Abstract
The aim of this cross-sectional study was to assess the associations of burnout with cortisol parameters in 197 police officers from the Buffalo Cardio-Metabolic Occupational Police Stress (BCOPS) study (2010–2014). The Maslach Burnout Inventory-General Survey assessed depersonalization, exhaustion, and professional efficacy. Officers provided salivary cortisol samples collected upon awakening, and 15, 30, and 45 min thereafter as well as three additional samples at lunchtime, dinnertime, and bedtime. Total area under the curve with respect to increase (AUCWI for waking and AUCDI for diurnal), total area under the curve with respect to ground (AUCWG for waking and AUCDG for diurnal), and diurnal slope were determined and used in this study. Unadjusted and adjusted (age, sex, and race/ethnicity) associations were examined using linear regression. The mean age of the officers was 48 years and 72% were males. The depersonalization component of burnout was negatively associated with AUCDG (β = −108.4; p = 0.036). Similarly, as exhaustion increased, AUCWI (β = −9.58, p = 0.038), AUCDG (β = −114.7, p = 0.029) and the diurnal slope (β = −0.000038; p = 0.017) decreased. The Professional efficacy was not associated with any of the cortisol parameters. These results suggest that certain characteristics of burnout may be associated with diminished cortisol secretion in this group of urban police officers. Our findings add to previous studies examining associations of burnout with the cortisol awakening response. Future longitudinal studies are needed to evaluate the temporal relationship between burnout and these cortisol parameters.
Keywords: CAR, Diurnal, Cortisol, Police officers, Burnout
Highlights
-
•
Burnout component scores and cortisol parameter values did not differ significantly between male and female officers.
-
•
Adjusted models demonstrated significant negative associations between the burnout components and cortisol parameters.
-
•
As depersonalization increased, AUCDG decreased.
-
•
As exhaustion increased, AUCWI, AUCDG, and the diurnal slope decreased.
-
•
Professional efficacy was not associated with either AUCWI or AUCDG.
1. Introduction
Police officers are frequently and repeatedly exposed to stressful events [1]. Chronic stress is associated with a number of biological and psychological outcomes [2,3]. Chronic stress can result in continuing activation of the stress response system, the hypothalamic-pituitary-adrenal (HPA) axis, with concomitant abnormally high levels of physiologically active substances such as catecholamines and cortisol as well as psychological issues including burnout. Several factors contribute to high stress and burnout in police. The goal of this cross-sectional study was to assess the association between burnout and cortisol parameters in police officers.
Police officers work under extreme circumstances [4]. They are regularly exposed to occupational hazards including needle stick injuries, HIV, body fluids and infections via exposure to blood or by being bitten. Officers can also sustain injuries when being shot at, arresting suspects, or while pursuing fleeing suspects. Vehicular accidents are also occupational hazards officers’ experience, both from being hit from lane deviations or during a police chase [4]. These factors along with administrative pressure, lack of resources, and low salary are related to physical and mental health issues [4]. One consequence of these issues are higher rates of burnout [1,[5], [6], [7], [8], [9], [10]].
Burnout is a deleterious form of impairment resulting from chronic stress in the workplace. It is characterized by three components including depersonalization, reduced professional efficacy, and exhaustion [11]. Depersonalization describes a lack of empathy, cynicism, and hostility toward members of the public. Reduced professional efficacy involves directing negative emotions towards oneself, resulting in feelings of inadequacy and a sense of failure. Exhaustion is a means of self-preservation arising from fatigue and emotional depletion. In a study conducted in Israeli police officers, the mean burnout score was found to be higher than the national average (3.05 vs 2.8) [12,13]. In police, burnout is associated with organizational and operational stress including lack of support from the community or the lack of promotion [8]. Exhaustion and depersonalization positively correlated to a number of psychological and somatic symptoms [14]. Reduced professional efficacy was negatively correlated to factors such as anxiety, insomnia, severe depression, and somatic symptoms. Among police the perception of danger and unfairness were also associated with burnout [15]. Detrimental consequences of burnout can include substance abuse, decreased quality of service, and impaired mental and physical health. Research also indicates that burnout may be associated with HPA axis dysregulation and level of cortisol [16,17].
Glucocorticoid hormones regulate the central nervous system and are controlled by the HPA axis neuroendocrine system [18]. Cortisol is the primary glucocorticoid in humans. The HPA axis receives direct and indirect neural input from a number of brain regions and is responsible for the secretion of cortisol, which is involved in energy mobilization, metabolic processes, immune response, and arousal [19]. The HPA axis and associated cortisol secretion has three distinct temporal patterns, which likely ensures optimal cellular, molecular, and systems level functioning and includes basal ultradian pulses, basal circadian fluctuation and stimulus [19]. Basal cortisol levels fluctuate with circadian rhythms. It also follows a diurnal pattern in which it is observed to rise around dawn with a peak surge upon awakening, referred to as the cortisol awakening response (CAR), then slowly decrease across the course of the day, leveling out around bedtime. The CAR is a discreet component of the cortisol circadian cycle that is not related to the cortisol secretion pattern observed across the rest of the day, but this diurnal pattern is considered to be a biomarker of HPA axis activity [20,21]. The HPA axis is one of the biological systems that responds to stress, with cortisol being a key component in that stress response system [22]. Acute stress typically elevates cortisol levels for several hours, while chronic stress may result in depleted cortisol and failure in cortisol functioning. Research evaluating both CAR and diurnal metrics have found that each may be associated with mental and physical health issues such as posttraumatic stress disorder, depression, fatigue, cardiovascular disease, and burnout [16,17,[23], [24], [25], [26]].
