Abstract
Terrestrial carbon cycling is largely mediated by soil food webs. Identifying the carbon source for soil animals has been desired to distinguish their roles in carbon cycling, but it is challenging for small invertebrates at low trophic levels because of methodological limitations. Here, we combined radiocarbon (14C) analysis with stable isotope analyses (13C and 15N) to understand feeding habits of soil microarthropods, especially focusing on springtail (Collembola). Most Collembola species exhibited lower Δ14C values than litter regardless of their δ13C and δ15N signatures, indicating their dependence on young carbon. In contrast with general patterns across all taxonomic groups, we found a significant negative correlation between δ15N and Δ14C values among the edaphic Collembola. This means that the species with higher δ15N values depend on C from more recent photosynthate, which suggests that soil-dwelling species generally feed on mycorrhizae to obtain root-derived C. Many predatory taxa exhibited higher Δ14C values than Collembola but lower than litter, indicating non-negligible effects of collembolan feeding habits on the soil food web. Our study demonstrated the usefulness of radiocarbon analysis, which can untangle the confounding factors that change collembolan δ15N values, clarify animal feeding habits and define the roles of organisms in soil food webs.
Keywords: Collembola, isotope analysis, root-derived carbon, soil fauna, terrestrial carbon cycle
1. Introduction
In terrestrial ecosystems, soil provides key ecosystem functions and services related to litter decomposition [1]. Decomposition is mainly driven by soil microorganisms and invertebrate animals via their transfers of energy and materials through the food web [2,3]. Thus, how to identify the food sources of these organisms has been a central theme for soil ecologists to understand their roles in the ecosystem. Despite difficulties inherent in the complexity of the soil matrix and limitations of conventional methods (e.g. gut content analysis and laboratory food choice experiments), stable isotope signatures have shown significant progress as a powerful tool [4,5]. The use of stable isotopes has revealed hidden trophic structures and trophic links in the soil. These studies provided evidence that food sources vary widely among organisms and include living plants and photoautotrophic microorganisms (e.g. cyanobacteria and algae) as well as decayed materials and related saprotrophic microorganisms [6–8]. Especially, studies using isotope tracers have revealed the underestimated importance of carbon inputs from living roots into the soil food web [9–12] and have raised questions over whether soil food webs are really based primarily on detritus.
Recently, an isotopic map that summarizes food sources and their effects on the bulk stable isotope values (δ13C and δ15N) of soil animals was proposed [13]. This map covers most of the variation in soil animal food sources and enables us to estimate the primary food source of each animal taxon based on natural variations in δ13C and δ15N values. However, the interpretation of isotope values partly remains ambiguous because multiple factors alter isotope values in the same directions [14–16]. 15N enrichment is a particular problem, since we cannot accurately distinguish between mycorrhizal effects and isotope enrichment by microbial decay processes, even for non-predatory taxa [13]. Because feeding on mycorrhiza could be a highly significant pathway for carbon input from living roots [15,17], the dependence of soil organisms on mycorrhiza or microbially decayed materials can lead to quite different consequences for their ecosystem functioning. Furthermore, assessing the degree of dependence on living roots is difficult. Soil animals, including many omnivores, can use both living and dead materials [18–20]. Most studies that assessed carbon inputs from living roots have used isotope tracers [10–12], which usually cannot be done simultaneously with the measurement of natural variations of the same isotope (but see [9]). Thus, we still cannot specify animal feeding habits directly related to their ecosystem functioning only from the δ13C and δ15N isotopic map.
To resolve that difficulty, we used radiocarbon (14C) analysis to add information on the carbon age in animals into the biplot of δ13C and δ15N. The radiocarbon technique provides in situ information about the age (i.e. the time elapsed after photosynthesis) of assimilated carbon, which can distinguish taxa dominated by carbon that is younger than litter from taxa that depend more on carbon from the litter and humus. This technique is based on the atmospheric 14CO2 peak in the early 1960s (the ‘bomb peak’) that resulted from nuclear bomb testing and the 1963 Nuclear Test Ban treaty. The 14C content of atmospheric CO2 has continued to decline since this peak, and this decay curve is reflected in the 14C content of photosynthate carbon. 14C analysis has been used to estimate the feeding habits of some soil animals, such as termites [21–24], ants [23,25], earthworms [24,26,27] and enchytraeids [28]. We can estimate the diet ages from the 14C contents in most soil animals, with exceptions for several wood-feeding termites, by using the decay curve since the peak in atmospheric 14CO2 [22]. In this study, we conducted the first trial that has used 14C to assess the feeding habits of soil microarthropods, especially focusing on Collembola. Given that Collembola are the primary prey of many soil animals at higher trophic positions, their carbon sources will clearly affect the whole soil food web [2,29]. We assessed animal feeding habits by revealing the relationships between the age of assimilated carbon and the stable isotope values for multiple collembolan species and other mesofaunal taxa. We then assessed the utility of the multi-dimensional isotopic map that includes 13C, 15N and 14C signatures.
