, Leroux JC, Soulez G, Gaboury L, Martel S 2011Co-encapsulatino of magnetic nanoparticles and doxorubicin into biodegradable microcarriers for deep tissue targeting by vascular MRI navigation Biomaterials 323481–3486 [DOI] [PubMed] [Google Scholar]
- [117].Bartling SH, Budhan J, Aviv H, et al. 2011First multimodal embolization particles visible on x-ray/computed tomography and magnetic resonance imaging Investigative Radiology 46178–186 [DOI] [PubMed] [Google Scholar]
- [118].Hagit A, Soenke B, Johannes B, Shlomo M 2010Synthesis and characterization of dual modality (CT/MRI) core-shell microparticles for embolization purposes Biomacromolecules 111600–1607 [DOI] [PubMed] [Google Scholar]
- [119].Stampfl U, Sommer CM, Bellemann N, et al. 2012Multimodal visibility of a modified polyzene-F-coated spherical embolic agent for liver embolization: Feasibility study in a porcine model J. Vasc. Interv. Radiol. 231225–1231 [DOI] [PubMed] [Google Scholar]
- [120].Zhang GY, Zhou XF, Zhou XY, et al. 2013Effect of alginate-chitosan sustained release microcapsules for transhepatic arterial embolization in VX2 rabbit liver cancer model J. Biomed. Mater. Res. A 1013192–3200 [DOI] [PubMed] [Google Scholar]
- [121].Wang YJ, Molin DGM, Sevrin C, Grandfils C, van den Akker NMS, Gagliardi M, Knetsch ML, Delhaas T, Koole LH 2016In vitro and in vivo evaluation of drug-eluting microspheres designed for transarterial chemoembolization therapy Int. J. Pharm. 503150–162 [DOI] [PubMed] [Google Scholar]
- [122].Wang YJ, Benzina A, Molin DGM, van den Akker N, Gagliardi M, Koole LH Preparation and structure of drug-carrying biodegradable microspheres designed for transarterial chemoembolization therapy J. Biomater. Sci. Polym. Ed. 2677–91 [DOI] [PubMed] [Google Scholar]
- [123].Choi JW, Park JH, Cho HR, Chung JW, Kim DD, Kim HC, Cho HJ 2017Sorafenib and 2,3,5-triiodobenzoic acid-loaded imageable microspheres for transarterial embolization of a liver tumor Sci. Rep. 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Gao Y, Wang Q, Cui X, et al. 2014Controlled release of stromal cell-derived factor-1α from silk fibroin-coated coils accelerates intra-aneurysmal organization and occlusion of neck remnant by recruiting endothelial progenitor cells Int. J. Clin. Exp. Pathol. 78366–8380 [PMC free article] [PubMed] [Google Scholar]
- [125].Waldau B, Fargen KM, Mack WJ, et al. 2012Axium MicroFX coil for the completing endovascular aneurysm surgery study (ACCESS): A prospective evaluation of the safety and durability of axium MicroFX PLGA coils Interv. Neuroradiol. 18200–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Murayama Y, Viñuela F, Suzuki Y, et al. 1999Development of the biologically active guglielmi detachable coil for the treatment of cerebral aneurysms part II: An experimental study in a swine aneurysm model AJNR Am. J. Neuroradiol. 201992–1999 [PMC free article] [PubMed] [Google Scholar]
- [127].Ahuja AA, Hergenrother RW, Strother CM, Rappe AA, Cooper SL, Graves VB Platinum coil coatings to increase thrombogenicity: A preliminary study in rabbits AJNR Am. J. Neuroradiol. 14794–798 [PMC free article] [PubMed] [Google Scholar]
- [128].Dawson RC, Krisht AF, Barrow DL, Joseph GJ, Shengelaia GG, Bonner G 1995Treatment of experimental aneurysms using collagen-coated microcoils Neurosurgery 36133–140 [DOI] [PubMed] [Google Scholar]
- [129].Kallmes DF, Williams AD, Cloft HJ, Lopes MB, Hankins GR, Helm GA 1998Platinum coil-mediated implantation of growth factor-secreting endovascular tissue grafts: An in vivo study Radiology 207519–523 [DOI] [PubMed] [Google Scholar]
- [130].El Feninat F, Laroche G, Fiset M, Mantovani D 2002Shape memory materials for biomedical applications Adv. Eng. Mater. 491–104 [Google Scholar]
- [131].Herting SM, Ding Y, Boyle AJ, et al. 2019In vivo comparison of shape memory polymer foam-coated and bare metal coils for aneurysm occlusion in the rabbit elastase J. Biomed. Mater. Res. B Appl. Biomater. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kunkel R, Laurence D, Wang J, et al. 2018Synthesis and characterization of biocompatible shape memory polymers with potential applications to endovascular embolization of intracranial aneurysms J. Mech. Behav. Biomed. Mater. 88 [DOI] [PubMed] [Google Scholar]