Several studies have evaluated the relationship between burnout and cortisol in various populations [16,26,27]. Studies in medical professionals found that higher burnout symptoms were associated with higher cortisol, including higher diurnal salivary cortisol measured six times over the course of the day [28,29]. Marchand et al. [25] found that specific time points of the diurnal cortisol profile were associated with burnout, depressive symptoms, and psychological distress in a random sample of Canadian workers. Similarly, a review article reported a negative relationship between CAR and burnout [16]. However, there are also studies that have not found a relationship between burnout and cortisol [26,27,30]. Furthermore, to our knowledge, there are no studies that have evaluated this relationship in police officers. To address this knowledge gap, the aim of this study was to determine if burnout was associated with atypical cortisol parameters in police officers.
2. Methods
2.1. Participants
The data for this study were collected as part of the Buffalo Cardio-Metabolic Occupational Police Stress (BCOPS) study at the Center for Health Research at the School of Public Health and Health Professions, State University of New York in Buffalo, NY. All active duty police officers from the Buffalo, NY police department were invited to participate in this study; a total of 281 officers participated and were examined between 2011 and 2014. There were no specific inclusion criteria other than being a sworn police officer and willing to participate in the study. There were no exclusion criteria. Questionnaires were administered to collect demographic, lifestyle information, and psychosocial measures. Participants also provided medical history information and all officers completed a clinical evaluation including blood chemistry and blood pressure. Of the 281 officers who participated, 197 police officers had complete data on the variables of interest, including burnout and cortisol. All participants provided informed consent and the study was approved by the State University of New York at Buffalo Institutional Review Board.
2.2. Measures
2.2.1. Burnout
The Maslach Burnout Inventory (MBI) assessed job-related burnout [11]. It is a 16-item instrument that records the frequency of job-related feelings, with each item ranging from 0 (never) to 6 (every day). The MBI has three components: depersonalization, exhaustion, and professional efficacy. Depersonalization refers to indifference or distance toward work. High levels of depersonalization are a coping mechanism for feeling overwhelmed at work. Exhaustion refers to feelings of being overextended and high levels of fatigue at work. Higher levels of exhaustion indicate higher burnout with work. Professional efficacy measures feelings of accomplishment and competence in one’s work. A high degree of burnout is reflected in high exhaustion and depersonalization scores and in a low professional efficacy score. Cronbach alpha was used to measure internal consistency for the Maslach sub-scales, which were 0.86, 0.91, and 0.78 for depersonalization, exhaustion and professional efficacy sub-scales, respectively.
2.2.2. Cortisol
Cortisol was measured from saliva samples. During the clinic visit (first day of data collection), the saliva samples consisted of those taken pre-and-post blood draw, a baseline saliva sample prior to ingestion of a protein challenge followed by four additional samples after the protein challenge (taken at 15 min interval). Those results are not shown here. However, on the second day (day 2), seven additional saliva samples were collected: four samples within the first hour after awakening, and three additional samples taken at lunch time, dinner, and bedtime (Supplemental Table 1). These samples were used for the assessment of CAR and diurnal cortisol in this study. For the assessment of CAR, subjects were instructed to collect saliva samples immediately upon awakening, and 15, 30, and 45 min thereafter. No specific protocols were placed for the lunch, dinner, and bedtimes except that the participants take the saliva samples at the respective times. The first morning saliva sample was to be collected immediately after waking before getting out of bed. During their clinic examination, participants were provided with a pre-printed log sheet on which they noted their time of waking, and times of each of the cortisol samples. Participants were also instructed on proper saliva sampling techniques, including adherence to specific sampling times, which were reiterated on the log sheet given to each participant. The officers were required to come to the clinic examination on the last day of their off workdays. The typical work schedule included 4 days of work, 4 days off work, 4 days of work, and 3 days off work. Therefore, the awakening salivary cortisol samples were collected the day after the clinic examination when the officers had been off-duty for at least 3 days. To avoid contamination of saliva with food or blood caused by micro-injuries of the oral cavity, participants were asked to refrain from taking stimulant medication, smoking, eating and drinking, and brushing their teeth before completing salivary sampling. The wakening saliva samples were collected during a single day and occurred on the day after the clinic examination. Participants were provided Medication Events Monitoring System (MEMS) bottles with salivettes (Sarstedt, USA), a commercially available collection device consisting of a dental roll and a centrifuge tube, for the collection of saliva samples.
At the designated collection time, the officers removed the dental roll from the centrifuge tube and placed it in their mouth for approximately 2 min allowing for saturation of the roll. The roll was then returned to the tube and samples were returned to the clinic and subsequently sent to the laboratory. The officers were told that times when the MEMS cap is opened and closed would be recorded by the device, thereby reinforcing protocol adherence. Upon delivery the tubes were centrifuged to provide a non-viscous saliva sample for assay of cortisol. Samples were stored in a freezer at −20 °C until sent to the Technical University of Dresden (Germany) for analysis. After thawing, salivettes were centrifuged at 3000 rpm for 5 min, which resulted in a clear supernatant of low viscosity. Salivary concentrations were measured using commercially available chemiluminescence immunoassay with high sensitivity (IBL International, Hamburg, Germany). Sample and reagent handling were semi-automated using a liquid handling robot (Genesis, Tecan, Switzerland) and quality control samples of low, medium, and high cortisol concentrations were run on each microtiter plate assayed. The intra and inter assay coefficients for cortisol were both below 8%.