2. Material and methods
The research site was a warm temperate natural forest of Japanese cypress (Chamaecyparis obtusa), one of the major species for timber production in Japan, at the Kamigamo Experimental Forest Station of Kyoto University, Japan (35°04′ N, 135°43′ E) [30]. The vegetation consisted of a canopy layer of C. obtusa and an understory of shrubs, such as Cleyera japonica, Eurya japonica and C. obtusa saplings. The soil has a moder humus with an organic layer (A0) 3 to 5 cm thick above a poorly developed A horizon that was 1 to 2 cm thick and BC horizons. In the A0 layer, densely distributed fine roots of C. obtusa formed a root mat. More than 30 papers have been published about the ecology of the soil microarthropod community, including its interactions with C. obtusa roots, at this research site (e.g. [31,32]).
We collected samples of the soil organic layer, including soil animals and C. obtusa fine roots, at monthly intervals from November 2018 to March 2019. Soil animals were extracted alive into deionized water using Tullgren funnels at 35°C. The extracted animals were stored in a fridge at 4°C and immediately identified and divided into taxonomic groups under a stereomicroscope to prevent decay. Most Collembola were grouped at the species level, whereas mites were grouped at the sub-order level and other animals were combined at the order or class level (table 1). These samples were stored frozen at –20°C and then freeze-dried for isotope analyses. Fine roots that were 1 mm in diameter or less and the first-order roots (i.e. root tips) were removed from the soil samples and ground into powder using an agate mortar. The soil organic layer was separated into the litter (L) layer and the fragmented litter and humus layer (i.e. the FH layer), and each layer (excluding roots) was ground into a powder using a ball mill. We obtained three samples of the dominant collembolan species (Folsomia octoculata, Tetracanthella sylvatica and Tomocerus varius): sample 1 in November and December 2018, sample 2 in January and February 2019, and sample 3 in March 2019. We combined the samples of the other taxa to obtain enough material (more than 2 mg) to permit the three isotopic analyses (i.e. δ13C, δ15N and Δ14C). For the substrates (i.e. roots and litter), we took small random samples from each harvesting date and combined them, then dried them at 70°C for 48 h. Details of isotopic analyses are in the electronic supplementary material, S1. We used Pearson's correlation coefficient (r) to analyse the relationships among pairs of the three isotopic signatures (i.e. δ13C, δ15N and Δ14C).
Table 1.
Soil animal taxa identified in this study. The vertical habitats of the collembolan species are based on studies by Takeda [33,34]. FH, fragmented litter and humus; L, litter.
| sample | ID | notes on family, order and class | habitat |
|---|---|---|---|
| Collembola | |||
| Folsomia octoculata | F_octoculata | Isotomidae | FH layer |
| Tetracanthella sylvatica | T_sylvatica | Isotomidae | L layer, but shows vertical migration |
| Isotoma carpenteri | I_carpenteri | Isotomidae | FH layer |
| Onychiurus flavescens | O_flavescens | Onychiuridae | FH layer |
| Onychiurus sibiricus | O_sibiricus | Onychiuridae | FH layer, but shows vertical migration |
| Oncopodura yosiiana | O_yosiiana | Oncopoduridae | FH layer |
| Tomocerus varius | T_varius | Tomoceridae | L layer |
| Tomocerus ocreatus | T_ocreatus | Tomoceridae | L layer |
| Entomobryidae | Entomobryidae | Entomobryidae: mix of Entomobrya spp., Lepidocyrtus spp., Homidia spp. and Sinella spp. | L layer |
| Neanurinae | Neanurinae | Neanuridae: mix of Neanura kitayamana, Vitronura pygmaea and Vitronura mandarina | L layer |