- [133].Nash LD, Monroe MBB, Ding YH, et al. 2017Increased X-ray visualization of shape memory polymer foams by chemical incorporation of iodine motifs Polymers 9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Boyle AJ, Landsman TL, Wierzbicki MA, et al. 2016In vitro and in vivo evaluation of a shape memory polymer foam-over-wire embolization device delivered in saccular aneurysm models J. Biomed. Mater. Res. B Appl. Biomater. 1041407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Wong YS, Salvekar AV, Da Zhuang K, Liu H, Birch WR, Tay KH, Huang WM, Venkatraman SS 2016Bioabsorbable radiopaque water-responsive shape memory embolization plug for temporary vascular occlusion Biomaterials 10298–106 [DOI] [PubMed] [Google Scholar]
- [136].Monroe MBB, Easley AD, Grant K, Fletcher GK, Boyer C, Maitland DJ 2017Multifunctional shape-memory polymer foams with bio-inspired antimicrobials Chem Phys Chem 191999–2008 [DOI] [PubMed] [Google Scholar]
- [137].Rodriguez JN, Miller MW, Boyle A, et al. 2014Reticulation of low density shape memory polymer foam with an in vivo demonstration of vascular occlusion J. Mech. Behav. Biomed. Mater. 40102–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Rodriguez JN, Clubb FJ, Wilson TS, et al. 2013In vivo response to an implanted shape memory polyurethane foam in a porcine aneurysm model J. Biomed. Mater. Res. A 1021231–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Rodriguez JN, Yu YJ, Miller MW, et al. 2012Opacification of shape memory polymer foam designed for treatment of intracranial aneurysms Ann. Biomed. Eng. 40883–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Dai F, Tang L, Yang J, et al. 2009Fast thermoresponsive BAB-type HEMA/NIPAAm triblock copolymer solutions for embolization of abnormal blood vessels J. Mater. Sci. Mater. Med. 20967–974 [DOI] [PubMed] [Google Scholar]
- [141].Chung JE, Yokoyama M, Yamato M, Aoyagi T, Sakurai Y, Okano T 1999Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly(N-isopropyl-acrylamide) and poly(butylmethacrylate) J. Control. Release 62115–127 [DOI] [PubMed] [Google Scholar]
- [142].Chung JE, Yokoyama M, Okano T 2000Inner core segment design for drug delivery control of thermo-responsive polymeric micelles J. Control. Release 6593–103 [DOI] [PubMed] [Google Scholar]
- [143].Qian K, Ma Y, Wan J, et al. 2015The studies about doxorubicin-loaded p(N-isopropylacrylamide-co-butyl methylacrylate) temperature-sensitive nanogel dispersions on the application in TACE therapies for rabbit VX2 liver tumor J. Control. Release 2841–49 [DOI] [PubMed] [Google Scholar]
- [144].Wang Y, Xu N, Luo Q, et al. 2011In vivo assessment of chitosan/β-glycerophosphate as a new liquid embolic agent Interv. Neuroradiol.1787–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Chenite A, Chaput C, Wang D, Combes C, Buschmann MD, Hoemann CD, Leroux JC, Atkinson BL, Binette F, Selmani A 2000Novel injectable neutral solutions of chitosan form biodegradable gels in situ Biomaterials 212155–2161 [DOI] [PubMed] [Google Scholar]
- [146].Zhao Y, Zheng C, Wang Q, et al. 2011Permanent and peripheral embolization: Temperature-sensitive p(N-isopropylacrylamide-co-butyl methylacrylate) nanogel as a novel blood-vessel-embolic material in the interventional therapy of liver tumors Adv. Funct. Mater. 212035–2042 [Google Scholar]
- [147].Huang L, Shen M, Li R, et al. 2016Thermo-sensitive composite hydrogels based on poloxamer 407 and alginate and their therapeutic effect in embolization in rabbit VX2 liver tumors Oncotarget 773280–73291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Schmaljohann D 2006Thermo- and pH-responsive polymers in drug delivery Adv. Drug Deliv. Rev. 581655–1670 [DOI] [PubMed] [Google Scholar]
- [149].Rizwan M, Yahya R, Hassan A, et al. 2017pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications Polymers 9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Nguyen QV, Lym JS, Huynh CT, et al. 2016A novel sulfamethazine-based pH-sensitive copolymer for injectable radiopaque embolic hydrogels with potential application in hepatocellular carcinoma therapy Polym. Chem. 75805–5818 [Google Scholar]