In terms of data cleaning of the cortisol values (at all time points) were set to missing if the value was ≥75 nmol/l while measurements between 0 and 0.44 were set to 0.44. When we suspected the accuracy of the dates and times of salivary sample collection reported by the study, we utilized information from MEMS CAP and Actiwatch (wrist worn device for assessment of sleep) to correct the dates and times of collection. Actiwatch data were used to verify and correct the date and time an officer woke up, date and time an officer went to bed (when we had some concerns regarding the accuracy of the dates and times reported by the study participants). Similarly, MEMS CAP date and times were used in some instances to verify or correct dates and times the subject reported. For the current manuscript, only participants with non-missing cortisol values and times of collection at all seven time points were utilized.
The four salivary cortisol samples and the corresponding times of collection were used to derive the following four parameters: total area under the curve with respect to ground for waking samples (AUCWG), total area under the curve with respect to increase for waking samples (AUCWI), total area under the curve with respect to ground for diurnal samples (AUCDG), and total area under the curve with respect to increase for diurnal samples (AUCDI). The derivation of the waking parameters was based on the four waking samples and is illustrated in Supplemental Fig. 1 while the derivation of the diurnal samples was based on all seven samples. Diurnal slope (SP4) was calculated using data only from the first waking, lunch, dinner, and bedtime samples.
2.3. Statistical analysis
Descriptive statistics were calculated to characterize the study population. Pearson correlation analysis was conducted to evaluate associations between study variables. Unadjusted and multivariable adjusted linear regression models were used to assess the association between the cortisol parameters AUCWI, AUCWG, AUCDI, AUCDG, SP4, and the three burnout components of depersonalization, exhaustion, and professional efficacy. Models were adjusted for age, sex and race/ethnicity. For descriptive purposes, we also created a graphical representation examining the relationship between burnout and salivary cortisol. To represent the timeline of salivary cortisol collection on the X-axis, we derived a variable called time since awakening as the number of minutes elapsed from the baseline sample (sample taken upon first awakening) to each subsequent sample; time 0 represents the baseline sample. We then divided the participants into two groups based on median values of the burnout components (Low: ≤ median and High: > median). For each group, the mean value of cortisol at each sampling point was computed using data from all subjects in that group and displayed on a Y-axis. These figures were created for both the CAR and diurnal response. All analyses were performed using SAS, version 9.4 [31].
3. Results
The mean age of the officers was 48 years and 72% were males (Table 1). The majority of officers were married (69.9%), never smoked (57.1%), and held the rank of police officer (52.3%). Most of the officers had some college education (48.7%). There were significant differences between men and women officers with regards to race/ethnicity, smoking status, marital status, and the amount of alcohol drunk per week, but not across any of the cortisol measures or burnout components.
Table 1.
Descriptive characteristics of the participating officers by gender.
|
Characteristics |
Total (N = 197) N (%) |
Female (N = 56) N (%) |
Male (N = 141) N (%) |
p-value |
|---|---|---|---|---|
| Race/Ethnicity | 0.005 | |||
| Caucasian | 155 (79.1) | 37 (66.1) | 118 (84.3) | |
| African American | 41 (20.9) | 19 (33.9) | 22 (15.7) | |
| Education | 0.630 | |||
| High school/GED | 15 (7.6) | 3 (5.3) | 12 (8.5) | |
| College <4 years | 96 (48.7) | 30 (53.6) | 66 (46.8) | |
| College ≥4 years | 86 (43.7) | 23 (41.1) | 63 (44.7) | |
| Smoking status | 0.026 | |||
| Current | 18 (9.2) | 7 (12.7) | 11 (7.8) | |
| Former | 66 (33.7) | 25 (45.5) | 41 (29.1) | |
| Never | 112 (57.1) | 23 (41.8) | 89 (63.1) | |
| Rank | 0.163 | |||
| Police officer | 103 (52.3) | 35 (62.5) | 68 (48.2) | |
| Sergeant/Lieutenant | 39 (19.8) | 10 (17.9) | 29 (20.6) | |
| Captain/Detective | 55 (27.9) | 11 (19.6) | 44 (31.2) | |
| Marital status | 0.002 | |||
| Single | 19 (9.7) | 8 (14.3) | 11 (7.9) | |
| Married | 137 (69.9) | 29 (51.8) | 108 (77.1) | |
| Divorced | 40 (20.4) | 19 (33.9) | 21 (15.0) | |
| Mean (SD) |
Mean (SD) |
Mean (SD) |
||
| Age (years) | 48.3 (7.9) | 47.7 (6.7) | 48.6 (8.3) | 0.480 |
| Alcohol (drinks/week) | 4.9 (8.3) | 2.9 (4.6) | 5.7 (9.2) | 0.033 |
| Cortisol Measures | ||||
| Waking AUCWI | 120.5 (479.2) | 166.2 (397.3) | 102.3 (508.3) | 0.400 |
| Waking AUCWG | 921.2 (770.9) | 901.7 (430.3) | 929.0 (871.2) | 0.823 |
| Diurnal AUCDI | −6737.8 (10275.4) | −6242.3 (9055.4) | −6934.5 (1,0745.5) | 0.671 |
| Diurnal AUCDG | 8644.4 (5444.8) | 8525.9 (5097.4) | 8691.5 (5593.6) | 0.848 |
| SP4 | −0.0024(0.002) | −0.0025(0.001) | −0.0023(0.002) | 0.534 |
| Burnout components | ||||
| Depersonalization | 11.8 (7.8) | 12.2 (7.7) | 11.7 (7.9) | 0.644 |
| Exhaustion | 11.6 (7.6) | 12.8 (8.5) | 11.1 (7.1) | 0.159 |
| Professional efficacy | 28.8 (6.2) | 29.4 (5.5) | 28.6 (6.4) | 0.377 |
Note: Waking AUCWI: Area under the curve with respect to increase from the awakening sample (samples on awakening, and 15, 30, and 45 min after).