| Neanuroidea | Neanuroidea | Neanuridae, Odontellidae: mix of Friesia japonica, Pseudachorutes spp. and Superodontella sp.1 | L layer |
| trophic position | |||
| others | |||
| Prostigmata | Prostigmata | Acari | predator |
| Mesostigmata | Mesostigmata | Acari | predator |
| Oribatida | Oribatida | Acari | decomposer |
| Araneae | Araneae | Araneae | predator |
| Geophilomorpha | Geophilomorpha | Chilopoda | predator |
| Lithobiomorpha | Lithobiomorpha | Chilopoda | predator |
| Diplopoda_adult | Diplopoda_ad | Diplopoda | decomposer |
| Diplopoda_juvenile | Diplopoda_ju | Diplopoda | decomposer |
| Pseudoscorpiones | Pseudoscorpiones | Pseudoscorpiones | predator |
| Symphyla | Symphyla | Symphyla | decomposer |
3. Results
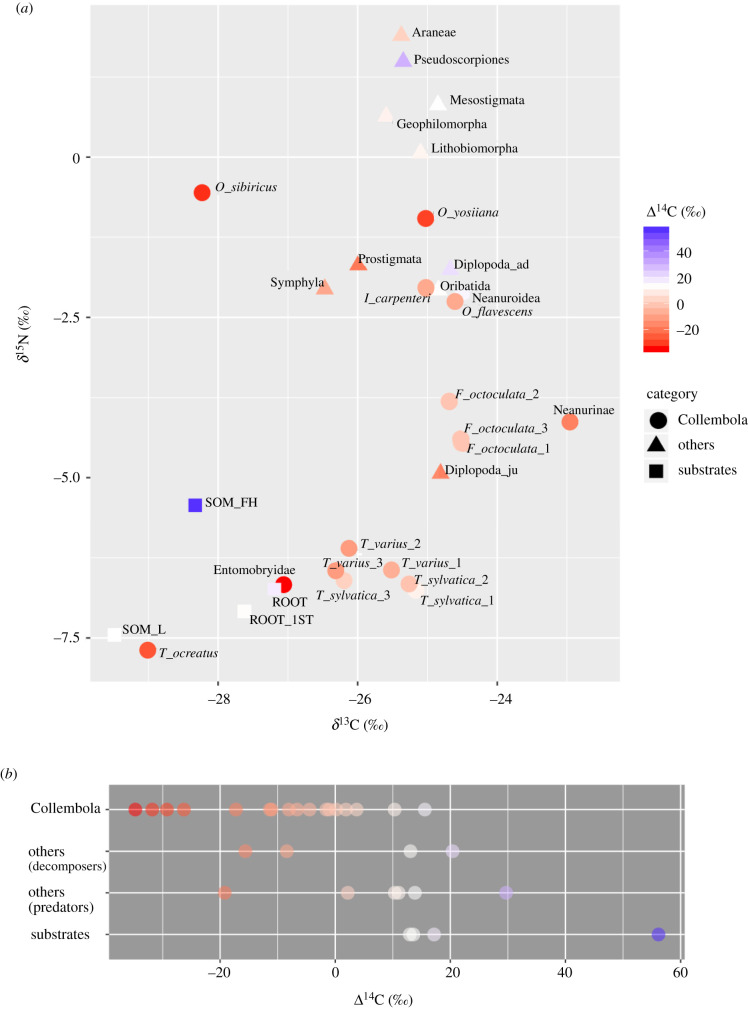
The δ13C and δ15N signatures of soil animal taxa spanned 6.1 and 9.6‰, respectively (figure 1a; electronic supplementary material, S2). The δ13C and δ15N signatures of the L layer were lower than those of most soil animals, except for the δ15N of Tomocerus ocreatus. The δ13C and δ15N signatures of soil organic matter in the FH layer were higher than those in the L layer but lower than those of most FH layer inhabitants. The δ13C signatures of the root substrates were higher than litter, whereas the δ15N signatures were between those in the L and FH layers. The δ13C and δ15N signatures were slightly lower in the first-order roots than in the fine roots.
Figure 1.
(a) Isotope values (i.e. δ13C, δ15N, Δ14C) of the soil fauna and of the litter and root substrates. Colours represent the Δ14C values: white indicates the Δ14C value for the litter layer (i.e. 13.6‰), with higher values as blue gradation and lower values as red gradation. The shape key is as follows: circles, Collembola; triangles, other fauna; squares, substrates. Abbreviations for soil fauna are shown in table 1; abbreviations for substrates are SOM_L for the litter (L) layer soil organic matter; SOM_FH for the FH layer; ROOT for the fine roots and ROOT_1ST for the first-order roots. (b) The ranges of Δ14C value for Collembola, other fauna and substrates. For other fauna, different trophic positions (i.e. decomposers and predators, table 1) are displayed separately. Each dot shows each sample. Colour representation is the same as figure 1a.
The Δ14C signatures of the soil animal taxa spanned 64.5‰, with values ranging from −34.8‰ for Entomobryidae to 29.7‰ for Pseudoscorpiones (figure 1a,b; electronic supplementary material, S2). The Δ14C signatures of the substrates showed a broad range from 13.0‰ for the first-order roots to 56.2‰ for soil organic matter in the FH layer. However, most Collembola (except for Neanuroidea) had a lower Δ14C value than the substrates. Based on the simulation calculated by Graven et al. [35], the atmospheric Δ14C value was declining and estimated to be about 5–10‰ at the global scale in 2019. Lower Δ14C values in soil microarthropods than the globally estimated values indicate that Δ14CO2 levels at our site were affected by local CO2 emissions derived from fossil fuels. Local variation in atmospheric 14CO2 has been widely reported and ascribed to local CO2 emissions derived from fossil fuel (Δ14C = −1000‰), which decreases the Δ14C values in the curve [36,37]. If we assume the lowest Δ14C signature of −34.8‰ for Entomobryidae to be the current photosynthate C at our site in 2019, 17.2‰ of Δ14C value in the tree fine roots is equivalent to the value of 12–13 year-aged C on the simulation curve [35]. The results are consistent with the findings that C in fine roots was aged 10 ± 1 years on average because fine roots are produced from stored non-structural carbohydrates [38].