- [151].Park W, Chen J, Cho S, et al. 2016Acidic pH-triggered drug-eluting nanocomposites for magnetic resonance imaging-monitored intra-arterial drug delivery to hepatocellular carcinoma ACS Appl. Mater. Interfaces 812711–12719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Lee BH, Bearat HH, Cheng V, et al. 2013In vitro and in vivo demonstration of physically and chemically in situ gelling NIPAAm-based copolymer system J. Biomater. Sci. Polym. Ed. 241575–1588 [DOI] [PubMed] [Google Scholar]
- [153].Bearat HH, Lee BH, Vernon BL 2012Comparison of properties between NIPAAm-based simultaneously physically and chemically gelling polymer systems for use in vivo Acta Biomater. 83629–3642 [DOI] [PubMed] [Google Scholar]
- [154].Bearat HH, Preul MC, Vernon BL 2013Cytotoxicity, in vitro models and preliminary in vivo study of dual physical and chemical gels for endovascular embolization of cerebral aneurysms J. Biomed. Mater. Res. A 1012515–2525 [DOI] [PubMed] [Google Scholar]
- [155].Becker TA, Kipke DR 2002Flow properties of liquid calcium alginate polymer injected through medical microcatheters for endovascular embolization J. Biomed. Mater. Res. 61533–540 [DOI] [PubMed] [Google Scholar]
- [156].Becker TA, Preul MC, Bichard WD, Kipke DR, McDougall CG 2007Preliminary investigation of calcium alginate gel as a biocompatible material for endovascular aneurysm embolization in vivo Neurosurgery 601119–1127 [DOI] [PubMed] [Google Scholar]
- [157].Qin L, Mei L, Shan Z, et al. 2016Phytantriol based liquid crystal provide sustained release of anticancer drug as a novel embolic agent Drug Dev. Ind. Pharm. 42307–316 [DOI] [PubMed] [Google Scholar]
- [158].Han K, Pan X, Chen M, et al. 2010Phytantriol-based inverted type bicontinuous cubic phase for vascular embolization and drug sustained release Eur J. Pharm. Sci. 41692–699 [DOI] [PubMed] [Google Scholar]
- [159].Du LR, Lu XJ, Guan HT, et al. 2014Development and evaluation of liquid embolic agents based on liquid crystalline material of glyceryl monooleate Int. J. Pharm. 471285–296 [DOI] [PubMed] [Google Scholar]
- [160].Johnson TD, Christman KL 2013Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction Expert Opin. Drug Deliv. 1059–72 [DOI] [PubMed] [Google Scholar]
- [161].Avery RK, Albadawi H, Akbari M, et al. 2016An injectable shear-thinning biomaterial for endovascular embolization Sci Transl. Med. 8365ra156 [DOI] [PubMed] [Google Scholar]
- [162].Gaharwar AK, Avery RK, Assmann A, et al. 2014Shear-thinning nanocomposite hydrogels for the treatment of hemorrhage ACS Nano 89833–9842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Moore MJ, Malaxos L, Doyle BJ 2019Development of a shear-thinning biomaterial as an endovascular embolic agent for the treatment of type B aortic dissection J. Mech. Behav. Biomed. Mater. 9966–77 [DOI] [PubMed] [Google Scholar]
- [164].Chen MH, Wang LL, Chung JJ, Kim YH, Atluri P, Burdick JA 2017Methods to assess shear-thinning hydrogels for application as injectable biomaterials ACS Biomater. Sci. Eng. 33146–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Overcashier DE, Chan EK, Hsu CC 2006Technical considerations in the development of prefilled syringes for protein products American Pharmaceutical Review 977–83 [Google Scholar]
- [166].Allmendinger A, Fischer S, Huwyler B, Mahler HC, Schwarb E, Zarraga IE, Mueller R 2014Rheological characterization and injection forces of concentrated protein formulations: An alternative predictive model for non-Newtonian solutions Eur. J. Pharm. Biopharms. 87318–328 [DOI] [PubMed] [Google Scholar]
- [167].Sutera SP, Skalak R 1993The history of Poiseuille’s law Annu. Rev. Fluid Mech. 251–19 [Google Scholar]
- [168].Becker TA, Kipke DR, Brandon T 2001Calcium alginate gel: A biocompatible and mechanically stable polymer for endovascular embolization J. Biomed. Mater. Res. 5476–86 [DOI] [PubMed] [Google Scholar]
- [169].Cilurzo F, Selmin F, Minghetti P, Adami M, Bertoni E, Lauria S, Montanari L 2011Injectability Evaluation: An Open Issue AAPS PharmSciTech 12604–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Coldwell DM, Stokes KR, Yakes WF 1994Embolotherapy: Agents, clinical applications, and techniques Radiographics 14623–643 [DOI] [PubMed] [Google Scholar]