Waking AUCWG: Total area under the curve for waking samples with respect to ground; Diurnal AUCDI: Total area under the curve with respect to increase for diurnal samples; Diurnal AUCDG: Total area under the curve with respect to ground for diurnal samples (on awakening, lunchtime, dinnertime, and bedtime).
SP4: Diurnal slope estimated by fitting a regression model using the first waking sample, lunch, dinner and bedtime samples (4 samples).P-values were obtained from Student’s t-tests for continuous variables and from Chi-square/Fisher’s exact tests for categorical variable.
Correlation coefficients indicated that exhaustion was positively correlated to depersonalization (r = 0.63; p < 0.001; Table 2). Professional efficacy was negatively correlated to depersonalization (r = −0.40; p < 0.001). There was a negative correlation between the diurnal slope (SP4) and exhaustion (r = −0.17; p < 0.05). AUCWI was positively correlated with SP4 (r = 0.23; p < 0.01). In contrast, AUCWG was negatively correlated with SP4 (r = −0.17; p < 0.05). AUCDG was negatively correlated with exhaustion (r = −0.16; p < 0.05) and depersonalization (r = −0.16; p < 0.05), but positively correlated to SP4 (r = 0.18; p < 0.05), AUCWI (r = 0.15; p < 0.05), and AUCWG (r = 0.36; p < 0.01). AUCDI was positively correlated to SP4 (r = 0.47; p < 0.001), AUCWI (r = 0.55; p < 0.01), and negatively correlated to AUCWG (r = −0.330 p < 0.01). Age was negatively correlated to depersonalization (r = −0.20; p < 0.01). Being Caucasian was positively correlated to depersonalization (r = 0.16; p < 0.05) and negatively correlated to being female (r = - 0.19; p < 0.01).
Table 2.
Pearson correlation coefficients for the main variables of interest.
| Exhaustion | Depersonalization | PE | SP4 | AUCWI | AUCWG | AUCDG | AUCDI | Age | Female† | Caucasian† | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhaustion | – | – | – | – | – | – | – | – | – | – | – |
| Depersonalization | 0.63∗∗∗ | – | – | – | – | – | – | – | – | – | – |
| Professional Efficacy (PE) | −0.09 | −0.40∗∗∗ | – | – | – | – | – | – | – | – | – |
| SP4 | −0.17∗ | −0.09 | −0.002 | – | – | – | – | – | – | – | – |
| AUCWI | −0.14 | −0.06 | −0.04 | 0.23∗∗ | – | – | – | – | – | – | – |
| AUCWG | 0.06 | −0.09 | 0.06 | −0.17∗ | −0.08 | – | – | – | – | – | – |
| AUCDG | −0.16∗ | −0.16∗ | 0.01 | 0.18∗ | 0.15∗ | 0.36∗∗ | – | – | – | – | – |
| AUCDI | −0.07 | 0.03 | −0.06 | 0.47∗∗∗ | 0.55∗∗ | −0.33∗∗ | 0.14 | – | – | – | – |
| Age | −0.13 | −0.20∗∗ | 0.17 | −0.03 | −0.03 | 0.05 | 0.08 | −0.08 | – | – | – |
| Female† | 0.10 | 0.03 | 0.06 | 0.04 | 0.06 | −0.02 | −0.01 | 0.03 | −0.05 | – | – |
| Caucasian† | 0.06 | 0.16∗ | −0.02 | −0.06 | −0.03 | 0.02 | 0.03 | −0.09 | 0.03 | −0.19∗∗ | – |
Note: †Sex (Female = 1, Male = 0); Race/Ethnicity (Caucasian = 1, Black = 0); ∗p-value <0.05, ∗∗p-value <0.01, ∗∗∗p-value <0.001.
Results from the regression models indicate that the depersonalization component of burnout was negatively associated with AUCDG (β = −108.4; p = 0.036); as depersonalization increased AUCDG decreased (Table 3). Similarly, as exhaustion increased, AUCWI (β = −9.58, p = 0.038), AUCDG (β = −114.7, p = 0.029), and SP4 (β = −0.000038; p = 0.017) decreased. Reduced professional efficacy was not associated with any of the cortisol parameters.
Table 3.
Adjusted associations of burnout components with cortisol parameters.
|
Burnout Components |
AUCWI β [SE] |
AUCWG β [SE] |
AUCDI β [SE] |
AUCDG β [SE] |
SP4 β [SE] |
|---|---|---|---|---|---|
| Depersonalization | −4.40 [4.6] | −8.73 [7.4] | 44.3 [97.7] | −108.4 [51.4]∗ | −0.000019 [0.000016] |
| Exhaustion | −9.58 [4.6]∗ | −6.12 [7.5] | −110.5 [98.6] | −114.7 [51.9]∗ | −0.000038 [0.00002]∗ |
| Professional Efficacy | −3.17 [5.7] | 7.09 [9.2] | −90.4 [121.7] | 0.579 [64.8] | 0.0000018 [0.00002] |
Note: β = regression coefficient, SE = standard error. ∗ p-value <0.05.