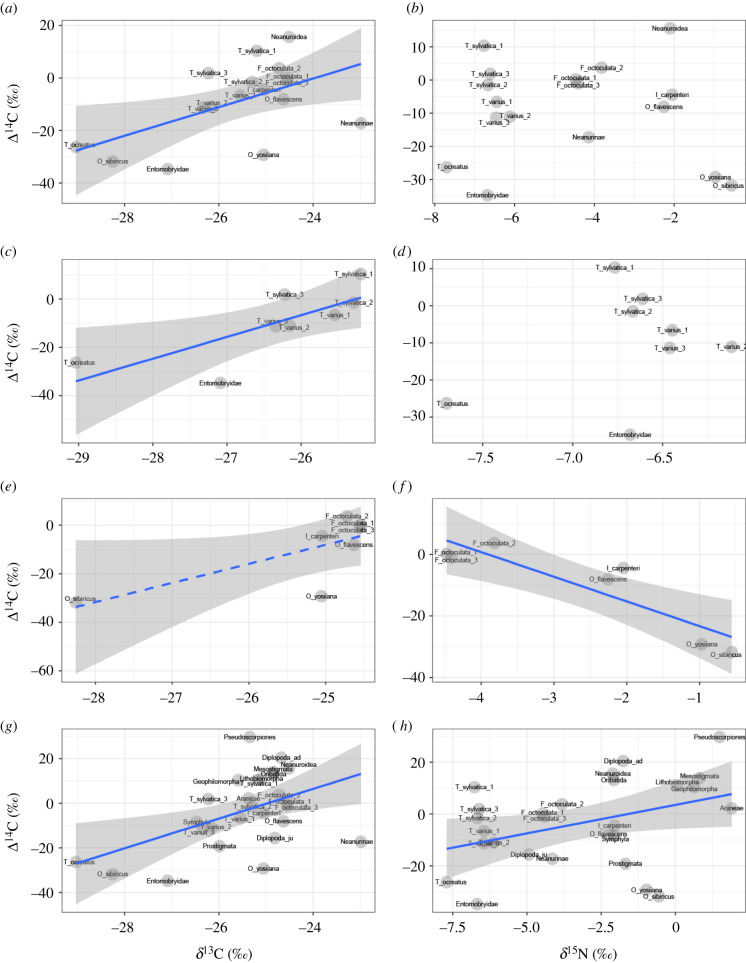
For all collembolan samples combined, Δ14C values were significantly positively correlated with δ13C values, but not with δ15N values (δ13C: r = 0.55, p < 0.05; δ15N: r = −0.09, p = 0.72, n = 17; figure 2a,b). We obtained similar results for the epigeic collembolan species (i.e. L-layer inhabitants), except for Neanurinae and Neanuroidea (which have blade-like mandibles); that is, Δ14C values were significantly positively correlated with δ13C values, but not with δ15N values (δ13C: r = 0.77, p < 0.05; δ15N: r = 0.33, p = 0.42, n = 8; figure 2c,d). By contrast, among the edaphic collembolan species (i.e. FH-layer inhabitants), the positive correlation between Δ14C and δ13C values was only marginally significant, but the Δ14C values were significantly negatively correlated with δ15N (δ13C: r = 0.73, p = 0.06; δ15N: r = −0.89, p < 0.01, n = 7; figure 2e,f). For all taxa combined, Δ14C values were significantly positively correlated with δ13C values and δ15N values (δ13C: r = 0.49, p < 0.01; δ15N: r = 0.39, p < 0.05, n = 27; figure 2g,h).
Figure 2.
Correlations between (a) δ13C and Δ14C and (b) δ15N and Δ14C for all collembolan species combined, between (c) δ13C and Δ14C and (d) δ15N and Δ14C for the epigeic collembolan species (i.e. L-layer inhabitants), between (e) δ13C and Δ14C and (f) δ15N and Δ14C for the edaphic collembolan species (i.e. FH-layer inhabitants), and between (g) δ13C and Δ14C and (h) δ15N and Δ14C for all taxa combined. Abbreviations for collembolan species are shown in table 1. Solid lines show significant relationships (p < 0.05), while a dashed line shows a marginally significant relationship (p < 0.1) between variables. Shaded areas represent 95% confidence intervals.
4. Discussion
Most Collembola showed lower Δ14C values than the litter, regardless of their δ13C and δ15N signatures. Only the Neanuroidea sample (which included Friesia japonica, which is known to be a carnivore [33]) had a higher Δ14C value than litter in the L layer. These results indicate that Collembola generally depend more on carbon younger than litter, such as carbon from living roots and algae. T. ocreatus, which is a litter surface dweller and exhibited lower δ15N than the litter, likely depends on algae as its main food source [6,39]. On the other hand, T. varius, F. octoculata and Onychiurus flavescens could depend on root-derived C as a main pathway for the younger carbon input, although each species showed different combinations of δ13C and δ15N values. These species all showed clear responses to recent photosynthate C in a 13CO2 pulse-labelling experiment using greenhouse pots with C. obtusa seedlings and soil collected from the same research site [11]. Differences in stable isotope signatures among these species could reflect differences in their habitat [14] (table 1), leading to differences in the foods they use in addition to root-derived C and the relative proportions of these multiple foods. The stable isotope signatures could also reflect which materials they feed on directly to obtain root-derived C; these include rhizodeposits such as mucilage, mycorrhizal fungi, microorganisms that propagated on the rhizodeposits, and the living root itself, although these sources are still controversial [8,17,40].
Positive correlations between δ15N and Δ14C values have often been reported for earthworms and termites [24,26,41]. This pattern has supported enrichment of their diet (i.e. soil organic matter) in 15N with humification by saprotrophic microorganisms, and this was often observed as a vertical isotopic gradient in the soil [4,14]. However, we did not find a similar relationship between the δ15N value and the carbon age for Collembola, likely because they feed more on microorganisms than on detritus. The significant positive correlation between δ13C and Δ14C values could be attributed to the same mechanism that is responsible for the soil 15N enrichment [21], since soil enrichment in heavier isotopes during the humification process has been reported for both 15N and 13C [13,23]. This supports the hypothesis that collembolan species with higher δ13C values could generally use more carbon derived from the litter and humus. By contrast, 15N enrichment by a mycorrhizal pathway [42–44] may obscure the relationship between δ15N and Δ14C values in Collembola. Both factors (i.e. microbial humification and mycorrhizae) can increase δ15N values with increasing soil depth [13,43], but have the opposite effect on Δ14C; that is, microbially humified soil exhibits a higher Δ14C value, whereas mycorrhizae have a lower value [45]. The significant negative correlation between δ15N and Δ14C values in the edaphic Collembola (i.e. FH-layer inhabitants; figure 2f) indicates that collembolan species with higher δ15N values depend more on recent photosynthate C, which means that the edaphic species generally feed on mycorrhizae to obtain root-derived C. For epigeic species, we cannot assess whether they feed on mycorrhiza or other materials such as microorganisms that are associated with rhizodeposits. Epigeic species may not feed on mycorrhiza from the point of their low δ15N values compared to mycorrhizal δ15N [40]. Whether Collembola have enough opportunities to encounter mycorrhizal mycelium in their living space could be critical in the first place, although it is likely influenced by the site-specific environment. Here, we can conclude that Collembola seem not to feed directly on fine roots at our coniferous forest site, because even the first-order roots had a much older carbon age than Collembola.
Predatory taxa with high δ15N values [13,46] mostly showed higher Δ14C values than Collembola, leading to the positive correlation between δ15N and Δ14C values across all taxa. Mesostigmata, Chilopoda and Pseudoscorpiones with high Δ14C values may also feed on other animals with higher Δ14C values than Collembola, such as Oribatida and adult Diplopoda. In addition to Oribatida and Diplopoda, which have been reported to feed mainly on litter or humus-derived C [11,17], animals we did not sample, such as Diptera larvae, could contribute to increasing the carbon age of predators [47]. Isotopic signatures of other animals should also be assessed at the species level in future studies. However, our findings that many predators, including one of the top predators, Araneae, exhibited a younger carbon age than the litter, indicate non-negligible effects of the feeding habits of Collembola on the whole soil food web. These results emphasize that the soil food web does not necessarily function exclusively as a brown food web derived from detritus [9].
In this study, we showed the utility of radiocarbon analysis, which can compensate for the effects of confounding factors that alter δ15N of Collembola (e.g. microbial humification and mycorrhizae). Although we should investigate the Δ14C values for potential food sources themselves (especially the sources made out of the current carbon) in the future, our findings provide an important step to improve the assessment of the feeding habits of soil animals and ecosystem functioning through their impacts on the food web.
Acknowledgements
We thank T. Kitajima for her help collecting collembolan samples under a stereomicroscope, Dr H. Kurokawa for her support regarding the experimental facility, and Dr K. Ozaki for the financial help. We also thank the staff of the Kamigamo Experimental Station, Field Science Education and Research Center, Kyoto University for their support.