Regression coefficients and respective p-values were obtained from linear regression; All models were adjusted for age, sex and race/ethnicity.
AUCWI: Area under the curve with respect to increase from the waking sample.
AUCWG: Total area under the curve for waking samples with respect to ground.
AUCDI: Area under the curve with respect to increase for diurnal sample.
AUCDG: Total area under the curve with respect to ground for diurnal sample.
SP4: Diurnal slope estimated by fitting a regression model using the first waking sample, lunch, dinner and bedtime samples (4 samples).
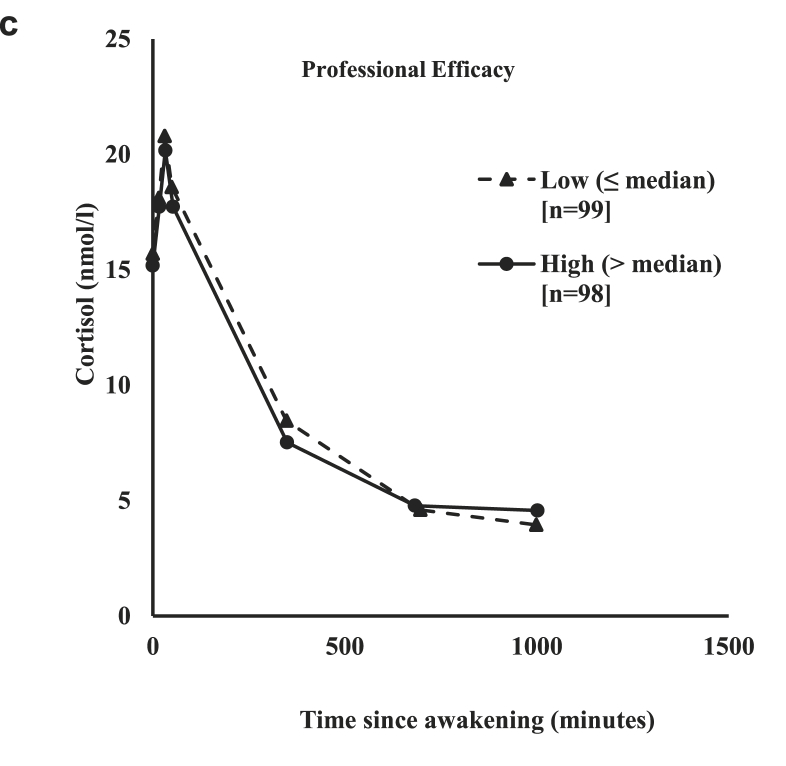
For demonstrative purposes, Fig. 1a, Fig. 1b, Fig. 1ca–c shows the mean cortisol level by low and high burnout components. As can be seen in all three figures cortisol levels peaked within 40 min of waking (Fig. 1a, Fig. 1b, Fig. 1ca–c). In all three figures, officers who reported high depersonalization, exhaustion, and professional efficacy had lower cortisol levels than those officers who reported low levels of depersonalization, exhaustion, and professional efficacy.
Fig. 1a.
Profile of the cortisol awakening response (CAR) by low and high categories of depersonalization.
Fig. 1b.
Profile of the cortisol awakening response (CAR) by low and high categories of exhaustion.
Fig. 1c.
Profile of the cortisol awakening response (CAR) by low and high categories of professional efficacy.
The mean level of diurnal cortisol was also plotted by high and low levels of exhaustion, depersonalization, and professional efficacy (Fig. 2a, Fig. 2b, Fig. 2ca–c). As can be seen in all three figures, diurnal cortisol increased with waking then declined sharply across the course of the day, leveling off after around the 6 p.m. measurement. Officers who reported higher exhaustion had slightly lower and steeper cortisol levels than those officers who reported lower levels of exhaustion (Fig. 2a). However, for the most part there was very little difference between mean diurnal cortisol levels in officers who reported high versus low exhaustion (Fig. 2a), depersonalization (Fig. 2b), and high versus low professional efficacy (Fig. 2c).
Fig. 2a.
Profile of the diurnal cortisol response by low and high categories of exhaustion.
Fig. 2b.
Profile of the diurnal cortisol response by low and high categories of depersonalization.
Fig. 2c.
Profile of the diurnal cortisol response by low and high categories of professional efficacy.
4. Discussion
Studies that have evaluated the effects of burnout on cortisol levels are inconsistent and to our knowledge none have evaluated cortisol levels in police officers, a highly stressed and potentially burnt out population [26,32]. The results indicate that cortisol levels, particularly the awakening cortisol profile (AUCWI), the total daily hormonal output (AUCDG), and diurnal slope may be negatively associated with burnout, particularly with depersonalization and exhaustion. Thus, these findings support the theory that higher burnout corresponds to a downregulation in the CAR and a flattening of diurnal cortisol. Our results are similar to several studies that have also found a negative relationship between burnout and cortisol [17,21,33,34], but contrast with a number of studies that have found a positive association, or no association between burnout and cortisol [29,30,35].
In a pilot study, Mommersteeg et al. [33], found that individuals with burnout had significantly lower salivary cortisol levels after awakening compared to controls, but there were no differences in the rise of cortisol between the two groups. Oosterholt et al. [[34], [36]] compared salivary cortisol levels in individuals with clinical burnout, non-clinical burnout, and healthy controls before and after treatment [34,36]. The authors reported that the CAR AUCG at 30 min post awakening was significantly lower in the clinical and non-clinical burnout groups compared to the control group [36]. At the second time point, following treatment for burnout, the AUCG30 was significantly lower only among the non-clinical burnout group compared to the healthy control group [34]. Similarly, research evaluating CAR and burnout in teachers found that the CAR pattern was lower in the high burnout teachers compared to teachers with low burnout [37], and that higher emotional exhaustion and reduced professional efficacy (lack of accomplishment) specifically were associated with lower CAR [38]. These results support our findinga and also suggest that high burnout, and exhaustion in particular, may be associated with a blunted CAR response.
In contrast to our results, there are also a number of studies that do not support an association between CAR and burnout, or have found an increased CAR response in association with burnout [26,30,35,39,40]. Chida et al. [16] conducted a meta-analysis using 147 studies to evaluate the relationship between several psychosocial factors including burnout and CARi (increase in cortisol following awakening) and CARauc (volume released over the waking period). Overall, they found a negative association between fatigue, burnout, or exhaustion and CARi. However, this difference did not reach statistical significance and two of the studies included in the meta-analysis, in fact, showed no association between burnout and the CAR [39,40].
Research indicates that the CAR response is under separate control from the cortisol output observed over the rest of the day [41]. For this reason, we also evaluated diurnal cortisol output. The AUCDG was negatively associated with depersonalization and exhaustion. The diurnal slope was also negatively associated with exhaustion. Similarly, burnout was reported to be negatively associated with cortisol levels at 2pm, 4pm and bedtime, but not overall cortisol concentrations [17,25]. These results were supported by another study that also evaluated salivary cortisol concentrations across the course of the day and found that burnout was negatively correlated with cortisol at 2pm, 4pm, and bedtime [36]. These results suggest that in individuals with burnout, diurnal cortisol might be down-regulated across the course of the day, resulting in a flattened curve. However, longitudinal studies are needed to clarify the temporal relationship between burnout and diurnal cortisol patterns.
In contrast to our findings, burnout has also been found to be associated with higher cortisol [29]. Measured salivary cortisol levels at four time points across the course of the day and found that people with at least two burnout components had higher levels of cortisol at each time point compared to individuals with either one or no burnout components [29]. In another study, cortisol measured six times across the course of the day, found that cortisol secretion was lower in individuals without burnout compared to those with high scores on at least one burnout component [28].
While most of these studies evaluated salivary cortisol, two studies, one that evaluated urine cortisol levels found that urinary free cortisol was significantly lower in patients with burnout and remained reduced following four months of treatment [42]. In contrast, cortisol measured in hair was positively associated with higher burnout symptoms particularly reduced professional efficacy [43]. However, not all studies report an association between HPA axis function and burnout [32,44]. Differences in the results across some of these studies and ours may be the result of differences in the composition of the study population, timing and type of cortisol samples, control of covariates, sample size, or burnout severity [20,45].
Our study indicates that discrepancies in cortisol levels are associated with the burnout components depersonalization and exhaustion. Similar results were observed in Marchand et al. [17] who suggested that the MBI’s emotional exhaustion may be a key component in HPA axis dysregulation. The fact that we observed associations with both AUCWI and diurnal cortisol levels suggest that it may be important to measure both when evaluating the association between mental and physical health and cortisol levels. Furthermore, our results indicate that evaluating the contribution of burnout components (depersonalization, exhaustion, professional efficacy) rather than total burnout alone may be important and may explain some of the discrepancies observed across studies. Discrepancies across studies may also be a result of which CAR is measured. Chida et al. [16] suggest that CARi (increase) may be a better measure of HPA activity than CARauc (total volume), because CARauc will be large even if the increase in cortisol upon awakening is small, and there is some evidence that the CARauc is correlated to the 12- hour diurnal pattern. We found that AUCWI, rather than the AUCWG was significantly associated with exhaustion and therefore may be a better measure of HPA activity and burnout. However, this hypothesis is not consistently supported in the literature, warranting more research [16,28,36].
4.1. Strengths and limitations
This study has several strengths including a unique occupational cohort, a unique population, a high response rate, the Actiwatch and MEMs cap to independently verify the times of sample collection, and the use of well-standardized and validated psychosocial instruments. However, when interpreting the results, there are also a few limitations that need to be considered. First, this is a cross-sectional study, which precludes causal inferences; the temporal pattern between the two variables of interest (burnout and cortisol) is not known. Second, burnout is a self-reported measure, which may introduce recall bias. Third, there is the potential for measurement error in the cortisol if participants didn’t comply with the collection protocol. Furthermore, generalizability is limited to police departments of similar size and geographic area.