Data accessibility
All data are provided in the electronic supplementary material [48].
Authors' contributions
S.F. conceived the ideas and collected samples for isotope analyses. S.F. and T.F.H. did isotope analyses under the supervision of I.T. S.F. analysed the data and wrote the manuscript with critical inputs from T.F.H and I.T. All authors were involved in revising the manuscript. All authors agree to be held accountable for the content therein and approve the final version of the manuscript.
Competing interests
We declare we have no competing interests
Funding
This study was supported by Japan Society for the Promotion of Science KAKENHI (grant nos. 18H06078, 19K21201 and 21H04784).
References
- 1.Wall DH. 2012Soil ecology and ecosystem services. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Coleman DC, Crossley DA Jr, Hendrix PF. 2004Fundamentals of soil ecology. Amsterdam, The Netherlands: Elsevier Academic Press. [Google Scholar]
- 3.Chapin FS, Matson PA, Vitousek PM. 2011Principles of terrestrial ecosystem ecology. New York, NY: Springer. [Google Scholar]
- 4.Tiunov AV III. 2007Stable isotopes of carbon and nitrogen in soil ecological studies. Biol. Bull. 34, 395-407. ( 10.1134/s1062359007040127) [DOI] [PubMed] [Google Scholar]
- 5.Hyodo F. 2015Use of stable carbon and nitrogen isotopes in insect trophic ecology. Entomol. Sci. 18, 295-312. ( 10.1111/ens.12128) [DOI] [Google Scholar]
- 6.Chahartaghi M, Langel R, Scheu S, Ruess L. 2005Feeding guilds in Collembola based on nitrogen stable isotope ratios. Soil Biol. Biochem. 37, 1718-1725. ( 10.1016/j.soilbio.2005.02.006) [DOI] [Google Scholar]
- 7.Schmidt O, Dyckmans J, Schrader S. 2016Photoautotrophic microorganisms as a carbon source for temperate soil invertebrates. Biol. Lett. 12, 20150646. ( 10.1098/rsbl.2015.0646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endlweber K, Ruess L, Scheu S. 2009Collembola switch diet in presence of plant roots thereby functioning as herbivores. Soil Biol. Biochem. 41, 1151-1154. ( 10.1016/j.soilbio.2009.02.022) [DOI] [Google Scholar]
- 9.Pollierer MM, Langel R, Körner C, Maraun M, Scheu S. 2007The underestimated importance of belowground carbon input for forest soil animal food webs. Ecol. Lett. 10, 729-736. ( 10.1111/j.1461-0248.2007.01064.x) [DOI] [PubMed] [Google Scholar]
- 10.Ostle N, Briones MJI, Ineson P, Cole L, Staddon P, Sleep D. 2007Isotopic detection of recent photosynthate carbon flow into grassland rhizosphere fauna. Soil Biol. Biochem. 39, 768-777. ( 10.1016/j.soilbio.2006.09.025) [DOI] [Google Scholar]
- 11.Fujii S, Mori AS, Kominami Y, Tawa Y, Inagaki Y, Takanashi S, Takeda H. 2016Differential utilization of root-derived carbon among collembolan species. Pedobiologia 59, 225-227. ( 10.1016/j.pedobi.2016.05.001) [DOI] [Google Scholar]
- 12.Potapov AM, Goncharov AA, Tsurikov SM, Tully T, Tiunov AV. 2016Assimilation of plant-derived freshly fixed carbon by soil collembolans: not only via roots? Pedobiologia 59, 189-193. ( 10.1016/j.pedobi.2016.07.002) [DOI] [Google Scholar]
- 13.Potapov AM, Tiunov AV, Scheu S. 2019Uncovering trophic positions and food resources of soil animals using bulk natural stable isotope composition. Biol. Rev. Camb. Phil. Soc. 94, 37-59. ( 10.1111/brv.12434) [DOI] [PubMed] [Google Scholar]
- 14.Hishi T, Hyodo F, Saitoh S, Takeda H. 2007The feeding habits of collembola along decomposition gradients using stable carbon and nitrogen isotope analyses. Soil Biol. Biochem. 39, 1820-1823. ( 10.1016/j.soilbio.2007.01.028) [DOI] [Google Scholar]
- 15.Pollierer MM, Langel R, Scheu S, Maraun M. 2009Compartmentalization of the soil animal food web as indicated by dual analysis of stable isotope ratios (15N/14N and 13C/12C). Soil Biol. Biochem. 41, 1221-1226. ( 10.1016/j.soilbio.2009.03.002) [DOI] [Google Scholar]
- 16.Pollierer MM, Scheu S, Tiunov AV. 2020Isotope analyses of amino acids in fungi and fungal feeding Diptera larvae allow differentiating ectomycorrhizal and saprotrophic fungi-based food chains. Funct. Ecol. 34, 2375-2388. ( 10.1111/1365-2435.13654) [DOI] [Google Scholar]
- 17.Pollierer MM, Dyckmans J, Scheu S, Haubert D, Treseder K. 2012Carbon flux through fungi and bacteria into the forest soil animal food web as indicated by compound-specific 13C fatty acid analysis. Funct. Ecol. 26, 978-990. ( 10.1111/j.1365-2435.2012.02005.x) [DOI] [Google Scholar]
- 18.Verhoef H, Prast J, Verweij R. 1988Relative importance of fungi and algae in the diet and nitrogen nutrition of Orchesella cincta (L.) and Tomocerus minor (Lubbock) (Collembola). Funct. Ecol. 2, 195-201. ( 10.2307/2389695) [DOI] [Google Scholar]
- 19.Scheu S, Folger M. 2004Single and mixed diets in Collembola: effects on reproduction and stable isotope fractionation. Funct. Ecol. 18, 94-102. ( 10.1046/j.0269-8463.2004.00807.x) [DOI] [Google Scholar]
- 20.Digel C, Curtsdotter A, Riede J, Klarner B, Brose U. 2014Unravelling the complex structure of forest soil food webs: higher omnivory and more trophic levels. Oikos 123, 1157-1172. ( 10.1111/oik.00865) [DOI] [Google Scholar]
- 21.Tayasu I, Nakamura T, Oda H, Hyodo F, Takematsu Y, Abe T. 2002Termite ecology in a dry evergreen forest in Thailand in terms of stable (δ13C and δ15N) and radio (14C, 137Cs and 210Pb) isotopes. Ecol. Res. 17, 195-206. ( 10.1046/j.1440-1703.2002.00479.x) [DOI] [Google Scholar]
- 22.Hyodo F, Tayasu I, Wada E. 2006Estimation of the longevity of C in terrestrial detrital food webs using radiocarbon (14C): how old are diets in termites? Funct. Ecol. 20, 385-393. ( 10.1111/j.1365-2435.2006.01081.x) [DOI] [Google Scholar]
- 23.Hyodo F, Matsumoto T, Takematsu Y, Itioka T, Harwood J. 2015Dependence of diverse consumers on detritus in a tropical rain forest food web as revealed by radiocarbon analysis. Funct. Ecol. 29, 423-429. ( 10.1111/1365-2435.12357) [DOI] [Google Scholar]
- 24.Hyodo F, Tayasu I, Konaté S, Tondoh JE, Lavelle P, Wada E. 2008Gradual enrichment of 15N with humification of diets in a below-ground food web: relationship between 15N and diet age determined using 14C. Funct. Ecol. 22, 516-522. ( 10.1111/j.1365-2435.2008.01386.x) [DOI] [Google Scholar]
- 25.Tanaka HO, Haraguchi TF, Tayasu I, Hyodo F. 2018Stable and radio-isotopic signatures reveal how the feeding habits of ants respond to natural secondary succession in a cool-temperate forest. Insectes Soc. 66, 37-46. ( 10.1007/s00040-018-0665-0) [DOI] [Google Scholar]
- 26.Briones MJI, Garnett MH, Piearce TG. 2005Earthworm ecological groupings based on 14C analysis. Soil Biol. Biochem. 37, 2145-2149. ( 10.1016/j.soilbio.2005.03.001) [DOI] [Google Scholar]
- 27.Hyodo F, Uchida T, Kaneko N, Tayasu I. 2012Use of radiocarbon to estimate diet ages of earthworms across different climate regions. Appl. Soil. Ecol. 62, 178-183. ( 10.1016/j.apsoil.2012.09.014) [DOI] [Google Scholar]
- 28.Briones MAJI, Ineson P. 2002Use of 14C carbon dating to determine feeding behaviour of enchytraeids. Soil Biol. Biochem. 34, 881-884. ( 10.1016/S0038-0717(02)00010-X) [DOI] [Google Scholar]
- 29.Potapov A, et al. 2020Towards a global synthesis of Collembola knowledge: challenges and potential solutions. Soil Organisms 92, 161-188. ( 10.25674/so92iss3pp161) [DOI] [Google Scholar]
- 30.Nakagawa H, et al. 2020Long-term monthly climate data at the forest stations of Kyoto University. Ecol. Res. 35, 733-741. ( 10.1111/1440-1703.12116) [DOI] [Google Scholar]
- 31.Fujii S, Saitoh S, Takeda H. 2014Effects of rhizospheres on the community composition of Collembola in a temperate forest. Appl. Soil. Ecol. 83, 109-115. ( 10.1016/j.apsoil.2014.03.018) [DOI] [Google Scholar]
- 32.Hishi T, Takeda H. 2008Soil microarthropods alter the growth and morphology of fungi and fine roots of Chamaecyparis obtusa. Pedobiologia 52, 97-110. ( 10.1016/j.pedobi.2008.04.003) [DOI] [Google Scholar]