4.2. Conclusions
The results from our study indicate that in this group of urban police officers, exhaustion may be associated with a dysregulated awakening profile, while both depersonalization and exhaustion may be associated with diminished cortisol secretion across the day. Besides the negative consequences of police burnout, due to cortisol’s importance in multiple biological functions throughout the body, dysregulated cortisol may have serious implications in a number of biological and psychological systems for these officers; affecting their mental and physical health and their ability to effectively work with the public. Further research evaluating the temporal relationship between burnout and cortisol and whether addressing burnout normalizes cortisol would be of interest.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by grants from the National Institute for Occupational Safety and Health (NIOSH) [1R01OH009640-01A1] and [1R01OH010807-01].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpnec.2020.100016.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Marmar C.R., McCaslin S.E., Metzler T.J., Best S., Weiss D.S., Fagan J., Liberman A., Pole N., Otte C., Yehuda R., Mohr D., Neylan T. Predictors of posttraumatic stress in police and other first responders. Ann. N. Y. Acad. Sci. 2006;1071:1–18. doi: 10.1196/annals.1364.001. [DOI] [PubMed] [Google Scholar]
- 2.Kim M.J., Loucks R.A., Palmer A.L., Brown A.C., Solomon K.M., Marchante A.N., Whalen P.J. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioral Brain Research. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Violanti J.M. Charles C. Thomas, Publisher, LTD; Springfield, IL: 2014. Dying for the Job: Police Work, Exposure and Heatlh. [Google Scholar]
- 4.Mona G.G., Chimbari M.J., Hongoro C. A systematic review on occupational hazards, injuries and diseases among police officers worldwide: policy implications for the South African Police Service. J. Occup. Med. Toxicol. 2019;14:2. doi: 10.1186/s12995-018-0221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltran C.A., Moreno M.P., Estrada J.G.S., Lopez T.M.T., Rodriguez M.G.A. Social support, burnout syndrome and occupational exhaustion among Mexican traffic police agents. Spanish J. Psychol. 2009;12:585–592. doi: 10.1017/s1138741600001955. [DOI] [PubMed] [Google Scholar]
- 6.Carlier I.V., Lamberts R.D., Gersons B.P. Risk factors for posttraumatic stress symptomatology in police officers: a prospective analysis. J. Nerv. Ment. Dis. 1997;185:498–506. doi: 10.1097/00005053-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Husain W., Sajjad R., ur Rehman A. Depression, anxiety and stress among female and male police officers. Pakistan Journal of Clinical Psychology. 2014;13:13–14. [Google Scholar]
- 8.Kula S. Occupational stress, supervisor support, job satisfaction, and work-related burnout: perceptions ofTurkish national police (TNP) members. Police Pract. Res. 2017;18:146–159. [Google Scholar]
- 9.Maran D.A., Varettoc A., Zedda M., Ieraci V. Occupational stress, anxiety and coping strategies in police officers. Occup. Med. 2015;65:466–473. doi: 10.1093/occmed/kqv060. [DOI] [PubMed] [Google Scholar]
- 10.McCarty W.P., Aldirawi H., Dewald S., Palacios M. Burnout in blue: an analysis of the extent and primary predictors of burnout among law enforcement officers in the United States. Police Q. 2019;22:278–304. [Google Scholar]
- 11.Maslach C., Jackson S.E. The measurement of experienced burnout. Journal of Occupational Behavior. 1981;2:99–113. [Google Scholar]
- 12.Malach-Pines A., Keinan G. Stress and burnout in Israeli police officers during a Palestinian uprising (Intifada) Int. J. Stress Manag. 2007;14:160–174. [Google Scholar]
- 13.Pines A.M., Keinan G. Stress and burnout: the significant difference. Pers. Indiv. Differ. 2005;39:625–635. [Google Scholar]
- 14.Talavera-Velasco B., Luceno-Moreno L., Martin-Garcia J., Garcia-Albuerne Y. Psychosocial risk factors, burnout and hardy personality as variables associated with mental health in police officers. Front. Psychol. 2018;9:1478. doi: 10.3389/fpsyg.2018.01478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarty W.P., Skogan W.G. Job-related burnout among civilian and sworn police personnel. Police Q. 2012;16:66–84. [Google Scholar]
- 16.Chida Y., Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol. Psychol. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Marchand A., Juster R.P., Durand P., Lupien S.J. Burnout symptom sub-types and cortisol profiles: what’s burning most? Psychoneuroendocrinology. 2014;40:27–36. doi: 10.1016/j.psyneuen.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Clow A., Hucklebridge F., Stalder T., Evans P., Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neurosci. Biobehav. Rev. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Spencer R.L., Deak T. A users guide to HPA axis research. Physiol. Behav. 2017;178:43–65. doi: 10.1016/j.physbeh.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adam E.K., Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Pruessner J.C., Wolf O.T., Hellhammer D.H., Buske-Kirschbaum A., von Auer K., Jobst S., Kaspers F., Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- 22.Herman J.P., McKlveen J.M., Ghosal S., Kopp B., Wulsin A., Makinson R., Scheimann J., Myers B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comp. Physiol. 2016;6:603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adam E.K., Quinn M.E., Tavernier R., McQuillan M.T., Dahlke K.A., Gilbert K.E. Diurnal cortisol slopes and mental and physical health outcomes: a systematic review and meta-analysis. Psychoneuroendocrinology. 2017;83:25–41. doi: 10.1016/j.psyneuen.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hannibal K.E., Bishop M.D. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 2014;94:1816–1825. doi: 10.2522/ptj.20130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchand A., Durand P., Juster R.P., Lupien S.J. Workers’ psychological distress, depression, and burnout symptoms: associations with diurnal cortisol profiles. Scand. J. Work. Environ. Health. 2014;40:305–314. doi: 10.5271/sjweh.3417. [DOI] [PubMed] [Google Scholar]