- 33.Takeda H. 1987Dynamics and maintenance of collembolan community structure in a forest soil ecosystem. Res. Popul. Ecol. 29, 291-346. ( 10.1007/BF02538892) [DOI] [Google Scholar]
- 34.Takeda H. 1978Ecological studies of collembolan population in a pine forest soil II. Vertical distribution of Collembola. Pedobiologia 18, 22-30. [Google Scholar]
- 35.Graven H, Keeling RF, Rogelj J. 2020Changes to carbon isotopes in atmospheric CO2 over the industrial era and into the future. Global Biogeochem. Cycles 34, e2019GB006170. ( 10.1029/2019GB006170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibata S, Kawano E, Nakabayashi T. 2005Atmospheric 14C CO2 variations in Japan during 1982–1999 based on 14C measurements of rice grains. Appl. Radiat. Isot. 63, 285-290. ( 10.1016/j.apradiso.2005.03.011) [DOI] [PubMed] [Google Scholar]
- 37.Riley W, Hsueh D, Randerson J, Fischer M, Hatch J, Pataki D, Wang W, Goulden M. 2008Where do fossil fuel carbon dioxide emissions from California go? An analysis based on radiocarbon observations and an atmospheric transport model. J. Geophys. Res. Biogeosci. 113, G04002. ( 10.1029/2007jg000625) [DOI] [Google Scholar]
- 38.Solly EF, et al. 2018Unravelling the age of fine roots of temperate and boreal forests. Nat. Commun. 9, 3006. ( 10.1038/s41467-018-05460-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Potapov AM, Korotkevich AY, Tiunov AV. 2018Non-vascular plants as a food source for litter-dwelling Collembola: field evidence. Pedobiologia 66, 11-17. ( 10.1016/j.pedobi.2017.12.005) [DOI] [Google Scholar]
- 40.Potapov AM, Tiunov AV. 2016Stable isotope composition of mycophagous collembolans versus mycotrophic plants: do soil invertebrates feed on mycorrhizal fungi? Soil Biol. Biochem. 93, 115-118. ( 10.1016/j.soilbio.2015.11.001) [DOI] [Google Scholar]
- 41.Toyota A, Tayasu I, Fujimaki R, Kaneko N, Uchida M, Shibata Y, Hiura T. 2010Effects of vegetation switch and subsequent change in soil invertebrate composition on soil carbon accumulation patterns, revealed by radiocarbon concentrations. Radiocarbon 52, 1471-1486. ( 10.1017/S0033822200046567) [DOI] [Google Scholar]
- 42.Kohzu A, Yoshioka T, Ando T, Takahashi M, Koba K, Wada E. 1999Natural 13C and 15N abundance of field-collected fungi and their ecological implications. New Phytol. 144, 323-330. ( 10.1046/j.1469-8137.1999.00508.x) [DOI] [Google Scholar]
- 43.Wallander H, Goransson H, Rosengren U. 2004Production, standing biomass and natural abundance of 15N and 13C in ectomycorrhizal mycelia collected at different soil depths in two forest types. Oecologia 139, 89-97. ( 10.1007/s00442-003-1477-z) [DOI] [PubMed] [Google Scholar]
- 44.Högberg P, Högbom L, Schinkel H, Högberg M, Johannisson C, Wallmark H. 199615N abundance of surface soils, roots and mycorrhizas in profiles of European forest soils. Oecologia 108, 207-214. ( 10.1007/BF00334643) [DOI] [PubMed] [Google Scholar]
- 45.Hobbie EA, Weber NS, Trappe JM, Van Klinken GJ. 2002Using radiocarbon to determine the mycorrhizal status of fungi. New Phytol. 156, 129-136. ( 10.1046/j.1469-8137.2002.00496.x) [DOI] [Google Scholar]
- 46.Wada E, Mizutani H, Minagawa M. 1991The use of stable isotopes for food web analysis. Crit. Rev. Food Sci. Nutr. 30, 361-371. ( 10.1080/10408399109527547) [DOI] [PubMed] [Google Scholar]
- 47.Haraguchi TF, Uchida M, Shibata Y, Tayasu I. 2013Contributions of detrital subsidies to aboveground spiders during secondary succession, revealed by radiocarbon and stable isotope signatures. Oecologia 171, 935-944. ( 10.1007/s00442-012-2446-1) [DOI] [PubMed] [Google Scholar]
- 48.Fujii S, Haraguchi TF, Tayasu I. 2021Radiocarbon signature reveals that most springtails depend on carbon from living plants. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Fujii S, Haraguchi TF, Tayasu I. 2021Radiocarbon signature reveals that most springtails depend on carbon from living plants. Figshare. [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data are provided in the electronic supplementary material [48].