- 26.Rothe N., Steffen J., Penz M., Kirschbaum C., Walther A. Examination of peripheral basal and reactive cortisol levels in major depressive disorder and the burnout syndrome: a systematic review. Neurosci. Biobehav. Rev. 2020;114:232–270. doi: 10.1016/j.neubiorev.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 27.Danhof-Pont M.B., van Veen T., Zitman F.G. Biomarkers in burnout: a systematic review. J. Psychosom. Res. 2011;70:505–524. doi: 10.1016/j.jpsychores.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Sanchez J.C., Perez-Marmol J.M., Blasquez A., Santos-Ruiz A.M., Peralta-Ramirez M.I. Association between burnout and cortisol secretion, perceived stress, and psychopathology in palliative care unit health professionals. Palliat. Support Care. 2018;16:286–297. doi: 10.1017/S1478951517000244. [DOI] [PubMed] [Google Scholar]
- 29.Wingenfeld K., Schulz M., Damkroeger A., Rose M., Driessen M. Elevated diurnal salivary cortisol in nurses is associated with burnout but not with vital exhaustion. Psychoneuroendocrinology. 2009;34:1144–1151. doi: 10.1016/j.psyneuen.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Grossi G., Theorell T., Jurisoo M., Setterlind S. Psychophysiological correlates of organizational change and threat of unemployment among police inspectors. Integr. Physiol. Behav. Sci. 1999;34:30–42. doi: 10.1007/BF02688708. [DOI] [PubMed] [Google Scholar]
- 31.SAS Institute Inc . SAS Institute Inc.; Cary, NC: 2008. SAS/STAT® 9.2 User’s Guide. [Google Scholar]
- 32.Jonsdottir I.H., Sjors Dahlman A. Mechanisms IN endocrinology: endocrine and immunological aspects of burnout: a narrative review. Eur. J. Endocrinol. 2019;180:R147–R158. doi: 10.1530/EJE-18-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mommersteeg P.M., Keijsers G.P., Heijnen C.J., Verbraak M.J., van Doornen L.J. Cortisol deviations in people with burnout before and after psychotherapy: a pilot study. Health Psychol. 2006;25:243–248. doi: 10.1037/0278-6133.25.2.243. [DOI] [PubMed] [Google Scholar]
- 34.Oosterholt B.G., Maes J.H.R., Van der Linden D., Verbraak M., Kompier M.A.J. Getting better, but not well: a 1.5 year follow-up of cognitive performance and cortisol levels in clinical and non-Clinical burnout. Biol. Psychol. 2016;117:89–99. doi: 10.1016/j.biopsycho.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 35.De Vente W., Olff M., Van Amsterdam J.G., Kamphuis J.H., Emmelkamp P.M. Physiological differences between burnout patients and healthy controls: blood pressure, heart rate, and cortisol responses. Occup. Environ. Med. 2003;60(Suppl 1):i54–61. doi: 10.1136/oem.60.suppl_1.i54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oosterholt B.G., Maes J.H.R., Van der Linden D., Verbraak M., Kompier M.A.J. Burnout and cortisol: evidence for a lower cortisol awakening response in both clinical and non-clinical burnout. J. Psychosom. Res. 2015;78:445–451. doi: 10.1016/j.jpsychores.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Pruessner J.C., Hellhammer D.H., Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom. Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Bellingrath S., Weigl T., Kudielka B.M. Cortisol dysregulation in school teachers in relation to burnout, vital exhaustion, and effort-reward-imbalance. Biol. Psychol. 2008;78:104–113. doi: 10.1016/j.biopsycho.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Langelaan S., Bakker A.B., Schaufeli W.B., van Rhenen W., van Doornen L.J. Do burned-out and work-engaged employees differ in the functioning of the hypothalamic-pituitary-adrenal axis? Scand. J. Work. Environ. Health. 2006;32:339–348. doi: 10.5271/sjweh.1029. [DOI] [PubMed] [Google Scholar]
- 40.Mommersteeg P.M., Heijnen C.J., Verbraak M.J., van Doornen L.J. Clinical burnout is not reflected in the cortisol awakening response, the day-curve or the response to a low-dose dexamethasone suppression test. Psychoneuroendocrinology. 2006;31:216–225. doi: 10.1016/j.psyneuen.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt-Reinwald A., Pruessner J.C., Hellhammer D.H., Federenko I., Rohleder N., Schurmeyer T.H., Kirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci. 1999;64:1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- 42.Moch S.L., Panz V.R., Joffe B.I., Havlik I., Moch J.D. Longitudinal changes in pituitary-adrenal hormones in South African women with burnout. Endocrine. 2003;21:267–272. doi: 10.1385/ENDO:21:3:267. [DOI] [PubMed] [Google Scholar]
- 43.Penz M., Stalder T., Miller R., Ludwig V.M., Kanthak M.K., Kirschbaum C. Hair cortisol as a biological marker for burnout symptomatology. Psychoneuroendocrinology. 2018;87:218–221. doi: 10.1016/j.psyneuen.2017.07.485. [DOI] [PubMed] [Google Scholar]
- 44.Parent-Lamarche A., Marchand A. Work stress, personality traits, and cortisol secretion: testing a model for job burnout. Work. 2018;60:485–497. doi: 10.3233/WOR-182755. [DOI] [PubMed] [Google Scholar]
- 45.Stalder T., Kirschbaum C., Kudielka B.M., Adam E.K., Pruessner J.C., Wust S., Dockray S., Smyth N., Evans P., Hellhammer D.H., Miller R., Wetherell M.A., Lupien S.J., Clow A. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.